 Open Access Article
Open Access ArticleSimultaneous analysis of residual prohibited doping substances in foods using gas chromatography-tandem mass spectrometry†
Yejin
Lee‡
ab,
Yoeseph
Cho‡
a,
Seongeun
Jeon
a,
Yinglan
Xu
a,
Kang Mi
Lee
a,
Ho Jun
Kim
a,
Dong-Woo
Lee
 b and
Junghyun
Son
b and
Junghyun
Son
 *a
*a
aDoping Control Center, Korea Institute of Science and Technology, Seoul 02792, Republic of Korea. E-mail: son.junghyun@kist.re.kr
bDepartment of Biotechnology, Yonsei University College of Life Science and Biotechnology, Seoul 03722, Republic of Korea
First published on 16th December 2024
Abstract
The continuous consumption of various foods increases the risk of unintentional exposure to residual contaminants. Thus, improving premonitoring procedures to ensure food safety is critical. Herein, a rapid and efficient assay was developed to monitor residual contaminants in food, with a focus on banned doping substances. First, 73 doping compounds, including anabolic agents that can be ingested from food were selected, after which a gas chromatography-tandem mass spectrometry (GC-MS/MS) method was developed for their simultaneous screening. Based on the GC-MS/MS-determined food-matrix characteristics and types, a sample-preparation module was developed to optimize the sample-preparation method. Thereafter, the developed analytical method was validated using representative food matrices, and the results confirmed that the developed method obtained good recoveries (80–123% (limit of quantification: 0.01–20 μg kg−1)). To monitor residual doping substances in commercially available foods, the established method was applied to the analysis of 40 food samples, including meat. Notably, endogenous hormones, such as testosterone, nandrolone, 19-norandrosterone, and 19-noretiocholanolone, were detected in the meat samples, although they did not exceed the maximum residue limits. This approach enables the assessment of potential exposure levels to food-borne endogenous hormones, thereby supporting food safety and preventing unintentional doping incidents in athletes.
Introduction
The globalization of the food market has enhanced the accessibility to varieties of foods, resulting in more diverse and active food consumption. However, this trend continuously increases the exposure risks to residual contaminants in such abundant food varieties.1,2 Veterinary-drug residues, such as growth promoters, account for major examples of contaminants in foods.3,4 Such drugs may have been originally deployed for the treatment of livestock or for controlling reproduction. However, they may have been illegally deployed to boost meat production and quality to match the continuous increase in global meat consumption.5–7 Additionally, some of these residues may have accumulated unintentionally via microbial contamination. For instance, zearalenone (a precursor for synthesizing zeranol) is produced by Fusarium species (fungi present in wheat and barley).8 Zearalenone can be detected in the meat and milk of animals that have consumed contaminated wheat or barley feeds.9,10 Upon consuming foods or meats containing these residual contaminants, they are absorbed into the human body and may cause endocrine disruption by mimicking endogenous hormones.11 This is particularly concerning for prepubescent children owing to their low endogenous-hormone-secretion levels, as it can cause severe conditions, such as precocious puberty.12 Moreover, adverse effects on the cardiovascular, nervous, and reproductive systems, including carcinogenicity, have also been reported.13Owing to concerns about these risks, the regulatory authorities of each country have established guidelines. However, these guidelines vary across countries, and this has resulted in varied regulations and maximum residue limits (MRLs) for these substances. For example, European Union countries have banned the use of growth promoters in livestock production.14 Regarding the MRL for veterinary drugs in food, the U.S. permits testosterone MRLs of 0.64–2.6 μg kg−1,15 although CODEX classifies it as an unnecessary substance.16 Further, the Republic of Korea has a designated MRL for nandrolone (∼2 μg kg−1), an anabolic steroid exhibiting a similar structure to testosterone.17 As only some of these potentially harmful substances are regulated, the exposure risks to them through food is always a concern. Moreover, verifying their presence in unofficially imported foods is even more challenging.
Exposure to growth promoters, including steroids, through food poses general health concerns and can severely affect athletes in particular. Increasing muscle mass, which is a well-known effect of steroids, is directly linked to enhanced sports performance;18 thus, their illegal use constitutes a form of doping that is strictly regulated by the World Anti-Doping Agency (WADA).19 However, it is also known that unintentional doping can proceed through the consumption of contaminated foods.20–22 During the 2011 FIFA U-17 World Cup in Mexico, over 100 players tested positive for clenbuterol most likely due to the consumption of meat contaminated by the substance. Notably, clenbuterol was detected at 0.06–11 μg kg−1 in 30% of meat samples obtained from restaurants serving food to the participating football teams, and 52% of urine samples collected from the players contained 0.001–1.56 ng mL−1 of clenbuterol.23 In another study, urine samples obtained from three male French volunteers who consumed 310 g of boar kidney, heart, liver, and meat tissue contained 3.1–7.5 and 0.5–1.2 ng mL−1 levels of 19-norandrosterone (19-NA) and 19-noretiocholanolone (19-NE), respectively, after 10 h of consumption.24
Unintentional doping related to food consumption extends beyond anabolic agents. Studies have reported the cases of exposure to substances such as beta-agonists (e.g., zilpaterol from meat consumption) and selective estrogen receptor modulators (e.g., tamoxifen from dietary supplements).25,26 These substances induce anabolic effects and function as hormone modulators, leading to their classification as prohibited substances. In addition, scenarios exist in which exposure to morphine and cannabinoids occurs through the consumption of foods such as hemp seeds and poppy seeds.27–30 The strict liability policy of WADA holds athletes responsible for any illegal substances found in their bodies regardless of their intent in ingesting such substances. Moreover, proving unintentional doping through food is quite challenging unless athletes verify the quality and ingredients of the food they consume in advance.
To mitigate the risk of exposure to potentially harmful substances in food, including unintentional doping scenarios, developing effective analytical approaches and premonitoring techniques for various foods is crucial. Regarding analytical techniques, advances in mass spectrometry (MS) have led to the widespread use of MS-based methods combined with gas chromatography (GC) or liquid chromatography (LC) owing to their exceptional analytical sensitivity and specificity.31,32 LC is widely used for detecting various multicomponent substances owing to its relatively simple sample preparation process. By contrast, GC offers superior resolution through gaseous-state separation, with high mobility and diffusion rates. When combined with derivatization, GC enhances the volatility and structural stability of analytes, enabling more accurate analysis.33 For the simultaneous profiling of substances such as steroids, GC-MS-based analysis remains the preferred approach because of its outstanding selectivity and sensitivity.34,35
WADA-accredited doping control laboratories worldwide conduct supervised sophisticated analyses of prohibited substances in biological samples.36 Doping-analysis methods cover a range of substances that could negatively impact the human body, e.g., growth promoters. Additionally, these methods measure trace amounts of residual substances, including metabolites of prohibited substances, with high precision. Specifically, the analyses generally target substances, such as clenbuterol, salbutamol, nandrolone, testosterone, zilpaterol, and zeranol, along with their metabolites, which have designated veterinary-drug MRLs in the Republic of Korea.17 These target substances also include over 20 anabolic agents listed in the banned ingredients for direct imports. Thus, by optimizing some doping control analysis techniques for food-matrix applications, the residuals of banned doping substances in food can be easily and rapidly monitored to ensure food safety.
Contemporary society is characterized by easy access to a wide variety of foods; however, this increased accessibility and consumption also heighten the risk of unintentional exposure to residual contaminants in these foods. As such, the procedures for verifying food safety must transcend the current practices. Based on the existing doping control analysis methods, we aimed to develop a new highly-effective sample preparation and analysis method using gas chromatography-tandem mass spectrometry (GC-MS/MS) that could simultaneously detect 73 controlled doping compounds, including steroids, cannabinoids, and opiates that may be unintentionally ingested through food to help people, particularly athletes verify such foods before consuming to avoid unwanted effects and penalties.
Experimental
Chemicals and reagents
All standards as well as their suppliers are reported in Table S1 in the ESI.† Additionally, deuterium-labeled d3-testosterone and d4-19-norandrosterone glucuronide, along with methyltestosterone, were employed as the internal standards (ISTDs). Further, high-performance liquid chromatography (HPLC)-grade methanol, methyl tert-butyl ether (MTBE), and ethyl acetate solvents were purchased from JT Baker (Phillipsburg, NJ, USA), and β-glucuronidase (isolated from E. coli) was obtained from Roche Diagnostics (Mannheim, Germany). Sodium phosphate dibasic, sodium phosphate monobasic, potassium carbonate, N-methyl-N-(trimethylsilyl) trifluoroacetamide (MSTFA), ammonium iodide (NH4I), and dithioerythritol (DTE) reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA). Furthermore, Serdolit polymeric adsorbent (PAD)-1 was purchased from Serva Electrophoresis (Heidelberg, Germany). The utilized water was purified with a Milli-Q water purification system from Millipore (Bedford, MA, USA).All the standards and ISTDs were dissolved in methanol at concentrations of 100 or 1000 μg mL−1 to prepare their respective stock solutions. These stock solutions were mixed appropriately to prepare a working solution, which was further diluted for analysis. The ISTD working solution was prepared to contain 5, 2, and 0.25 μg mL−1 of methyltestosterone, d3-testosterone, and d4-19-norandrosterone glucuronide, respectively. All the standard solutions were stored at −20 °C.
Modular sample preparation
To ensure the efficient extraction of the target substances from food matrices, we implemented a sample-preparation module that was tailored to the characteristics of the food sample (Fig. 1). First, a homogenized solid (1 g) or liquid (1 mL) sample was spiked with the ISTD solution (20 μL). For the fatty solid, water (1 mL) was added to the sample and irradiated at 280 W for 30 s in a microwave oven. Thereafter, the sample was ultrasonically extracted using methanol (6 mL) at 50 °C for 20 min, followed by centrifugation for 5 min at 2000g. Furthermore, the extracted sample was stored in a freezer at −30 °C and subsequently filtered using a Whatman grade 4 filter paper (pore size 20–25 μm) to remove the frozen lipids. The nonfat sample was filtered immediately without freezing. The filtered extract was concentrated using nitrogen gas at 50 °C for 40 min, and the concentrated extract was evaporated to dryness using a rotary vacuum evaporator for 10 min at 50 °C. The resulting residue was reconstituted in 10% methanol (1 mL), and a PAD-1 resin was packed into a Pasteur pipette and conditioned with water (2 mL) before sample loading. The reconstituted sample was loaded into the pipette, washed with water (2 mL), and eluted with methanol (4 mL). Thereafter, the eluted sample was dried and reconstituted in 10% methanol (1 mL). Next, enzymatic hydrolysis was performed, by referring to procedures in the literature.37,38 The pH was adjusted by the addition of a sodium phosphate buffer (1 mL) and β-glucuronidase (50 μL, 140 U mL−1) to promote the reaction. The resulting sample was incubated at 55 °C for 1 h in a heating block. For the liquid–liquid extraction, MTBE (5 mL) was added to the sample, shaken for 10 min, and centrifuged for 5 min at 2000g. Subsequently, the sample was stored at −30 °C to separate the organic-solvent layer from the aqueous layer, after which the extract-containing supernatant was transferred to another tube. Next, the extract was evaporated using nitrogen at 50 °C for 15 min, and the residual moisture was removed in a desiccator containing phosphorus pentoxide and dry silica for 30 min. To increase the volatility and sensitivity of the analytes, they were derivatized via trimethylsilylation using MSTFA/NH4I/DTE (50 μL; 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4
4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, v/w/w) in a heat block at 60 °C for 20 min. Thereafter, the derivatized sample (1 μL) was injected into the GC-MS/MS for the analysis.
2, v/w/w) in a heat block at 60 °C for 20 min. Thereafter, the derivatized sample (1 μL) was injected into the GC-MS/MS for the analysis.
Gas chromatography-tandem mass spectrometry
GC separation was performed using an Agilent 7890B GC device (Agilent Technologies, Santa Clara, CA, USA) equipped with an HP-Ultra 1 column (17 m × 0.2 mm i.d., 0.11 μm film thickness) from Agilent. A derivatized sample (1 μL) was injected by an Agilent 7683 autosampler in the split mode (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), at an injection-port temperature of 280 °C. Helium was served as the carrier gas at a constant flow rate of 0.7 mL min−1. Furthermore, the GC oven temperature was initially programmed to start at 180 °C, increasing to 200 °C at 20 °C min−1 and then to 210 °C at 5 °C min−1, holding at 210 °C for 4 min. Subsequently, the temperature was increased at a rate of 5 °C min−1 to 220 °C, held for 2.5 min, and then ramped up at 50 °C min−1 to the final temperature of 320 °C, which was maintained for 2.5 min. The total run time was 16 min. The employed mass spectrometer was an Agilent 7010 triple quadrupole mass spectrometer, which was operated in the electron ionization mode. The ion-source and quadruple temperatures were set to 250 °C and 150 °C, respectively. Furthermore, we divided the runtime into seven segments and analyzed one set of MRM transitions in each segment. Table S2 in the ESI† presents the optimized MRM conditions and retention times for the target analytes.
1), at an injection-port temperature of 280 °C. Helium was served as the carrier gas at a constant flow rate of 0.7 mL min−1. Furthermore, the GC oven temperature was initially programmed to start at 180 °C, increasing to 200 °C at 20 °C min−1 and then to 210 °C at 5 °C min−1, holding at 210 °C for 4 min. Subsequently, the temperature was increased at a rate of 5 °C min−1 to 220 °C, held for 2.5 min, and then ramped up at 50 °C min−1 to the final temperature of 320 °C, which was maintained for 2.5 min. The total run time was 16 min. The employed mass spectrometer was an Agilent 7010 triple quadrupole mass spectrometer, which was operated in the electron ionization mode. The ion-source and quadruple temperatures were set to 250 °C and 150 °C, respectively. Furthermore, we divided the runtime into seven segments and analyzed one set of MRM transitions in each segment. Table S2 in the ESI† presents the optimized MRM conditions and retention times for the target analytes.
Method validation
The developed method was validated using the ISO/IEC 17025 and WADA guidelines, as it applied to food analysis and doping control. A representative matrix including all the sample-preparation steps was selected, and a matrix-matched validation approach was used to evaluate the limit of quantification (LOQ), recovery, precision, and matrix effect (ME) of the developed method. The LOQ was determined as the lowest concentration with a signal-to-noise ratio of ≥10 in the samples spiked with serially diluted standards. Further, the recovery and precision were evaluated using a minimum of three replicate samples at specific concentrations not less than the LOQ for each substance. Recovery was determined by comparing the detection levels of the standard-spiked samples at the initial sample-preparation step and after liquid–liquid extraction. Furthermore, the precision of the method was estimated as the relative standard deviation (RSD) among the detection intensities for the replicated samples. ME was determined by comparing the peak areas of the target substances in the representative and blank matrices (solvent). For the endogenous steroids, the validation was performed using a steroid-free matrix. This matrix was prepared by loading the samples with a PAD-1 resin.39 First, the PAD-1 resin was conditioned using water (2 mL), and the reconstituted sample (after ultrasonic extraction and lipid filtration) was loaded onto the resin. Consequently, the steroids were retained in the PAD-1 resin, and the unretained fraction was collected. The collected fraction was screened for the residual steroids (Fig. S1, ESI†) and used as a matrix for validating endogenous steroids.Monitoring samples
The monitored samples were selected from foods with renowned histories of reported residual doping substances or the potential to contain such contaminants. The selected foods included meat containing potential residues of growth-promoting substances, grains with potential zeranol contamination from microbial sources, and other foods with the possibility of containing narcotics and cannabinoids.20,40–43 Thus, 40 samples from 23 different food items were purchased from local markets as well as online food stores in the Republic of Korea (Table S3, ESI†). Next, the collected samples were homogenized by selecting the edible parts, after which they were stored at −20 °C until analysis. All the samples were screened for the presence of the target substances using the developed qualitative method; the detected substances were subsequently subjected to quantitative analysis.Results and discussion
Optimization of the sample preparation
First, we optimized the extraction and cleanup processes. After optimizing the sample-preparation methods, we constructed a sample-analysis module using the food-matrix characteristics and types. Fig. 1 depicts the proposed sample-preparation method. Previous studies have reported the direct extraction of meat using a solvent.10,44,45 To effectively extract the target analytes simultaneously from the sample, we optimized the water-to-methanol ratio, leading to the use of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6 (v/v) ratio of water to methanol (Fig. 2A). However, in this study, the recoveries were low due to protein and lipid precipitation clogging the ion-exchange resin during cleanup. To address this challenge, we implemented the microwave-based irradiation of the fatty solid sample before extraction. Studies have demonstrated that microwave irradiation improves the extraction efficiency of various analytes, as well as lipids, from meat samples.46–48 The results of this study indicated that the addition of the microwave step was more effective for extracting the target substances (Fig. 2B). Furthermore, we separated the analytes from the lipids using microwave and ultrasonic extraction with methanol, followed by freezing. The difference between the freezing points of lipids (4 °C) and methanol (−98 °C) was sufficient for this separation. Most of the lipids coagulated as a white mass on the surface, and a cold extract was filtered at −30 °C. The lipid content was quantified using the Soxhlet extraction method.49 This cryolipid filtration method proved to be efficient for lipid removal, eliminating approximately 90% of the lipids (Fig. 2C). For efficient sample preparation, the nonfat samples, such as fruits and vegetables, were filtered without freezing after the methanol-driven extraction. Considering the target analytes, further cleanup step was performed using PAD-1 resin, which is conventionally deployed in doping control and has proven to be effective.50 Samples processed with PAD-1 resin exhibited enhanced removal of interferences such as pigments and higher recoveries for most analytes compared with those subjected only to simple syringe filtration after lipid removal (Fig. 2D). Consequently, we used the internationally accredited doping control method certified by the Korea Laboratory Accreditation Scheme, which involved enzymatic hydrolysis and liquid–liquid extraction using MTBE after PAD-1 cleanup, resulting in adequate extraction efficiency for the target compounds. Enzymatic hydrolysis was used to convert the conjugated form of residues, which were generated through metabolic processes in animals, into their free forms. In animal muscle tissue, conjugated testosterone has been reported to account for less than 20% of the total testosterone.51 Some studies assert that enzymatic hydrolysis is essential, whereas others indicate that it can be omitted for samples other than urine or liver without substantially affecting the results.52,53 Herein, the enzymatic hydrolysis step was incorporated to ensure precise quantification of analytes, even at trace levels, and to broaden the applicability of the method to tissues such as the liver, which are consumed as food. Therefore, the proposed method enables rapid and efficient simultaneous analysis of 73 target substances and also considers the scalability for various sample types.
6 (v/v) ratio of water to methanol (Fig. 2A). However, in this study, the recoveries were low due to protein and lipid precipitation clogging the ion-exchange resin during cleanup. To address this challenge, we implemented the microwave-based irradiation of the fatty solid sample before extraction. Studies have demonstrated that microwave irradiation improves the extraction efficiency of various analytes, as well as lipids, from meat samples.46–48 The results of this study indicated that the addition of the microwave step was more effective for extracting the target substances (Fig. 2B). Furthermore, we separated the analytes from the lipids using microwave and ultrasonic extraction with methanol, followed by freezing. The difference between the freezing points of lipids (4 °C) and methanol (−98 °C) was sufficient for this separation. Most of the lipids coagulated as a white mass on the surface, and a cold extract was filtered at −30 °C. The lipid content was quantified using the Soxhlet extraction method.49 This cryolipid filtration method proved to be efficient for lipid removal, eliminating approximately 90% of the lipids (Fig. 2C). For efficient sample preparation, the nonfat samples, such as fruits and vegetables, were filtered without freezing after the methanol-driven extraction. Considering the target analytes, further cleanup step was performed using PAD-1 resin, which is conventionally deployed in doping control and has proven to be effective.50 Samples processed with PAD-1 resin exhibited enhanced removal of interferences such as pigments and higher recoveries for most analytes compared with those subjected only to simple syringe filtration after lipid removal (Fig. 2D). Consequently, we used the internationally accredited doping control method certified by the Korea Laboratory Accreditation Scheme, which involved enzymatic hydrolysis and liquid–liquid extraction using MTBE after PAD-1 cleanup, resulting in adequate extraction efficiency for the target compounds. Enzymatic hydrolysis was used to convert the conjugated form of residues, which were generated through metabolic processes in animals, into their free forms. In animal muscle tissue, conjugated testosterone has been reported to account for less than 20% of the total testosterone.51 Some studies assert that enzymatic hydrolysis is essential, whereas others indicate that it can be omitted for samples other than urine or liver without substantially affecting the results.52,53 Herein, the enzymatic hydrolysis step was incorporated to ensure precise quantification of analytes, even at trace levels, and to broaden the applicability of the method to tissues such as the liver, which are consumed as food. Therefore, the proposed method enables rapid and efficient simultaneous analysis of 73 target substances and also considers the scalability for various sample types.
Method validation
The developed method was subjected to a validation procedure. Briefly, pork was selected as the representative matrix for this study because it undergoes all the proposed modular sample-preparation processes as well as has a history of containing the detected residues, including growth promoters. Thus, a pork sample was prepared, as described in Modular sample preparation section, and the validation results (Table S4, ESI†) confirmed the suitability of the proposed method for monitoring prohibited substances and their metabolites in food samples. All the target substances exhibited distinct, independent peaks that were not affected by matrix interferences or interactions between the analytes. The LOQ results of the 73 substances were 0.01–20 μg kg−1, indicating that sufficient detection sensitivity was achieved for the verification of the MRL of nandrolone, zilpaterol, and zeranol in foods.17 Further, compared to existing analytical methods for foods, our developed method improved the detection sensitivity for clenbuterol from 9.8 ng kg−1 (in previous studies)54 to 5 ng kg−1. The recoveries of 80–123% were obtained for all the analytes, indicating sufficient extraction of the analytes from the samples. The precision rate was 3–18%, and the ME was −38% to +20%.Compared with the reported recoveries, those obtained in this study for testosterone, nandrolone, and clenbuterol were significantly higher. Testosterone was extracted using acidified acetonitrile (1% acetic acid in acetonitrile) and analyzed via HPLC-MS/MS, yielding an extraction recovery of 73%.55 Employing GC-MS based on a C18 solid-phase extraction cartridge, Xu et al. noted that the recovery of nandrolone in pork was 64%.56 Additionally, Zhang et al. analyzed the presence of clenbuterol in porcine muscle by combining the QuEChERS method with LC-MS, obtaining a recovery of 78%.57 Notably, the extraction-recoveries for testosterone, nandrolone, and clenbuterol obtained using our method were higher than those previously reported (Fig. S2, ESI†).
Application of the developed method to real sample
Next, the proposed method was used to analyze commercially available food samples that might contain ingredients potentially threatening to health or affecting doping results. The method facilitated the qualitative screening of the presence of 73 target substances. The qualitative screening confirmed the presence of endogenous anabolic steroids, such as testosterone and nandrolone, along with their metabolites (19-NA and 19-NE), in the meat samples (Fig. 3). Residues of doping substances were only detected in meat, and no traces were found in other samples. To determine the concentrations of the four substances that were detected in the meat samples, we performed quantification using calibration curves that covered the relevant concentration ranges. The results of the quantitative analysis revealed that 0.15–3.89 μg kg−1 testosterone were detected in the sow, barrow, and porcine testes (Table 1). Nandrolone was detected at a concentration of 2.04 μg kg−1 in porcine testes. The sow samples contained 0.87 and 0.41 μg kg−1 19-NA and 19-NE, respectively. Except for the pig-testicle samples, the residual contaminants in the other meat samples, including pork, beef, and chicken, did not exceed the MRL for veterinary drugs established in the Republic of Korea. Pig testicles are specialty tissues without an established MRL. However, as they are sometimes consumed as food, caution must be exercised regarding the potential exposure to endogenous steroids by consuming pig testicles.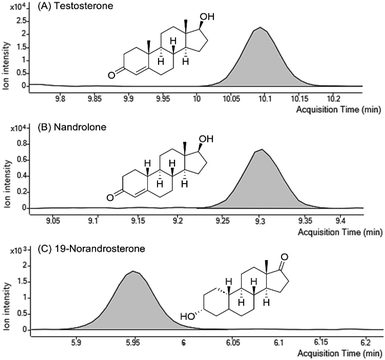 | ||
| Fig. 3 Representative ion chromatograms of (A) testosterone, (B) nandrolone, and (C) 19-norandrosterone extracted from pork samples with positive results. | ||
| Testosterone (μg kg−1) | Nandrolone (μg kg−1) | 19-NA (μg kg−1) | 19-NE (μg kg−1) | Zilpaterol (μg kg−1) | Zeranol (μg kg−1) | Clenbuterol (μg kg−1) | Salbutamol (μg kg−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| a MRL, maximum residue limits; ND, not detected *based on the MRLs for Veterinary Drugs in the Republic of Korea (2023). | |||||||||
| Food sample | Sow | 0.15 | ND | 0.87 | 0.41 | ND | ND | ND | ND |
| Barrow | 0.15 | ND | 0.56 | ND | ND | ND | ND | ND | |
| Porcine testes | 3.89 | 2.04 | ND | ND | ND | ND | ND | ND | |
| Beef | ND | ND | ND | ND | ND | ND | ND | ND | |
| MRL* | Muscle | Exemption | 2 (pig, cattle) | Undesignated | Undesignated | 1 (cattle) | 2 (cattle) | Prohibited | Prohibited |
Based on the detected results, we calculated the estimated daily intake (EDI) of endogenous hormones through meat consumption across the Republic of Korea. The EDIs for different age groups were determined based on the National Nutrition Statistics of the Republic of Korea58 using the following equation:
| EDI (ng per kg per b.w. per day) = [substances content (μg kg−1) × food intake (g per day)]/average body weight (kg b.w.) | (1) |
The statistical data used to calculate the EDI did not specify the intake amounts of sow or barrow within total pork consumption, nor the intake amounts of porcine testes within pork variety meats. Thus, the calculated EDI values were presented as a range (Table 2). Additionally, the effect of cooking on analyte levels was not considered in the EDI calculation. However, food processing steps, such as cooking, have little effect on endogenous hormone levels, or a decrease of 5–30% has been reported depending on the fat content.59,60 Therefore, the actual intake may be lower than the estimated value. For testosterone, the calculated EDI was compared with the acceptable daily intake (ADI) levels established by CODEX.16 The EDI of testosterone for age groups 1–2, 3–5, and 6–11 years were 0.16–0.59, 0.23–0.64, and 0.21–0.53 ng per kg per b.w per day, respectively. Although these values were significantly lower than the ADI (0–2 μg per kg b.w.) for testosterone, caution must still be exercised, particularly for prepubescent children (children at this development stage exhibit relatively low levels of endogenous-hormone production). As the research on the effects of chronic low-dose exposure on these vulnerable population is lacking, we did not conduct thorough risk assessments.
| Age group (years) | Food intakea (g per day) | Body weightb (kg) | Estimated daily intake (ng per kg b.w. per day) | |||||
|---|---|---|---|---|---|---|---|---|
| Pork | Variety meat (pork) | Testosterone | Nandrolone | 19-NA | 19-NE | |||
| a Based on the average intake in the Republic of Korea (2021). b Applying an average weight for survey participants. | ||||||||
| Total | (n = 5940) | 46 | 3.7 | 62.5 | 0.11–0.34 | ≤0.12 | 0.42–0.65 | ≤0.30 |
| 1–2 | (n = 73) | 13 | 1.4 | 12.7 | 0.16–0.59 | ≤0.23 | 0.59–0.91 | ≤0.43 |
| 3–5 | (n = 154) | 28 | 1.9 | 18.0 | 0.23–0.64 | ≤0.21 | 0.86–1.34 | ≤0.63 |
| 6–11 | (n = 383) | 49 | 2.9 | 35.5 | 0.21–0.53 | ≤0.17 | 0.78–1.21 | ≤0.57 |
| 12–18 | (n = 357) | 67 | 3.2 | 60.4 | 0.17–0.37 | ≤0.11 | 0.62–0.96 | ≤0.45 |
| 19–29 | (n = 584) | 68 | 3.7 | 66.9 | 0.15–0.37 | ≤0.11 | 0.57–0.88 | ≤0.41 |
| 30–49 | (n = 1359) | 56 | 6.3 | 69.6 | 0.12–0.48 | ≤0.19 | 0.45–0.71 | ≤0.33 |
| 50–64 | (n = 1405) | 39 | 2.8 | 65.2 | 0.09–0.25 | ≤0.09 | 0.33–0.52 | ≤0.24 |
| ≥65 | (n = 1625) | 18 | 1.5 | 60.7 | 0.04–0.14 | ≤0.05 | 0.17–0.26 | ≤0.12 |
Furthermore, the results of the food sample analysis confirmed that unintentional doping can occur through food consumption. The National Nutrition Statistics of the Republic of Korea revealed that age group 19–29 years consumes >198 g of meat products per day, with the top 10% consuming >500 g.58 According to the analysis results, the consumption of 500 g of contaminated meat can be accompanied by the unintentional ingestion of 640 ng of nandrolone metabolites (19-NA and 19-NE). Nandrolone and its metabolites are generally excreted from urine as 19-NA and 19-NE, with reported peak concentrations emerging after 2–12 h of ingestion; they can be detected for up to 72–96 h.61 This means that 19-NA and 19-NE can be detected above the minimum required performance limit (2 ng mL−1) in doping control urine analysis,62 especially when consuming large amounts or via a sustained consumption of contaminated meat. Thus, to prevent such unintentional doping, we argue that, at the very least, monitoring for prohibited doping substances in food should be conducted in areas where such foods are served to athletes (e.g., athletes' villages). Moreover, the developed method could efficiently monitor prohibited substances in food; it could play an important role in preventing unintentional doping via the consumption of contaminants in food.
Conclusions
Here, we developed a screening method comprising GC-MS/MS analysis and modular sample preparation for the simultaneous detection and analysis of 73 prohibited doping substances in food, e.g., anabolic agents. We first optimized the sample-preparation modules based on the type of food matrices (fatty liquids, nonfat liquids, fatty solids, and nonfat solids), as determined by GC-MS/MS, thereby extending the applicability of the developed method to various food types. The proposed method was also validated for LOQ, recovery, precision, and ME. We applied this analytical method to the detection of residual contaminants in food, and no residual substances were detected at higher than MRL in the 40 food samples examined. However, the levels of endogenous hormones detected in some meat tissues indicated that athletes could be victims of unintentional doping owing to their consumption of large quantities or sustained consumption of such meats. Thus, our method can be used to monitor residual contaminants in food as well as to detect prohibited doping substances, thus playing an important role in ensuring food safety and preventing unintentional doping due to the consumption of contaminants in unlikely sources.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Yejin Lee: conceptualization, methodology, validation, formal analysis, investigation, writing – original draft. Yoeseph Cho: methodology, validation, formal analysis, investigation, writing – original draft, writing – review & editing. Seongeun Jeon: validation, formal analysis, investigation. Yinglan Xu: resources. Kang Mi Lee: resources. Ho Jun Kim: resources. Dong-Woo Lee: writing – review & editing, supervision. Junghyun Son: conceptualization, resources, writing – review & editing, supervision, project administration, funding acquisition.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study was supported by the Korea Institute of Science and Technology [grant numbers 2V09270, 2V09691, and 2V10011].References
- L. A. Thompson and W. S. Darwish, J. Toxicol., 2019, 2019, 2345283 CrossRef PubMed.
- L. N. He, Q. Li, J. M. Ma, M. R. Cao, L. X. Fan, X. W. Ren, G. H. Shi and Y. Zhang, J. Food Qual., 2024, 2024, 3859819 Search PubMed.
- A. H. Atta, S. A. Atta, S. M. Nasr and S. M. Mouneir, Environ. Sci. Pollut. Res. Int., 2022, 29, 15282–15302 CrossRef PubMed.
- T. Chen, B. Le Bizec and G. Dervilly, Steroids, 2024, 206, 109420 CrossRef PubMed.
- C. Cloteau, Z. Kaabia, B. Le Bizec, L. Bailly-Chouriberry and G. Dervilly, Food Control, 2023, 148, 109601 CrossRef CAS.
- G. Dervilly-Pinel, F. Courant, S. Chereau, A. L. Royer, F. Boyard-Kieken, J. P. Antignac, F. Monteau and B. Le Bizec, Drug Test. Anal., 2012, 4(Suppl 1), 59–69 CrossRef CAS PubMed.
- K. Skoupa, A. Batik, K. St’astny and Z. Sladek, Animals, 2023, 13, 2141 CrossRef PubMed.
- M. Gajęcki, M. Gajęcka, E. Jakimiuk, Ł. Zielonka and K. Obremski, in Mycotoxins in Food, Feed and Bioweapons, ed. M. Rai and A. Varma, Springer Berlin Heidelberg, Berlin, Heidelberg, 2010, pp. 131–144, DOI:10.1007/978-3-642-00725-5_9.
- A. F. Erasmuson, B. G. Scahill and D. M. West, J. Agric. Food Chem., 1994, 42, 2721–2725 CrossRef.
- P. Zollner, J. Jodlbauer, M. Kleinova, H. Kahlbacher, T. Kuhn, W. Hochsteiner and W. Lindner, J. Agric. Food Chem., 2002, 50, 2494–2501 CrossRef PubMed.
- M. M. Qaid and K. A. Abdoun, J. Appl. Anim. Res., 2022, 50, 426–439 CrossRef.
- F. Massart, V. Meucci, G. Saggese and G. Soldani, J. Pediatr., 2008, 152, 690–695 CrossRef PubMed.
- E. B. Lust, C. Barthold, M. A. Malesker and T. O. Wichman, J. Emerg. Med., 2011, 40, 198–207 CrossRef PubMed.
- European Commission, Commission Regulation (EU) No 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin, http://data.europa.eu/eli/reg/2010/37(1)/oj, accessed August 25, 2024.
- Food and Drug Administration, Code of Federal Regulations, Title 21, Chapter I, Subchapter E, Part 556—Tolerances for Residues of New Animal Drugs in Food, https://www.ecfr.gov/current/title-21/part-556, accessed August 25, 2024.
- Codex Alimentarius Commission, Maximum Residue Limits (MRLs) and Risk Management Recommendations (RMRs) for Residues of Veterinary Drugs in Foods, https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1%26url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXM%2B2%252FMRL2e.pdf, accessed August 25, 2024.
- Ministry of Food and Drug Safety, MRLs in Veterinary drugs, https://residue.foodsafetykorea.go.kr/vd/mrl, accessed August 25, 2024.
- R. I. G. Holt, I. Erotokritou-Mulligan and P. H. Sönksen, Growth Horm. IGF Res., 2009, 19, 320–326 CrossRef PubMed.
- World Anti-Doping Agency, World anti-doping code 2021, https://www.wada-ama.org/sites/default/files/resources/files/2021_wada_code.pdf, accessed August 25, 2024.
- K. Walpurgis, A. Thomas, H. Geyer, U. Mareck and M. Thevis, Foods, 2020, 9, 1012 Search PubMed.
- J. M. Martinez-Sanz, I. Sospedra, C. M. Ortiz, E. Baladia, A. Gil-Izquierdo and R. Ortiz-Moncada, Nutrients, 2017, 9, 1093 Search PubMed.
- M. Thevis, T. Kuuranne, M. Fedoruk and H. Geyer, Drug Test. Anal., 2021, 13, 1814–1821 CrossRef PubMed.
- M. Thevis, L. Geyer, H. Geyer, S. Guddat, J. Dvorak, A. Butch, S. S. Sterk and W. Schänzer, Drug Test. Anal., 2013, 5, 372–376 CrossRef PubMed.
- K. De Wasch, B. Le Bizec, H. De Brabander, F. Andre and S. Impens, Rapid Commun. Mass Spectrom., 2001, 15, 1442–1447 CrossRef PubMed.
- L. Euler, F. Wagener, A. Thomas and M. Thevis, Rapid Commun. Mass Spectrom., 2022, 36, e9357 CrossRef PubMed.
- K. Tsarouhas, N. Kioukia-Fougia, P. Papalexis, A. Tsatsakis, D. Kouretas, F. Bacopoulou and C. Tsitsimpikou, Food Chem. Toxicol., 2018, 115, 447–450 CrossRef PubMed.
- M. Thevis, G. Opfermann and W. Schanzer, J. Anal. Toxicol., 2003, 27, 53–56 CrossRef PubMed.
- W. Van Thuyne, P. Van Eenoo and F. T. Delbeke, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2003, 785, 245–251 CrossRef PubMed.
- E. Johnson, M. Kilgore and S. Babalonis, Drug Alcohol Depend., 2022, 237, 109522 CrossRef PubMed.
- E. Jang, H. Kim, S. Jang, J. Lee, S. Baeck, S. In, E. Kim, Y. U. Kim and E. Han, Forensic Sci. Int., 2020, 306, 110064 CrossRef PubMed.
- I. Domínguez, A. G. Frenich and R. Romero-González, Anal. Methods, 2020, 12, 1148–1162 RSC.
- A. Thomas and M. Thevis, Expert Rev. Proteomics, 2024, 21, 27–39 CrossRef PubMed.
- N. Krone, B. A. Hughes, G. G. Lavery, P. M. Stewart, W. Arlt and C. H. L. Shackleton, J. Steroid Biochem., 2010, 121, 496–504 CrossRef PubMed.
- G. Micalizzi, K. Huszti, Z. Palinkas, F. Mandolfino, E. Martos, P. Dugo, L. Mondello and M. Utczas, Drug Test. Anal., 2021, 13, 128–139 CrossRef PubMed.
- M. R. Fuh, S. Y. Huang and T. Y. Lin, Talanta, 2004, 64, 408–414 CrossRef PubMed.
- World Anti-Doping Agency, International Standard for Laboratories (ISL), https://www.wada-ama.org/sites/default/files/resources/files/isl_2021.pdf, accessed August 25, 2024.
- K. M. Lee, H. J. Kim, E. S. Jeong, H. H. Yoo, O. S. Kwon, C. Jin, D. H. Kim and J. Lee, Rapid Commun. Mass Spectrom., 2011, 25, 2261–2267 CrossRef PubMed.
- E. S. Jeong, S. H. Kim, E. J. Cha, K. M. Lee, H. J. Kim, S. W. Lee, O. S. Kwon and J. Lee, Rapid Commun. Mass Spectrom., 2015, 29, 367–384 CrossRef PubMed.
- R. Gonzalo-Lumbreras, D. Pimentel-Trapero and R. Izquierdo-Hornillos, J. Chromatogr. Sci., 2003, 41, 261–266 Search PubMed.
- C. M. Selavka, J. Forensic Sci., 1991, 36, 685–696 CrossRef PubMed.
- M. Thevis, G. Fusshöller and W. Schänzer, Drug Test. Anal., 2011, 3, 777–783 CrossRef PubMed.
- M. X. Xie, Y. Liu and M. Jiang, Chin. J. Anal. Chem., 2002, 30, 1308–1311 Search PubMed.
- M. Thevis, C. Görgens, S. Guddat, A. Thomas and H. Geyer, Scand. J. Med. Sci. Sports, 2024, 34, e14228 CrossRef PubMed.
- M. R. Fuh, S. Y. Huang and T. Y. Lin, Talanta, 2004, 64, 408–414 CrossRef PubMed.
- D. P. Zeng, C. P. Lin, Z. L. Zeng, X. H. Huang and L. M. He, Agric. Sci. China, 2010, 9, 306–312 CrossRef.
- A. L. Medina, M. A. O. da Silva, H. de Sousa Barbosa, M. A. Z. Arruda, A. Marsaioli Jr and N. Bragagnolo, Food Res. Int., 2015, 78, 124–130 CrossRef PubMed.
- B. Kaufmann and P. Christen, Phytochem. Anal., 2002, 13, 105–113 CrossRef PubMed.
- K. Ganzler, A. Salgo and K. Valko, J. Chromatogr., 1986, 371, 299–306 CrossRef PubMed.
- J. Seo, H. Y. Kim, B. C. Chung and J. K. Hong, J. Chromatogr. A, 2005, 1067, 303–309 CrossRef PubMed.
- M. H. Choi and B. C. Chung, Analyst, 2001, 126, 306–309 RSC.
- C. Blasco, C. Van Poucke and C. Van Peteghem, J. Chromatogr. A, 2007, 1154, 230–239 CrossRef PubMed.
- G. Kaklamanos, G. Theodoridis and T. Dabalis, J. Chromatogr. A, 2009, 1216, 8072–8079 CrossRef PubMed.
- H. Noppe, B. Le Bizec, K. Verheyden and H. F. De Brabander, Anal. Chim. Acta, 2008, 611, 1–16 CrossRef PubMed.
- Y. P. Xiao, H. Y. Lu, S. J. Lü, S. X. Xie, Z. Z. Wang and H. W. Chen, Chin. J. Anal. Chem., 2016, 44, 1633–1637 Search PubMed.
- E. Attalah, Y. S. Nasr, H. A. El-Gammal and F. A. Nour El-Dien, Food Addit. Contam.,:Part A, 2016, 33, 1545–1556 CrossRef PubMed.
- C. L. Xu, C. F. Peng, K. Hao, Z. Y. Jin and X. G. Chu, Anal. Lett., 2006, 39, 555–568 CrossRef.
- Z. H. Zhang, H. Yan, F. Y. Cui, H. Yun, X. H. Chang, J. H. Li, X. Liu, L. J. Yang and Q. R. Hu, Food Anal. Methods, 2016, 9, 915–924 CrossRef.
- Korea Disease Control and Prevention Agency, Korea National Health and Nutrition Examination Survey, https://knhanes.kdca.go.kr/knhanes/sub03/sub03_02_05.do, accessed August 25, 2024.
- E. Braekevelt, B. P. Lau, B. Tague, S. Popovic and S. A. Tittlemier, J. Agric. Food Chem., 2011, 59, 915–920 CrossRef PubMed.
- S. Hartmann, M. Lacorn and H. Steinhart, Food Chem., 1998, 62, 7–20 CrossRef.
- Y. L. Tseng, C. Y. Sun and F. H. Kuo, Steroids, 2006, 71, 817–827 CrossRef PubMed.
- World Anti-Doping Agency, WADA Technical Document – TD2022MRPL, https://www.wada-ama.org/sites/default/files/2022-01/td2022mrpl_v1.1_eng_0.pdf, accessed August 25, 2024.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ay01606h |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |


