 Open Access Article
Open Access ArticleSynthesis and optimization of a 2-isopropenyl-2-oxazoline based polymer for enhanced phenolic acid extraction
Lena Sophia
Nigsch
 a,
Christoph
Kappacher
a,
Dominik
Hense
b,
Christian Wolfgang
Huck
a,
Christoph
Kappacher
a,
Dominik
Hense
b,
Christian Wolfgang
Huck
 a,
Oliver I.
Strube
a,
Oliver I.
Strube
 b and
Matthias
Rainer
b and
Matthias
Rainer
 *a
*a
aInstitute of Analytical Chemistry and Radiochemistry, Leopold-Franzens University of Innsbruck, Innrain 80-82, A-6020 Innsbruck, Austria. E-mail: m.rainer@uibk.ac.at; Fax: +43-512-507-57399; Tel: +43-512-507-57307
bInstitute of Chemical Engineering, Leopold-Franzens University of Innsbruck, Innrain 80-82, A-6020 Innsbruck, Austria
First published on 20th October 2025
Abstract
Phenolic acids, widely present in plants, plant-based foods, and human metabolites, are well known for their potent antioxidant properties, which are mainly attributed to their phenolic hydroxyl groups. The number and position of these hydroxyl groups, together with methoxy and carboxylic acid groups significantly influence their antioxidant capacity. In this study, five different phenolic acids were studied: chlorogenic acid, caffeic acid, 2,3-dihydroxybenzoic acid, ferulic acid and trans-cinnamic acid. They were analyzed by solid phase extraction and high-performance liquid chromatography hyphenated to ultraviolet detection. Different polymers for solid phase extraction were synthesized using ultraviolet photoinitiation with ethylene glycol dimethacrylate and thermal initiation with divinylbenzene as crosslinkers. The monomer utilized in this process was 2-isopropenyl-2-oxazoline, which exhibited analogous properties to the commonly known monomers employed in the polymerization process for commercially available solid phase extraction cartridges. The most effective polymer was optimized and applied to two different types of honey, demonstrating its ability to extract phenolic acids from matrix compounds.
Introduction
Phenolic acids are a class of bioactive compounds known for their significant antioxidant properties and their role in managing oxidative stress-related damage.1–4 These compounds have been the focus of extensive research due to their health benefits, especially in terms of preventing free radical-induced body damage and reducing the risk of cognitive decline and chronic diseases, including diabetes, cancer and cardiovascular disorders.2–5Recent studies have revealed the potential of specific phenolic compounds for neuroprotective and anti-inflammatory applications, as well as their antioxidant properties. Phenolic acids' diverse benefits have led to their commercial utilization in the health, cosmetic and pharmaceutical industries, where they are frequently incorporated into dietary supplements, skincare products and food, further demonstrating their importance.6,7
Nonetheless, despite their broad applications, efficient extraction from complex natural matrices remains challenging. This difficulty stems from their natural variability, the presence of interfering compounds and the limited selectivity of conventional extraction methods.6,8,9
As a result, new approaches must be developed or existing techniques refined to optimize extraction efficiency, selectivity and reproducibility. Solid phase extraction (SPE) is a widely used technique for isolating phenolic acids, due to its advantages, such as increased analyte concentration and reduced solvent consumption.10–12
SPE has gained significant attention in recent years due to its versatility, efficiency and adaptability across a wide range of analytical contexts. Emerging materials, such as molecularly imprinted polymers (MIPs),13–15 mesoporous silica,16 carbon-based nanomaterials17 and metal–organic frameworks (MOFs)14 have expanded the applicability of SPE by offering improved selectivity, higher surface area and enhanced binding capacity.
In line with the ongoing development of advanced functional SPE materials, 2-isopropenyl-2-oxazoline (iPOX) has emerged as a highly versatile monomer that undergoes ring-opening isomerization to yield well-defined polymers suitable for analytical applications. Its polymerization can proceed via both ionic and covalent pathways,18 and the resulting polymers can undergo efficient post-polymerization modification with nucleophiles such as carboxylic acids and thiols.19 Poly(iPOX) coatings exhibit resistance to non-specific protein adsorption, rendering them suitable for biomedical devices and sensors.20 Collectively, these characteristics establish iPOX as a robust platform for designing functional polymers with extensive biomedical relevance.
Moreover, the choice of crosslinker in the polymerization of the stationary phase for SPE cartridges plays a critical role in defining the physicochemical properties of the resulting sorbent. Different crosslinkers offer unique structural and chemical characteristics that help address specific challenges in SPE, such as selectivity and capacity. Since the sorbent directly influences the retention behavior of the target analytes, optimizing the type and ratio of crosslinker is essential for enhancing extraction performance.21 Additionally, the ratio between the crosslinker and the porogen significantly impacts the polymer's surface morphology, including its porosity and surface area. These parameters in turn affect the adsorption efficiency and extraction capacity of the polymer.22 A well-balanced porogen-crosslinker system is therefore important for producing sorbents with high selectivity, reproducibility and durability under different extraction conditions.
It has been demonstrated that (ethylene glycol dimethacrylate) EGDMA functions as an effective di-functional crosslinker in polymerization processes, forming moderately crosslinked networks. Its relatively hydrophilic nature enhances swelling in aqueous matrices and allows milder elution, a property that can be advantageous for aqueous SPE applications.23,24 In addition, the crosslinking ability of EGDMA facilitates the formation of well-defined binding sites that can selectively interact with target molecules such as phenolic acids, making it highly suitable for applications requiring specificity, such as molecular imprinting.25,26 Collectively, these properties highlight the versatility and effectiveness of EGDMA as a crosslinker in polymerization processes.
In contrast, DVB is a widely used aromatic crosslinker in commercial SPE sorbents such as Oasis® HLB. It forms highly rigid, hydrophobic networks with a large surface area, providing strong retention, mechanical stability, and chemical resistance, making it ideal for non-polar and moderately polar analytes.27 However, its pronounced hydrophobicity may reduce performance with highly polar analytes unless the polymer is co-functionalized or combined with hydrophilic monomers. DVB has been demonstrated to act as an effective crosslinker for polymers utilized in SPE of phenolic acids.28 Furthermore, DVB contributes to maintaining the polymer's structural integrity and porosity, even in the presence of polar or acidic solutions.29 The rigid DVB-crosslinked network preserves well-defined functional cavities when functional monomers are incorporated, enabling selective interactions with phenolic acids through hydrogen bonding and π–π interactions.30 Careful optimization of DVB content is therefore crucial to balance rigidity and binding specificity, ensuring efficient sorption of phenolic acids.31,32 Additionally, the excellent thermal and chemical stability of DVB-crosslinked polymers ensures reproducibility and long-term usability of the SPE sorbent over multiple extraction cycles and under both aqueous and organic solvent conditions.33 However, the pronounced hydrophobicity of DVB may reduce performance with highly polar analytes unless the polymer is co-functionalized or combined with hydrophilic monomers.
Trimethylolpropane trimethacrylate (TRIM) has been shown to possess a higher functionality in comparison to EGDMA, resulting in enhanced imprinting and higher binding capacities.23,34 TRIM is relatively hydrophobic, which can result in stronger analyte retention, making it superior to EGDMA for high-capacity sorbents. However, this can also make elution more challenging, requiring stronger solvents.24 In contrast, hydrophilic crosslinkers such as methylenebisacrylamide (MBA), ethylene bisacrylamide (EBA), and bis-acryloylpiperazine (BAP) are frequently employed in hydrogel-based or protein-targeted MIPs. These crosslinkers provide excellent water compatibility, high swelling, and suitable networks for biomacromolecule binding.35
When it comes to complex biological samples, conventional SPE materials may suffer from non-selective interactions with matrix components resulting in diminished extraction yields and lower analytical accuracy.
To conquer these limitations, this research focuses on the development and optimization of a polymer sorbent for the efficient SPE of phenolic acids. In this study polymers were synthesized using ultraviolet (UV) photoinitiation and thermal initiation polymerization techniques, incorporating crosslinkers such as EGDMA and divinylbenzene (DVB), as well as a porogen solution to improve the stability and porosity of the resulting polymers. This was important to improve the polymers' binding selectivity towards phenolic acid molecules, which leads to better extraction performance.
As a proof of concept, honey was selected as a real-world sample to evaluate the performance of the synthesized and optimized polymer. Honey is well known for its composition, which includes bioactive compounds, especially phenolic acids, which contribute to its antioxidant capacity.36,37
By optimizing the polymer composition and extraction parameters, this research contributes to the advancement of analytical techniques for natural product analysis and has potential applications in pharmaceutical and environmental research, where efficient isolation of bioactive compounds is important.
Materials and methods
Chlorogenic acid (Chlor A, ≥95.0%), caffeic acid (Caff A, ≥98.0%), 2,3-dihydroxybenzoic acid (DHB, 99%), trans-cinnamic acid (Cin A, ≥99.0%), 2,2-dimethoxy-2-phenylacetophenone (99%), 2-isopropenyl-2-oxazoline (iPOX, 98%), ethylene glycol dimethacrylate (EGDMA, 98%), N-methylformamide (99%), as well as basic, activated aluminum oxide (Al2O3, approx. 150 mesh, standard grade) were purchased from Sigma-Aldrich (St. Louis, MI, USA), an affiliate of Merck (Darmstadt, Germany). 1-Decanol (97%), divinylbenzene (DVB), α,α-azoisobutyronitrile (AIBN, ≥98.0%), were purchased from Merck (Darmstadt, Germany). Acetic acid (AA, 100%), formic acid (FA, 98%) and hydrochloric acid (HCl, fuming, 37%) were obtained from ROTIPURAN®, an affiliate from Carl Roth® GmbH + Co. KG (Karlsruhe, Germany). Trans-ferulic acid (Fer A, ≥99.0%) was purchased from Rotichrom®, another affiliate from Carl Roth® GmbH + Co. KG (Karlsruhe, Germany). Methanol (MeOH) was obtained from Thermo Fisher Scientific (Waltham, MA, USA).The empty SPE cartridges (1 mL) and their matching polyethylene (PE) frits with 20 μm porosity were obtained from Supelco® (Bellefonte, PA, USA). The pre-packed Oasis® HLB cartridges (1 cc, 30 mg sorbent, 30 μm) were purchased from Waters™ (Milford, MA, USA). The pre-packed Strata™-X cartridges (33 μm Polymeric Reversed Phase, 30 mg/1 mL, Tubes) were from Phenomenex (Torrance, CA, USA). Water was obtained from a Milli-Q water purification system from Merck Millipore (Burlington, MA, USA).
The honey sample from Röthis (Vorarlberg, Austria) was acquired from the beekeeper A. Nigsch from the year 2023. The liquid blossom honey was obtained from the brand “JedenTag”, which is a mixture of honey from EU-countries and non-EU-countries.
The phenolic acid standard solution mixture used for the comparison of the recovery rates of the different SPE types, contained 100 mg L−1 of each analyte, including Chlor A, Caff A, DHB, Fer A and Cin A in 4% MeOH in water. These solutions were stored at 4 °C. Different concentrations of the phenolic acid standard solution mixtures were created, ranging from 0.1 mg L−1 to 200 mg L−1.
The DVB was extracted three times with 10 (w/v) NaOH solution and distilled under vacuum. The EGDMA and the iPOX were extracted with activated basic Al2O3 to remove inhibitors prior to its use.
Instrumentation and chromatographic separation
The polymerization with UV light was executed by exposing the mixture to an UV lamp operating at 8 W and 254 nm (CX-2000 UV Crosslinker, UVP Upland, CA, USA) for 30 minutes. The thermal polymerization was performed by using the C12 CS water bath by Lauda scientific (Lauda-Königshofen, Germany) at 70 °C for 19 hours and 30 minutes. Centrifugation was carried out by a Hermle Universal Centrifuge (Wehingen, Germany) to sediment the residue from the dissolved honey samples at the bottom of the tube. The standard solutions and honey samples were dissolved using a VWR Ultrasonic Cleaner USC-TH. The removal of the inhibitors present in the polymerization solutions was achieved through the utilization of activated Al2O3 using an Eppendorf thermomixer (Hamburg, Germany).The scanning electron microscope (SEM) measurements were performed on a Tescan Clara Ultra High Resolution (UHR) system with a field emission gun (FEG). Before the measurements, the samples were coated with a 7 nm layer of platinum. The topography images were captured with an acceleration voltage of 10 keV using an Everhart–Thornley detector, which detects secondary electrons.
Energy dispersive X-ray spectroscopy (EDX) was performed using an Oxford Ultim Extreme detector with an acceleration voltage of 10 keV. The error in the percentage values is ±2% of the given value.
The attenuated total reflection (ATR) measurements were performed using a PerkinElmer Spectrum 400 FT-IR/FT-NIR device.
The HPLC measurements were conducted using an Agilent 1100 Series system equipped with a G1314A VWD detector. The stationary phase was a YMC-Pack Pro C18 RS column (150 × 4.6 mm I.D., S-5 μm, 8 nm pore size) maintained at 40 °C. A 5 μL injection volume was used, with the autosampler temperature set to 10 °C. The mobile phase comprised eluent A (water with 0.1% formic acid) and eluent B (methanol with 0.1% formic acid). The flow rate was set to 0.650 mL min−1. The following gradient was performed when using the method for the phenolic acids: 0 min/10% B, 16 min/70% B, 16.01 min/99% B, 18 min/99% B, 18.01 min/10% B, 22 min/10% B. When using the method for ferulic acid, the following gradient was performed: 0 min/10% B, 16 min/70% B, 16.01 min/99%, 18 min/10% B, 19 min/10% B.
Method validation
The HPLC-VWD method and SPE measurements were validated in accordance with the guidelines set by the “Society of Toxicological and Forensic Chemistry” (GTFCh) and the DIN 32645 standard.38,39 A linear regression model was used to assess the HPLC method's linearity and precision. Additionally, the stability of both the HPLC autosampler and the standard solutions was monitored. The limit of detection (LOD) and limit of quantification (LOQ) were also determined.Linearity of calibration
Throughout this study, external calibrations were performed to ensure the accuracy of the HPLC measurements. The calibration concentrations were adapted to match the analyte concentrations being analyzed. To prove the linearity of calibration, a dilution series from 25 mg L−1 to 200 mg L−1 was prepared by diluting a 200 mg per L phenolic acid standard solution mixture. Each standard concentration was measured in triplicate.Recovery rate
To prove the effectiveness of the SPE method, the synthesized and optimized polymer, along with the Oasis® HLB cartridge and the Strata™-X cartridge were analyzed. After performing the SPE protocols, the resulting recoveries were compared to examine the adsorption efficiency of the produced polymer.Reusability of the cartridges
The adsorption quality was evaluated by comparing the performance of the synthesized polymer to that of the Oasis® HLB and Strata™-X cartridges. The SPE procedures were carried out and repeated four times for each cartridge. The resulting recoveries for each cartridge were then compared.Stability test
To verify the stability of both the HPLC autosampler and the standard solutions, a 24-hour stability test was performed. Two 100 mg per L phenolic acid mixture solutions were measured every hour over a 24-hour period. The resulting peak areas were compared by normalizing the initial measurement to 100%. All subsequent results were then calculated relative to this baseline, with the first measurement serving as the reference point for accuracy.Determination of LOD and LOQ
To determine the LOD and LOQ a dilution series, reaching from 25 mg L−1 to 200 mg L−1 was prepared by diluting a 200 mg per L phenolic acid standard solution mixture, resulting in five distinct calibration curves. Each standard concentration was measured in triplicate to ensure reliability. The LOD (XLOD) values were calculated according to DIN 32645, whereas the LOQ (XLOQ) values were derived using a rapid estimation approach based on the simplified calibration method, as described by eqn (1) and (2).38 | (1) |
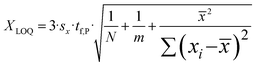 | (2) |
![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) demonstrates the mean value of the x-values of the regression line, with Σ(xi −
demonstrates the mean value of the x-values of the regression line, with Σ(xi − ![[x with combining macron]](https://www.rsc.org/images/entities/i_char_0078_0304.gif) )2 being the sum of squared deviations from x.
)2 being the sum of squared deviations from x.
Thermally initiated polymerization
For the thermally initiated polymerization, iPOX and DVB were combined in an amber glass flask in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Before use, iPOX was treated with basic activated Al2O3 (75 mg per 1.5 mL of untreated solution) and incubated for 15 minutes. Afterwards, a porogen mixture consisting of 1-decanol and N-methylformamide in a 2
1 ratio. Before use, iPOX was treated with basic activated Al2O3 (75 mg per 1.5 mL of untreated solution) and incubated for 15 minutes. Afterwards, a porogen mixture consisting of 1-decanol and N-methylformamide in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was added to the solution. Next, the initiator AIBN (2 w%) was added into the mixture and the solution was placed in an ultrasonic bath to ensure complete dissolution of the initiator. After degassing the solution with argon for 10 minutes, the glass flask was placed in a water bath at 70 °C for 19 hours and 30 minutes (reaction scheme: Fig. 1).
1 ratio was added to the solution. Next, the initiator AIBN (2 w%) was added into the mixture and the solution was placed in an ultrasonic bath to ensure complete dissolution of the initiator. After degassing the solution with argon for 10 minutes, the glass flask was placed in a water bath at 70 °C for 19 hours and 30 minutes (reaction scheme: Fig. 1).
Once the polymerization was completed, the polymer was removed from the glass flask by breaking it. The monolithic polymer was then ground in a mortar and thoroughly washed with water, ACN and MeOH. After drying overnight, it was sieved to achieve a particle size range of 50 to 500 μm.
UV photoinitiated polymerization
For the UV photoinitiated polymerization, two glass plates were prepared by rinsing them with acetone and water. Afterwards, iPOX and EGDMA were combined at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio. Before use, both solutions were treated with basic activated Al2O3 (75 mg per 1.5 mL of untreated solution) and incubated for 15 minutes. Next, a porogen mixture of 1-decanol and N-methylformamide in a 2
1 ratio. Before use, both solutions were treated with basic activated Al2O3 (75 mg per 1.5 mL of untreated solution) and incubated for 15 minutes. Next, a porogen mixture of 1-decanol and N-methylformamide in a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio was added to the solution mixture. Then, the initiator 2,2-dimethoxy-2-phenylacetophenone (2 w%) was added to the polymer solution. The mixture was then placed in an ultrasonic bath to ensure complete dissolution of the initiator. The polymer solution was then injected between two glass plates, which were fixed in place using two separators positioned along the edges and four clamps securing the glass plates. The polymer solution was exposed to a UV light for 30 minutes at 8 W and 254 nm (Fig. 1).
1 ratio was added to the solution mixture. Then, the initiator 2,2-dimethoxy-2-phenylacetophenone (2 w%) was added to the polymer solution. The mixture was then placed in an ultrasonic bath to ensure complete dissolution of the initiator. The polymer solution was then injected between two glass plates, which were fixed in place using two separators positioned along the edges and four clamps securing the glass plates. The polymer solution was exposed to a UV light for 30 minutes at 8 W and 254 nm (Fig. 1).
After the polymerization process, the polymer was removed from the glass plates by scraping it off. The resulting monolithic polymer was then thoroughly washed with water, ACN and MeOH. The polymer was dried overnight and then sieved to obtain a particle size range of 50 to 500 μm.
Porogen and crosslinker ratio
Various ratios of porogen and crosslinker of the thermally and UV initiated polymers have been investigated ranging from 50 to 80 percent of porogen and 50 to 20 percent of crosslinker (Tables S1 and S2).Cartridge preparation
To perform an SPE, 30 mg of polymer was loaded into a pre-fitted 1 mL polypropylene (PP) cartridge. A polyethylene (PE) frit with a porosity of 20 μm was then placed on top of the polymer. The cartridge was packed under approximately 9 to 10 kg of pressure to ensure proper preparation for the subsequent SPE procedures.SPE protocol for phenolic acids
For the extraction of phenolic acids using the synthesized polymers, a five-step SPE protocol was established. The protocol consists of conditioning, equilibration, sample loading, washing and analyte elution. The flow rate for the SPE procedure was maintained at 3–4 seconds per drop. The conditioning step was performed using 0.5 mL of MeOH, followed by equilibration with 0.5 mL of 4% MeOH in water. Then, 1 mL of a 100 mg per L solution containing the previously mentioned five phenolic acids was loaded onto the cartridge. After washing with 1 mL of 10% MeOH in water, the analyte was eluted in two steps using 1 mL of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2% FA) per step. The collected eluates were then directly injected into the HPLC system for analysis.
1, 2% FA) per step. The collected eluates were then directly injected into the HPLC system for analysis.
For the honey samples, a modified SPE protocol was adapted, from a previously published method to evaluate the effectiveness of the synthesized polymers with real-world samples at a flow rate of 3–4 seconds per drop.40
The conditioning and equilibration steps were performed using 1 mL of MeOH followed by 1 mL of water (pH 2, adjusted with concentrated HCl). The polymer was then loaded with 1 mL of a honey solution, prepared by dissolving 30 g of honey in 120 mL of water (pH 2, adjusted with concentrated HCl). The washing step was carried out with 1 mL of water (pH 2, adjusted with concentrated HCl) to remove sugars and other polar components that were not retained by the sorbent. The analyte elution was performed in one step using 1 mL of a FA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MeOH (1
MeOH (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) solution. The elution solution was then evaporated and re-dissolved in 0.5 mL of MeOH (2% AA) to concentrate the sample. The concentrated solution was subsequently filtered through a 0.20 μm PTFE-filter before being injected into the HPLC system.40
9) solution. The elution solution was then evaporated and re-dissolved in 0.5 mL of MeOH (2% AA) to concentrate the sample. The concentrated solution was subsequently filtered through a 0.20 μm PTFE-filter before being injected into the HPLC system.40
To compare the performance of the synthesized polymers in SPE, commercially available cartridges were used. These included 30 mg Oasis® HLB 1 cc (hydrophilic-lipophilic balance) cartridges with 30 μm sorbent and 30 mg Strata™-X cartridges with 33 μm sorbent. The SPE protocols for these commercially available cartridges were strictly adhered during the extraction process to ensure consistency in the evaluation of their performance against the synthesized polymers. Detailed protocols for the Oasis® HLB and Strata™-X cartridges are provided in the Table S3 (SI).
Optimization of the elution solution
The elution was optimized by testing two different elution solutions and comparing their effectiveness for SPE with the synthesized polymers. The first elution solution, consisting of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN
ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 2% FA, was initially used. However, after evaluating the recoveries, a second composition was introduced, which yielded more satisfactory results. The optimal elution solution, as previously mentioned, was MeOH:water (1
1) with 2% FA, was initially used. However, after evaluating the recoveries, a second composition was introduced, which yielded more satisfactory results. The optimal elution solution, as previously mentioned, was MeOH:water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 2% FA. The recovery-results for the different elution solutions are shown in Fig. S1 (SI).
1) with 2% FA. The recovery-results for the different elution solutions are shown in Fig. S1 (SI).
Optimization of the particle size
An attempt was made to use smaller polymer particle sizes, ranging from 25 to 50 μm, for the SPEs. The polymer was synthesized as previously described, with the only variation being sieved to achieve smaller particle sizes. However, due to excessive back pressure and unacceptably high flow rate during the SPE process, the larger particle size range of 50 to 500 μm was ultimately retained.Dynamic loading capacity study
A dynamic loading capacity study was conducted to assess the capacity of the polymers and to compare the performance of the various synthesized polymers which differed in porogen and crosslinker ratios. Therefore, a cartridge was prepared using the previously described protocol, employing the synthesized polymers with varying porogen and crosslinker ratios. The polymer was then conditioned and equilibrated with 0.5 mL of MeOH and 0.5 mL of 4% MeOH in water. Solutions ranging from 50 to 200 mg L−1 of a Fer A-solution were added in 1 mL increments for up to 60 cycles on the various cartridges. After each loading step, the residues were collected and analyzed using HPLC. The eluate concentrations were plotted to compare the performance of the different polymers.To calculate the dynamic loading capacity, a Boltzmann model was fitted to the data, resulting in the following eqn (3).41
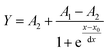 | (3) |
With eqn (3) the column's capacity can be calculated, including the breakthrough volume VB and the hold-up volume VM, which can be determined using eqn (4) and (5):41
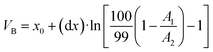 | (4) |
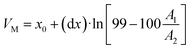 | (5) |
As already mentioned, the retention volume VR corresponds to the inflexion point x0, where the solute is in equilibrium between being absorbed onto the sorbent and eluted from it (VR = x0). With eqn (6) and (7) the number of theoretical plates N and the retention factor k can be calculated. The dynamic capacity QB is determined by eqn (8).41
 | (6) |
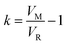 | (7) |
 | (8) |
Results and discussion
A 2-isopropenyl-2-oxazoline-based polymer was synthesized and optimized for the SPE of phenolic acids, followed by characterization and analysis using various instruments. To ensure the accuracy and reliability of the obtained data, all SPE experiments and HPLC measurements were conducted in triplicate providing a robust basis for the evaluation of the polymer's performance.EGDMA or DVB?
As mentioned previously, both thermally and UV initiated polymers with different porogen and crosslinker ratios (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 70
40, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30, 80
30, 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20) were synthesized and their adsorption properties were compared. After synthesis, initial differences were observed, with the 80
20) were synthesized and their adsorption properties were compared. After synthesis, initial differences were observed, with the 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio polymers proving difficult to work with. These polymers were too viscous to handle properly, adhering to all used utensils, which made it impossible to load them into the cartridge. Consequently, the 80
20 ratio polymers proving difficult to work with. These polymers were too viscous to handle properly, adhering to all used utensils, which made it impossible to load them into the cartridge. Consequently, the 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 ratio polymers were discontinued, and further experiments focused on the 50
20 ratio polymers were discontinued, and further experiments focused on the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, and 70
40, and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratios. Three SPEs were conducted for each porogen-crosslinker ratio (50
30 ratios. Three SPEs were conducted for each porogen-crosslinker ratio (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 70
40, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30) for both UV and thermally initiated polymers. The SPE process for the synthesized polymers was already mentioned in “SPE protocol for phenolic acids” in the “Materials and methods” part of this paper. However, it should be noted that, at this stage, the polymers were only compressed with 3 to 4 kg of pressure. While this did not affect their comparability, it did prevent them from achieving a 100% adsorption rate. This limitation was later addressed by increasing the compression pressure to 9 to 10 kg, which allowed for more efficient packing and improved adsorption performance.
30) for both UV and thermally initiated polymers. The SPE process for the synthesized polymers was already mentioned in “SPE protocol for phenolic acids” in the “Materials and methods” part of this paper. However, it should be noted that, at this stage, the polymers were only compressed with 3 to 4 kg of pressure. While this did not affect their comparability, it did prevent them from achieving a 100% adsorption rate. This limitation was later addressed by increasing the compression pressure to 9 to 10 kg, which allowed for more efficient packing and improved adsorption performance.
When comparing the three porogen crosslinker ratios in terms of recovery and relative standard deviation (RSD) values across the loading, washing and elution steps, the UV-initiated polymer exhibited the best performance. In contrast, the thermally initiated polymers demonstrated the highest efficiency at a 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratio, followed closely by the 60
30 ratio, followed closely by the 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 formulation. These findings suggest that the optimal porogen-crosslinker ratio depends on the initiation method, influencing the polymer's structural properties and adsorption efficiency.
40 formulation. These findings suggest that the optimal porogen-crosslinker ratio depends on the initiation method, influencing the polymer's structural properties and adsorption efficiency.
However, this study also concluded that these UV-initiated polymers are not suitable for the SPE of phenolic acids, as none achieved 100% adsorption, as evidenced by residual recoveries in the washing and loading steps. This outcome may be attributed to the crosslinker EGDMA, which has a high polarity and lacks the ability to form π–π stacking interactions. In contrast, thermally initiated polymers were able to reach full adsorption. Since the performance of DVB polymers at 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 70
40 and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 was comparable, no definitive conclusion could be drawn regarding the optimal ratio.
30 was comparable, no definitive conclusion could be drawn regarding the optimal ratio.
The recovery values of the analyte elution and their corresponding RSD values are presented in Table 1. A detailed comparison of the UV- and thermally initiated polymers is provided in the Fig S2–S4 (SI), where bar charts offer a visual representation for easier interpretation of the results. Regarding the choice between EGDMA and DVB, DVB was selected as the preferred crosslinker due to its superior performance in SPE. As expected, DVBs aromatic structure facilitates π–π stacking interactions with the aromatic rings of phenolic compounds, which significantly enhances retention and selectivity, resulting in higher recoveries. These non-covalent interactions contribute to the high recovery rates observed in SPE, as the DVB-based polymer matrices can effectively “capture” phenolic acids through complementary by the polymer network. This combination of rigid crosslinked structure and π–π interactions makes DVB particularly effective for SPE applications targeting aromatic analytes such as phenolic acids, which has been backed up by various studies.42,43
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 70
40 and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 UV photoinitiated (EGDMA) and thermally initiated (DVB) polymers. To determine recovery rates, 1 mL of a 100 mg per L phenolic acid mixture was loaded onto each SPE cartridge, and the eluate was collected at a flow rate of 3–4 s per drop. The procedure was repeated three times for each polymer formulation (50
30 UV photoinitiated (EGDMA) and thermally initiated (DVB) polymers. To determine recovery rates, 1 mL of a 100 mg per L phenolic acid mixture was loaded onto each SPE cartridge, and the eluate was collected at a flow rate of 3–4 s per drop. The procedure was repeated three times for each polymer formulation (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, and 70
40, and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 DVB/EGDMA), using three independently packed cartridges per run
30 DVB/EGDMA), using three independently packed cartridges per run
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 polymer 50 polymer |
60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 polymer 40 polymer |
70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 polymer 30 polymer |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EGDMA | DVB | EGDMA | DVB | EGDMA | DVB | |||||||
| Analytes | RE E/% | RSD/% | RE E/% | RSD E/% | RE E/% | RSD E/% | RE E/% | RSD E/% | RE E/% | RSD E/% | RE E/% | RSD E/% |
| Chlor A | 72.04 | 22.68 | 103.29 | 12.24 | 86.99 | 11.51 | 94.81 | 4.38 | 84.48 | 29.74 | 96.81 | 1.02 |
| Caff A | 84.31 | 23.53 | 104.03 | 10.38 | 97.94 | 7.71 | 96.94 | 4.89 | 96.99 | 27.78 | 99.28 | 2.00 |
| DHB | 60.47 | 27.91 | 70.65 | 4.86 | 71.58 | 0.11 | 69.32 | 6.92 | 69.01 | 17.53 | 73.70 | 0.51 |
| Fer A | 84.15 | 22.54 | 107.30 | 14.45 | 97.72 | 9.51 | 97.45 | 3.40 | 97.51 | 29.46 | 99.22 | 1.69 |
| Cin A | 90.21 | 21.48 | 113.03 | 9.76 | 102.14 | 8.71 | 110.39 | 1.34 | 102.10 | 28.55 | 108.22 | 2.70 |
Dynamic loading capacity
To further investigate the different thermally initiated polymers, a dynamic loading capacity study was conducted. Initially, a 100 mg per L Fer A solution was used in incremental loading steps to compare the three polymer formulations. The varying slopes of the functions in the resulting graph indicated that the polymers with 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 70
40 and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratios were optimal, as both achieved 100% adsorption of Fer A in the first loading step. The 70
30 ratios were optimal, as both achieved 100% adsorption of Fer A in the first loading step. The 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratio maintained full adsorption even in the second step, whereas the 50
30 ratio maintained full adsorption even in the second step, whereas the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 polymer exhibited a much shallower slope indicating slower adsorption kinetics. In contrast, the 60
50 polymer exhibited a much shallower slope indicating slower adsorption kinetics. In contrast, the 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 70
40 and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 polymers displayed steeper slopes, suggesting faster and more efficient adsorption. These characteristics make them more promising candidates for further investigations into adsorption capacity (Fig. S5).
30 polymers displayed steeper slopes, suggesting faster and more efficient adsorption. These characteristics make them more promising candidates for further investigations into adsorption capacity (Fig. S5).
A direct comparison between the 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 and 70
40 and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 polymers using a 100 mg per L Fer A solution over 30 loading steps revealed nearly identical adsorption trends (Fig. S6). However, since the 70
30 polymers using a 100 mg per L Fer A solution over 30 loading steps revealed nearly identical adsorption trends (Fig. S6). However, since the 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 thermally initiated polymer exhibited smaller standard deviations, an additional dynamic loading study was conducted with this polymer to determine its saturation point.
30 thermally initiated polymer exhibited smaller standard deviations, an additional dynamic loading study was conducted with this polymer to determine its saturation point.
Saturation was reached after 60 loading steps using a 100 mg per L Fer A solution. The peak areas were converted into concentrations using external calibration, and the dynamic loading capacity was determined by fitting the data to a Boltzmann model (Fig. S7). The final calculations revealed a dynamic loading capacity of 31.61 mg g−1, which is ten times higher than the value reported for a 30 mg Oasis® HLB 1 cc cartridge in the literature.44
Optimization of the elution solution
Upon evaluating the recovery rates of the phenolic acids during the SPE process, particularly the suboptimal recovery of DHB, a new elution solution was investigated. The second elution solution, consisting of MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) with 2% FA, demonstrated superior effectiveness in enhancing the recovery of DHB compared to the first elution solution (MeOH
1) with 2% FA, demonstrated superior effectiveness in enhancing the recovery of DHB compared to the first elution solution (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN
ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 1
water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with 2% FA). Although ACN is typically considered to have a higher elution strength, the enhanced performance of the MeOH-based solution can be attributed to several key factors. Methanol (MeOH), with its higher polarity compared to ACN, promotes stronger interactions with DHB, a highly polar analyte. Additionally, as a protic solvent, MeOH is capable of forming hydrogen bonds with DHB, which facilitates its desorption from the polymer surface. In contrast, ACN, being an aprotic solvent, lacks the ability to form such interactions, thereby resulting in reduced desorption efficiency for DHB.
1 with 2% FA). Although ACN is typically considered to have a higher elution strength, the enhanced performance of the MeOH-based solution can be attributed to several key factors. Methanol (MeOH), with its higher polarity compared to ACN, promotes stronger interactions with DHB, a highly polar analyte. Additionally, as a protic solvent, MeOH is capable of forming hydrogen bonds with DHB, which facilitates its desorption from the polymer surface. In contrast, ACN, being an aprotic solvent, lacks the ability to form such interactions, thereby resulting in reduced desorption efficiency for DHB.
Using the previous elution solution (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ACN
ACN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 1
water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with 2% FA) in the SPE protocol, the following recoveries were obtained (n = 3): Chlor A: 105.53 ± 2.52%, Caff A: 103.03 ± 3.09%, DHB: 90.20 ± 2.63%, Fer A: 108.46 ± 2.23% and Cin A: 105.00 ± 2.36%. However, when the new elution solution (MeOH
1 with 2% FA) in the SPE protocol, the following recoveries were obtained (n = 3): Chlor A: 105.53 ± 2.52%, Caff A: 103.03 ± 3.09%, DHB: 90.20 ± 2.63%, Fer A: 108.46 ± 2.23% and Cin A: 105.00 ± 2.36%. However, when the new elution solution (MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water, 1
water, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with 2% FA) was applied, improved recoveries were observed, particularly for DHB: Chlor A: 105.88 ± 1.96%, Caff A: 104.74 ± 4.00%, DHB: 104.49 ± 3.05%, Fer A: 110.44 ± 3.02% and Cin A: 108.68 ± 2.62%. For all SPE experiments, 1 mL of a 100 mg per L phenolic acid mixture was loaded, and each measurement was performed in triplicate. The recovery-results for the different elution solutions are shown in Fig. S1 (SI).
1 with 2% FA) was applied, improved recoveries were observed, particularly for DHB: Chlor A: 105.88 ± 1.96%, Caff A: 104.74 ± 4.00%, DHB: 104.49 ± 3.05%, Fer A: 110.44 ± 3.02% and Cin A: 108.68 ± 2.62%. For all SPE experiments, 1 mL of a 100 mg per L phenolic acid mixture was loaded, and each measurement was performed in triplicate. The recovery-results for the different elution solutions are shown in Fig. S1 (SI).
Method validation
The HPLC method was successfully validated by assessing the linearity of the calibration curve, evaluating sample stability, and determining the limits of detection (LOD) and quantification (LOQ).For the linearity of calibration, concentrations ranging from 25 to 200 mg L−1 were tested, demonstrating a linear relationship within the examined concentration range, with correlation coefficients (R2) between 0.9998 and 0.9999. The maximum RSD value observed was 0.239%, while the minimum RSD value was 0.150%. The detailed data for the linearity of calibration are provided in Table S4 (SI).
After performing a 24-hour stability test on two standard solutions of a 100 mg per L phenolic acid mixture, it was concluded that both the HPLC system's autosampler and the phenolic acid mixture exhibited excellent stability. This was evidenced by the consistent values across all measurements (Fig. S8). The RSD values remained low, indicating minimal variation over time (Table S5). Therefore, measurements can be reliably conducted over a 24-hour period without significant fluctuations.
The LOD and LOQ were determined by using an external calibration with concentrations ranging from 25 to 200 mg L−1. The calculated LOD, as low as 0.5 mg L−1, and LOQ, down to 2 mg L−1, demonstrate the high sensitivity and reliability of the analytical method. The low LOD ensures that each analyte can be easily identified, while the LOQ allows for accurate quantification of analyte concentrations as low as 2 mg L−1. Consequently, the HPLC-UV method is highly suitable for precise and reproducible measurements. The calculated LOD and LOQ values for the five analyzed analytes are provided in Table S6 (SI).
Recovery studies
To assess the recoveries from the SPEs conducted with a 100 mg per L phenolic acid mixture, the synthesized and optimized polymer, along with the Oasis® HLB and Strata™-X cartridges were evaluated in triplicate. An external calibration curve was constructed using concentrations ranging from 25 to 200 mg L−1, enabling the calculation of recovery efficiencies (RE) and relative standard deviation (RSD) values.Recoveries (RE) were determined by quantifying each analyte's concentration from its peak area using an external calibration curve. RE (%) was calculated as the ratio of the measured concentration to the theoretical concentration, multiplied by 100. The relative standard deviation (RSD, %) of the recoveries was calculated as the standard deviation divided by the mean recovery, multiplied by 100. The SPE recovery study revealed that all three stationary phases – Oasis® HLB, Strata™-X and the synthesized and optimized polymer – demonstrated excellent performance, with recoveries around 100%. The highest recoveries were observed for Strata™-X (up to 116%), while the synthesized polymer achieved recoveries of up to 110%. These results confirm the effectiveness of the synthesized polymer as a stationary phase for phenolic acid extraction (Fig. 2).
The RSD values provide further validation of the reliability of the SPE process across all investigated sorbents. While the Strata™-X cartridge exhibited the lowest standard deviation, the polymer phase demonstrated slightly higher variability, particularly for Caff A, likely due to inconsistencies in manual cartridge preparation. Despite this, all RSD values remained below 3%, except for Caff A in the polymer SPE, where the value marginally exceeded this threshold.
These findings support the conclusion that the optimized polymer is a reliable and effective stationary phase for the SPE of phenolic acids (Table S7).
Polymer reusability
The reusability of the polymer for multiple SPE cycles was evaluated using a 100 mg L−1 mixture of the five previously mentioned phenolic acids. Following the established protocol for the optimized polymer and the existing SPE protocols for Oasis® and Strata™-X, five consecutive SPE cycles were performed. The recoveries and standard deviations for each stationary phase were determined. To ensure reliability, the study was repeated three times. The obtained values are presented in Fig. 3 for easier comparison.Recoveries were determined and calculated as described in the “Recovery studies” section of this manuscript.
The results from five consecutive SPE cycles demonstrated that the polymer maintained high recovery rates and low standard deviations across all analytes, surpassing the performance of the commercial materials. Strata™-X showed variability and became unreliable after the third cycle, while Oasis® HLB maintained consistent performance through the third cycle but showed increased deviations in the fourth. In contrast, the optimized polymer exhibited superior stability and reusability, making it a more effective material for extended SPE cycles. These findings highlight the polymer's potential for sustained performance in repeated analytical procedures.
Real-life sample study
To assess the effectiveness of the polymers in SPE, honey, a natural source of phenolic acids, was used as a real-world sample. Two different types of honey were analyzed following the SPE methods outlined before. The collected flow-through was subsequently analyzed by HPLC-UV using the established method. For comparative purposes, Oasis® HLB was also utilized as the stationary phase in parallel with the optimized polymer. To ensure reproducibility and reliability, the experiment was conducted in triplicate.Determination of phenolic acids in “JedenTag”-honey
One of the analyzed samples, “JedenTag”-honey, was found to contain Fer A, a compound which is known for its antioxidant, anti-inflammatory, antimicrobial and anticancer properties. Given its importance in traditional medicine, the study aimed to determine the actual concentration of the Fer A in the sample. HPLC analysis confirmed its presence, and SPE was performed using both the optimized polymer and a commercially available Oasis® HLB cartridge for comparative studies. External calibration resulted in an average Fer A concentration of 9.72 ± 0.04 ppm (0.972 mg per 100 g of honey), based on three different measurements (9.73 ppm, 9.74 ppm, 9.67 ppm) that had been conducted. This average value is moderately higher than the typical range of Fer A concentrations in honey (0.02 to 0.82 mg per 100 g).45 The SPE recoveries exceeded 90% for both materials, demonstrating the efficiency of the polymers (polymer: RE: 99.42%, RSD: 1.61% and Oasis® HLB: RE: 107.45%, RSD: 2.56%). Since “JedenTag” honey is sourced from multiple regions, including Argentina, Brazil, Hungary and Greece, determining the exact floral origin of the phenolic acids was challenging. However, comparison of the HPLC chromatograms reveals that the concentration process was effective, as evidenced by a peak area that was twice as large after SPE compared to before (Fig. 4). | ||
| Fig. 4 Demonstration of the found ferulic acid in the honey samples (left: “JedenTag”-honey, right: honey 2023) before the SPE process (above) and after the SPE process (below) (n = 3). | ||
Determination of phenolic acids in honey from Röthis (2023)
A honey sample collected in 2023 from Röthis (Vorarlberg, Austria) was analyzed, revealing the presence of Fer A, one of the five phenolic acids previously examined. Due to the close proximity of the chromatographic peaks, the identification of Fer A presented initial challenges. However, the application of SPE significantly improved peak resolution, thereby enabling precise quantification and reliable recovery calculations (Fig. 4).To further evaluate the effectiveness of the synthesized polymer, a commercially available Oasis® HLB cartridge was also tested for comparison. HPLC-UV analysis of the concentrated samples revealed that the separation was successful, confirming the SPE methods efficiency. Recovery rates exceeding 90% were achieved for both the polymer (RE: 107.80%, RSD: 0.90%) and Oasis® HLB (RE: 125.66%, RSD: 2.89%) demonstrating excellent performance. The average Fer A concentration in the Röthis honey sample was determined to be 1.79 ± 0.01 ppm (0.179 mg per 100 g honey), based on three different measurements (1.79 ppm, 1.80 ppm, 1.79 ppm) that had been conducted. Although this value is on the lower end, it falls within the established range of 0.02–0.82 mg per 100 g typically found in honey.45
Characterization of the polymer
Various techniques were utilized to characterize the thermally initiated polymers synthesized 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, and 70
40, and 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 ratios. Attenuated total reflectance (ATR) spectroscopy confirmed the successful integration of both the monomer and crosslinker into the polymer network. Additionally, scanning electron microscopy (SEM) combined with energy-dispersive X-ray spectroscopy (EDXS) was employed to analyze the pore size distribution and elemental composition of the different polymers.
30 ratios. Attenuated total reflectance (ATR) spectroscopy confirmed the successful integration of both the monomer and crosslinker into the polymer network. Additionally, scanning electron microscopy (SEM) combined with energy-dispersive X-ray spectroscopy (EDXS) was employed to analyze the pore size distribution and elemental composition of the different polymers.
ATR measurements
The resulting ATR spectra, along with their corresponding data tables, are provided in Table S8 and Fig. S9 to S13 (SI).The ATR spectra reveal that all the polymers share a consistent composition, a result that was later confirmed by EDXS measurements. Specific bands associated with the monomer and the crosslinker were clearly identified. Notably, bands present in both the crosslinker, and the polymer were detected at 800 cm−1 (olefinic, δ(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H)) and 910 cm1 (olefinic, δ(
C–H)) and 910 cm1 (olefinic, δ(![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H)). Additionally, bands common to both the monomer and the polymer appeared at 1100 cm−1 (amine, δ(C–N)), 1200 cm−1 (ether, ν(–C–O–C)) and 1650 cm−1 (olefinic, ν(–C
C–H)). Additionally, bands common to both the monomer and the polymer appeared at 1100 cm−1 (amine, δ(C–N)), 1200 cm−1 (ether, ν(–C–O–C)) and 1650 cm−1 (olefinic, ν(–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C)).
C)).
SEM and EDXS measurements
SEM and EDXS measurements were conducted to examine the pore structure and elemental composition of the thermally synthesized polymers with 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, 60
50, 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40, 70
40, 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 monomer-to-crosslinker ratios. The corresponding images and data are presented in Fig. S14 (SI). However, since the results for all thermally synthesized polymers were comparable, only the polymer with the 70
30 monomer-to-crosslinker ratios. The corresponding images and data are presented in Fig. S14 (SI). However, since the results for all thermally synthesized polymers were comparable, only the polymer with the 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 porogen-to-crosslinker ratio is shown. The synthesized 70
30 porogen-to-crosslinker ratio is shown. The synthesized 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 polymer exhibited pore sizes ranging from 55 nm to 75 nm with uniform distribution and circular agglomerates, indicating a well-controlled synthesis process. The elemental composition of around 92.8% C, 4.5% N and 2.6% O confirmed the successful incorporation of the iPOX monomer, as evidenced by the presence of the nitrogen. The detected Si and Pt peaks were attributed to the silicon wafer substrate and the platinum coating used for SEM analysis.
30 polymer exhibited pore sizes ranging from 55 nm to 75 nm with uniform distribution and circular agglomerates, indicating a well-controlled synthesis process. The elemental composition of around 92.8% C, 4.5% N and 2.6% O confirmed the successful incorporation of the iPOX monomer, as evidenced by the presence of the nitrogen. The detected Si and Pt peaks were attributed to the silicon wafer substrate and the platinum coating used for SEM analysis.
For the 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 40 polymer, the pore size ranged from 30 nm to 50 nm exhibiting circular agglomerates and a homogeneous structure, further indicating effective synthesis. The elemental composition was around 92.2% C, 4.8% N and 2.9% O, closely resembling that of the 70
40 polymer, the pore size ranged from 30 nm to 50 nm exhibiting circular agglomerates and a homogeneous structure, further indicating effective synthesis. The elemental composition was around 92.2% C, 4.8% N and 2.9% O, closely resembling that of the 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 polymer, confirming the successful incorporation of the iPOX monomer. Again, Si and Pt were observed due to the silicon wafer substrate and platinum coating.
30 polymer, confirming the successful incorporation of the iPOX monomer. Again, Si and Pt were observed due to the silicon wafer substrate and platinum coating.
For the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 polymer, the average pore size ranged from 50 nm to 95 nm, exhibiting a homogeneous structure with circular agglomerates. Elemental analysis revealed around 91.5% C, 5.6% N and 2.7% O, with the presence of nitrogen confirming the successful incorporation of iPOX. The Si and Pt peaks were again due to the substrate and coating.
50 polymer, the average pore size ranged from 50 nm to 95 nm, exhibiting a homogeneous structure with circular agglomerates. Elemental analysis revealed around 91.5% C, 5.6% N and 2.7% O, with the presence of nitrogen confirming the successful incorporation of iPOX. The Si and Pt peaks were again due to the substrate and coating.
In summary, EDXS and SEM analyses revealed a consistent composition across all three polymers, with carbon, nitrogen, and oxygen contents aligning with the expected incorporation of the iPOX monomer. The nitrogen content in particular served as direct evidence of successful monomer integration into the polymer matrix. Additionally, the detection of Si and Pt signals was attributed to the silicon wafer substrate and platinum coating used during SEM analysis. These findings collectively validate the effectiveness of the thermal polymerization method in producing well-defined polymer structures with predictable elemental compositions.
Conclusion
In conclusion, the thermally synthesized 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 polymer demonstrated superior performance in the SPE of phenolic acids, achieving a high dynamic loading capacity of around 31.61 mg g−1. This capacity significantly surpasses that of the commercially available Oasis® HLB cartridge, highlighting the polymer's enhanced adsorption efficiency. The polymer's effectiveness was further demonstrated through recovery tests, which showed comparable results to widely used commercial alternatives such as, Oasis® HLB and Strata™-X, along with remarkable reusability over multiple extraction cycles. Even when applied to real-life samples the polymer maintained high efficiency in extracting phenolic acids, reinforcing its practical applicability in complex sample matrices.
30 polymer demonstrated superior performance in the SPE of phenolic acids, achieving a high dynamic loading capacity of around 31.61 mg g−1. This capacity significantly surpasses that of the commercially available Oasis® HLB cartridge, highlighting the polymer's enhanced adsorption efficiency. The polymer's effectiveness was further demonstrated through recovery tests, which showed comparable results to widely used commercial alternatives such as, Oasis® HLB and Strata™-X, along with remarkable reusability over multiple extraction cycles. Even when applied to real-life samples the polymer maintained high efficiency in extracting phenolic acids, reinforcing its practical applicability in complex sample matrices.
Comprehensive characterization through ATR spectroscopy, SEM, and EDXS confirmed the successful incorporation of both the monomer and crosslinker into the polymer matrix, validating the controlled synthesis process and the polymer's structural integrity. The uniform pore structure and consistent elemental composition further support the reliability and reproducibility of the developed material.
While the polymer demonstrated excellent efficiency in SPE applications, further optimizations could be explored to enhance its performance. Future studies could investigate variations in crosslinker-to-monomer ratios to fine-tune porosity, surface area, and selectivity. Additionally, testing the polymer with a broader range of analytes and different sample conditions could further validate its versatility and expand its application potential.
Overall, the synthesized polymer represents a promising alternative to conventional SPE cartridges, combining high extraction efficiency, excellent reusability, and cost-effectiveness. Its superior performance in both controlled and real-life sample conditions positions it as a viable candidate for routine analytical and preparative extractions of phenolic acids and potentially other target compounds in environmental, food, and pharmaceutical analyses.
Author contributions
L. S. Nigsch: conceptualization, formal analysis, methodology, validation, investigation, writing – original draft. C. Kappacher: investigation, writing – review & editing. D. Hense: investigation, writing – review & editing. C. W. Huck: writing – review & editing. O. I. Strube: writing – review & editing. M. Rainer: project administration, supervision, visualization, resources, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Data availability
The data supporting this article have been included as part of the supplementary information (SI). Supplementary information: detailed information on polymer synthesis, calibration curves, stability studies, validation parameters, recovery and capacity studies, ATR spectra and SEM images. See DOI: https://doi.org/10.1039/d5ay00885a.Acknowledgements
We would like to thank Mr A. Nigsch for kindly providing the honey samples from Röthis.References
- J. Chen, J. Yang, L. Ma, J. Li, N. Shahzad and C. K. Kim, Structure-antioxidant activity relationship of methoxy, phenolic hydroxyl, and carboxylic acid groups of phenolic acids, Sci. Rep., 2020, 10, 2611 CrossRef PubMed.
- W. Liu, X. Cui, Y. Zhong, R. Ma, B. Liu and Y. Xia, Phenolic metabolites as therapeutic in inflammation and neoplasms: Molecular pathways explaining their efficacy, Pharmacol. Res., 2023, 193, 106812 CrossRef PubMed.
- G. Caruso, J. Godos, A. Privitera, G. Lanza, S. Castellano, A. Chillemi, O. Bruni, R. Ferri, F. Caraci and G. Grosso, Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer's Disease, Nutrients, 2022, 14, 819 CrossRef PubMed.
- N. Kumar and N. Goel, Phenolic acids: Natural versatile molecules with promising therapeutic applications, Biotechnol. Rep., 2019, 24, e00370 CrossRef PubMed.
- H. B. Rashmi and P. S. Negi, Phenolic acids from vegetables: A review on processing stability and health benefits, Food Res. Int., 2020, 136, 109298 CrossRef PubMed.
- Q.-W. Zhang, L.-G. Lin and W.-C. Ye, Techniques for extraction and isolation of natural products: a comprehensive review, Chin. Med. J., 2018, 13, 20 CrossRef PubMed.
- S. Al Jitan, S. A. Alkhoori and L. F. Yousef, in Studies in Natural Products Chemistry, ed. A.-U. Rahman, Elsevier, 2018, vol. 58, pp. 389–417 Search PubMed.
- L. M. Thornton Hampton, H. De Frond, K. Gesulga, S. Kotar, W. Lao, C. Matuch, S. B. Weisberg, C. S. Wong, S. Brander, S. Christansen, C. R. Cook, F. Du, S. Ghosal, A. B. Gray, J. Hankett, P. A. Helm, K. T. Ho, T. Kefela, G. Lattin, A. Lusher, L. Mai, R. E. McNeish, O. Mina, E. C. Minor, S. Primpke, K. Rickabaugh, V. C. Renick, S. Singh, B. van Bavel, F. Vollnhals and C. M. Rochman, The influence of complex matrices on method performance in extracting and monitoring for microplastics, Chemosphere, 2023, 334, 138875 CrossRef PubMed.
- C. Bitwell, S. S. Indra, C. Luke and M. K. Kakoma, A review of modern and conventional extraction techniques and their applications for extracting phytochemicals from plants, Sci. Afr., 2023, 19, e01585 Search PubMed.
- M. Mencin, M. Mikulic-Petkovsek, R. Veberič and P. Terpinc, Development and Optimisation of Solid-Phase Extraction of Extractable and Bound Phenolic Acids in Spelt (Triticum spelta L.) Seeds, Antioxidants, 2021, 10, 1085 CrossRef PubMed.
- A. Michalkiewicz, M. Biesaga and K. Pyrzynska, Solid-phase extraction procedure for determination of phenolic acids and some flavonols in honey, J. Chromatogr. A, 2008, 1187, 18–24 CrossRef CAS PubMed.
- X. Hou, X. Lu, S. Tang, L. Wang and Y. Guo, Graphene oxide reinforced ionic liquid-functionalized adsorbent for solid-phase extraction of phenolic acids, J. Chromatogr. B, 2018, 1072, 123–129 CrossRef CAS PubMed.
- S. H. Hashemi and M. Kaykhaii, Porous Polymer Sorbents in Micro Solid Phase Extraction: Applications, Advantages, and Challenges, Top. Curr. Chem., 2024, 382, 37 CrossRef CAS PubMed.
- P. Chen, Q. Zhang, H. Yin, S. Di, H. Liu, H. Qin, M. Liu, Y. Liu, Z. Li and S. Zhu, Recent Progress and Applications of Advanced Nanomaterials in Solid-Phase Extraction, Electrophoresis, 2025, 46, 582–601 CrossRef PubMed.
- T. Hu, R. Chen, Q. Wang, C. He and S. Liu, Recent advances and applications of molecularly imprinted polymers in solid-phase extraction for real sample analysis, J. Sep. Sci., 2021, 44, 274–309 CrossRef CAS PubMed.
- D. Wang, X. Chen, J. Feng and M. Sun, Recent advances of ordered mesoporous silica materials for solid-phase extraction, J. Chromatogr. A, 2022, 1675, 463157 CrossRef CAS PubMed.
- C. D. Bosco, M. G. De Cesaris, N. Felli, E. Lucci, S. Fanali and A. Gentili, Carbon nanomaterial-based membranes in solid-phase extraction, Microchim. Acta, 2023, 190, 175 CrossRef CAS PubMed.
- K. Aoi and M. Okada, Polymerization of oxazolines, Prog. Polym. Sci., 1996, 21, 151–208 CrossRef CAS.
- C. Weber, T. Neuwirth, K. Kempe, B. Ozkahraman, E. Tamahkar, H. Mert, C. R. Becer and U. S. Schubert, 2-Isopropenyl-2-oxazoline: A Versatile Monomer for Functionalization of Polymers Obtained via RAFT, Macromolecules, 2012, 45, 20–27 CrossRef CAS.
- M. Singh, L. Poláková, A. D. S. Pereira, O. Pop-Georgievski, J. Svoboda, T. Riedel, S. Gupta, Z. Sedláková, V. Raus and R. Poręba, Well-defined poly(2-isopropenyl-2-oxazoline) brushes provide fouling resistance and versatility in surface functionalization, Polym. Chem., 2024, 15, 3300–3310 RSC.
- F. Meischl, C. G. Kirchler, S. E. Stuppner and M. Rainer, Comparative study of substituted poly(4-vinylbenzyl chloride/ethylene glycol dimethacrylate) sorbents for enrichment of selected pharmaceuticals and estrogens from aqueous samples, J. Hazard. Mater., 2018, 355, 180–186 CrossRef CAS PubMed.
- N. Morales, S. C. Thickett and F. Maya, Effect of crosslinker/porogen ratio on sponge-nested polymer monoliths for solid-phase extraction, J. Chromatogr. A, 2024, 1730, 465124 CrossRef CAS PubMed.
- M. Cegłowski, J. Kurczewska, A. Lusina, T. Nazim and P. Ruszkowski, EGDMA- and TRIM-Based Microparticles Imprinted with 5-Fluorouracil for Prolonged Drug Delivery, Polymers, 2022, 14, 1027 CrossRef PubMed.
- R. Viveiros, S. Rebocho and T. Casimiro, Green Strategies for Molecularly Imprinted Polymer Development, Polymers, 2018, 10, 306 CrossRef PubMed.
- Q.-Z. Feng, L.-X. Zhao, W. Yan, J.-M. Lin and Z.-X. Zheng, Molecularly imprinted solid-phase extraction combined with high performance liquid chromatography for analysis of phenolic compounds from environmental water samples, J. Hazard. Mater., 2009, 167, 282–288 CrossRef CAS PubMed.
- M. Mora-Granados, D. González-Gómez, J. S. Jeong and A. Gallego-Picó, A Molecularly Imprinted Polymer for Selective Extraction of Phenolic Acids from Human Urine, Appl. Sci., 2021, 11, 1577 CrossRef CAS.
- M. N. Qureshi, G. Stecher, C. Huck and G. Bonn, Preparation of polymer based sorbents for solid phase extraction of polyphenolic compounds, Cent. Eur. J. Chem., 2011, 9, 206–212 CAS.
- M. Harder, R. Bakry, F. Lackner, P. Mayer, C. Kappacher, C. Grießer, S. Neuner, C. W. Huck, G. K. Bonn and M. Rainer, The Crosslinker Matters: Vinylimidazole-Based Anion Exchange Polymer for Dispersive Solid-Phase Extraction of Phenolic Acids, Separations, 2022, 9, 72 CrossRef CAS.
- A. Wibowo, N. Makhnunah, D. Irawati, C. Purnawan, N. Nurhayati and H. Storz, Synthesis and Thermal-Stability Study of Polybutylene Itaconate Modified with Divinyl Benzene and Glycerol, Indones. J. Chem., 2014, 14, 283–289 CrossRef CAS.
- L. Agibayeva, Y. Melnikov, D. Kubiyeva and R. Kondaurov, Impact of Crosslinking Agent on Sorption Properties of Molecularly Imprinted Polymers in Relation to Silver, Polym., 2025, 17, 2055 CAS.
- A. Mueller, A Note about Crosslinking Density in Imprinting Polymerization, Molecules, 2021, 26, 5139 CrossRef CAS PubMed.
- K. Golker and I. A. Nicholls, The effect of crosslinking density on molecularly imprinted polymer morphology and recognition, Eur. Polym. J., 2016, 75, 423–430 CrossRef CAS.
- M. A. Gauthier, J. Luo, D. Calvet, C. Ni, X. X. Zhu, M. Garon and M. D. Buschmann, Degree of crosslinking and mechanical properties of crosslinked poly(vinyl alcohol) beads for use in solid-phase organic synthesis, Polymer, 2004, 45, 8201–8210 CrossRef CAS.
- S. Shoravi, G. D. Olsson, B. C. G. Karlsson and I. A. Nicholls, On the Influence of Crosslinker on Template Complexation in Molecularly Imprinted Polymers: A Computational Study of Prepolymerization Mixture Events with Correlations to Template-Polymer Recognition Behavior and NMR Spectroscopic Studies, Int. J. Mol. Sci., 2014, 15, 10622–10634 CrossRef CAS PubMed.
- M. J. Whitcombe, I. Chianella, L. Larcombe, S. A. Piletsky, J. Noble, R. Porter and A. Horgan, The rational development of molecularly imprinted polymer-based sensors for protein detection, Chem. Soc. Rev., 2011, 40, 1547–1571 RSC.
- S. Ahmed, S. A. Sulaiman, A. A. Baig, M. Ibrahim, S. Liaqat, S. Fatima, S. Jabeen, N. Shamim and N. H. Othman, Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action, Oxid. Med. Cell. Longev., 2018, 2018, 8367846 CrossRef PubMed.
- M. A. Al-Kafaween, M. Alwahsh, A. B. Mohd Hilmi and D. H. Abulebdah, Physicochemical Characteristics and Bioactive Compounds of Different Types of Honey and Their Biological and Therapeutic Properties: A Comprehensive Review, J. Antibiot., 2023, 12, 337 CrossRef CAS PubMed.
- Austrian Standards International, DIN 32645: Chemische Analytik – Nachweis-, Erfassungs- und Bestimmungsgrenze unter Wiederholbedingungen – Begriffe, Verfahren, Auswertung, 2008, 30 Search PubMed.
- F. T. Peters, M. Hartung, T. Daldrup, F. Mußhoff and L. D. Paul, GTFCh: Requirements for the validation of analytical methods, 2009, vol. 1, p. 23 Search PubMed.
- C. Sun, H. Tan, Y. Zhang and H. Zhang, Phenolics and abscisic acid identified in acacia honey comparing different SPE cartridges coupled with HPLC-PDA, J. Food Compos. Anal., 2016, 53, 91–101 CrossRef CAS.
- E. Bacalum, M. Radulescu, E.-E. Iorgulescu and V. David, Breakthrough parameters of spe procedure on C18 cartridges for some polar compounds, Rev. Roum. Chim., 2011, 56, 137–143 CAS.
- N. Fontanals, M. Galià, R. M. Marcé and F. Borrull, Solid-phase extraction of polar compounds with a hydrophilic copolymeric sorbent, J. Chromatogr. A, 2004, 1030, 63–68 CrossRef CAS PubMed.
- M. Sobiesiak and B. Podkoscielna, Preparation and characterization of porous DVB copolymers and their applicability for adsorption (solid-phase extraction) of phenol compounds, Appl. Surf. Sci., 2010, 257, 1222–1227 CrossRef CAS.
- What is the loading capacity for Oasis Solid Phase Extraction devices?, https://support.waters.com/KB_Chem/Sample_Preparation/WKB25301_What_is_the_loading_capacity_for_Oasis_HLB_devices, accessed 8 February 2025.
- J. Lachman, A. Hejtmánková, J. Sykora, J. Karban, M. Orsák and B. Rygerova, Contents of Major Phenolic and Flavonoid Antioxidants in Selected Czech Honey, Czech J. Food Sci., 2010, 28, 412–426 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |



