Open Access Article
Open Access ArticleMonitoring plastic pollution using bioindicators: a global review and recommendations for marine environments
Matthew S.
Savoca
 *ab,
Neil Angelo
Abreo
c,
Andres H.
Arias
*ab,
Neil Angelo
Abreo
c,
Andres H.
Arias
 de,
Laura
Baes
f,
Matteo
Baini
de,
Laura
Baes
f,
Matteo
Baini
 gh,
Elisa
Bergami
hi,
Susanne
Brander
j,
Miquel
Canals
gh,
Elisa
Bergami
hi,
Susanne
Brander
j,
Miquel
Canals
 klm,
C. Anela
Choy
klm,
C. Anela
Choy
 n,
Ilaria
Corsi
n,
Ilaria
Corsi
 gh,
Bavo
De Witte
o,
Camila
Domit
p,
Sarah
Dudas
gh,
Bavo
De Witte
o,
Camila
Domit
p,
Sarah
Dudas
 q,
Emily M.
Duncan
q,
Emily M.
Duncan
 r,
Claudia E.
Fernández
s,
Maria Cristina
Fossi
r,
Claudia E.
Fernández
s,
Maria Cristina
Fossi
 gh,
Ostin
Garcés-Ordóñez
gh,
Ostin
Garcés-Ordóñez
 ktu,
Brendan J.
Godley
ktu,
Brendan J.
Godley
 r,
Daniel
González-Paredes
r,
Daniel
González-Paredes
 vw,
Victoria González
Carman
vw,
Victoria González
Carman
 xy,
Bonnie M.
Hamilton
xy,
Bonnie M.
Hamilton
 z,
Britta Denise
Hardesty
z,
Britta Denise
Hardesty
 aa,
Sang Hee
Hong
aa,
Sang Hee
Hong
 ab,
Shirel
Kahane-Rapport
ac,
Lauren M.
Kashiwabara
j,
Mariana Baptista
Lacerda
ad,
Guillermo
Luna-Jorquera
aeaf,
Clara
Manno
ab,
Shirel
Kahane-Rapport
ac,
Lauren M.
Kashiwabara
j,
Mariana Baptista
Lacerda
ad,
Guillermo
Luna-Jorquera
aeaf,
Clara
Manno
 ag,
Sarah E.
Nelms
ag,
Sarah E.
Nelms
 r,
Cristina
Panti
r,
Cristina
Panti
 gh,
Diego J.
Pérez-Venegas
ah,
Christopher K.
Pham
ai,
Jennifer F.
Provencher
z,
Sara
Purca
gh,
Diego J.
Pérez-Venegas
ah,
Christopher K.
Pham
ai,
Jennifer F.
Provencher
z,
Sara
Purca
 aj,
Harunur
Rashid
aj,
Harunur
Rashid
 ak,
Yasmina
Rodríguez
ai,
Conrad
Sparks
ak,
Yasmina
Rodríguez
ai,
Conrad
Sparks
 al,
ChengJun
Sun
al,
ChengJun
Sun
 am,
Martin
Thiel
am,
Martin
Thiel
 aeafan,
Catherine
Tsangaris
ao and
Robson G.
Santos
aeafan,
Catherine
Tsangaris
ao and
Robson G.
Santos
 ap
ap
aHopkins Marine Station, Stanford University, Pacific Grove, CA, USA. E-mail: msavoca13@gmail.com
bCalifornia Marine Sanctuary Foundation, Monterey, CA, USA
cCollege of Health Sciences, Mapua Malayan Colleges Mindanao, Philippines
dDepartamento de Química, Universidad Nacional del Sur, Bahía Blanca, 8000, Argentina
eInstituto Argentino de Oceanografía (IADO), CONICET, Argentina
fLaboratório de Ecologia de Interações, Departamento de Ecologia e Biologia Evolutiva, Universidade Federal de São Carlos, São Carlos, SP, Brazil
gDepartment of Physical, Earth and Environmental Sciences, University of Siena, Via P.A. Mattioli, 4, Siena, Italy
hNBFC, National Biodiversity Future Center, Palermo 90133, Italy
iDepartment of Life Sciences, University of Modena and Reggio Emilia, Italy
jDepartment of Fisheries, Wildlife and Conservation Sciences, Oregon State University, USA
kSustainable Blue Economy Chair, CRG Marine Geosciences, Department of Earth and Ocean Dynamics, Earth Sciences Faculty, University of Barcelona, E-08028 Barcelona, Spain
lReial Acadèmia de Ciències i Arts de Barcelona (RACAB), La Rambla 115, 08002 Barcelona, Spain
mInstitut d’Estudis Catalans (IEC), Secció de Ciències i Tecnologia, Carme 47, 08001 Barcelona, Spain
nScripps Institution of Oceanography, University of California San Diego, La Jolla, CA, USA
oAquatic Environment and Quality, Animal Sciences Unit, Flanders Research Institute for Agriculture, Fisheries and Food, Ostend, Belgium
pLaboratório de Ecologia e Conservação, Universidade Federal do Paraná, PR, Brazil
qFisheries and Oceans Canada, British Columbia, Canada
rCentre for Ecology and Conservation, University of Exeter, Penryn Campus, Penryn, Cornwall TR10 9EZ, UK
sEscuela de Ciencias Biológicas, Universidad Nacional, Heredia, Costa Rica
tMarine Environmental Quality Research Group, Marine and Coastal Research Institute José Benito Vives de Andréis – INVEMAR, Santa Marta, Colombia
uGrupo de Investigación Territorios Semiáridos del Caribe, Universidad de La Guajira, Colombia
vJames Cook University, QLD, Australia
wKarumbé NGO, Montevideo, Uruguay
xInstituto de Investigaciones Marinas y Costeras (CONICET-UNMdP), Mar del Plata, Argentina
yInstituto Nacional de Investigación y Desarrollo Pesquero (INIDEP), Mar del Plata, Argentina
zEnvironment and Climate Change Canada, Science, Technology Branch, Ottawa, Canada
aaCSIRO Environment, Hobart, Tasmania, Australia
abRisk Assessment Research Center, Korea Institute of Ocean Science and Technology, Geoje, Republic of Korea
acDepartment of Biological Sciences, Old Dominion University, USA
adLaboratório de Ecologia e Conservação, Universidade Federal do Paraná, PR, Brazil
aeDepartamento de Biología Marina, Facultad Ciencias del Mar, Universidad Católica del Norte, Larrondo 1281, Coquimbo, Chile
afCenter for Ecology and Sustainable Management of Oceanic Island (ESMOI), Coquimbo, Chile
agBritish Antarctic Survey, Natural Environment Research Council, Cambridge, UK
ahCentro de Investigación y Gestión de Recursos Naturales (CIGREN), Instituto de Biología, Facultad de Ciencias, Universidad de Valparaíso, Valparaíso, Chile
aiInstituto de Investigação em Ciências do Mar – OKEANOS, Universidade dos Açores, 9900-138 HORTA, Portugal
ajInstituto del Mar del Peru (IMARPE), Callao, Peru
akDepartment of Fisheries Management, Bangladesh Agricultural University, Mymensingh-2202, Bangladesh
alCentre for Sustainable Oceans, Cape Peninsula University of Technology, South Africa
amMarine Bioresource and Environment Research Center, First Institute of Oceanography, Ministry of Natural Resources, China
anMarineGEO Program, Smithsonian Environmental Research Center, Edgewater, Maryland, USA
aoInstitute of Oceanography, Hellenic Centre for Marine Research (HCMR), Anavyssos 19013, Greece
apInstituto de Ciências Biológicas e da Saúde, Universidade Federal de Alagoas, Maceió, AL, Brazil
First published on 3rd October 2024
Abstract
Monitoring the movement of plastic into marine food webs is central to understanding and mitigating the plastic pollution crisis. Bioindicators have been a component of the environmental monitoring toolkit for decades, but how, where, and which bioindicators are used in long-term monitoring programs has not yet been assessed. Moreover, these programs have yet to be synthesized and evaluated globally. Doing so is imperative if we are to learn from these pioneering programs and expand on their efforts. We reviewed global monitoring programs using bioindicators that focus on plastic pollution and found 11 worldwide that met our definition of long-term monitoring. Limited data availability and few programs in the Global South hinder progress on tracking global trends. Most commonly, long-term programs either tracked macroplastics with opportunistic sampling of large vertebrates or monitored microplastics with targeted sampling of invertebrates. These long-term bioindicators could be incorporated as essential ocean variables in the global ocean observing system, and thus provide critical insights into the trajectory and effects of plastic pollution on marine ecosystems. However, to enhance the effectiveness and inclusivity of these monitoring efforts, there is a pressing need for the implementation of harmonized and standardized methods, increased collaboration between regions, and greater support for data sharing and open science practices. By addressing these challenges and expanding the geographic scope of monitoring programs, we can better inform evidence-based policies and interventions aimed at mitigating plastic pollution on a global scale.
Environmental significancePlastic pollution is a threat to ecosystems worldwide. Understanding its extent and impacts is essential for effective mitigation efforts. Our review highlights the scarcity of long-term monitoring programs using bioindicators, crucial tools for tracking plastic pollution's effects on marine food webs. By synthesizing existing initiatives and identifying gaps, we underscore the urgent need for standardized methodologies, enhanced collaboration, and data sharing to strengthen global monitoring efforts. Incorporating bioindicators as essential ocean variables in the global ocean observing system can provide invaluable insights into plastic pollution trends and its ecological consequences. Strengthening these monitoring frameworks will inform evidence-based policies that address the plastic pollution crisis in the global ocean. |
Introduction
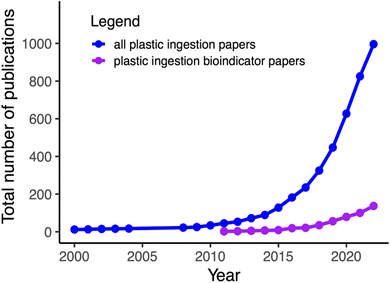
Over the past decade, plastic items of various morphologies (e.g., macro litter items, fragments, fibers, beads, etc.) and sizes—macro (>25 mm in the longest dimension), meso (25–5 mm), micro (5 mm−1 μm), and nano (<1 μm)—have emerged as pervasive pollutants of global concern due to their negative impacts on organisms, ecosystems, and human livelihoods.1,2 Marine fauna were among the earliest sentinels through which people became aware of marine plastic debris (e.g., Laysan albatross, Phoebastria immutabilis3). Several years later, in the early 1970s, plastic “micro-spherules,” also known as nurdles, were identified in ocean water and in the ingesta of eight fish species.4 Over the past decade, hundreds of studies have reported plastic ingestion by more than 1000 marine and coastal species5 (Fig. 1). These studies have provided valuable data and a deep understanding of the affected species and have highlighted some of those that might serve as bioindicators of plastic pollution in marine food webs. However, much of this early research occurred before standardized protocols, methods, and reporting guidelines were established and agreed upon for a variety of taxa, limiting the ability to compare findings across spatial and temporal scales. Standardization is crucial for evaluating progress and for designing and integrating bioindicators into monitoring systems at local, regional, and global scales.6 In recent years, there have been numerous recommendations for these methodologies and guidelines for a variety of taxa.7–10Currently, the United Nations Environment Programme (UNEP; see Box 1 for acronym descriptions and organization links) is developing a legally binding international agreement, known as the UN Plastics Treaty, aimed at eliminating plastic pollution (https://www.unep.org/inc-plastic-pollution). This treaty adopts a comprehensive approach to managing the entire life cycle of plastics, with the goals of safeguarding human health, protecting the environment, and promoting sustainable development. As the UN Plastics Treaty moves closer to finalization and ratification, the need for bioindicators becomes increasingly clear. Bioindicators are vital for assessing the spread and impacts of plastic pollution in the food chain, understanding how ingested plastic affects individuals and populations, and evaluating the effectiveness of legislative measures in addressing these impacts. This urgency aligns with the objectives of the UN Decade of Ocean Science for Sustainable Development, particularly Challenge 01: “to end marine pollution of all kinds, including plastic and nutrient pollution”. The Ocean Decade provides a framework for concerted global action to address the challenges facing our ocean, emphasizing the importance of collaborative efforts and scientific innovation.
Here, as a diverse group of international plastic pollution scientists, we conduct a horizon scan on the global state of plastic pollution monitoring programs using bioindicators for plastic ingestion. We identify what species are used, the plastic size classes they monitor, and the ecosystem compartments they surveil. Finally, we assess roadblocks and suggest solutions to chart a path forward. We hope this will serve as an initiative toward coordinated monitoring and standardized reporting of marine plastic pollution using bioindicators.
Different types of monitoring
Aligning the type of monitoring needed with scientific or policy goals is fundamental to program development and indicator species selection. Baseline mapping is conducted prior to monitoring to establish the initial levels of the stressor. The results serve as a starting point for studying spatial and temporal trends. Results from pollutant baseline studies are rarely zero in the modern world, and this is especially true for plastic pollution. After a baseline is established for the region or system, monitoring with standardized or harmonized methods can take several forms (Box 2).As part of monitoring program initiation, effort should be taken to set the best possible benchmarks to compare with future data collections. Ideally, monitoring also delivers data to practitioners and the wider community in a timely fashion to be relevant to informing management decisions, ideally via an online and close-to-real time database platform. If and how monitoring data are used in management depends on local or regional policies. Designing programs that align with the concerns of relevant rightsholders and stakeholders will maximize the use of the information. For monitoring using bioindicators, this includes appropriate selection of the species that capture the key process(es) of interest while balancing ethical and logistical considerations on species collections.
Monitoring details to consider
The Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection (GESAMP) – a group of independent scientific experts that provides advice to the UN system on scientific aspects of marine environmental protection – emphasizes tailoring strategies, protocols, and indicators to specific questions, often driven by policy considerations, and advocates for flexible, situation-specific approaches.11 Detecting changes of interest (e.g., effect size, morphology) should guide the sampling effort to capture spatial and temporal trends, accounting for inherent variability. To identify trends and assign risk, monitoring studies need to report ingestion data beyond the frequency of occurrence (e.g., selectivity, rate of ingestion, egestion, effects on organism health) whenever possible. In addition to counts, plastic ingestion studies should ideally report shapes (i.e., morphology), sizes including minimum detection limit, polymer types, and mass; however, estimating mass for items smaller than mesoplastics may be impracticable, and particle counts may be used instead.12 Importantly, if polymer type or shape are important characteristics to address management questions, then monitoring programs need to be designed to capture these metrics (e.g., polymer types are being considered within the UN plastics treaty framework).Similarly, the frequency of data collection must consider ecological, financial, and logistical factors as well as the regulatory or management drivers for monitoring. In certain cases, monitoring frequency is determined by the bioindicator's phenology, as is the case for many seabirds, where numerous specimens can be collected during the nesting period at breeding colonies.13 Where resources are limited, even monitoring once every several years can yield important findings. Ecologically and economically, monitoring coastal and epipelagic areas is easier comparatively to more inaccessible systems such as the meso- and bathypelagic zones. Harder-to-monitor ecosystems and threatened species or those predicted to be at high risk should be prioritized for baseline assessments, whereas it is likely that only systems and species that are relatively easy to sample—either because of accessibility, abundance, or both—are candidates for long-term monitoring.
The success of a monitoring program often lies in using cost-effective methods while simultaneously ensuring high reliability and accuracy of the results. Some studies have tested and implemented different methods of sample collection and processing to explore the time and fiscal costs and implications that accompany choices within monitoring frameworks. For example, Brawn et al. compared lab-based methods used to detect microplastics in fish for differences in detection, cross-contamination, fiscal costs, and human resource time.14 Numerous studies have compared field methods in relation to particle number, size, and morphology.15,16 Thus, a critical analysis of field and lab methods needs to be included when a monitoring program is considered.
Selecting bioindicator species
For decades, biota have served as sentinels of environmental pollution, including plastic pollution.12,17,18 These species accumulate plastic particles in relation to their environmental abundance and are accessible for studies at the scale of interest to facilitate sample acquisition and the development of robust datasets for trend analyses. Other considerations for a good bioindicator may include the availability of past data or specimens (e.g., via archived specimen banks) to assess trends more quickly at the start of a program, and if possible, a small home range so pollution can be tracked to a specific location.To select the optimal bioindicator species, knowledge and understanding of plastic occurrence in a given species is critical.19–23 Plastics can be detected in a large variety of species, including invertebrates such as mollusks, arthropods, annelids, echinoderms, and a variety of smaller, even microscopic, zooplankton, as well as vertebrates such as fishes, marine turtles, birds, or mammals across life stages. The size, shape, and number of plastic items that can be found in a single individual is also dependent on the body size, feeding behavior, and digestive morphology and retention of the species. Selection of the appropriate bioindicator species is therefore inherently linked to the size and shape of plastic particles under consideration by the monitoring program. The spatial and temporal scales and areas for sampling should also be clearly defined. For example, ingestion of plastics can vary seasonally and may reflect significant shifts in environmental conditions.
Within the UN Sustainable Development Goal (UN SDG) 14 “Life Below Water”, target 14.1 states: “by 2025, prevent and significantly reduce marine pollution of all kinds, in particular from land-based activities, including marine debris and nutrient pollution”. It explicitly calls for indicators to monitor progress towards these goals. Moreover, it establishes a tier system to explain and assess the levels of indicator development and application:
• Tier 1: “Indicator is conceptually clear, has an internationally established methodology and standards are available, and data are regularly produced by countries for at least 50% of countries and of the population in every region where the indicator is relevant”.
• Tier 2: “Indicator is conceptually clear, has an internationally established methodology and standards are available, but data are not regularly produced by countries”.
• Tier 3: “No internationally established methodology or standards are yet available for the indicator, but methodology/standards are being (or will be) developed or tested”.
Several regional organizations have identified ideal bioindicator species. The North Pacific Marine Science Organization (PICES) and the Arctic and Assessment Monitoring Program (AMAP) have undertaken reviews of plastic ingestion at regional scales, and then used standardized processes to recommend species for plastic pollution monitoring.22,24 Similarly in the North Atlantic, the Oslo/Paris (OSPAR) Convention for the Protection of the Marine Environment of the North-East Atlantic has implemented long-term monitoring programs to address the abundance, trends, distribution, and composition of plastics, and a Regional Action Plan (RAP) for marine litter prevention and management.25 In parallel, the European Union (EU) Marine Strategy Framework Directive (MSFD) has developed specific guidelines for harmonized monitoring of marine litter requiring EU Member States to report information about this contaminant in EU waters.26 Other initiatives in the European region involve international collaborative projects in which systematic reviews have been done to identify analytical methods for plastic determination in environmental matrices, including biota.27,28
A global review of monitoring programs by region
Arctic Ocean
In 2017, AMAP released an assessment of chemicals of emerging concern in the Arctic, including plastics pollution. In 2019, the Arctic Council's working group Protection of the Arctic Marine Environment (PAME) conducted a desktop study on marine litter including microplastics in the Arctic, representing the first Arctic-wide evaluation of the occurrence and impacts of plastic pollution across the circumpolar North (PAME, 2019). That study highlighted the need to create a regional action plan on marine litter in the Arctic. AMAP's Litter and Microplastics Expert Group (LMEG) was formed in 2019 with the aims to: (a) design a monitoring program for plastic pollution in the Arctic environment, (b) develop necessary guidelines for this monitoring program, and (c) create recommendation frameworks and identify areas of future research priorities.AMAP-LMEG has since released the Litter and Microplastics Monitoring Plan, which provides recommendations that will lead to a coordinated, ecosystem-scale pan-Arctic monitoring program to collect information for future spatial and temporal assessments. This monitoring program includes priority monitoring compartments, including seabirds, while other biota groups were recommended for further baseline and methodology work before implementation of widespread monitoring programs.29 Following this, LMEG also released the Litter and Microplastics Monitoring Guidelines, a technical document that reviews litter and microplastics protocols and research techniques paired with technical recommendations for harmonized monitoring efforts across the Arctic.30 The technical guidelines include recommendations for sampling methods including sample sizes, locations, and frequency, as well as advice on sample processing and data handling, which reflect published harmonized protocols.
While international bodies such as the Arctic Council's AMAP-LMEG have released monitoring guidelines and recommendations, these efforts are coordinated at the national level. Some Arctic countries such as Norway and Sweden use northern fulmars (Fulmarus glacialis) as bioindicator species to monitor plastic pollution in the region, following the OSPAR Convention.31 The target size of plastics pollution using northern fulmars is >1 mm. However, while pan-Arctic recommendations are in place, there is a need for coordinated biotic, ecosystem-level monitoring efforts,24,32 both above and below the Arctic circle.
Mediterranean Sea
In the Mediterranean Sea, several EU projects have supported two main legislative frameworks: the European MSFD (2008/56/EC, and in particular descriptor 10, criteria C3) and the Integrated Monitoring and Assessment Programme (IMAP),33 adopted by COP 19 of the UN Environment Program-Barcelona Convention (Decision IG.22/7). The MSFD – IMAP monitoring programs include assessments of plastic ingestion. Several Mediterranean countries including Spain, France, Italy, Slovenia, Croatia, Greece, and Cyprus implemented national plastic ingestion monitoring programs under MSFD. The selection of bioindicator species varies among countries and includes the loggerhead sea turtle (Caretta caretta)34 as well as fish species such as the red mullet (Mullus barbatus) and the bogue (Boops boops).20 Loggerhead turtles are designated as a bioindicator species both by the MSFD and the Barcelona Convention and are the most widely used indicator in the region. Their implementation as an indicator of marine litter was supported by the EU funded INDICIT and INDICIT II projects (Implementation of Indicators of Marine Litter on Sea Turtles and Biota in Regional Sea Conventions and Marine Strategy Framework Directive Areas), which collected a large set of standardized data on ingested litter by sea turtles in the Mediterranean region (Spain, Italy, France, Greece, Cyprus, Turkey and Tunisia).35These projects supported existing and newly forming networks for measuring litter impacts on loggerhead sea turtles, developed a standard protocol for data collection,36 and compiled a harmonized dataset on 1121 necropsied turtles.35 The dataset consisted of historic data collected from 1988 and standard data collected from 2016 in eight Mediterranean and North-East Atlantic countries and showed ingested litter and ingested plastic in 69.2% and 56.6% of the sampled turtles, respectively. However, an important consideration for future monitoring is the inclusion of green turtles (Chelonia mydas) for the eastern Mediterranean, which will reveal interspecific differences in frequency of ingestion.34
Additional projects were implemented in the Mediterranean area, including the Plastic Busters MPAs (coastal marine protected areas) InterregMed project, which defined and implemented a harmonized approach against marine litter. The project addressed the overarching management cycle of marine litter, from monitoring and assessment to prevention and mitigation, as well as actions to strengthen networks between and among pelagic and coastal MPAs. The project applied marine litter monitoring approaches using common bioindicator species (up to 46 marine species in four pilot areas across the Mediterranean Basin), which also included endangered species (cetaceans, monk seals, sea turtles, seabirds, and elasmobranchs) and commercially harvested species (invertebrates and fish), sampled inside and outside the MPAs.37,38 Risk analysis in hotspot areas and MPAs for each habitat and ecological compartment was conducted with a threefold monitoring approach: (1) analysis of gastro-intestinal content to evaluate the marine litter ingested by the organisms, (2) quantitative and qualitative analysis of plastic additives, and (3) analysis of the effects of litter ingestion by biomarker responses at different levels of biological organization.19
North Atlantic
Species-focused monitoring programs have been instituted in the Northeast Atlantic to support OSPAR and the European MSFD. Monitoring of plastic ingestion by the northern fulmar began in the 1980s in the Netherlands.39 Officially implemented as an bioindicator species in 1992,40 it continues to be used to monitor floating plastic litter by several countries in the North Sea region based on a sample size of 50–100 individuals over at least five years.41 This enduring initiative has played a pivotal role in assessments within the OSPAR framework and, more recently, the MSFD.40 In 2015, the loggerhead sea turtle (Caretta caretta) was also suggested as a bioindicator species for southern Europe,35,42 with a proposed minimum of 30 individuals per year in the OSPAR region.43 However, its widespread implementation is pending due to insufficient availability of turtle casualties per annum in some of the OSPAR region.The Portuguese administration initiated a long-term monitoring program in 2015 that considered Cory's shearwaters (Calonectris borealis) as a target species in the reporting descriptor 10[1]—“properties and quantities of marine litter do not cause harm to the coastal and marine environment”—under the MSFD.44 A recent analysis of the data obtained from this program concluded that Cory's shearwaters fledglings have many favorable characteristics—such as regular ingestion of plastic debris and ease of access of specimens that died of other causes—to act as a bioindicators for both OSPAR and the MSFD throughout its breeding range, which is beyond the distribution range of northern fulmars. A detailed assessment of more than 1200 deceased birds over eight years supported the definition of plastic ingestion metrics, and essential parameters including target age, collection methodology, sampling approach, and a threshold value.45 In southwestern Europe where the two previous bioindicators are not present, the monitoring of several seabird species allowed the identification of other potential bioindicators to support the MSFD. The common guillemot (Uria aalge) and the Atlantic puffin (Fratercula arctica) are the most suitable candidates, with the northern gannet (Morus bassanus) also having the potential to act as an indicator specifically to track fishing activities.46
In the Caribbean region, studies have measured the presence and the effects of microplastics in various groups of marine organisms, such as fish,47–49 mollusks,50 crustaceans,51 echinoderms,52 and nematodes.53 However, no region-wide, regular monitoring program of any given marine species is yet in place. In collaboration with the International Atomic Energy Agency (IAEA), research groups from 18 countries in the region are currently working on the development of technical capabilities to harmonize methodologies for monitoring microplastics in both marine organisms and the environment. The goal is reporting on environmental indicators within the Research Network on Marine-Coastal Stressors in Latin America and the Caribbean (REMARCO) (https://remarco.org/contaminacion-por-microplasticos/). Additionally, in Colombia, the Marine and Coastal Research Institute (INVEMAR) coordinates the national marine environmental quality monitoring network (REDCAM) (https://siam.invemar.org.co/redcam), which semi-annually monitors various contaminants, including microplastics, in water, sediments, and organisms. While the current focus of microplastic monitoring is abundance and characteristics in habitats, future efforts may include monitoring these pollutants in commercially important fish and mollusks.
Canada mirrored OSPAR's northern fulmar monitoring in its own Canadian Environmental Sustainability Index program (ECCC 2020). The range of the northern fulmar in Canada extends from the Arctic to the southern border, with annual collections of fulmars occurring as far south as Sable Island, Nova Scotia, which provides coverage along the Atlantic–Arctic gradient in the region. This program reports on plastic particles above 1 mm. While polymer type has not been a focus of efforts so far, to align with policy needs, all future reporting will include polymer types of the particles detected.
South Atlantic
In Brazil, a Beach Monitoring Program (PMP) of stranded megafauna (seabirds, sea turtles, and marine mammals) was established in 2009,54 expanding to reach more than 3000 km of coastline in 2015. In connection with PMP, the Brazilian Institute of Environment and Renewable Natural Resources (IBAMA) has required all necropsied individuals analyzed through that program to be assessed for plastic ingestion. Although the expansion of PMP makes it highly interesting as a potential building block for a future regional plastic ingestion monitoring program, the PMP has some limitations due to data architecture (see Oliveira et al. 2024 for details) and the lack of a formal standardized protocol for data collection regarding plastic interactions.54 Despite this, data collected during PMP have helped to build local baselines regarding plastic pollution identifying two seabird species, the great shearwater (Ardenna gravis) and the white-chinned petrel (Procellaria aequinoctialis), as potential bioindicators in the region (Oliveira et al., 2024).55 In Uruguay, the non-governmental organization (NGO) Karumbé has been monitoring plastic ingestion by green turtles (Chelonia mydas) since 2005.56,57 The organization systematically monitors an average of 120 turtles annually, including both dead and live animals, using established procedures and standard methodologies to assess incidence levels, as well as the characterization and quantification of ingested plastics >1 mm.56,57In Argentina, at least five monitoring programs are led by local NGOs or government agencies, most of which began in the last decade. They focus on the occurrence of either micro, meso- or macroplastic on shorelines and in the neritic marine environment, and their primary goal is to establish a baseline and identify trends. However, none of these programs focuses on any indicator species. Monitoring of plastics in a wide variety of marine organisms is conducted by different research groups working individually and on an opportunistic basis.58–61 Recently, efforts to identify marine megafaunal species as potential indicators of plastic pollution were performed in the Río de la Plata and adjacent waters of Argentina and Uruguay.58 These monitoring programs and research groups could provide the foundations for a future, country-wide plastic monitoring plan using bioindicators while taking advantage of preexisting field logistics. Shared protocols would be needed, and additional base level funds furnished.
In the Southeast Atlantic region, namely along the western coast of Africa, baseline studies and assessments have shown interactions between plastics and marine organisms including seabirds, fishes, polychaetes, mussels, and bivalves,62,63 some of which can serve as indicator species.62 Akindele and Alimba (2021) reviewed 59 research articles on the prevalence of plastic pollution in Africa between 1987 and 2020. Of these, 13 (22%) were from West Africa and 25 (42%) from South Africa.63 Within the limited number of publications on the impacts of marine litter on organisms in West Africa, 78% corresponded to South Africa, 12% to Nigeria, 7% to Ghana, and 3% to Mauritania.64 Over time, this region has seen increases in plastic ingestion by loggerhead turtles,65 but no significant changes in plastic ingestion by tube-nosed seabirds.66
South Africa is the only country with an ongoing plastic monitoring program using bioindicator species in the Southeast Atlantic region. The Microplastics Laboratory at the Cape Peninsula University of Technology has been monitoring microplastics in sediments and the Mediterranean mussel (Mytilus galloprovincialis) since 2021. Mussels are collected during the dry and wet seasons from three sites representing the warmer waters of the south coast of Cape Town (Strandfontein), the colder waters of Table Bay (Lagoon Beach), and an industrial/aquaculture harbor/bay, 100 km north of Cape Town (Saldanha Bay).
North Pacific
Along the North American west coast, plastic ingestion by marine fauna has been well documented across all major taxonomic groups. The majority of data comes from herbivores, forage species, and mesopredators,67–72 with less data from top predators.73,74 There are a variety of sampling programs in place where plastic ingestion is measured. Every five years, the Southern California Coastal Water Research Project (SCCWRP) coordinates the Southern California Bight Regional Monitoring Program (SCBRMP).75 More recently, this collaboration has resulted in ongoing efforts to standardize sampling protocols for microplastics across different environmental matrices. While the SCBRMP has been monitoring macrodebris opportunistically since its inception in 1994, systematic sampling of plastic ingestion by bioindicator species, specifically oysters (Crassostrea gigas) and mussels (M. californianus and/or M. galloprovincialis), only began in 2023.76 In British Columbia the program ‘PollutionTracker’ was established in 2015 and included microplastic monitoring in blue mussels (M. edulis).77In the coastal northeast Pacific (Oregon, Washington, British Columbia, and Alaska), the resources available to establish new longer-term programs for plastic pollution monitoring are currently sparse and regionally focused. However, there may be opportunities to leverage ongoing sampling for other contaminants, such as the National Oceanic and Atmospheric Administration's (NOAA) Mussel Watch program (https://coastalscience.noaa.gov/science-areas/pollution/mussel-watch/). Published research on occurrence in biota and other environmental matrices has often relied on strong collaborations with state agencies, such as the Oregon Dept. of Fish and Wildlife's Marine Reserves program,78 which already sample regularly in potentially impacted areas. For macro debris, the longstanding Coastal Observation and Seabird Survey Team (COASST; https://coasst.org) could sample plastic debris ingested by northern fulmar, one of their most commonly collected species and a well-established plastic indicator species.40,79
Across the North Pacific, NOAA's National Seabird Program collects hundreds of seabirds that perish as bycatch in commercial fishing operations each year. Many of these birds either have, or could be, monitored for plastic ingestion.80 Species that are regularly caught include fulmars as well black-footed (P. nigripes) and Laysan albatrosses that forage across the North Pacific and breed in Hawaii. All three species have been highlighted as having high potential to be excellent bioindicators of plastic ingestion;22 however, no official monitoring programs on these species exist in this region outside of Canada.
There are several ongoing monitoring programs in the subtropical North Pacific. One uses the longnose lancetfish (Alepisaurus ferox), which is a common bycatch species in the Hawaii-based longline tuna fishery (Fig. 2). This program is a collaboration of the Scripps Institution of Oceanography and NOAA's Pacific Islands Regional Office (PIRO) and the Pacific Islands Fisheries Science Center (PIFSC), with regular collections by fishery observers beginning in 2014 (Table 1). Each monthly collection of lancetfish specimens yields about 20–25 stomachs, providing valuable data on plastic ingestion over large temporal and spatial scales. Observers record fish length, date/time, and a general location. In the laboratory, stomachs are defrosted and visually examined for diet contents. Plastic items are sorted, categorized, counted, weighed, and measured for further analysis, following established protocols.83–85 Another long-term program based in the tropical North Pacific is the Biological and Environmental Monitoring and Archival of Sea Turtle Tissues (BEMAST) that began collecting samples annually in 2012 (ref. 82) (Fig. 2 and Table 1). BEMAST is a collaboration between the NOAA Longline Observer Program, PIFSC, the U.S. Geological Survey (USGS), and the National Institute of Standards and Technology (NIST). Samples are collected year-round from stranding or fisheries bycatch monitoring programs. Recommended sample size is at least 20 individuals per species per sampling method per year, if possible.22
 | ||
| Fig. 2 Species used as plastic ingestion bioindicators around the world. 1: Loggerhead sea turtle Caretta caretta, one of five species used in the BEMAST and KIOST/MABIK/NIE programs, and also by MMA/IBAMA and the MSFD. 2: Longnose lancetfish Alepisaurus ferox, used by NOAA/UCSD program. 3: Blue mussel Mytilus edulis, used by PollutionTracker and KOEM. 4: Northern fulmar Fulmarus glacialis, used by AMAP and OSPAR. 5: Cory's shearwater Calonectris borealis, used by the MFSD. The North Pacific has the most bioindicator monitoring programs of any large marine region. There are currently no known monitoring programs from the South Pacific, Indian, or Southern oceans. See Table 1 for more details on the monitoring programs using these bioindicators. World map from https://marineregions.org/sources.php. | ||
| Name of program (organizational lead) | Region | Year started | Species monitored | Collection frequency | Plastic size monitored | Data availability | UN tier classification for global indicators | Major program findings to date | References |
|---|---|---|---|---|---|---|---|---|---|
| Plastic particles in fulmar stomachs in the North sea (OSPAR) | Northeast Atlantic Ocean; North Sea | 1992 | Northern fulmar (F. glacialis) | 100 individuals per region per year | Macro-, meso-, micro- (>1 mm) | https://oap.ospar.org/en/ospar-assessments/quality-status-reports/qsr-2023/indicator-assessments/plastic-in-fulmar/ | 1 | Decrease in industrial plastics, increase in user plastics; projected MSFD threshold reduction achieved by mid-century | 39–41 |
| Plastic particles in fulmar stomachs in Canada (ECCC) | Arctic Ocean; Northwest Atlantic Ocean; Northeast Pacific Ocean | 2008 | Northern fulmar (F. glacialis) | 40 individuals per region annually | Macro-, meso-, micro- (>1 mm) | https://www.canada.ca/en/environment-climate-change/services/environmental-indicators/plastic-particles-northern-fulmar.html | 1 | Canadian fulmars ingest less plastic as compared to fulmars in the European Arctic | 24, 30 and 81 |
| BEMAST (NIST/NOAA/USGS) | Northeast Pacific Ocean | 2012 | Loggerhead sea turtle (Caretta caretta), Green sea turtle (Chelonia mydas), leatherback sea turtle (D. coriacea), olive ridley sea turtle (L. olivacea), hawksbill sea turtle (E. imbricata) | 20 individuals per species annually | Macro-, meso-, micro- (>1 mm) | NA | 2 | Plastic ingestion prevalence highest in olive ridley sea turtles, Green sea turtles ingest the most plastic | 82 |
| Beach monitoring project, PMP (MMA/IBAMA) | Southwest Atlantic Ocean | 2013 | All megafauna species, resident and migratory | Daily/opportunistic | Meso-, macro- | https://simba.petrobras.com.br/simba/web/sistema/ | 2 | 54 | |
| Longnose lancetfish trophic and plastic monitoring in the central North Pacific pelagic ecosystem (NOAA PIFSC/UCSD Scripps Institution of Oceanography) | Northeast Pacific Ocean; North Pacific Subtropical Gyre | 2014 | Longnose lancetfish (A. ferox) | Monthly | Meso-, macro- | https://www.fisheries.noaa.gov/inport/item/70277 | 2 | Approximately one-third of all specimens examined contained meso- and macro-plastics, likely feeding across the water column. No strong trends over time | 83–86 |
| Marine litter ingested by sea turtles (OSPAR/MSFD) | Northeast Atlantic Ocean; Bay of Biscay, Iberian coast | 2015 | Loggerhead sea turtle (C. caretta) | 30 individuals per contracting party annually | Macro-, meso-, micro- (>1 mm) | NA | 2 | Identification of threshold value: “there should be less than 33% of sea turtles having more than 0.05 g of ingested plastic in the GI” | 35, 36, 43 and 87 |
| PollutionTracker (Ocean Wise) | Northeast Pacific coast | 2015 | Blue mussel (M. edulis) | Annually (funding dependent) | Micro (>100μm) | NA | 2 | Polyester microfibers most commonly ingested particle type | 77 |
| Plastic in stomachs of Cory's shearwater fledglings (MSFD) | Northwest Atlantic Ocean | 2015 | Cory's shearwater (C. borealis) | 40 individuals per assessment area/region annually | Meso-, micro- (>1 mm) | NA | 1 | Threshold value exceeded and worsening since 2015; ingested plastic number, but not mass, increasing over time; fisheries identified as a potential source of ingested litter | 45 |
| Sea Turtle conservation joint research (KIOST/MABIK/NIE) | Northwest Pacific Ocean; Korean coast | 2018 | Loggerhead sea turtle (C. caretta), Green sea turtle (C. mydas), leatherback sea turtle (D. coriacea), olive ridley sea turtle (L. olivacea), hawksbill sea turtle (E. imbricata) | 7 to 24 stranded or by-caught individuals, annually | Macro-, meso-, micro- (>20μm) | NA | 2 | Green sea turtles ingest plastic most commonly; some recovered debris items can be sourced to country of origin | 88 |
| Korea National marine Microplastic monitoring program (KOEM) | Northwest Pacific Ocean; Korean coast | 2020 | Oyster (C. gigas), blue mussel (M. edulis) | 50 coastal sites, annually | Micro- (>20μm) | NA | 2 | Fragment-type plastic particles are the most common. The temporal trend remains uncertain | KOEM 2023, annual report on the nationwide survey on coastal microplastic pollution |
| Southern California bight monitoring program (SCCWRP) | Northeast Pacific Ocean; Southern California Bight | 1994; monitoring biota starting in 2023 | Coastal fish and invertebrates | Every 5 years | Macro-, meso-, micro- | https://www.sccwrp.org/about/research-areas/data-portal/ | 2 | NA | 75 |
In the western North Pacific, a growing number of publications has reported on the biomonitoring of plastic ingestion by marine species since 2015. Most biomonitoring efforts have been conducted through short-term research projects. South Korea is the only western North Pacific nation to establish long-term monitoring programs using bioindicators. The monitoring and assessment protocols of plastic debris ingestion (including macro-, meso-, and microplastics) by marine organisms, were developed and established by the Korea Institute of Ocean Science and Technology (KIOST). The selected bivalve bioindicators are oysters (C. gigas), mussels (M. edulis), Manila clam (Ruditapes philippinarum). Considered vertebrates include the blackmouth angler (Lophiomus setigerus), black scraper (Thamnaconus modestus), Swinhoe's storm petrel (Hydrobates monorhis), black-tailed gull (Larus crassirostris), as well as loggerhead (Caretta caretta) and green sea turtles (Chelonia mydas).
In 2020, South Korea initiated the National Marine Microplastic Monitoring Program, encompassing biotic and abiotic matrices. Biotic sampling involves blue mussels (M. edulis) and/or oysters (C. gigas) at 50 coastal sites spanning the western, southern, and eastern coasts of Korea to gain a comprehensive understanding of nationwide status and trends. Alongside bivalves, seawater and seabed sediment are collected at the same sites, linking biota and their abiotic environments. Bivalves and seabed sediment are concurrently collected annually, while seawater is sampled semiannually. The target size range for plastic particles ranged from 20 μm to 5 mm. Abundance, shape, size, polymer types, and color of plastic particles are recorded. The mass of each particle is estimated based on length, width, and polymer type.
The Korea Ocean Environment Management (KOEM) operates this monitoring program on behalf of the Korean government. KIOST, the National Marine Biodiversity Institute of Korea (MABIK), and the National Institute of Ecology (NIE) established a collaborative research team for sea turtle conservation and have conducted joint autopsies on stranded or by-caught sea turtles since 2018.88 KIOST investigates plastic ingestion, MABIK focuses on ecology and genetics, and NIE investigates disease and cause of death. The contents of the esophagus, stomach, small intestine, and large intestine are used for plastic analysis. Weight, shape, size, weight, color, polymer type, and origin (if possible) of each plastic are recorded. The abundance of plastics ingested by sea turtles is reported using both weight and count. Loggerhead and green turtles are the dominant species found in Korean waters, accounting for 94% of the total, while leatherbacks (Dermochelys coriacea), olive ridley (Lepidochelys olivacea), and hawksbills (Eretmochelys imbricata) are found less frequently.
In China, biomonitoring studies have been conducted piecemeal since the first publications on microplastics in bivalves.89,90 The State Oceanic Administration of China conducted a pilot microplastic monitoring in bivalves in 2016 and reported the results in the Bulletin of China Marine Environmental Status. Since then, bivalves such as mussels, oysters, and clams have been frequently studied as microplastic bioindicators.91–94 Fish, barnacles, and seabirds have also been investigated.95–98 The China National Center for Food Safety Risk Assessment also carried out microplastic monitoring on seafood from 2021–2022. There might be other on-going biomonitoring programs, but no open access information is available so far.
In Japan, a robust legal and policy framework has been established to combat marine plastics pollution. Key initiatives include the Act on Promoting the Treatment of Marine Debris (enacted in 2009 and amended in 2018), the Japan Action Plan for Marine Plastic Litter (launched in 2019), and the Act on Promotion of Resource Circulation for Plastics (enacted in 2021). Notably, in August 2023, the Ministry of the Environment (Japan) convened an International Workshop on Marine Debris Data Harmonization. One of this workshop's goals was to facilitate harmonization for developing crucial marine debris indicators, such as biota ingestion and marine life activity levels, aiding in better understanding and mitigating this environmental issue. Despite numerous studies covering the biomonitoring potential of seabirds, fish, and crustaceans for plastic debris,99–102 no long-term programs exist.
Although the marine litter problem has been studied for decades in some regions, many developing countries are only beginning to address the issue. In the Philippines, for example, the National Plan of Action for the Prevention, Reduction and Management of Marine Litter (NPOA-ML) was launched in November 2021. As such, research is currently limited on quantifying marine litter in different coastal habitats, identifying what species ingest marine litter, and establishing baselines.103 Currently, the Philippine government is working with international partners to develop and implement a baseline of mismanaged waste across the Philippines, with an eye to establishment of an ongoing monitoring program. Similarly, other countries within the region are embarking on similar programs, supported by the United Nations Coordinating Body on the Seas of East Asia (COBSEA). These are largely land-based programs, whereas species-specific research projects are less common, and, to our knowledge, there is not a regional-based monitoring program using marine or coastal taxa as bioindicators for plastic pollution.
South Pacific
In the Southeast Pacific, initial efforts were supported by the Comisión Permanente del Pacífico Sur (CPPS), and baseline studies have been conducted for a number of different taxa, including invertebrates,104–106 fishes,107–112 sea turtles,113 seabirds,114–118 and pinnipeds.119,120 However, these are individual, single-year studies. Currently, there is no regular monitoring of plastic ingestion, though some research groups may have sampled their study organisms opportunistically in different years. Several government institutions (IMARPE in Peru, SERNAPESCA & IFOP in Chile, IPIAP in Ecuador, INVEMAR and CIOH-DIMAR in Colombia) conduct regular surveys of gut contents or contaminants (metals, hydrocarbons) of commercial species of fishes, crustaceans, and mollusks, offering the potential for regular monitoring, but this has not yet been established and implemented.Baseline studies in this area indicate high prevalence of microplastics in some bivalves and crabs, which are commercially fished and easily available for monitoring purposes.104,105,121 Fishes have high incidences and plastic loads in Rapa Nui,108,122 and in immediate coastal waters,107,123 whereas small pelagic fishes from the Southeast Pacific, especially the Humboldt Current Large Marine Ecosystem, have very low incidences of plastic ingestion.109,124 All these species (invertebrates and small coastal fishes) are harvested in large quantities for human consumption and/or fishmeal production and would therefore be easily available for monitoring plastic ingestion. Seabirds with a wide distribution range, but different foraging behaviors, can monitor different ecosystems. Storm-petrels that forage in the open ocean have a low incidence of plastic ingestion.125 Monitoring plastic litter from urban areas is conducted by collecting pellets and sampling nests of kelp gulls (Larus dominicanus), which forage in landfills, coves, and urban environments.125–127 The nests of red-legged cormorant (Phalacrocorax gaimardi) and the pellets of Guanay cormorant (Leucocarbo bougainvilliorum), both of which forage in coastal marine environments, can be used to monitor the plastic entering the sea.118,128 In sea lions from the Southeast Pacific, microplastics were found in scats from all studied rookeries, often with high incidence.129 Thus, there are several species that could be useful and easily accessible indicator species for the South–East Pacific. Especially for several of the larger vertebrates, non-invasive sampling techniques (e.g. sampling nests or feces) appears a feasible approach for monitoring interactions with plastics.
In Australia, the national government established a Threat Abatement Plan (TAP) for the impacts of marine debris on vertebrate marine species which falls under the 1999 Environment Protection and Biodiversity Conservation Act (EPBC). Because marine plastic pollution was listed as a ‘key threatening process,’ there is a national imperative to address the potential harm from plastic pollution to threatened vertebrate fauna within Australian waters. A key threatening process is identified as ‘a process that threatens or may threaten the survival, abundance or evolutionary development of a native species or ecological community’.130 Both ingestion and entanglement were specifically listed in the TAP and harmful marine debris is noted explicitly to include both land- and sea-based garbage in addition to recreational and commercial fishing gear, whether lost or discarded intentionally. The TAP also makes specific mention of actions required, including developing an improved understanding of the potential impacts resulting from microplastics and technologies that may aid in improving management of threatened marine vertebrate taxa. Despite the national mandate, there is no corresponding federal program that use bioindicators, though there are multiple individual or independent studies which have assessed plastic ingestion and/or entanglement within or among various taxa, including pinnipeds, sea turtles, and seabirds over the past decade or more.
Indian Ocean
Although there are no dedicated plastic monitoring programs using indicator species in the Western Indian Ocean (WIO), there are substantive efforts to record and monitor marine litter in WIO countries.131 Most monitoring programs in the WIO focus on recording standing stocks and accumulation surveys of coastal litter in sandy beaches. The efforts are mainly the result of work (and funding) by the United Nations Environment Programme/Nairobi Convention Secretariat and the Western Indian Ocean Marine Science Association (WIOMSA), which established a Group of Experts in Marine Litter and Microplastics in 2018. This Expert Group conducted an extensive review on litter and microplastics in the WIO, which was published in 2022.132 The authors reviewed 136 studies in the WIO region and of these, 38% reported on interactions between coastal litter or microplastics and organisms. In addition, there is a growing body of evidence of plastic interaction with large marine vertebrates, but it is often not published or collated at a national level.133 Plastic ingestion was reported in 111 species, including seabirds, turtles, and fish. These authors found that 94% of seabird, 100% of turtle, 100% of bony fish, and 71% of shark species analyzed in the WIO had ingested plastics.132 Microplastics have been found in the guts of coastal (e.g., mussels, oysters, crabs, and sea anemones) and offshore invertebrates (zooplankton) in the WIO region. Most of the invertebrates were reported to have ingested fibers.134 Filter-feeding coastal invertebrates are reported to have higher microplastics ingestion rates, primarily because filter-feeders sieve high volumes of seawater to concentrate filtrates, which makes them well suited as bioindicators.Coastal litter in Africa (including WIO countries) is mainly due to mismanaged waste, ranging from 58% in South Africa135 to 99% in Mozambique.136 Furthermore, the WIO is downstream from Southeast Asia, where western boundary current systems are predicted to significantly increase the amount of mismanaged waste in the WIO. Numerous studies have reported on the concentrations and type (shape, and more recently polymer type) of plastics and microplastics across Africa. However, most of this published research was from South Africa132 indicating the need for a holistic approach to monitoring across the region. To address this need, WIOMSA developed a protocol to monitor litter and microplastics in the coastal region and open ocean of the WIO.
The Marine Litter and Microplastics Project at the National Centre for Coastal Research in India is actively working to monitor and manage plastic pollution in the Indian Ocean and adjacent seas. Key activities include developing methodologies for sampling, analysis, and quantification of marine litter and microplastics; assessing the transport and fate of plastics through numerical modeling and remote sensing; and raising public awareness through citizen science initiatives. The project aims to provide essential data for a National Marine Litter Policy. Key initiatives include generating baseline data on microplastic pollution along the east coast of India, drafting standard operating procedures for sampling and analysis, and conducting beach clean-up and awareness campaigns. Collaborative efforts with international entities like Cefas (UK), CSIRO (Australia), JAMSTEC (Japan), and Norwegian organizations have bolstered their research and mitigation strategies.
There is also research monitoring plastic pollution in Bangladesh. In the Bay of Bengal, scientists funded by the Bangladesh Fisheries Research Institute (BFRI) and the Asia Pacific Network for Global Change Research (APN) have been examining the occurrence of microplastics in fish gastrointestinal tracts. Additionally, a study on transboundary microplastic contaminations in the Sundarbans mangrove region located in the northern Bay of Bengal, is supported by the APN.137 On the southeast coast of Bangladesh, research on microplastic pollution in the Karnaphuli River was also supported by the BFRI.138 These studies highlight international collaborative efforts to tackle microplastic pollution in critical aquatic environments along the Bay of Bengal.138–140
Southern Ocean
Entanglement, ingestion, and widespread reports of microplastics in various ecosystems underscore the alarming extent of plastic pollution in Antarctica and the Southern Ocean.141,142 However, there is no consensus on Antarctic specific sampling methods and bioindicator species.143 To date, Antarctic krill (Euphausia superba), clams (Laternula elliptica), penguins, and albatrosses have been proposed as plastic ingestion bioindicators,23,141,144–146 resulting from evidence of plastic ingestion, their documented sensitivity to other anthropogenic stressors (e.g. fisheries, chemical pollution, and habitat reduction), and their key roles in Antarctic trophic webs. The circumpolar distribution and abundance of Antarctic krill make long-term, comprehensive monitoring relatively easy to carry out.147,148 Albatross and Gentoo penguins (Pygoscelis papua) are valuable bioindicators as well-known mesopredators with the possibility to study non-invasive samples (e.g., scats, regurgitated boluses).141,144,149 Plastic ingestion by brown skuas (Stercorarius antarcticus) show tight relationships with plastic debris exposure,150 suggesting a high bioindicator potential.Antarctica has no specific regulations, nor any continent-wide monitoring program on plastic pollution.151,152 The presence and impacts of macroplastics on marine species, including entanglement, have been monitored by the Commission for the Conservation of Antarctic Marine Living Resources (CCAMLR) since 1989 (https://www.ccamlr.org/en/science/marine-debris). The issue of microplastic pollution was first presented in a CCAMLR Working Group meeting in 2016.153 In 2019, the Committee for Environmental Protection (CEP) of the Antarctic Treaty encouraged parties to reduce plastic pollution in Antarctica and the Southern Ocean (resolution 5, https://www.ats.aq/devAS/Meetings/Measure/705).
The CEP has also identified specific science needs and noted the current lack of plastics monitoring data to inform decision-making. The CEP and the Antarctic Treaty system include a scientific body to provide advice on plastic pollution: the Plastic in Polar Environments Action Group (PLASTIC-AG) (https://www.scar.org/science/plastic/home/) of the Scientific Committee on Antarctic Research (SCAR). The key aims of the PLASTIC-AG are to collate information, establish baselines, understand the impacts of plastic pollution, establish standardized procedures for sampling and monitoring, and propose new measures to reduce and/or limit any potential negative impacts on polar environments.
Conclusion and recommendations
In our global review, we found existing monitoring programs for plastic pollution using bioindicators only in a limited number of regions, specifically the North Pacific (Korean and North American Coasts), and the eastern North Atlantic (Mediterranean and North Seas) (Table 1). In the Southern Hemisphere we found only one long-term monitoring program using bioindicators in the Southwest Atlantic (Table 1). In general, programs that use seabirds or sea turtles focus on mesoplastics (e.g. BEMAST, Plastic Particles in Fulmar Stomachs in the North Sea), while programs that use bivalves monitor microplastics (e.g., PollutionTracker and Korea National Marine Microplastic Monitoring Program).Public data availability for many of these programs are limited or lacking (Table 1). These data, often controlled and fully accessible only to those who work with them, may span decades, with northern fulmar data available in Europe and Canada dating back to the 1990s and the early 2000s, respectively. While we did identify 11 long-term bioindicator programs for plastic pollution (Table 1), most work to date on plastic ingestion bioindicators appears to be initiating or proposing monitoring, using a variety of methods and approaches, with limited traction and funding to implement long-standing programs.
This piecemeal information gained from patchwork research restricts our ability to monitor and understand plastic ingestion as a global phenomenon. Notably, most current indicators are regional in nature, and except for the fulmar, are not compared beyond ocean basin or across more than three large marine ecosystem zones (LMEs). A clear, unifying, and standardized theoretical framework for bioindicator species would be beneficial and could provide useful guidance for practitioners looking to examine trends over time and space. Understanding what makes useful bioindicator species is central to these efforts.11,19,20,22 Once suitable bioindicators are identified, monitoring programs can be designed that are appropriate for the species' ecology, and data collections can be aligned to support regional policies and needs.
OSPAR's use of northern fulmars to monitor plastic in the North Sea is an example of a premier monitoring program.41 Standardized methods have been applied since 1979 and expanded since 2002, examining plastic loads in thousands of individuals, which has allowed long-term trend analyses of small floating plastics in the North Atlantic.13,40,154 With the informal expansion of this bioindicator to Canada in the 2000s, and its formal adoption in 2020, trend analyses of mesoplastic pollution in the North Atlantic, Arctic, and eastern north Pacific are now underway. Other governments have invested in similar long-term programs, as is the case of Portugal and the biomonitoring of Cory's shearwaters since 2015, which has allowed the assessment of spatiotemporal trends of floating plastics and the evaluation of ‘Good Environmental Status’ to respond to the MSFD.11 Several Mediterranean and Atlantic Europe countries have implemented other monitoring programs using the loggerhead turtle as a bioindicator to support the MSFD and OSPAR.35,42,43 Despite its pitfalls, the PMP in Brazil serves as another example of a long-lasting monitoring program that has emerged from collaboration between government and the private sector. However, care must be taken with these types of partnerships; guidelines for independence of the monitoring agents/institutions should be established. BEMAST and the Sea Turtle Conservation Joint Research programs in the North Pacific are other examples of several organizations cooperating to monitor plastic ingestion by sea turtles. We recommend that future programs use these existing examples to learn from past efforts of collaboration.
Evaluating and improving monitoring programs
Only a few bioindicators used in long-term plastic monitoring programs could be considered Tier 2 at present, and even less could be considered Tier 1, according to the UN SDG Target 14.1 (Table 1). Globally, most plastic ingestion bioindicator research falls under Tier 3. Researchers around the world need to coalesce quickly if we are to meet the target for effective monitoring of plastic pollution in marine and coastal environments. Lack of resources hampers these efforts, preventing continuity of monitoring work with access to personnel, expertise, instrumentation, capacity building, and collaboration. A major challenge moving forward will be adequate funding of the labor needed to process samples using acceptable quality control procedures and spectroscopy approaches to accurately identify material types.155 One way forward may be “disaggregated monitoring”, wherein the same species or groups are recommended as bioindicators, and methods are standardized across regions and research teams independently. This has been suggested with mussels, which could serve as a global bioindicator of coastal microplastic pollution.156 Once bioindicators are identified and standardized or harmonized methods are established and adopted, retrospective baseline assessments and trend monitoring can be developed (e.g., government bycatch collections such as the BEMAST repository, species targeted by commercial fisheries, museum collections).The microplastic challenge
Microplastics are extremely pervasive, reaching even the most remote marine ecosystems.151,157 However, the sizes, shapes and colors of microplastics in the ocean is almost as disparate as the natural food sources of the organisms exposed to these microplastics.158 Large marine vertebrates (fishes, sea turtles, seabirds) prefer larger food items and therefore are more likely to ingest microplastics >1 mm in size.7 On the other hand, many invertebrates, especially smaller species and suspension feeders (e.g. mussels and oysters), handle and consume large quantities of small food items such as e.g. microalgae or detritus particles and therefore commonly ingest microplastics ≪1 mm, among which microfibers are very common.While these differences in food preferences and microplastic ingestion offer opportunities for the selection of suitable bioindicator species, they also impose important challenges for monitoring programs. In order to evaluate the microplastic types and loads found in particular organisms, essential information about their foraging biology, energy budget, and retention of indigestible items (e.g. microplastics) is required. The smaller the bioindicator species, the more difficult it is to (i) extract the very small microplastic particles from biological samples, and (ii) reliably confirm that these small particles are indeed synthetic plastics. For example, many microfibers are composed of natural materials like cotton or processed cellulose (e.g., rayon), rather than synthetic polymers,143,159 which calls for highly specific approaches in their extraction and identification.
Since microplastics (as well as larger plastics) often contain chemical additives, including dyes, flame retardants, antimicrobial agents, and UV stabilizers160 and are pervasive across levels of biological organization due to their small size, they can have significant toxicological effects. However, since the extraction of small microplastics (including microfibers) from bioindicators and their polymer characterization is logistically challenging and costly, we recommend to carefully evaluate if the benefits of reporting very small microplastics (including microfibers) are worth the costs in terms of time, effort, and equipment/expertise needed. If small microplastics (≪1 mm) are included, the polymer type must be confirmed with standard methods (e.g. μFTIR),143 and the number of microfibers (synthetic and natural) and the number of plastic fragments should be reported separately.
Benefits of coordinated monitoring over space and time
If the resources are available to apply the appropriate methods for extraction and polymer identification, evaluating trends in plastic pollution in their habitats, including the influence of regional or global policies, is a key benefit of long-term monitoring. There is a growing body of literature demonstrating the value40,79,161 and the pitfalls162–164 of using plastic ingestion by marine fauna as an indicator for plastic concentrations in abiotic compartments of the environment (e.g., water and sediments). Animals are not passive samplers for plastic, and in most cases should not be treated as such. Overall, caution is advised when using bioindicators to evaluate plastic pollution in the environment, as current understanding of the relationship between plastic in the environment and plastic ingestion is still limited for most species. Importantly, monitoring programs need to be fit for purpose, and how information on plastics pollution must be considered within the ecological context. Nevertheless, monitoring plastic ingested by wildlife is important to demonstrate the incorporation of plastics into food webs, which in many cases, lead to people.Improving conservation assessments by considering the threats posed by plastic ingestion will, in turn, benefit from coordinated monitoring.165 Bioindicator species can highlight whether specific polymers (including chemical additives) or item types are preferentially ingested. Regulations and policies can target those especially harmful to wildlife and the natural environment, and often ultimately to human populations. Understanding and monitoring the amount and types of plastic ingested by a given species may help better classify the risk of mortality to individuals or population consequences for species.166,167 Selecting common species to monitor can also assist in delineating threats to much rarer, threatened species with similar life history strategies and distributions.
Critical elements of monitoring programs using bioindicators
Successful monitoring programs must have clearly outlined, feasible goals. It would be helpful if these goals are co-developed with partner organizations and relevant rightsholders and stakeholders to best align with management or other regional priorities. Goals might include evaluating trends, conducting species risk assessments, or pursuing policy-driven or nature conservation targets for present and future generations. Cooperation between organizations that have related goals is another ingredient for success; the United States' BEMAST and South Korea's National Marine Microplastic Monitoring programs are good examples. Associations and collaboration of research groups for biomonitoring can also facilitate exchange of technologies, training and education of researchers, and the acquisition of resources with projects involving international cooperation agencies, as is the case for Latin America and the Caribbean with the IAEA. Partnerships between organizations could maximize the ability of the program to secure long-term funding. Partnerships between governments and the private sector could emerge where governments could establish the rules, while the private sector provides funding. A key component of both funding and feasibility is knowledge of what data will produce the most useful information toward the stated program goals. The realities of the funding landscape in many regions, however, means that disaggregated monitoring may be the best way forward in some cases.When using bioindicators, ethical considerations during sampling are paramount. Non-lethal sampling (e.g., scat, blood, regurgitated pellet, seabird nest) is preferable. In cases where that is not possible, working with existing data and utilizing dead animals (stranded, caught for human consumption, or bycaught in fisheries operations) is preferred over sacrificing specimens. In all cases, the “Three Rs” of institutional animal care are important: replacement, reduction, and refinement.168,169 Replacement in this case may be substituting the study of rare species for more common proxies with similar ecologies. Using power analyses during the development of monitoring plans to determine the optimal number of specimens required to detect the effects of interest exemplifies the principle of reduction. Refinement is the continued development of non-lethal sampling methods that minimize the impact of research activities to individuals and populations. Lastly, a fundamental question should also be whether to simply monitor abiotic ecosystem compartments, and specifically what additional information bioindicators contribute to the monitoring program.
A monitoring and assessment program requires a data management plan covering standards for data collection, quality control, storage, sharing, analysis, reporting, and communication.11 Further, data sharing should follow FAIR principles to be Findable, Accessible, Interoperable, Reusable.170 Inaccessible data not only hinders scientific discovery but also poses a significant barrier to evidence-based conservation and environmental management.171 Most conservation science fails to translate into practical actions, a challenge known as the “knowledge-action gap”.172 FAIR data practices can help bridge this gap and should be considered at the conception of monitoring programs, rather than created post-hoc.
Outlook
We found few programs (n = 11) with bioindicators that meet Tier 1 or 2 classification (Table 1) of the UN SDG despite increasing research efforts and calls for the use of bioindicators in plastic pollution monitoring (Fig. 1). The few programs that have been collecting these data for years have produced results demonstrating the promise of these programs (Table 1). Seabirds such as the northern fulmar, turtles such as green and loggerhead turtle, fish such as longnose lancetfish, and bivalves including mussels and oysters are examples of species that are used in plastic pollution monitoring programs (Table 1 and Fig. 2). Seabirds and sea turtles are most commonly used to track plastics visible to the naked eye (>1 mm) in epipelagic environments,45,173 whereas invertebrates and small fish are used to monitor microscopic plastics—especially fibers—in nearshore intertidal and benthic environments18,93,156 (Fig. 3).Plastic ingestion is not random. Some consumers ingest plastic inadvertently, while others mistake plastic for food items.5,174–176 As such, plastic ingestion bioindicators can be most directly applied to monitor the incorporation of plastic into food webs, rather than tracking plastic levels in abiotic compartments of the environment. However, plastic exposure and ingestion levels have a positive association in some taxa,177,178 suggesting that certain species can be used to monitor plastic levels in abiotic compartments of marine environments indirectly. Moreover, certain species may pursue specific items (e.g., sea turtles with single-use food packaging and plastic bags),35,88,179,180 making them extremely sensitive monitors for these items in the environment.
Ideal bioindicator species have several shared characteristics. These include (i) being relatively common with (ii) a wide geographic range and (iii) commercial or ecological significance, as well as (iv) regularly ingesting plastic, thereby facilitating assessments of exposure risk and bioaccumulation.11,20,22,45 Beyond species concerns, monitoring programs should have clear goals, broad collaborations, plans for data sharing from program inception, and explicit pathways to long-term funding. While many difficult-to-access ecosystems still need baseline assessments, (e.g., bathypelagic and seafloor ecosystems; Fig. 3) these needs are distinct from monitoring. Our review highlights the possibilities and roadblocks to establishing a monitoring strategy of plastic ingestion by wildlife with a global reach. The World Health Organization's One Health approach and the UN Ocean Decade for Sustainable Development both acknowledge the connections between human and environmental health, providing further motivation to drastically reduce plastic pollution. Bioindicators will be central to monitoring progress towards that goal.
Box 1: List of acronyms
AMAP – Arctic and Assessment Monitoring Program: https://www.amap.no/APN – Asia Pacific Network for Global Change Research: https://www.apn-gcr.org/
BFRI – Bangladesh Fisheries Research Institute: https://fri.gov.bd/
CCAMLR – Commission for the Conservation of Antarctic Marine Living Resources: https://www.ccamlr.org/en/organisation/home-page
CEP – Antarctic Treaty System's Committee for Environmental Protection: https://www.ats.aq/e/committee.html
CIOH-DIMAR – Oceanographic and Hydrographic Research Center of General Maritime Directorate: https://cioh.dimar.mil.co/index.php/es/
CPPS – Comisión Permanente del Pacífico Sur: https://cpps-int.org
EPMC – Australia's Environment Protection and Biodiversity Conservation Act: https://www.dcceew.gov.au/environment/epbc
GES – E.U.'s Good Environmental Status under the Marine Directive: https://environment.ec.europa.eu/topics/marine-environment_en
GESAMP – The Joint Group of Experts on the Scientific Aspects of Marine Environmental Protection: http://www.gesamp.org/
GOOS – Global Ocean Observing System: https://goosocean.org/
IAEA – International Atomic Energy Agency: https://www.iaea.org/
IBAMA – Brazilian Institute of Environment and Renewable Natural Resources: https://www.abc.gov.br/training/informacoes/InstituicaoIBAMA_en.aspx
IFOP – Fisheries Development Institute: https://www.ifop.cl/en/
IMAP – European Integrated Monitoring and Assessment Programme: https://www.medqsr.org/integrated-monitoring-and-assessment-programme-mediterranean-sea-and-coast/
IMARPE – Institute of the Sea of Peru: https://www.gob.pe/imarpe.
INVEMAR – Institute of Marine and Coastal Research José Benito Vives de Andréis: https://www.invemar.org.co/
IPIAP – Public Institute for Aquaculture and Fisheries Research: https://institutopesca.gob.ec/
KIOST – Korea Institute of Ocean Science and Technology: https://www.kiost.ac.kr/eng.do
KOEM – Korea Ocean Environment Management: https://www.koem.or.kr/site/eng/main.do
LMEG – AMAP's Litter and Microplastics Expert Group: https://litterandmicroplastics.amap.no/
MABIK – National Marine Biodiversity Institute of Korea: https://www.mabik.re.kr/eng/
MSFD – European Marine Strategy Framework Directive: https://environment.ec.europa.eu/topics/marine-environment_en
NIE – Korea National Institute of Ecology: https://www.nie.re.kr/nieEng/main/main.do
NIST – U.S. National Institute of Standards and Technology: https://www.nist.gov/
NGO – Non-governmental organization
NOAA – U.S. National Oceanic and Atmospheric Administration: https://www.noaa.gov/
OSPAR Convention – Oslo/Paris Convention for the Protection of the Marine Environment of the North–East Atlantic: https://www.ospar.org/
PICES – The North Pacific Marine Science Organization: https://meetings.pices.int/
PIRO – NOAA's Pacific Islands Regional Office: https://www.fisheries.noaa.gov/about/pacific-islands-regional-office
PIFSC – NOAA's Pacific Islands Fisheries Science Center: https://www.fisheries.noaa.gov/about/pacific-islands-fisheries-science-center
PMP – Brazil's Beach Monitoring Program: https://simba.petrobras.com.br/simba/web/sistema/
REDCAM – Surveillance network for the conservation and protection of marine and coastal waters of Colombia: https://siam.invemar.org.co/redcam.
REMARCO – Research Network of Marine-Coastal Stressors in Latin America and the Caribbean: https://remarco.org/en/remarco/
SCBRMP – SCCWRP's Southern California Bight Regional Monitoring Program: https://www.sccwrp.org/about/research-areas/regional-monitoring/southern-california-bight-regional-monitoring-program/
SCCWRP – Southern California Coastal Water Research Project: https://www.sccwrp.org/
SCAR – Scientific Committee on Antarctic Research: https://scar.org/
SERNAPESCA – National Fisheries and Aquaculture Service: https://www.sernapesca.cl/
TAP – Threat Abatement Plan: https://www.dcceew.gov.au/environment/biodiversity/threatened/threat-abatement-plans
UNEP – United Nations Environment Programme: https://www.unep.org/
UN SDG – United Nations Sustainable Development Goals: https://sdgs.un.org/goals
USGS – U.S. Geological Survey: https://www.usgs.gov/
WIOMSA – Western Indian Ocean Marine Science Association: https://www.wiomsa.org/
Box 2: Glossary
Bioindicator: a species or group of species that provides data on a specific ecosystem stressor.45,181 Example: blue mussels are a bioindicator of microfiber pollution.Sentinel: a species or group of species that tracks an ecosystem state or process. Example: blue mussels are sentinels of marine pollution and ecosystem/human health.
Monitoring: the repeated measurement of a characteristic of the environment, or of a process, in order to detect a trend in space or time.11 Monitoring should define and/or use standardized sampling, analysis, and reporting guidelines.
Trend monitoring: designed to detect changes across temporal and/or spatial scales.
Surveillance monitoring: used to identify a change in conditions that may need to be addressed through management.182
Source monitoring: developed to identify potential point sources/specific pressures.24
Effects monitoring: monitoring of effects caused by plastic pollution and related contaminants on designated sentinel species.
Risk-based monitoring: uses thresholds developed by laboratory experiments or prior effects monitoring to assess contamination levels critical for certain species, human health, or food safety.24
Compliance monitoring: if regulatory or management action is taken, this form of monitoring can use bioindicators to ensure that regulatory requirements/standards are being met.24
Efficacy monitoring: can use bioindicators to assess if a policy, or regulatory action is effective in reaching the stated goal.182
Negative effects monitoring: evaluating for unintended consequences of the management activity.
Biomonitoring: the use of a bioindicator to assess the health or contamination of an environment over time or space.
Monitoring program: an organized program that tracks a specific indicator using standardized methods. To be considered a long-term program, it must have been operating for at least three years with plans and support (funding, personnel, training) to continue in the future.
Standardization: the application of certain methods according to robust criteria, with limited flexibility, to allow for comparability between laboratories.183
Harmonization: when methods used by different studies have been rigorously tested to the point that results can be viewed as comparable despite differences in methodologies.183
Data availability
The data and code used to generate Fig. 1 can be found at: https://github.com/mssavoca/GPIB_monitoring_review.Author contributions
MSS conceived the paper and led the writing with help from MC, BJG, OGO, YR, MT, and RGS. All authors wrote sections on their respective geographic regions. All authors reviewed and edited the manuscript.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
GLJ acknowledges support from the Chilean Millennium Initiative through Millennium Nucleus Ecology and Sustainable Management of Oceanic Islands ESMOI, and the CIMAR-Islas Oceánicas program from Comité Oceanográfico Nacional (CONA), Chile. RGS was funded by National Council for Scientific and Technological Development (CNPq) (research productivity grant # 312099/2023-1). The authors would like to express their gratitude for the support provided by their respective institutions. MSS thanks Rachel Anderson for editorial assistance. CAC is grateful for the efforts of NOAA's Pacific Islands Region Observer Program for their collection of lancetfish stomachs, and to the scientists of the Pacific Islands Fisheries Science Center for their efforts in keeping this program running. MC and OG acknowledge GRC Geociències Marines, which is funded by the Catalan Government (grant 2021 SGR 01195) as part of its program for excellence in research groups. SHH is grateful for the financial support provided by the Ministry of Oceans and Fisheries, Republic of Korea, for research project number 20220357. EMD, BJG and SEN would like to acknowledge the support of NERC (Ref. NE/V005448/1, NE/V009354/1).References
- P. J. Landrigan, J. J. Stegeman, L. E. Fleming, D. Allemand, D. M. Anderson, L. C. Backer, F. Brucker-Davis, N. Chevalier, L. Corra, D. Czerucka, M.-Y. D. Bottein, B. Demeneix, M. Depledge, D. D. Deheyn, C. J. Dorman, P. Fénichel, S. Fisher, F. Gaill, F. Galgani, W. H. Gaze, L. Giuliano, P. Grandjean, M. E. Hahn, A. Hamdoun, P. Hess, B. Judson, A. Laborde, J. McGlade, J. Mu, A. Mustapha, M. Neira, R. T. Noble, M. L. Pedrotti, C. Reddy, J. Rocklöv, U. M. Scharler, H. Shanmugam, G. Taghian, J. A. J. M. van de Water, L. Vezzulli, P. Weihe, A. Zeka, H. Raps and P. Rampal, Ann. Glob. Health, 2020, 86, 151 CrossRef PubMed.
- Z. Yuan, R. Nag and E. Cummins, Sci. Total Environ., 2022, 823, 153730 Search PubMed.
- K. W. Kenyon and E. Kridler, Auk, 1969, 86, 339–343 CrossRef.
- E. J. Carpenter, S. J. Anderson, G. R. Harvey, H. P. Miklas and B. B. Peck, Science, 1972, 178, 749–750 Search PubMed.
- R. G. Santos, G. E. Machovsky-capuska and R. Andrades, Science, 2021, 373, 56–60 CrossRef CAS PubMed.
- E. V. Satterthwaite, N. J. Bax, P. Miloslavich, L. Ratnarajah, G. Canonico, D. Dunn, S. E. Simmons, R. J. Carini, K. Evans, V. Allain, W. Appeltans, S. Batten, L. Benedetti-Cecchi, A. T. F. Bernard, S. Bristol, A. Benson, P. L. Buttigieg, L. C. Gerhardinger, S. Chiba, T. E. Davies, J. E. Duffy, A. Giron-Nava, A. J. Hsu, A. C. Kraberg, R. M. Kudela, D. Lear, E. Montes, F. E. Muller-Karger, T. D. O’Brien, D. Obura, P. Provoost, S. Pruckner, L.-M. Rebelo, E. R. Selig, O. S. Kjesbu, C. Starger, R. D. Stuart-Smith, M. Vierros, J. Waller, L. V. Weatherdon, T. P. Wellman and A. Zivian, Front. Mar. Sci., 2021, 8, 737416 CrossRef.
- J. Provencher, A. Bond, S. Avery-Gomm, S. Borrelle, E. Bravo Rebolledo, S. Hammer, S. Kühn, J. Lavers, M. Mallory, A. Trevail and J. van Franeker, Anal. Methods, 2017, 1454–1469 RSC.
- J. F. Provencher, S. B. Borrelle, A. L. Bond, J. L. Lavers, J. A. van Franeker, S. Kühn, S. Hammer, S. Avery-Gomm and M. L. Mallory, Facets, 2019, 4, 111–130 CrossRef.
- W. Cowger, A. M. Booth, B. M. Hamilton, C. Thaysen, S. Primpke, K. Munno, A. L. Lusher, A. Dehaut, V. P. Vaz, M. Liboiron, L. I. Devriese, L. Hermabessiere, C. Rochman, S. N. Athey, J. M. Lynch, H. De Frond, A. Gray, O. A. H. Jones, S. Brander, C. Steele, S. Moore, A. Sanchez and H. Nel, Appl. Spectrosc., 2020, 74, 1066–1077 CrossRef CAS PubMed.
- C. Tsangaris, C. Panti, M. Compa, C. Pedà, N. Digka, M. Baini, M. D’Alessandro, C. Alomar, D. Patsiou, D. Giani, T. Romeo, S. Deudero and M. C. Fossi, Mar. Pollut. Bull., 2021, 164, 111992 CrossRef CAS.
- GESAMP, GESAMP Reports Stud, 2019, vol. 99, pp. 138 Search PubMed.
- P. D. Boersma, Science, 1986, 231, 373–376 CrossRef CAS PubMed.
- J. A. van Franeker, S. Kühn, T. Anker-nilssen, E. W. J. Edwards, F. Gallien, N. Guse, J. E. Kakkonen, M. L. Mallory, W. Miles, K. Olav, J. Pedersen, J. Provencher, M. Roos, E. Stienen, D. M. Turner and W. M. G. M. van Loon, Mar. Pollut. Bull., 2021, 166, 112246 CrossRef CAS PubMed.
- C. Brawn, B. M. Hamilton, M. S. Savoca, M. L. Mallory and J. F. Provencher, Mar. Environ. Res., 2024, 106785 CrossRef CAS PubMed.
- C. Hung, N. Klasios, X. Zhu, M. Sedlak, R. Sutton and C. M. Rochman, Integrated Environ. Assess. Manag., 2021, 17, 282–291 CrossRef CAS PubMed.
- A. Rodríguez, F. Ramírez, M. N. Carrasco and A. Chiaradia, Environ. Pollut., 2018, 243, 1750–1757 CrossRef PubMed.
- J. A. Franeker, C. Blaize, J. Danielsen, K. Fairclough, J. Gollan, N. Guse, P. Hansen, M. Heubeck, J. Jensen, G. Le, B. Olsen, K.-O. Olsen, J. Pedersen, E. W. M. Stienen, D. M. Turner, G. Le Guillou, B. Olsen, K.-O. Olsen, J. Pedersen, E. W. M. Stienen and D. M. Turner, Environ. Pollut., 2011, 159, 2609–2615 CrossRef PubMed.
- J. Beyer, N. W. Green, S. Brooks, I. J. Allan, A. Ruus, T. Gomes, I. L. N. Bråte and M. Schøyen, Mar. Environ. Res., 2017, 130, 338–365 CrossRef CAS PubMed.
- C. M. Fossi, C. Peda, M. Compa, C. Tsangaris, C. Alomar, F. Claro, C. Ioakeimidis, F. Galgani, T. Hema, S. Deudero, T. Romeo, P. Battaglia, F. Andaloro, I. Caliani, S. Casini, C. Panti and M. Baini, Environ. Pollut., 2018, 237, 1023–1040 CrossRef PubMed.
- L. Bray, N. Digka, C. Tsangaris, A. Camedda, D. Gambaiani, G. A. de Lucia, M. Matiddi, C. Miaud, L. Palazzo, A. Pérez-del-Olmo, J. A. Raga, C. Silvestri and H. Kaberi, Environ. Pollut., 2019, 247, 1071–1077 Search PubMed.
- B. De Witte, A. I. Catarino, L. Vandecasteele, M. Dekimpe, N. Meyers, D. Deloof, S. Pint, K. Hostens, G. Everaert and E. Torreele, Front. Mar. Sci., 2022, 8, 794636 Search PubMed.
- M. S. Savoca, S. Kühn, C. Sun, S. Avery-gomm, C. A. Choy, S. Dudas, S. Hee, K. D. Hyrenbach, T. Li, C. K. Ng, J. F. Provencher and J. M. Lynch, Environ. Pollut., 2022, 310, 119861 Search PubMed.
- I. Corsi, A. Bellingeri and E. Bergami, Ecol. Indicat., 2023, 154, 110836 CrossRef.
- J. Provencher, T. Kögel, A. Lusher, K. Vorkamp, A. Gomiero, I. Peeken, M. Granberg, S. Hammer, J. Baak, J. R. Larsen and E. Farmen, Arct. Sci., 2022, 8, 1067–1081 Search PubMed.
- OSPAR, Second Regional Action Plan for the Prevention and Management of Marine Litter in the North-East Atlantic (2022 – 2030), 2022 Search PubMed.
- T. G.-M. L. MSFD, Guidance on Monitoring Marine Litter in European Seas, 2013 Search PubMed.
- S. Aliani, A. Lusher, F. Galgani, D. Herzke, V. Nikiforov, S. Primpke, L. Roscher, V. H. da Silva, J. Strand, G. Suaria, D. Vanavermaete, K. Verlé, B. De Witte and B. van Bavel, Nat. Rev. Earth Environ., 2023, 4, 290–291 CrossRef.
- D. Vanavermaete, A. Lusher, J. Strand, E. Abad, M. Farré, E. Kallenbach, M. Dekimpe, K. Verlé, S. Primpke, S. Aliani and B. De Witte, Micropl. Nanopl., 2024, 4, 6 CrossRef.
- A. L. Lusher, J. F. Provencher, J. E. Baak, B. M. Hamilton, K. Vorkamp, I. G. Hallanger, L. Pijogge, M. Liboiron, M. P. T. Bourdages, S. Hammer, M. Gavrilo, J. C. Vermaire, J. F. Linnebjerg, M. L. Mallory and G. W. Gabrielsen, Arct. Sci., 2022, 8, 1217–1235 Search PubMed.
- E. Farmen, J. Provencher, S. Aliani, J. Baak, M. Bergmann, A. M. Booth, M. P. T. Bourdages, L. Buhl-Mortensen, L. Feld, G. W. Gabrielsen, F. Galgani, G. Gerdts, A. Gomiero, M. Granberg, H. D. Guls, I. G. Hallanger, H. P. Halldorsson, B. Hamilton, S. Hammer, D. Herzke, M. Huserbråten, L. M. Jantunen, T. Kögel, M. Liboiron, J. F. Linnebjerg, A. Lusher, K. Magnusson, M. L. Mallory, F. R. Merkel, P. Murphy, D. Orihel, I. Peeken, L. Pijogge, S. Primpke, C. M. Rochman, J. Strand, B. M. Scholz-Böttcher, J. C. Vermaire, K. Vorkamp, J. R. Larsen and R. Hurley, AMAP Litter and Microplastics Monitoring Guidelines, Version 1.0, Arctic Monitoring and Assessment Programme, https://www.amap.no/documents/download/6761/inline Search PubMed.
- J. E. Baak, J. F. Provencher and M. L. Mallory, Mar. Pollut. Bull., 2020, 158, 111386 CrossRef CAS PubMed.
- B. M. Hamilton, L. Jantunen, M. Bergmann, K. Vorkamp, J. Aherne, K. Magnusson, D. Herzke, M. Granberg, I. G. Hallanger, A. Gomiero and I. Peeken, Arct. Sci., 2022, 8, 1116–1126 Search PubMed.
- IMAP, Integrated Monitoring and Assessment Programme of the Mediterranean Sea and Coast and Related Assessment Criteria, UN Environment Programme, Greece, 2016 Search PubMed.
- E. M. Duncan, H. D. Akbora, P. Baldi, D. Beton, A. C. Broderick, B. A. Cicek, C. Crowe-Harland, S. Davey, T. DeSerisy, W. J. Fuller, J. C. Haywood, Y. J. Hsieh, E. Kaya, L. C. M. Omeyer, M. Ozkan, J. L. Palmer, E. Roast, D. Santillo, M. J. Schneider, R. T. E. Snape, K. C. Sutherland and B. J. Godley, Mar. Pollut. Bull., 2024, 201, 116141 CrossRef CAS PubMed.
- G. Darmon, M. Schulz, M. Matiddi, A. L. Loza, J. Tomás, A. Camedda, O. Chaieb, H. A. El Hili, M. N. Bradai, L. Bray, F. Claro, T. Dellinger, F. Dell'Amico, G. A. de Lucia, E. M. Duncan, D. Gambaiani, B. Godley, H. Kaberi, Y. Kaska, J. Martin, C. Moreira, P. Ostiategui, C. K. Pham, R. Piermarini, O. Revuelta, Y. Rodríguez, C. Silvestri, R. Snape, D. Sozbilen, C. Tsangaris, M. Vale, F. Vandeperre and C. Miaud, Mar. Pollut. Bull., 2022, 185, 114364 CrossRef CAS PubMed.
- M. Matiddi, S. Hochsheid, A. Camedda, M. Baini, C. Cocumelli, F. Serena, P. Tomassetti, A. Travaglini, S. Marra, T. Campani, F. Scholl, C. Mancusi, E. Amato, P. Briguglio, F. Maffucci, M. C. Fossi, F. Bentivegna and G. A. de Lucia, Environ. Pollut., 2017, 230, 199–209 CrossRef CAS PubMed.
- G. Hernandez-Milian, C. Tsangaris, A. Anestis, M. C. Fossi, M. Baini, I. Caliani, C. Panti, L. Bundone and A. Panou, Mar. Pollut. Bull., 2023, 193, 115227 CrossRef CAS PubMed.
- D. Patsiou, N. Digka, M. Galli, M. Baini, M. C. Fossi and C. Tsangaris, Mar. Environ. Res., 2024, 196, 106438 CrossRef CAS PubMed.
- J. A. van Franeker and A. Meijboom, Litter NSV; Marine Litter Monitoring by Northern Fulmars (A Pilot Study), Alterra, Green World Research, Wageningen, NL, 2002 Search PubMed.
- J. A. van Franeker, C. Blaize, J. Danielsen, K. Fairclough, J. Gollan, N. Guse, P. Hansen, M. Heubeck, J. Jensen, G. Le, B. Olsen, K.-O. Olsen, J. Pedersen, E. W. M. Stienen, D. M. Turner, G. Le Guillou, B. Olsen, K.-O. Olsen, J. Pedersen, E. W. M. Stienen and D. M. Turner, Environ. Pollut., 2011, 159, 2609–2615 CrossRef CAS PubMed.
- S. Kühn, J. A. Van Franeker and W. M. G. M. van Loon, in OSPAR, 2023: The 2023 Quality Status Report for the Northeast Atlantic, OSPAR Commission, London, 2023 Search PubMed.
- M. Matiddi, G. A. deLucia, C. Silvestri, G. Darmon, J. Tomás, C. K. Pham, A. Camedda, F. Vandeperre, F. Claro, Y. Kaska, H. Kaberi, O. Revuelta, R. Piermarini, R. Daffina, M. Pisapia, D. Genta, D. Sözbilen, M. N. Bradai, Y. Rodríguez, D. Gambaiani, C. Tsangaris, O. Chaieb, J. Moussier, A. L. Loza and C. Miaud, JoVE, 2019, e59466 Search PubMed.
- F. Galgani, G. Darmon, C. Pham, F. Claro, N. Marques, T. Dellinger and O. Gerigny, in OSPAR, 2023: The 2023 Quality Status Report for the North-East Atlantic, OSPAR Commission, London, UK, 2022 Search PubMed.
- Consulta Pública – Relatório do 2.o Ciclo das Estratégias Marinhas da DQEM - DGRM, https://www.dgrm.pt/iniciativa, accessed February 8, 2024 Search PubMed.
- Y. Rodríguez, A. Rodríguez, W. M. G. M. van Loon, J. M. Pereira, J. Frias, E. M. Duncan, S. Garcia, L. Herrera, C. Marqués, V. Neves, C. Domínguez-Hernández, J. Hernández-Borges, B. Rodríguez and C. K. Pham, Environ. Int., 2024, 186, 108595 CrossRef PubMed.
- J. Franco, J. Fort, I. García-Barón, P. Loubat, M. Louzao, O. del Puerto and I. Zorita, Mar. Pollut. Bull., 2019, 146, 387–392 CrossRef CAS PubMed.
- E. A. Calderon, P. Hansen, A. Rodríguez, M. C. M. Blettler, K. Syberg and F. R. Khan, Water, Air, Soil Pollut., 2019, 230, 1–9 Search PubMed.
- O. Garcés-Ordóñez, K. A. Mejía-Esquivia, T. Sierra-Labastidas, A. Patiño, L. M. Blandón and L. F. Espinosa Díaz, Mar. Pollut. Bull., 2020, 154, 111085 CrossRef PubMed.
- O. Garcés-Ordóñez, J. F. Saldarriaga-Vélez, L. F. Espinosa-Díaz, A. D. Patiño, J. Cusba, M. Canals, K. Mejía-Esquivia, L. Fragozo-Velásquez, S. Sáenz-Arias, T. Córdoba-Meza and M. Thiel, Sci. Total Environ., 2022, 829, 154643 CrossRef PubMed.
- D. A. Aranda, H. A. Oxenford, J. Medina, G. Delgado, M. E. Díaz, C. Samano, V. C. Escalante, M. Bardet, E. Mouret and C. Bouchon, Mar. Pollut. Bull., 2022, 178, 113582 CrossRef CAS PubMed.
- M. Cabarcas, C. Mercado-Molares, A. Cortes Aguilar, I. Acosta Coley, L. Sierra-Marquez, A. Henao-Castro and C. Castellanos, Global Pollutants Assessment: Mercury Bioaccumulation and Microplastic Bioadhesion in Acanthopleura Granulata (Gmelin, 1791) in the Colombian Caribbean, 2023 Search PubMed.
- C. Coc, A. Rogers, E. Barrientos and H. Sanchez, Chin. J. Otorhinolaryngology-Skull Base Surg., 2021, 51, 166–174 Search PubMed.
- I. Acosta-Coley, M. Duran-Izquierdo, E. Rodriguez-Cavallo, J. Mercado-Camargo, D. Mendez-Cuadro and J. Olivero-Verbel, Mar. Pollut. Bull., 2019, 146, 574–583 Search PubMed.
- B. S. S. P. Oliveira, R. G. Santos and B. A. Santos, J. Environ. Manage., 2024, 351, 119815 CrossRef PubMed.
- L. Baes, C. D. Santiago, L. Roman, P. C. dos Santos Costa, É. Pugliesi and C. Reigada, Mar. Pollut. Bull., 2024, 199, 115847 CrossRef CAS PubMed.
- G. M. Vélez-Rubio, N. Teryda, P. E. Asaroff, A. Estrades, D. Rodriguez and J. Tomás, Mar. Pollut. Bull., 2018, 127, 603–611 CrossRef PubMed.
- V. González Carman, P. Denuncio, M. Vassallo, M. P. Berón, K. C. Álvarez and S. Rodriguez-Heredia, Front. Mar. Sci., 2021, 8, 699100 CrossRef.
- V. González Carman, P. Denuncio, M. Vassallo, M. P. Berón, K. C. Álvarez and S. Rodriguez-Heredia, Front. Mar. Sci., 2021, 8, 699100 CrossRef.
- A. C. Ronda, A. H. Arias, G. N. Rimondino, A. F. Pérez, A. Harte and J. E. Marcovecchio, Curr. Environ. Health Rep., 2021, 8, 212–222 CrossRef PubMed.
- D. M. Truchet, A. D. F. López, M. G. Ardusso, G. N. Rimondino, N. S. Buzzi, F. E. Malanca, C. V. Spetter and M. D. F. Severini, Mar. Pollut. Bull., 2021, 173, 113023 CrossRef CAS PubMed.
- T. Recabarren-Villalón, A. C. Ronda, L. La Sala, C. Sanhueza, L. Díaz, L. S. Rodríguez Pirani, A. L. Picone, R. M. Romano, P. Petracci and A. H. Arias, Mar. Pollut. Bull., 2023, 188, 114628 CrossRef PubMed.
- V. Perold, M. Connan, G. Suaria, E. A. Weideman, B. J. Dilley and P. G. Ryan, Mar. Pollut. Bull., 2024, 203, 116400 CrossRef CAS PubMed.
- E. O. Akindele and C. G. Alimba, Environ. Sci. Pollut. Res., 2021, 28, 7636–7651 CrossRef CAS PubMed.
- S. Arabi, Y. Neehaul and C. Sparks, in The African Marine Litter Outlook, ed. T. Maes and F. Preston-Whyte, Springer International Publishing, Cham, 2023, pp. 91–136 Search PubMed.
- P. G. Ryan, G. Cole, K. Spiby, R. Nel, A. Osborne and V. Perold, Mar. Pollut. Bull., 2016, 107, 155–160 CrossRef CAS PubMed.
- P. G. Ryan, L. Pichegru, V. Perold and C. L. Moloney, S. Afr. J. Mar. Sci., 2020, 116, 1–9 Search PubMed.
- P. Davison and R. Asch, Mar. Ecol. Prog. Ser., 2011, 432, 173–180 CrossRef.
- J.-P. Desforges, A. Hall, B. McConnell, A. Rosing-Asvid, J. L. Barber, A. Brownlow, S. De Guise, I. Eulaers, P. D. Jepson, R. J. Letcher, M. Levin, P. S. Ross, F. Samarra, G. Víkingson, C. Sonne and R. Dietz, Science, 2018, 361, 1373–1376 CrossRef CAS PubMed.
- C. A. Choy, B. H. Robison, T. O. Gagne, B. Erwin, E. Firl, R. U. Halden, J. A. Hamilton, K. Katija, S. E. Lisin, C. Rolsky and K. S. Van Houtan, Sci. Rep., 2019, 9, 7843 CrossRef CAS PubMed.
- J. A. Brandon, A. Freibott and L. M. Sala, Limnol. Oceanogr. Lett., 2020, 5, 46–53 CrossRef.
- B. Hamilton, C. Rochman, T. Hoellein, B. Robison, K. Van Houtan and C. Choy, Mar. Ecol. Prog. Ser., 2021, 675, 23–33 CrossRef.
- J. M. Chavarry, K. L. Law, A. D. Barton, N. M. Bowlin, M. D. Ohman and C. A. Choy, Environ. Res. Lett., 2022, 17, 064038 CrossRef CAS.
- L. Hermabessiere, C. Thaysen, C. Sherlock, C. A. Choy and C. M. Rochman, Mar. Ecol. Prog. Ser., 2023, 724, 17–32 CrossRef CAS.
- L. G. Torres, S. M. Brander, J. I. Parker, E. M. Bloom, R. Norman, J. E. Van Brocklin, K. S. Lasdin and L. Hildebrand, Front. Mar. Sci., 2023, 10, 1201078 CrossRef.
- SCCWRP, 2023 Southern California Bight Regional Monitoring Survey, Southern California Coastal Water Research Program, 2023 Search PubMed.
- SCCWRP, Shellfish Assessment Field and Laboratory Plan, Southern California Coastal Water Research Program, 2023 Search PubMed.
- M. Noël, C. Wong, P. S. Ross, S. Patankar, A. Etemadifar, C. Morales-Caselles, S. Lyons and K. Delisle, Mar. Pollut. Bull., 2022, 185, 114273 CrossRef PubMed.
- K. S. Lasdin, M. Arnold, A. Agrawal, H. W. Fennie, K. Grorud-Colvert, S. Sponaugle, L. Aylesworth, S. Heppell and S. M. Brander, PeerJ, 2023, 11, e14564 CrossRef PubMed.
- S. Avery-Gomm, P. D. O'Hara, L. Kleine, V. Bowes, L. K. Wilson and K. L. Barry, Mar. Pollut. Bull., 2012, 64, 1776–1781 CrossRef CAS PubMed.
- S. M. Fitzgerald and J. E. Dolliver, Alaska Fisheries Science Center Coordinated Seabird Studies Strategic Plan 2022-2026, NOAA Fisheries, 2023 Search PubMed.
- J. E. Baak, K. E. Hanifen, M. L. Maddox, M. L. Mallory, K. H. Elliott, S. Keegan and J. F. Provencher, Mar. Pollut. Bull., 2024, 206, 116800 CrossRef CAS PubMed.
- J. M. Keller, R. S. Pugh and P. R. Becker, Biological and Environmental Monitoring and Archival of Sea Turtle Tissues (BEMAST): Rationale, Protocols, and Initial Collections of Banked Sea Turtle Tissues, National Institute of Standards and Technology Internal Report, vol. 7996, 2014 Search PubMed.
- C. Choy and J. Drazen, Mar. Ecol. Prog. Ser., 2013, 485, 155–163 CrossRef.
- C. A. Choy, E. Portner, M. Iwane and J. C. Drazen, Mar. Ecol. Prog. Ser., 2013, 492, 169–184 CrossRef.
- E. J. Portner, J. J. Polovina and C. A. Choy, Deep Sea Res. Oceanogr. Res. Pap., 2017, 125, 40–51 CrossRef.
- NOAA Pacific Islands Fisheries Science Center, Longnose Lancetfish Data Set, 2024, https://www.fisheries.noaa.gov/inport/item/70277.
- M. Matiddi, T. Valente, A. Camedda, C. Centelleghe, C. Cocumelli, S. Dara, G. A. De Lucia, L. Di Renzo, N. Ferri, G. Gioacchini, S. Hochscheid, G. Lucifora, F. Maffucci, V. Monteverde, T. Pelamatti, A. Petrella, G. Pietroluongo, C. Roncari, G. Terracciano and C. Silvestri, Mar. Pollut. Bull., 2024, 205, 116647 CrossRef CAS PubMed.
- Y. Moon, W. J. Shim, G. M. Han, J. Jeong, Y. Cho, I. H. Kim, M. S. Kim, H. R. Lee and S. H. Hong, Environ. Pollut., 2022, 298, 118849 CrossRef CAS PubMed.
- J. Li, D. Yang, L. Li, K. Jabeen and H. Shi, Environ. Pollut., 2015, 207, 190–195 CrossRef CAS PubMed.
- J. Li, X. Qu, L. Su, W. Zhang, D. Yang, P. Kolandhasamy, D. Li and H. Shi, Environ. Pollut., 2016, 214, 177–184 CrossRef CAS PubMed.
- X. Qu, L. Su, H. Li, M. Liang and H. Shi, Sci. Total Environ., 2018, 621, 679–686 CrossRef CAS PubMed.
- J. Ding, J. Li, C. Sun, F. Jiang, C. He, M. Zhang, P. Ju and N. X. Ding, Sci. Total Environ., 2020, 739, 139887 CrossRef CAS PubMed.
- J. Ding, C. Sun, C. He, J. Li, P. Ju and F. Li, Sci. Total Environ., 2021, 782, 146830 CrossRef CAS PubMed.
- J. Teng, J. Zhao, C. Zhang, B. Cheng, A. A. Koelmans, D. Wu, M. Gao, X. Sun, Y. Liu and Q. Wang, Sci. Total Environ., 2020, 745, 140815 CrossRef CAS PubMed.
- L. Su, H. Deng, B. Li, Q. Chen, V. Pettigrove, C. Wu and H. Shi, J. Hazard. Mater., 2019, 365, 716–724 CrossRef CAS PubMed.
- L. Zhu, H. Wang, B. Chen, X. Sun, K. Qu and B. Xia, Sci. Total Environ., 2019, 677, 493–501 CrossRef CAS PubMed.
- X.-Y. Xu, C. Y. Wong, N. F. Y. Tam, H. M. Liu and S. G. Cheung, Mar. Pollut. Bull., 2020, 154, 111081 CrossRef CAS PubMed.
- C. Zhang, S. Wang, D. Sun, Z. Pan and J. Zou, Sustainability, 2023, 15, 4193 CrossRef CAS.
- K. Tanaka and H. Takada, Sci. Rep., 2016, 6, 34351 CrossRef CAS PubMed.
- K. Tanaka, J. A. van Franeker, T. Deguchi and H. Takada, Mar. Pollut. Bull., 2019, 145, 36–41 CrossRef CAS PubMed.
- B. Nishizawa, J.-B. Thiebot, F. Sato, N. Tomita, K. Yoda, R. Yamashita, H. Takada and Y. Watanuki, Sci. Rep., 2021, 11, 1–7 CrossRef PubMed.
- M. Yagi, T. Kobayashi, Y. Maruyama, S. Hoshina, S. Masumi, I. Aizawa, J. Uchida, T. Kinoshita, N. Yamawaki, T. Aoshima, Y. Morii and K. Shimizu, Mar. Pollut. Bull., 2022, 174, 113304 CrossRef CAS PubMed.
- Department of Environment and Natural Resources, Philippines National Plan of Action for the Prevention, Reduction and Management of Marine Litter, 2021, https://seaknowledgebank.net/sites/default/files/2024-04/Jan%202022%20Final%20Philippines%20NPOA-ML%20%281%29.pdf.
- C. Andrade and F. Ovando, An. Inst. Patagonia, 2017, 45, 59–65 CrossRef.
- A. Aguirre-Sanchez, S. Purca and A. G. Indacochea, Air Soil. Water Res., 2022, 15, 117862212211245 Search PubMed.
- Y. Gong, Y. Wang, L. Chen, Y. Li, X. Chen and B. Liu, Mar. Pollut. Bull., 2021, 169, 112509 CrossRef CAS PubMed.
- R. Mizraji, C. Ahrendt, D. Perez-venegas, J. Vargas, J. Pulgar, M. Aldana, F. P. Ojeda, C. Duarte and C. Galbán-malagón, Mar. Pollut. Bull., 2017, 116, 498–500 CrossRef CAS PubMed.
- N. C. Ory, P. Sobral, J. L. Ferreira and M. Thiel, Sci. Total Environ., 2017, 586, 430–437 CrossRef CAS PubMed.
- C. Fernández-Ojeda, M. C. Muniz, R. P. Cardoso, R. M. dos Anjos, E. Huaringa, C. Nakazaki, A. Henostroza and O. Garcés-Ordóñez, Mar. Pollut. Bull., 2021, 173, 113039 CrossRef PubMed.
- C. Chagnon, M. Thiel, J. Antunes, J. L. Ferreira, P. Sobral and N. C. Ory, Environ. Pollut., 2018, 243, 127–133 CrossRef CAS PubMed.
- C. Fernández and A. Anastasopoulou, Mar. Pollut. Bull., 2019, 149, 110501 CrossRef PubMed.
- J. Vivas-Sánchez, E. Gamboa-Garcia and G. Duque, Rev. Acad. Colomb. Cienc. Exactas Fis. Nat., 2023, 47, 571–590 CrossRef.
- J. Quiñones, S. Quispe, M. Manrique and E. Paredes, Boletin. Instituto del Mar del Peru, 2021, 36, 85–105 CrossRef.
- D. Miranda-Urbina, M. Thiel and G. Luna-Jorquera, Mar. Pollut. Bull., 2015, 96, 235–244 CrossRef CAS PubMed.
- G. Luna-Jorquera, M. Thiel, M. Portflitt-Toro and B. Dewitte, Aquat. Conserv.: Mar. Freshw. Ecosyst., 2019, 29, 245–259 CrossRef.
- V. Hidalgo-Ruz, G. Luna-Jorquera, M. Eriksen, H. Frick, D. Miranda-Urbina, M. Portflitt-Toro, M. M. Rivadeneira, C. J. R. Robertson, R. P. Scofield, J. Serratosa, C. G. Suazo and M. Thiel, Aquat. Conserv.: Mar. Freshw. Ecosyst., 2020, 1–19 Search PubMed.
- S. Mendez-Sanhueza, M. Torres, K. Pozo, G. Del Aguila, F. Hernandez, C. Jacobsen and D. Echeverry, Animals, 2023, 13, 2840 CrossRef PubMed.
- I. Diaz-Santibañez, B. L. Clark and C. B. Zavalaga, Mar. Pollut. Bull., 2023, 192, 115104 CrossRef PubMed.
- D. J. Perez-Venegas, M. Seguel, H. Pavés, J. Pulgar, M. Urbina, C. Ahrendt and C. Galbán-Malagón, Mar. Pollut. Bull., 2018, 136, 50–54 CrossRef CAS PubMed.
- F. Ayala, M. Cardeña-Mormontoy and S. Cardenas, Rev. Int. Contam. Ambiental, 2021, 37, 273–279 Search PubMed.
- A. Aguirre-Sanchez, S. Purca, M. Cole, A. G. Indacochea and P. K. Lindeque, Mar. Pollut. Bull., 2024, 200, 116075 CrossRef CAS PubMed.
- A. Markic, C. Niemand, J. H. Bridson, N. Mazouni-Gaertner, J. C. Gaertner, M. Eriksen and M. Bowen, Mar. Pollut. Bull., 2018, 136, 547–564 CrossRef CAS PubMed.
- K. Pozo, V. Gomez, M. Torres, L. Vera, D. Nuñez, P. Oyarzún, G. Mendoza, B. Clarke, M. Cristina, M. Baini, P. Petra and J. Klánová, Mar. Pollut. Bull., 2019, 140, 315–319 CrossRef CAS PubMed.
- N. Ory, C. Chagnon, F. Felix, C. Fernández, J. Lia, C. Gallardo, O. Garcés, A. Henostroza, E. Laaz, R. Mizraji, H. Mojica, V. Murillo, L. Ossa, M. Preciado, P. Sobral, M. A. Urbina and M. Thiel, Mar. Pollut. Bull., 2018, 127, 211–216 CrossRef CAS PubMed.
- C. E. Fernández, G. Luna-Jorquera, V. González Encinas, A. Auger Lancelloti, C. Lantadilla, R. Aguilar-Pulido, T. Kiessling, K. Knickmeier, A. I. Varela and M. Thiel, Sci. Total Environ., 2024, 952, 175938 CrossRef PubMed.
- K. Ludynia, S. Garthe and G. Luna-Jorquera, Waterbird, 2005, 28, 359–365 CrossRef.
- P. Yorio, C. Marinao, T. Kasinsky, C. Ibarra and N. Suárez, Mar. Pollut. Bull., 2020, 156, 111240 CrossRef CAS PubMed.
- A. M. Garcia-Cegarra, R. Ramirez and R. Orrego, Mar. Pollut. Bull., 2020, 160, 111632 CrossRef CAS PubMed.
- D. J. Perez-Venegas, C. Toro-Valdivieso, F. Ayala, B. Brito, L. Iturra, M. Arriagada, M. Seguel, C. Barrios, M. Sepúlveda, D. Oliva, S. Cárdenas-Alayza, M. A. Urbina, A. Jorquera, E. Castro-Nallar and C. Galbán-Malagón, Mar. Pollut. Bull., 2020, 153, 110966 CrossRef CAS PubMed.
- Department of Climate Change, Energy, the Environment and Water, Key threatening processes under the EPBC Act – DCCEEW, https://www.dcceew.gov.au/environment/biodiversity/threatened/key-threatening-processes, accessed May 18, 2024 Search PubMed.
- D. Honorato-Zimmer, E. A. Weideman, P. G. Ryan and M. Thiel, in Oceanography and Marine Biology, CRC Press, 2022 Search PubMed.
- WIOMSA, Marine plastic litter in the WIO region: Status, implications on the environment, human populations and effectiveness of measures and opportunities, A Synthesis Report, UN Environment Programme, Nairobi, Kenya, 2022 Search PubMed.
- L. C. M. Omeyer, E. M. Duncan, N. A. S. Abreo, J. M. V. Acebes, L. A. AngSinco-Jimenez, S. T. Anuar, L. V. Aragones, G. Araujo, L. R. Carrasco, M. A. H. Chua, M. R. Cordova, L. P. Dewanti, E. Q. Espiritu, J. B. Garay, E. S. Germanov, J. Getliff, E. Horcajo-Berna, Y. S. Ibrahim, Z. Jaafar, J. I. B. Janairo, T. K. Gyi, D. Kreb, C. L. Lim, Y. Lyons, P. L. K. Mustika, M. L. Neo, S. Z. H. Ng, B. Pasaribu, A. Pariatamby, C. Peter, L. Porter, N. P. Purba, E. T. Santa Cruz, S. Shams, K. F. Thompson, D. S. Torres, R. Westerlaken, T. Wongtawan and B. J. Godley, Sci. Total Environ., 2023, 874, 162502 CrossRef CAS PubMed.
- B. S. Mayoma, C. Sørensen, Y. Shashoua and F. R. Khan, Bull. Environ. Contam. Toxicol., 2020, 105, 513–521 CrossRef CAS PubMed.
- IUCN-EA-QUANTIS, National Guidance for Plastic Pollution Hotspotting and Shaping Action, South Africa, 2020 Search PubMed.
- IUCN-EA-QUANTIS, Orientação Nacional para Pontos de Acesso e Acções de Modelação da Poluição por Plástico: Resultados de Moçambique, Mozambique, 2020 Search PubMed.
- K. Fatema, M. A. Hawa, S. Masnoon, Md. J. Alam, M. J. Islam, Md. M. Hasan, M. A. M. Siddiquee, Md. H. Uddin, K. A. Sumon, R. K. Bhandari and H. Rashid, Reg. Stud. Mar. Sci., 2023, 67, 103226 Search PubMed.
- K. Fatema, T. Rahman, M. J. Islam, K. A. Sumon, Md. H. Uddin, S. J. Hasan, S. M. A. Kawsar, H. Arakawa, Md. M. Haque and H. Rashid, Environ. Sci. Pollut. Res., 2023, 30, 38853–38868 CrossRef CAS PubMed.
- K. Fatema, M. J. Islam, M. A. I. Sarker, K. S. Elahi, Md. J. Alam, S. J. Hasan and H. Rashid, Environ. Sci. Pollut. Res., 2024, 31, 24329–24343 CrossRef PubMed.
- K. Fatema, K. A. Sumon, S. M. Moon, M. J. Alam, S. J. Hasan, M. H. Uddin, H. Arakawa and H. Rashid, J. Sediment. Environ., 2023, 8, 231–246 CrossRef.
- R. A. Phillips and C. M. Waluda, Environ. Int., 2020, 136, 105443 CrossRef PubMed.
- G. Caruso, E. Bergami, N. Singh and I. Corsi, Water Biol. Secur., 2022, 1, 100034 CrossRef.
- G. Suaria, A. Achtypi, V. Perold, J. R. Lee, A. Pierucci, T. G. Bornman, S. Aliani and P. G. Ryan, Sci. Adv., 2020, 6, 1–9 Search PubMed.
- J. Fragão, F. Bessa, V. Otero, A. Barbosa, P. Sobral, C. M. Waluda, H. R. Guímaro and J. C. Xavier, Sci. Total Environ., 2021, 788, 147698 CrossRef PubMed.
- D. Taurozzi and M. Scalici, Front. Mar. Sci., 2024, 11, 1343617 CrossRef.
- M. González-Aravena, C. Rotunno, C. A. Cárdenas, M. Torres, S. A. Morley, J. Hurley, L. Caro-Lara, K. Pozo, C. Galban and R. Rondon, Mar. Pollut. Bull., 2024, 201, 116257 CrossRef PubMed.
- W. Zhu, W. Liu, Y. Chen, K. Liao, W. Yu and H. Jin, Sci. Total Environ., 2023, 870, 161880 CrossRef CAS PubMed.
- L. Wilkie Johnston, E. Bergami, E. Rowlands and C. Manno, R. Soc. Open Sci., 2023, 10, 221421 CrossRef PubMed.
- F. Bessa, N. Ratcliffe, V. Otero, P. Sobral, J. C. Marques, C. M. Waluda, P. N. Trathan and J. C. Xavier, Sci. Rep., 2019, 9, 1–7 CrossRef CAS PubMed.
- A. E. Ibañez, L. M. Morales, D. S. Torres, P. Borghello, N. S. Haidr and D. Montalti, Mar. Pollut. Bull., 2020, 157, 111351 CrossRef PubMed.
- C. L. Waller, H. J. Griffiths, C. M. Waluda, S. E. Thorpe, I. Loaiza, B. Moreno, C. O. Pacherres and K. A. Hughes, Sci. Total Environ., 2017, 598, 220–227 CrossRef CAS PubMed.
- M. Zhang, M. Haward and J. McGee, Polar Rec., 2020, 56, e36 CrossRef.
- Outcomes from a review of the CCAMLR Marine Debris Program | Meetings, https://meetings.ccamlr.org/en/sc-camlr-38/09, accessed May 18, 2024 Search PubMed.
- J. A. van Franeker and K. Lavender, Environ. Pollut., 2015, 203, 89–96 CrossRef CAS PubMed.
- S. M. Brander, V. C. Renick, M. M. Foley, C. Steele, M. Woo, A. Lusher, S. Carr, P. Helm, C. Box, S. Cherniak, R. C. Andrews and C. M. Rochman, Appl. Spectrosc., 2020, 74, 1099–1125 CrossRef CAS PubMed.
- J. Li, A. L. Lusher, J. M. Rotchell, S. Deudero, A. Turra, I. L. N. Bråte, C. Sun, M. Shahadat Hossain, Q. Li, P. Kolandhasamy and H. Shi, Environ. Pollut., 2019, 244, 522–533 CrossRef CAS PubMed.
- L. C. Woodall, A. Sanchez-vidal, G. L. J. Paterson, R. Coppock, V. Sleight, A. Calafat, A. D. Rogers, B. E. Narayanaswamy and R. C. Thompson, R. Soc. Open Sci., 2014, 1, 140317 CrossRef PubMed.
- A. Cózar, M. Sanz-Martín, E. Martí, J. I. González-Gordillo, B. Ubeda, J. Á. Gálvez, X. Irigoien and C. M. Duarte, PLoS One, 2015, 10, e0121762 CrossRef PubMed.
- S. Santini, E. De Beni, T. Martellini, C. Sarti, D. Randazzo, R. Ciraolo, C. Scopetani and A. Cincinelli, Toxics, 2022, 10, 391 CrossRef CAS PubMed.
- S. N. Athey and L. M. Erdle, Environ. Toxicol. Chem., 2022, 41, 822–837 CrossRef CAS PubMed.
- M. Thibault, L. Hoarau, L. Lebreton, M. Le Corre, M. Barret, E. Cordier, S. Ciccione, S.-J. Royer, A. Ter Halle, A. Ramanampamonjy, C. Jean and M. Dalleau, Mar. Pollut. Bull., 2023, 194, 115343 CrossRef CAS PubMed.
- Q. Schuyler, B. D. Hardesty, C. Wilcox and K. Townsend, PLoS One, 2012, 7, e40884 CrossRef CAS PubMed.
- R. G. Santos, R. Andrades, L. M. Fardim and A. S. Martins, Environ. Pollut., 2016, 214, 585–588 CrossRef CAS PubMed.
- J. E. Ward, S. Zhao, B. A. Holohan, K. M. Mladinich, T. W. Griffin, J. Wozniak and S. E. Shumway, Environ. Sci. Technol., 2019, 53, 8776–8784 CrossRef CAS PubMed.
- S. Avery-Gomm, S. B. Borrelle and J. F. Provencher, Sci. Total Environ., 2018, 637–638, 1492–1495 CrossRef CAS PubMed.
- L. Roman, B. D. Hardesty, M. A. Hindell and C. Wilcox, Sci. Rep., 2019, 9, 1–7 CrossRef PubMed.
- N. Marn, M. Jusup, S. A. L. M. Kooijman and T. Klanjscek, Ecol. Lett., 2020, 23, 1479–1487 CrossRef PubMed.
- H. J. Curzer, G. Perry, M. C. Wallace and D. Perry, Sci. Eng. Ethics, 2016, 22, 549–565 CrossRef PubMed.
- R. C. Hubrecht and E. Carter, Animals, 2019, 9, 754 CrossRef PubMed.
- M. D. Wilkinson, M. Dumontier, Ij. J. Aalbersberg, G. Appleton, M. Axton, A. Baak, N. Blomberg, J.-W. Boiten, L. B. da Silva Santos and P. E. Bourne, et al. , Sci. Data, 2016, 3, 1–9 Search PubMed.
- J. R. B. Fisher, S. A. Wood, M. A. Bradford and T. R. Kelsey, Conserv. Sci. Pract., 2020, 2, e210 CrossRef.
- D. G. Roche, R. E. O'Dea, K. A. Kerr, T. Rytwinski, R. Schuster, V. M. Nguyen, N. Young, J. R. Bennett and S. J. Cooke, Conserv. Biol., 2022, 36, e13835 CrossRef PubMed.
- J. A. van Franeker and K. L. Law, Environ. Pollut., 2015, 203, 89–96 CrossRef CAS PubMed.
- M. S. Savoca, M. E. Wohlfeil, S. E. Ebeler and G. A. Nevitt, Sci. Adv., 2016, 2, e1600395 CrossRef PubMed.
- S. R. Kahane-Rapport, M. F. Czapanskiy, J. A. Fahlbusch, A. S. Friedlaender, J. Calambokidis, E. L. Hazen, J. A. Goldbogen and M. S. Savoca, Nat. Commun., 2022, 13, 6327 CrossRef CAS PubMed.
- N. C. Ory, C. Gallardo, M. Lenz and M. Thiel, Environ. Pollut., 2018, 240, 566–573 CrossRef CAS PubMed.
- L. C. Young, C. Vanderlip, D. C. Duffy, V. Afanasyev and S. A. Shaffer, PLoS One, 2009, 4, e7623 CrossRef PubMed.
- C. Wilcox, E. Van Sebille and B. D. Hardesty, Proc. Natl. Acad. Sci. U. S. A., 2015, 112, 11899–11904 CrossRef CAS PubMed.
- M. Rizzi, F. L. Rodrigues, L. Medeiros, I. Ortega, L. Rodrigues, D. S. Monteiro, F. Kessler and M. C. Proietti, Mar. Pollut. Bull., 2019, 140, 536–548 CrossRef CAS PubMed.
- A. Camedda, M. Matiddi, A. Vianello, S. Coppa, J. Bianchi, C. Silvestri, L. Palazzo, G. Massaro, F. Atzori, A. Ruiu, R. Piermarini, C. Cocumelli, P. Briguglio, S. Hochscheid, R. Brundu and G. A. de Lucia, Environ. Pollut., 2022, 292, 118274 CrossRef CAS PubMed.
- G. Bonanno and M. Orlando-bonaca, Mar. Pollut. Bull., 2018, 137, 209–221 CrossRef CAS PubMed.
- R. L. Hutto and R. T. Belote, For. Ecol. Manag., 2013, 289, 183–189 CrossRef.
- J. F. Provencher, G. A. Covernton, R. C. Moore, D. A. Horn, J. L. Conkle and A. L. Lusher, Sci. Total Environ., 2020, 748, 141426 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2025 |