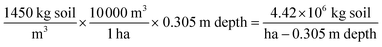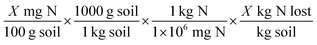Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Evaluation of nitrogen fate from land-application wastewater treatment for cheese making and vegetable processing facilities
Geoffrey S.
Siemering
 *a,
Francisco J.
Arriaga
a,
Clay P.
VanderLeest
ab and
Sarah L.
Naatz
a
*a,
Francisco J.
Arriaga
a,
Clay P.
VanderLeest
ab and
Sarah L.
Naatz
a
aDepartment of Soil Science, University of Wisconsin Madison, Madison, WI 53706, USA. E-mail: geoff.siemering@wisc.edu
bVanderleest Soil Testing, Sturgeon Bay, WI 54235, USA
First published on 27th November 2023
Abstract
Cheese making and vegetable processing are vital industries worldwide, but their operations generate billions of liters of wastewater annually that must be managed in an environmentally safe yet cost-effective manner. For small to medium sized facilities or those without access to wastewater treatment plants, land application systems are commonly utilized. These systems rely primarily on plant uptake and denitrification to remove nitrogen (N) from the effluent. Quantification of soil denitrification is difficult because of the challenges in differentiating between the N2 produced by microbial soil action and atmospheric N. At the behest of industry and regulators, we developed a full N mass balance for six industry facilities to evaluate their systems effectiveness in protecting local water resources. A fully automated acetylene inhibition technique (AIT) soil gas collection system was deployed at each site over two years. These data combined with effluent parameters, lysimeter and plant uptake data and continuously collected soil parameter data allowed mass balance calculation. A laboratory-based soil incubation study provided correction factors for known AIT limitations and evaluation over a greater temperature range. Lab study results indicate that the AIT underestimates system denitrification by 12.4× in the wetland-like cheese making treatment systems and 4.4× in the managed grassland vegetable processing treatment systems. While the wide variability between system performance limits method application at a single facility for short time periods, average values are indicative of general system design performance and utility in wastewater treatment when highly engineered options are unavailable or cost prohibitive.
Environmental significanceVegetable processing and cheese making facilities use land application systems to treat billions of liters of wastewater annually. Soil denitrification processes will remove wastewater nitrate if the systems are functioning correctly. Regulators are concerned that current practices may lead to nitrate groundwater contamination. Industry wants to retain land application as highly engineering solutions are cost prohibitive and nearby water treatment plants infrequent. This study developed a nitrogen mass balance for six facilities to evaluate treatment effectiveness. Findings indicate that systems are treating the wastewater applied during the warmer months. However, during the winter when soil microbial action slows, system performance suffers. This seasonal variation may need accounted for in system and regulatory permit design. |
Introduction
Cheese making and vegetable processing are vital industries worldwide, but their operations inevitably generate large wastewater volumes that must be managed.1 In 2021, the Wisconsin (USA) vegetable processing industry ranked second nationally, producing 2.9 × 105 mt of snap beans, 4.1 × 105 mt of sweet corn, and 5.4 × 104 mt of green peas.2 Ölmez estimates that for each metric ton of specialty crop processed, between 9.8 × 103 and 2.3 × 104 L of wastewater are generated—therefore in 2021 Wisconsin processors generated between 7.4 × 109 and 17.3 × 109 L of wastewater effluent in processing these three crops alone.3 Wisconsin also leads the U.S. in cheese production with 1.5 × 109 kg produced in 2021.4 Each kilogram of cheese produced generates an average of 9.4 L of effluent or 14.1 × 109 L annually statewide.5 In Europe it is estimated that 94.3 × 109 L of cheese making wastewater is produced annually.6Worldwide the rural locations of most cheese making and vegetable processing facilities prevent wastewater disposal via municipal treatment systems. Instead, many facilities utilize land application since soil-based systems are simple in design and operate at a lower cost than highly engineered approaches.7,8 Through careful characterization and monitoring of wastewater and application site parameters, long-term wastewater land application can be performed in an environmentally sustainable manner.9 Effluent volume and characteristics, soil type, slope, drainage class and hydraulic loading are all factors that limit the amount and type of wastewater that can be safely land applied.10
Wastewater land application permits issued by the Wisconsin Department of Natural Resources (WDNR) typically follow the industry standard limiting nitrogen (N) discharges to the agronomic need of the system cover crop (Napplied = Nplant uptake ≈448 kg N per ha per year). However, Wisconsin state statute NR214 states, “Total pounds of nitrogen applied per acre per year shall be limited to the annual nitrogen needs of the cover crop, plus demonstrable nitrogen losses, such as from denitrification or ammonia volatilization occurring in the treatment system”.11 In practice, if facility-installed groundwater monitoring systems can demonstrate no increase in groundwater nitrate levels then the “demonstrable nitrogen loss…” component of the statute can be claimed to be met and discharge limits calculated as Napplied = Nplant uptake + Ndemonstrable losses. Many cheese making facilities and some vegetable processing facilities have received increased discharge limits via this process, but nitrate (NO3−) loading to groundwater is still a concern for state regulators where land treatment is utilized. To confirm land treatment efficacy and industry compliance with statute, the Wisconsin Cheese Makers Association, Midwest Food Products Association, and the WDNR jointly funded this research study to develop full nitrogen budgets (including “demonstrable N losses such as from denitrification…”) for six land application systems operating in different regions of the state.
Land based wastewater treatment systems primarily rely on plant uptake and denitrification to eliminate nitrogen (N) from effluent. Minor N elimination pathways include ammonia (NH3) volatilization, anaerobic ammonium (NH4+) oxidation, and long-term soil storage as soil humus.8 Plants take up N as NO3−, but also NH4+ through their root system and incorporating it into their tissue. Plant uptake of N is well understood and readily measured, making it the most conservative and easily quantified discharge permit limit. During denitrification, NO3− is converted primarily into nitrous oxide (N2O) and dinitrogen or molecular N (N2) by soil bacteria and then released to the atmosphere as N2 gas. However, the amount of N denitrified in land treatment systems is not easily quantified because of the difficulty in differentiating between N2 produced during denitrification and that normally present in the atmosphere.12 This makes denitrification rates difficult to include in industry discharge permits.
Soil denitrification has been measured with the acetylene inhibition technique (AIT) for decades despite implementation challenges and acknowledgement of its likely underestimation.13–18 This study used AIT despite known limitations as Groffman et al. identified the conditions found in industry land application systems (small scale, saturated soil, and high NO3−) as optimal for the method as part of an integrated mass balance approach.12 This method builds on the work of Ryden et al. (in-field AIT), Parkin & Venterea (USDA GraceNET protocols), and van der Weerden et al. (remote monitoring).19–21 Siemering et al. details the autonomous portable multi chamber sampling array and automated lysimeter collection system utilized in this study.22
Industry stakeholders also asserted that significant denitrification occurs in soil at all depths above the water table (“deep denitrification”) and that in studying only the upper 0–91 cm of soil, most denitrification research studies underestimate system treatment capacity. While there is very limited deep denitrification data, theoretically it could occur if suitable soil conditions exist (e.g., sufficient soluble N and C sources, saturated conditions, and above freezing temperatures). In theory instrumentation could be installed as deep as is of interest, but extension of the AIT to groundwater is likely impractical as it would be difficult to inject a sufficient volume of acetylene gas to inhibit denitrification in such a large volume of soil.
We hypothesize that the land application systems studied would successfully treat nitrogen from wastewater applied following current permit limits during warmer months but that seasonal temperatures may limit system effectiveness during colder periods. The combination of using an autonomous AIT system in a field mass balance approach provides a system capable of monitoring a wide range of soil and aquatic environments while accommodating the limitations inherent in the AIT method.
Experimental
Field monitoring
Six industry facilities in different regions of the state were monitored over two years, three cheese making (4×/year) and three vegetable processing facilities (3×/year). All sites operated under normal permitted conditions. One cheese making and one vegetable processing site were in region of sandy soil at industry and regulator request as leaching potential in sand is much higher. The cheese making sites utilized ridge and furrow (RF) systems; a series of closed-end asymmetrical furrows typically 90 m long and 5 m wide planted with water tolerant grasses (e.g., Echinochloa crusgalli L., Polygonum persicaria, P. coccineum, Cyperus esculentus L. and Amaranthus spinosus). The furrow grasses are burned each fall and allowed to regrow. Wastewater was pumped into one furrow per day at 13 or 30 day intervals (Table 1) to a depth of 10–20 cm and allowed to percolate into the soil. Cheese plant effluent is highly variable depending on facility processes, but averages 150 mg N per L.23| Site ID/soil type | Wastewater hydraulic limit (L per ha per day) | Permit N rate (kg N per ha per year) | Wastewater pretreatment and application cycle |
|---|---|---|---|
| a Note: data provided by facility operators. Cheese maker facility application rates per area are higher because all wastewater is discharged to a single 450 or 750 m2 cell in 0.8–2 ha facilities compared to 65–121 ha vegetable processor spray fields. | |||
| RF Sand loamy sand | 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 154 154 |
896 | Ultrafiltration and reverse osmosis. Single pulse, 30 day cycle |
| RF 1 silt loam | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 769 769 |
2129 | Ultrafiltration and reverse osmosis. Anaerobic and aerobic treatment lagoons. Single pulse, 30 day cycle |
| RF 2 silt loam | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 539 539 |
504 | Small mixing tank. Multiple pulses, 13 day cycle. 750 m2 cell |
| SF1 silt loam | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 185 |
672 | Storage lagoon. Center pivot continuous spray as conditions allow |
| SF 2 silt loam | 29![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 652 652 |
336 | Settling tank and lagoon. Center pivot continuous spray as conditions allow |
| SF Sand loamy sand | 84![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 185 185 |
392 | No pretreatment. Center pivot continuous spray as conditions allow |
The vegetable processing facilities applied wastewater to multi hectare spray fields (SF) via center pivot irrigation systems equipped with nozzles on 12- or 48 hour cycles (Table 1) calibrated to not exceed site soil field moisture capacity. The sites are planted with a mix of perennial forage grasses (Loleum perenne, Bromus inermis, Poe annua, and Elymus repens) and harvested 2–3 times per growing season for use as animal forage. Vegetable processing industry average wastewater nitrogen levels are moderate (12–50 mg N per L), but the volumes are high and constant for the entire June–November processing season.24,25
The site monitoring system description and proof of concept data is detailed in Siemering et al.22 Briefly, an autonomous system of four soil gas collection chambers networked to a photoacoustic gas analyser was rotated between treatment fields to collect and analyse soil gas continuously for seven-day periods associated with single load/rest cycles at each site at least once per season as site conditions allowed. Chamber bases were permanently installed on the soil surface at each site with one acetylene injection nozzle inserted to a depth of 1.5 m underneath each chamber (Fig. 1). Soil volumetric water content, temperature, and electrical conductivity sensors were permanently installed at 0.9 and 1.5 m below the surface near each gas-sampling chamber, and readings were collected every 60 minutes with a datalogger during the gas collection periods. Nitrate (NO3−), ammonium (NH4+), and dissolved organic C (DOC) leaching was monitored from soil pore water collected quarterly from porous cup lysimeters installed at a depth of 1.5 m at each site near each of the gas sampling chambers. Soil cores (1.5 m deep) were collected and analysed annually for soil NO3−, DOC, chloride (Cl−), and total Kjeldahl nitrogen (TKN). Chloride is part of the wastewater stream and comparing nitrate and chloride (as a conservative tracer) can help assess nitrate and water movement with depth in soil. Plant tissue was collected immediately prior to burning (RF systems) or at harvest (SF systems).
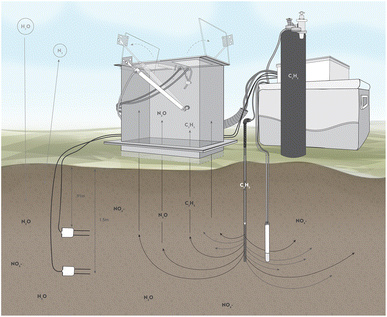 | ||
| Fig. 1 Field denitrification measurement system. Note: lysimeter and acetylene injection at 1.5 m, and buried sensors at 0.9 m and 1.5 m. | ||
At each RF field site, one treatment cell was monitored with four instrument clusters, two at furrow bottom and two at the midpoint between furrow bottom and ridge top with a minimum of 1 m from furrow edge to ensure the zone of denitrification inhibition was entirely within the treatment cell. RF site data was collected from Spring 2016-Winter 2018.
At each SF system, a single location within the field was monitored with four instrument clusters. The clusters were placed 7.6 m apart radially from the central data logger in areas of typical cover crop growth, avoiding pivot tire track ridges or low areas where water could pool. Site data was collected over the 2016 and 2017 processing seasons.
Laboratory incubation study
Following field data analysis showing a large percentage of system N unaccounted for, a lab-based incubation study component using facility soil and wastewater was conducted to provide a corollary dataset to allow estimation of AIT potential denitrification undermeasurement. This component determined denitrification rates by mass balance following incubation in a closed system allowing a correction factor to be calculated for the assumed underestimation of field-based data by comparison to closed system behavior.26,27 This lab component allowed measurement of soil treatment system efficiency at a wider range of temperatures and comparison to the predominantly lab-based denitrification literature.15In summary, wastewater with known ammonium (NH4+) and nitrate (NO3−) was added to characterized soil to bring it almost to saturation in a closed vessel. Following incubation NH4+ and NO3− were extracted from the soil and water mixture and quantified. By difference, the N loss by denitrification was determined.
Four facilities (RF 1 and 2, SF 2, and SF Sand) were sampled using an 8.3 cm diameter soil hand auger at 4–6 random locations in each treatment cell (cheese) or field (vegetable processing) used in the field monitoring component at 0–30.5 cm and 30.5–61 cm depth layers. RF Sand and SF 1 facilities declined incubation study participation. Each sample was composited, kept moist in sealed 19 L plastic buckets, and stored at 2.1 °C prior to study use.
The moisture content of the collected soils was determined by weight difference after oven drying overnight at 105 °C. Soil water holding capacity was determined from weight of water retained by samples in Gooch crucibles wetted from below and drained to constant weight (modified from Bollen and Wright) and the 92% water holding capacity was calculated.28 Soil particle size distribution of each sample was analysed using the hydrometer method.29 Soil texture was determined from these results using a standard texture triangle. Soil was analysed for total C and N using a FlashEA 1112 Analyzer (Thermo Fisher, Waltham, MA) eight weeks following collection immediately prior to incubation. Total C and N analysis was repeated 15 months after initial analysis to confirm sample C and N stability under storage conditions.
At the time of soil collection, 10 L facility wastewater grab samples were collected from the wastewater streams (RF 1 yogurt and semisoft cheese, SF 2 green beans and SF Sand carrots) or holding tank mixtures (RF 2 semisoft cheese) in polyethylene carboys and stored at 2.1 °C until use. The samples were collected mid-day during typical production and not a cleaning or disinfection cycle. Facility design and wastewater volume prevented the collection of composite samples. As needed for experimental work, wastewater was decanted into 1 L glass jars with HDPE lined lids. Wastewater samples were analysed for TKN (USEPA351.2), nitrite (EPA300.0) and DOC (SM5310C) at a commercial laboratory. Wastewater NH4+ was determined using the modified Berthelot method from Stackpoole et al.30 Wastewater NO3− was determined using the vanadium chloride method modified for a microplate reader.31,32
Seven-day incubations were conducted at regional seasonal average soil temperatures, 2.1 °C (winter), 8 °C (spring/fall), 20.4 °C (summer), as measured at a U.S. National Oceanographic and Atmospheric Administration weather monitoring station in Necedah, WI, (central) and a U.S. Department of Agriculture weather monitoring station in Lancaster, WI (southern). Since denitrification slows with decreasing temperatures, 2.1 °C was studied to mimic cheese making facility treatment cell bottoms where discharge of heated wastewater creates localized environments where denitrification can occur. Incubations were also conducted at two elevated temperatures, 30 °C (summer average daytime high), and 35 °C (summer single day record high), as these can be reached in Wisconsin in the upper 5–7 cm soil layer during summer months if vegetative cover is limited and allow study results to be applied to a wider range of climates.
At each incubation temperature, two sets of triplicate water-tight, 300 mL flip-top plastic vials were filled with field moist soil equal to 100 g dry weight soil for each site and soil layer (0–30.5 cm and 30.5–61 cm). Soil water content was adjusted to 92% soil water holding capacity (mimicking saturated system operating conditions) with facility wastewater (experimental set) or deionized water (DI) (control set). Following incubation, 200 mL of 2 M KCl (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 extractant to soil ratio) was added to the vials and they were shaken in the horizontal position for 60 minutes. After a 5 minute settling period, the supernatant was filtered under vacuum through Whatman 42 filter paper. The filtered samples were stored at 2.5 °C until analysis for NH4+ and NO3− within seven days of extraction.30,31
1 extractant to soil ratio) was added to the vials and they were shaken in the horizontal position for 60 minutes. After a 5 minute settling period, the supernatant was filtered under vacuum through Whatman 42 filter paper. The filtered samples were stored at 2.5 °C until analysis for NH4+ and NO3− within seven days of extraction.30,31
Data analysis
Using the field data from each industry facility, an estimated nitrogen budget was calculated using collected field data using the bass balance equation: Nwastewater = Nplant uptake + Nleached + Ndenitrified + Δsoil storage. It is important to note that multiple factors affect the way a soil-plant system functions, including specific weather patterns, characteristics of applied wastewater, and individual site management, among others. These factors contribute to the relatively high variability within and between sites. Nitrogen budget estimates, therefore, have a degree of uncertainty associated with them. Previous efforts to estimate denitrification in these systems have relied on a nitrogen budget approach by attempting to measure leaching and assuming that any unaccounted nitrogen was the denitrified pool because measuring denitrification is difficult and expensive.33 Another drawback of this approach is that nitrogen leaching measurements are difficult, and most approaches rely on measuring concentrations in the soil solution, and not actual nitrogen mass.For the laboratory study, the percent loss of nitrogen from incubation was calculated for each sample as follows:
Inorganic nitrogen in soil:
| Ninorganic (mg) = (NH4-Ninitial (mg kg−1) + NO3-Ninitial (mg kg−1))× soilmass (kg) |
Nitrogen in wastewater added to soil:
| Nwastewater (mg) = TKNwastewater (mg kg−1) × H2Omass@92%WHC (kg) |
Mineralizable organic nitrogen in soil:
| Nmineralizable (mg) = ((NH4-NDI incubation (mg kg−1) + NO3-NDI incubation (mg kg−1)) × soilmass (kg)) − Ninorganic (mg) |
Note: DI = deionized water. If the mineralizable organic nitrogen in soil was less than 0, it was assumed to be 0.
Total initial nitrogen:
| Ntotal (mg) = Ninorganic (mg) + Nwastewater (mg) + Nmineralizable (mg) |
Amount of nitrogen after incubation:
| Npost (mg) = (NH4-Npost (mg kg−1) + NO3-Npost (mg kg−1)) × soilmass (kg) |
Percent loss of nitrogen:
| %Nloss = (Ntotal (mg) − Npost (mg)) ÷ Ninorganic (mg) |
Note: if the percent loss of nitrogen from incubation was less than 0, it was assumed to be 0. This was observed in approximately 5 percent of the samples.
Percent loss results between cheese and vegetable sites at both soil depths (0–30.5 cm and 30.5–61 cm) were analysed using the analysis of variance (ANOVA) (p-value 5%) fitting platform of SAS JMP Version 16 software.
To compare the annualized estimated denitrification measured in the facility treatment fields and the lab incubation study results, the denitrification loss measured in the 100 g dry weight incubation vials was converted to a theoretical loss from a soil mass 30.5 cm thick using the following equations assuming a soil bulk density of 1450 kg m−3:
Results and discussion
Field denitrification study
SF site plant uptake averaged 234 kg N per ha (215–256 kg N per ha, STD = 52.6, CV = 25) and RF site plant uptake averaged 107 kg N per ha (76–138 kg N per ha, STD = 47, CV = 49). Plant uptake during the study period matched that of previous years per facility records. Differing plant species and crop management (e.g., multiple harvesting events vs. single burn) are likely causes of the system differences. Soil nutrient analysis indicated no deficiencies that might limit plant growth. Nitrogen plant uptake represents a substantial removal of the total nitrogen applied to these land treatment sites although well below the ≈448 kg N per ha per year generally accepted rate. This is likely due to the short mid-May to late-September regional growing season.Soil core data showed no change in total nitrogen within the soil profile at any depth over the study period. Soil carbon varied with depth but the relative change between sampling times was less than 1%. All six field study sites had a greater decrease in nitrate with depth relative to that of chloride indicating that denitrification and nitrogen uptake by plants is occurring within the soil profile.
Denitrification measured at the six sites displayed similar limited denitrification occurring both preceding and following wastewater application events.22 During warm season months, as wastewater entered the RF treatment cells creating anaerobic soil conditions the increased nitrogen and DOC led to NO3− enhanced denitrification for approximately five days following application. During those five days, diurnal peaks in denitrification followed daily temperature fluctuations. As water drained through the profile, the amount of oxygen present increased, disrupting optimal denitrification conditions. The denitrification profiles measured during winter at the three RF sites displayed a larger initial increase (2× the other seasons) followed by a drop to baseline denitrification after 24 hours. This is likely caused by opportunistic microbes taking advantage the heat supplied by the nutrient-rich wastewater. Denitrification is typically reduced below 4 °C because of reduced microbial activity in the soil.34 Measured soil temperatures at 0.9 and 1.5 m depths were less than 4 °C for most of the winter months.
In the SF systems smaller amounts of wastewater applied more often, resulting in more frequent but shorter periods of anaerobic conditions. Denitrification peaks are observed shortly after a wastewater application event with rates returning to baseline in under 12 hours.
Denitrification measurements were collected for one week once per season per site. The average measurement before application was considered the baseline denitrification rate. The baseline denitrification rate was extrapolated for non-treatment days at each site and the cumulative denitrification for non-treatment days was included in the total denitrification amount. Denitrification during wastewater treatment was calculated based on the average measured rate following application until the rate returned to baseline (days for RF sites, hours for SF sites) extrapolated for the number of applications per season. Using data from the different application events within a season, average denitrification rates were estimated (Table 2). The annual total average denitrification rate was calculated by averaging the seasonal denitrification rates for available years and summing the seasonal average to obtain an annual total for each site. The RF systems have more total nitrogen uptake than SF systems in part because RF sites operate year-round while SFs operate only during the processing season.
| Site | N applied in wastewatera (kg N per ha per year) | Crop uptake (kg N per ha per year) | Denitrification (kg N per ha per year) | N unaccounted (kg N per ha per year) | N unaccounted (%) |
|---|---|---|---|---|---|
| a Wastewater N data from facility operators. During study period, some facilities had negotiated new permit levels that included demonstrable losses and WDNR allowed other facilities to exceed permitted limits while new permits were negotiated. Where permit exceedances were allowed, additional groundwater NO3− monitoring was required. | |||||
| RF Sand | 1744 | 215 | 49 | 1480 | 85 |
| RF 1 | 1450 | 231 | 62 | 1157 | 80 |
| RF 2 | 1020 | 236 | 63 | 721 | 71 |
| SF 1 | 534 | 109 | 22 | 403 | 75 |
| SF 2 | 113 | 76 | 27 | 10 | 9 |
| SF Sand | 349 | 138 | 28 | 183 | 52 |
In RF 1 and RF Sand there were three wastewater applications per cell per season (both sites each have 30 cells), while RF 2 had six wastewater applications per cell per season (site has 15 cells). Between the three RF sites, there was a small difference in total denitrification between the two silt loam sites while denitrification was lower for the sand site. Coarse textured soils have a lower water holding capacity and typically have greater drainage rates, therefore anaerobic soil conditions needed for denitrification are more difficult to maintain. Annual total denitrification rates (kg N per ha per year) for RF 1, 2, and Sand sites, respectively, were 50 (CV = 18), 57 (CV = 15), and 3 (CV = 35).
Vegetable processing is conducted approximately June–November depending on the type of vegetables processed and seasonal variability. A wastewater application season of 182 days was used for SF 1 and SF Sand, 91 days for summer and 91 days for fall. At SF 2 no processing occurred in either fall season so the wastewater application season as considered 91 days. Annual total denitrification rates (kg N per ha per year) for the SF 1, 2, and Sand, respectively, were 22 (CV = 34), 27 (CV = 77), and 27 (CV = 29). The large variability between SF sites was most likely related to differences in weather conditions and wastewater differences depending on what was processed. The SF 1 site had slightly lower denitrification which could be attributed to the use of an open-air retention pond to store wastewater before application at this site, which can result in some of the N in the wastewater to be lost to the atmosphere before it is field applied.
Nitrogen budget at wastewater application sites
Table 2 shows measured denitrification from the top 1.5 m of soil, plant uptake and assumes the unaccounted N is potentially leachable. The change in soil N storage was zero, thus data were not included here. The nitrogen leached component in the equation was a less reliable parameter in this work given that the soil solution samplers only provide concentration data of the soil pore water collected at that point.The high percentage of unaccounted for system N prompted additional literature review to ascertain likely N underestimation with the use of the AIT for this purpose. As a USDA validated method for the measurement of gaseous nitrogen loss from soil, this denitrification underestimation was initially not anticipated. Table 3 compares field study data with AIT calculated underestimation factors from four publications found that addressed this issue in somewhat comparable systems. One cited study was published after data collection for this project had begun, two after data collection was complete and only one had a field component so were not included in the initial literature review.
| Parameters | AIT underestimation | Location | Reference |
|---|---|---|---|
| a AIT underestimation average of six industry sites assuming 100% of unaccounted N leaves the system through denitrification. | |||
| Soil, semitropical forest and semi-arid mountain | 5–26× | Lab | 18 |
| Soil, managed grassland | 4× | Field | 13 |
| Sediment, wetland | 5.3× | Lab | 35 |
| Soil, riparian | 10× | Lab | 36 |
| Soil, ridge and furrow systems | 19.2× (average)a | Field | Current study |
| Soil, spray field systems | 7.7× (average)a | Field | Current study |
The realization of systemic underestimation by the AIT method prompted the design and implementation of a lab-based study component to attempt to calculate AIT underestimation for these industry wastewater disposal systems. Two industry sites (SF 1 and RF Sand) opted to not continue further participation in the research so additional soil and wastewater could not be collected from these sites.
Deep denitrification potential
In response to industry request, potential denitrification at soil depths below 1.5 m was calculated stoichiometrically as nitrate-N leftover after all potentially available DOC is consumed = [NO3-N − (0.91 × DOC)].37 A negative value represents sufficient dissolved organic carbon is available for denitrification to occur, while a positive number would indicate that dissolved organic carbon may be limiting denitrification thereby increasing the possibility of nitrate leaching.Analysis shows the potential for additional denitrification below 1.5 m (Table 4) in four of the sites (RF 1, RF 2, SF 1, and SF Sand). All seasonal potential denitrification calculated values for the RF Sand site were positive, indicating a potential risk of nitrate leaching. However, the RF Sand site was unique in its wastewater characteristics relative to other RF systems with lower dissolved carbon applied. Additionally, a soil clay layer at 3.7–4.3 m depth results in a perched water table which may have diluted the extracted pore water sample due to excess capillary water. SF 2 indicates a positive risk of leaching in one year and negative in the other but was only used for treatment for three months of each year. The high %CV is indicative of the impact of seasonal operations on wastewater characteristics.
| Site | NO3-N (ppm) | CV | DOC (ppm) | CV | NO3-N after potential denitrification (ppm) |
|---|---|---|---|---|---|
| a Note: potential denitrification was calculated as nitrate-N leftover after all DOC is consumed = [NO3-N − (0.91 × DOC)].37 | |||||
| RF Sand | 25 | 56 | 17 | 89 | 10 |
| RF 1 | 27 | 105 | 36 | 47 | −6 |
| RF 2 | 3 | 98 | 37 | 26 | −31 |
| SF 1 | 3 | 32 | 48 | 126 | −41 |
| SF 2 | 45 | 130 | 46 | 55 | 3 |
| SF Sand | 35 | 42 | 48 | 52 | −9 |
Soil temperature measurements from central and southern Wisconsin show that at 50 cm depth the annual low temperatures do not go below 1.5 °C and summer temperatures averaged 20 °C. Groundwater temperature measurements from wells across Wisconsin 9–106 m in depth average 10 °C year-round.38 From these data it can be assumed the temperatures of the unsaturated zone above the water table remain above freezing and fluctuate close to 10 °C thereby creating the potential for denitrification to occur.
Van Cleemput found that deep denitrification in agricultural soils 3 m in depth may account for 60–70 kg per ha per year but could not determine if the denitrification was due to microbial or chemical pathways.39 Yu et al. utilized 15N methods on forest catchments to estimate that groundwater discharge zones accounted for 31 to 97% of total N loss from the top to the bottom of the catchment but did not estimate nitrogen removal by soil depth or process.40 Jahangir et al. found denitrification accelerated by adding C directly into permeable reactive barriers and/or indirectly, by irrigating with dirty water in a lab study with 1.3 m soil cores from an intensively grazed grassland.41
Available N and C below 1.5 m in these systems is unknown, however lysimeters and water content sensors could be installed as deep as is of interest and the collected soil pore water analyzed. This data would allow for an estimation of deep denitrification potential and if system management could be optimized to promote deep denitrification.
Laboratory denitrification study
Collected wastewater parameters are shown in Table 5. High variability is not unusual in grab samples as wastewater content will vary greatly depending on the current facility operations. Soil textural analysis confirmed RF 1 and 2 and SF 2 sites to be silty clay loam soils and SF Sand site a loamy sand.| Site | TKN | NH3-N | DOC | COD | BOD5 |
|---|---|---|---|---|---|
| RF 1 | 27 | 3.7 | 340 | 789 | 809 |
| RF 2 | 118 | 19 | 843 | 5136 | 2870 |
| SF 2 | 14 | 5.6 | 467.5 | 1118 | 1192 |
| SF Sand | 1.7 | 0.27 | 97.31 | 174 | 173 |
Initial soil C&N ratios ranged from 5.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to 8.8
1 to 8.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Carbon to nitrogen ratios (C
1. Carbon to nitrogen ratios (C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N) significantly impact the rate of organic matter decomposition and the mineralization of organic nitrogen. Organic nitrogen can be easily mineralized and support both decomposition and vegetation growth at a C
N) significantly impact the rate of organic matter decomposition and the mineralization of organic nitrogen. Organic nitrogen can be easily mineralized and support both decomposition and vegetation growth at a C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio of 15
N ratio of 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and below. Above C
1 and below. Above C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N 15
N 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, nitrogen is needed to support C compound decomposition almost exclusively thereby binding the N (N immobilization).26,27 Immobilization continues until further decomposition and loss of CO2 through microbial respiration lowers the C
1, nitrogen is needed to support C compound decomposition almost exclusively thereby binding the N (N immobilization).26,27 Immobilization continues until further decomposition and loss of CO2 through microbial respiration lowers the C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio. Given the high amount of carbon already present in the systems soils, after wastewater addition no C
N ratio. Given the high amount of carbon already present in the systems soils, after wastewater addition no C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio exceeded 10
N ratio exceeded 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 regardless of wastewater C
1 regardless of wastewater C![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) N ratio. Limmer and Steele found that microbial activity may continue at 4 °C in stored soil samples leading to rapid degradation of available C and inaccurate laboratory denitrification study results, but our experience was counter to this.42 Carbon and N analysis was repeated 12 months after collection and storage at 2.5 °C and showed no significant change in elemental C and N levels from the initial analysis.
N ratio. Limmer and Steele found that microbial activity may continue at 4 °C in stored soil samples leading to rapid degradation of available C and inaccurate laboratory denitrification study results, but our experience was counter to this.42 Carbon and N analysis was repeated 12 months after collection and storage at 2.5 °C and showed no significant change in elemental C and N levels from the initial analysis.
Incubation denitrification
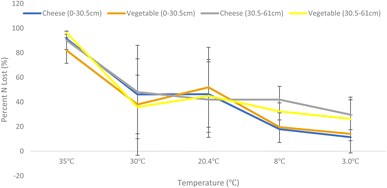
Total system nitrogen at the start of each incubation equalled the sum of the amount of inorganic nitrogen in soil, the amount of mineralizable organic nitrogen in soil, and the amount of nitrogen in wastewater. ANOVA analysis of soil NH4+ and NO3− extraction following incubation shows that change in incubation temperature has a statistically significant effect on denitrification rates within the industry facility soils (F ratio = 35.5182, prob > F ≤ 0.0001). Differences in soil source (RF versus SF) and soil depth did not result in statically different denitrification rates.Decreasing temperature resulted in decreased denitrification (Fig. 2). Hattori et al. also found that lower seasonal temperatures decrease denitrification in shallow soils concluding that forest watersheds may be vulnerable due to season environmental changes such as snow melt.43 They also found that denitrification hotspots within the systems varied over the course of the year.
The samples incubated at 35 °C exhibited both the highest denitrification rates likely due to uniform metabolic increase of all microorganisms present. This differs from Bremner and Shaw which found that maximum denitrification occurred at 25 °C with little rate at temperatures above 25 °C.44 However, that study used glucose as the C source and potassium nitrate (KNO3) as the N source and differences in C and N bioavailability may explain the discrepancy between study findings. Glucose and KNO3 are readily available for soil microbe activity, industry facility wastewater C and N sources are typically much more recalcitrant with C present as lignin and cellulose and N present in cheese proteins and other organic compounds. These more complex sources may require higher temperatures to achieve maximum denitrification rates.
Samples incubated at 30 °C (summer high) and 20.4 °C (summer) showed high variability likely due to differences in soil microbial composition between samples and suboptimal microbial metabolic increase. The 8 °C (fall) and 2.1 °C (winter) incubation temperature showed both lower denitrification rates and lower variability than the summer temperatures likely due to uniform microbial metabolic suppression. This temperature effect is consistent with findings from Dong et al. in estuarine sediment and Stanford in agricultural soil but this study is the first to confirm the effect in these types of wastewater systems.45,46
Comparison of laboratory-derived data to field-collected data
Table 6 shows the hypothetical annual denitrification rates from the average of the four participating industry site soils at two soil depths. These hypothetical losses are then summed for the two layers and divided by the annualized N losses measured during the field monitoring to give an underestimation factor between the two systems (Table 7).| Temp. (°C) | RF sites | SF sites | ||||||
|---|---|---|---|---|---|---|---|---|
| 0–30.5 cm | 30.5–61 cm | 0–30.5 cm | 30.5–61 cm | |||||
| (kg ha−1) | ± | (kg ha−1) | ± | (kg ha−1) | ± | (kg ha−1) | ± | |
| 35 | 317 | 219 | 312 | 214 | 47 | 22 | 54 | 20 |
| 30 | 82 | 49 | 93 | 52 | 45 | 6 | 12 | 13 |
| 20.4 | 118 | 49 | 85 | 35 | 60 | 5 | 15 | 9 |
| 8 | 67 | 69 | 131 | 90 | 44 | 40 | 11 | 1 |
| 2.1 | 51 | 50 | 77 | 31 | 38 | 42 | 9 | 5 |
| Season (°C) | RF sites | SF sites | ||||
|---|---|---|---|---|---|---|
| kg ha−1 | ± | Factor | kg ha−1 | ± | Factor | |
| Summer (20.4) | 19.4 | 5.8 | 10.4 | 16.8 | 12.8 | 4.5 |
| Spring (8) | 11.6 | 6.6 | 17.1 | n/a | n/a | |
| Fall (8) | 20.4 | 15.0 | 9.7 | 12.9 | 9.7 | 4.2 |
| Winter (2.1) | 3.9 | 2.2 | 32.6 | n/a | n/a | |
RF cheese making wastewater is discharged year-round so an annual underestimation factor between the lab incubation study and the field data these systems is 17.5×. The spring, summer and fall average underestimation factor is 12.4×. Both factors are within the one-two orders of magnitude estimated undermeasurement of field-based acetylene inhibition methods observed in wetland soils by Watts and Seitzinger.36 The RF treatment sites are constructed and managed such that they essentially create regularly timed ephemeral wetlands.
The RF site winter underestimation factor was the largest (32.6×), but the amount of measured denitrification in both the field and incubation studies was small. While system denitrification rates are undoubtedly lower at winter temperatures, the soil warming due to wastewater application is likely to increase localized surface soil denitrification and will contribute to increased soil temperatures at deeper soil strata depths where freezing does not occur. Further study of winter denitrification is needed to elucidate low-temperature system capacity more fully. This might entail installation and year-round monitoring of temperature probes at multiple depths to ascertain true system operating temperatures.
The SF sites which are constructed and managed as grasslands harvested for forage both had an average underestimation factor of 4.4×. This value corresponds closely to the 3-5× factor determined by Sgouridis et al. using 15N methods in grazed and fertilized grasslands.13
The estimated denitrification at the six study locations was calculated using industry-specific underestimation factors (Table 8). After applying the underestimation factor, the average cheese facility percent unaccounted N is 4.3%, and the vegetable sites 4.7% albeit with wide inter-site variability. This range in unaccounted system N fate is typical of studies of nutrient behaviour in agricultural systems.
| Site | N applied (kg N per ha per year) | Crop uptake (kg N per ha per year) | Denitrification | N unaccounted | %N unaccounted | |||
|---|---|---|---|---|---|---|---|---|
| Field (kg N per ha per year) | Adjusted (kg N per ha per year) | Field (kg N per ha per year) | Adjusted (kg N per ha per year) | Field (kg N per ha) | Adjusted (kg N per ha) | |||
| a Note: negative values do not indicate nitrification but are artifacts of mathematical estimations of system performance. | ||||||||
| RF Sand | 1744 | 215 | 49 | 873 | 1480 | 656 | 85 | 38 |
| RF SL 1 | 1450 | 231 | 62 | 1091 | 1158 | 128 | 80 | 9 |
| RF SL 2 | 1020 | 256 | 63 | 1111 | 702 | −347 | 69 | −34 |
| SF SL1 | 534 | 109 | 22 | 99 | 402 | 326 | 75 | 61 |
| SF SL2 | 113 | 76 | 27 | 118 | 10 | −81 | 9 | −72 |
| SF Sand | 349 | 138 | 28 | 123 | 183 | 87 | 52 | 25 |
Conclusions
Despite validation as a part of the USDA Gracenet protocol and under the optimal conditions as described by Groffman et al. the AIT method results in systemic denitrification underestimation in these wastewater treatment systems.12,20 Almaraz et al. indicates that 15N measurement is currently the most accurate method for field measurement of denitrification with most studies using enriched fertilizer as a tracer.15 However, this method is not practical in these industry wastewater systems as the milk protein and plant-structure bound N present in these systems is not directly comparable to the readily bioavailable fertilizer-15N. For 15N methods to be used effectively, the isotopic enrichment would need to be introduced at the milk and vegetable production stages for realistic enriched wastewater to eventually be produced.The field denitrification study results show a positive correlation between ambient temperature and denitrification rates in these treatment with denitrification and plant uptake the primary N loss pathways. It can also be concluded that near surface (0–91 cm) denitrification rates decrease with decreasing ambient temperature which may limit treatment efficiency in cheese making systems that operate year-round. It is acknowledged that warm wastewater discharges may temporarily increase localized denitrification during colder months. This variable efficiency should be considered by both industry and regulators when considering year-round wastewater disposal. Data from temperature probes installed at multiple depths around the point of discharge could clarify the extent of the warming and low temperature system efficiency.
Laboratory batch incubations provided denitrification estimations for a wide range of temperatures, although with wide variability similar to that observed in natural systems. Denitrification underestimation factors calculated using incubation data and applied to the field data indicate that, on average, less than 5% of system nitrogen is unaccounted for when all potential N loss pathways are considered. This indicates that optimally designed and managed land applications systems can successfully remove N from land applied cheese making and vegetable processing wastewater at rates above cover crop needs while minimizing risk to groundwater.
Author contributions
Geoffrey S. Siemering: conceptualization, funding acquisition, investigation, methodology, project administration, formal analysis, writing-original draft, writing-review and editing. Francisco J. Arriaga: conceptualization, funding acquisition, methodology, supervision, formal analysis, writing-review and editing. Clay P. Vanderleest: investigation, methodology, formal analysis. Sarah L. Naatz: investigation, methodology, formal analysis.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was support with grants from the Wisconsin Department of Natural Resources, the Midwest Food Products Association, and the Wisconsin Cheese Makers Association. We thank Janelle Naab, Nik Hawkins, and Danielle Philipp for their assistance.References
- L. K. Wang, Y. T. Hung, H. H. Lo and C. Yapijakis, Waste Treatment in the Food Processing Industry, CRC press, 2005 Search PubMed.
- USDA/NASS 2022 State Agriculture Overview for Wisconsin, cited 2023 Mar 13, available from: https://www.nass.usda.gov/Quick_Stats/Ag_Overview/stateOverview.php?state=WISCONSIN.
- H. Ölmez, Water consumption, reuse and reduction strategies in food processing, in Sustainable Food Processing, John Wiley & Sons, Ltd, Chichester, UK, 2013, pp. 401–34 Search PubMed.
- USDA/NASS 2021 Wisconsin Agricultural Statistics, cited 2023 Mar 13, available from: https://www.nass.usda.gov/Statistics_by_State/Wisconsin/Publications/Annual_Statistical_Bulletin/2021AgStats-WI.pdf.
- The European Dairy Association, Product Environmental Footprint Category Rules for Dairy Products, 2018, available from: https://ec.europa.eu/environment/eussd/smgp/pdf/PEFCR-DairyProducts_2018-04-25_V1.pdf Search PubMed.
- A. S. Stasinakis, P. Charalambous and I. Vyrides, Dairy wastewater management in EU: Produced amounts, existing legislation, applied treatment processes and future challenges, J. Environ. Manage., 2022, 303(114152), 114152, DOI:10.1016/j.jenvman.2021.114152.
- R. W. Crites, J. E. Middlebrooks and S. C. Reed, Natural Wastewater Treatment Systems, London, England, CRC Press, 2006 Search PubMed.
- USEPA, Process Design Manual. Land Treatment of Municipal and Industrial Wastewater Effluents, EPA/625/R-06/016, USEPA, Cincinnati, Ohio, 2006 Search PubMed.
- P. Isosaari, S. W. Hermanowicz and Y. Rubin, Sustainable natural systems for treatment and disposal of food processing wastewater, Crit. Rev. Environ. Sci. Technol., 2010, 40(7), 662–697, DOI:10.1080/10643380802359396.
- S. X. Liu, Food and Agriculture Wastewater Utilization and Treatment, Blackwell Publishing, Ames, Iowa, 2007 Search PubMed.
- Wisconsin Legislative Reference Bureau, Chapter NR214 Land Treatment of Industrial Liquid Wastes, by Product Solids and Sludges, 2014, available from: https://docs.legis.wisconsin.gov/code/admin_code/nr/200/214.pdf Search PubMed.
- P. M. Groffman, M. A. Altabet, J. K. Böhlke, K. Butterbach-Bahl, M. B. David and M. K. Firestone, et al., Methods for measuring denitrification: diverse approaches to a difficult problem, Ecol. Appl., 2006, 16(6), 2091–2122, DOI:10.1890/1051-0761(2006)016[2091:mfmdda]2.0.co;2.
- F. Sgouridis, S. Ullah and A. Stott, Application of the 15N-gas flux method for measuring in situ N2 and N2O fluxes due to denitrification in natural and semi-natural terrestrial ecosystems and comparison with the acetylene inhibition technique, Biogeosci. Discuss., 2015, 12, 12653–12689 Search PubMed.
- M. S. Smith, M. K. Firestone and J. M. Tiedje, The acetylene inhibition method for short-term measurement of soil denitrification and its evaluation using nitrogen-131, Soil Sci. Soc. Am. J., 1978, 42(4), 611, DOI:10.2136/sssaj1978.03615995004200040015x.
- M. Almaraz, M. Y. Wong and W. H. Yang, Looking back to look ahead: a vision for soil denitrification research, Ecology, 2020, 101(1), e02917, DOI:10.1002/ecy.2917.
- A. Bollmann and R. Conrad, Acetylene blockage technique leads to underestimation of denitrification rates in oxic soils due to scavenging of intermediate nitric oxide, Soil Biol. Biochem., 1997, 29(7), 1067–1077, DOI:10.1016/s0038-0717(97)00007-2.
- R. Felber, F. Conen, C. R. Flechard and A. Neftel, Theoretical and practical limitations of the acetylene inhibition technique to determine total denitrification losses, Biogeosciences, 2012, 9(10), 4125–4138, DOI:10.5194/bg-9-4125-2012.
- H. Yuan, Z. Zhang, S. Qin, S. Zhou, C. Hu and T. Clough, et al., Effects of nitrate and water content on acetylene inhibition technique bias when analysing soil denitrification rates under an aerobic atmosphere, Geoderma, 2019, 334, 33–36, DOI:10.1016/j.geoderma.2018.07.039.
- J. C. Ryden, L. J. Lund and D. D. Focht, Direct measurement of denitrification loss from soils: I. laboratory evaluation of acetylene inhibition of nitrous oxide reduction, Soil Sci. Soc. Am. J., 1979, 43(1), 104–110, DOI:10.2136/sssaj1979.03615995004300010019x.
- T. B. Parkin and R. T. Venterea, USDA-ARS GRACEnet Project Protocols, Chapter 3. Chamber-based trace gas flux measurements, Sampling Protocols, USDA-ARS, Beltsville, MD, 2010, pp. 1–39 Search PubMed.
- T. J. van der Weerden, A. Manderson, F. M. Kelliher and C. A. M. de Klein, Spatial and temporal nitrous oxide emissions from dairy cattle urine deposited onto grazed pastures across New Zealand based on soil water balance modelling, Agric. Ecosyst. Environ., 2014, 189, 92–100, DOI:10.1016/j.agee.2014.03.018.
- G. S. Siemering, C. P. Vanderleest and F. J. Arriaga, Autonomous high-throughput in situ soil nitrogen flux measurement system, Environ. Monit. Assess., 2022, 194(10), 680, DOI:10.1007/s10661-022-10351-x.
- M. Watkins and D. Nash, Dairy factory wastewaters, their use on land and possible environmental impacts-a mini review, Open Agric. J., 2010, 4, 1–9, DOI:10.2174/1874331501004010001.
- C. Wei, T. Zhang, C. Feng, H. Wu, Z. Deng and C. Wu, et al., Treatment of food processing wastewater in a full-scale jet biogas internal loop anaerobic fluidized bed reactor, Biodegradation, 2011, 22(2), 347–357, DOI:10.1007/s10532-010-9405-5.
- G. R. Miller, Y. Rubin, K. U. Mayer and P. H. Benito, Modeling vadose zone processes during land application of food-processing waste water in California's Central Valley, J. Environ. Qual., 2008, 37(suppl. 5), S43–S57, DOI:10.2134/jeq2007.0320.
- J. T. Gilmour, The effects of soil properties on nitrification and nitrification inhibition, Soil Sci. Soc. Am. J., 1984, 48(6), 1262–1266, DOI:10.2136/sssaj1984.03615995004800060056x.
- J. T. Gilmour, C. G. Cogger, L. W. Jacobs, G. K. Evanylo and D. M. Sullivan, Decomposition and plant-available nitrogen in biosolids: laboratory studies, field studies, and computer simulation, J. Environ. Qual., 2003, 32(4), 1498–1507, DOI:10.2134/jeq2003.1498.
- W. B. Bollen and E. Wright, Microbes and nitrates in soils from virgin and young-growth forests, Can. J. Microbiol., 1961, 7(5), 785–792, DOI:10.1139/m61-093.
- G. W. Gee, 2.4 Particle Size Analysis, Methods of Soil Analysis: Part 4 Physical Methods, 2002, pp. 255–293 Search PubMed.
- S. M. Stackpoole, B. A. A. Workmaster, R. D. Jackson and K. R. Kosola, Nitrogen conservation strategies of cranberry plants and ericoid mycorrhizal fungi in an agroecosystem, Soil Biol. Biochem., 2008, 40(11), 2736–2742, DOI:10.1016/j.soilbio.2008.07.017.
- T. A. Doane and W. R. Horwáth, Spectrophotometric determination of nitrate with a single reagent, Anal. Lett., 2003, 36(12), 2713–2722, DOI:10.1081/al-120024647.
- G. K. Sims, T. R. Ellsworth and R. L. Mulvaney, Microscale determination of inorganic nitrogen in water and soil extracts, Commun. Soil Sci. Plant Anal., 1995, 26(1–2), 303–316, DOI:10.1080/00103629509369298.
- F. J. Doran, Treatment of cheese processing wastewater by ridge and furrow disposal: nitrogen transformations, Doctoral dissertation, University of Wisconsin Madison, 1985.
- S. A. F. Bonnett, M. S. A. Blackwell, R. Leah, V. Cook, M. O'Connor and E. Maltby, Temperature response of denitrification rate and greenhouse gas production in agricultural river marginal wetland soils, Geobiology, 2013, 11(3), 252–267, DOI:10.1111/gbi.12032.
- C. W. Lindau, A. E. Scaroni, V. H. Rivera-Monroy and J. A. Nyman, Comparison of 15N2 flux and acetylene inhibition denitrification methods in Atchafalaya River basin sediments, J. Freshw. Ecol., 2011, 26(3), 337–344 CrossRef CAS.
- S. H. Watts and S. P. Seitzinger, Denitrification rates in organic and mineral soils from riparian sites: a comparison of N2 flux and acetylene inhibition methods, Soil Biol. Biochem., 2000, 32(10), 1383–1392, DOI:10.1016/s0038-0717(00)00056-0.
- K. R. Brye, J. M. Norman, L. G. Bundy and S. T. Gower, Nitrogen and carbon leaching in agroecosystems and their role in denitrification potential, J. Environ. Qual., 2001, 30(1), 58–70, DOI:10.2134/jeq2001.30158x.
- R. G. Hennings and J. P. Connelly, Average Ground-Water Temperature Map, Wisconsin, Wisconsin Geological and Natural History Survey, Madison, Wisconsin, 1980 Search PubMed.
- O. Van Cleemput, Subsoils: chemo-and biological denitrification, N2O and N2 emissions, Nutrient Cycl. Agroecosyst., 1998, 52, 187–194 CrossRef CAS.
- L. Yu, J. Mulder, J. Zhu, X. Zhang, Z. Wang and P. Dörsch, Denitrification as a major regional nitrogen sink in subtropical forest catchments: Evidence from multi-site dual nitrate isotopes, Global Change Biol., 2019, 25(5), 1765–1778, DOI:10.1111/gcb.14596.
- M. M. R. Jahangir, M. I. Khalil, P. Johnston, L. M. Cardenas, D. J. Hatch and M. Butler, et al., Denitrification potential in subsoils: A mechanism to reduce nitrate leaching to groundwater, Agric. Ecosyst. Environ., 2012, 147, 13–23, DOI:10.1016/j.agee.2011.04.015.
- A. W. Limmer and K. W. Steele, Denitrification potentials: Measurement of seasonal variation using a short-term anaerobic incubation technique, Soil Biol. Biochem., 1982, 14(3), 179–184, DOI:10.1016/0038-0717(82)90020-7.
- S. Hattori, Y. Nuñez Palma, Y. Itoh, M. Kawasaki, Y. Fujihara and K. Takase, et al., Isotopic evidence for seasonality of microbial internal nitrogen cycles in a temperate forested catchment with heavy snowfall, Sci. Total Environ., 2019, 690, 290–299, DOI:10.1016/j.scitotenv.2019.06.507.
- J. M. Bremner and K. Shaw, Denitrification in soil. II. Factors affecting denitrification, J. Agric. Sci., 1958, 51(1), 40–52, DOI:10.1017/s0021859600032779.
- L. F. Dong, D. C. O. Thornton, D. B. Nedwell and G. J. C. Underwood, Denitrification in sediments of the River Colne estuary, England, Mar. Ecol. Prog. Ser., 2000, 203, 109–122, DOI:10.3354/meps203109.
- G. Stanford, S. Dzienia and R. A. Vander Pol, Effect of temperature on denitrification rate in soils, Soil Sci. Soc. Am. J., 1975, 39(5), 867–870, DOI:10.2136/sssaj1975.03615995003900050024x.
| This journal is © The Royal Society of Chemistry 2024 |