Introduction to neutron techniques for cultural heritage
Analytical Methods Committee, AMCTB No. 118
First published on 19th June 2025
Abstract
Since the early 2000s, neutron techniques have become available for the non-invasive characterisation of cultural heritage artefacts, especially those made of metal, ceramic or stone. This is thanks to the ability of neutrons to penetrate deeply into objects, to study their inner volume non-invasively. The neutron methods available can efficiently provide information on the composition and micro-structural properties of materials, along with 2D and 3D images of an artefact's internal structure, helping to answer questions related to manufacturing processes, state of degradation, authenticity and provenance. This Technical Brief presents an overview of the main neutron-based methods and the different types of neutrons suitable for the characterisation of materials of interest in cultural heritage and includes case studies highlighting the impact of these applications in archaeological and historical research.
1. Why neutrons?
Neutrons are an ideal probe for the scientific investigations of materials in many fields, including heritage science. In many respects, they can be used in a similar way to X-rays to produce images and plots reflecting the structure and composition of objects, but there are important differences.The advantage of neutrons is that they can penetrate deeply but non-invasively into an object and can interact strongly with some light atoms, such as hydrogen, unlike X-rays which only interact weakly. This is a useful property for the analysis of organic materials or the detection of moisture. Additionally, bulk elemental analysis can be performed using gamma-ray emission techniques following neutron irradiation.
Table 1 compares some of the properties of neutrons and X-rays, and a practical example involving a cultural heritage object is shown in Fig. 1.
| Element of comparison | X-rays | Neutrons |
|---|---|---|
| Interaction with | Electron shells of atoms | Nuclei of atoms |
| Interaction proportional to the mass of atoms | Yes | No |
| Sensitive to light elements | No | Yes (some) |
| Interference from surface corrosion/patina | Yes | No |
| Penetration through dense materials | No | Yes |
Neutron techniques can be applied to objects with complex shapes. The main requirements are that objects must fit in the instrument's experimental area and should not be so thick that they absorb all of the neutron beam – areas analysed can span several tens of centimetres, up to a few meters in the vertical direction, with thicknesses not exceeding 10 cm. For most materials, neutrons can access the inner volume of an object up to a thickness of several centimetres, even when corrosion layers or patinas are present on the surface.
Neutrons can reveal the inner structure, composition and microstructural properties of objects and can provide information about their state of conservation and method of manufacture (ref. 1 and references cited therein), without requiring sampling.
The main restriction on the use of these techniques in cultural heritage is that neutrons are only accessible at large-scale facilities, such as the ISIS Neutron and Muon Source in the UK, ILL (Institut Laue-Langevin) in France, SNS (Spallation Neutron Source) in the USA, J-PARC in Japan, etc., and operation requires highly specialised personnel. Usually, access to the facilities (‘beam-time’) is awarded by a panel. Access is free of charge for academic purposes, and in many cases funds to cover travel and accommodation are available. Non-academic institutions can apply alone or with an academic partner. Most neutron sources assign a beamline scientist to non-technical applicants, to help with the application and submission process. Portable Isotopic Neutron Spectroscopy (PINS), which enables on-site measurements by bringing a portable instrument directly to the field, has the potential to eliminate the need for transporting objects to large-scale facilities, and could be valuable for future cultural heritage applications.
2. Types of neutron interactions
The interactions of neutrons with the nuclei in the material under study depend on the structure of those nuclei, rather than (as with X-rays) their mass. Usually, elements close in atomic number Z (where Z is the number of protons) can be more easily distinguished with neutrons than with X-rays. Neutron techniques can also distinguish isotopes of the same element.Neutrons interact with materials via scattering – which can be elastic or inelastic – and via absorption (see Fig. 2 and 3).
2.1 Scattering
In a scattering interaction, a neutron hits the object and is scattered at a certain angle, which depends on how the atoms in the object itself are arranged. This neutron may or may not change its energy, in what is called inelastic or elastic scattering, respectively.The diffraction pattern obtained via neutron diffraction provides quantitative information on phase composition and other microstructural properties such as grain size, texture and residual stresses in the materials investigated. These properties can be unequivocally derived from the position, shape and intensity of the diffraction peaks. For example, in the case of metals, the technique provides clues on the efficiency of the smelting process, carburisation level, state of conservation, quantity of alloying elements and thermal and mechanical treatments applied during manufacturing and use, all leaving permanent features in the diffraction pattern.
Additionally, with small angle scattering, where the scattering angle is small with respect to common scattering (also called wide angle neutron scattering), it is possible to investigate the interface between ‘softer’ materials, such as painting surfaces and polymers, but also the porosity of objects, which has relevant applications in the study of ceramics.
Neutron diffraction from amorphous materials such as glass can also give information about the local interaction of atoms in the structure, in the absence of long-range order, as is the case of a crystalline structure.
2.2 Absorption
In an absorption interaction, a neutron is captured by a nucleus in the object under investigation and is therefore removed from the incident beam. This enables imaging applications, such as radiography and tomography (a three-dimensional image of the internal structure of a solid object that allows the visualisation of any 2D slice of it). Whilst its resolution is lower than that of X-ray tomography,3 it still provides better results with thick objects, or objects offering low contrast between different components, and for visualising hydrogen atoms (Fig. 1).Recently, a more advanced energy selective technique, NRTI (Neutron Resonance Transmission Imaging) has been gaining popularity. Based on the resonant neutron absorption reaction, NRTI combines sensitivity to elemental and isotopic composition with detailed morphological information and can provide quantitative isotope-selective 2D (radiography) and possibly 3D (tomography) elemental maps.1
After neutron capture, the resulting combined absorbed neutron and nucleus, referred to as a compound nucleus, is in an excited state, and must decay to a more stable state through a number of different processes, which includes the emission of γ-rays. These are fingerprints of elements/isotopes, making γ-spectroscopy following neutron capture an effective elemental analysis method, with added isotope sensitivity. The available techniques for elemental analysis with γ-spectroscopy are PGAA (Prompt Gamma Activation Analysis), NRCA (Neutron Resonance Capture Analysis) and NAA (Neutron Activation Analysis); the first two rely on γ-ray measurements during irradiation, the last on delayed γ-ray measurements after irradiation.1
3. How many types of neutrons are there?
Neutrons are loosely classified according to their energy (given in millielectronvolts – meV, or electron volts – eV), with different applications for each energy band. Only the lower three are useful for heritage applications: cold neutrons (neutron energy less than 25 meV) are suitable for imaging applications; thermal neutrons (neutron energy around 25 meV, up to 0.3 eV) are suitable for diffraction, small angle scattering and imaging; epithermal neutrons (or resonance region, neutron energy higher than 0.3 eV) are useful for elemental analysis and mapping with isotope sensitivity.4. Case studies
Diffraction, imaging and elemental analysis are the most established neutron-based techniques to address questions about the composition, state of conservation and production method of an artefact.2 These three case studies provide examples of practical cultural heritage applications.4.1 The Vittoria Alata of Brescia
The analysis of fragments from the Vittoria Alata,4 an archaeological bronze, showed that neutron diffraction can easily differentiate between superficial layers (due to corrosion, chemical treatment, gilding, silvering, etc.) and the bulk material. Where other elemental techniques overestimated the amount of tin due to surface corrosion and loss of copper (decuprication), neutron diffraction reliably derived the tin content of the original alloy.Fig. 4 shows the diffraction patterns obtained from two fragments. The peaks correspond to bronze, lead and cuprite (a corrosion product), and it is possible to quantify their relative amounts, thereby giving information on both the fragments' compositions and their state of conservation. Crucially, the signal from the original, intact copper alloy (bronze) is separate from that of the surface corrosion product (cuprite). The peaks' intensities reveal that the lead and cuprite contents vary in different areas of the statue. Also, the position of the peaks corresponding to the bronze phase is shifted, showing that the two fragments have different tin contents. Neutron imaging on other fragments revealed that one of them is most likely not original.
4.2 Wootz steel
Neutron diffraction is the only analytical non-destructive technique able to identify wootz steel,5 a type of high-carbon crucible steel with a distinctive pattern, developed in India during the mid-1st millennium BCE. The most interesting microstructural feature to identify wootz steel artefacts is the texture (the preferred orientation) present in the carbon-containing cementite phase, but not in the ferrite phase. In the case shown in Fig. 5, diffraction patterns collected at different scattering angles exhibit a strong change in intensity of the cementite peaks.Information from the carbon content, obtained from the ferrite/cementite ratio, and the texture (preferred orientation) of the cementite phase, indicated the temperature range at which the steel had been produced. For both of these reasons, neutron diffraction can also be used to discriminate between authentic and reproduction wootz steel.
4.3 Bimetallic Chinese swords
In one of the first examples of a non-destructive bulk elemental map, two ancient Chinese bimetallic swords were characterised6 in unprecedented detail using NRTI and ND. NRTI enabled the presence and distribution of iron and copper to be mapped.The main difference between the two swords was the assembly/manufacturing method and the composition. In one of the swords (Fig. 6), the iron blade extends deep into the bronze hilt, contrary to the other sword that seems to be built favouring aesthetics over functionality.
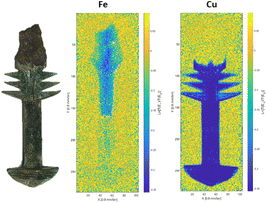 | ||
| Fig. 6 Fe and Cu distribution in a Chinese bimetallic sword fragment. The colour scale indicates the level of absorption – the darker the colour, the higher the absorption. | ||
Only iron could be detected in the blades, while the bronze hilts show the presence of secondary and trace elements. They both show similar amounts of copper and relevant traces of silver and antimony, but different amounts of tin and arsenic.
This Technical Brief was prepared by Antonella Scherillo (ISIS Neutron and Muon Source, STFC Rutherford Appleton Laboratory, UK) on behalf of the Heritage Science Expert Working Group of the Analytical Methods Committee and approved by the AMC on 15th May 2025.
References
-
Neutron Methods for Archaeology and Cultural Heritage, ed. N. Kardjilov and G. Festa, Springer, 2017, DOI:10.1007/978-3-319-33163-8
.
- Heritage Science Highlights, https://www.isis.stfc.ac.uk/Pages/Heritage-Science.aspx, access date 15 May 2025.
- Analytical Methods Committee, X-ray micro computed tomography in cultural heritage, AMC Technical Brief No. 98, Anal. Methods, 2020, 12, 4496–4500, 10.1039/d0ay90112a
.
- F. Cantini, A. Scherillo and A. Fedrigo,
et al., The Vittoria Alata from Brescia: a combined neutron techniques and SEM-EDS approach to the study of the alloy of a bronze Roman statue. 2023, J. Archaeol. Sci. Rep., 2023, 51, 104112, DOI:10.1016/j.jasrep.2023.104112
.
- F. Grazzi, E. Barzagli and A. Scherillo,
et al., Determination of the manufacturing methods of Indian swords through neutron diffraction, Microchem. J., 2016, 125, 273–278, DOI:10.1016/j.microc.2015.11.035
.
- A. Fedrigo, D. Raspino, F. Grazzi and A. Scherillo, An integrated approach between neutron diffraction and elemental imaging through neutron resonance transmission imaging: preliminary results on Chinese bimetallic sword fragments, J. Anal. At. Spectrom., 2019, 34(12), 2420–2427, 10.1039/c9ja00300b
.
| This journal is © The Royal Society of Chemistry 2025 |







