Open Access Article
Open Access ArticleA steam-mediated isothermal amplification and flocculation-based detection platform for electricity-free point of care diagnostics†
Kissan D.
Achary‡
a,
Satheesh
Natarajan‡
 b and
Aashish
Priye
b and
Aashish
Priye
 *ac
*ac
aDepartment of Chemical and Environmental Engineering, University of Cincinnati, Cincinnati, OH 45221, USA. E-mail: priyeah@uc.edu; Tel: +1-979-676-0953
bHealthcare Technology Innovation Center, Indian Institute of Technology, Madras, Chennai, India
cDigital Futures, University of Cincinnati, OH 45221, USA
First published on 24th February 2025
Abstract
Approximately 9% of the global population lacks access to reliable electricity, and the absence of affordable, electricity-free diagnostic tools hinders early detection of infectious diseases, exacerbating public health burdens in resource-limited settings. We introduce SteamFloc-LAMP – an electricity-free molecular diagnostic platform engineered for instrument-free detection of pathogenic nucleic acid targets. The platform leverages steam-mediated heating from boiling water to sustain the isothermal conditions required for Loop-Mediated Isothermal Amplification (LAMP). Thermal characterization of the steam-mediated heating system identified parameters that enable the consistent maintenance of optimal temperatures for LAMP reactions with minimal fluctuations. Visual end-point detection was achieved through a bridging flocculation mechanism, which exploits the interaction between LAMP amplicons, spermine, and charcoal particles, leading to visible aggregation in positive samples, thus enabling naked-eye detection without the need for specialized equipment or expensive reagents like fluorophores or colorimetric dyes. The SteamFloc-LAMP assay targeted the lipL32 gene, recognized for its exclusivity to pathogenic Leptospira strains. The assay achieved a detection limit of 100 fg of genomic DNA per reaction (∼90 genome copies). Specificity tests using lipL32-specific primers demonstrated the assay's ability to distinguish pathogenic Leptospira accurately, with no cross-reactivity with ligB, ligA, or lipL41 genes found in nonpathogenic strains. A blind test involving DNA extracted from Leptospira reference cultures further validated the assay's diagnostic accuracy, aligning with PCR results. These findings demonstrate the SteamFloc-LAMP assay as a reliable, simple, and cost-effective field deployable diagnostic tool with significant implications for point-of-care detection.
Introduction
Pathogenic diseases caused by bacteria such as Leptospira, Salmonella, Escherichia coli, and Mycobacterium tuberculosis pose significant global health challenges, particularly in resource-limited settings.1,2 These diseases affect millions annually, often with high morbidity and mortality rates, especially in regions with inadequate healthcare infrastructure. Rapid and accurate detection is critical as many pathogens have limited viability in clinical and environmental samples.3 However, traditional diagnostic methods, such as bacterial culture, are often slow and insensitive, making them unsuitable for early-stage detection. Serological assays like the Microscopic Agglutination Test (MAT)4,5 and Enzyme-Linked Immunosorbent Assay (ELISA)6 are faster but primarily detect antibodies, leading to reduced sensitivity and specificity in the early stages of infection. Molecular diagnostics, such as polymerase chain reaction (PCR), offer highly sensitive and specific detection by targeting pathogen DNA, yet their widespread implementation is hindered by high costs, complexity, and reliance on sophisticated equipment, rendering them impractical for point-of-care applications in resource-constrained environments.7,8 Efforts to improve diagnostics include Loop-Mediated Isothermal Amplification (LAMP), which amplifies DNA at a constant temperature (∼65 °C) within 30 minutes, offering a simpler alternative to PCR for point-of-care use.9,10 LAMP has enabled the direct detection of pathogens in biological samples with visual and fluorescence-based methods. However, its reliance on power-intensive heaters poses a barrier to adoption in regions with unreliable electricity. This challenge is particularly concerning, given that approximately ∼9% of the global population—∼700 million people—still lack access to reliable electricity.11 Addressing this limitation is critical for ensuring the broader applicability of LAMP diagnostics in low-resource settings.A promising approach to eliminating electricity-dependent heaters for LAMP is to harness exothermic chemical reactions (e.g., magnesium–iron alloys, calcium oxide, and lithium) that, when triggered by water or air, generate enough heat to maintain the assay's incubation temperature in low-resource settings without electricity.12 Early demonstrations by LaBarre et al. showed that adding water to calcium oxide (CaO) could reliably heat LAMP reaction vessels at ∼65 °C for 30–45 minutes, all housed in a simple thermos-like container.13,14 This concept was extended by buffering exothermic heat with a phase-change material (PCM), which stabilizes the temperature at the PCM's melting point, thereby preventing enzymatic damage from overheating.15,16 Subsequent work by Buser et al. enhanced these designs by carefully regulating the Mg–Fe + water reaction, adding wicks and insulation to achieve faster heat-up and longer temperature hold times.17 Other variants use commercial hand/toe-warmer pouches (iron powder oxidizing in the air),18 lithium–water devices that allow rapid heat generation in very small packages,19 or mixed PCM/fuel powders to simplify heater construction.20,21 Such electricity-free approaches have also proven effective in actuating biochemical reactions, including CRISPR-based diagnostics.22 Meanwhile, cost-effective detection formats such as lateral flow strips23 or simple turbidimetric assays24 avoid the need for expensive probes or dyes, making them attractive for low-resource settings. Despite these advances, many solutions still rely on multiple reagents or require intricate assembly and design, highlighting the need for simpler, broadly accessible heating methods in resource-limited environments.
Here, we introduce SteamFloc-LAMP (Steam-Mediated Heating and Bridging Flocculation-Based Detection for LAMP Diagnostics), a straightforward approach that uses heat from boiling water—readily available in most low-resource environments—to maintain the temperatures required for LAMP. The assay is initiated and sustained by the consistent isothermal environment provided by steam (Fig. 1A). For simple visual detection of assay results, the bridging flocculation mechanism employs spermine-induced coiling of amplified DNA strands, which then binds to a mixture of activated charcoal (AC) and diatomaceous earth (DE) particles.25 AC, with its distinct black color and insolubility in water, aids in the visual confirmation of flocculation, while DE helps form a stable particle mixture that improves sedimentation. A positive amplification is evident when these particles settle, leaving a clear supernatant, whereas a negative result maintains a black colloidal suspension, providing an immediate visual cue (Fig. 1B). This combined diagnostic approach – integrating steam-mediated heating and visual detection of sedimented particles in a suspension – holds significant promise for point-of-care diagnostics.
Materials and methods
Steam-mediated isothermal heating setup
To enable isothermal amplification in resource-limited settings, a custom steam-mediated heating setup was designed to maintain the temperature required for LAMP (approximately 65 °C). Borosilicate glass beakers (Karter Scientific) with diameters of 6 cm (cat. #: 213F13), 8 cm (cat. #: 213F14), and 10 cm (cat. #: 213F13) were used to generate steam. These beakers were filled with water to a height such that the steam wake region was 9 cm in all beakers. They were heated using a portable propane Bunsen burner (Cybring store; cat. #: 780744724614) set to a low, consistent flame. Thin-walled 0.2 mL PCR tubes with flat caps (Bio-Rad, cat. #: TBS0201) were used to contain the LAMP reaction mixtures. The tubes were suspended in the “steam wake” region above the boiling water using a custom-built tube holder (ESI Fig. S1†). This holder allowed precise adjustment of the tube's vertical height and horizontal position relative to the beaker rim. Temperatures were recorded using 8 Type K thermocouples (PerfectPrime; cat. #: TL0260, 0.13 mm diameter) interfaced with an Arduino Mega 2560 microcontroller (Arduino; Model: A000067) through MAX6675 thermocouple amplifier modules (Adafruit; Product ID: 269). The MAX6675 modules amplified and digitized the thermocouple signals, interfacing with the Arduino via three pins: CS (chip select), SCK (serial clock), and SO (serial output). The Arduino Mega 2560 was programmed to read temperature data from the MAX6675 modules at 1-second intervals. Data were monitored in real-time using the Arduino IDE's Serial Monitor and exported via the Arduino Excel Data Plugin for analysis. Each thermocouple was labeled according to its height above the boiling water surface (e.g., 3 cm, 6 cm, and 9 cm), enabling precise mapping of the thermal gradient in the steam wake region. To evaluate the robustness of the steam-mediated heating setup under varying environmental conditions, we used a table fan (Dreo, cat. #: DR-HAF002) to simulate an average ambient wind speed of ∼4 m s−1 around the beaker.Standard LAMP assay
The LAMP reactions were conducted following established protocols. A 25 μl reaction mixture was prepared, comprising 1.6 μM each of FIP and BIP primers, 0.4 μM each of LF and LB primers, 0.2 μM each of F3 and B3 primers, along with 1× ThermoPol buffer, 6 mM MgSO4, a 1.4 mM deoxynucleotide triphosphate (dNTP) mixture (New England Biolabs, UK), 8 U Bst DNA polymerase (New England Biolabs), and 1 μl of DNA template. The primer sequences were obtained from prior studies26 (ESI Table S1†). The reaction mixture underwent incubation at 65 °C for 30 minutes. LAMP amplification products were analyzed using a 2% agarose gel prepared in 1× TAE buffer. Samples (5 μL) were mixed with 6× loading dye and loaded alongside a DNA ladder. Electrophoresis was performed at 60 V for ∼45 minutes using a blueGel™ electrophoresis system (miniPCR). Gels were visualized with the system's built-in transilluminator to confirm the amplification product size.Bridging-flocculation LAMP assay
Flocculation solution is made from spermine, activated charcoal (AC), diatomaceous earth (DE) particles, Tris, and polyethylene glycol (PEG). 10 mL reaction volume was used for LAMP reactions with lambda DNA as a target in positive samples. To make the flocculation solution, 800 mg of AC and 1200 mg of DE particles were broken down to finer particles in a coffee grinder and added to 50 mL solution containing 50 mM Tris (pH 7.4), 10 mM spermine and 1% (v/v) PEG 1450. A higher proportion of DE particles was used (DE![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AC = 1.5
AC = 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) to eliminate finer AC particles from sticking to the walls and allow rapid settling of clumped particles (15). 1% (v/v) PEG made the solution more viscous and delayed sedimentation in negative controls. 30 mL of this flocculation solution was then added to 10 mL of the postamplification sample. Results were observed by holding the tubes vertically. The flocculation solution comprises activated charcoal, sized 100 mesh (Sigma, St. Louis, MO, USA), and powdered diatomaceous earth. In a 50 ml solution containing 50 mM Tris (pH 8), 10 mM spermine, and 1% (w/v) PEG8000, 400 mg of activated charcoal and 600 mg of diatomaceous earth are thoroughly mixed.
1) to eliminate finer AC particles from sticking to the walls and allow rapid settling of clumped particles (15). 1% (v/v) PEG made the solution more viscous and delayed sedimentation in negative controls. 30 mL of this flocculation solution was then added to 10 mL of the postamplification sample. Results were observed by holding the tubes vertically. The flocculation solution comprises activated charcoal, sized 100 mesh (Sigma, St. Louis, MO, USA), and powdered diatomaceous earth. In a 50 ml solution containing 50 mM Tris (pH 8), 10 mM spermine, and 1% (w/v) PEG8000, 400 mg of activated charcoal and 600 mg of diatomaceous earth are thoroughly mixed.
Analytical sensitivity is determined through tenfold serial dilutions of LAMP amplification products. The limit of detection is established by assessing the SteamFloc-LAMP assay's ability to amplify and detect target DNA in these diluted samples. To assess the assay's analytical specificity, we screened non-specific DNA targets—including lipL41, ligB, and ligA—and examined the potential cross-reactivity of the lipL32 LAMP primers. To assess the SteamFloc-LAMP's effectiveness, a blinded study was conducted using DNA extracted from Leptospira reference cultures. Reference cultures were maintained under the supervision of the Indian Council of Medical Research (ICMR)-Regional Medical Research Centre (RMRC), Port Blair, and stored at the Leptospirosis Research Laboratory, ICAR-NIVEDI, Bengaluru (Institutional Biosafety Committee (IBSC)-F. No. 6-52/NIVEDI/Biosafety/2019/10/05 dated 7 September 2021).
Results and discussion
Thermal characterization of the electricity-free convective heating system
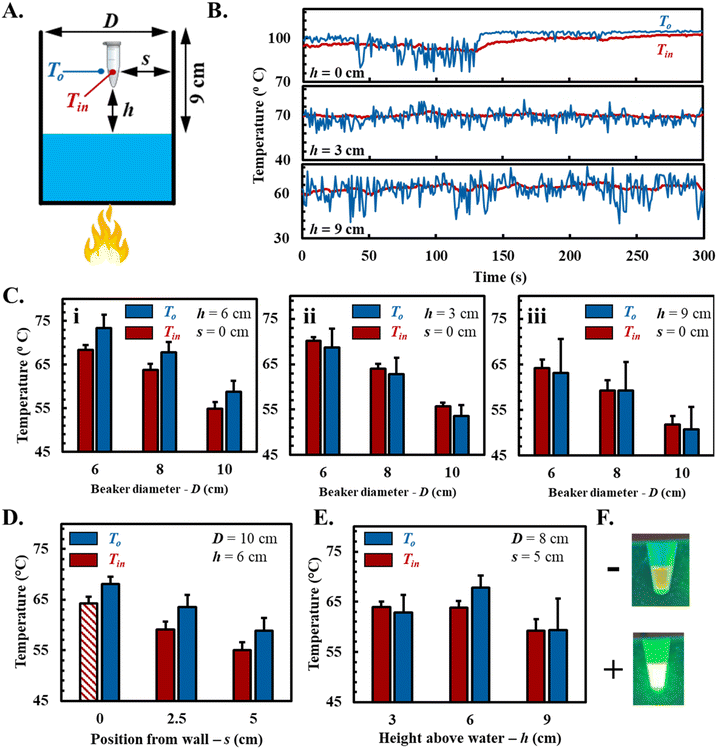
We developed a “steam-mediated isothermal heating” setup using a low flame to boil water in a glass beaker (Fig. 1A). As the water boils, hot steam rises to the beaker brim, mixing with ambient air. The temperature of the steam above the boiling water can be harnessed to sustain temperatures relevant to LAMP reactions. We measured transient temperatures inside (Tin) and outside (To) a LAMP tube placed in the steam wake region – the area above the boiling water until the beaker rim where steam rises and convective currents form. The vial's vertical (h) and horizontal (s) positions, along with the beaker diameter (D), were varied to assess the thermal characteristics for sustaining the LAMP reaction (Fig. 2A).Our results showed that air temperature outside the vial fluctuated more than inside, with higher variance outside and lower variance inside (Fig. 2B). This trend was consistent across all vial positions and beaker diameters. Reduced fluctuation inside the vial is due to water's high heat capacity, which stabilizes the temperature by absorbing and releasing heat gradually, acting as a thermal buffer. The beaker diameter significantly influenced the vial temperature in the steam wake region. As the beaker diameter increased from 6 cm to 10 cm, the vial temperature (Tin) in the central steam wake region (h = 6 cm; s = 0) decreased from 68 ± 1.08 °C to 55 °C ± 1.5 (ΔT = 13 °C) due to increased exposure to cooler ambient air, which infiltrates the larger opening and reduces the steam wake temperature (Fig. 2Ci). Additionally, increasing the beaker diameter reduces the steam velocity in the wake region, as the constant mass flow rate is distributed over a larger area, which further lowers the temperature. Temperature changes at different vial heights (h = 3 and 9 cm) followed a similar trend as the beaker diameter changed (Fig. 2Cii and iii).
Horizontal positioning affected the vial temperature (Tin), with an increase from 64 ± 1.3 °C at the beaker wall to 55 ± 1.5 °C at the beaker center (ΔT = 9 C) (Fig. 2D). Higher temperatures near the wall result from reduced convective currents here compared to the center. Increasing the vial height in the steam wake region led to a slight temperature decrease from 64 ± 1.05 °C to 60 ± 2.2 °C (ΔT = 4 °C) as the vial was raised from 3 cm to 9 cm (D = 8 cm; s = 0) (Fig. 2E). Similar trends were observed across other beaker sizes, indicating that the height has minimal impact on the temperature in the steam wake region, likely due to consistent steam coverage. The standard deviation (SD) of temperature increased with height, suggesting greater fluctuations caused by increased exposure to ambient air, which introduces variability as the vial moves further from the water surface. Simulated ambient wind, generated using a table fan, caused fluctuations in LAMP tube temperatures, highlighting the impact of convective currents on thermal stability. However, the addition of a windshield around the beaker restored temperature consistency within the tubes, matching conditions without wind (ESI Fig. S2†). This demonstrates that our SteamFloc-LAMP system functions robustly under variable conditions, as the windshield ensures consistent LAMP tube temperatures for reliable amplification. Based on our target temperature of 65 °C, we selected a beaker diameter of 10 cm, with the vial positioned near the wall (s = 0) in the central steam wake region (h = 6 cm). In this setup, the temperature inside the vial was maintained at 64.3 °C ± 1.2 °C, suitable for LAMP (Fig. 2D, shaded bar). Two vials were suspended at this position, and after 30 minutes of boiling, amplification in the positive DNA template confirmed the system's ability to support LAMP without electrical power (Fig. 2F).
Flocculation-LAMP assay
Following DNA amplification via LAMP, the assay employs the bridging flocculation mechanism to visually distinguish between positive and negative samples.25 This process hinges on the surface adsorption of spermine, a polyamine that acts as a polymer capable of cross-linking numerous particles. In the presence of post-reaction amplified DNA products, spermine binds to the phosphate backbone of DNA, inducing it to coil. This coiling facilitates the attachment of DNA to activated charcoal (AC) and diatomaceous earth (DE) particles, which are also present in the reaction mixture. The AC is chosen for its high surface area and non-solubility in water, which aids in the effective adsorption of the coiled DNA. Furthermore, AC particles have a distinct black color and can potentially stay suspended in aqueous solutions. DE helps in forming a dense particle mix that enhances the settling process. Together, these particles create a matrix that, in the presence of target DNA, aggregates and settles rapidly due to the weight and interactions of the bound complexes, resulting in the sedimentation of particles and a clear supernatant (Fig. 1B). In contrast, without DNA amplification, the lack of DNA-AC-DE binding prevents flocculation, keeping the particles in suspension and resulting in a turbid, black colloidal solution.Using the steam-mediated isothermal heating setup, we conducted both standard and bridging flocculation LAMP assays to compare their diagnostic sensitivities and selectivities. Serial dilutions of lipL32 genomic DNA (1 ng to 10 fg per reaction) were amplified and analyzed via gel electrophoresis and the flocculation method (Fig. 3A). Both assays detected as low as 100 fg of DNA (∼90 genome copies), demonstrating equivalent sensitivity. This confirms that the flocculation mechanism does not compromise detection capabilities, which is crucial for diagnosing infections with low bacterial loads. To evaluate specificity, we used primers targeting the pathogenic lipL32 gene and compared the results with amplifications of ligB, ligA, and lipL41 genes as negative controls. The lipL32 gene is highly conserved and targeted in leptospirosis diagnostics for its specificity to pathogenic strains, making it a crucial marker for diagnostics.27,28 In contrast, the ligB and lipL41 genes are also present in nonpathogenic strains, making them suitable for ensuring that the assay does not cross-react with non-target Leptospira species. Positive lipL32 samples exhibited visible flocculation and sedimentation, whereas tubes containing ligB, lipL41, or no DNA remained uniformly black, indicating no flocculation (Fig. 3B). These results mirror gel electrophoresis findings, confirming that our LAMP-flocculation assay reliably identifies pathogenic Leptospira and is suitable for field deployment. Taken together, these findings demonstrate that the bridging flocculation LAMP assay is both highly sensitive and specific for detecting pathogenic Leptospira, providing a practical and field-deployable diagnostic tool suitable for resource-limited settings.
Detection of Leptospira in reference samples
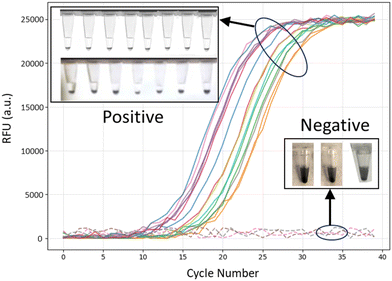
To assess the SteamFloc-LAMP's effectiveness, a blinded study was conducted using DNA extracted from Leptospira reference cultures. Laboratory personnel performing the LAMP assays were blinded to the identity of the samples. The results were compared against the samples’ known PCR test only after the assays were completed. The comparison demonstrated complete agreement between the results of the SteamFloc-LAMP and PCR assays (Fig. 4). Specifically, all PCR-positive samples yielded positive results with LAMP, while PCR-negative samples consistently returned negative results. These findings underscore the assay's high specificity and sensitivity for Leptospira detection.Conclusion
In this study, we introduce SteamFloc-LAMP, an electricity-free molecular diagnostic platform engineered for inexpensive, sensitive, and specific detection of pathogenic Leptospira. By utilizing steam-mediated heating from boiling water, we successfully maintained the isothermal conditions required for LAMP reactions without reliance on electricity or sophisticated laboratory equipment. The bridging flocculation detection mechanism enabled simple and immediate visual differentiation between positive and negative samples without the need for expensive fluorophores or colorimetric dyes. Our findings demonstrated that the SteamFloc-LAMP assay matches the sensitivity and specificity of standard PCR assays.While the steam-mediated heating setup offers a practical solution for electricity-independent diagnostics, it is susceptible to environmental factors. The boiling point of water is influenced by salt concentration and atmospheric pressure. While seawater (3.5% salinity) can raise the boiling point by only ∼0–2 °C, higher elevations reduce it substantially, with a drop of up to 15 °C at altitudes of 5000 meters. Despite this, the boiling point remains above the incubation temperature for the LAMP reaction (∼65 °C). Adjustments in the experimental setup, such as the beaker diameter and vial height, would ensure that the system remains effective in actuating LAMP reactions even at higher altitudes. Enhancing thermal regulation—by incorporating insulating materials or designing a more enclosed heating chamber—could mitigate these fluctuations, ensuring more reliable amplification across various conditions and improving the assay's robustness for consistent use in diverse, resource-limited settings. Similarly, the bridging flocculation detection method, although effective, requires opening the reaction tubes to add reagents. Opening the tubes to add detection reagents increases the risk of contamination and exposes amplified DNA to the environment, potentially leading to false positives or biosafety concerns. To address this limitation, future improvements could involve custom vials with integrated detection reagents in the cap, allowing reagent release by inversion or pressing, enabling a true one-pot reaction without opening the tube. Since this study used purified genomic DNA, further validation with clinical samples is needed to address sample complexity, inhibitors, and extraction efficiency, along with integrating simple, field-deployable DNA extraction methods for practical use. With these advancements, SteamFloc-LAMP holds significant potential as a robust, low-cost, and electricity-free diagnostic platform for rapid disease detection in resource-limited settings.
Data availability
The data underlying this study are available in the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the NSF (NSF TI-2422468; A. Priye). We thank Digital Futures at the University of Cincinnati for providing valuable resources.References
- F. Costa, J. E. Hagan, J. Calcagno, M. Kane, P. Torgerson, M. S. Martinez-Silveira, C. Stein, B. Abela-Ridder and A. I. Ko, Global Morbidity and Mortality of Leptospirosis: A Systematic Review, PLoS Negl. Trop. Dis., 2015, 9, e0003898, DOI:10.1371/journal.pntd.0003898.
- K. S. Ikuta, L. R. Swetschinski, G. Robles Aguilar, F. Sharara, T. Mestrovic, A. P. Gray, N. Davis Weaver, E. E. Wool, C. Han and A. Gershberg Hayoon, et al., Global Mortality Associated with 33 Bacterial Pathogens in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019, Lancet, 2022, 400, 2221–2248, DOI:10.1016/S0140-6736(22)02185-7.
- W. Tantibhedhyangkul, E. Wongsawat, P. Chongtrakool, S. Tiengrim, J. Thaipadungpanit and Y. Suputtamongkol, Case Report: Recovery of Pathogenic Leptospira Spp. from Routine Aerobic Blood Culture Bottles, Am. J. Trop. Med. Hyg., 2020, 103, 1834–1837, DOI:10.4269/ajtmh.20-0204.
- J. J. Waggoner, I. Balassiano, A. Mohamed-Hadley, J. M. Vital-Brazil, M. K. Sahoo and B. A. Pinsky, Reverse-Transcriptase PCR Detection of Leptospira: Absence of Agreement with Single-Specimen Microscopic Agglutination Testing, PLoS One, 2015, 10, e0132988, DOI:10.1371/journal.pone.0132988.
- A. Kannan, C. Priya, L. Prajna and S. Rathinam, Efficiency of Two Commercial Kits in Serodiagnosis of Leptospiral Uveitis, Indian J. Med. Microbiol., 2012, 30, 418–422, DOI:10.4103/0255-0857.103762.
- H. L. Smits, Y. V. Ananyina, A. Chereshsky, L. Dancel, A. F. Lai and H. D. Chee, et al., International Multicenter Evaluation of the Clinical Utility of a Dipstick Assay for Detection of Leptospira-Specific Immunoglobulin M Antibodies in Human Serum Specimens, J. Clin. Microbiol., 2000, 38, 2901–2907, DOI:10.1128/JCM.38.8.2901-2907.2000.
- A. Ahmed, M. F. Engelberts, K. R. Boer, N. Ahmed and R. A. Hartskeerl, Development and Validation of a Real-Time PCR for Detection of Pathogenic Leptospira Species in Clinical Materials, PLoS One, 2005, 3, e709, DOI:10.1371/journal.pone.0002081.
- R. A. Stoddard, J. E. Gee, P. P. Wilkins, K. McCaustland and A. R. Hoffmaster, Detection of Pathogenic Leptospira Spp. through TaqMan Polymerase Chain Reaction Targeting the LipL32 Gene, Diagn. Microbiol. Infect. Dis., 2009, 64, 247–255, DOI:10.1016/j.diagmicrobio.2009.03.014.
- T. Notomi, Y. Mori, N. Tomita and H. Kanda, Loop-Mediated Isothermal Amplification (LAMP): Principle, Features, and Future Prospects, J. Microbiol., 2015, 53, 1–5 CrossRef CAS PubMed.
- T. Notomi, H. Okayama, H. Masubuchi, T. Yonekawa, K. Watanabe, N. Amino and T. Hase, Loop-Mediated Isothermal Amplification of DNA, Nucleic Acids Res., 2000, 28, e63 CrossRef CAS PubMed.
- H. Ritchie, P. Rosado and M. Roser, Access to Energy, in Our World Data, 2024 Search PubMed.
- M. G. Mauk, F. Ansah and M. El-Tholoth, Chemical Heating for Minimally Instrumented Point-of-Care (POC) Molecular Diagnostics, Biosensors, 2024, 14, 554, DOI:10.3390/bios14110554.
- P. LaBarre, K. R. Hawkins, J. Gerlach, J. Wilmoth, A. Beddoe, J. Singleton, D. Boyle and B. Weigl, A Simple, Inexpensive Device for Nucleic Acid Amplification without Electricity—toward Instrument-Free Molecular Diagnostics in Low-Resource Settings, PLoS One, 2011, 6, e19738 CrossRef CAS PubMed.
- P. Labarre, J. Gerlach, J. Wilmoth, A. Beddoe, J. Singleton and B. Weigl, Non-Instrumented Nucleic Acid Amplification (NINA): Instrument-Free Molecular Malaria Diagnostics for Low-Resource Settings, in Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. IEEE Eng. Med. Biol. Soc. Annu. Int. Conf., 2010, vol. 2010, pp. 1097–1099. DOI:10.1109/IEMBS.2010.5627346.
- J. Singleton, C. Zentner, J. Buser, P. Yager, P. LaBarre and B. H. Weigl, Instrument-Free Exothermic Heating with Phase Change Temperature Control for Paper Microfluidic Devices, Proc. SPIE, 2013, 8615, 86150R, DOI:10.1117/12.2005928.
- S. Fu, Y. Jiang, X. Qin, T. Yang, S. Chen, X. Yang, W. Zhang, Y. Qu and C. Man, Electricity-Free Amplification and Visual Detection of Cronobacter Species in Powdered Infant Formula, J. Dairy Sci., 2020, 103, 6882–6893, DOI:10.3168/jds.2019-17661.
- J. R. Buser, S. Diesburg, J. Singleton, D. Guelig, J. D. Bishop, C. Zentner, R. Burton, P. LaBarre, P. Yager and B. H. Weigl, Precision Chemical Heating for Diagnostic Devices, Lab Chip, 2015, 15, 4423–4432, 10.1039/C5LC01053E.
- S. Huang, J. Do, M. Mahalanabis, A. Fan, L. Zhao, L. Jepeal, S. K. Singh and C. M. Klapperich, Low Cost Extraction and Isothermal Amplification of DNA for Infectious Diarrhea Diagnosis, PLoS One, 2013, 8, e60059, DOI:10.1371/journal.pone.0060059.
- B. Udugama, P. Kadhiresan and W. C. W. Chan, Tunable and Precise Miniature Lithium Heater for Point-of-Care Applications, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 4632–4641, DOI:10.1073/pnas.1916562117.
- C. Liu, M. G. Mauk, R. Hart, X. Qiu and H. H. Bau, A Self-Heating Cartridge for Molecular Diagnostics, Lab Chip, 2011, 11, 2686–2692, 10.1039/C1LC20345B.
- R. J. Li, M. G. Mauk, Y. Seok and H. H. Bau, Electricity-Free Chemical Heater for Isothermal Nucleic Acid Amplification with Applications in COVID-19 Home Testing, Analyst, 2021, 146, 4212–4218, 10.1039/D1AN00309G.
- Z. Li, X. Ding, K. Yin, L. Avery, E. Ballesteros and C. Liu, Instrument-Free, CRISPR-Based Diagnostics of SARS-CoV-2 Using Self-Contained Microfluidic System, Biosens. Bioelectron., 2022, 199, 113865, DOI:10.1016/j.bios.2021.113865.
- D. Das, M. Masetty and A. Priye, Paper-Based Loop Mediated Isothermal Amplification (LAMP) Platforms: Integrating the Versatility of Paper Microfluidics with Accuracy of Nucleic Acid Amplification Tests, Chemosensors, 2023, 11, 163, DOI:10.3390/chemosensors11030163.
- Y. Mori, K. Nagamine, N. Tomita and T. Notomi, Detection of Loop-Mediated Isothermal Amplification Reaction by Turbidity Derived from Magnesium Pyrophosphate Formation, Biochem. Biophys. Res. Commun., 2001, 289, 150–154 CrossRef CAS PubMed.
- M. Glenn Mason and J. Ramón Botella, A Simple, Robust and Equipment-Free DNA Amplification Readout in Less than 30 Seconds, RSC Adv., 2019, 9, 24440–24450, 10.1039/C9RA04725E.
- Y.-H. Hsu, S.-J. Chou, C.-C. Chang, M.-J. Pan, W.-C. Yang, C.-F. Lin and K.-W. Chan, Development and Validation of a New Loop-Mediated Isothermal Amplification for Detection of Pathogenic Leptospira Species in Clinical Materials, J. Microbiol. Methods, 2017, 141, 55–59, DOI:10.1016/j.mimet.2017.07.010.
- N. Koizumi, C. Nakajima, T. Harunari, T. Tanikawa, T. Tokiwa and E. Uchimura, et al., A New Loop-Mediated Isothermal Amplification Method for Rapid, Simple, and Sensitive Detection of Leptospira Spp. in Urine, J. Clin. Microbiol., 2012, 50, 2072–2074 CrossRef CAS PubMed.
- P. Sonthayanon, W. Chierakul, V. Wuthiekanun, J. Thaipadungpanit, T. Kalambaheti and S. Boonsilp, et al., Accuracy of Loop-Mediated Isothermal Amplification for Diagnosis of Human Leptospirosis in Thailand, Am. J. Trop. Med. Hyg., 2011, 84, 614–620 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4an01526f |
| ‡ Equal contributions. |
| This journal is © The Royal Society of Chemistry 2025 |