Open Access Article
Open Access ArticleA rapid method for the determination of methylmercury and inorganic mercury species in whole blood by liquid chromatography with detection using vapor generation ICP-MS/MS†
Emily J.
Pacer
 ab,
Christopher D.
Palmer
ab,
Christopher D.
Palmer
 ab and
Patrick J.
Parsons
ab and
Patrick J.
Parsons
 *ab
*ab
aWadsworth Center, New York State Department of Health, Empire State Plaza Albany, NY 12237, USA. E-mail: patrick.parsons@health.ny.gov
bDepartment of Environmental Health Sciences, University at Albany, Rensselaer, NY 12144, USA
First published on 4th February 2025
Abstract
Speciation methods provide a more detailed picture regarding human exposure to toxic metals/metalloids and their effects on human health. The toxicity of methylmercury (MeHg) differs considerably from inorganic mercury (iHg), such that their separation and quantification in whole blood is helpful in identifying sources and possible pathways of exposure. Liquid chromatography (LC) has several advantages over gas chromatography (GC) for the separation of iHg from MeHg due to the former's compatibility with uptake rates of common nebulizer systems used with ICP-MS and the latter's requirement for a derivatization step to produce gaseous Hg species for an effective separation. Here we report an improved method that was developed to separate and quantify MeHg and iHg species in whole blood using isocratic LC elution with determination by vapor generation (VG) coupled with ICP-MS/MS. Chromatographic separation of MeHg and iHg is achieved in ∼4 minutes on a C8 reversed phase column. In those rare cases where there may be human exposure to ethylmercury (EtHg), or where a certified reference material (CRM) is known to contain EtHg (e.g., NIST SRM 955c), all three Hg species can be separated by extending the LC elution time to 8 minutes. Adding VG post column boosts the signal-to-noise ratio, and lowers the LOD. With optimized sample preparation, the LC-VG-ICP-MS/MS method LOD for both iHg and MeHg is 0.2 μg L−1. Method validation was conducted using NIST SRM 955c Toxic Metals in Caprine Blood and NIST SRM 955d Toxic Elements and Metabolites in Frozen Human Blood. Additional validation data were generated using archived blood reference materials from multiple Proficiency Testing programs and External Quality Assessment schemes. Blood-based quality control materials, previously analyzed for Hg species using isotope dilution with GC coupled to ICP-MS, were provided by the US CDC.
Introduction
Mercury (Hg) is a naturally occurring element that is found in several chemical species in biological matrices. A “chemical species” refers to the specific form of an element defined by its complex or molecular structure, electronic or oxidation state, or its isotopic composition.1 While total elemental determination is important for biological and environmental investigations, they do not provide the complete picture on bioavailability and potential toxic effects on biological systems. For Hg, bioavailability and toxicity depend on the individual chemical species present.2 Exposure to Hg can have adverse effects on the central nervous system and the renal system, although different Hg species target different organ systems. Organomercury compounds, such as methylmercury (MeHg), tend to be more toxic than inorganic mercury (iHg), i.e., Hg2+ or elemental Hg (Hg0).3Biomonitoring provides an opportunity to measure direct toxic exposures from the environment through analysis of human specimens, like blood or urine.4 This approach provides a direct way to assess internal exposure (dose) compared to environmental analysis of air, soil, or water, which provides information on sources and pathways of exposure. Both iHg and MeHg bind to hemoglobin, which makes whole blood an ideal biomarker for speciation analysis.3,5 As some Hg species are more harmful than others, it is important to develop reliable and rugged speciation methods that are fit for purpose. Speciation analysis typically involves two complementary techniques, one for separation and the other for detection.1 Hyphenated techniques are advantageous for identifying and quantifying individual species.2 Only a few workers have used chromatographic separation coupled with ICP-MS for Hg speciation. Yet there is a growing need for improved Hg speciation methods of analysis that are not only rapid and rugged, but are suitable for use in human biomonitoring studies.
Some Hg speciation methods can be instrumentally challenging, costly, and time consuming, which can be impractical to implement for large population-based biomonitoring studies. Ongoing public health surveillance for Hg exposures is essential for identifying high-risk groups, tracking exposures over time, and evaluating interventions. The US National Health and Nutrition Examination Survey (NHANES) has included assessing exposure to Hg species in whole blood since 2011. The analyzing laboratory at US Centers for Disease Control and Prevention (CDC) uses a method based on Solid Phase Micro Extraction-Isotope Dilution-Gas Chromatography-Inductively Coupled Plasma Mass Spectrometry (SPME-ID-GC-ICP-MS) for Hg speciation in whole blood.6 This method requires derivatization of the Hg species in blood samples and ID with enriched Hg isotopes.7,8 While limits of detection (LODs) for Hg speciation may be lower with GC compared to current LC methods, the latter has several important advantages including its compatibility with the nebulizer uptake rate of the ICP-MS. Additionally, there is no need for a derivatization step prior to separation of Hg species or a heated transfer interface between the LC and the ICP-MS.8 One LC-ICP-MS method for blood Hg speciation reported separating iHg, MeHg, and EtHg in 10 minutes.9
Sensitivity enhancements for Hg with LC methods are feasible by increasing sample introduction efficiency using a vapor generation (VG) technique. This well-established technique falls into two categories, hydride generation (HG) or cold vapor (CV), the latter is only applicable to Hg2+. The VG technique is based on converting dissolved analytes into gaseous hydrides with a reducing agent, which can significantly improve the efficiency of sample introduction into the instrument and achieve better, i.e., lower detection limits.10,11 VG or cold vapor (CV) techniques have been coupled to AAS, AFS and ICP-MS are well established to determine Hg in many different sample matrices. Two previous reports have described using VG with LC post column for Hg speciation in human plasma, blood, and hair.12,13
Most VG efficiencies are species dependent, and analytes need to be reduced after elution from the LC column.14 Since SnCl2 only reacts with Hg2+ to form Hg0, and does not react with the organoalkyl forms of Hg, NaBH4 must be used as the reducing agent.15 Traditional VG produces Hg0 from Hg2+, but organomercury compounds MeHg and EtHg must first be converted into their respective volatile hydrides, MeHgH and EtHgH for determination.13 Different Hg species show different behaviors in the CV generation process, which can lead to different sensitivities, and the need for species-specific calibration standards.16
Different designs have been proposed to couple LC with VG before the analytes can enter the ICP torch. One such design uses a simple T-piece to combine the LC eluent with the reducing agent prior to transfer to the nebulizer, using the mass spectrometer's peristaltic pump.12 Another design leverages a T-piece to add the reducing agent to the LC eluent post-column, followed by a gas–liquid separator (GLS) before reaching the spray chamber of the ICP-MS.13 In both designs, the flow rates of acid and the reducing agent were optimized and controlled by peristaltic pumps. Most manual systems are constructed in-house and are unique to each laboratory. Therefore, we compared a commercially available, continuous flow VG system as a more efficient means of vapor generation to the ICP-MS/MS to a conventional nebulizer/spray chamber-based approach.
Published LC-ICP-MS speciation methods have included determination of EtHg in human blood.9,13,17–19 Historically, the US NHANES has included the determination of EtHg in addition to iHg and MeHg, due to public health concerns with its use in thimerosal, a vaccine preservative.20 However, the current NHANES report from 2013–2018 that includes data for Hg species shows that there were no detects for EtHg out of the thousands of samples analyzed in the US population.21 These data are not unexpected due to the limited use of thimerosal vaccines, a suspected source of EtHg exposure, and the short half-life of the species in blood.6 Consequently, concerns have since diminished and measuring EtHg is no longer deemed necessary, as it is suspected that it converts to other Hg species in a matter of days.6,9 For these reasons, the method developed here was optimized primarily for the rapid determination of iHg and MeHg species in blood using LC coupled to VG-ICP-MS/MS.
Novel aspects of the current method for Hg speciation in whole blood includes using a simple alkaline sample pre-treatment step, isocratic LC elution on a C8 column with determination by VG coupled to ICP-MS/MS. The sample preparation procedure was optimized for a high throughput of samples.
The U.S. EPA has established an estimated reference dose (RfD) of 5.8 μg L−1 for MeHg in blood below which is unlikely to cause harmful effects in humans.6,22–24 The New York State Department of Health (NYS DOH) Heavy Metals Registry (HMR) mandates total blood Hg concentrations at or above 5 μg L−1 to be reported electronically by the clinical laboratory.25 Thus, even if a VG step were not used, the LC method proposed here could still serve as a confirming clinical method for capturing iHg and MeHg, in those cases where the total Hg level was previously found to be elevated (≥5 μg L−1). However, the addition of a VG step increases sensitivity for a better (i.e., lower) LOD such that it is suitable for human biomonitoring studies and is competitive in performance with current GC-ID-ICP-MS methods. The proposed LC-VG-ICP-MS/MS method offers a simpler, and more cost-effective solution that is both reliable, and suitable for a high throughput of samples. Method validation data (accuracy and precision) are provided based on analysis of National Institute of Standards and Technology (NIST) standard reference materials (SRM) 955c and 955d. Performance is assessed via analysis of archived proficiency testing (PT) samples.
Experimental
Materials and methods
| LC conditions Agilent 1260 infinity series | |
|---|---|
| Column (optimal) | C8 Zorbax StableBond (5 μm, 150 mm × 5 mm) |
| Guard column | C8 Zorbax StableBond (5 μm, 12.5 mm × 4.6 mm) |
| Mobile phase | 0.06 mol L−1 ammonium acetate, 0.05% (v/v) mercaptoethanol, 0.4% (m/v) L-cysteine, 5% (v/v) methanol, pH ∼6.7 |
| Flow rate | 1.0 mL min−1 |
| Sample injection volume | 60 μL |
| Temperature | Ambient |
| Internal standard | 0.1 μg L−1 MeHg |
| Sample loop | 20 μL |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| VG parameters Elemental Scientific hydrideICP | |
| Carrier acid | 0.01 mol L−1 HCl |
| Reductant | 0.1% m/v NaOH and 0.5% m/v NaBH4 |
| Flow rates (carrier & reductant) | 0.5 mL min−1 |
| Nebulizer gas flow rate | 1.1 L min−1 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|
| ICP-MS/MS parameters Agilent 8900 | |
| ICP power (W) | 1550 W |
| Carrier gas flow (L min−1) | 1.1 L min−1 |
| Nebulizer pump (rps) | 0.50 rps |
| Spray chamber temperature | 2 °C |
| Monitored signals | 202Hg → 202Hg |
| Gas mode | 40% O2 |
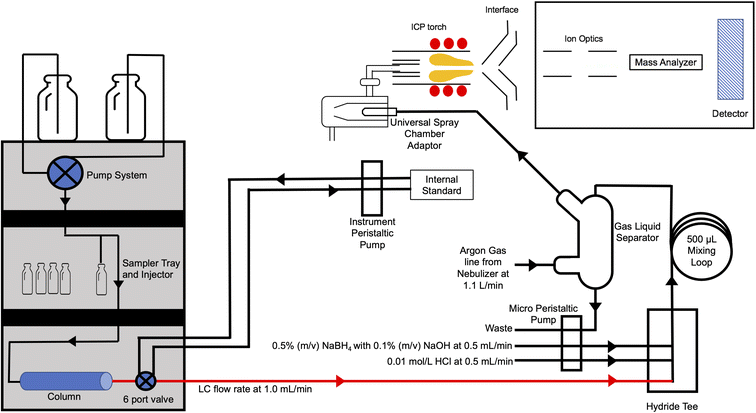
For post column VG studies, a compact Elemental Scientific hydrideICP system (Elemental Scientific Inc., Omaha, NE) was evaluated. The Elemental Scientific design integrates a gas liquid separator (GLS) with a precision micro peristaltic pump (MP2). The GLS design is low volume and has frosted internal thimble which promotes a fast reaction rate. Compared to a traditional peristaltic pump, the MP2 pump has smaller roller sizes which provide a lower pulsation to the carrier flow, compared to traditional peristaltic pumps with larger rollers. Argon gas from the ICP-MS/MS is swept through the bottom of the GLS, which helps transport the volatile species to the spray chamber. The standard ICP-MS/MS nebulizer is replaced with an Elemental Scientific universal spray chamber adaptor. A schematic of the VG commercial manifold integrated with the LC-ICP-MS/MS is shown in Fig. 1. Table 1 shows the optimized conditions for the LC-VG-ICP-MS/MS with the Elemental Scientific hydrideICP system.
Additional reagents for speciation analysis included HPLC grade methanol, 2-mercaptoethanol (≥99.0%), ammonium acetate (99.999% trace metals basis), and L-cysteine (Sigma-Aldrich, USA). The concentrations of the mobile phase reagents were previously studied and optimized as reported by Rodrigues et al. and were used in this work too (Table 1).9 LC columns were stored with acetonitrile when not used for analysis (Sigma-Aldrich, USA). Solutions of NaBH4 (Sigma-Aldrich, USA) in 0.1% (m/v) NaOH (GFS Chemicals, USA) were used as the reducing agent.
Results and discussion
Optimization of Hg species extraction from blood matrices
One of the most challenging steps in developing a speciation method for Hg in blood samples is the efficient extraction of all Hg species without changing their original composition. Sample preparation should be minimal and yet sufficiently fast to avoid interspecies conversion during analysis.12 The principal goals of the sample preparation step are to ensure the final injected sample extract will not damage the LC column, be relatively free of physical and chemical interferences, and be compatible with the intended analytical method.29 Sample preparation for whole blood tends to be a more challenging task compared to other sample matrices. Injecting undiluted blood onto an LC column would cause catastrophic damage, blockages, and lead to decreased column lifetimes.Previous studies reported analyzing diluted blood samples with a mixture of mercaptoethanol, L-cysteine and acid, followed by sonication and filtration.12,13 Rather than use an acidic diluent, we explored using TMAH, an alkaline diluent that has been used by previous workers for Hg speciation analysis of biological tissues.30,31 For example, while TMAH has been used for analyzing fish matrices for Hg species, its application to whole blood using LC coupled to ICP-MS has not been reported.30,32,33 We found the optimized TMAH concentration for the extraction solvent to be 0.1% (v/v) (ESI, Fig. 1†). This concentration was selected based on optimal sensitivity coupled with method accuracy when analyzing SRM 955c, as shown in the chromatograms of ESI, Fig. 1.† At higher TMAH concentrations (1% and 5% v/v), the signal increase for iHg and decrease for EtHg, suggesting interconversion of EtHg into iHg species.
Blood samples were sonicated and then centrifuged to separate the cell debris from the extract containing Hg species. Previous workers reported using 0.2 μm nylon filters12 or 0.45 μm polyvinylidene difluoride (PVDF) filters,13 to clean up the blood extract. However, due to an incompatibility issue between TMAH and the nylon filters, 0.45 μm PES disposable syringe filters were used. Refine™ Ultrafiltration columns were also investigated for cleaning up the blood extracts. For the Refine™ Ultrafiltration columns, a positive pressure manifold with the capability of filtering up to 48 samples in a single batch was investigated to evaluate a higher sample throughput. Compared to the syringe filters, the Refine™ Ultrafiltration columns produced similar recoveries and analytical performance for all Hg species, as seen in the chromatograms shown in ESI, Fig. 2.†
PES disposable syringe filters achieved a throughput of approximately 1000 injections per single LC column before the column backpressure increased and poorer resolution was evident. Experience has also shown that a C8 guard column is mandatory, and it should be changed after every 250 injections to protect and extend the life of the analytical column. However, when using the Refine™ Ultrafiltration columns the prefilter and guard column must be changed more frequently, (∼100 injections). For a more rapid and potentially lower-cost sample extraction process, Refine™ Ultrafiltration columns might be more suitable, even though LC column lifetimes may be limited to ∼800 samples per column. Details for optimized sample preparation using the Refine™ Ultrafiltration columns are shown in ESI, Fig. 3.†
Optimization of LC separation
Sulfur-containing compounds such as L-cysteine, 2-mercaptoethanol, glutathione and APDC have a strong affinity for binding Hg and have been used in previous studies.30,34,35 For this study, we used L-cysteine and 2-mercaptoethanol, combined with ammonium acetate and methanol for the final optimized mobile phase as recommended previously by Rodrigues et al.9 The mobile phase components includes 0.05% (v/v) 2-mercaptoethanol, 0.4% (m/v) L-cysteine, 0.06 mol L−1 ammonium acetate, and 5% (v/v) methanol at a pH of 6.7, and delivered at a flow rate of 1 mL min−1.9 The optimized sample injection volume was found to be 60 μL (see ESI, Fig. 4†). While the signal response for each Hg species increases with higher sample injection volumes, poorer resolution is evident at 80 μL. Sufficient resolution between the iHg and MeHg peaks is achieved using 60 μL along with an increase in peak height as shown in ESI, Fig. 4.†For large biomonitoring studies where a high throughput of samples is needed, analysis times need to be optimized. While the principal Hg species of interest, i.e., iHg and MeHg, elute in ∼5 minutes, EtHg (if present) is a limiting factor eluting at ∼10 minutes. Various options were explored to achieve shorter analysis times. For example, a gradient elution with increasing methanol concentration would require the addition of oxygen to the carrier gas-flow as an optional plasma gas, and platinum cones, to ensure the additional organic solvent could be introduced into a stable plasma while minimizing the risk of carbon residue build up on the sampler/skimmer cones.36 The additional gas coupled with longer column re-equilibration times, would prolong the analysis. As the methanol concentration remained low (<5%), gradient elution was not necessary for this work. In preliminary studies to reduce the analysis time, a 5 cm C18 column (Zorbax StableBond, 5 μm, 50 mm × 4.6 mm) was investigated. While the elution time for the Hg species was significantly shorter, the resolution between iHg and MeHg was compromised. Even after adjusting the concentrations of mercaptoethanol and methanol, flow rates and integration times, baseline resolution between the two principal Hg species was still not possible. Another option was to reduce the column particle size. This would be expected to improve the separation efficiency and achieve better resolution. However, increased backpressure and reduced column lifetime were a major concern working with a blood-based matrix. Therefore, we investigated the separation of these Hg species on a 15 cm C8 column.
While separation of Hg species on a C8 column was previously reported with human plasma12 and breast milk,37 to our knowledge, whole blood has not been explored. Zou et al., found the retention of phenyl Hg, a species that elutes after EtHg in environmental samples, to be too strongly retained on a C18 column and they used a C8 column with a weaker retention capacity.38 In our method development studies, we explored using a 15 cm C8 column (Zorbax StableBond, 5 μm, 150 mm × 4.6 mm) with a guard column containing a similar packing material. The retention times for all three Hg species on the C8 column were slightly shorter compared to the 15 cm C18 column, where EtHg eluted in just under 10 minutes, compared to under 8 minutes on the C8 column. Thus, the total analysis time is faster by ∼2 minutes (ESI, Fig. 5†). Additionally, resolution of iHg and MeHg is much improved on a 15 cm C8 column, with a narrower iHg peak, i.e., less peak tailing. The two principal Hg species elute in under 5 minutes on a 15 cm C18 column, compared to under 4 minutes on the 15 cm C8 column. ESI, Fig. 5† shows the chromatogram for NIST SRM 955c level 4 on a 15 cm C18 column (blue) compared to the 15 cm C8 (red) column, without using VG.
If the sample analysis is stopped at 5 minutes capturing the two species of interest (MeHg and iHg), any EtHg present in the sample would appear at a unique retention time in the subsequent chromatogram, at approximately 1.5 minutes, and would not co-elute with either iHg or MeHg in the next sample. Recognizing this as EtHg potentially eluting from the previous sample injection, the analyst could re-analyze that sample (along with the appropriate EtHg calibration standards) and adjust the chromatogram time for an 8 minute elution. After evaluating a 5 cm C18 column, a 15 cm C18 column, and a 15 cm C8 column, the latter was selected as the optimal stationary phase for the method.
Optimization of hydrideICP and hydride reagents
Vapor generation after LC separation yielded better sensitivities for all three Hg species. However, some workers report disadvantages that include increased concentrations of organic solvents and acids, that increase analysis times and degrade the signal-to-noise ratio.16,39 Gao et al., reported that additional sample preparation steps could overcome such interferences, but may introduce more uncertainties.14 For blood sample analysis specifically, a foaming effect in the gas–liquid separator was reported as troublesome and affected method performance when using a Flow Injection Mercury System (FIMS).40 Plasma stability is not only a concern from increased hydride vapor but also from hydrogen gas that is generated from NaBH4 and passed into to the ICP.1 Given these challenges, a commercial hydrideICP system was investigated. The sample preparation procedure developed initially was found to be fit for purpose, either with or without addition of the VG system. As only the hydride vapor travels into the universal spray chamber adaptor and then into the ICP, dry plasma conditions are stable.An investigation of optimal VG reagent concentrations was conducted using the continuous flow hydrideICP system. The effect of HCl concentration was studied in the range 0.001 mol L−1 to 0.05 mol L−1. The concentration range of NaBH4 was investigated from 0.01% to 0.5% (m/v). The VG reagent flow rates were optimized at 0.5 mL min−1. A standard containing 10 μg L−1 iHg and MeHg was prepared, and the VG reagents were investigated across 16 different variables. Results are shown in Fig. 2. On the y-axis, counts from the 10 μg L−1 Hg standard are normalized to the internal standard counts. On the x-axis, HCl concentration is shown in mol L−1. Each of the colored lines/symbols represent NaBH4 concentration (v/v): 0.01% (black); 0.05% (red); 0.1% (blue); and 0.5% (green).
 | ||
| Fig. 2 Effect of HCl and NaBH4 concentrations at 0.5 mL min−1 on the Hg signal with VG coupled to LC-ICP-MS/MS for (A) iHg and (B) MeHg. Standard solution contained 10 μg L−1 of each species. | ||
Sensitivity for iHg is improved as the HCl concentration increases but is poorer as NaBH4 concentration increases. In contrast, MeHg sensitivity is better at lower HCl concentrations and at higher NaBH4 concentrations. Others have reported similar findings, i.e., that different Hg species have different sensitivities when measured with a VG system.16 Therefore, the VG reagent concentrations were optimized at 0.01 mol L−1 HCl and 0.5% m/v NaBH4, specifically for MeHg, as it is typically the primary species found in blood.
The additional tubing and GLS required for the hydrideICP system, slightly increased retention times for the 3 Hg species by ∼0.5 min on the C8 column. Elution of iHg and MeHg occurs in under 4.5 minutes, followed by EtHg at 8.5 minutes when using the hydrideICP system, as shown in Fig. 3.
Validation and stability studies
Method LODs for Hg species in whole blood by LC-ICP-MS/MS, with and without post column VG are summarized in Table 2. The LODs were calculated based on the IUPAC/ISO harmonized protocol, i.e., 3 × SD for n = 7 measurements, i.e., collected over 7 independent analytical runs using NIST SRM 955c level 2.41| LC-ICP-MS/MS | LC-VG-ICP-MS/MS | |
|---|---|---|
| iHg (μg L−1) | 0.45 | 0.22 |
| MeHg (μg L−1) | 0.42 | 0.23 |
| EtHg (μg L−1) | 0.84 | 0.33 |
Typical calibration curves (and associated chromatograms) are presented in ESI, Fig. 6a and b† for the three Hg species and show good linearity. Chromatograms for NIST 955c level 2 and 4 with and without VG are shown in Fig. 3.
A limited, short-term stability study was carried out by storing TMAH-extracted NIST 955c blood samples post filtration, at 4 °C for 7 days. NIST 955c level 2 samples were analyzed over 3 days, and then again on day 7. Stability criteria were based on modified Westgard rules, (i.e., ±3SD).42 Results falling outside of the ±3SD quality assurance limit were deemed unacceptable. On days 1–3 and day 7, the TMAH extracted samples were diluted into new LC vials and analyzed (results shown in ESI, Fig. 7†). TMAH-extracted blood samples were sufficiently stable for up to 3 days when stored at 4 °C but were found to be unstable at 7 days. Based on these experimental data, we can say that the working calibration standards are also stable for up to 3 days at 4 °C as they are of the same chemical composition.
Overnight stability of extracted blood samples was explored. If the analytical run must be repeated, e.g., due to an instrumental issue, the stability of extracted Hg species needs to be verified. For this study, extracted blood samples diluted with mobile phase were maintained at room temperature (∼22 °C) on the instrument autosampler for 24 hours, and re-analyzed for Hg species. There were no statistically significant differences (p > 0.05) in the results (iHg p = 0.377, MeHg p = 0.439, EtHg p = 0.111) and the results showed the samples were stable overnight at room temperature.
Analysis of blood-based SRMs and proficiency testing materials
Both human and caprine blood SRMs were analyzed to assess method accuracy and repeatability (precision). NIST SRM 955c and 955d were analyzed using the proposed LC method and results are shown in Table 3. NIST certified values for total Hg were determined by ICP-MS, while certified or reference values for Hg species were determined by GC-ID-ICP-MS at NIST. Data obtained with the proposed LC method, both with and without VG, are shown in Table 3 along with assigned values provided by NIST.| Assigned value (μg L−1 ± USRM) | LC-ICP-MS/MS (μg L−1 ± UMeas) | LC-VG-ICP-MS/MS (μg L−1 ± UMeas) | ||
|---|---|---|---|---|
| a Calculated as the sum of species, ND = nondetectable. Certified values, except where indicated as a † reference value. | ||||
| NIST 955c level 2 | iHg† | 2.33 ± 0.61 | 2.0 ± 0.7 | 1.7 ± 0.6 |
| MeHg† | 1.82 ± 0.14 | 1.6 ± 0.7 | 1.7 ± 0.2 | |
| EtHg† | 1.84 ± 0.81 | 1.2 ± 0.8 | 1.1 ± 0.8 | |
| Total | 5.42 ± 0.66 | 4.8 ± 1.4a | 4.6 ± 0.7a | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| NIST 955c level 3 | iHg† | 9.0 ± 1.3 | 9.2 ± 2.2 | 8.0 ± 1.5 |
| MeHg† | 4.5 ± 1.0 | 4.0 ± 2.5 | 5.0 ± 1.1 | |
| EtHg† | 4.42 ± 0.78 | 3.2 ± 1.2 | 3.2 ± 0.9 | |
| Total | 17.8 ± 1.6 | 16.4 ± 4.8a | 16.3 ± 1.7a | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| NIST 955c level 4 | iHg† | 18.4 ± 5.2 | 19.9 ± 8.0 | 15.4 ± 5.6 |
| MeHg† | 7.70 ± 0.37 | 6.9 ± 2.0 | 7.3 ± 1.1 | |
| EtHg† | 9.4 ± 3.9 | 7.3 ± 4.2 | 6.2 ± 4.0 | |
| Total | 35.4 ± 2.0 | 34.1 ± 9.3a | 28.9 ± 4.2a | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| NIST 955d level 1 | iHg | 0.405 ± 0.024 | ND | 0.30 ± 0.1 |
| MeHg | 0.626 ± 0.02 | 0.52 ± 0.2 | 0.52 ± 0.1 | |
| EtHg | 0.392 ± 0.034 | ND | ND | |
| Total | 1.373 ± 0.081 | ND | ND | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||
| NIST 955d level 2 | iHg | 2.135 ± 0.043 | 1.7 ± 0.5 | 1.7 ± 0.2 |
| MeHg | 3.844 ± 0.077 | 3.2 ± 1.0 | 3.8 ± 0.3 | |
| EtHg | 0.649 ± 0.028 | ND | 0.5 ± 0.1 | |
| Total | 6.83 ± 0.33 | ND | 6.0 ± 0.5a | |
The mean and expanded uncertainty (U) for reported values (n = 7) were calculated following the GUM and EUROLAB guidelines.43,44 Values reported that were above the method LOQ, both with and without VG, were within ±30% or ±USRM (whichever is greater) of the assigned values for the NIST SRMs. For the Hg species of interest (iHg and MeHg), all detectable values both with and without VG were found to be within ±20% or ±USRM (whichever is greater) of the assigned values of the NIST SRMs.
As no routine proficiency testing (PT) program exists for Hg species in blood at this time, other blood-based materials from the NYSDOH, QMEQAS, and PCI programs were analyzed for additional confidence. These PT samples were originally supplemented with iHg and MeHg by the scheme organizers but assigned values were only available for total Hg. Nevertheless, the sum of Hg species as determined by the proposed LC method was calculated with and without VG and compared to the total Hg value assigned by the PT program provider. A difference plot depicted in ESI, Fig. 8† shows found values for the sum of species with and without VG in good agreement with the assigned values. Total Hg values calculated as the “sum of the species” fell within the NYSDOH PT program quality specifications (±3 μg L−1 or ±30% criteria). The PT materials available were archived from previous rounds and so this precluded blind analysis. An additional benefit from characterizing these PT materials for Hg species, is that these data are now available for comparison with other laboratories using different methods.
Conclusions
A rapid, sensitive and reliable method for Hg speciation in whole blood was developed based on LC-VG-ICP-MS/MS and validated for use in human biomonitoring studies. A similar LC-ICP-MS/MS method but without VG was found to be fit for clinical purposes, i.e., for quantifying Hg species in blood samples previously found to have total Hg levels exceeding 5 μg L−1. Method accuracy was characterized as ±20% or ±USRM (whichever is greater) for iHg and MeHg using blood based NIST SRMs for both methods. Method LODs are better, i.e., lower, when using a VG setup (LC-VG-ICP-MS/MS), making it competitive with GC-ID-ICP-MS methods. With chromatographic separation achieved in ∼4 minutes and the simplicity of the TMAH sample preparation approach, sample throughput can reach ∼100 samples per 7 hour shift, and more than 1000 samples can be analyzed before the LC analytical column needs to be replaced. The LC-VG-ICP-MS/MS method is fit for purpose for use in large scale biomonitoring studies.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization (PP, EP, CP); investigation (EP, PP, CP); methodology (PP, EP, CP); formal analysis (PP, EP, CP); writing original draft (EP); writing – review and editing (PP, EP, CP); project administration (PP); supervision (PP, CP); funding acquisition (PP).Conflicts of interest
The authors report that there are no conflicts to declare.Acknowledgements
This work was supported in part by grant funding from the National Institute of Environmental Health Sciences (NIEHS) of the National Institutes of Health (NIH) under Award Number U2CES026542 (Parsons), and funding from the Centers for Disease Control and Prevention (CDC) under Award Number NU88EH001329-01-00 (Parsons). We are grateful to the CDC's Division of Laboratory Sciences for providing blood-based reference materials to assist with method validation and comparability studies.Notes and references
- X. Yu, C. Liu, Y. Guo and T. Deng, Molecules, 2019, 24, 926 CrossRef PubMed.
- J. Delafiori, G. Ring and A. Furey, Talanta, 2016, 153, 306–331 CrossRef CAS PubMed.
- National Research Council, in Toxicological Effects of Methylmercury, National Academies Press, Washington, D.C, 2000, DOI:10.17226/9899.
- C. D. Palmer, M. E. Lewis, C. M. Geraghty, F. Barbosa and P. J. Parsons, Spectrochim. Acta, Part B, 2006, 61, 980–990 CrossRef.
- E. M. Krupp, Z. Gajdosechova, T. Schwerdtle and H. Lohren, in Metallomics: Analytical Techniques and Speciation Methods, ed. Bernhard Michalke, Wiley-VCH, Weinheim, Germany, 2016, ch. 9, pp. 285–304, DOI:10.1002/9783527694907.
- M. E. Mortensen, S. P. Caudill, K. L. Caldwell, C. D. Ward and R. L. Jones, Environ. Res., 2014, 134, 257–264 CrossRef CAS PubMed.
- Y. L. Sommer, C. P. Verdon, M. R. Fresquez, C. D. Ward, E. B. Wood, Y. Pan, K. L. Caldwell and R. L. Jones, Anal. Bioanal. Chem., 2014, 406, 5039–5047 CrossRef CAS PubMed.
- V. L. Dressler, F. G. Antes, C. M. Moreira, D. Pozebon and F. A. Duarte, Int. J. Mass Spectrom., 2011, 307, 149–162 CrossRef CAS.
- J. L. Rodrigues, S. S. de Souza, V. C. de Oliveira Souza and F. Barbosa, Talanta, 2010, 80, 1158–1163 CrossRef CAS PubMed.
- B. Welz and M. Sperling, in Atomic Absorption Spectrometry, Wiley-VCH, Weinheim, Germany, 3rd edn, 1999 Search PubMed.
- C.-S. Chiou, S.-J. Jiang and K. S. Kumar Danadurai, Spectrochim. Acta, Part B, 2001, 56, 1133–1142 CrossRef.
- S. S. de Souza, A. D. Campiglia and F. Barbosa, Anal. Chim. Acta, 2013, 761, 11–17 CrossRef CAS PubMed.
- I. Petry-Podgórska, V. Schrenková, M. Migašová, T. Matoušek and J. Kratzer, Microchem. J., 2021, 170, 106606 CrossRef.
- Y. Gao, R. Liu and L. Yang, Chin. Sci. Bull., 2013, 58, 1980–1991 CrossRef CAS.
- M. Migašová, T. Matoušek, V. Schrenková, R. Žídek, I. Petry-Podgórska and J. Kratzer, Anal. Chim. Acta, 2020, 1119, 68–76 CrossRef PubMed.
- C.-C. Wan, C.-S. Chen and S.-J. Jiang, J. Anal. At. Spectrom., 1997, 12, 683–687 RSC.
- Y. Sogame and A. Tsukagoshi, J. Chromatogr. B, 2020, 1136, 121855 CrossRef CAS PubMed.
- M. Iwai-Shimada, Y. Kobayashi, T. Isobe, S. F. Nakayama, M. Sekiyama, Y. Taniguchi, S. Yamazaki, T. Michikawa, M. Oda, H. Mitsubuchi, M. Sanefuji, S. Ohga, N. Mise, A. Ikegami, R. Suga and M. Shimono, Toxics, 2021, 9, 82 CrossRef CAS PubMed.
- A. Abedinlah, S. M. Elias, S. M. Praveena, S. A. Bakar, Z. Rejali and J. Jalaludin, Pertanika J. Sci. & Technol., 2021, 29(3), 2028–2044 Search PubMed.
- US Food & Drug Administration, Thimerosal and Vaccines, 2024, https://www.fda.gov/vaccines-blood-biologics/safety-availability-biologics/thimerosal-and-vaccines, (accessed January 21, 2025 Search PubMed.
- Centers for Disease Control and Prevention (CDC), in National Report on Human Exposure to Environmental Chemicals, National Center for Environmental Health (NCEH), Atlanta, 2021, https://www.cdc.gov/environmental-exposure-report/index.html, (accessed January 21, 2025) Search PubMed.
- R. Mahaffey Kathryn, P. Clickner Robert and C. Bodurow Catherine, Environ. Health Perspect., 2004, 112, 562–570 CrossRef CAS PubMed.
- W. McKelvey, B. Alex, C. Chernov, P. Hore, C. D. Palmer, A. J. Steuerwald, P. J. Parsons and S. E. Perlman, J. Urban Health, 2018, 95, 813–825 CrossRef PubMed.
- U.S. Environmental Protection Agency, U.S. EPA, Reference Dose for Methylmercury, Washington, D.C., 2000 Search PubMed.
- New York State Department of Health, Heavy Metals Surveillance: New York State Heavy Metals Registry, https://www.health.ny.gov/environmental/workplace/heavy_metals_registry/, (accessed January 21, 2025) Search PubMed.
- S. T. Lancaster, T. Prohaska and J. Irrgeher, J. Anal. At. Spectrom., 2023, 38, 1135–1145 RSC.
- B. K. Lasorsa, G. A. Gill and M. Horvat, in Mercury in the Environment, ed. M. S. Bank, University of California Press, Berkeley, CA, 2012, ch. 3, pp. 27–54, DOI:10.1525/9780520951396-007.
- L. L. Yu, G. C. Caceres, S. E. Long, K. E. Murphy, C. E. Bryan Sallee, T. W. Vetter, C. Hagwood, M. A. Franklin, J. Castro Georgi, J. M. Jarrett, D. M. Jones, R. L. Jones, Z. Li, Y. L. Sommer, D. S. Tevis, K. Wallon, C. D. Ward, M. Maras, S. Erdahl, C. Gilmer, M. Wermers, H. Woldeysus, K. F. Mehigan, M. Morrissette, C. D. Palmer and P. J. Parsons, Certification of Standard Reference Material® 955d, in Toxic Elements and Metabolites in Frozen Human Blood, NIST SP 260-206, National Institute of Standards and Technology, Gaithersburg, USA, 2020 DOI:10.6028/NIST.SP.260-206, (Accessed January 21, 2025).
- R. Majors, in Sample Preparation Fundamentals for Chromatography, Agilent Technologies, Wilmington, USA, 2013, pp. 1–364 Search PubMed.
- T. Narukawa, T. Iwai, K. Chiba and J. Feldmann, Anal. Sci., 2018, 34, 1329–1334 CrossRef CAS PubMed.
- S. N. Willie, D. Conrad Grégoire and R. E. Sturgeon, Analyst, 1997, 122, 751–754 RSC.
- L. H. Reyes, G. M. Mizanur Rahman, T. Fahrenholz and H. M. Skip Kingston, Anal. Bioanal. Chem., 2008, 390, 2123–2132 CrossRef CAS PubMed.
- J. Qvarnström and W. Frech, J. Anal. At. Spectrom., 2002, 17, 1486–1491 RSC.
- B. Vallant, R. Kadnar and W. Goessler, J. Anal. At. Spectrom., 2007, 22, 322–325 RSC.
- X. Chen, C. Han, H. Cheng, Y. Wang, J. Liu, Z. Xu and L. Hu, J. Chromatogr. A, 2013, 1314, 86–93 CrossRef CAS PubMed.
- Z. Hu, S. Hu, S. Gao, Y. Liu and S. Lin, Spectrochim. Acta, Part B, 2004, 59, 1463–1470 CrossRef.
- V. Vacchina, F. Séby, R. Chekri, J. Verdeil, J. Dumont, M. Hulin, V. Sirot, J.-L. Volatier, R. Serreau, A. Rousseau, T. Simon and T. Guérin, Talanta, 2017, 167, 404–410 CrossRef CAS PubMed.
- H. Zou, C. Zhou, Y. Li, X. Yang, J. Wen, C. Li, S. Song and C. Sun, Anal. Bioanal. Chem., 2020, 412, 2829–2840 CrossRef CAS PubMed.
- L.-Y. Lin, L.-F. Chang and S.-J. Jiang, J. Agric. Food Chem., 2008, 56, 6868–6872 CrossRef CAS PubMed.
- F. Barbosa Jr, C. D. Palmer, F. J. Krug and P. J. Parsons, J. Anal. At. Spectrom., 2004, 19, 1000–1005 RSC.
- M. Thompson, S. L. R. Ellison and R. Wood, Pure Appl. Chem., 2002, 74, 835–855 CrossRef CAS.
- Y. L. Sommer, C. D. Ward, Y. Pan, K. L. Caldwell and R. L. Jones, J. Anal. Toxicol., 2016, 40, 222–228 CrossRef CAS PubMed.
- BIPM, IEC, IFCC, ILAC, ISO, IUPAC, IUPAP and OIML, Evaluation of measurement data — Guide to the expression of uncertainty in measurement, Joint Committee for Guides in Metrology, JCGM 100:2008, DOI:10.59161/JCGM100-2008E, (accessed January 2025).
- Eurolab, Measurement uncertainty revisited: Alternative approaches to uncertainty evaluation, in Technical Report No. 1/2007, EUROLAB, Paris, 2007, (accessed January 21, 2025 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ay02116a |
| This journal is © The Royal Society of Chemistry 2025 |