 Open Access Article
Open Access ArticleUnusual response behavior of ion exchange-based potassium ion-selective nano-optodes based on bis(crown ether) neutral ionophores and a cationic solvatochromic dye†
Naoya
Matsumoto
,
Kenji
Sueyoshi‡
,
Tatsuro
Endo
 and
Hideaki
Hisamoto
and
Hideaki
Hisamoto
 *
*
Department of Applied Chemistry, Graduate School of Engineering, Osaka Metropolitan University, 1-1 Gakuen-cho, Naka-ku, Sakai, OSAKA, 599-8531 Japan. E-mail: hisamoto@omu.ac.jp
First published on 7th July 2025
Abstract
In this study, we used three different potassium ionophores developed for ion-selective electrodes to fabricate nanoemulsion-type ion-selective optodes (NE-ISOs) with a pH-independent response mechanism based on cation exchange between a polarity-responsive cationic dye in the organic phase and potassium ions in the aqueous phase and compared their responses. As a result, the NE-ISO prepared with valinomycin as the typical ionophore showed an ideal response in which the initial fluorescence intensity decreased with increasing ion concentration, as the cationic dye was extruded out into the aqueous phase following extraction of potassium ions by valinomycin. On the other hand, when the same experiment was performed using a bis-crown-type ionophore with a long alkyl chain, the initial fluorescence intensity became extremely small and increased with increasing potassium ion concentration, indicating a completely opposite response behavior that cannot be explained by the conventional ion exchange model. As a result of extraction experiments of hydrophobic organic cations and investigation of ionophore concentrations in nanodroplets, we obtained some results that suggest that the interaction between the ionophore molecule and the dye molecule at the organic–aqueous interface and the accumulation behavior of the ion–ionophore complex at the organic–aqueous interface greatly affect the response behavior to ions, depending on the molecular structure of the ionophore. These results contradict the conventional perception in the development of ion-selective optodes that the same principle can be applied to measure different ions by changing the ionophore. It clearly demonstrates the importance of considering the chemical structures of the ionophores and dye molecules and their interaction, in addition to the response mechanism when designing NE-ISOs.
Introduction
Ion sensors that can measure ion concentrations in water with high sensitivity and selectivity will contribute to the development of medical diagnosis and environmental analysis, and so many ion-selective electrodes (ISEs) and ion-selective optodes (ISOs) based on neutral ionophores have been studied to date.1–3 Recently, ISOs have been applied to research on new principles,4–7 micro- and nano-analysis devices,8–12 paper-based devices,13 thin film devices14,15 and other devices, as well as pipette-type sensing using liquid ISO.16In recent years, film-type ion-selective optodes (film-ISOs) that contain dye molecules or ionophores in a hydrophobic thin film have been expanded to nanoemulsion (NE)-type ion-selective optodes (NE-ISOs) in which the constituent molecules and materials are made into oil droplets using a surfactant and dispersed in water.17,18 This is pioneering research by Xie and Bakker et al., and it has attracted attention for its fast and pH-independent response.19,20 NE-ISOs are also applicable to direct measurements in vivo, enabling measurements that were not possible with conventional film-ISOs.21,22
Recently, studies have been reported on the surfactants used in NE-ISOs23,24 and on the comparison of film-ISOs and NE-ISOs.25 Although there are some differences in performance in terms of detection limit, response range, and selectivity coefficient, the response mechanism and behavior of both film-ISOs and NE-ISOs are basically the same, and it is generally believed that ionophores developed for ISEs can be directly applied to the selective measurement of the corresponding ions.
However, among the many ionophores for ISEs that have been developed so far, most of the NE-ISOs reported so far use specific ionophores. For example, valinomycin (potassium ionophore I) is used as the potassium ionophore and sodium ionophore X (tetraethyl 4-tert-butylcalix[4]arene-O,O′,O′′,O′′′-tetraacetate) is used as the sodium ionophore. However, there are few reports comparing these specific ionophores with other ionophores.
Recently, we have been attempting to develop highly sensitive sensors based on film-ISOs and NE-ISOs that utilize the lipophilic dye liquids that we have developed26–29 and the interactions between fluoroalkyl chains.30 In the course of this research, we frequently experienced that the response behavior of NE-ISOs using some ionophore molecules deviates significantly from the theoretical response model. In particular, in the case of NE-ISOs based on the exchange of pH-independent cationic solvatochromic dyes reported by Xie and Bakker et al., the deviation from the conventionally known ion exchange mechanism was particularly large. Therefore, in this study, we aimed to gain insight into the deviating response behavior.
In this study, we focused on valinomycin, which has been widely reported in potassium ion sensing, and two other bis(crown ether) type ionophores, which have been less reported for NE-ISOs, and compared the response behavior of a pH-independent sensor based on the change in fluorescence intensity associated with the extrusion of a cationic solvatochromic dye from an organic phase to the aqueous phase upon extraction of potassium ions from the aqueous phase. In order to consider the response behavior, we carried out an extraction experiment of hydrophobic cations and an experiment in which the amount of ionophore in nano-oil droplets was systematically changed, and we report here the findings obtained regarding the response behavior.
Experimental
Materials and instruments
Acridine orange 10-nonyl bromide ([9-AO+]Br−), potassium ionophore I (valinomycin, K-I), potassium ionophore III (2-dodecyl-2-methyl-1,3-propanediylbis[N-[5′-nitro(benzo-15-crown-5)-4′-yl]carbamic acid], K-III), and Pluronic®F-127 (F-127) were purchased from Sigma-Aldrich Japan (Tokyo, Japan). Tetrakis[3,5-bis(trifluoromethyl)phenyl]borate, sodium salt (Na+ [TFPB−]) and bis(benzo-15-crown-5) (bis[(benzo-15-crown-5)-4-methyl]pimelate, K-II) were purchased from Dojindo (Kumamoto, Japan). Bis(2-ethylhexyl) sebacate (DOS) was purchased from Kanto Chemical (Tokyo, Japan). All other reagents of the highest grade were used for sample preparation.Due to the presence of sodium cations and bromide anions in the starting materials of Na+ [TFPB−] and [9-AO+]Br−, an ion-exchange procedure was carried out to prepare the dye ion pair ([9-AO+][TFPB−]) using the same procedure described in the previous report.29 Briefly, 0.1 mmol of both reagents were dissolved in 40 mL of dichloromethane, 10 mL of pure water was added, and the mixture was stirred for approximately 30 minutes. The organic phase was washed with pure water and brine, dried over anhydrous sodium sulfate, and the solvent was removed under reduced pressure to obtain the desired [9-AO+][TFPB−].
Fluorescence spectra were recorded using a JASCO FP-8550 instrument (JASCO, Tokyo, Japan). The particle size and polydispersity of each NE-ISO prepared were measured using a nanoparticle analyzer (NanoPartica SZ-100V2, HORIBA, Kyoto, Japan) following the dynamic light scattering (DLS) principle.
Preparation of NE-ISOs and sample solutions for fluorescence measurements
The NE-ISOs (K-I, K-II, and K-III) were prepared through the following procedure unless otherwise stated. First, THF solutions were prepared by mixing 0.8 mg of the dye ion pair [9-AO+][TFPB−], 1.2 mg of the ionophore (either K-I, K-II, or K-III), 8.0 mg of the matrix component, DOS, and 5.0 mg of the surfactant, F-127, in 3.0 mL of THF. Next, 0.9 mL of each THF solution was mixed with 9 mL of ultrapure water and sonicated for 5 minutes to obtain the corresponding crude NE-ISO. Finally, nitrogen gas was purged over each crude NE-ISO for approximately 15 minutes to remove the residual THF, with the evaporation confirmed by monitoring the weight difference before and after the purge, resulting in the formation of the NE-ISOs (K-I, K-II, and K-III). In this experiment, the amount of ionophore was set to 1.2 mg. In this case, assuming that the specific gravity of DOS is 1, the molar concentrations in DOS, taking into account the molecular weight of the ionophore, were 0.108 M (K-I), 0.166 M (K-II), and 0.124 M (K-III), respectively. In addition, the molar concentration of [9-AO+][TFPB−] in DOS was 0.064 M based on the same assumption.50 μL of the prepared NE-ISO solution was mixed with 4500 μL of sample solution in buffer (0.1 M Tris-HCl pH 7.4) and 4450 μL of ultrapure water (180 times dilution). Then, the fluorescence spectrum was recorded (ex. 500 nm, em. 520 nm).
The prepared NE was used for ion response measurements on the same day. No sedimentation of oil droplets or deposition on the wall during measurements was observed in this experiment.
Results and discussion
Response behavior of NE-ISOs with three types of potassium ionophores
Present NE-ISOs contain a potassium ionophore and a pH-insensitive (polarity-responsive) cationic fluorescent dye ion-pair ([9-AO+][TFPB−]) in the DOS oil droplet. As the concentration of K+ in the sample solution increases, the amount of K+ extracted into the oil droplet increases by forming a complex with the potassium ionophore. At this time, in order to maintain electroneutrality in the oil droplet, 9-AO+ is extruded out from the oil droplet (the low polarity environment) to the sample solution (the high polarity environment), and the fluorescence intensity of the dye decreases significantly (cation exchange type K+ response mechanism, see Fig. 1). The alkylacridine orange used in this study is a molecule that has been used in plasticized PVC membrane-type fluorescent ion sensors in the past. It is known that due to molecular partitioning near the oil/water interface, the fluorescence intensity is high in a highly hydrophobic environment and low in a hydrophilic environment such as water.31,32 Therefore, it can be applied to the ion exchange type ion-selective optode with a pH-independent response reported recently by Xie and Bakker et al.17 Among these, the commercially available 9-AO+ has been used in the past in our laboratory for enzyme-responsive NE-ISOs.29 Although 9-AO+ has an alkyl chain with a carbon number of 9, its salt with bromide ions is water-soluble and always cationic without deprotonation, making it suitable for use in non-pH-responsive cation exchange mechanisms. In addition, because the alkyl chain introduced has a certain degree of hydrophobicity, it can form an electrically neutral ion pair with TFPB−, a highly hydrophobic anion, and can be retained within the oil droplet. In this study, an ion pair with a molar ratio of approximately 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 was prepared in advance and used.
1 was prepared in advance and used.
 | ||
| Fig. 1 Typical response mechanism of ion exchange-based pH-independent nano-optodes, and optode components used in this work. | ||
Bis(2-ethylhexyl) sebacate (DOS), which is widely used as a plasticizer in film-ISOs and NE-ISOs, is used as the matrix that mainly constitutes the oil droplets. Nonionic Pluronic®F-127 (F-127) is used as a surfactant to disperse and stabilize the oil droplets in water. The cations used to compare the response behavior are potassium ion (K+) as the target cation, sodium ion (Na+) as the interfering cation, and tetrabutylammonium ion (TBA+) as the hydrophobic cation that is forcibly extracted into the oil droplets without complexing with the ionophore to verify the cation exchange mechanism shown in Fig. 1.
Fig. S1–S3† show the fluorescence spectrum changes when various ionophores are used and the response curves for K+ (Fig. S1), Na+ (Fig. S2), and TBA+ (Fig. S3†).
The fluorescence spectra in Fig. S1–S3† show that all NE-ISOs exhibited fluorescence spectra originating from 9-AO+. However, the initial fluorescence intensity (when mixed with Tris-HCl buffer) and the behavior of the change in fluorescence intensity when the K+ concentration was increased differed greatly depending on the type of ionophore. It should be noted that since the absolute amount of dye used was the same in all experiments, the differences in fluorescence intensity are worthy of discussion.
These results are summarized for each ionophore in Fig. 2. In the case of the ion exchange response according to the model shown in Fig. 1, cation exchange should occur as the K+ concentration in the aqueous phase increases, and the fluorescence intensity should decrease, but only NE-ISO (K-I) showed such a response. The fluorescence intensity of NE-ISO (K-II) did not decrease even at high K+ concentrations, and the fluorescence intensity of NE-ISO (K-III) had already decreased significantly before the ion response, and at higher K+ concentrations, the fluorescence intensity increased and then decreased again.
 | ||
| Fig. 2 Response curves of NE-ISOs based on each ionophore to Na+, K+, and TBA+ (F.I. denotes fluorescence intensity). | ||
These results suggest that NE-ISO (K-I) selectively extracts K+ and shows the cation exchange response according to the model in Fig. 1, while NE-ISO (K-II) only undergoes cation exchange based on K+ extraction at high K+ concentrations (1 M K+), and that NE-ISO (K-III) has some kind of quenching process (a factor that greatly increases the polar environment around 9-AO+) that is not dependent on K+ extraction or cation exchange. Since there was almost no change in the fluorescence intensity with respect to the Na+ concentration change in either ionophore, it is considered that these changes in fluorescence intensity are related to the complex formation between the ionophore and potassium ions. In addition, to confirm that the reason for the lower cation exchange response in NE-ISOs (K-II, K-III) compared to NE-ISO (K-I) is due to the ionophore, the response results when TBA+ was forcibly extracted into the NE-ISO oil droplet without complex formation with the ionophore were examined, and the fluorescence intensity showed a tendency to decrease in both cases. In NE-ISO (K-II), the fluorescence intensity decreased significantly with increasing concentration from around [TBA+] = 10−4 M. On the other hand, in NE-ISO (K-III), the initial fluorescence intensity was very small, so the change was relatively small compared to NE-ISO (K-II), but the fluorescence intensity decreased slightly and then increased.
These results indicate that a certain amount of cation exchange of 9-AO+ into the aqueous phase occurs in NE-ISOs (K-II, K-III) and suggest that the reason why NE-ISOs (K-II, K-III) do not respond as shown in the model in Fig. 1 is due to ionophores.
Effect of the amount of ionophore used in the preparation of NE-ISOs on response behavior
To understand why the initial fluorescence intensity of NE-ISO (K-III) was extremely low, we tried a similar experiment by increasing or decreasing the amount of ionophore used in the preparation of NE-ISO. The amount of ionophore added was changed from 0.3 mg to 4.8 mg, based on the standard amount of ionophore (1.2 mg) described in the Experimental section. The amounts of components other than the ionophore were identical to those described in the Experimental section. The particle size and polydispersity of the prepared NE-ISO are shown in Table S1.† The particle size was about 340 nm when 2.4 mg of K-III was added, which was slightly larger, but the other particles were within the range of about 130–170 nm. Therefore, we believe that the difference in particle size does not significantly affect the potassium ion response.Fig. 3 shows the response curves to potassium ions for NE-ISOs prepared with different amounts of each ionophore added. The K+ response behavior of NE-ISOs (K-I) showed K+ concentration dependence at all K-I addition amounts, and the fluorescence intensity began to decrease at lower K+ concentration ranges with increasing K-I addition amounts. From this result, K-I is considered to be an ionophore suitable for NE-ISOs in the ion exchange response model shown in Fig. 1. In addition, since there was almost no change in the initial fluorescence intensity with increasing ionophore addition amounts, it is considered that K-I itself has little effect on the polar environment around 9-AO+ in the oil droplets.
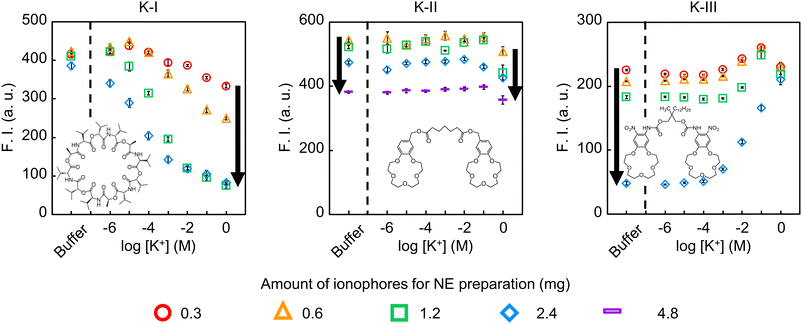 | ||
| Fig. 3 Response curves of NE-ISOs based on each ionophore when the amount of ionophore added in the nanodroplets is varied. | ||
The K+ response behavior of NE-ISOs (K-II) showed a decrease in fluorescence intensity in response to K+ only in a high K+ concentration range (0.1–1 M) at all K-II addition amounts. In addition, because the initial fluorescence intensity decreased with increasing amounts of K-II, it is considered that K-II itself may affect the polar environment around 9-AO+ in the oil droplets. In the case of NE-ISO (K-II), there was no significant change in fluorescence intensity in the concentration range of 10−6 M to 10−1 M K+. In this case, it is considered that K-II forms a complex with K+ at the oil/water interface, but it is presumed that it is not extracted into the bulk of the NE-ISO oil droplets. Since K-II has a bis(crown ether) molecular structure, when it forms a complex with K+, even if the potassium ion is sandwiched between two crown ether moieties, it becomes a complex in which the potassium ion is partially exposed, and it is difficult to completely cover it with hydrophobic groups, unlike large cyclic molecules such as K-I, which fully encapsulate K+ by the many hydrophobic isopropyl functional groups of valinomycin. Therefore, electrostatic repulsion between the ion–ionophore complex cations localized at the oil/water interface and the 9-AO+ existing in the bulk of the oil droplets prevailed, and cation exchange could not occur. However, if the K+ concentration was sufficiently high, the proportion of K+-K-II complexes localized at the interface increased, leading to bulk extraction into the oil droplets, and a decrease in fluorescence intensity associated with cation exchange was observed. This assumption is supported by the fact that the fluorescence intensity was decreased in the TBA+ extraction experiment shown in Fig. 2. In addition, the initial fluorescence intensity of NE-ISO (K-II) was slightly higher than that of NE-ISO (K-I) with good reproducibility. This is probably because, unlike the relatively hydrophobic K-I, K-II can exist at the oil/water interface with its relatively hydrophilic bis(crown ether) moiety facing the aqueous phase, and therefore the polarity of the oil droplet interior is relatively lower in NE-ISO (K-II) than in NE-ISO (K-I).
The K+ response behavior of NE-ISOs (K-III) shows that the initial fluorescence intensity decreases significantly with increasing K-III loading. In the case of NE-ISO (K-III/2.4), the amount of K-III is in excess of 3.4 molecules per molecule of 9-AO+ in the oil droplet, which suggests that the interaction between 9-AO+ and K-III is very strong. Unlike K-II, K-III has a surfactant-type molecular structure with a long alkyl chain, so it is more likely to localize at the oil/water interface with a micelle-like orientation than K-II. In addition, since 9-AO+ also has a long alkyl chain, it is thought that localization at the oil/water interface becomes more pronounced with increasing K-III loading. The fact that the localization of dyes and ionophores with long alkyl chains present in the oil droplets of NE-ISOs at the oil/water interface affects the sensor response has been found in our previous study of chloride ion-responsive optodes and in the study of carbonate anion-responsive optodes by Bakker et al.27,33 In such cases, the sensor performance was affected by the background signal and the change in the response concentration range, but there are few reports of change in the response of the fluorescence intensity to the concentration change of the target ion fully reversed, as in this case. Another point of view is that the 9-AO+ we used here has a hydrophobic linear alkyl chain, unlike the dye used by Bakker et al., and has a molecular structure similar to that of a surfactant. We believe that this surfactant-like molecular structure is also one of the important factors that led to the results we obtained. Since it was thought that 9-AO+ might form a complex with the bis(crown ether) moiety of K-III, we attempted to analyze the interaction by proton NMR experiments, but almost no chemical shifts of the protons from 9-AO+ and K-III were observed (data not shown). However, when dyes or ionophores with long-chain alkyl groups are oriented and accumulated at the oil/water interface, it is speculated that the interaction between 9-AO+ and K-III is much stronger than in the bulk solution. In addition, the increase in fluorescence intensity with an increase in K+ concentration from 10−3 M to 10−1 M K+ in Fig. 2 (K-III) is probably due to an increase in the K+-K-III complex at the oil/water interface, and 9-AO+, which was quenched probably by the interaction between 9-AO+ and K-III at the oil/water interface, might be “pushed back into the bulk oil phase” by electrostatic repulsion and begin to emit strong fluorescence again. Since K-III has a long alkyl chain, the K+-K-III complex has an amphiphilic molecular structure and is more likely to be oriented and localized at the interface than the K+-K-II complex. As for the reason why the fluorescence intensity decreases when the K+ concentration is 1 M in Fig. 2 (K-III), as in the case of K-II, it is considered that the K+ concentration becomes high enough that 9-AO+ might start extruding out into the aqueous phase by cation exchange, resulting in a decrease in the fluorescence intensity. In addition, the fluorescence intensity at a K+ concentration of 1 M was almost the same regardless of the amount of K-III added. This might be because the excess K+ formed K+-K-III complexes with almost all of the K-III present in the oil droplets of each NE-ISO (K-III) at the oil/water interface, essentially consuming all the K-III that can directly interact with 9-AO+, and the amount of ionophore might no longer be involved in the distribution equilibrium of 9-AO+.
Considering the above discussion, we speculate that the possible response mechanism of NE-ISOs using K-II and K-III can be shown schematically in Fig. 4.
 | ||
| Fig. 4 Possible response mechanism to NE-ISOs based on (a) K-II and (b) K-III toward K+ under the experimental conditions of Fig. 2. | ||
Conclusions
In this study, we prepared NE-ISOs based on three types of potassium ionophores for ISEs and NE-ISOs with different amounts of potassium ionophore added to the oil droplets and compared the K+ response behavior of the ion-exchange-type NE-ISOs. The experimental results revealed that NE-ISOs containing K-I showed a typical ion-exchange response to K+ regardless of the amount of K-I added, as shown in the model in Fig. 1, while NE-ISOs containing K-II and K-III showed completely different fluorescence response behavior, and especially, NE-ISOs containing K-III showed completely opposite response behavior. Ionophore molecules for ISEs have been used as highly selective sensing materials in various sensors based on various principles, including electrochemical and optical sensors, and it was believed that a certain level of response and ion selectivity was guaranteed in any sensor application. Therefore, it has been believed by many researchers that by changing the ionophore molecule, different ions can be measured based on the same principle. However, the results of this study clearly show that conventional wisdom does not necessarily hold true. Although this research did not completely clarify the mechanism experimentally, the preliminary results of this study suggest that it is speculated that the cause is probably related to the molecular structures of the ionophore and dye molecules and their interactions within the nanodroplets and at the oil–water interface. Therefore, it might be important to consider the above interactions in addition to the response mechanism when designing NE-ISOs.Author contributions
N. M., K. S., T. E., and H. H. conceptualised, planned, and designed the experiments, and drafted the manuscript. N. M. performed the experiments and analysed the data. All authors have approved the manuscript and agreed with its submission to this journal.Conflicts of interest
There are no conflicts to declare.Data availability
All the experimental data are presented in the main text and the ESI.† Other pieces of information are available upon reasonable request to the corresponding author.Acknowledgements
This study was partly supported by the Japan Society for the Promotion of Science through a Grant-in-Aid for Scientific Research (no. 23H01990) and the Takahashi Industrial and Economic Research Foundation.References
- E. Bakker, P. Bühlmann and E. Pretsch, Chem. Rev., 1997, 97, 3083–3132 CrossRef CAS PubMed.
- P. Bühlmann, E. Pretsch and E. Bakker, Chem. Rev., 1998, 98, 1593–1688 CrossRef PubMed.
- H. Hisamoto and K. Suzuki, Trends Anal. Chem., 1999, 18, 513–524 CrossRef CAS.
- H. Shibata, Y. Hiruta and D. Citterio, Analyst, 2019, 144, 1178–1186 RSC.
- Y. Soda, D. Citterio and E. Bakker, ACS Sens., 2019, 4, 670–677 CrossRef CAS PubMed.
- A. Konefał, P. Piątek, K. Maksymiuk and A. Michalska, Sens. Actuators, B, 2023, 391, 134022 CrossRef.
- F. Steininger, J. Palmfeldt, K. Koren and A. V. Kalinichev, ACS Sens., 2024, 9, 4555–4559 CrossRef CAS PubMed.
- H. Hisamoto, T. Horiuchi, K. Uchiyama, M. Tokeshi, A. Hibara and T. Kitamori, Anal. Chem., 2001, 73, 5551–5556 CrossRef CAS PubMed.
- H. Hisamoto, Y. Nakashima, C. Kitamura, S. Funano, M. Yasuoka, K. Morishima, Y. Kikutani, T. Kitamori and S. Terabe, Anal. Chem., 2004, 76, 3222–3228 CrossRef CAS PubMed.
- S. Aki, T. Endo, K. Sueyoshi and H. Hisamoto, Anal. Chem., 2014, 86, 11986–11991 CrossRef CAS PubMed.
- X. Wang, M. Sun, S. A. Ferguson, J. D. Hoff, Y. Qin, R. C. Bailey and M. E. Meyerhoff, Angew. Chem., Int. Ed., 2019, 58, 8092–8096 CrossRef CAS PubMed.
- R. Wang, Y. Zhou, G. N. Ghanbari, Y. Mawaldi and X. Wang, Anal. Chem., 2021, 93, 13694–13702 CrossRef CAS PubMed.
- H. Shibata, T. G. Henares, K. Yamada, K. Suzuki and D. Citterio, Analyst, 2018, 143, 678–686 RSC.
- X. Wang, Q. Zhang, C. Nam, M. Hickner, M. Mahoney and M. E. Meyerhoff, Angew. Chem., Int. Ed., 2017, 56, 11826–11830 CrossRef CAS PubMed.
- Q. Zhang, X. Wang, V. Decker and M. E. Meyerhoff, ACS Appl. Mater. Interfaces, 2020, 12, 25616–25624 CrossRef CAS PubMed.
- N. G. Ghalehjoughi, R. Wang, S. Kelley and X. Wang, Anal. Chem., 2023, 95, 12557–12564 CrossRef PubMed.
- X. Xie and E. Bakker, Anal. Bioanal. Chem., 2015, 407, 3899–3910 CrossRef CAS PubMed.
- X. Xie, G. Mistlberger and E. Bakker, Anal. Chem., 2013, 85, 9932–9938 CrossRef CAS PubMed.
- X. Xie, J. Zhai and E. Bakker, Anal. Chem., 2014, 86, 2853–2856 CrossRef CAS PubMed.
- X. Xie, A. Gutiérrez, V. Trofimov, I. Szilagyi, T. Soldati and E. Bakker, Anal. Chem., 2015, 87, 9954–9959 CrossRef CAS PubMed.
- X. Xie, A. Gutiérrez, V. Trofimov, I. Szilagyi, T. Soldati and E. Bakker, Chimia, 2015, 69, 196–198 CrossRef CAS PubMed.
- W. Di and H. A. Clark, Anal. Methods, 2020, 12, 1441–1448 RSC.
- A. A. Mendonsa, T. Z. Sodia and K. J. Cash, Analyst, 2024, 149, 4615–4622 RSC.
- K. Robinson, C. Mao and E. Bakker, Anal. Chem., 2021, 93, 15941–15948 CrossRef CAS PubMed.
- M. Villanueva, J. Vega-Chacón and G. Picasso, Anal. Methods, 2024, 16, 4710–4723 RSC.
- T. Mizuta, K. Sueyoshi, T. Endo and H. Hisamoto, Anal. Chem., 2021, 93, 4143–4148 CrossRef CAS PubMed.
- K. Maki, R. Oishi, T. Mizuta, K. Sueyoshi, T. Endo and H. Hisamoto, Analyst, 2022, 147, 1529–1533 RSC.
- S. Oka, K. Sueyoshi, T. Endo and H. Hisamoto, Anal. Sci., 2023, 39, 1249–1256 CrossRef CAS PubMed.
- N. Wada, K. Sueyoshi, T. Endo and H. Hisamoto, Bunseki Kagaku, 2024, 73, 351–357 CrossRef CAS.
- S. Iwamoto, K. Sueyoshi, T. Endo and H. Hisamoto, Anal. Sci., 2024, 40, 1787–1792 CrossRef CAS PubMed.
- Y. Kawabata, R. Tahara, T. Kamichika, T. Imasaka and N. Ishibashi, Anal. Chem., 1990, 62, 1528–1531 CrossRef CAS.
- Y. Kawabata, T. Kamichika, T. Imasaka and N. Ishibashi, Anal. Chem., 1990, 62, 2054–2055 CrossRef CAS.
- N. Y. Tiuftiakov, K. J. Robinson and E. Bakker, Electroanalysis, 2023, 35, e202200507 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5an00516g |
| ‡ Current affiliation: Department of Chemistry, School of Science, Kitasato University, 1-15-1, Kitazato, Minami-ku, Sagamihara-shi, Kanagawa 252-0373, Japan. |
| This journal is © The Royal Society of Chemistry 2025 |
