 Open Access Article
Open Access ArticleExposing active sites in zeolites with fluoropyridines as NMR probe molecules†
Joseph
Hurd
a,
Yujie
Ma
 b,
Paolo
Cerreia Vioglio
c,
Run
Zou
a,
Dinu
Iuga
d,
Sihai
Yang
be,
Xiaolei
Fan
b,
Paolo
Cerreia Vioglio
c,
Run
Zou
a,
Dinu
Iuga
d,
Sihai
Yang
be,
Xiaolei
Fan
 af and
Daniel
Lee
af and
Daniel
Lee
 *a
*a
aDepartment of Chemical Engineering, The University of Manchester, Oxford Road, Manchester M13 9PL, UK. E-mail: daniel.lee@manchester.ac.uk
bDepartment of Chemistry, The University of Manchester, Oxford Road, Manchester M13 9PL, UK
cSir Peter Mansfield Imaging Centre, The University of Nottingham, Nottingham NG7 2RD, UK
dDepartment of Physics, The University of Warwick, Coventry CV4 7AL, UK
eCollege of Chemistry and Molecular Engineering, BNLMS, Peking University, 202 Chengfu Road, Haidian, Beijing 100871, China
fNingbo China Beacons of Excellence Research and Innovation Institute, University of Nottingham Ningbo China, 211 Xingguang Road, Ningbo 315048, China
First published on 30th June 2025
Abstract
Active sites in zeolites are crucial for catalysis, but their identification remains challenging due to structural complexity. Solid-state NMR, a key tool for studying zeolites, can struggle with directly detecting acidic properties due to the quadrupolar nature of ubiquitous metal dopants. Here, we use fluorinated pyridine as a probe to identify active sites in HY and HZSM-5, two important solid acid catalysts. 19F NMR effectively detects binding environments, while 19F–27Al polarization transfer reveals elusive penta-coordinated AlV Brønsted acid sites and shows that the dominant active sites are distorted framework-associated AlIV and AlV sites with large quadrupolar coupling constants, shedding light on catalytic properties. Low-temperature (100 K) NMR proves essential for capturing these interactions. This work enhances the understanding of zeolite active sites and highlights the broad applicability of fluorinated probe molecules for surface characterization, offering a cost-effective, highly sensitive approach for catalytic studies.
Introduction
Zeolites are the most widely used catalyst class for the cracking of hydrocarbons.1–4 They also show promise for methanol-to-olefin (MTO) fuel production and for the processing of other types of renewable biomass into usable fuels and products,5–8 as well as for the upcycling of polyolefin plastics.9–11 Starting from a porous silica (SiO2) structure, doping of metals such as Al into the zeolite framework introduces acid sites that can be active for catalysis.6,12–15 Much work over many decades has been undertaken to try to optimise the framework structures and pore sizes,16–20 the amount of acid sites, the ratio of types of acid sites (Brønsted![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lewis),21,22 and also the synergy between different acid sites and dopants (such as single atoms sites23–25 or nanoparticles26,27) for particular applications. Therefore, knowledge of how the atomic-level structure relates to catalytic mechanisms is essential.
Lewis),21,22 and also the synergy between different acid sites and dopants (such as single atoms sites23–25 or nanoparticles26,27) for particular applications. Therefore, knowledge of how the atomic-level structure relates to catalytic mechanisms is essential.
Solid-state NMR (ssNMR) spectroscopy can provide local information about 1H,28–3029Si,31–3317O,34–36 and 27Al28–32,37 sites. Although Al (and other metals) can be inserted into the framework in tetrahedral (AlIV) positions, it is well known that there are a number of different extra-framework aluminium (EFAl) sites present in such materials: AlIV, penta-coordinated AlV, and hexa-coordinated AlVI.14,32,38 Different synthesis/doping methods and post synthetic treatments have been applied to change the silicon![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) aluminium ratio (SAR) of zeolites as well as their pore structures, thus varying the Brønsted acid site (BAS)
aluminium ratio (SAR) of zeolites as well as their pore structures, thus varying the Brønsted acid site (BAS)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Lewis acid site (LAS) ratio and their accessibilities.30 This leads to different functional properties.
Lewis acid site (LAS) ratio and their accessibilities.30 This leads to different functional properties.
Computational chemistry techniques have been applied to model how reagents bind, elucidating reaction pathways,39–41 and to investigate the types of active site present,42–45 so that structure can be related to function. Nevertheless, the nature of some of these sites remains ambiguous even after frequent investigations. One such site is the penta-coordinated EFAlV moiety;32,46 it has generally been treated as a LAS and its distorted geometry leads to it being a highly active species.47–49 However, recent work from Deng and coworkers suggests that there is also the potential for BAS-type activity for AlV sites.32 Structural ambiguities such as these hinder a comprehensive understanding of the catalytic activity of these vital porous materials.
Analysis using adsorption of probe molecules can give the types and relative strengths of the acid sites present. Probe molecules are commonly used with IR spectroscopy, temperature programmed desorption (TPD), as well as NMR spectroscopy. For NMR, the main probe molecules can be split into two categories derived from their NMR-active isotopes used to characterise surfaces: 31P-based, e.g. trimethylphosphine (TMP) and trimethylphosphine oxide (TMPO),50,51 and 15N-based, e.g. pyridine (Py).50 These are used because they interact strongly with active sites and their spy nuclei have high sensitivities to environmental changes owing to large chemical shift ranges. However, TMP and TMPO are hazardous (TMP is highly toxic and flammable).21,51,52 Py is commonly used with FT-IR to give rapid assignment of acid sites and their strengths. Ammonia and Py have proven to be particularly useful for the characterization of acid sites through the combination of FT-IR and TPD for quasi-quantitative assignment of site coverage.53–59 Ammonia can be preferred for TPD owing to its lower cost, smaller size, lower desorption temperatures, and reduced hazards compared to Py. The utility of Py as a probe molecule with NMR spectroscopy has been recently highlighted for example by Copéret and coworkers who used adsorption of 15N-enriched Py combined with variable temperature 15N NMR and DFT studies to elucidate the presence of a pseudo tricoordinated framework-associated LAS in the zeolite mordenite.46 Its distorted nature suggests that it is likely very active in catalysis, but this needs to be further investigated.46 The use of isotopically-enriched Py was required as 15N has low NMR receptivity (0.04% compared to 1H) but 15N-Py is costly (∼2 k€ per g).
Both categories of probe molecule exhibit multiple binding modes. TMPO adsorbs to both BAS and LAS and shows a total of 4 LAS and BAS coordination modes/strengths for zeolite Y (H form, HY)52,60 and binding to 5 different BASs for HZSM-5.51 The relative spatial proximities between the active sites can be determined using 2D correlation NMR spectroscopy involving 31P from TMPO.60 Similarly, monomethylamine (MMA) studies for HZSM-5 and HY have been used to examine spatial correlations whilst showing multiple binding sites for both zeolites.53,61 Combining NMR spectroscopy of probe molecules with synchrotron resonant soft X-ray diffraction and neutron powder diffraction has very recently enabled the identification of the specific locations of tetrahedral framework aluminium in HZSM-5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 but not framework-associated or extra-framework sites owing to their lack of long-range order.
62 but not framework-associated or extra-framework sites owing to their lack of long-range order.
Similar to 31P, 19F has favourable NMR properties, including high receptivity (>12 times that of 31P) and a wide chemical shift range. Fluorine is also predominantly found naturally only in minerals, so it rarely contributes to signals from most synthetic zeolitic analytes and thus resonance overlap is minimal, and resolution is high. This means that it is in principle an ideal spy nucleus; indeed, 4-fluoronitrobenzene and 4-fluoroaniline have been used to measure zeolite acidity,63 4-fluoroacetophenone has been used to do so quantitatively,64,65 and CF4 has been used to probe pore dimensions and accessibility in a similar manner to 129Xe NMR,66 while ammonium hexafluorosilicate has been used to investigate dealumination processes.67 Functionalised pyridines containing 19F such as 2- and 3-fluoropyridine (2FP and 3FP, respectively) are widely commercially available at low cost (∼10 € per g). TPD experiments have shown that they bind to active sites in zeolites68 and therefore they have the potential to be useful probe molecules for NMR.
Herein, the utility of fluoropyridines as NMR probe molecules is demonstrated. Zeolites HY (SAR = 2.6) and HZSM-5 (SAR = 40) were selected as the focus of this investigation due to their wide industrial relevance69–73 as well as both having similar active sites but different pore sizes, structures, and active site distributions. Pyridine has a kinetic diameter of ∼5.3 Å, so it can fit into the medium-sized pores of the 10-membered rings (10-MR) (∼5.5 Å) of HZSM-5 (ref. 74) but will be tightly constrained by the small size (see Fig. 1). Pyridine fits more easily into the large-pore 12-MR supercages of HY (pore opening ∼7.4 Å). However, pyridine is too large to fit into the smaller sodalite cages of HY. Here, high-field NMR and low temperature NMR are used for increased resolution and sensitivity, respectively, enabling multiple binding modes/strengths to be detected for fluoropyridines adsorbed on HY and HZSM-5 via19F NMR. Notably, polarisation transfer from the probe molecule (19F) to the zeolites (27Al) has shown the unexpected presence of pentacoordinated framework-associated BAS and that these are the preferred adsorption sites for both zeolites. This highlights the importance of using probe molecules to gain detailed insights into the active sites so that structure can be related to function and thus so that structures can be effectively rationally optimised.
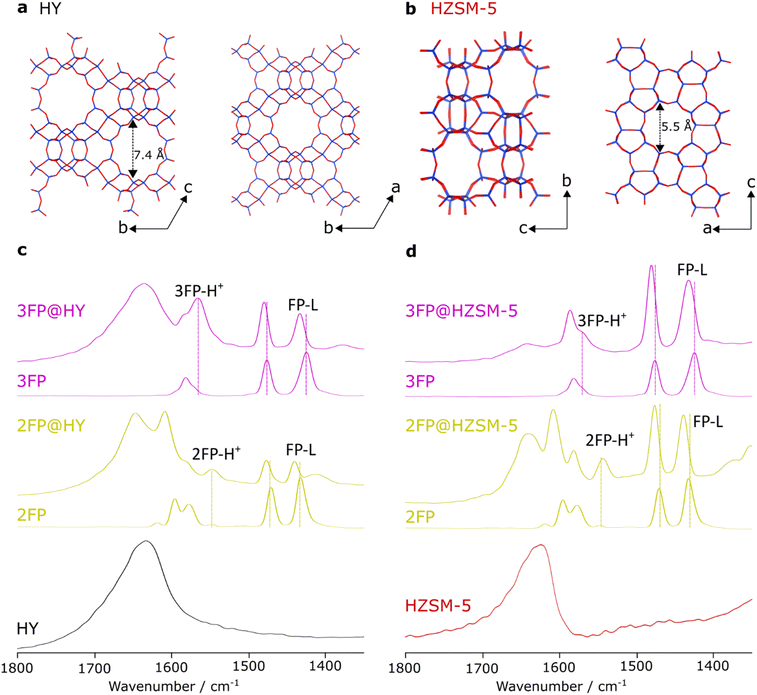 | ||
| Fig. 1 Structures of zeolite Y (a) and ZSM-5 (b); only oxygen (red) and silicon/aluminium (blue) sites are represented. FT-IR spectra (c and d) of zeolite HY (c) and zeolite HZSM-5 (d), dosed with 3FP (top), 2FP (middle) and undosed (bottom) with relevant acid site bindings shown on the spectra. Wavenumbers and assignment are tabulated in Table S7.† | ||
Results and discussion
Pyridine is commonly used as a probe molecule for IR studies of active sites in zeolites since the resulting spectra can be used to discriminate between protonated pyridine (Py-H+), pyridine adsorbed at a weak BAS through H-bonding (Py-B), and pyridine adsorbed at a LAS (Py-L).75,76Fig. 1 shows the absorbance-mode FT-IR spectral region of 1800–1350 cm−1 for HY and HZSM-5 dosed with 2FP and 3FP as well as the pure zeolites and fluoropyridines, for comparison. The peaks observed for the 2FP-dosed zeolites are very similar to those expected for equivalent Py adsorption. These show clear protonated species (2FP-H+@HY and 2FP-H+@HZSM-5) and adsorption at LAS (2FP-L@HY and 2FP-L@HZSM-5) for both zeolites. 3FP does not have the same spectral separation of (ν8a) vibrational modes as Py or 2FP but the 3FP-dosed zeolites exhibit bands corresponding to 3FP-H+ with higher wavenumbers (1565 cm−1 for HY and 1571 cm−1 for HZSM-5; cf. 1547 and 1543 cm−1 for 2FP-dosed zeolites, respectively) and bands corresponding to adsorption at weak LAS with lower wavenumbers (1434 cm−1 for HY and 1433 cm−1 for HZSM-5; cf. 1440 and 1439 cm−1 for 2FP-dosed zeolites, respectively). This suggests that 2FP and 3FP have different adsorption geometries and/or binding strengths and these are dependent on the type of active site. These variations highlight the sensitivity of fluoropyridines as probe molecules.As the FT-IR spectra demonstrated that both 3FP and 2FP bind to active sites in zeolites HY and HZSM-5, they were both investigated using conventional 19F solid-state NMR spectroscopy at 9.4 T field strength. A TPD study shows different preferential bindings for 2FP and 3FP to zeolite acid sites in HZSM-5 and H-Mordenite with 3FP having a higher adsorption enthalpy.68 Disappointingly, the {1H–}19F cross-polarisation magic angle spinning (CPMAS) NMR spectra of 2FP@HY and 2FP@HZSM-5 are almost identical (see Fig. S1†). They both show a large component (δ{19F} ≈ −70 ppm) and two smaller components (δ{19F} ≈ −70 and −80 ppm), with the smaller components exhibiting sizeable chemical shift anisotropies (CSA). Cross-polarisation was used to be selective to species that are strongly adsorbed; CPMAS uses the dipolar interaction (here between 1H and 19F) to transfer polarisation between spins and this can be averaged-out under fast isotropic motion, leading to poor transfer efficiencies. Nevertheless, the larger peak displays negligible CSA, indicating dynamical averaging (see Fig. S1 and Table S8†). Thus, this larger peak can be assigned to physisorbed species and the smaller peaks to species bound to active sites. Although CPMAS is not inherently quantitative, 19F direct excitation with careful sample preparation can enable a quantitative analysis of acid sites, as has been shown previously.64
The {1H–}19F CPMAS NMR spectra of 3FP@HY and 3FP@HZSM-5 are substantially different, even at moderate field strengths (9.4 T, see Fig. 2, S1, and S2†). This is a first indication that 3FP can be used to differentiate active sites between similar zeolite solid acid catalysts. For 3FP, however, the smaller, broader, higher anisotropy peaks are deshielded (i.e. higher ppm) compared to the narrower peaks from physisorbed species (δ{19F} ≈ −130 ppm). This infers, as expected, that the position of the F on the pyridine ring (e.g. 2FP vs. 3FP) plays a role in the electronic interaction of the probe molecule with the zeolite surface and this is reflected in its chemical shielding. The direct excitation 19F MAS NMR spectra of 3FP@HY and 3FP@HZSM-5 (Fig. 2) are similar to the respective CPMAS NMR spectra but it is clear from the reduced relative presence of spinning side bands that there is a large proportion of dynamic species that are not detected via CP. However, comparing these spectra to those of 3FP@silicalite-1 (a pure silica MFI-type zeolite) and 3FP@γ-alumina (a nanoparticulate Lewis acid catalyst) shows that 3FP interacts strongly with acid sites in the porous acid catalysts since the 19F resonances for 3FP@silicalite-1 and 3FP@γ-alumina correspond to mainly physisorbed species (Fig. 2a and b). Since 3FP shows a greater difference for 19F NMR spectra between HY and HZSM-5 compared to 2FP, 3FP will be the focus of the rest of the study.
To reduce the dynamics of adsorbed 3FP, the doped zeolites were cooled to 260 K. Very similar {1H–}19F CPMAS NMR spectra are observed compared to those recorded at ambient temperatures (Fig. S2†). However, there are some notable differences. The 19F resonances assigned to physisorbed 3FP have become broader and now present a larger CSA, as would be expected with reduced dynamics. The highest shifted (most down-field) 19F isotropic resonance observed for 3FP@HZSM-5 appears at a lower chemical shift compared to 3FP@HY (cf. δ{19F} ≈ −118 ppm for HZSM-5 and −112 ppm for HY) but it has very similar CSA parameters: δCSA and η (see Fig. 2, S1, and S2, and Table S8†). This indicates that 3FP is adsorbed in a similar mode, but with a different strength. Notably, there are 19F resonances observed with intermediate chemical shifts (δ{19F} ≈ −120 to −128 ppm), and the associated CSA tensors have asymmetry parameters, η, close to 1, whereas the highest shifted resonances have asymmetry parameters closer to 0; this indicates that the binding mode that results in the corresponding 19F CSA tensor is very different. Previous NH3-TPD measurements on HY (with the silicon-to-aluminium ratio, SAR, = 2.6) show a balanced weak![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) strong acidity (0.8
strong acidity (0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0) where the total active acidity is from a 1.0
1.0) where the total active acidity is from a 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 BAS
1.1 BAS![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LAS ratio and that the majority of LAS show weak acidity whereas the BAS show strong acidity.77 For HZSM-5 with lower Al content (SAR = 38), NH3-TPD studies show a greater proportion of BAS compared to LAS and weaker LAS but stronger BAS than HY.78 Therefore, it is likely that the downfield 19F resonances stem from 3FP-L and the intermediate 19F resonances stem from 3FP-B owing to their relative amounts in each zeolite (Table S8†) and that downfield shifts (higher ppm) indicate greater acid strengths. The 19F spectrum and resonances are generally broader for 3FP@HY compared to 3FP@HZSM-5. This is consistent with the NH3-TPD analysis that highlights the complexity and variation of acidic strength of HY,78,79 leading to a distribution of 19F chemical shifts that are not dynamically averaged. Therefore, NMR chemical shifts and their anisotropies from 3FP 19F resonances can be used to distinguish LAS and BAS in zeolites, as well as their strengths, with higher deshielding (more positive ppm) stemming from both stronger binding and from 3FP-L.
LAS ratio and that the majority of LAS show weak acidity whereas the BAS show strong acidity.77 For HZSM-5 with lower Al content (SAR = 38), NH3-TPD studies show a greater proportion of BAS compared to LAS and weaker LAS but stronger BAS than HY.78 Therefore, it is likely that the downfield 19F resonances stem from 3FP-L and the intermediate 19F resonances stem from 3FP-B owing to their relative amounts in each zeolite (Table S8†) and that downfield shifts (higher ppm) indicate greater acid strengths. The 19F spectrum and resonances are generally broader for 3FP@HY compared to 3FP@HZSM-5. This is consistent with the NH3-TPD analysis that highlights the complexity and variation of acidic strength of HY,78,79 leading to a distribution of 19F chemical shifts that are not dynamically averaged. Therefore, NMR chemical shifts and their anisotropies from 3FP 19F resonances can be used to distinguish LAS and BAS in zeolites, as well as their strengths, with higher deshielding (more positive ppm) stemming from both stronger binding and from 3FP-L.
The impact of the 3FP doping can also be observed via27Al MAS NMR spectra (Fig. S3†). Framework tetrahedral aluminium-27 resonances (δ{27Al} ≈ 60 ppm) are perturbed upon adsorption of 3FP. For both zeolites there is a slight broadening of these signals, particularly at the high-field (lower ppm) side, and this effect is larger for HY than HZSM-5. Furthermore, the resonances corresponding to hexa-coordinated extra-framework aluminium (EFAlVI) species (δ{27Al} ≈ 0 ppm) for both zeolites, which are much more prevalent in HY, are reduced upon adsorption of 3FP. This is likely due to direct binding of 3FP to these LAS (vide supra) or 3FP may lead to an annealing effect on the zeolites as pyridines can help relax distorted Al centres.46
To gain higher spectral resolution from 3FP adsorbed on the zeolites, high-field (20.0 T) NMR analysis was employed and combined with fast MAS (33 kHz). It is clear from high-resolution 1H MAS NMR spectra recorded at ∼260 K (Fig. S4†) that a highly acidic proton is observed (δ{1H} ≈ 15 ppm) upon adsorption of 3FP to zeolite HY. A similar chemical shift is observed (δ{1H} ≈ 15 ppm) for d5-pyridine adsorbed to HY and is assigned to Py-H+.32 The assignment to 3FP-H+ is confirmed here through a 1H–1H dipolar correlation spectrum (Fig. S5†), which shows a correlation for this resonance with 3FP protons. The absence of a similar peak for 3FP@HZSM-5 is attributed to the lower amount of BAS compared to HY. It is also clear that the 1H from 3FP are more dynamic for 3FP@HZSM-5 compared to 3FP@HY, as demonstrated by the narrower associated resonances (δ{1H} ≈ 6–10 ppm). This is consistent with the analysis of the 9.4 T 260 K {1H–}19F CPMAS NMR spectra (vide supra). This could be considered counterintuitive since the pores of HZSM-5 are smaller than those of HY and thus 3FP should be more tightly constrained in the former. This infers that 3FP interacts more strongly with the pores in HY, either due to the nature of the active sites or to the larger proportion of these. Ambient temperature high-field acquisition of {1H–}19F CPMAS NMR spectra of 3FP@HY and 3FP@HZSM-5 was challenging. This again highlights the mobility of 3FP in the pores. With cooling (∼260 K), to counteract frictional sample heating under MAS, high-resolution {1H–}19F CPMAS NMR spectra could be obtained (Fig. 3). Deconvolution of the 3FP@HY 19F NMR spectrum shows two clusters of peaks (δ{19F} ≈ −104 to −116 ppm and −118 to −134 ppm). Even at high-field with fast MAS, the 19F resolution is not sufficient to resolve individual peaks; the deconvolution is non-definitive but has been fit with the minimum number of peaks required for a suitable reproduction of the spectrum (full fitting parameters can be found in Table S10†). This deconvolution demonstrates the benefits of high-field as it is evident that there are multiple 19F resonances and thus adsorption modes/strengths. As noted above, the downfield (higher ppm) cluster is assigned to 3FP-L and the upfield cluster is from 3FP-B and physisorbed 3FP. For 3FP@HZSM-5 the observed 19F chemical shift range is more limited but there are still two discrete clusters (δ{19F} ≈ −114 to −120 ppm and −120 to −134 ppm), with the downfield and upfield clusters assigned to 3FP-L and 3FP-B/physisorbed, respectively (vide supra). The sample cooling has provided further evidence for 3FP adsorption to strong BAS through the formation of 3FP-H+ and, combined with high-fields, the 19F NMR spectra show that there are a variety of binding modes and strengths at both LAS and BAS and that the variety is greater for HY compared to HZSM-5, as is the strength of LAS.
 | ||
| Fig. 3 20 T {1H–}19F CPMAS NMR spectra recorded with cooling (∼260 K) and using a MAS frequency of ∼33 kHz. Spectra shown are of (a) 3FP-dosed zeolite HY (black) and (b) 3FP-dosed HZSM-5 (red), with deconvolutions (purple) and the envelope (green). Fitted parameters are given in Table S10.† | ||
Pyridine has previously been shown to adsorb to common zeolites with multiple binding modes.58,76 This is consistent with the number of major adsorption sites found for 3FP for the two zeolites studied herein. To further reduce the dynamics of adsorbed 3FP and also to increase the NMR sensitivity, MAS NMR spectra were collected at 100 K and 14.1 T. This enabled {19F–}27Al CPMAS NMR spectra to be recorded (Fig. 4), which show where 3FP is adsorbed in the zeolites; this was not possible previously at higher temperatures owing to reduced experimental sensitivity (Fig. S6†). As expected, 27Al resonances are detected near 19F spins at δ{27Al} ≈ 60 ppm for both HY and HZSM-5, which correspond to 3FP-B since these BAS arise from framework tetrahedral AlIV. These 27Al resonances can be deconvoluted into two contributions: (i) framework AlIV-1 (CQ ≈ 2.9 MHz, δiso{27Al} ≈ 64 ppm) and (ii) framework-associated AlIV-2 (CQ ≈ 6.4 MHz, δiso{27Al} ≈ 65 ppm).38,47,80 Their relative contributions to the {19F–}27Al CPMAS NMR spectra (1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.2 for HY and 1
1.2 for HY and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 for HZSM-5, see Table S11†) are different compared to the quantitative direct excitation 27Al MAS NMR spectra (1.0
2 for HZSM-5, see Table S11†) are different compared to the quantitative direct excitation 27Al MAS NMR spectra (1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5 for undosed HY and 1.0
0.5 for undosed HY and 1.0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4 for undosed HZSM-5, Fig. S9 and Table S12†). This demonstrates that 3FP adsorbs near AlIV-2 sites more preferentially than near AlIV-1 sites. This could be expected since these sites protrude into the pores and have been shown to be responsible for enhanced catalytic activity.80 It is notable that the relative intensity of 3FP adsorption to AlIV-2 compared to AlIV-1 is greater for HZSM-5 compared to HY; this may explain the results of calorimetry studies of the two zeolites which show greater pyridine adsorption enthalpy for HZSM-5 than HY.81
0.4 for undosed HZSM-5, Fig. S9 and Table S12†). This demonstrates that 3FP adsorbs near AlIV-2 sites more preferentially than near AlIV-1 sites. This could be expected since these sites protrude into the pores and have been shown to be responsible for enhanced catalytic activity.80 It is notable that the relative intensity of 3FP adsorption to AlIV-2 compared to AlIV-1 is greater for HZSM-5 compared to HY; this may explain the results of calorimetry studies of the two zeolites which show greater pyridine adsorption enthalpy for HZSM-5 than HY.81
 | ||
| Fig. 4 14.1 T, 100 K {19F–}27Al CPMAS NMR spectra (a and b) and DNP-enhanced {1H–}15N CPMAS NMR spectrum (c) of 3FP-dosed zeolites HY (black) (a and c) and HZSM-5 (red) (b). The corresponding 27Al direct excitation MAS NMR spectra are shown with dashed lines in (a and b) and spectral deconvolutions are given in Fig. S8.† The MAS frequency was 8 kHz for all spectra and 27Al background correction is shown in Fig. S7.† | ||
Surprisingly, and importantly, a different 27Al resonance is detected (δ{27Al} ≈ 20 ppm) as the primary adsorption site for 3FP in both zeolites (∼8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 compared to AlIV-1). This resonance is not detected in direct excitation 27Al MAS NMR spectra nor in a 1H–27Al dipolar correlation MAS NMR spectrum, even at high field (see Fig. S10 and S11†); only an AlV site stemming from EFAlV is detected for HY (δ{27Al} ≈ 30 ppm). This demonstrates the utility of {19F–}27Al CP with adsorbed 3FP to reveal previously undetectable Al sites; they are likely scarce yet have a high propensity for adsorption. To assign this resonance to 3FP-B or 3FP-L, a complementary 15N NMR spectrum was recorded for 3FP@HY (Fig. 4c). Owing to the low NMR receptivity of 15N (e.g. natural isotopic abundance of 0.4%), the {1H–}15N CPMAS NMR spectrum was enhanced using dynamic nuclear polarization.82–84 The resulting spectrum shows a single 15N resonance (δ{15N} ≈ 205 ppm) that corresponds to Py-B.58,59 There is no observed signal for Py-L (expected δ{15N} ≈ 240 ppm46,85). Therefore, the primary adsorption site for 3FP is a BAS with low 27Al chemical shift.
1 compared to AlIV-1). This resonance is not detected in direct excitation 27Al MAS NMR spectra nor in a 1H–27Al dipolar correlation MAS NMR spectrum, even at high field (see Fig. S10 and S11†); only an AlV site stemming from EFAlV is detected for HY (δ{27Al} ≈ 30 ppm). This demonstrates the utility of {19F–}27Al CP with adsorbed 3FP to reveal previously undetectable Al sites; they are likely scarce yet have a high propensity for adsorption. To assign this resonance to 3FP-B or 3FP-L, a complementary 15N NMR spectrum was recorded for 3FP@HY (Fig. 4c). Owing to the low NMR receptivity of 15N (e.g. natural isotopic abundance of 0.4%), the {1H–}15N CPMAS NMR spectrum was enhanced using dynamic nuclear polarization.82–84 The resulting spectrum shows a single 15N resonance (δ{15N} ≈ 205 ppm) that corresponds to Py-B.58,59 There is no observed signal for Py-L (expected δ{15N} ≈ 240 ppm46,85). Therefore, the primary adsorption site for 3FP is a BAS with low 27Al chemical shift.
Recent studies have presented active sites in zeolites that could be responsible for the primary adsorption site for 3FP. Yakimov et al. used adsorption of pyridine on mordenite zeolite combined with low temperature MAS NMR spectroscopy and DFT calculations and showed that Py-B (CQ ≈ 1.8 MHz, δiso{27Al} ≈ 54 ppm) were prevalent and that framework-associated pseudo tri-coordinated Al LAS existed and could also adsorb Py (CQ ≈ 9.4 MHz, δiso{27Al} ≈ 58 ppm).46 Zheng et al. also used Py-adsorption but for HY and with 2D correlation MAS NMR spectroscopy demonstrated the existence of unprecedented penta-coordinated AlV-BAS (CQ ≈ 5.6 MHz, διso{27Al} ≈ 35 ppm).32 The latter study was performed at room temperature, and it is known that quadrupolar parameters can be dynamically averaged.46 The values extracted from our low temperature measurements (Table S11†) for the primary adsorption site for 3FP on HY and HZSM-5 (CQ ≈ 7.5 MHz, δiso{27Al} ≈ 30 ppm) match well to those reported for AlV-BAS, accounting for reduced dynamics. Moreover, a very recent theoretical and experimental study by Wang et al.86 showed that “NMR-invisible” tri-coordinated Al sites in ultrastable Y (USY) zeolite become detectable upon hydration and that this adsorption of water leads to framework-associated AlIV-2 and, importantly, AlV-BAS (CQ ≈ 9.3 MHz, δiso{27Al} ≈ 32 ppm). USY is prepared from HY via calcination that dealuminates the framework, creating tri-coordinated Al defects. Therefore, the evidence here suggests that these sites can also be found in HY and HZSM-5 in low concentrations and that adsorption of 3FP is preferentially to these difficult-to-detect AlV-BAS structures and {19F–}27Al CPMAS NMR facilitates this observation. This highlights the challenge of correlating structure with activity in heterogeneous catalysts. However, probe molecules can effectively target active sites and, when combined with solid-state NMR analysis, provide detailed insights into the accessibility and acidity of these sites.
Conclusion
This study demonstrates the efficacy of 3-fluoropyridine (3FP) as a powerful probe molecule for elucidating the nature of active sites in zeolites (exemplified by HY and HZSM-5). 2-fluoropyridine (2FP) was also investigated but shows inferior properties compared to 3FP in terms of adsorption strength and resulting 19F chemical shift perturbations. Nevertheless, it may find value for certain applications. The advantages of 3FP are that: (i) it is readily applicable as a probe molecule for a variety of analytical tools including TPD, IR, and NMR, facilitating complementary characterisations; (ii) it is relatively inexpensive; (iii) it is relatively small so can fit in micropores; (iv) it can bind (as Py does) to both BAS and LAS; and (v) it can differentiate acid strengths through chemical shifts of both 15N and 19F. The limitations are that it does not fit inside the smallest zeolite pores, it could block moderately-sized pores preventing a full quantitative analysis, and that it displays substantial dynamics at ambient temperature. Through conventional, high-field, and low-temperature 19F MAS NMR techniques various active sites have been identified, including previously hard-to-detect AlV Brønsted acid sites that are in low concentrations but apparently crucial for adsorption and thus catalytic activity. The local aluminium environments, which are generally deemed as the active sites or adjacent to active sites (BAS), are very similar for zeolites HY and HZSM-5 in terms of where 3-fluoropyridine is bound; their 27Al NMR signatures after polarisation transfer from 19F are almost identical. However, the 19F NMR response from the fluoropyridine is very different. This method provides valuable insight into different activity for seemingly similar sites. This concept is well known for zeolites, but not so well understood. Such findings provide further evidence for the nuanced role of zeolite active site environments in determining molecular interactions, reinforcing the need for detailed spectroscopic characterisation to fully understand structure–function relationships in zeolite catalysis.Experimental procedures
Details regarding the experimental procedures can be found in the ESI.†Data availability
Data for this article, including NMR and FT-IR data are available at Figshare at https://doi.org/10.48420/28378607.v1.Author contributions
D. L. conceptualized the original idea. J. H., Y. M., and R. Z. designed the adsorption conditions and performed the standard characterization of all samples. J. H. collected, interpreted, and finalized the solid-state NMR data with input and supervision from P. C. V. for the low temperature MAS NMR and from D. I. for the high-field MAS NMR. S. Y. and X. F. supervised the synthetic and adsorption work and D. L. supervised the solid-state NMR spectroscopy. J. H. wrote the first draft of the manuscript and the ESI.† S. Y., X. F., and D. L. revised the article for submission, with input from all coauthors.Conflicts of interest
The authors declare no competing interests.Acknowledgements
The UK High-Field Solid-State NMR Facility used in this research was funded by EPSRC and BBSRC (EP/T015063/1), as well as the University of Warwick including via part funding through Birmingham Science City Advanced Materials Projects 1 and 2 supported by Advantage West Midlands (AWM) and the European Regional Development Fund (ERDF). The low temperature (100 K) MAS NMR experiments were performed at the Nottingham DNP MAS NMR Facility which is funded by the University of Nottingham and EPSRC (EP/L022524/1 and EP/R042853/1). We also thank the EPSRC and The University of Manchester for funding the 16.4 T NMR spectrometer (EP/V007580/1) used in this work.References
- K. Li, J. Valla and J. Garcia-Martinez, ChemCatChem, 2014, 6, 46–66 CrossRef CAS.
- M. Niwa, K. Suzuki, N. Morishita, G. Sastre, K. Okumura and N. Katada, Catal. Sci. Technol., 2013, 3, 1919–1927 RSC.
- N. Rahimi and R. Karimzadeh, Appl. Catal., A, 2011, 398, 1–17 Search PubMed.
- A. Primo and H. Garcia, Chem. Soc. Rev., 2014, 43, 7548–7561 Search PubMed.
- M. Dusselier, M. A. Deimund, J. E. Schmidt and M. E. Davis, ACS Catal., 2015, 5, 6078–6085 CrossRef CAS.
- S. Wang, Z. Qin, M. Dong, J. Wang and W. Fan, Chem Catal., 2022, 2, 1657–1685 CrossRef CAS.
- J. Liang, G. Shan and Y. Sun, Renewable Sustainable Energy Rev., 2021, 139, 110707 CrossRef CAS.
- M. Hu, C. Wang, Y. Chu, Q. Wang, S. Li, J. Xu and F. Deng, Angew. Chem., Int. Ed., 2022, 61, e202207400 CrossRef CAS.
- Z. Qie, H. Xiang, H. Xiang, R. Zou, A. Alhelali, H. Alhassawi, S. Ding, Y. Jiao, S. M. Holmes, A. A. Garforth, X. Gao, J. Wang and X. Fan, Fuel, 2024, 368, 131532 Search PubMed.
- Z. Dong, W. Chen, K. Xu, Y. Liu, J. Wu and F. Zhang, ACS Catal., 2022, 12, 14882–14901 Search PubMed.
- J. Sun, J. Dong, L. Gao, Y.-Q. Zhao, H. Moon and S. L. Scott, Chem. Rev., 2024, 124, 9457–9579 CrossRef CAS.
- A. Abraham, S.-H. Lee, C.-H. Shin, S. B. Hong, R. Prins and J. A. van Bokhoven, Phys. Chem. Chem. Phys., 2004, 6, 3031–3036 RSC.
- J. Dědeček, Z. Sobalík and B. Wichterlová, Catal. Rev., 2012, 54, 135–223 CrossRef.
- S. Ramdas, J. M. Thomas, J. Klinowski, C. A. Fyfe and J. S. Hartman, Nature, 1981, 292, 228–230 CrossRef CAS.
- S. Wang, Y. He, W. Jiao, J. Wang and W. Fan, Curr. Opin. Chem. Eng., 2019, 23, 146–154 CrossRef.
- P. G. Blakeman, E. M. Burkholder, H.-Y. Chen, J. E. Collier, J. M. Fedeyko, H. Jobson and R. R. Rajaram, Catal. Today, 2014, 231, 56–63 Search PubMed.
- J. Jae, G. A. Tompsett, A. J. Foster, K. D. Hammond, S. M. Auerbach, R. F. Lobo and G. W. Huber, J. Catal., 2011, 279, 257–268 CrossRef CAS.
- C. D. Chudasama, J. Sebastian and R. V. Jasra, Ind. Eng. Chem. Res., 2005, 44, 1780–1786 Search PubMed.
- Y. Li, T.-S. Chung, C. Cao and S. Kulprathipanja, J. Membr. Sci., 2005, 260, 45–55 CrossRef CAS.
- U. Olsbye, S. Svelle, M. Bjørgen, P. Beato, T. V. W. Janssens, F. Joensen, S. Bordiga and K. P. Lillerud, Angew. Chem., Int. Ed., 2012, 51, 5810–5831 CrossRef CAS.
- B. Xu, C. Sievers, S. B. Hong, R. Prins and J. A. van Bokhoven, J. Catal., 2006, 244, 163–168 CrossRef CAS.
- A. Palčić and V. Valtchev, Appl. Catal., A, 2020, 606, 117795 CrossRef.
- J.-Z. Qiu, J. Hu, J. Lan, L.-F. Wang, G. Fu, R. Xiao, B. Ge and J. Jiang, Chem. Mater., 2019, 31, 9413–9421 CrossRef CAS.
- B. Wei, X. Liu, Q. Chang, S. Li, H. Luo, K. Hua, S. Zhang, J. Chen, Z. Shao, C. Huang, H. Wang and Y. Sun, Chem Catal., 2022, 2, 2066–2076 CrossRef CAS.
- T. Zhang, Z. Chen, A. G. Walsh, Y. Li and P. Zhang, Adv. Mater., 2020, 32, 2002910 CrossRef CAS.
- M. Rahimi, E.-P. Ng, K. Bakhtiari, M. Vinciguerra, H. A. Ahmad, H. Awala, S. Mintova, M. Daghighi, F. Bakhshandeh Rostami, M. de Vries, M. M. Motazacker, M. P. Peppelenbosch, M. Mahmoudi and F. Rezaee, Sci. Rep., 2015, 5, 17259 CrossRef CAS.
- M. A. Snyder and M. Tsapatsis, Angew. Chem., Int. Ed., 2007, 46, 7560–7573 CrossRef CAS.
- Z. Yu, S. Li, Q. Wang, A. Zheng, X. Jun, L. Chen and F. Deng, J. Phys. Chem. C, 2011, 115, 22320–22327 CrossRef CAS.
- S. Li, O. Lafon, W. Wang, Q. Wang, X. Wang, Y. Li, J. Xu and F. Deng, Adv. Mater., 2020, 32, 2002879 CrossRef CAS.
- N. Thomas, J. Dhainaut, A. Moissette, O. Lafon and G. N. Manjunatha Reddy, J. Phys. Chem. C, 2024, 128, 10428–10439 CrossRef CAS.
- D. Ma, F. Deng, R. Fu, X. Han and X. Bao, J. Phys. Chem. B, 2001, 105, 1770–1779 CrossRef CAS.
- M. Zheng, Q. Wang, Y. Chu, X. Tan, W. Huang, Y. Xi, Y. Wang, G. Qi, J. Xu, S. B. Hong and F. Deng, J. Am. Chem. Soc., 2024, 146, 29417–29428 CrossRef CAS.
- C. A. Fyfe, G. C. Gobbi, G. J. Kennedy, J. D. Graham, R. S. Ozubko, W. J. Murphy, A. Bothner-By, J. Dadok and A. S. Chesnick, Zeolites, 1985, 5, 179–183 CrossRef CAS.
- Y. Ji, K. Chen, A. Hao and G. Hou, J. Magn. Reson. Open, 2025, 22, 100182 CrossRef.
- L. Peng, Y. Liu, N. Kim, J. E. Readman and C. P. Grey, Nat. Mater., 2005, 4, 216–219 CrossRef CAS.
- R. Verma, C. Singhvi, A. Venkatesh and V. Polshettiwar, Nat. Commun., 2024, 15, 6899 CrossRef.
- P. Xiao, L. Wang, H. Toyoda, Y. Wang, K. Nakamura, J. Huang, R. Osuga, M. Nishibori, H. Gies and T. Yokoi, J. Am. Chem. Soc., 2024, 146, 31969–31981 CrossRef CAS.
- S. Li, A. Zheng, Y. Su, H. Fang, W. Shen, Z. Yu, L. Chen and F. Deng, Phys. Chem. Chem. Phys., 2010, 12, 3895–3903 RSC.
- H. Fang, P. Kamakoti, J. Zang, S. Cundy, C. Paur, P. I. Ravikovitch and D. S. Sholl, J. Phys. Chem. C, 2012, 116, 10692–10701 CrossRef CAS.
- H. Soscún, O. Castellano, J. Hernandez, F. Arrieta, Y. Bermúdez, A. Hinchliffe, M. R. Brussin, M. Sanchez, A. Sierraalta and F. Ruette, J. Mol. Catal. A: Chem., 2007, 278, 165–172 CrossRef.
- S. Wannakao, C. Warakulwit, K. Kongpatpanich, M. Probst and J. Limtrakul, ACS Catal., 2012, 2, 986–992 CrossRef CAS.
- D. L. Bhering, A. Ramírez-Solís and C. J. A. Mota, J. Phys. Chem. B, 2003, 107, 4342–4347 CrossRef CAS.
- X. Rozanska, R. A. van Santen, T. Demuth, F. Hutschka and J. Hafner, J. Phys. Chem. B, 2003, 107, 1309–1315 CrossRef CAS.
- C. Liu, G. Li, E. J. M. Hensen and E. A. Pidko, J. Catal., 2016, 344, 570–577 CrossRef CAS.
- A. Zheng, L. Chen, J. Yang, M. Zhang, Y. Su, Y. Yue, C. Ye and F. Deng, J. Phys. Chem. B, 2005, 109, 24273–24279 CrossRef CAS.
- A. V. Yakimov, M. Ravi, R. Verel, V. L. Sushkevich, J. A. van Bokhoven and C. Copéret, J. Am. Chem. Soc., 2022, 144, 10377–10385 CrossRef CAS.
- Z. Yu, A. Zheng, Q. Wang, L. Chen, J. Xu, J. P. Amoureux and F. Deng, Angew. Chem., Int. Ed., 2010, 49, 8657–8661 CrossRef CAS.
- C. A. Fyfe, J. L. Bretherton and L. Y. Lam, J. Am. Chem. Soc., 2001, 123, 5285–5291 CrossRef CAS.
- J. Jiao, J. Kanellopoulos, W. Wang, S. S. Ray, H. Foerster, D. Freude and M. Hunger, Phys. Chem. Chem. Phys., 2005, 7, 3221–3226 Search PubMed.
- A. Zheng, S.-B. Liu and F. Deng, Solid State Nucl. Magn. Reson., 2013, 55–56, 12–27 CrossRef CAS.
- Q. Zhao, W.-H. Chen, S.-J. Huang, Y.-C. Wu, H.-K. Lee and S.-B. Liu, J. Phys. Chem. B, 2002, 106, 4462–4469 CrossRef CAS.
- H.-M. Kao and C. P. Grey, Chem. Phys. Lett., 1996, 259, 459–464 CrossRef CAS.
- W. L. Earl, P. O. Fritz, A. A. V. Gibson and J. H. Lunsford, J. Phys. Chem., 1987, 91, 2091–2095 CrossRef CAS.
- G. I. Kapustin, T. R. Brueva, A. L. Klyachko, S. Beran and B. Wichterlova, Appl. Catal., 1988, 42, 239–246 CrossRef CAS.
- F. Benaliouche, Y. Boucheffa, P. Ayrault, S. Mignard and P. Magnoux, Microporous Mesoporous Mater., 2008, 111, 80–88 Search PubMed.
- F. Jin and Y. Li, Catal. Today, 2009, 145, 101–107 Search PubMed.
- E. Selli and L. Forni, Microporous Mesoporous Mater., 1999, 31, 129–140 CrossRef CAS.
- W. R. Gunther, V. K. Michaelis, R. G. Griffin and Y. Roman-Leshkov, J. Phys. Chem. C, 2016, 120, 28533–28544 CrossRef CAS.
- I. G. Shenderovich, G. Buntkowsky, A. Schreiber, E. Gedat, S. Sharif, J. Albrecht, N. S. Golubev, G. H. Findenegg and H.-H. Limbach, J. Phys. Chem. B, 2003, 107, 11924–11939 CrossRef CAS.
- X. Yi, H. H. Ko, F. Deng, S. B. Liu and A. Zheng, Nat. Protoc., 2020, 15, 3527–3555 CrossRef CAS.
- H.-M. Kao and C. P. Grey, J. Phys. Chem., 1996, 100, 5105–5117 CrossRef CAS.
- G. Li, C. Foo, R. Fan, M. Zheng, Q. Wang, Y. Chu, J. Li, S. Day, P. Steadman, C. Tang, T. W. B. Lo, F. Deng and S. C. E. Tsang, Science, 2025, 387, 388–393 CrossRef CAS.
- J. B. Nicholas, J. F. Haw, L. W. Beck, T. R. Krawietz and D. B. Ferguson, J. Am. Chem. Soc., 1995, 117, 12350–12351 CrossRef CAS.
- A. Simon, L. Delmotte, J.-M. Chezeau and L. Huve, Chem. Commun., 1997, 263–264 Search PubMed.
- A. Simon, L. Delmotte, J.-M. Cheźeau, A. Janin and J.-C. Lavalley, Phys. Chem. Chem. Phys., 1999, 1, 1659–1664 RSC.
- J.-H. Yang, L. A. Clark, G. J. Ray, Y. J. Kim, H. Du and R. Q. Snurr, J. Phys. Chem. B, 2001, 105, 4698–4708 CrossRef CAS.
- H.-M. Kao and Y.-C. Chen, J. Phys. Chem. B, 2003, 107, 3367–3375 CrossRef CAS.
- C. Lee, D. J. Parrillo, R. J. Gorte and W. E. Farneth, J. Am. Chem. Soc., 1996, 118, 3262–3268 CrossRef CAS.
- M. Arabiourrutia, M. Olazar, R. Aguado, G. López, A. Barona and J. Bilbao, Ind. Eng. Chem. Res., 2008, 47, 7600–7609 CrossRef CAS.
- J. Qi, Y. Guo, H. Jia, B. Fan, H. Gao, B. Qin, J. Ma, Y. Du and R. Li, Fuel Process. Technol., 2023, 240, 107586 CrossRef CAS.
- A. Boréave, A. Auroux and C. Guimon, Microporous Mater., 1997, 11, 275–291 CrossRef.
- E. Taarning, C. M. Osmundsen, X. Yang, B. Voss, S. I. Andersen and C. H. Christensen, Energy Environ. Sci., 2011, 4, 793–804 RSC.
- S. C. Cardona and A. Corma, Appl. Catal., B, 2000, 25, 151–162 CrossRef CAS.
- W. Song, R. E. Justice, C. A. Jones, V. H. Grassian and S. C. Larsen, Langmuir, 2004, 20, 8301–8306 Search PubMed.
- K. Hadjiivanov, in Advances in Catalysis, ed. F. C. Jentoft, Academic Press, 2014, vol. 57, pp. 99–318 Search PubMed.
- V. Zholobenko, C. Freitas, M. Jendrlin, P. Bazin, A. Travert and F. Thibault-Starzyk, J. Catal., 2020, 385, 52–60 CrossRef CAS.
- R. Zhang, S. Xu, D. Raja, N. B. Khusni, J. Liu, J. Zhang, S. Abdulridha, H. Xiang, S. Jiang, Y. Guan, Y. Jiao and X. Fan, Microporous Mesoporous Mater., 2019, 278, 297–306 CrossRef CAS.
- C. V. Hidalgo, H. Itoh, T. Hattori, M. Niwa and Y. Murakami, J. Catal., 1984, 85, 362–369 CrossRef CAS.
- J. Cattanach, E. L. Wu and P. B. Venuto, J. Catal., 1968, 11, 342–347 Search PubMed.
- K. Chen, S. Horstmeier, Vy. T. Nguyen, B. Wang, S. P. Crossley, T. Pham, Z. Gan, I. Hung and J. L. White, J. Am. Chem. Soc., 2020, 142, 7514–7523 CrossRef CAS.
- D. J. Parrillo and R. J. Gorte, J. Phys. Chem., 1993, 97, 8786–8792 CrossRef CAS.
- J. W. Harris, W.-C. Liao, J. R. Di Iorio, A. M. Henry, T.-C. Ong, A. Comas-Vives, C. Copéret and R. Gounder, Chem. Mater., 2017, 29, 8824–8837 Search PubMed.
- W.-C. Liao, B. Ghaffari, C. P. Gordon, J. Xu and C. Copéret, Curr. Opin. Colloid Interface Sci., 2018, 33, 63–71 CrossRef CAS.
- I. B. Moroz, K. Larmier, W.-C. Liao and C. Copéret, J. Phys. Chem. C, 2018, 122, 10871–10882 CrossRef CAS.
- S. Chapman, M. Carravetta, I. Miletto, C. M. Doherty, H. Dixon, J. D. Taylor, E. Gianotti, J. Yu and R. Raja, Angew. Chem., Int. Ed., 2020, 59, 19561–19569 CrossRef CAS.
- X. Wang, Q. Wang, C. Wang, Y. Chu, M. Hu, F. Deng, J. Yu and J. Xu, J. Am. Chem. Soc., 2025, 147, 17829–17838 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ay00507h |
| This journal is © The Royal Society of Chemistry 2025 |

