Open Access Article
Open Access ArticleAQbD-based development of a stability-indicating UHPLC-PDA-QDa method for triptorelin in parenteral formulations†
Jesús Alberto
Afonso Urich
 *ab,
Viktoria
Marko
b,
Anna
Fedorko
b and
Dalibor
Jeremic
c
*ab,
Viktoria
Marko
b,
Anna
Fedorko
b and
Dalibor
Jeremic
c
aInstitute of Process and Particle Engineering, Graz University of Technology, 8010 Graz, Austria. E-mail: afonsourich@student.tugraz.at; jesus.afonso@rcpe.at; Tel: +43-316-873-30988
bResearch Center Pharmaceutical Engineering GmbH, 8010 Graz, Austria. E-mail: viktoria.marko@rcpe.at; anna.fedorko@rcpe.at
cDepartment of Health Studies—Biomedical Science, FH JOANNEUM, 8020 Graz, Austria. E-mail: dalibor.jeremic@fh-joanneum.at
First published on 30th July 2025
Abstract
A robust, stability-indicating analytical method for the quantification of triptorelin in suspension formulations was developed using reverse-phase ultra (high)-performance liquid chromatography (RP-UHPLC) under the Analytical Quality by Design (AQbD) framework. This systematic, risk-based approach enabled the efficient identification and optimization of critical method parameters, reducing reliance on traditional trial-and-error procedures. Key variables such as column type, temperature, gradient profile, and organic modifier composition were evaluated. Optimal chromatographic conditions were achieved using a YMC Triart C18 column (50 × 2.1 mm, 1.9 μm) at 53.8 °C. The mobile phase consisted of 10 mM ammonium formate buffer (pH 5.0) as phase A and acetonitrile containing 0.1% formic acid as phase B. A short 5 minute gradient elution at 0.48 mL min−1, with UV detection at 280 nm, was applied. The method was subjected to forced degradation studies under hydrolytic (acidic and basic), oxidative, and thermal stress conditions to demonstrate its stability-indicating capability. These results support its overall suitability for routine quality control and regulatory applications in peptide drug analysis.
1. Introduction
Triptorelin, a synthetic decapeptide analog of gonadotropin-releasing hormone (GnRH), has established therapeutic significance in managing hormone-responsive conditions, including advanced prostate cancer, endometriosis, uterine fibroids, and central precocious puberty.1–8 Its pharmacological action involves sustained pituitary downregulation, leading to decreased secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), thereby inhibiting gonadal steroidogenesis.9,10 The complex physicochemical properties of peptide structures, such as triptorelin, susceptibility to hydrolysis and oxidation, and low dosage in injectable formulations, pose significant challenges for accurate quantification in quality control environments.11The molecular structure of triptorelin is depicted in Fig. 1. Its pKa values are approximately 9.49 for the acetate salt and 2.8 for the pamoate salt, reflecting differences in their ionization profiles.12,13
Current analytical methods for the quantification of triptorelin primarily involve high-performance liquid chromatography (HPLC) with ultraviolet (UV)14,15 or mass spectrometry (MS) detection.16–19 While HPLC-UV methods are widely used and generally effective, they typically involve longer run times and offer lower resolution and sensitivity compared to UPLC-based techniques.20–22 LC-MS/MS approaches provide high sensitivity and selectivity but require expensive instrumentation and complex sample preparation, and are therefore less practical for routine QC applications.23 Additionally, these traditional methods often lack a systematic development strategy that ensures robustness under varied conditions. In this context, no methodology developed in accordance with Analytical Quality by Design (AQbD) principles, as required by current regulatory guidelines, has been identified as suitable for pharmaceutical filling.24–27
Quality by Design (QbD) is a strategic framework endorsed by regulatory agencies such as the FDA and EMA for the development and manufacturing of pharmaceutical products (Yu, 2008). Its core objective is to design a product with an emphasis on predefined quality attributes to ensure consistent performance.28–31 In recent years, QbD principles have been increasingly adopted in analytical method development, leading to the evolution of AQbD, a framework aimed at designing robust analytical methods that reliably meet performance criteria.32–37
AQbD provides a knowledge-driven, risk-based, and cost-effective approach to analytical development, aligning well with the broader goals of regulatory flexibility and lifecycle management such as ICH Q8(R2), Q9, Q10, and Q14 guidelines27–30 and USP <1220>.38 The AQbD workflow parallels that of product QbD, as outlined in the ICH Q8 guideline,31 and culminates in the definition of a Method Operable Design Region (MODR), a multidimensional space within which a method remains robust and fit for purpose.37
A critical component of AQbD is the Analytical Target Profile (ATP), which defines the method's intended purpose and performance requirements, analogous to the Quality Target Product Profile (QTPP) in QbD.31,37 The ATP typically addresses regulatory expectations such as those outlined in ICH Q2(R2), including attributes like specificity, linearity, accuracy, precision, range, limit of detection (LOD), and limit of quantification (LOQ).39
Once the ATP is established, critical method attributes (CMAs) are identified alongside acceptance criteria and specifications.40 Through Quality Risk Management (QRM) and tools such as Design of Experiments (DoE), critical method parameters (CMPs) are evaluated for their impact on method performance.24,33,41 This systematic, data-driven approach facilitates the experimental linking of CMAs and CMPs and supports a better understanding of method variability.
Ultimately, analytical methods developed under the AQbD framework are more robust and less susceptible to out-of-trend (OOT) and out-of-specification (OOS) results, which can lead to improved regulatory compliance and operational efficiency.32,37,42 The integration of AQbD into method development is gaining traction in the pharmaceutical industry as part of broader initiatives in risk management, pharmaceutical development, and pharmaceutical quality systems.25,43,44
2. Materials and methods
2.1. Reagents and consumables
Acetonitrile (Reag. Ph. Eur., HPLC grade) was sourced from M&B Stricker Laborfachhandel GbR (Germany) and used for mobile phase and sample dilution. Formic acid (≥98%), nylon membrane filters (0.22 μm), and other filtration supplies were obtained from Carl Roth GmbH (Germany). Ammonium formate (≥99%, LC-MS grade) was provided by VWR Chemicals (USA). Sodium hydroxide pellets, hydrochloric acid (37%), and hydrogen peroxide (>30%) were supplied by Merck-Supelco (Germany). All samples were filtered using 0.22 μm nylon syringe filters from YETI Merz Brothers GmbH (Austria) before analysis.2.2. Standards, samples, and excipients
Triptorelin acetate (≥98%) from Sigma-Aldrich (USA) served as the reference standard. The test product, Pamorelin® LA (triptorelin pamoate, 11.25 mg/2 mL), was obtained from Ipsen Pharma Austria GmbH (Austria). Excipients included mannitol (Caesar & Loretz GmbH, Germany), sodium carboxymethyl cellulose and polysorbate 80 (Sigma-Aldrich), and Resomer® RG 653 H (Evonik, Germany). For method accuracy studies, the excipient composition of Trelstar®45 was used to simulate the formulation matrix (Table S1†). Although Pamorelin® LA was the product analyzed, it was pharmaceutically identical to Trelstar®, with both representing the same triptorelin pamoate formulation developed by Debiopharm. The distinction lies solely in regional licensing and branding: Pamorelin® is commercialized in the EU/Switzerland through partners such as Ipsen Pharma46,47 and Vifor Pharma,48 while Trelstar® is marketed in the US and Canada via Watson Pharmaceuticals.49,50 The use of Trelstar® excipient data was based on the availability of publicly disclosed composition, which accurately reflects the formulation characteristics of Pamorelin® LA.2.3. Equipment and software
A reversed-phase ultra (high)-performance liquid chromatograph (UHPLC) Acquity H-Class from Waters Corp. (Milford, CT, USA), paired with a photodiode array detector (PDA) and a single quadrupole detector (QDa), was employed for method development. The configuration for the QDa analysis included an MS scan range of 100–700 Da in negative mode, a probe temperature set at 600 °C, a cone voltage of 23 V, and capillary voltages of 0.8 kV for positive and 0.8 kV for negative modes. The system was controlled using Empower 3 software from Waters Corp. The pH adjustments were carried out with a FiveEasy™ pH meter from Mettler Toledo GmbH (Columbus, OH, USA). Water purification and filtration were achieved with a Triton lab water system from Envirofalk (Germany). The chromatographic columns used for column screening in the DoE included an Acquity HSS T3 (2.1 × 50 mm; 1.8 μm), Acquity BEH C18 (2.1 × 50 mm; 1.7 μm), and Cortecs Premier C18 from Waters Corp. (USA), along with a Triart C18 (2.1 × 50 mm; 1.9 μm) from YMC Europe GmbH (Germany).DoE evaluation and statistical analyses were conducted using Design-Expert® version 13 (Stat-Ease Inc., USA). The linearity evaluation was performed by JASP statistical software version 0.19.3 (University of Amsterdam, The Netherlands).
2.4. Analytical preparations
The mobile phase consisted of a 10 mM ammonium formate buffer, adjusted to pH 5.0 with diluted formic acid, used in combination with acetonitrile as the organic modifier. This buffer–organic mixture was applied in a gradient elution system. The diluent used for sample and standard preparation was a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) mixture of acetonitrile and water. A reference stock solution of triptorelin was prepared by dissolving 20.0 mg of triptorelin acetate in the diluent and diluting to 100 mL to yield a concentration of 200 μg mL−1, which was further diluted as required for analysis. For precision testing, Pamorelin® suspension (5.625 mg mL−1) was diluted by transferring 0.1 mL into a 10 mL volumetric flask, resulting in a final concentration of approximately 56 μg mL−1.
50 (v/v) mixture of acetonitrile and water. A reference stock solution of triptorelin was prepared by dissolving 20.0 mg of triptorelin acetate in the diluent and diluting to 100 mL to yield a concentration of 200 μg mL−1, which was further diluted as required for analysis. For precision testing, Pamorelin® suspension (5.625 mg mL−1) was diluted by transferring 0.1 mL into a 10 mL volumetric flask, resulting in a final concentration of approximately 56 μg mL−1.
2.5. Analytical method validation
• Acidic hydrolysis: treatment with 1.25 mL of 1 N hydrochloric acid (HCl) for 18 hours,
• Alkaline hydrolysis: exposure to 1.25 mL of 1 N sodium hydroxide (NaOH) for 1 hour,
• Oxidative stress: reaction with 0.7 mL of 30% hydrogen peroxide (H2O2) for 30 minutes,
• Thermal stress: heating at 65 °C in a steam bath for 18 hours.
Stress durations were determined based on preliminary experiments aimed at inducing measurable degradation without complete analyte breakdown. Following the exposure period, acid and base-stressed samples were neutralized using equimolar NaOH or HCl, respectively, to quench further degradation. All stressed solutions were then diluted with a 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 (v/v) acetonitrile–water mixture to a final test concentration equivalent to 100% of the nominal value.
50 (v/v) acetonitrile–water mixture to a final test concentration equivalent to 100% of the nominal value.
An unstressed standard solution was prepared under identical dilution conditions for comparison. Chromatograms of stressed samples were overlaid with that of the standard to visualize the formation and separation of degradation products. Peak purity was assessed using PDA detection, with a purity flag algorithm confirming the absence of co-eluting impurities in the main triptorelin peak. To support impurity identification, mass spectrometric data were also acquired using a QDa detector. While not capable of full structural elucidation, the QDa provided useful m/z data that, combined with the literature, allowed assignment of plausible degradation products. Together, PDA and QDa data offered a more comprehensive evaluation of peak identity and method specificity.
3. Results and discussion
3.1. Method development based on AQbD principles
Given triptorelin's complex peptide structure, susceptibility to hydrolysis, and low dosage strength, the method was designed to achieve high specificity, stability-indication, and compatibility with mass detection, while remaining efficient, reproducible and fast under routine use. Considering the peptide nature of triptorelin and its amphiphilic profile, reverse-phase UHPLC was selected as the preferred technique. This choice was driven by its enhanced resolution, shorter analysis time, and reduced solvent consumption. UHPLC, coupled not only with PDA detection but also with a QDa mass detector, provided orthogonal detection capabilities to support peak identity confirmation and impurity profiling. While QDa does not provide full structural elucidation, it offers valuable mass-based confirmation that enhances the method's stability-indicating performance. Overall, this integrated approach is better suited for routine Good Manufacturing Practices (GMP) workflows and robust regulatory compliance. At the foundation of the AQbD process, the ATP was established to define the method's intended purpose and performance criteria (Table 1). The ATP also incorporated stability-indicating capabilities to ensure method suitability during degradation studies and over the product shelf life.| ATP element | Target | Requirement reference |
|---|---|---|
| Chromatographic features | ||
| Tailing factor | 0.8–1.8 | 53,54 |
| Resolution | >2 | 54 |
| Capacity factor (k′) | >2 | 54 |
| Peak purity | Acceptable | 54,55 |
| Plate count | >20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
54 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| Validation parameters | ||
| Linearity and range | R 2 ≥ 0.995 | 39,54 |
| 70–130% of the test concentration | ||
| Specificity | Absence of interference | 39,54 |
| Accuracy | 95.0–105.0% recovery within the established range | 39,54 |
| Repeatability | RSD less than or equal to 2.0% | 39,54 |
| Intermediate precision | Complies with repeatability and is not significantly different | 39,54 |
| Robustness | Not statistically different | 27,39 |
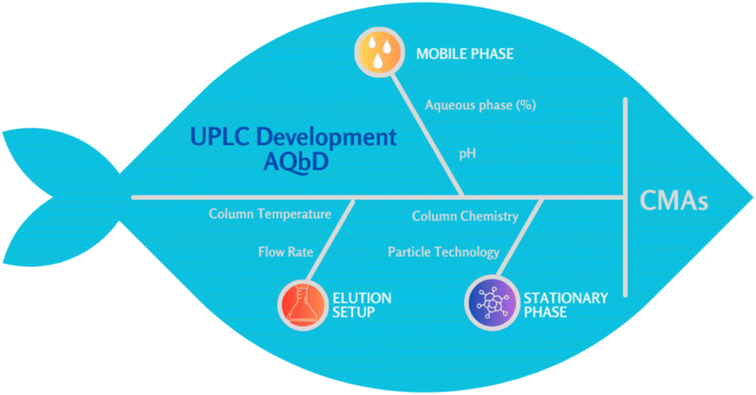
A QRM approach was used to identify the CMPs likely to influence the CMAs such as peak symmetry and chromatographic retention represented on k′. An Ishikawa (fishbone) diagram was developed to systematically visualize potential sources of method variability, including column chemistry, pH, aqueous percentage in the mobile phase gradient, and column temperature (Fig. 2).
To complement the Ishikawa diagram, a semi-quantitative risk matrix was constructed to prioritize method parameters based on their potential influence on CMAs; it is included in the ESI (Table S2).† Based on the results, a DoE was implemented to evaluate the influence of selected parameters on chromatographic performance. A Response Surface Methodology (RSM) was chosen, using an I-optimal design that allowed modeling of both the main effects and two-factor interactions. The study included 34 randomized runs to explore the influence of selected method parameters across a defined experimental space. The experimental factors investigated are displayed in Table 2.
| Chromatographic column | pH of the aqueous phase | Column temperature (°C) | Initial aqueous phase (%) | Flow (mL min−1) |
|---|---|---|---|---|
| a As fixed method parameters, all injections were performed with a 3 μL injection volume and UV detection at 280 nm. The gradient program for mobile phase B (acetonitrile) was set as follows: 20–30% at 0.0 min, increased to 45% at 2.0 min, returned to 20–30% at 2.1 min, and maintained until 5.0 min. The exact gradient range was adjusted based on the initial composition of the aqueous phase (Table 2). | ||||
| Waters BEH C18 | 4–6 | 45–55 | 70–80 | 0.49–0.51 |
| Waters Cortecs C18 | ||||
| Waters HSS T3 | ||||
| YMC Triart C18 | ||||
The developed method was assessed based on key chromatographic response variables, including tailing factor, capacity factor, and peak purity. Evaluation of peak purity was specifically conducted using degraded triptorelin acetate samples, as described in Section 2.5.2, to ensure the method's capability to distinguish the active pharmaceutical ingredient from potential degradation products. This approach verified the method's suitability as a stability-indicating procedure under forced degradation conditions.
In addition, two further responses were included. One was a binary separation indicator, assessing whether potential impurities were fully resolved (1) or not (0), instead of modeling chromatographic resolution directly. This response was evaluated via logistic regression which estimates the probability of successful impurity separation based on experimental conditions, offering a simplified yet effective selectivity criterion. The other variable was the overall sensitivity, which was treated as a numerical response and maximized during optimization, though it was not a formally defined quality attribute and therefore not subject to a specification limit.
The quality of the statistical model was assessed using a Fraction of Design Space (FDS) analysis. At an FDS of 0.8, the standard error of the mean was 0.709, indicating that the model provided reliable predictions across the majority of the design space. All continuous responses were evaluated using linear regression models with interaction terms, while the binary response was modeled using a logistic approach.
To identify the optimal method conditions that balanced all relevant responses, a desirability function-based optimization was conducted. Each response was transformed into an individual desirability value ranging from 0 (undesirable) to 1 (ideal), based on its predefined targets. These individual desirabilities were then combined into an overall desirability index using a geometric mean. This approach enabled simultaneous optimization across conflicting response goals.
The resulting desirability profiles (Fig. 3) revealed that initial gradient and column selection had the greatest impact on method performance, while pH, temperature, and flow rate showed relatively low influence—suggesting good method robustness across these latter parameters within the tested range.
The optimized set of conditions that simultaneously fulfilled all specification criteria and maximized method performance is shown in Table 3.
A confirmation run using these settings demonstrated that all measured tailing and k′ values were within the 95% prediction intervals of the model (Table 4). The observed values were closely aligned with the model predictions, with only minor underestimation of the tailing factor in the untreated standard and oxidative samples – yet still within statistical limits. This indicates good model reliability and predictive accuracy for the intended application range.
| Sample | Response variable | Predicted mean | 95% PI low | Data mean | 95% PI high |
|---|---|---|---|---|---|
| a In accordance with ICH Q2(R2)39 and the newly established ICH Q14 (ref. 27) guidelines, a robustness assessment can be integrated into the method development phase, representing a shift from the approach outlined in ICH Q2(R1).56 To proactively evaluate method robustness, we employed a DoE strategy, allowing for the simultaneous investigation of potential interactions among critical method parameters. | |||||
| Control sample | Tailing | 0.9 | 0.5 | 1.1 | 1.2 |
| k′ | 6.1 | 4.8 | 6.8 | 7.3 | |
| Acid hydrolysis | Tailing | 1.1 | 0.6 | 1.1 | 1.6 |
| k′ | 6.9 | 5.5 | 6.8 | 8.4 | |
| Alkaline hydrolysis | Tailing | 1.0 | 0.8 | 1.1 | 1.3 |
| k′ | 6.9 | 5.0 | 6.8 | 8.8 | |
| Thermal stress | Tailing | 0.9 | 0.6 | 1.1 | 1.3 |
| k′ | 6.9 | 4.9 | 6.8 | 8.8 | |
| Oxidative stress | Tailing | 0.9 | 0.5 | 1.0 | 1.4 |
| k′ | 7.1 | 5.0 | 6.8 | 9.2 | |
A quadratic RSM using an I-optimal design was applied to assess the influence of small, deliberate variations in method settings. Unlike the optimization phase, no further model-based adjustment of factor levels was performed. The primary responses monitored were k′ and tailing factor, serving as indicators of chromatographic performance. A total of 28 randomized experimental runs were conducted to capture the effects of parameter fluctuations and assess the method's resilience under realistic variation. The experimental factors investigated on the robustness DoE are displayed in Table 5.
| Chromatographic column | pH of the aqueous phase | Column temperature (°C) | Flow (mL min−1) |
|---|---|---|---|
| a Across all runs, the method has met its predefined specifications (k′ > 2 and tailing 0.8–1.8), demonstrating excellent robustness. Observed k′ values ranged from 5.5 to 7.0, while tailing remained between 0.9 and 1.5 for all tested conditions, including stress degradation scenarios (Table 6). These limited variations in response indicate that the method is robust and remains unaffected by small fluctuations in critical parameters. | |||
| Waters BEH C18 (batch 1) | 4.7–5.3 | 51.1–56.5 | 0.473–0.523 |
| Waters BEH C18 (batch 2) | |||
| YMC Triart C18 | |||
| Conditions | k′ Range | Tailing range |
|---|---|---|
| a The robustness study demonstrates a high degree of method consistency, with no indications of failure or drift, even under minor variations in system parameters and column batches. | ||
| Control sample | 5.5–6.9 | 1.0–1.4 |
| Acid hydrolysis | 5.5–6.9 | 0.9–1.5 |
| Alkaline hydrolysis | 5.5–7.0 | 1.1–1.4 |
| Thermal stress | 5.5–6.9 | 1.0–1.4 |
| Oxidative stress | 5.5–6.9 | 0.9–1.4 |
The statistical analysis of the quadratic model revealed several significant terms (see ESI Tables S3–S7†), most notably the column type, which consistently contributed the largest share of variation in both k′ and tailing (based on sums of squares). The flow rate also had a notable effect in some cases. Additionally, a few quadratic terms (e.g., for temperature and pH) were statistically significant but lacked practical interpretability, likely due to subtle curvatures in the response surface. Importantly, none of these effects led to any violation of the method's acceptance criteria.
Given the goal of robustness testing, statistical significance without practical impact was interpreted conservatively, confirming the stability and reliability of the method across the tested range. Therefore, no further optimization was deemed necessary.
3.2. Validation of the analytical method
Based on the results obtained, the following conditions were established as the final analytical method parameters for validation (Table 7).| Flow | 0.498 mL min−1 |
| Injection volume | 3 μL |
| Gradient organic modifier | t = 0 min, 20%; t = 2.0 min, 45%; t = 2.1 min, 20%; t = 5.0 min, 20% |
| Column | YMC Triart C18 or Waters BEH C18 |
| Column temperature | 53.8 °C |
| Wavelength | 280 nm |
 | ||
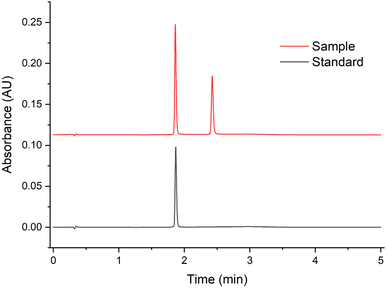
| Fig. 4 Chromatogram at 280 nm of the specificity test of the developed triptorelin analytical method. | ||
This confirmed that the method can reliably distinguish triptorelin from other potential sample constituents, and the specificity requirement is outlined in ICH Q2(R2).39 The absence of interfering peaks ensures the method's suitability for stability-indicating applications, where degradation products or excipients may be present in complex matrices.
The forced degradation study was performed in triplicate, and all degradation products were adequately resolved, with the corresponding peaks eluting within acceptable retention windows (Fig. 5). The goal of the study was not to drive extensive degradation, but rather to generate partial degradation under realistic stress conditions to assess the method's specificity, including its ability to resolve and detect both the intact API and its degradation products. Peak purity assessments confirmed the absence of co-eluting impurities for the main Triptorelin peak under all stress conditions (see Fig. S1–S5 in the ESI†). A summary of the degradation outcomes is provided in Table 8.
| Testing conditions | % Degradation |
|---|---|
| Control sample | 0.6 ± 0.2 |
| Acid hydrolysis | 8.2 ± 0.1 |
| Alkaline hydrolysis | 27.6 ± 0.2 |
| Thermal stress | 8.0 ± 0.1 |
| Oxidative stress | 27.3 ± 1.2 |
Triptorelin has a relative molecular mass of 1311.5 Da, which corresponds to a primary molecular ion at m/z 654.95 in the mass spectrum, observed as the doubly charged ion [M − 2H]2− in negative ionization mode. This signal was detected in the control sample, representing the unstressed triptorelin acetate solution. As expected, the acetate counterion was not observed, likely due to its high volatility and limited detectability in negative ionization mode.17
Under accelerated degradation conditions, Triptorelin acetate exhibited degradation across all stress scenarios. Among these, oxidative degradation produced a distinct impurity peak, which was confirmed via UV and MS spectra as a genuine degradation product (Fig. S6 in the ESI†). This finding highlights the susceptibility of triptorelin to oxidative stress and underscores the importance of monitoring degradation pathways during stability assessments.
A degradation peak observed at a retention time (RT) of 2.198 minutes with m/z 669.44 is likely attributable to a doubly oxidized form of triptorelin, corresponding to the ion [M + 2O − 2H]2−, possibly resulting from oxidation at both tryptophan residues.57
During acid-induced stress testing, two additional degradation products were detected, eluting at 1.755 min and 2.055 min, respectively (Fig. S7 in the ESI†). These findings suggest that triptorelin is susceptible to structural modification under strongly acidic conditions, leading to the formation of distinct degradation species.
In this case, the peak at an RT of 1.755 min with m/z 663.87 is attributed to the mono-oxidized form of triptorelin, corresponding to the [M + O − 2H]2− ion.57 The peak at an RT of 2.055 min with m/z 654.76 displays both the same molecular ion and UV spectrum as the intact triptorelin molecule. This suggests that while the core structure remains largely unaltered, a subtle modification has occurred. The most likely explanation is deamidation of the N-terminal amino acid, resulting in the formation of a deaminated Triptorelin with the composition [M + OH − NH2 – 2H]2−.
Under alkaline stress conditions, two degradation products were identified (Fig. S8 in the ESI†). Upon exposure to sodium hydroxide (NaOH), a peak at an RT of 1.840 min was observed with m/z 668.93, corresponding to the sodium adduct of Triptorelin ([M + Na − 2H]2−). This suggests cation exchange at the carboxyl terminus of the peptide, a typical reaction in alkaline environments.
A second peak at an RT of 2.064 min, exhibiting m/z 655.25, is attributed to the deamidated triptorelin. This species likely results from hydrolytic cleavage of the N-terminal amide, forming deamidated triptorelin with the proposed structure [M + OH − NH2 − 2H]2−.58 These findings indicate that deamidation is a common degradation pathway.
Exposure to thermal stress resulted in the formation of two degradation products, detected at retention times (RT) of 1.841 min and 2.063 min (Fig. S9 in the ESI†). Elevated temperatures are known to promote peptide hydrolysis. In this case, mass spectrometric analysis revealed a primary ion at m/z 355.25, consistent with a tripeptide fragment potentially composed of His–Ser–Leu.59 Notably, this sequence is not contiguous within the native triptorelin structure, suggesting that the degradation involves multiple cleavage events and the formation of a stable fragment composed of residues originating from distinct regions of the molecule. The associated UV absorbance maximum at 278.8 nm supports the presence of aromatic amino acids, particularly histidine.
The second peak at an RT of 2.063 min corresponds to the previously identified deamidated Triptorelin [M + OH − NH2 – 2H]2−, indicating that thermal degradation also promotes this transformation.
Furthermore, on evaluation of the residuals (Fig. S11 in the ESI†), there is no evidence of outliers or influential points across the entire concentration range, indicating homoscedasticity and model reliability. The normality of the residuals for both analytes was confirmed using the Shapiro–Wilk test (p = 0.054), supporting the validity of the linear regression model assumptions.
Accuracy was evaluated by analyzing three concentration levels (70%, 100%, and 130%) of the target concentration, each prepared in triplicate. The recovery values at each level were calculated by comparing the measured concentrations to the nominal values (Table 9). The results indicate the accuracy of the method and compliance with the established ATP.
| Concentration level | Recovery % | Average % | RSD % |
|---|---|---|---|
| a The method demonstrated high precision and reproducibility for the analytes, as evidenced by relative standard deviations (RSDs) below 2.0% for both repeatability and intermediate precision. Statistical comparison of the two precision levels showed no significant difference, with p-values greater than 0.05 (ANOVA), confirming equivalence at the 95% confidence level (Table 10). | |||
| 70% | 96.7 | 96.7 | 1.0 |
| 97.7 | |||
| 96.0 | |||
| 100% | 99.4 | 99.1 | 1.3 |
| 100.2 | |||
| 97.6 | |||
| 130% | 98.6 | 97.8 | 1.2 |
| 96.5 | |||
| 98.4 | |||
| Parameter | Operator I | Operator II |
|---|---|---|
| Mean | 104.2 | 106.8 |
| SD | 0.73 | 0.95 |
| RSD | 0.7 | 0.9 |
| N | 6 | 6 |
| P-value | 0.9545 | |
An exemplary chromatogram is presented in Fig. 6, illustrating a comparison between the triptorelin standard (RT 1.9 minutes) (acetate form) and the Pamorelin sample (pamoate form). The distinct peak observed at an RT of approximately 2.5 minutes corresponds to the pamoate counterion, indicating its successful separation from the active pharmaceutical ingredient.
As an additional assessment, the stability of the sample solution was evaluated by comparing the chromatographic peak areas of samples analyzed immediately after preparation and again after 24 hours of storage at room temperature. The difference in peak area was found to be less than 1% (Table S8 in the ESI†), indicating that the solution remained chemically stable over the tested period.
3.3. Analytical method lifecycle
The analytical method developed for the quantification of triptorelin in injectable dosage form was established in alignment with the principles outlined in USP <1220> and ICH Q14,27,38 embracing a full analytical procedure lifecycle approach.60 This methodology ensures that the procedure remains reliable, fit-for-purpose, and capable of consistently meeting the predefined ATP across its lifecycle, from initial design through routine application and continuous monitoring.By implementing these lifecycle elements, the triptorelin analytical method should achieve sustained control, enabling reliable quantification across batch releases and stability studies. This structured lifecycle framework not only reinforces regulatory compliance but also supports proactive method management and continual improvement.
4. Conclusions
In this study, a single UHPLC method was developed for the quantitative and stability-indicating analysis of triptorelin in its injectable dosage form. The method was established following AQbD principles, supported by a statistical DoE and validated in accordance with ICH Q2(R2) and Q14 guidelines. It demonstrated compliant linearity, accuracy, precision, specificity, and robustness. This work exemplifies a structured approach to analytical method development grounded in QbD concepts, offering enhanced method understanding and lifecycle control. Moreover, it supports regulatory compliance, reduces development time and resource use, and ensures high-quality, reproducible analytical performance throughout routine application.40Abbreviations
| AQbD | Analytical quality by design |
| ATP | Analytical target profile |
| CMAs | Critical method attributes |
| CMPs | Critical method parameters |
| DoE | Design of experiments |
| EMA | European medicine agency |
| FDA | United states food and drug administration |
| FDS | Fraction of design space |
| FSH | Follicle-stimulating hormone |
| GMP | Good manufacturing practices |
| GnRH | Gonadotropin-releasing hormone |
| HPLC | High-performance liquid chromatography |
| ICH | International council of harmonization |
| LH | Luteinizing hormone |
| MODR | Method operable design region |
| MS | Mass spectrometry |
| OOS | Out of specification |
| OOT | Out of trend |
| PDA | Photodiode array |
| Ph.Eur. | European pharmacopoeia |
| QbD | Quality by design |
| QDa | Single quadrupole mass detector from waters corp |
| QRM | Quality risk management |
| QTPP | Quality target product profile |
| RP | Reverse phase |
| RSM | Response surface methodology |
| RT | Retention time |
| UHPLC | Ultra (high)-performance liquid chromatography |
| USP | United states pharmacopoeia |
| UV | Ultraviolet |
Data availability
All data and materials are present in the manuscript and ESI.†Author contributions
Jesús Alberto Afonso Urich: conceptualization; methodology; formal analysis; resources; writing – original draft preparation; visualization; supervision; project administration; funding acquisition. Viktoria Marko: methodology; validation; formal analysis; data curation; writing – review and editing; visualization. Anna Fedorko: methodology; formal Analysis; data Curation; writing – review and editing. Dalibor Jeremic: formal analysis; writing – original draft preparation.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This research was funded within the framework of COMET – Competence Centers for Excellent Technologies by BMK, BMAW, Land Steiermark, and SFG. The COMET program is managed by the FFG.References
- A. Malhotra, R. Singh, P. C. Acharya and R. Bansal, in Medicinal Chemistry of Chemotherapeutic Agents, Elsevier, 2023, pp. 589–613 Search PubMed.
- B. L. Furman, Biomed. Sci., 2016, 1–3 Search PubMed.
- A. S. Merseburger and M. C. Hupe, Adv. Ther., 2016, 33, 1072–1093 CrossRef CAS PubMed.
- T. Lebret, M. Rouanne, O. Hublarov, V. Jinga, L. Petkova, R. Kotsev, I. Sinescu and P. Dutailly, Ther. Adv. Urol., 2015, 7, 125–134 CrossRef CAS PubMed.
- E. Yoo, S. Kim, H. L. Jung, J. Y. Shim, J. W. Shim, D. S. Kim, J. H. Kwak, E. S. Kim and A. Yang, Front. Endocrinol., 2023, 14, 1–7 Search PubMed.
- L. Zhu, Z. Guan, Y. Huang, K. Hua, L. Ma, J. Zhang, D. Yang, V. Perrot, H. Li and X. Zhang, Medicine, 2022, 101, e28766 CrossRef CAS PubMed.
- W. Choktanasiri, W. Boonkasemsanti, T. Sittisomwong, S. Kunathikom, S. Suksompong, U. Udomsubpayakul and A. Rojanasakul, Int. J. Gynecol. Obstet., 1996, 54, 237–243 CrossRef CAS PubMed.
- C. F. Heyns, Am. J. Cancer, 2005, 4, 169–183 CrossRef CAS.
- P. Kumar and A. Sharma, J. Hum. Reprod. Sci., 2014, 7, 170 CrossRef CAS PubMed.
- A. G. Bachem, Product Information Triptorelin Acetate & Triptorelin Pamoate, https://www.bachem.com/all-generic-apis/triptorelin-acetate-and-triptorelin-pamoate/, accessed 20 February 2025 Search PubMed.
- Y. Zhang, H. Zhang, D. Ghosh and R. O. Williams, Int. J. Pharm., 2020, 587, 119491 CrossRef CAS PubMed.
- DrugBank Online, Triptorelin Acetate, https://go.drugbank.com/salts/DBSALT001171, accessed 14 April 2025 Search PubMed.
- DrugBank Online, Triptorelin Pamoate, https://go.drugbank.com/salts/DBSALT001256, accessed 14 April 2025 Search PubMed.
- A.-P. Forsback, P. Noppari, J. Viljanen, J. Mikkola, M. Jokinen, L. Leino, S. Bjerregaard, C. Borglin and J. Halliday, Nanomaterials, 2021, 11, 1578 CrossRef CAS PubMed.
- S. S. Hosseiny Davarani, A. Pourahadi and P. Ghasemzadeh, Electrophoresis, 2019, 40, 1074–1081 CrossRef CAS PubMed.
- J. Han, J. Sun, C. Sha, J. Zhang, Y. Gai, Y. Li and W. Liu, J. Pharm. Biomed. Anal., 2012, 66, 334–338 CrossRef CAS PubMed.
- Y. Zan, Y. Chen, F. Xie, S. Shang, N. Chen and Y. Ding, J. Chromatogr. Open, 2025, 7, 100218 CrossRef.
- N. Saardpun, R. Songsaeng, P. Tanratana, T. Kusamran and D. Pinthong, Molecules, 2023, 28, 4572 CrossRef CAS PubMed.
- N. Saardpun, C. Asawesna, S. Kaewklam, P. Sangkhum, W. Kongchareonsombat, T. Kusamran and D. Pinthong, Drug Test. Anal., 2025 DOI:10.1002/dta.3849.
- M. Gumustas, S. Kurbanoglu, B. Uslu and S. A. Ozkan, Chromatographia, 2013, 76, 1365–1427 CrossRef CAS.
- A. Cichocki, HPLC vs. UPLC - What's the Difference?, https://www.chromatographytoday.com/news/hplc-uhplc/31/breaking-news/hplc-vs-uplc-whats-the-difference/56814, accessed 1 July 2025 Search PubMed.
- Separation Science, Comparing HPLC and UPLC: Which Analytical Technique is Right for Your Lab?, https://www.sepscience.com/automated-sample-preparation-for-multi-residue-pesticide-analysis-by-lcms-and-gcms-11473, accessed 1 July 2025 Search PubMed.
- M. Khalikova, J. Jireš, O. Horáček, M. Douša, R. Kučera and L. Nováková, Mass Spectrom. Rev., 2024, 43, 560–609 CrossRef CAS PubMed.
- A. Bairagi, R. Kothrukar, H. Chikhale, S. Kosanam and L. Borse, Futur. J. Pharm. Sci., 2024, 10, 138 CrossRef.
- Medicines and Healthcare products Regulatory Agency, Consultation on the application of Analytical Quality by Design (AQbD) principles to pharmacopoeial standards for medicines, https://www.gov.uk/government/consultations/consultation-on-the-application-of-analytical-quality-by-design-aqbd-principles-to-pharmacopoeial-standards-for-medicines, accessed 2 March 2025 Search PubMed.
- D. M. Zagalo, J. Sousa and S. Simões, Eur. J. Pharm. Biopharm., 2022, 178, 1–24 CrossRef CAS PubMed.
- International Coucil of Harmonisation, (Concept Paper) Q14: Analytical Procedure Development and Revision of Q2(R1) Analytical Validation, 2022, Draft Document.
- International Coucil of Harmonisation, Guideline Q8 (R2) Pharmaceutical Development, 2009, vol. 8 Search PubMed.
- International Coucil of Harmonisation, Guideline Q9 Quality Risk Management, 2017, vol. 44 Search PubMed.
- International Coucil of Harmonisation, Guideline Q10 on Pharmaceutical Quality System, 2015, vol. 44 Search PubMed.
- T. Verch, C. Campa, C. C. Chéry, R. Frenkel, T. Graul, N. Jaya, B. Nakhle, J. Springall, J. Starkey, J. Wypych and T. Ranheim, AAPS J., 2022, 24, 34 CrossRef PubMed.
- J. A. Afonso Urich, V. Marko, K. Boehm, R. A. Lara Garcia, A. Fedorko, S. Salar-Behzadi and D. Jeremic, Sci. Pharm., 2024, 92, 22 CrossRef CAS.
- A. Gaggero, V. Marko, D. Jeremic, C. Tetyczka, P. Caisse and J. A. Afonso Urich, Molecules, 2025, 30, 486 CrossRef CAS PubMed.
- J. Kochling, W. Wu, Y. Hua, Q. Guan and J. Castaneda-Merced, J. Pharm. Biomed. Anal., 2016, 125, 130–139 CrossRef CAS PubMed.
- D. M. Zagalo, B. M. A. Silva, C. Silva, S. Simões and J. J. Sousa, J. Drug Deliv. Sci. Technol., 2022, 70, 103207 CrossRef.
- H. Kasem, M. Ntorkou, P. D. Tzanavaras and C. K. Zacharis, Anal. Methods, 2025, 17, 3459–3470 RSC.
- P. Ramalingam and B. Jahnavi, QbD Considerations for Analytical Development, Elsevier Inc., 2019 Search PubMed.
- USP43-NF38, <1220> Analytical Procedure Life Cycle, https://doi.usp.org/USPNF/USPNF_M10975_02_01.html, accessed 25 February 2025 Search PubMed.
- International Council for Harmonisation, Guideline Q2 (R2) Validation of Analytical Procedures, 2022 Search PubMed.
- S. Beg and M. Rahman, in Handbook of Analytical Quality by Design, Elsevier, 2021, pp. 87–97 Search PubMed.
- J. A. Afonso Urich, V. Marko, K. Boehm, R. A. Lara García, D. Jeremic and A. Paudel, Molecules, 2021, 26, 6597 CrossRef CAS PubMed.
- T. Tome, N. Žigart, Z. Časar and A. Obreza, Org. Process Res. Dev., 2019, 23, 1784–1802 CrossRef CAS.
- Medicines and Healthcare products Regulatory Agency, Analytical Quality by Design (AQbD): Questions and Answers, https://mhrainspectorate.blog.gov.uk/2019/08/21/analytical-quality-by-design-aqbd-questions-and-answers/, accessed 14 April 2025 Search PubMed.
- British Pharmacopoeia, Analytical Quality by Design Guidance (AQbD), https://www.pharmacopoeia.com/guidance/aqbd, accessed 14 April 2025 Search PubMed.
- P. C. John, Trelstar LA (Triptorelin Pamoate for Injectable Suspension): Technical Leaflet, https://www.rxlist.com/trelstar-la-drug.htm#description, accessed 6 May 2025 Search PubMed.
- Debiopharm, Debiopharm and Ipsen Extend Their Strategic Decapeptyl® (Triptorelin) Partnership for Another 15 Years, https://www.debiopharm.com/drug-development/press-releases/debiopharm-and-ipsen-extend-their-strategic-decapeptyl-triptorelin-partnership-for-another-15-years/, accessed 30 June 2025 Search PubMed.
- Debiopharm, Ipsen and Debiopharm Conclude an Exclusive Worldwide License Agreement for The Development And Commercialisation of The Ipsen's Proprietary CDC25 Inhibitor (IRC-083864 or Debio 0931), An Anti-Cancer Agent - Debiopharm, https://www.debiopharm.com/drug-development/press-releases/ipsen-and-debiopharm-conclude-an-exclusive-worldwide-license-agreement-for-the-development-and-commercialisation-of-the-ipsens-proprietary-cdc25-inhibitor-irc-083864-or-debio-0931-an-anti/, accessed 30 June 2025 Search PubMed.
- PRNewswire, Debiopharm and Vifor Pharma Sign an Exclusive Agreement for the Distribution and Commercialization of Pamorelin®LA in Switzerland - A Drug Treatment for Prostate Cancer and Endometriosis - BioSpace, https://www.biospace.com/debiopharm-and-vifor-pharma-sign-an-exclusive-agreement-for-the-distribution-and-commercialization-of-pamorelin-la-in-switzerland-a-drug-treatment-f?utm_source=chatgpt.com, accessed 28 June 2025 Search PubMed.
- Debiopharm, Debiopharm Group Regains The Commercial Rights to Trelstar® (Triptorelin pamoate) and Looks for New Partners in North America, https://www.debiopharm.com/drug-development/press-releases/debiopharm-group-regains-the-commercial-rights-to-trelstar-triptorelin-pamoate-and-looks-for-new-partners-in-north-america/?utm_source=chatgpt.com, accessed 28 June 2025 Search PubMed.
- Debiopharm, Debiopharm and Watson Pharmaceuticals Enter Into an Exclusive Licensing Agreement for Two Approved urology Products - Trelstar® DEPOT and Trelstar® LA, https://www.debiopharm.com/drug-development/press-releases/debiopharm-and-watson-pharmaceuticals-enter-into-an-exclusive-licensing-agreement-for-two-approved-urology-products-trelstar-depot-and-trelstar-la/?utm_source=chatgpt.com, accessed 28 June 2025 Search PubMed.
- M. Blessy, R. D. Patel, P. N. Prajapati and Y. K. Agrawal, J. Pharm. Anal., 2014, 4, 159–165 CrossRef CAS PubMed.
- T. Zelesky, S. W. Baertschi, C. Foti, L. R. Allain, S. Hostyn, J. R. Franca, Y. Li, S. Marden, S. Mohan, M. Ultramari, Z. Huang, N. Adams, J. M. Campbell, P. J. Jansen, D. Kotoni and C. Laue, J. Pharm. Sci., 2023, 112, 2948–2964 CrossRef CAS PubMed.
- USP43-NF38, <621> Chromatography, https://doi.usp.org/USPNF/USPNF_M99380_09_01.html, accessed 20 January 2025 Search PubMed.
- O. of R. A. U.S. Food and Drug Administration, Laboratory Manual Volume II Methods, Method Verification and Validation, 2020 Search PubMed.
- USP43-NF38, <2> Oral Drug Products—Product Quality Tests, https://doi.usp.org/USPNF/USPNF_M3211_06_01.html, accessed 3 February 2025 Search PubMed.
- International Conference of Harmonisation, Guideline Q2 (R1) Validation of Analytical Procedures: Text and Methodology, 2005 Search PubMed.
- L. R. Asmus, J.-C. Tille, B. Kaufmann, L. Melander, T. Weiss, K. Vessman, W. Koechling, G. Schwach, R. Gurny and M. Möller, J. Controlled Release, 2013, 165, 199–206 CrossRef CAS PubMed.
- M. C. Manning, J. Liu, T. Li and R. E. Holcomb, Advances in Protein Chemistry and Structural Biology, 2018, pp. 1–59 Search PubMed.
- K. Shafiee, S. Bazraei, A. Mashak and H. Mobedi, J. Polym. Environ., 2024, 32, 3591–3608 CrossRef CAS.
- M. Maziarz, P. Rainville and S. Naughton, Continued Performance Verification of Analytical Procedures Using Control Charts of Empower Chromatography Data Software, Milford, 2019 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ay00919g |
| This journal is © The Royal Society of Chemistry 2025 |