Open Access Article
Open Access ArticleDevelopment of an innovative optical sensor to detect extremely low levels of chromium in real samples using colorimetric methods
Eman R.
Darwish
a,
Reem F.
Alshehri
b,
Alaa S.
Amin
 *c and
Mai
Aish
a
*c and
Mai
Aish
a
aChemistry Department, Faculty of Science, Port Said University, Port Said, Egypt
bChemistry Department, College of Science, Taibah University, Saudi Arabia
cChemistry Department, Faculty of Science, Benha University, Benha, Egypt. E-mail: asamin2005@hotmail.com
First published on 16th December 2023
Abstract
The investigation focused on a methodology for concentrating and analyzing Cr(VI) in aqueous samples. This objective was accomplished through the creation of a cellulose triacetate (CTA) matrix-based membrane optode. This optode was constructed by physically integrating a specific chromophore, 1,3-benzenediamine,N,N′-bis(2-furanylmethylene) (BDBFM), known for its selectivity towards Cr(VI), alongside the plasticizer dioctylphthalate (DOP). The effectiveness of integrating Aliquat 336, an anion exchanger, was evaluated in the process of immobilizing both BDBFM and the Cr(VI)–BDBFM complex within the optode matrix. The progressive intensification of the violet color observed on the optodes, directly correlating with the amount of loaded Cr(VI), highlights the potential of this method for colorimetric screening of Cr(VI) in aqueous samples. The developed optode was also employed for the determination of the total chromium content by converting Cr(III) to Cr(VI) via oxidation using 0.1 M hydrogen peroxide. The concentration of Cr(III) can be quantified by subtracting the amount of Cr(VI) from the total chromium content. This optode enabled the quantitative detection of Cr(VI), even at levels as low as 2.85 ng mL−1. The suggested sensor displayed a low detection limit, fast response time, cost effectiveness, ease of preparation and also remarkable selectivity regarding some anions and cations. Regeneration of the optode can be easily accomplished by employing 0.05 M HNO3, while demonstrating remarkable reproducibility and reversibility in its response, with a relative standard deviation (RSD) below 1.9. The suggested method was effectively utilized to measure chromium levels in a diverse range of samples, such as food, water, and environmental, and biological samples.
Environmental significanceThe optode developed in this study offers a straightforward and efficient method for detecting Cr(VI) ions. Its utilization of cellulose triacetate as a matrix ensures favorable optical and mechanical properties. Notably, the optode exhibits a reversible color change from yellow to violet upon exposure to Cr(VI) ions, and it can be easily regenerated using a 0.05 M HNO3 solution with complete reversibility. Compared to previously reported optical sensors for Cr(VI) detection, the proposed optode demonstrates a significantly faster response time. An important aspect of this study is the novel application of BDBFM in combination with the proposed optode for the determination of Cr(VI) ions. This approach has not been documented in the existing literature, making this study the first to employ BDBFM in conjunction with the proposed optode. Furthermore, the versatility of the optode sensor extends to its successful implementation in monitoring BDBFM across various sample types, including water, food, and biological, and environmental samples. This wide range of applications showcases the potential of the optode sensor beyond its primary use in Cr(VI) determination. |
1 Introduction
Environmental pollutants such as heavy metals and organic and inorganic compounds in recent years have received a lot of attention.1–4 Inadequate wastewater treatment facilities in various industries lead to the discharge of heavy metals into aquatic environments.5,6 Chromium, in particular, is a significant environmental pollutant commonly associated with industrial activities such as steelworks, tanning factories, wood preservation, and industrial electroplating. Chromium is an element that necessitates assessment in environmental and pharmaceutical products, primarily due to the health risks it poses. Additionally, it has been found to cause various types of DNA lesions, as indicated by studies.7–9 Compounds of Cr(III) are recognized for their important role in glucose and lipid metabolism and are regarded as essential trace elements that contribute to maintaining efficient protein metabolism in humans.10–15Chromium has the ability to enter the human body through inhalation or ingestion via drinking water, and its presence in water, air, and biological samples is typically at low levels. Typically, the concentration of chromium in drinking water is found to be below 2.0 µg mL−1.15,16 According to the World Health Organization (WHO), the guideline values of 50 µg mL−1 for Cr(VI) are deemed excessively high given its genotoxic effects. In line with this, the National Health and Nutrition Examination Survey (NHANES) has provided data indicating that normal chromium levels in blood fall within the range of 0.1 to 1.7 µg mL−1, while urine samples typically exhibit chromium concentrations ranging from 0.24 to 1.8 µg mL−1.17 After entering the bloodstream, the transportation of Cr(VI) compounds occurs through nonspecific anionic channels into red blood cells (RBCs). Once inside the RBCs, they undergo rapid reduction to Cr(III), which then binds to hemoglobin in RBCs (Cr3/Cr4/Cr5/Cr6). So, the levels of chromium present in red blood cells serve as a reliable indicator of exposure to Cr(VI), as it undergoes conversion to Cr(III) within these cells.18 In order to promptly identify chromium exposure, it is essential to develop suitable methodologies for determining the speciation of chromium.
The predominant oxidation states of chromium commonly encountered in the environment are Cr(III) and Cr(VI).19 In comparison to Cr(III), Cr(VI) is widely recognized for its high oxidizing capacity, high toxicity, carcinogenicity, and resistance to biodegradation. These characteristics pose significant risks to both the environment and human health.20 Exposure to even low concentrations of Cr(VI) can lead to serious health issues such as hemolysis, renal and liver failure, and various types of cancer.21 Industries such as steel production, electroplating, leather tanning, and the manufacturing of alloys and pigments extensively utilize chromium, leading to the release of significant quantities of liquid or solid waste containing chromium into the environment.
This leads to pollution of groundwater, surface water, soil, and plants.22–24 Wastewaters from industries like electroplating or leather tanning typically contain chromium concentrations ranging from 3.0 to 30 µg mL−1 for Cr(VI) and 5.0 to 100 µg mL−1 for total chromium.25,26 The development of a straightforward and practical method for detecting Cr(VI) holds significant importance, considering the aforementioned levels and its potential impact.
The selective determination of Cr(VI) has been a persistent analytical challenge and holds significant importance due to its considerably higher toxicity compared to Cr(III).27 Historically, various approaches have been devised to detect Cr(VI), encompassing methods utilizing bulky instrumentation such as inductively coupled plasma mass spectrometry (ICP-MS),28 atomic absorption spectroscopy (AAS),29 X-ray fluorescence (XRF), and solid phase extraction.30 While methods based on large equipment offer superior accuracy and stability, they often come with drawbacks such as the need for complex sample pretreatment, specialized operation, and high costs. As a result, they are not suitable for real-time field monitoring applications. In the meantime, colorimetric assay based on metal optical chemical sensors (optodes) has attracted increasing attention recently, because of requiring no complex instrumentations and signal recognition can be achieved by the naked eye in the form of a color change.31–35
Chemical sensor technology can provide low-cost devices that can be tuned to a wide field of applications by coating mass-sensitive or optical transducers with a chemically sensitive layer.36 Optical sensors, specifically, operate on two fundamental principles within the realm of optical chemical sensors: (i) the absorption characteristics of the chromophore are directly influenced by its interaction with the analyte, and/or (ii) the optical properties change as a result of conformational shifts in the sensor, which are responsive to the analyte interaction.37,38 Significant research has been conducted in our laboratory to develop colorimetric sensors for detecting metal ions.32,39–43 These sensors provide distinct advantages in terms of sensitivity, selectivity and response time. Moreover, the theoretical basis of such optical membranes based on plasticized polyvinyl chloride (PVC) has been well established.44–47
To design an optical chemical sensor, a sensitive layer comprising dye indicators such as porphyrin compounds is applied onto a polymer matrix such as PVC and cellulose acetate.37,38 These polymers not only serve as solid supports for immobilizing indicator dyes, but also provide permeation selectivity for certain species while rejecting others.48 Several methods have been reported for chromium measurement using optical sensors based on colorimetric techniques.33–35,46–55
The objective of this study was to create a film-based sensor (optode), for detecting Cr(VI) by observing color changes and its applications for real samples. The optode employed BDBFM as the indicator, which was immobilized in a CTA matrix with DOP as the plasticizer. While PVC is frequently chosen for membrane sensors due to its cost-effectiveness and desirable mechanical characteristics, it is prone to water absorption, resulting in opacity. In contrast, CTA-based membranes offer favorable optical properties and do not absorb water. Thus, CTA was selected as the matrix-forming polymer over PVC. In order to evaluate the stability of the Cr(VI)–BDBFM complex, Aliquat 336 as an anion exchanger and dinonylnaphthalene sulfonic acid as a cation exchanger were investigated as additives in the optode matrix. The incorporation of Aliquat 336 into the optode membrane proved to be crucial in achieving the desired properties for an optical sensor. Extensive optimization efforts were undertaken to ensure accurate extraction of Cr(VI) within the optode, thereby optimizing the optical response and expanding the working range. The proposed optode was subjected to testing for the detection of Cr(VI) in water, food, and biological, and environmental samples that were spiked with the analyte.
2 Materials and methods
2.1. Reagents
All chemicals utilized in this study were of analytical reagent grade and employed without the need for further purification. For all dilutions, double deionized water with a resistivity of 18.2 MΩ cm−1 (Milli-Q Millipore) was employed. Plastic and glassware were meticulously cleaned by immersing them in a diluted solution of HNO3 (1 + 9) and subsequently rinsing them with distilled water prior to their usage. Analytical grade chemicals including Na2SO4, KCl, NaNO3, KH2PO4, CH3COONa, Na2CO3, CHCl3 and K2Cr2O7 from BDH, as well as supra pure grade HCl from Merck (Germany), were consistently used throughout the study. 2-Nitrophenyl octyl ether (NPOE) from Fluka, cellulose triacetate with a M. wt 72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–74
000–74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (Alfa Biochem), bis-(2-ethylhexyl) terephthalate (DOTP) from Acros, dioctyl phthalate (DOP) (99%, Sigma-Aldrich), di-(2-ethylhexyl) phosphoric acid (D2EHPA) (97%, Sigma-Aldrich), dioctyl sebacate (DOS) from Fluka, 1,5-diphenylcarbazide (DPC) from Merck, and trioctyltrimellitate (TOT) from Aldrich were used as received. Dinonylnaphthalene sulfonic acid (DNNS) obtained from Fluka and trioctylmethyl ammonium chloride (Aliquat-336) were both utilized in their received form without further modification.
000 (Alfa Biochem), bis-(2-ethylhexyl) terephthalate (DOTP) from Acros, dioctyl phthalate (DOP) (99%, Sigma-Aldrich), di-(2-ethylhexyl) phosphoric acid (D2EHPA) (97%, Sigma-Aldrich), dioctyl sebacate (DOS) from Fluka, 1,5-diphenylcarbazide (DPC) from Merck, and trioctyltrimellitate (TOT) from Aldrich were used as received. Dinonylnaphthalene sulfonic acid (DNNS) obtained from Fluka and trioctylmethyl ammonium chloride (Aliquat-336) were both utilized in their received form without further modification.
To prepare stock solutions of Cr(VI), K2CrO4 was dissolved in water, resulting in a concentration of 1000 mg L−1 for both Cr(VI) and Cr(III). For Cr(III) stock solutions, Cr(NO3)3 was dissolved in 0.5 M HNO3. Model and standard solutions were then prepared by diluting the stock solutions accordingly. Borate, acetate, thiel, phosphate, and universal buffer solutions of pH 2.0 to pH 12 were prepared to create different pH conditions using established methods.56 To generate stock solutions of interfering ions, salts from Merck were dissolved in double-distilled water to achieve a concentration of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 µg mL−1.
000 µg mL−1.
The oxidation of Cr(III) to Cr(VI) was conducted utilizing a previously described method in the literature.36,57 The solution's pH was adjusted to 10, and a 3.0% hydrogen peroxide solution was added, followed by heating at 80 °C for 30 minutes. Any excess hydrogen peroxide was eliminated by boiling the solution for 10 minutes. The aforementioned test procedure was subsequently employed for this solution. Subsequent to the conversion of Cr(III) to Cr(VI) via hydrogen peroxide oxidation under alkaline conditions, the total chromium content was determined. The concentration of Cr(III) was then calculated by subtracting the concentration of Cr(VI) from the total chromium concentration.
Upon oxidizing Cr(III) to Cr(VI) via the alkaline oxidation process using hydrogen peroxide, the method was applied to determine the total chromium content. Subsequently, the concentration of Cr(III) was calculated by deducting the concentration of Cr(VI) from the overall chromium concentration.
2.2. Apparatus
Absorbance measurements and spectral analysis were conducted using the UV-vis spectrophotometer model V-670 from JASCO (Tokyo, Japan).The acidity or alkalinity of the solutions was measured using a pH meter (Orion research model 601 A/digital ionalyzer pH meter). Absorbance measurements were taken by placing the samples of the sensor membrane within a quartz cuvette relative to the surrounding air and blank samples of the optode. The thickness of the optode was determined using a digital microscope (Ray Vision Y 103) that was connected to a video camera (JVC TK-C 751 EG) and a digital micrometer (Mitutoyo, Japan) with a precision of ±0.001 mm.For the analysis utilizing atomic absorption, a Shimadzu model 670 atomic absorption spectrometer equipped with flame atomization was utilized. The operational parameters were established based on the recommendations of the manufacturer. Measurements were conducted using a flame generated by a mixture of nitrous oxide and acetylene gas.
2.3. Preparation of the ligand
To synthesize BDBFM, a reaction was carried out by combining a solution of substituted benzene-1,3-amine (1.08 g) (10 mmol) and furan-2-carbaldehyde (1.65 mL) (20 mmol) (density = 1.16) in 10 mL of absolute ethanol. The mixture was refluxed for 5.0 hours, followed by cooling and dilution with ice-cold water. The resulting solid was purified through recrystallization from ethanol, leading to the isolation of pure BDBFM.The chemical structure of BDBFM was confirmed using various techniques such as FTIR, elemental analysis (C, H, and N), and 1H-NMR spectroscopy. To prepare a stock solution of BDBFM, an accurately weighed quantity of the solid reagent is dissolved in the minimum required volume of ethanol, and then diluted to the desired volume in a 100 mL measuring flask (Scheme 1).
2.4. Preparation of optodes
For the preparation of the optode, cellulose triacetate (CTA) was dissolved in 10 mL of CHCl3. Separate solutions of chloroform were prepared, each containing a known quantity of a plasticizer, such as DOP, NPOE, T2EHP, TOT, DOS, or DOTP, and an additive (Aliquat-336 or DNNS). A solution of BDBFM with a known concentration was also prepared by dissolving the compound in ethanol. The casting solution for the optode was then created by combining appropriate volumes of the CTA solution, plasticizer solution, and BDBFM solution. If an additive was necessary, CHCl3 solution of Aliquat-336 or DNNS was added to the casting solution. The resulting casting solution was thoroughly mixed through ultra-sonication for 2.0 minutes and then spread onto a flat-bottom glass Petri dish with a diameter of 9 cm. The Petri dish was placed on a leveled surface to facilitate uniform film formation and solvent evaporation, which typically took around 24 hours. Once the solvent had completely evaporated, the optode film was carefully detached from the Petri dish surface. The resulting optode samples had dimensions of approximately 1 cm × 3 cm, providing a suitable size for subsequent analysis and testing. To ensure precise and accurate measurements, the thickness of the membrane was determined using a digital microscope (Ray Vision Y 103) in combination with a video camera (JVC TK-C 751 EG). This meticulous measurement process allowed for the reliable determination of the optode membrane's thickness, a critical parameter for evaluating and characterizing its performance.2.5. Uptake of Cr(VI) and the stability of the Cr(VI)–BDBFM complex
A systematic examination was conducted to assess the influence of pH on the absorption of Cr(VI), the stability of the Cr(VI)–BDBFM complex within the optodes, and the absorbance of the corresponding blanks. To investigate this, the optode film was immersed in 2.5 mL of aqueous solutions with pH values ranging from 2.0 to 8.0, each containing 125 ng mL−1 of Cr(VI), for a duration of 10 min. Under acidic conditions, acetate buffer was utilized, while borate buffer was employed under basic conditions to achieve pH adjustments. Following color development, the films were maintained at a temperature of 25 ± 2.0 °C. Cr(VI) uptake at different pH levels was evaluated by submerging the optode and measuring the absorbance when maximum sorption was achieved. The absorbance changes in the Cr(VI)-loaded optode were monitored at fixed intervals using a wavelength of 627 nm. The optode films were placed on the inner wall of a 1 cm × 1 cm × 3 cm quartz cell. To evaluate the impact of pH on the blank, the optodes were immersed in 2.5 mL solutions with pH values ranging from 2.0 to 8.0, without the presence of Cr(VI), for the same duration.2.6. The influence of plasticizers on Cr(VI) in optodes
To investigate the impact of different plasticizers on the optical properties and the capacity of optodes to absorb Cr(VI), six distinct optodes were prepared, each incorporating a specific plasticizer. The plasticizers used in this study included DOP, NPOE, T2EHP, TOT, DOTP, and DOS. The optode films were immersed in stirred aqueous solutions (2.5 mL) with a pH of 4.25, containing 125 ng mL−1 of Cr(VI), for a duration of 10 minutes. The color development in the optode was monitored by placing it on the inner wall of a spectrophotometric cell, and the absorbance was measured at a specific wavelength of 627 nm. This allowed for the assessment of the progress of color development in the optode films.2.7. Stoichiometric determination of the Cr(VI)–BDBFM complex
The stoichiometry of the Cr(VI)–BDBFM complex was investigated using several methods, including the continuous-variation (Job's), the molar ratio, and the conductometric titration methods. These experiments were conducted under specific conditions, with a pH of 4.25 and concentrations of 3 × 10−4 M for both Cr(VI) and BDBFM. Absorbance measurements were taken at a wavelength of 627 nm, which corresponds to the maximum absorbance of the complex. These measurements were utilized to determine the precise stoichiometric ratio between Cr(VI) and BDBFM in the complex.2.8. The optode tolerance to different anions in relation to Cr(VI) sorption
The tolerance of the optode to various anions, such as NO3−, Cl−, PO43−, SO42−, CH3COO−, and CO32− on the sorption of Cr(VI) was investigated. For this study, a fixed size of the optode was used along with a concentration of 125 ng mL−1 of Cr(VI). Solutions containing known concentrations of the respective salts with anions or cations were mixed with the Cr(VI) solution, and the resulting solution's pH was adjusted to 4.25 using acetate buffer. It was ensured that the contribution of CH3COO− ions added for pH adjustment was taken into account. The optode samples were submerged in the solutions, and the absorption of Cr(VI) was assessed by comparing the concentration of Cr(VI) before and after the immersion of the optode samples. The volume of the solution in contact with the optode remained constant at 2.5 mL.2.9. The quantification of Cr(VI) in the optode
The optode films, sized at 1 cm × 3 cm, were dried and securely affixed to the inner wall of a quartz cell with dimensions of 1 cm × 1 cm × 3 cm. In order to directly quantify the concentration of Cr(VI) in the optodes, synthetic samples were prepared by adding various concentrations of Cr(VI) (ranging from 25 to 550 ng mL−1) to 2.5 mL of acetate buffer solution at a pH of 4.25. Each sample was thoroughly stirred for 10 min to ensure consistent and uniform development of a violet color on the optode film. This procedure was repeated using fresh optode films for each concentration. Spectrophotometric analysis of the Cr(VI)–BDBFM complex within the optode samples was conducted at a specific wavelength of 627 nm (λmax). The resulting data were utilized to construct a calibration curve, facilitating the quantitative determination of Cr(VI) concentrations. The effectiveness of the method was further evaluated by applying it to environmental samples, where the samples were treated with known quantities of Cr(VI) ions before being exposed to the optodes.In addition, the developed method was utilized for the determination of the total chromium content. This involved the conversion of Cr(III) to Cr(VI) through the oxidation process using hydrogen peroxide. By comparing the measured level of Cr(VI) in the sample with the overall concentration of chromium, the concentration of Cr(III) could be calculated. This approach allowed for the quantification of both Cr(III) and Cr(VI) in the sample, providing valuable information about the total chromium content.
2.10. The procedure for determining Cr(VI) using GFAAS
To prepare the real sample solutions, the samples were treated with a small amount of sulfuric acid (H2SO4) and a 0.05% hydrogen peroxide (H2O2) solution. The mixture was then gently heated for 5.0 min to facilitate the oxidation of Cr(III) to Cr(VI). After cooling, a drop of hydrochloric acid (HCl) at a concentration 2.0 M was added to eliminate any excess permanganate. Once the oxidation process was completed, the samples were analyzed GFAAS, with the absorbance measured at a wavelength of 357.9 nm. The concentration of Cr(VI) in the samples was determined by referencing a calibration plot constructed using standard solutions of known Cr(VI) concentrations.2.11. Statistical analysis
The experiments were conducted in six replicates, and the obtained data were presented as the mean ± standard deviation (SD). Statistical analysis was performed using the Student's t-test and F-value. Microsoft Excel 2007 was utilized to create datasheets for data processing and analysis, including calculations of average values and recovery rates. This comprehensive statistical evaluation enabled a thorough examination of the experimental results.3 Results and discussion
3.1. Determining the stoichiometric ratio of the Cr(VI)–BDBFM complex
The stoichiometry of the Cr(VI)–BDBFM complex was investigated using two methods: Job's method and the molar ratio procedure. The absorption spectra of BDBFM and the Cr(VI)–BDBFM complex were initially recorded and are illustrated in Fig. 1. The maximum absorption wavelength for BDBFM was found to be 493 nm, while the complex with Cr(VI) exhibited its peak absorption at 627 nm. Both methods were subsequently carried out at 627 nm, the wavelength at which the complex demonstrated the highest absorbance.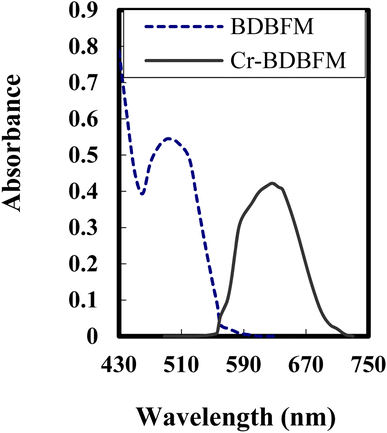 | ||
| Fig. 1 Absorption spectra of 3 × 10−4 mol L−1 of BDBFM and its complex with 125 ng per mL Cr(VI) ions at pH 4.25. | ||
In Job's method, absorbance was plotted against the mole fraction of Cr(VI), with varying concentrations of BDBFM and Cr(VI). The plot exhibited an inflection point at 0.35, indicating the presence of two BDBFM molecules in the formed complex. Furthermore, the molar ratio method yielded a BDBFM to Cr(VI) ratio of 2.0, providing additional evidence for the stoichiometric ratio of (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for BDBFM to Cr(VI). To further validate these findings, conductometric titration was employed.
1) for BDBFM to Cr(VI). To further validate these findings, conductometric titration was employed.
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K, which is the conditional formation constant, was determined by employing the Harvey and Manning equation with the data obtained from the aforementioned methods. The calculated log
K, which is the conditional formation constant, was determined by employing the Harvey and Manning equation with the data obtained from the aforementioned methods. The calculated log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) K value was determined to be 4.46, while the true constant was found to be 4.30. The interaction between Cr(VI) and BDBFM leads to the formation of a distinctive violet-colored complex known as the cationic complex [Cr(VI)–(BDBFM)2]n+. This complex formation is highly specific to Cr(VI) and is widely utilized for the colorimetric determination of Cr(VI) in aqueous samples.
K value was determined to be 4.46, while the true constant was found to be 4.30. The interaction between Cr(VI) and BDBFM leads to the formation of a distinctive violet-colored complex known as the cationic complex [Cr(VI)–(BDBFM)2]n+. This complex formation is highly specific to Cr(VI) and is widely utilized for the colorimetric determination of Cr(VI) in aqueous samples.
For the specific detection of Cr(VI), a membrane optode was developed by incorporating BDBFM into a CTA matrix that was plasticized with DOP. Upon exposure of this optode to an aqueous solution containing Cr(VI) at pH 4.25, a vibrant violet color develops within both the membrane and the surrounding solution. This color change serves as a visual indicator for the presence of Cr(VI) and allows for its sensitive and selective detection.
To ensure the optimal performance of the optode in extracting Cr(VI) from aqueous solutions, it is crucial for the membrane to possess certain characteristics such as homogeneity, flexibility, thinness, and mechanical strength. These criteria served as the foundation for the optimization of the membrane composition. Various combinations of supporting polymers, extractants, and reagents were initially tested to identify the most suitable combination for sensing Cr(VI) ions. The concentrations of Aliquat 336, DOP, and CTA, which served as the optimal carrier, plasticizer, and polymer for the chromophore BDBFM, were adjusted to enhance the sensitivity, selectivity, and stability of the optode. Among the critical components, the base polymer and the plasticizer play a significant role in determining the strength and flexibility of the membrane. Thus, different concentrations of these components were tested at three levels, while maintaining the Aliquat 336 and BDBFM concentrations at 40% and 1.0%, respectively, in all three setups. A combination of 6.0% DOP and 53% CTA resulted in minimal chromophore leakage, but the membrane exhibited rigidity due to the closely packed CTA strands caused by the low amount of plasticizer. On the other hand, the membrane containing 16% DOP was flexible but overly oily due to the excessive amount of plasticizer. The issue of leaching of the chromophore was addressed during the optimization process. It was observed that the optode composition consisting of 10% DOP and 49% CTA exhibited the best stability and flexibility while minimizing leaching. The ratios of DOP and CTA, along with the corresponding characteristics of the resulting optodes, are provided in Table 1. Once the base polymer and plasticizer concentrations were optimized, the BDBFM concentration was adjusted between 0.5 and 1.5 wt%. It was found that increasing the immobilized BDBFM content beyond 1.0 wt% resulted in a darker starting membrane color, which significantly reduced the sensitivity of the optode despite enhancing the reaction rate with Cr(VI). Therefore, the optimal BDBFM level was determined to be 1.0 wt%, leading to the adjustment of CTA to 49%. By using the composition of Aliquat 336 (40%), DOP (10%), CTA (49%), and BDBFM (1.0%), a stable and flexible membrane was obtained without any observed leaching.
| Conditions | PIM characteristic |
|---|---|
| 6.0% DOP and 53% TCA | No leakage observed – rigid membrane |
| 16% DOP and 43% TCA | Oily membrane – leaching observed |
| 10% DOP and 49% PVC | Stable, no leaching observed – flexible |
Optimizing the membrane composition, controlling room temperature at 25 ± 2.0 °C, managing humidity, and prolonging the casting solution's evaporation time from 24 to 48 hours were crucial for achieving an optode with high-quality. These findings emphasize the significant impact of external factors such as humidity and temperature on the membrane development process. Notably, the optode membrane's physical appearance remained unchanged even after two weeks of storage in a sealed bag, indicating its stability and durability.
The average thickness of the prepared membrane was found to be 31 ± 2 mm, which is considered optimal for facilitating effective ion mobility in the reaction involving the Cr(VI)–BDBFM complex. This thickness falls within the desired range, as it is neither too thick (greater than 100 mm) nor too thin (less than 5 mm). It is considered suitable for use as a transducer in an optical optode membrane based on the co-extraction principle.58
The pH value plays a crucial role in the selectivity and sensitivity of the sensing optode for Cr(VI) ions. Various buffer solutions spanning a pH range of 2.25 to 10, including borate, acetate, thiel, phosphate, and universal buffers, were evaluated. Among them, the acetate buffer solution exhibited the highest effectiveness. Furthermore, an optimal pH value of 4.25 was determined for the reaction, as depicted in Fig. 2. These findings indicate that the presence of Aliquat 336 within the membrane does not influence the ideal pH range for the formation of the BDBFM and Cr(VI) complex.
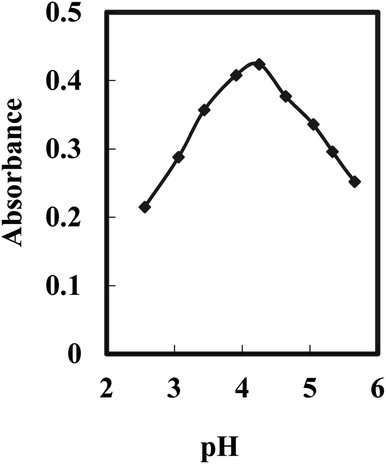 | ||
| Fig. 2 Effect of the pH value on the optode response for 125 ng mL−1 of Cr(VI) under optimum conditions. | ||
The complex formation between BDBFM and Cr(VI) still occurs within the optimal pH range of 4.0–4.5, which is known for Cr(VI) complexation in aqueous solutions. This suggests that the interaction between BDBFM and Aliquat 336 does not affect the functional groups of BDBFM involved in complex formation with Cr(VI). It was observed that the absorbance values decreased at lower and higher pH values. The decrease at lower pH values is attributed to the competition between H+ and Cr(VI) ions for binding with BDBFM. On the other hand, at higher pH values, the formation of solid CrO4− impedes the binding of Cr(VI) to immobilized BDBFM.
The performance of the optode is influenced not only by the immobilization technique and pH value but also by the type and quantity of the immobilized reagent.59Fig. 3 illustrates the effect of varying BDBFM concentrations on the optode preparation while keeping the Cr(VI) concentration constant at 125 ng mL−1 and maintaining a pH of 4.25. The response of the optode increases with increasing initial BDBFM concentration, reaching its peak at 1.0%. This enhanced response is achieved because the BDBFM percentage is sufficient to react with Cr(VI) ions in the presence of Aliquat 336, facilitating optimal performance of the optode. The proposed mechanism for the interaction between the BDBFM sensor and Cr(VI) ions and its complexation is depicted in Scheme 2. The images of the sensors in the schematic illustration are photos of the sensors.
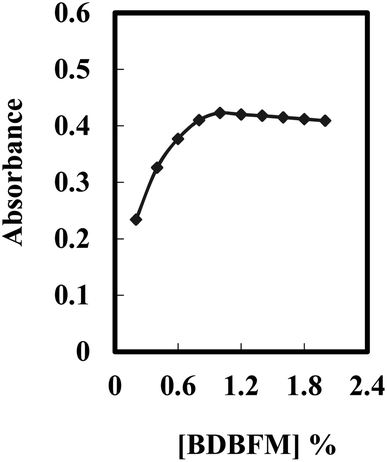 | ||
| Fig. 3 Effect of the BDBFM percentage on the optode membrane immersed in 125 ng mL−1 of Cr(VI) under optimum conditions. | ||
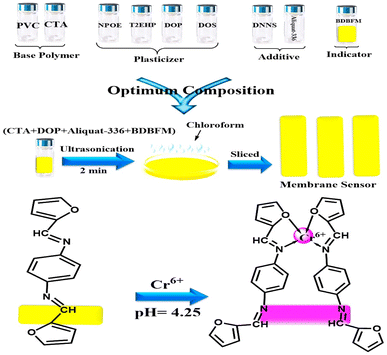 | ||
| Scheme 2 Schematic representation for the preparation and complexation of the formed optical sensor. The images are real photos of the sensor. | ||
The response time of the optode is predominantly influenced by its physical parameters.60 To determine the ideal duration for complete complexation of BDBFM–Cr(VI), an experiment was conducted. Fig. 4 depicts the response of the optode to two different concentrations of Cr(VI). The steady-state response varied based on the concentration of Cr(VI) ions, with response times of ten minutes for low concentrations and seven minutes for high concentrations.
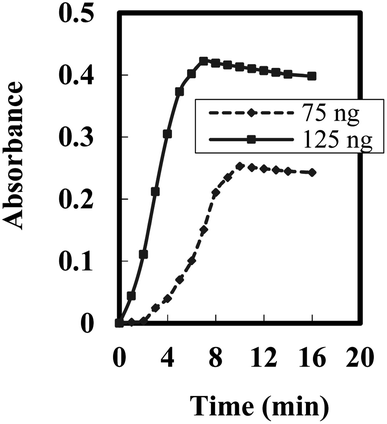 | ||
| Fig. 4 Steady state response time of the optode towards different [Cr(VI)] --- 75 ng mL−1 and – 125 ng mL−1. | ||
To overcome the issue of a long response time, a kinetic approach was implemented. Instead of measuring the steady-state response, the intensity difference of the optode was measured at a fixed time. This kinetic approach proved to be a more favorable method for determining the concentration of Cr(VI) ions. By measuring the absorbance after 10 min of reaction time, the time required to generate a significant signal from the initial reaction between BDBFM and Cr(VI) was significantly reduced. The stirring of the Cr(VI) solution had a significant impact on the response of the formed optode. Stirring the solution resulted in an eight-fold enhancement compared to the non-stirred solution. This enhancement can be attributed to the movement of Cr(VI) ions towards the immobilized BDBFM. Stirring facilitates the diffusion of Cr(VI) ions across the membrane, allowing them to reach BDBFM more quickly and promoting the reaction between Cr(VI) ions and BDBFM. In contrast, in the non-stirred process, the diffusion of Cr(VI) ions across the membrane relies solely on the concentration gradient.61
The proposed optode exhibits a notable feature of regenerability, allowing for multiple reuses of a single optode while minimizing reagent consumption. Various regenerating reagents, including EDTA, HNO3, SCN, and thiourea, were investigated. Regeneration with EDTA proved to be time-consuming, while regeneration with SCN and thiourea did not yield consistent results. However, 0.05 M nitric acid demonstrated complete regeneration within a short duration (less than 40 seconds) and exhibited high reproducibility. The membrane could be regenerated over 12 times without any loss of its characteristics, leading to the selection of 0.05 M nitric acid as the preferred regenerating reagent.
The precision and reliability of the optode are critical considerations in the development of chemical sensors. Precision refers to the ability of the optode to produce consistent results when used repeatedly, while reliability is related to the consistency of results obtained from different sets of optodes.62 Precision was evaluated by independently constructing eight optodes under similar conditions and measuring the absorbance at λmax 627 nm (with three repeated determinations) using a Cr(VI) ion solution of 125 ng mL−1 at pH 4.25. The relative standard deviation (RSD) value for precision was determined to be 1.65%, indicating good repeatability. The reproducibility, which measures consistency across different sets of optodes, was found to be 2.25%. The slight variation observed can be attributed to differences in the construction process, such as variations in the concentration of immobilized BDBFM and the thickness of the polymeric ion membrane (PIM).
To evaluate the reversibility of the sensor, a concentration of 125 ng mL−1 of Cr(VI) was introduced to the optode, followed by treatment with 0.05 M HNO3 for regeneration. The results demonstrated that the optode exhibited complete reversibility, with an average regeneration time of approximately three minutes. The reproducibility of the optode after regeneration was also investigated, and satisfactory results were obtained with a relative standard deviation (RSD) of 2.89%. The regenerability of the optode can be attributed to the properties of BDBFM, including the azomethane group in the BDBFM molecule that minimizes leaching issues, and the lipophilic nature of BDBFM that prevents leaching into the analyte solution. The incorporation of Aliquat 336 as a plasticizer effectively retains BDBFM within the optode, reducing the likelihood of leaching even after multiple regeneration cycles.
The stability of the optode was assessed by observing the reduction in Cr(VI) ions from the optode samples in contact with an aqueous solution over a two-week period. The decrease in absorbance of the formed Cr(VI)–BDBFM complex indicated the loss of Cr(VI). Additionally, the dimensions and weight of the optode were monitored throughout the same duration to detect any changes. The stability of the optode was assessed over a period of three weeks in an equilibrating solution at pH 4.25. Throughout the testing period, the optode remained stable and consistently exhibited the highest absorbance values at pH 4.25, with a calculated RSD value of 1.95%. These results indicate that the sensitivity of the optode remains constant even after exposure to the equilibrating solution and atmospheric conditions. The stability can be attributed to the presence of Aliquat, which contributes to the robustness and reliability of the optode.
3.2. Interferences
The optode’s capability to tolerate common anions and cations typically found in aqueous systems, as well as its specific affinity for Cr(VI) uptake and color development, was thoroughly investigated. The summarized outcomes presented in Table 2 indicate that the optode displays a strong selectivity towards Cr(VI) over a wide concentration range, even in the presence of various anions and cations. These additional ions do not have any discernible impact on the optode’s optical properties. This was confirmed through experiments where optode samples were added to solutions containing the same concentrations of these salts but lacking Cr(VI). In such instances, the absorbance of the optodes at the characteristic wavelength of 627 nm did not show any increase. The optode’s exceptional selectivity for Cr(VI), both in terms of uptake and color development, suggests its potential for precise analysis of Cr(VI) in real-world samples, even when they contain high concentrations of other ions.| Foreign ion | Tolerance limita (µg mL−1) | Foreign ion | Tolerance limita (µg mL−1) |
|---|---|---|---|
| a Tolerance limit was defined as the ratio that causes less than ±5.0% interference. b With 100 mg per L tartrate. | |||
| Na+, Li+, and CH3COO− | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Pb2+, Cd2+, and tartrate | 4250 |
| K+, PO43−, and oxalate | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
Ag+, Sn2+, and thiourea | 4000 |
| Glucose and fructose | 16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Cr3+ and CO32− | 3500 |
| Urea and ascorbic acid | 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Cr6+, W6+, and Cl− | 3000 |
| Ba2+, N3−, and BrO3− | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
Cr6+, Se4+, and F− | 2750 |
| Al3+, NO3−, and citrate | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
As3+, I−, and Br− | 2400 |
| Ca2+, Mg2+, and SO42− | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
As5+, Ti3+, and HCO3− | 2000 |
| La3+, Au3+, and NO2− | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Mo6+ and IO4− | 1600 |
| Fe2+, Zn2+, and Bi3+ | 8500 | La3+, Y3+, and Sc3+ | 1400 |
| Co2+, Cu2+, and NH4+ | 7500 | Be2+ and Mn2+ | 1200 |
| Cr3+, Bi3+, and Ga3+ | 6750 | Th4+ and UO22+ | 900 |
| Zr4+ and Hf4+ | 6000 | V5+b | 750 |
| Ce3+ and Ce4+ | 5250 | Pd2+ and Pt4+ | 600 |
| Hg2+, Sn4+, and S2O32− | 4750 | EDTA and CN− | 500 |
3.3. Quantitative determination of Cr(VI) in the optode sample
The membrane optode developed in this study offers practical applications for the visual colorimetric sensing of Cr(VI) in aqueous samples. The optode samples display a distinct violet color that corresponds to the concentration of Cr(VI) present in the samples. Leveraging the sensitivity of the human eye to subtle color changes, this optode enables the determination of Cr(VI) concentration ranges in aqueous samples through visual observation.Under optimal experimental conditions, the proposed optode demonstrates a linear dynamic range for Cr(VI) concentrations from 10 to 220 ng mL−1, as indicated in Table 3. Additionally, the optode exhibits a rapid response time of five minutes. The calculated detection limit (LOD) and quantification limit63 show values of 2.85 and 9.50 ng mL−1, respectively. These findings highlight the significantly improved detection limit of the optode compared to previously reported methods.33–35,46–55 The observed enhancement in sensitivity can be ascribed to the inclusion of Aliquat 336, which enhances the extraction of Cr(VI) ions from the aqueous phase, leading to improved sensitivity of the optode. In comparison to other methods, the optode exhibits superior sensitivity in detecting Cr(VI) ions.
| Parameters | Proposed optode | Parameters | Proposed optode |
|---|---|---|---|
| a For six replicate determination of 125 ng per mL Cr(VI). | |||
| pH | 4.25 | Quantification limit (ng mL−1) | 9.50 |
| λ max (nm) | 627 | Reproducibility (RSD%)a | 1.33 |
| Beer's range (ng mL−1) | 10–220 | Regression equation | |
| Ringbom range (ng mL−1) | 25–200 | Slope (ng mL−1) | 16.4 |
| Molar absorptivity (L mol−1 cm−1) | 4.36 × 107 | Intercept | −0.07 |
| Detection limit (ng mL−1) | 2.85 | Correlation coefficient (r) | 0.9985 |
3.4. Comparison of the proposed optode with other reported sensors and procedures
A comprehensive comparison is made between the proposed Cr(VI) optode and previously reported membrane-based sensors as well as other techniques for Cr(VI) determination (Table 4). The performance of the proposed optode surpasses that of previous sensors in terms of its working concentration range and limit of detection (LOD). However, it does exhibit interference from specific ions such as Fe2+, Cu2+, and Sn2+. Nevertheless, the proposed optode demonstrates satisfactory sensitivity and selectivity for the detection of trace amounts of Cr(VI) in real samples.| Method | Detection | LOD, ng mL−1 | RSD, % | Range, ng mL−1 | Sample volume | Ref. |
|---|---|---|---|---|---|---|
| a Task specific ionic liquid-based in situ dispersive liquid–liquid microextraction. b Single drop microextraction. c Diffuse reflectance Fourier transform infrared spectroscopy. d Dual electro-membrane extraction. e Cloud point assisted dispersive ionic liquid–liquid microextraction. f Cloud point extraction. g Solidified floating organic drop microextraction. h Ultrasound assisted-deep eutectic solvent based emulsification liquid phase microextraction. i Directly suspended single droplet microextraction. | ||||||
| TSIL-DLLMEa | FAAS | 5.7 | 1.1 | 25–750 | 10 mL | 64 |
| SPE | ETAAS | 0.056 | 2.5 | 2.0–160 | 25 mL | 65 |
| SDMEb | DRS-FTIRc | 800 | 2.6 | 5–500 | 8.0 mL | 66 |
| DEMEd | HPLC | 5.4 | 9.8 | 20–500 | 2.1 mL | 67 |
| CP-DLLMEe | ETAAS | 0.005 | 3.8 | 0.02–1.75 | 5.0 mL | 68 |
| CPEf | ETAAS | 0.02 | 2.6 | — | 10 mL | 69 |
| SFODMEg | ETAAS | 0.006 | 5.1 | 0.03–0.13 | 10 mL | 70 |
| UA-DES-ELPMEh | FAAS | 5.5 | 6.0 | — | 10 mL | 71 |
| DSDMEi | ETAAS | 0.03 | 4.7 | 0.10–2.0 | 30 mL | 72 |
| Optode | Spectra | 2.85 | 1.9 | 10–220 | 2.5 | This work |
In the field of Cr(VI) determination, there is a scarcity of reported optodes, with only a limited number of articles available on optodes designed for Cr(VI) and total chromium analysis. The use of BDBFM as a chromophoric reagent in the proposed optode sets it apart from other optodes for chromium monitoring, displaying comparable or even superior performance. The proposed sensor is superior to paper-based analytical devices based on the selectivity, simplicity, rapidity, and a wider range of detection.73–76 Moreover, the proposed optode offers a lower limit of detection compared to optodes incorporating alternative supporting materials. To the best of the authors’ knowledge, this study presents the inaugural utilization of BDBFM in an optode membrane for the purpose of Cr(VI) ion determination.
The characteristics of the proposed Cr(VI) optode are further compared to those of other techniques reported in the literature for Cr(VI) analysis. The optode’s linear range and detection limit are considered acceptable when compared to those of these methods. Notably, there are no previous reports on optode membranes incorporating chip reagents specifically for the determination of Cr(VI) ions in solution. The proposed procedure stands out for its simplicity, speed, and elimination of time-consuming reagent and optode preparation. With a response time of 5.0 minutes for each concentration, efficient calibration curve generation is facilitated. The procedure involves adding buffer and Cr(VI) to the optode in the measuring cell, followed by measuring the absorption spectra after 5.0 min.
3.5. Speciation of chromium
The high selectivity exhibited by the developed optical sensor in this study allows for its application in the speciation of Cr(III)/Cr(VI). This means that, even in solutions containing both Cr(VI) and Cr(III) ions, the optode can accurately determine the concentration of Cr(VI). Furthermore, by oxidizing Cr(III) to Cr(VI) using H2O2 as an oxidizing agent, the optode can also be used to determine the total chromium concentration in the same solution. The concentration of Cr(III) ions can then be calculated by subtracting the amount of Cr(VI) from the total chromium concentration.In order to oxidize Cr(III) to Cr(VI), a mixture of Cr(VI) and Cr(III) ions is subjected to a treatment involving 2.0 mL of 0.1 M H2O2 and 0.5 mL of 0.1 M HNO3 in a beaker. The beaker is heated at approximately 80 °C for 30 minutes, while being covered with a watch glass during the oxidation process. After the solution has cooled down, its pH is adjusted to pH 4.25 using acetate buffer. Subsequently, the solution is transferred to a calibrated 10 mL flask and diluted with water up to the mark. The recommended procedure for Cr(VI) determination using the optode is then followed. Additionally, the total chromium content is determined using flame atomic absorption spectroscopy (FAAS) for comparison purposes. The results of chromium speciation at various Cr(III)/Cr(VI) ratios are summarized in Table 5.
| Added (µg) | Founda (µg) | Recoverya (%) | Total Cr, FAAS | |||||
|---|---|---|---|---|---|---|---|---|
| Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Total Cr | Cr(III) | Cr(VI) | Total Cr | |
| a Average of six determinations. | ||||||||
| 0.00 | 2.00 | 0.00 | 1.98 | 1.98 | 0.00 | 99.00 | 99.00 | 2.05 |
| 0.50 | 1.50 | 0.49 | 1.52 | 2.01 | 98.00 | 101.33 | 100.50 | 1.97 |
| 1.00 | 1.00 | 1.01 | 1.01 | 2.02 | 101.00 | 101.00 | 101.00 | 1.95 |
| 1.50 | 0.50 | 1.48 | 0.49 | 1.97 | 98.67 | 98.00 | 98.50 | 2.04 |
| 2.00 | 0.00 | 2.01 | 0.00 | 2.01 | 100.50 | — | 100.50 | 1.97 |
| 2.25 | 0.75 | 2.27 | 0.76 | 3.03 | 100.89 | 101.33 | 101.00 | 2.95 |
| 3.00 | 0.00 | 2.99 | 0.00 | 2.99 | 99.67 | — | 99.67 | 3.05 |
The data presented in Table 5 reveal satisfactory agreement between the total chromium measurements obtained using the proposed optode membrane and those obtained using the GFAAS method, considering the reported experimental uncertainties. Furthermore, accurate measurements of both Cr(III) and Cr(VI) ions present in the initial solutions can be achieved using the proposed optode for all tested Cr(VI)/Cr(III) mixtures.
3.6. Analytical applications
The developed method was successfully utilized to perform the speciation analysis of Cr(III) and Cr(VI) in various natural water samples, including tap water, Nile river water from Benha City, and a sea water sample from the Mediterranean Sea at Port Said City. To evaluate the reliability of the method, chromium species were deliberately spiked into these samples.Meticulous precautions were taken during the collection and preparation of the water samples to ensure precise and reliable analysis. Specially pre-washed polyethylene bottles were utilized to prevent any potential contamination. To eliminate any particulate matter, the samples underwent filtration through a Millipore cellulose membrane that had a pore size of 0.45 µm. To maintain sample integrity and minimize chemical changes, the samples were stored in acidified polyethylene bottles at a temperature of 4.0 °C. Nitric acid was added to achieve a concentration of 1.0%, thereby stabilizing the samples and preventing degradation or alteration of the analytes during storage. The acidification step is vital for sample preservation.
For tap water samples, an additional step was incorporated to remove chlorine. Activated charcoal was introduced into the samples and allowed to interact for a duration of 5.0 minutes. Subsequently, the samples were subjected to a secondary filtration process to eliminate any residual charcoal particles.
Before conducting the analysis, the pH of the samples was carefully adjusted to 4.25. This specific pH value was chosen to ensure optimal conditions for the subsequent analysis of Cr(VI) using the developed method. By precisely controlling the pH, the accuracy and reliability of the analysis were significantly enhanced.
The results obtained, carefully documented in Table 6, exhibit excellent agreement between the measured amounts of the analytes and the amounts added, thereby confirming the accuracy and validity of the proposed optode for detecting trace levels of chromium in both spiked and natural water samples. The relative standard deviations for Cr(III) and Cr(VI) in the water samples demonstrate a commendable range of 1.15% to 2.48% and 1.56% to 2.84%, respectively. Furthermore, the proposed procedure was successfully extended to solid samples, which underwent microwave digestion, yielding relative standard deviations ranging from 3.5% to 8.0%.
| Samples | Added (µg) | Founda (µg) | Recovery (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Total | Cr(III) | Cr(VI) | Total | |
| a Average of six determinations. | ||||||||
| Tap water from Benha City, Egypt | — | — | 0.220 | 0.400 | 0.620 | — | — | — |
| 0.3 | 0.5 | 0.522 | 0.910 | 1.432 | 100.38 | 101.11 | 100.85 | |
| 0.5 | 0.30 | 0.720 | 0.695 | 1.415 | 100.00 | 99.29 | 99.65 | |
| 0.4 | 0.4 | 0.616 | 0.81 | 1.426 | 99.35 | 101.25 | 100.42 | |
| River water from Shobra, Egypt | — | — | 0.430 | 0.880 | 1.310 | — | — | — |
| 0.5 | 0.75 | 0.925 | 1.627 | 2.552 | 99.46 | 99.82 | 99.69 | |
| 0.75 | 0.50 | 1.170 | 1.370 | 2.540 | 99.15 | 99.28 | 99.22 | |
| 0.60 | 0.60 | 1.020 | 1.480 | 2.500 | 99.03 | 100.00 | 99.60 | |
| Sea water from Port Said, Egypt | — | — | 0.510 | 1.130 | 1.640 | — | — | — |
| 0.30 | 0.20 | 0.818 | 1.333 | 2.151 | 100.99 | 100.23 | 100.51 | |
| 0.25 | 0.25 | 0.755 | 1.370 | 2.125 | 99.34 | 99.28 | 99.30 | |
| 0.20 | 0.30 | 0.717 | 1.433 | 2.15 | 100.99 | 100.21 | 100.47 | |
To further demonstrate the practical applicability of the proposed system, a variety of food samples were carefully analyzed. Beverage samples, including orange juice, soda, cola, apple juice, and Sprite drink, were carefully collected from a local market in Port Said, Egypt. To prepare these samples for analysis, a degassing step was performed by vortexing them for 2.0 minutes. Subsequently, the samples were diluted five times with high purity water. After filtration using a 0.45 µm membrane filter, the solutions were refrigerated at 5 °C to guarantee their preservation and stability for future utilization. Solid samples such as coffee, tea, and tobacco were obtained from supermarkets in Benha City for analysis. Before subjecting these solid samples to the proposed optode testing, a digestion process was carried out utilizing a microwave system. The digestion procedure involved specific time intervals and power levels. The samples were microwaved for 2.0 minutes at 250 W, followed by 2.0 minutes at 0 W, 6.0 minutes at 250 W, 5.0 minutes at 400 W, and 8.0 minutes at 550 W. Venting was then conducted for an additional 8.0 min. During the digestion process, 1.0 gram of the tobacco, coffee, or tea samples was combined with 6.0 mL of 65% HNO3 and 2.0 mL of 30% H2O2 in the microwave system. The resulting mixture was subsequently diluted to a total volume of 25 mL using bi-distilled water. A blank sample was prepared following a similar procedure, excluding the addition of the solid sample. By adhering to these meticulous preparation procedures, both the beverage and solid samples were properly treated and made ready for subsequent analysis using the proposed optode method.
Food products, particularly those of wheat, play a significant role in the human diet. It is crucial to detect the presence of toxic Cr in the form of Cr(VI). In this study, a total of 50 commercially available bread samples were collected from a local market in Benha, Egypt. The extraction of Cr(VI) from these samples was performed using a 0.01 M NaOH solution at room temperature for a duration of 17 hours.77 Following the extraction process, the supernatant was obtained by centrifugation, and its composition was examined using the graphite furnace atomic absorption spectrometry (GFAAS) technique. The aforementioned procedure was applied to the resulting solutions, with the pH carefully adjusted to 4.25, allowing for the determination of chromium content.
For the analysis of a soil sample collected from Benha, Egypt, a precise procedure was followed. Initially, 2.0 grams of the soil sample were carefully transferred to a beaker. To initiate the digestion process, 20 mL of aqua regia was added to the beaker. The solution obtained was evaporated in a fume hood until dryness. This evaporation process was repeated twice to ensure thorough digestion of the sample. Next, 10 mL of bi-distilled water was added to the remaining residue in the beaker. The mixture was mixed well and then filtered using a high-quality blue band filter paper from Macherey-Nagel, a reputable company based in Düren, Germany. To remove any remaining impurities, the insoluble portion was meticulously washed with bi-distilled water.
The final solutions obtained from the filtration process were subjected to the proposed procedure, as previously described, to determine the chromium (Cr) content. Additionally, the Cr content was assessed using a sensitive technique utilized for analyzing trace elements which is GFAAS. During the analysis of real soil samples, the total chromium content was assessed as Cr(III) by converting any present Cr(VI) to Cr(III) utilizing the prescribed procedure as recommended.78,79
By utilizing the optimized parameters as described earlier, the system provided robust results for the food samples, as presented in Table 7. The accuracy of the method was evaluated by comparing these results with those obtained using GFAAS. Rigorous statistical tests, including the calculation of F-values and t-tests at a 95% confidence level, were performed, revealing no significant deviations between the two methods.80
| Sample | Added (µg g−1) | Founda (µg g−1) | RSD (%) | t-Testb | F-Valueb | |
|---|---|---|---|---|---|---|
| Optode | GF-AAS | |||||
| a Average of six determinations. b The theoretical values of t- and F- at P = 0.05 are 2.57 and 5.05, respectively. c [ng mL−1]. | ||||||
| Tobacco | — | 1.48 | 1.45 | |||
| 0.50 | 2.00 | 1.85 | 2.74 | 1.34 | ||
| 1.00 | 2.45 | 2.60 | 2.22 | 2.87 | ||
| Coffee | — | 0.27 | 0.26 | |||
| 0.75 | 1.00 | 0.98 | 3.15 | 1.58 | ||
| 1.50 | 1.75 | 1.80 | 2.76 | 3.21 | ||
| Red tea | — | 0.18 | 0.18 | |||
| 0.25 | 0.38 | 0.36 | 2.48 | 1.21 | ||
| 0.50 | 0.70 | 0.65 | 2.25 | 2.58 | ||
| Green tea | — | 0.15 | 0.16 | |||
| 0.40 | 0.56 | 0.53 | 3.27 | 1.77 | ||
| 0.80 | 0.93 | 1.00 | 2.57 | 3.43 | ||
| Soil from Benha City | — | 24.7 | 24.5 | |||
| 1.50 | 26.35 | 25.75 | 1.98 | 0.84 | ||
| 3.00 | 27.50 | 27.80 | 2.32 | 2.46 | ||
| Orange juicec | — | 0.25 | 0.25 | |||
| 6.0 | 6.40 | 6.15 | 2.53 | 1.54 | ||
| 12.0 | 12.05 | 12.50 | 1.97 | 3.16 | ||
| Sodac | — | 0.65 | 0.70 | |||
| 8.0 | 8.75 | 8.60 | 2.16 | 1.37 | ||
| 16.0 | 16.90 | 16.40 | 2.44 | 2.95 | ||
| Colac | — | 0.80 | 0.75 | |||
| 3.0 | 3.70 | 3.90 | 2.65 | 1.85 | ||
| 6.0 | 6.95 | 6.60 | 2.33 | 3.48 | ||
| Apple juicec | — | 0.25 | 0.25 | |||
| 9.0 | 9.40 | 9.10 | 1.87 | 1.62 | ||
| 18.0 | 18.00 | 18.55 | 2.05 | 3.23 | ||
| Sprite drinkc | — | 0.45 | 0.50 | |||
| 7.0 | 7.30 | 7.70 | 1.77 | 1.73 | ||
| 14.0 | 14.70 | 14.25 | 2.11 | 3.37 | ||
The analytical performance of the developed optode was thoroughly assessed for the detection of Cr(VI) in human blood (serum) and urine samples. Human serum and urine samples were collected from the Benha University Hospital to perform analytical investigations. In the case of the serum samples, the protein components were precipitated by subjecting 5.0 mL of the serum to centrifugation at 4000 rpm for 5.0 minutes. Trichloroacetic acid was used in a small volume during the centrifugation process to aid in protein precipitation. Following centrifugation, the serum portion was carefully decanted, and then diluted with deionized water at a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. For calibration purposes, a spiked sample was prepared by adding precise amounts of a Cr(III) standard solution to 0.1 mL of the human serum sample. The samples, including the spiked sample, were thoroughly mixed, and additional dilutions were made as required. The chromium concentrations in these samples were directly analyzed using the proposed optode technique, enabling accurate determination of the chromium levels.
10. For calibration purposes, a spiked sample was prepared by adding precise amounts of a Cr(III) standard solution to 0.1 mL of the human serum sample. The samples, including the spiked sample, were thoroughly mixed, and additional dilutions were made as required. The chromium concentrations in these samples were directly analyzed using the proposed optode technique, enabling accurate determination of the chromium levels.
The optode demonstrated effective detection of Cr(VI) in these samples, as well as in dietary supplements and artificially spiked human serum and urine samples. The samples were carefully prepared following the experimental procedures outlined in the study and analyzed using the standard addition method. The results, presented in a systematic manner in Table 8, exhibited excellent recoveries ranging from 97.67% to 102.67% and low relative standard deviations (RSDs) of less than 2.12%.
| Sample | Added (ng mL−1) | Founda (ng mL−1) | Total | Recovery (%) | |||
|---|---|---|---|---|---|---|---|
| Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | Cr(III) | Cr(VI) | ||
a Mean of three determinations ± confidence interval (P = 0.95, n = 5).
b Whole blood (WB) diluted with DW (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5), and the concentration is out of linear range, final Cr conc. = found Cr conc. × 5 (dilution factor). 5), and the concentration is out of linear range, final Cr conc. = found Cr conc. × 5 (dilution factor).
|
|||||||
| Whole blood | — | — | 0.655 ± 0.026 | 0.090 ± 0.003 | 0.745 ± 0.028 | — | — |
| 0.3 | — | 0.960 ± 0.048 | 0.095 ± 0.004 | 1.055 ± 0.056 | 100.52 | — | |
| — | 0.2 | 0.660 ± 0.028 | 0.285 ± 0.013 | 0.945 ± 0.045 | — | 98.28 | |
| Whole blood | — | — | 0.960 ± 0.055 | 0.205 ± 0.021 | 1.165 ± 0.064 | — | — |
| 0.4 | — | 1.350 ± 0.073 | 0.210 ± 0.011 | 1.560 ± 0.078 | 99.26 | — | |
| — | 0.3 | 0.975 ± 0.041 | 0.520 ± 0.018 | 1.495 ± 0.065 | — | 101.96 | |
| Whole bloodb | — | — | 0.215 ± 0.009 | 1.075 ± 0.057 | 1.290 ± 0.069 | — | — |
| 0.5 | — | 0.725 ± 0.031 | 1.060 ± 0.047 | 1.785 ± 0.084 | 101.40 | — | |
| — | 0.4 | 0.210 ± 0.011 | 1.485 ± 0.075 | 1.695 ± 0.092 | — | 101.71 | |
| Serum | — | — | 0.725 ± 0.033 | 0.130 ± 0.006 | 0.855 ± 0.043 | — | — |
| 0.6 | — | 1.310 ± 0.062 | 0.135 ± 0.005 | 1.445 ± 0.071 | 98.87 | — | |
| — | 0.5 | 0.735 ± 0.036 | 0.645 ± 0.016 | 1.380 ± 0.048 | — | 101.57 | |
| Serum | — | — | 1.250 ± 0.058 | 0.080 ± 0.002 | 1.330 ± 0.064 | — | — |
| 0.7 | — | 1.925 ± 0.077 | 0.075 ± 0.003 | 2.000 ± 0.082 | 98.72 | — | |
| — | 0.6 | 1.235 ± 0.061 | 0.690 ± 0.012 | 1.925 ± 0.078 | — | 102.22 | |
The superiority of the proposed method was thoroughly validated through a comprehensive comparison with the GFAAS method. Statistical tests such as F-values (for precision) and t-tests (for accuracy) were employed,80 and the calculated values consistently aligned with the theoretical values, as evidenced by the results presented in Tables 7 and 8. The developed procedure offers several notable advantages over other existing approaches. It provides a broader range of determination, reduces analysis time, improves stability, and enhances accuracy. Moreover, the proposed method eliminates the need for extraction or heating steps, which further enhances its practicality and appeal.
4 Conclusion
The optode developed in this study offers a straightforward and efficient method for detecting Cr(VI) ions. Its utilization of cellulose triacetate as a matrix ensures favorable optical and mechanical properties. Notably, the optode exhibits a reversible color change from yellow to violet upon exposure to Cr(VI) ions, and it can be easily regenerated using a 0.05 M HNO3 solution with complete reversibility. Compared to previously reported optical sensors for Cr(VI) detection, the proposed optode demonstrates a significantly faster response time. The results of the present sensor has the same accuracy and precession compared with those of the previous methods in addition to highly selectivity and low range of determination which is superior to those of the previously reported sensors.An important aspect of this study is the novel application of BDBFM in combination with the proposed optode for the determination of Cr(VI) ions. This approach has not been documented in the existing literature, making this study the first to employ BDBFM in conjunction with the proposed optode. Furthermore, the versatility of the optode sensor extends to its successful implementation in monitoring BDBFM across various sample types, including water, food, and biological, and environmental samples. This wide range of applications showcases the potential of the optode sensor beyond its primary use in Cr(VI) determination.
Ethical statement
All biological studies were carried out in strict accordance with the animal welfare guidelines of the World Organization for Animal Health. All biological experiments were performed using protocols approved by the Laboratory Animal Ethics Committee of Egypt, which was approved by Commission on the Ethics of Scientific Research, Faculty of Medicine, Benha University. In all cases, informed written consent was obtained from each participant.Author contributions
Eman R. Darwish and Reem F. Alshehri: conceptualization, data curation, investigation, methodology, visualization, validation, writing – original draft, writing – review & editing. Alaa Amin: conceptualization, data curation, investigation, supervision, writing – original draft, writing – review & editing. Mai Aish: conceptualization, investigation, methodology, validation, writing – original draft, writing – review & editing.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
The authors would like to acknowledge the Department of Chemistry, Faculty of Science, Benha, Taibah and Port Said Universities, for financial support and providing instrumental facilities.References
- K. Alizadeh, B. Rezaei and E. Khazaeli, A new triazene-1-oxide derivative, immobilized on the triacetyl cellulose membrane as an optical Ni2+ sensor, Sens. Actuators, B, 2014, 193, 267–272 CrossRef CAS.
- R. Zare-Dorabei, S. M. Ferdowsi, A. Barzin and A. Tadjarodi, Highly efficient simultaneous ultrasonic-assisted adsorption of Pb(II), Cd(II), Ni(II) and Cu(II) ions from aqueous solutions by graphene oxide modified with 2,2′-dipyridylamine: central composite design optimization, Ultrason. Sonochem., 2016, 32, 265–276 CrossRef CAS PubMed.
- R. R. Nair, A. M. Paul, E. S. Raju, D. N. Srivastava and P. B. Chatterjee, Can mechano-chemical synthesis be utilized in the progress of chemosensing technology? A case study with a series of Cu2+ selective receptors, Sens. Actuators, B, 2017, 253, 213–223 CrossRef CAS.
- Y. Ren, H. A. Abbood, F. He, H. Peng and K. Huang, Magnetic EDTA-modified chitosan/SiO2/Fe3O4 adsorbent: preparation, characterization, and application in heavy metal adsorption, Chem. Eng. J., 2013, 226, 300–311 CrossRef CAS.
- P. K. Rai and B. D. Tripathi, Heavy metals in industrial wastewater, soil and vegetables in Lohta village, India, Toxicol. Environ. Chem., 2008, 90, 245–257 CrossRef.
- C. Alquezar, et al., Heavy metals contaminating the environment of a progressive supranuclear palsy cluster induce tau accumulation and cell death in cultured neurons, Sci. Rep., 2020, 10, 1–12 CrossRef PubMed.
- J. Duruibe, M. Ogwuegbu and J. Egwurugwu, Heavy metal pollution and human biotoxic effects, Int. J. Phys. Sci., 2007, 2, 112–118 Search PubMed.
- U.D.O. Health and H. Services, Toxicological Profile for Chromium, Public Health Services Agency for Toxic Substances and Diseases Registry, Washington, DC, 1991 Search PubMed.
- O. Safety and H. Administration, Occupational Safety and Health Standards Occupational Noise Exposure, 2011 Search PubMed.
- K. M. Diniz and C. R. T. Tarley, Speciation analysis of chromium in water samples through sequential combination of dispersive magnetic solid phase extraction using mesoporous amino-functionalized Fe3O4/SiO2 nanoparticles and cloud point extraction, Microchem. J., 2015, 123, 185–195 CrossRef CAS.
- S. Sivakumar and C. Subbhuraam, Toxicity of chromium (III) and chromium (VI) to the earthworm Eisenia fetida, Ecotoxicol. Environ. Saf., 2005, 62, 93–98 CrossRef CAS PubMed.
- B. Markiewicz, I. Komorowicz, A. Sajnog, M. Belter and D. Barałkiewicz, Chromium and its speciation in water samples by HPLC/ICP-MS–technique establishing metrological traceability: a review since 2000, Talanta, 2015, 132, 814–828 CrossRef CAS PubMed.
- P. Hemmatkhah, A. Bidari, S. Jafarvand, M. R. M. Hosseini and Y. Assadi, Speciation of chromium in water samples using dispersive liquid-liquid microextraction and flame atomic absorption spectrometry, Microchim. Acta, 2009, 166, 69–75 CrossRef CAS.
- M. Habila, Y. E. Unsal, Z. A. Alothman, A. Shabaka, M. Tuzen and M. Soylak, Speciation of chromium in natural waters, tea, and soil with membrane filtration flame atomic absorption spectrometry, Anal. Lett., 2015, 48, 2258–2271 CrossRef CAS.
- U.E.P. Agency and D.O. Water, Drinking Water Standards and Health Advisories, Agencia de Protection Ambiental de los Estados Unidos (EPA), Washington, 2011 Search PubMed.
- EPA, Priority pollutant list, https://www2.epa.gov/eg/toxicand-priority-pollutants-under-clean-water-act, 6.12.2015, 2014 Search PubMed.
- U.D.O. Health and H. Services, National Health and Nutrition Examination Survey, Health Professionals Home Interview, Centers for Disease Control and Prevention, National Centers for Health Statistics, Hyattsville, MD, 2004 Search PubMed.
- C. Parish, Agency for Toxic Substances and Disease Registry 2013 Search PubMed.
- S. I. Hughes, S. S. R. Dasary, A. K. Singh, Z. Glenn, H. Jamison, P. C. Ray and H. Yu, Sensitive and selective detection of trivalent chromium using hyper Rayleigh scattering with 5,5′-dithio-bis-(2-nitrobenzoic acid)-modified gold nanoparticles, Sens. Actuators, B, 2013, 178, 514–519 CrossRef CAS PubMed.
- A. Deep, A. L. Sharma, S. K. Tuteja and A. K. Paul, Phosphinic acid functionalized carbon nanotubes for sensitive and selective sensing of chromium(VI), J. Hazard. Mater., 2014, 278, 559–565 CrossRef CAS PubMed.
- J.-H. Ye, Y. Wang, Y. Bai, W. Zhang and W. He, A highly selective turn-on fluorescent chemo dosimeter for Cr(VI) and its application in living cell imaging, RSC Adv., 2014, 4, 2989–2992 RSC.
- Y. Zhu, D. Deng, L. Xu, Y. Zhu, L. Wang, B. Qi and C. Xu, Ultrasensitive detection of lead ions based on a DNA-labelled DNAzyme sensor, Anal. Methods, 2015, 7, 662–666 RSC.
- P. Hoet, Speciation of chromium in occupational exposure and clinical aspects, in Handbook of Elemental Speciation II: Species in the Environment, Food, Medicine & Occupational Health, ed. R. Cornelis, H. Crews, J. Caruso and K. G. Heumann, 2005 Search PubMed.
- J. Kotaś and Z. Stasicka, Chromium occurrence in the environment and methods of its speciation, Environ. Pollut., 2000, 107, 263–283 CrossRef PubMed.
- X. Wu, Y. Xu, Y. Dong, X. Jiang and N. Zhu, Colorimetric determination of hexavalent chromium with ascorbic acid capped silver nanoparticles, Anal. Methods, 2013, 5, 560–565 RSC.
- H. Li, J. Li, W. Wang, Z. Yang, Q. Xu and X. Hu, A sub-nanomole level photo-electrochemical sensing platform for hexavalent chromium based on its selective inhibition of quercetin oxidation, Analyst, 2013, 138, 1167–1173 RSC.
- S. A. Steiner, M. D. Porter and J. S. Fritz, Ultrafast concentration and speciation of chromium(III) and (VI), J. Chromatogr. A, 2006, 1118, 62–67 CrossRef CAS PubMed.
- B. Wen, X. Q. Shan and J. Lian, Separation of Cr(III) and Cr(VI) in river and reservoir water with 8-hydroxyquinoline immobilized polyacrylonitrile fiber for determination by inductively coupled plasma mass spectrometry, Talanta, 2002, 56, 681–687 CrossRef CAS PubMed.
- J. Chwastowska, W. Skwara, E. Sterlinska and L. Pszonicki, Speciation of chromium in mineral waters and salinas by solid-phase extraction and graphite furnace atomic absorption spectrometry, Talanta, 2005, 66, 1345–1349 CrossRef CAS PubMed.
- I. Tsuyumoto and Y. Maruyama, X-ray fluorescence analysis of hexavalent chromium using K beta satellite peak observed as counterpart of X-ray absorption near-edge structure pre-edge peak, Anal. Chem., 2011, 83, 7566–7569 CrossRef CAS PubMed.
- H. Hesham, S. M. El-Bahy, A. M. E. Hassan and A. S. Amin, Utility of a novel optical sensor design for ultra-trace detection of chromium colorimetrically in real environmental samples, Int. J. Environ. Anal. Chem., 2023, 103, 4031–4048 CrossRef.
- S. Menon, S. P. Usha, H. Manoharan, P. V. N. Kishore and V. V. R. Sai, Metal–organic framework-based fiber optic sensor for chromium(VI) detection, ACS Sens., 2023, 8, 684–693 CrossRef CAS PubMed.
- J. Zhang and S. Li, Sensors for detection of Cr(VI) in water: a review, Int. J. Environ. Anal. Chem., 2021, 101, 1051–1073 Search PubMed.
- W. Feng, C. Yuan, T. Li and X. Yang, Ion-imprinted-polymer coated fiber-optic SPR sensor for detection of trace Cr3+ in water, Optik, 2023, 272, 170259 CrossRef CAS.
- H. A. Mohamed, B. F. Abdel-Wahab, M. N. M. Yousif and R. M. Abdelhameed, Novel allyl-hydrazones including 2,4-dinitrophenyl and 1,2,3-triazole moieties as optical sensor for ammonia and chromium ions in water, BMC Chem., 2022, 16, 26–34 CrossRef CAS PubMed.
- M. Nourbakhsh, Y. Sag, D. Ozer, Z. Aksu, T. Kutsal and A. Çaglar, A comparative study of various biosorbents for removal of chromium (VI) ions from industrial waste waters, Process Biochem., 1994, 29, 1–5 CrossRef CAS.
- A. R. Firooz, M. Movahedi and A. A. Ensafi, Selective and sensitive optical chemical sensor for the determination of Hg(II) ions based on tetrathia-12-crown-4 and chromo-ionophore I, Sens. Actuators, B, 2012, 171–172, 492–498 CrossRef CAS.
- Z. Yan, M. F. Yuen, L. Hu, P. Sun and C. S. Lee, Advances for the colorimetric detection of Hg2+ in aqueous solution, RSC Adv., 2014, 4, 48373–48388 RSC.
- I. M. I. Moustafa, A. S. Amin and E. Darwish, A novel bulk optode for ultra-trace detection of antimony coupled with spectrophotometry in food and environmental samples, Talanta Open, 2023, 7, 100197 CrossRef.
- A. S. Amin, Application of a triacetylcellulose membrane with immobilizated of 5-(2′,4′-dimethylphenylazo)-6-hydroxypyrimidine-2,4-dione for mercury determination in real samples, Sens. Actuators, B, 2015, 221, 1342–1347 CrossRef CAS.
- A. S. Amin, S. El-Bahy and H. H. El-Feky, Utility of 5-(2′,4′-dimethylphenyl-azo)-6-hydroxypyrimidine-2,4-dione in PVC membrane for a novel green optical chemical sensor to detect zinc ion in environmental samples, Anal. Biochem., 2022, 643, 114579 CrossRef CAS PubMed.
- A. S. Amin, Optical chemical sensor of lutetium(III) in water based on 2-nitro-6-(thiazol-2-yldiazenyl)phenol immobilized on polymethyl methacrylate and 2-nitrophenyl octyl ether matrix, Eurasian J. Anal. Chem., 2018, 13, 1–12 Search PubMed.
- A. A. Gouda and A. S. Amin, Design of a novel optical sensor for determination of trace amounts of tin in food and in environmental samples, Int. J. Environ. Anal. Chem., 2022, 102, 7313–7328 CrossRef CAS.
- H. H. El-Feky, A. S. Amin and E. M. I. Moustafa, Utilization of a plasticized PVC optical sensor for the selective and efficient detection of cobalt(II) in environmental samples, RSC Adv., 2022, 12, 18431–18440 RSC.
- H. H. El-Feky, S. M. El-Bahy and A. S. Amin, Sensitive optical thin film sensor based on incorporation of 2-(2′-hydroxynaphthylazo)-benzothiazole in a sol–gel matrix for detection of manganese(II) in environmental samples, Anal. Biochem., 2022, 651, 114720 CrossRef CAS PubMed.
- A. S. Amin and H. El-Feky, A novel optode for vanadium speciation: Sol–gel based optical sensor for vanadium determination, Iran. J. Anal. Chem., 2021, 8, 65–77 CAS.
- A. S. Amin, H. H. El-Feky and N. Hassan, Novel sensor for selective monitor-ring of trace ytterbium ions based on an agarose based optical membrane, RSC Adv., 2022, 12, 26620–26629 RSC.
- I. Oehme and O. S. Wolfbeis, Optical sensors for determination of heavy metal ions, Mikrochim. Acta, 1997, 126, 177–192 CrossRef CAS.
- E. Castillo, M. Granados and J. L. Cortina, Liquid-supported membranes in chromium(VI) optical sensing: transport modeling, Anal. Chim. Acta, 2002, 464, 197–208 CrossRef CAS.
- E. Castillo, M. Granados and J. L. Cortina, Chemically facilitated chromium (VI) transport throughout ananion-exchange membrane: Application to an optical sensor for chromium(VI) monitoring, J. Chromatogr. A, 2002, 963, 205–211 CrossRef CAS PubMed.
- J. Guo, D. Huo, M. Yanga, C. Hou, J. Li, H. Fa, H. Luo and P. Yang, Colorimetric detection of Cr(VI) based on the leaching of gold nanoparticles using a paper-based sensor, Talanta, 2016, 161, 819–825 CrossRef CAS PubMed.
- M. Ismail, M. I. Khan, K. Akhtar, M. A. Khan, A. M. Asiri and S. B. Khan, Biosynthesis of silver nanoparticles: A colorimetric optical sensor for detection of hexavalent chromium and ammonia in aqueous solution, Phys. E, 2018, 103, 367–376 CrossRef CAS.
- R. Guell, C. Fontas, V. Salvado and E. Antico, Development of a selective optical sensor for Cr(VI) monitoring in polluted waters, Anal. Chim. Acta, 2007, 594, 162–168 CrossRef PubMed.
- Y. M. Scindia, A. K. Pandey, A. V. R. Reddy and S. B. Manohar, Chemically selective membrane optode for Cr(VI) determination in aqueous samples, Anal. Chim. Acta, 2004, 515, 311–321 CrossRef CAS.
- J. Zhang and S. Li, Sensors for detection of Cr(VI) in water: a review, Int. J. Environ. Anal. Chem., 2021, 101, 1051–1073 CrossRef.
- H. T. S. Britton, Hydrogen Ions, Chapman and Hall, London, 4th edn, 1952 Search PubMed.
- J. Ma, B. Yang and R. H. Byrne, Determination of nanomolar chromate in drinking water with solid phase extraction and a portable spectrophotometer, J. Hazard. Mater., 2012, 219–220, 247–252 CrossRef CAS PubMed.
- C. C. Li and M. S. Kuo, Application of the acetylacetone chelation solid-phase extraction method to measurements of trace amounts of beryllium in human hair by GFAAS, Anal. Sci., 2002, 18, 607–611 CrossRef CAS PubMed.
- K. Wygladacz and E. Bakker, Imaging fiber microarray fluorescent ion sensors based on bulk optode microspheres, Anal. Chim. Acta, 2005, 532, 61–66 CrossRef CAS.
- C. McDonagh, C. S. Burke and B. D. MacCraith, Optical chemical sensors, Chem. Rev., 2008, 108, 400–421 CrossRef CAS PubMed.
- M. Ahmad and R. Narayanaswamy, Optical fibre Al(III) sensor based on solid surface fluorescence measurement, Sens. Actuators, B, 2002, 81, 259–266 CrossRef CAS.
- R. Narayanaswamy and O. S. Wolfbeis, Optical Sensors for Industrial, Environmental and Diagnostics Applications, Springer, Berlin, 2004 Search PubMed.
- IUPAC, Spectrochim. Acta, Part B, 1978, 33, 241–245 CrossRef.
- S. Sadeghi and A. Z. Moghaddam, Task-specific ionic liquid based in situ dispersive liquid–liquid microextraction for the sequential extraction and determination of chromium species: optimization by experimental design, RSC Adv., 2015, 5, 60621 RSC.
- Y. Gu and X. Zhu, Speciation of Cr(III) and Cr(VI) ions using a β-cyclodextrin-crosslinked polymer micro-column and graphite furnace atomic absorption spectrometry, Microchim. Acta, 2011, 173, 433–437 CrossRef CAS.
- D. Verma, S. K. Verma and M. K. Deb, Single-drop micro-extraction and diffuse reflectance Fourier transform infrared spectroscopic determination of chromium in biological fluids, Talanta, 2009, 78, 270–274 CrossRef CAS PubMed.
- M. Safari, S. Nojavan and S. S. H. Davarani, Speciation of chromium in environmental samples by dual electro-membrane extraction system followed by high performance liquid chromatography, Anal. Chim. Acta, 2013, 789, 58–64 CrossRef CAS PubMed.
- H. Shirkhanloo, M. Ghazaghi and M. M. Eskandari, Cloud point assisted dispersive ionic liquid-liquid microextraction for chromium speciation in human blood samples based on isopropyl 2-[(isopropoxy-carbothiolyl)disulfanyl] ethane thioate, Anal. Chem. Res., 2016, 10, 18–24 CrossRef CAS.
- M. Sun and Q. Wu, Cloud point extraction combined with graphite furnace atomic absorption spectrometry for speciation of Cr(III) in human serum samples, J. Pharm. Biomed. Anal., 2012, 60, 14–19 CrossRef CAS PubMed.
- M. R. Moghadam, S. Dadfarnia and A. M. H. Shabani, Speciation and determination of ultra trace amounts of chromium by solidified floating organic drop microextraction (SFODME) and graphite furnace atomic absorption spectrometry, J. Hazard. Mater., 2011, 186, 169–274 CrossRef CAS PubMed.
- E. Yilmaz and M. Soylak, Ultrasound assisted-deep eutectic solvent based on emulsification liquid phase microextraction combined with micro-sample injection flame atomic absorption spectrometry for valence speciation of chromium(III/VI) in environmental samples, Talanta, 2016, 160, 680–685 CrossRef CAS PubMed.
- S. Wen, X. Zhu, X. Wu and X. Qin, Directly suspended droplet micro-extraction coupled with electrothermal atomic absorption spectrometry for the speciation of chromium(III)/chromium(VI), Anal. Methods, 2014, 6, 9777 RSC.
- Z. Chen, Z. Zhang, J. Qi, J. You, J. Ma and L. Chen, Colorimetric detection of heavy metal ions with various chromogenic materials: strategies and applications, J. Hazard. Mater., 2023, 441, 129889 CrossRef CAS PubMed.
- H. A. Silva-Neto, I. V. S. Arantes, A. L. Ferreira, G. H. M. do Nascimento, G. N. Meloni, W. R. de Araujo, T. R. L. C. Paixao and W. K. T. Coltro, Recent advances on paper-based microfluidic devices for bioanalysis, TrAC, Trends Anal. Chem., 2023, 158, 116893 CrossRef CAS.
- B. Selvakumar and A. Kathiravan, Sensory materials for microfluidic paper based analytical devices-A review, Talanta, 2021, 235, 122733 CrossRef CAS PubMed.
- T. Ozer, C. McMahon and C. S. Henry, Advances in paper-based analytical devices, Annu. Rev. Anal. Chem., 2020, 13, 85–109 CrossRef PubMed.
- M. E. Soares, E. Vieira and M. J. De Lourdes Bastos, Quantification of total and hexavalent chromium in lager beers: variability between styles and estimation of daily intake of chromium from beer, J. Agric. Food Chem., 2010, 58, 1366–1370 CrossRef CAS PubMed.
- I. Narin, M. Tuzen and M. Soylak, Comparison of sample preparation procedures for the determination of trace heavy metals in house dust, tobacco and tea samples by atomic absorption spectrometry, Ann. Chim., 2004, 94, 867–874 CrossRef CAS PubMed.
- M. Soylak, M. Tuzen, I. Narin and H. Sari, Comparison of microwave, dry and wet digestion procedures for the determination of trace metal contents in spice samples produced in Turkey, J. Food Drug Anal., 2004, 12, 254–348 CAS.
- J. N. Miller and J. C. Miller, Statistics and Chemometrics for Analytical Chemistry, Prentice-Hall, England, 5th edn, 2005 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |