Open Access Article
Open Access ArticleFirst-in-class hydrazone–pyrazoline sensors for selective detection of Zn2+, Cd2+, and Hg2+ in aqueous environments†
Alexander
Ciupa
 *
*
Materials Innovation Factory, University of Liverpool, 51 Oxford Street, Liverpool L7 3NY, UK. E-mail: ciupa@liverpool.ac.uk
First published on 18th June 2025
Abstract
Six first-in-class hydrazone–pyrazoline fluorescent sensors that selectively detect Zn2+, Cd2+, and Hg2+ at three distinct wavelengths in aqueous environments are reported. These novel hybrid sensors are synthesized in just two steps from inexpensive, commercially available materials, enabling rapid generation and screening for desirable photophysical properties. Lead sensor P1 was validated for in situ detection of group 12 metals in river and pond water using a portable, low-cost device. Crucially, this system enabled rapid, naked-eye detection of toxic metals directly in the environment without the need for expensive instrumentation or mains electricity. These findings establish a new direction in pyrazoline sensor research and set a high benchmark for future sensor development.
Introduction
The first three metals in group 12 of the periodic table are zinc, the second most abundant transition metal in the human body critical to life,1 cadmium, a highly toxic industrial pollutant linked to numerous cancers,2 and mercury, with well-established neurotoxicity.3 The European Union (EU) maximum drinking water limits for cadmium and mercury are 44 nM and 5 nM respectively.4 The World Health Organisation (WHO) has a recommended zinc drinking water limit of 46 μM.5 The regular monitoring of group 12 metals in aqueous environments such as drinking water and external water courses is paramount. The ability to distinguish between Zn2+, Cd2+, and Hg2+ in aqueous environments is a major challenge requiring expensive and complex equipment not widely available, for example, ICP-MS. Fluorescence spectroscopy offers several advantages6 over traditional techniques including high selectivity, low limits of detection (LoD), and rapid analysis. Fluorescent sensors are “turned on” when the analyte increases fluorescence emission (λem)7 or “turned off” when the analyte decreases λem.8 Inhibition of photoinduced electron transfer (PET)9 or the chelation enhancement effect (CHEF)10 can increase λem. Decreased λem may result from fluorescence resonance energy transfer (FRET)11 or chelation-enhanced quenching effect (CHEQ).12 While numerous fluorescent sensors have been reported for Zn2+ and Cd2+,13 few can differentiate between these metals at different λem.14 The pyrazoline scaffold15 (blue in Fig. 1) is highly advantageous due to its unique fluorescence properties16 and modular structure from readily accessible chalcone precursors.17 Chalcones, while utilized as precursors for pyrazolines, have also been reported to display useful sensing properties.18 Pyridine is a well-established metal chelator often integrated into fluorescent sensors for Zn2+, Hg2+ and Fe3+ detection.19 Simple pyrazoline A displayed a “turn on” response only in the presence of Zn2+ and Cd2+;20B is a “turn on” sensor21 for Fe3+/Fe2+ whereas C displayed a “turn off” response in the presence of Fe3+/Fe2+.21 Pyrazole D (red in Fig. 1)22 demonstrated that an additional acetyl group greatly improved Zn2+/Cd2+ selectivity. A–D reveal that structural complexity is not essential for complex functionality20–22 highlighting the potential of these scaffolds in sensor development. During a recent pyrazoline synthesis study (see ESI S1†) side product P1 was identified (Scheme 1), isolated, and discovered as a “turn on” sensor for Zn2+ at λem 560 nm and Cd2+ at 510 nm and a “turn off” sensor for Hg2+ at λem 460 nm. This unexpected side product is a highly attractive first-in-class hydrazone–pyrazoline multi-analyte group 12 sensor in aqueous environments. | ||
| Fig. 1 Pyrazoline and pyrazole sensors; images reproduced from ref. 20–22 with permission from RSC. | ||
Experimental
Chemicals and instruments
Chemicals, solvents and reagents were purchased from commercial sources and used without further purification. PE refers to petroleum ether, bp 40–60 °C. Spectroscopy was performed with CHROMASOLV® gradient grade acetonitrile for HPLC, ≥99.9%, from Sigma-Aldrich. The metal complexes used in this study were LiCl, NaCl, KCl, CaCl2, MgCl2, CuCl2, NiCl2, ZnCl2, CdCl2, RuCl3, CoCl2, MnCl2, PbCl2, ZnCl2 and HgI2.UV/vis spectroscopy was performed on an Agilent Cary5000 using quartz cuvettes with a 1 cm pathlength using HPLC grade MeCN, in the 250–500 nm range with 0.2 s dwell time. Detector switchover occurred at 350 nm. FTIR spectroscopy was performed on a Bruker VERTEX 70 spectrometer. Fluorescence spectroscopy was performed on an Edinburgh Instruments FLS1000 with a xenon excitation source, 2 nm bandwidths for both excitation and emission monochromators, a scan speed of 1 nm and a dwell time of 0.2 s. Fluorescence quartz cuvettes with a 1 cm pathlength were used throughout with HPLC grade MeCN. A 100 watt 365 nm Analytik Jena high intensity UV lamp was used to image the sensors.
Synthesis of chalcones C1–C4
The following general procedure was followed (see the ESI† for more detailed information), using a method adapted from a previous synthesis,22 6 mmol 2,6-acetylpyridine was added to a stirred solution of 3.0 mmol aldehyde in MeOH followed by the addition of 3.0 mmol NaOH and stirring was continued at room temperature. After 24 hours the precipitate was filtered, washed with copious amounts of cold H2O collected and dried to afford the desired chalcone without further purification.Synthesis of hydrazone–pyrazolines P1–P6
The following general procedure was followed: 4.0 mmol of 2-hydrazinopyridine was added to a stirred solution of 1.0 mmol of required chalcone in 50 mL MeOH and heated to 60 °C. After 24 hours, the solvent was removed under reduced pressure, 100 mL H2O was added and it was extracted into 3 × 50 mL ethyl acetate. The ethyl acetate fractions were combined and the solvent was removed under reduced pressure to give an oil which was then purified by column chromatography with 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ethyl acetate
4 ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) petroleum ether to afford the desired product.
petroleum ether to afford the desired product.
Results and discussion
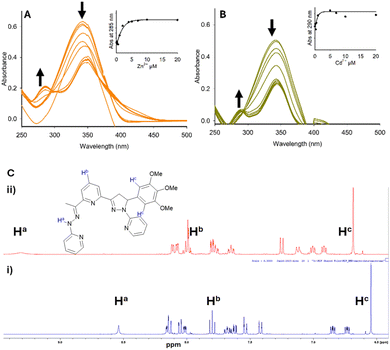
Chalcones C1–C4 were prepared using a previous procedure22 in acceptable yields (13–60%). A direct chalcone to hydrazone–pyrazoline synthesis was developed to give novel compounds P1–P4 (Scheme 2) in good yield (26–61%).This two-step synthesis from inexpensive commercially available starting materials unlocks a new class of previously unexplored pyrazoline sensors. Furthermore, the modular nature of the pyrazoline scaffold enables a high degree of modification unlocking a deeper understanding of their sensing properties. With four novel hydrazone–pyrazolines in hand, we investigated their photophysical properties against the group 12 metals using well-established protocols.22–24 UV/vis studies confirmed that P1 undergoes chelation with both Zn2+ and Cd2+ with the formation of a new band at 285 and 290 nm respectively (Fig. 2A and B). A 1H NMR titration study for P3 in the presence of the group 12 metals indicated broadening and downfield movement for multiple signals (Fig. 2C for P3 with Hg2+). The broad singlet at 8.54 ppm attributed to the hydrazone proton Ha shifted to 9.31 ppm on the addition of 2.0 equivalents Hg2+. Significant shifts in the remaining protons were also observed; for example, the pyridine signal from Hb moved from 7.79 ppm to 7.99 pm and the aryl singlet for Hc moved from 6.54 to 6.68 ppm. This is a well-established observation with previous pyrazoline sensors undergoing chelation.20–23,24a,b
The fluorescence response of P1 (φf 4.0%, τ 3.4 ns) was confirmed with the formation of new emission bands at 560 nm with Zn2+ (φf 1.0%, τ 14 ns) and 510 nm with Cd2+ (φf 1.0%, τ 13 ns) in 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 MeCN
3 MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O (Fig. 3A). On addition of Hg2+ a “turn off” response at λem 460 nm (φf < 1.0% τ 4.0 ns) is observed. Of note is that the response is retained in an aqueous solution demonstrating the real-world application of this sensor. A similar fluorescence profile was obtained with P2–P4 suggesting that substitution on the aryl ring was well-tolerated a range of both electron donating and electron withdrawing substituents. To determine the importance of hydrazone, two further pyrazolines were synthesised; P5 using phenyl hydrazine and P6 with methyl hydrazine (Scheme 3). P5 displayed no distinguishing features suggesting that the pyridine ring in P1–P4 was critical for group 12 selectivity. P6 with methyl units displayed insignificant fluorescence on addition of the group 12 metals suggesting that an aromatic unit was required. The contrast between P1 and P5 is striking confirming that pyridine units are essential for group 12 selectivity (Fig. 4).
H2O (Fig. 3A). On addition of Hg2+ a “turn off” response at λem 460 nm (φf < 1.0% τ 4.0 ns) is observed. Of note is that the response is retained in an aqueous solution demonstrating the real-world application of this sensor. A similar fluorescence profile was obtained with P2–P4 suggesting that substitution on the aryl ring was well-tolerated a range of both electron donating and electron withdrawing substituents. To determine the importance of hydrazone, two further pyrazolines were synthesised; P5 using phenyl hydrazine and P6 with methyl hydrazine (Scheme 3). P5 displayed no distinguishing features suggesting that the pyridine ring in P1–P4 was critical for group 12 selectivity. P6 with methyl units displayed insignificant fluorescence on addition of the group 12 metals suggesting that an aromatic unit was required. The contrast between P1 and P5 is striking confirming that pyridine units are essential for group 12 selectivity (Fig. 4).
 | ||
Fig. 4 Fluorescence studies of P5 (A) and P6 (B) 15 μM, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 MeCN 3 MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O at λex 285 nm; cps is counts per second. Cuvette images were obtained under the irradiation of a 100 W λex 365 nm lamp. H2O at λex 285 nm; cps is counts per second. Cuvette images were obtained under the irradiation of a 100 W λex 365 nm lamp. | ||
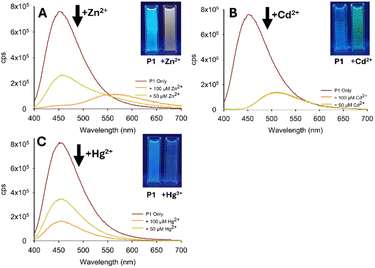
P1 was selected as the lead compound and submitted for further investigation. A titration study confirmed λem at 560 nm (yellow emission) which peaked with 5.0 equivalents of Zn2+ (Fig. 5B). A Zn2+ limit of detection (LoD) of 6.4 μM was calculated which is well below the WHO recommended drinking water limit. A similar study with Cd2+ confirmed λem at 510 nm (green emission) which reached a plateau with 5.0 equivalents of Cd2+ (LoD 2.4 μM). The 50 nm difference in λem between Zn2+ and Cd2+ is significant with very few examples reporting such a distinction between two group 12 metals.14 To our surprise Hg2+ generated an opposite response, a “turn off” response, with a decrease in λem at 460 nm, Hg2+ LoD 10.0 μM. These LoD values agree with those of previously reported pyrazoline sensors for Zn2+ and Cd2+ (ESI S6†). Competition assays were performed to determine the response of P1 in the presence of a range of interferents (Fig. 6 for P1 with Zn2+).
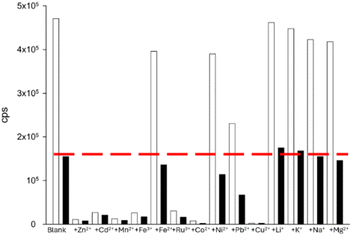
Several paramagnetic metals including Fe3+, Ru3+, Co2+, and noticeably Cu2+ disrupted the “turn on” response to Zn2+ at 560 nm (Fig. 6). This is a common phenomenon for pyrazolines with paramagnetic metals.13b,c,22,23 Interestingly, the biological group 1 and 2 metals, Li+, K+, Na+ and Mg2+, resulted in very minor disruption suggesting that P1 could be used for the monitoring of Zn2+ in living systems high in these metals, an active research area.13a,26 A similar profile was observed with Cd2+; paramagnetic metals Mn2+, Ru3+, Co2+ and Cu2+ hampered λem 510 nm but a good response remained with the group 1 and 2 metals (Fig. 7). A further assay of P1 with Hg2+ showed that paramagnetic metals disrupted the “turn off” response (Fig. 8). Under these conditions, the group 1 and 2 metals did not significantly influence the “turn off” response to Hg2+ suggesting that P1 could be a useful sensor for the “turn on” detection of Zn2+ at 560 nm and the “turn off” detection of Hg2+ at 460 nm in biological systems.
To determine the real-world application of P1 in large-scale monitoring, several studies of a variety of water sources were conducted including drinking water (tap and mineral water see Fig. 9 and ESI S5†). A strong response to 100 μM Zn2+ in tap water (Fig. 9) with a visible colour change from blue to yellow was observed at λex 365 nm (Fig. 9A inset). A similar study with Cd2+ in tap water produced a more noticeable response at 50 μM Cd2+ (Fig. 9B) and a green colour change under λex 365 nm irradiation (Fig. 9B inset). The “turn off” response to Hg2+ was detectable in tap water (Fig. 9C) with a reduction in λem at 460 nm with both 50 μM and 100 μM Hg2+. Mineral water (see ESI S5†) yielded a comparable result. The visible colour change on the addition of Zn2+ (yellow), Cd2+ (green) and Hg2+ (blue) suggests that P1 could form a prototype for a low-cost and portable field-based device. A low-cost prototype was constructed consisting of a portable excitation source and a suitable dark box (ESI S8†).25 Samples from two environmental locations (river and pond water) were taken and analysed in the laboratory and in situ using a portable device (Fig. 10 for pond water).
 | ||
Fig. 10 Group 12 metal triggered fluorescence response of P1 (20 μM, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 MeCN 3 MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) in pond water; +Zn2+ (panel A), +Cd2+ (panel B), +Hg2+ (panel C) and location (D). H2O) in pond water; +Zn2+ (panel A), +Cd2+ (panel B), +Hg2+ (panel C) and location (D). | ||
At two different concentrations, 50 μM and 100 μM of Zn2+, Cd2+, and Hg2+, a fluorescence response was triggered analogous to the drinking water study. The decrease in λem with Zn2+ (Fig. 10A) was less than expected possibly due to additional interferents present in the environment, possibly sediment or bacteria (Fig. 10A). In the presence of Cd2+ the change in λem is noticeable and a visible change to green is observed (Fig. 10B inset). A decrease in λem at 460 nm was observed on addition of Hg2+ (Fig. 10C inset) confirming that P1 can distinguish Cd2+ from Hg2+ in the field that can be observed with the naked eye. A similar result was obtained in river water (Fig. 11). This study confirmed that P1 can be used outside of a laboratory environment with naked eye detection of the group 12 metals in both stagnant pond and free flowing river waters. To confirm the robustness of P1, a reversibility study was performed which confirmed that P1 can be used for multiple cycles for Zn2+ detection (Fig. 12A). The proposed binding mechanism of P1 with Zn2+ (Fig. 12B) is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and agrees with previous studies on A–D and the Job plot (ESI S7†).20–23 While hydrazone units have been incorporated into multi-analyte fluorescent sensors previously,27P1–P4 are the first reported sensors combining hydrazone and pyrazoline units which distinguish Zn2+, Cd2+, and Hg2+ at different λem in aqueous environments.
1 and agrees with previous studies on A–D and the Job plot (ESI S7†).20–23 While hydrazone units have been incorporated into multi-analyte fluorescent sensors previously,27P1–P4 are the first reported sensors combining hydrazone and pyrazoline units which distinguish Zn2+, Cd2+, and Hg2+ at different λem in aqueous environments.
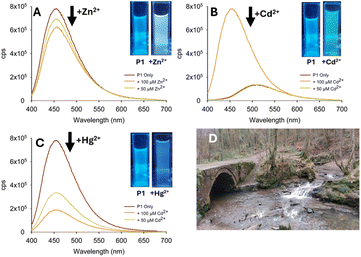 | ||
Fig. 11 Group 12 metal triggered fluorescence response of P1 (20 μM, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 MeCN 3 MeCN![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2O) in river water; +Zn2+ (panel A), +Cd2+ (panel B), +Hg2+ (panel C) and location (D). H2O) in river water; +Zn2+ (panel A), +Cd2+ (panel B), +Hg2+ (panel C) and location (D). | ||
 | ||
| Fig. 12 A reversibility study for P1 in the presence of multiple cycles of Zn2+ and EDTA (panel A) and the proposed binding mode of P1 with Zn2+ (panel B). | ||
Conclusions
Six first-in-class hydrazone–pyrazoline sensors were synthesised with P1–P4 displaying excellent selectivity towards group 12 metals. The aryl ring was well-tolerated to both electron donating and withdrawing substituents; however, substitution of pyridine for a phenyl unit in P5 abolished group 12 selectivity (Fig. 13). Substitution of the aryl ring on hydrazone and pyrazoline for methyl units in P6 significantly disrupted the fluorescence intensity. P1 was selected for further investigation and while a few heavy metals were significant interferents to the fluorescent response for P1, its ability to detect group 12 metals in real-world samples was confirmed. Combining P1 with a low-cost portable device enabled rapid visual detection by the naked eye of the 12 metals in rural environments without expensive apparatus or mains electricity. The group 1 and 2 metals exhibited very little influence on the fluorescence response indicating that P1 could also be a highly useful sensor for the selective detection of Zn2+ and Hg2+ in biological systems; for example cell culture and living systems.26 This highly unexpected discovery establishes a new subset of pyrazoline sensors, hydrazone–pyrazoline hybrid sensors. These hybrid sensors composed of both hydrazone and pyrazoline functional units set a high benchmark for future group 12 sensors. Further work is underway to transform this discovery into a second generation of sensors that retain the group 12 metal selectivity of P1 but with improved properties (increased λem intensity and reduced interference). The high tolerance of the aryl R1 group to substitution enables the attachment of these sensors to solid substrates as reported previously by Magri et al.28 This provides valuable applications for enhanced sensor design and molecular logic gate development.29 These are priority objectives for ongoing work and will be reported in due course. | ||
| Fig. 13 Summary of P1–P6; EDG is the electron donating group and EWG is the electron withdrawing group. | ||
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Alexander Ciupa designed, synthesized, characterised, and performed all spectroscopy studies and authored the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The author acknowledges Steven Robinson for assistance with time-of-flight high resolution mass spectrometry and Krzysztof Pawlak for assistance with fluorescence spectroscopy. Emily Cunliffe is acknowledged for valuable discussions. This work made use of shared equipment located at the Materials Innovation Factory, created as part of the UK Research Partnership Innovation Fund (Research England) and co-funded by the Sir Henry Royce Institute.References
- C. T. Chasapis, C. A. Spiliopoulou, A. C. Loutsidou and M. E. Stefanidou, Arch. Toxicol., 2012, 86, 521 CrossRef CAS PubMed.
- G. Genchi, S. M. Sinicropi, G. Lauria, A. Carocci and A. Catalano, Int. J. Environ. Res. Public Health, 2020, 17, 3782 CrossRef CAS PubMed.
- Y. S. Wu, A. I. Osman, M. Hosny, A. M. Elgarahy, A. S. Eltaweil, D. W. Rooney, Z. Chen, N. S. Rahim, M. Sekar, S. C. B. Gopinath, N. N. Mat Rani, K. Batumalaie and P. S. Yap, ACS Omega, 2024, 9, 5100 CrossRef CAS PubMed.
- Directive (EU) 2020/2184 Of The European Parliament And Of The Council of 16 December 2020, on the quality of water intended for human consumption, 2015, http://data.europa.eu/eli/dir/2020/2184/ Search PubMed.
- Zinc in Drinking-water, WHO/SDE/WSH/03.04/17, https://cdn.who.int/media/docs/default-source/wash-documents/wash-chemicals/zinc.pdf?sfvrsn=9529d066_4 Search PubMed.
- H. N. Kim, W. X. Ren, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2012, 41, 3210 RSC.
- G. Wu, M. Li, J. Zhu, K. W. Chiu Lai, Q. Tong and F. Lu, RSC Adv., 2016, 6, 100696 RSC.
- S. Manickam and S. K. Lyer, RSC Adv., 2020, 10, 11791 RSC.
- H. Nui, J. Liu, H. M. O'Connor, T. Gunnlaugsson, T. D. James and H. Zhang, Chem. Soc. Rev., 2023, 52, 2322 RSC.
- J. W. Nugent, H. Lee, H.-S. Lee, J. H. Reibenspies and R. D. Hancock, Chem. Commun., 2013, 49, 9749 RSC.
- L. G. T. A. Duarte, F. L. Coelho, J. C. Germino, G. G. da Costa, J. F. Berbigier, F. S. Rodembusch and T. D. Z. Atvars, Dyes Pigm., 2020, 181, 108566 CrossRef.
- L. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X. P. He, H. Tian, J. Yoon, J. L. Sessler and T. D. James, Chem. Soc. Rev., 2020, 49, 5110 RSC.
- Selected examples: (a) Z. Zhang, F.-W. Wang, S.-Q. Wang, F. Ge, B.-X. Zhao and J.-Y. Miao, Org. Biomol. Chem., 2012, 10, 8640 RSC; (b) Z. L. Gong, F. Ge and B.-X. Zhao, Sens. Actuators, B., 2011, 159, 148 CrossRef CAS; (c) M. M. Li, F. Wu, X. Y. Wang, T. T. Zhang, Y. Wu, Y. Xiao, J. Y. Miao and B.-X. Zhao, Anal. Chim. Acta, 2014, 826, 77 CrossRef CAS PubMed.
- Selected examples: (a) Z. Xu, K.-H. Baek, H. N. Kim, J. Cui, X. Qian, D. R. Spring, I. Shin and J. Yoon, J. Am. Chem. Soc., 2010, 132, 601 CrossRef CAS PubMed; (b) Y. Tan, J. Gao, J. Yu, Z. Wang, Y. Cui, Y. Yang and G. Qian, Dalton Trans., 2013, 42, 11465 RSC; (c) G. Tian, Y.-Z. Han and Q. Yang, J. Mol. Struct., 2023, 1273, 134341 CrossRef CAS.
- B. Varghese, S. N. Al-Busa, F. O. Suliman and S. M. Z. Al- Kindy, RSC Adv., 2017, 7, 46999 RSC.
- H. El Karout, C. Labassi, K. Waszkowska, N. Fournier-le Ray, R. Gatri, F. Gauffre, A. Fihey, B. Sahraoui and J.-L. Fillaut, New J. Chem., 2024, 48, 18709 RSC.
- A. Ciupa, P. A. De Bank, M. F. Mahon, P. J. Wood and L. Caggiano, MedChemComm, 2013, 4, 956 RSC.
- Selected examples: (a) S. A. Khan, M. Z. Alam, M. Mohasin, S. Ahmad, U. Salma, H. Parveen, S. Mukhtar, M. Al-Anazi, F. A. Alotaibi and M. A. Abdelaziz, J. Fluoresc., 2024, 34, 723 CrossRef CAS PubMed; (b) S. Prajapati, P. Sinha, S. Hindore and S. Jana, Spectrochim. Acta, Part A, 2023, 287, 122107 CrossRef CAS PubMed; (c) L. E. Santos-Figueroa, A. Llopis-Lorente, S. Royo, F. Sancenón, R. Martínez-Máñez, A. M. Costero, S. Gil and M. Parra, ChemPlusChem, 2015, 80, 800 CrossRef CAS PubMed.
- Selected examples: (a) M. Z. Alam, S. Ahmad, A. Alimuddin and S. A. Khan, J. Fluoresc., 2025, 35(3), 1241 CrossRef PubMed; (b) M. Z. Alam, M. Mohasin, S. Ahmad, U. Salma and S. A. Khan, J. Coord. Chem., 2025, 48, 367 CrossRef; (c) M. Mohasin, M. Z. Alam, S. Ahmad, U. Salma, Q. Ullah and S. A. Khan, J. Mol. Struct., 2025, 1336, 142034 CrossRef CAS.
- A. Ciupa, M. F. Mahon, P. A. De Bank and L. Caggiano, Org. Biomol. Chem., 2012, 10, 8753 RSC.
- A. Ciupa, RSC Adv., 2024, 14, 34918 RSC.
- A. Ciupa, RSC Adv., 2024, 14, 3519 RSC.
- A. Ciupa, New J. Chem., 2024, 48, 13900 RSC.
- Selected examples: (a) A. Sahana, A. Banerjee, S. Das, S. Lohar, D. Karak, B. Sarkar, S. K. Mukhopadhyay, A. K. Mukherjee and D. Das, Org. Biomol. Chem., 2011, 9, 5523 RSC; (b) S. Santhoshkumar, K. Velmurugan, J. Prabhu, G. Radhakrishnan and R. Nandhakumar, Inorg. Chim. Acta, 2016, 439, 105 CrossRef; (c) N. Bhuvanesh, S. Suresh, K. Kannan, V. R. Kannan, N. Maroli, P. Kolandaivel and R. Nandhakumar, New J. Chem., 2019, 43, 2519 RSC; (d) Y. Mise, K. Imato, T. Ogi, N. Tsunoji and Y. Ooyama, New J. Chem., 2021, 45, 4164 RSC; (e) G. Mun, S. H. Jung, A. Ahn, S. S. Lee, M. Y. Choi, D. H. Kim, J.-Y. Kim and J. H. Jung, RSC Adv., 2016, 6, 53912 RSC.
- R. Iftikhar, I. Parveen, A. Ayesha, A. Mazhar, M. S. Iqbal, G. M. Kamal, F. Hafeez, A. L. Pang and M. Ahmadipour, J. Environ. Chem. Eng., 2023, 11, 109030 CrossRef CAS.
- Selected examples: (a) S. Melo-Hernández, M. Camila Ríos and J. Portilla, RSC Adv., 2024, 14, 39230 RSC; (b) Y. Y. Gao, J. He, X.-H. Li, J.-H. Li, H. Wu, T. Wen, J. Li, G.-F. Hao and J. Yoon, Chem. Soc. Rev., 2024, 53, 6992 RSC.
- Selected examples: (a) X. Su and I. Aprahamian, Chem. Soc. Rev., 2014, 43, 1963 RSC; (b) W. N. Wu, H. Wu, Y. Wang, X. J. Mao, B. Z. Liu, X. L. Zhao, Z. Q. Xu, Y. C. Fan and Z. H. Xu, RSC Adv., 2018, 8, 5640 RSC; (c) Y. Yang, W. W. Wang, W. Z. Xue, W. N. Wu, X. L Zhao, Z. Q. Xu, Y. C. Fan and Z. H. Xu, J. Lumin., 2019, 212, 191 CrossRef; (d) A. Ciupa, RSC Adv., 2025, 15, 3465 RSC.
- N. Zerafa, M. Cini and D. C. Magri, Mol. Syst. Des. Eng., 2021, 6, 93 RSC.
- A. Ciupa, RSC Adv., 2025, 15, 10565 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ay00639b |
| This journal is © The Royal Society of Chemistry 2025 |