DOI:
10.1039/D4TC00463A
(Paper)
J. Mater. Chem. C, 2024,
12, 6131-6139
Multiferroicity in 2D MSX2 (M = Nb and Zr; X = Cl, Br, and I)†
Received
2nd February 2024
, Accepted 28th March 2024
First published on 29th March 2024
Abstract
Recently, two-dimensional (2D) multiferroic materials have aroused intensive interest owing to the combination of ferroelectric and magnetic properties. In this work, we design a family of 2D MSX2 (M = Zr and Nb; X = Cl, Br, and I) and investigated their structural, mechanical, magnetic, electronic, ferroelectric, and piezoelectric properties based on first-principles calculations. We find that ferroelectric (FE) ZrSX2, paraelectric (PE) ZrSX2 and FE NbSX2 are stable. ZrSX2 materials are nonmagnetic, while NbSX2 materials have antiferromagnetic (AFM) ground states. NbSX2 materials demonstrate multiferroic behaviors, which can be explained by the pseudo Jahn–Teller distortion (PJTE) mechanism. Our work reveals the intrinsic multiferroicity of MSX2, which may provide a guidance on the design of 2D functional materials.
1. Introduction
2D multiferroics have been attracting much attention due to their promising properties such as high spontaneous FE polarization,1–4 photovoltaic effects,5–8 and high-temperature magnetism.9 FE materials, such as BiFeO310 and BaTiO3,11–13 have demonstrated outstanding potential for memory storage and integrated microelectrons. 2D GeS showed a tunable Curie temperature and spontaneous polarization of 1 C m−2, and mutliferroic behaviors under small strain provided high freedom for modulating ferroelectric devices.3 2D CrI3-based magnetic tunneling junctions exhibit layered-dependent magnetism, in which an outstanding magnetoresistance was reported.14,15 Commonly, there is an off-center displacement in FE phases other than the PE phase, indicating the break of symmetry for FE polarization.10,16–18 Taking 2D VOCl2 as an example, the off-center displacement of V ions contributes to FE characteristics, and the energy barrier of the FE flip is 0.18 eV, which stabilizes the ferroelectricity.19 2D TiOBr2 exhibits significant piezoelectricity with d11 = 37.758 pm V−1, resulting from low in-plane elastic constants and atomic sensitivity to an applied strain.4 Young's moduli (Cij) reflect the ability to stretch and deform. The structures also have large piezoelectric strain coefficients (dij) because of their large piezoelectric stress coefficients (eij) and low Young's moduli. The CrOF2 monolayer exhibits a half-metallic nature with intrinsic ferromagnetism (FM) and a high phase transition temperature. The Pmna-MnOCl2 monolayer is an FM-stabilized semiconductor with an indirect band gap of 0.152 eV, while the Pmmn-MnOCl2 monolayer is an FM Dirac semimetal with a Curie temperature of 910 K.20 Uniaxial strain drives the magnetic phase transition in CrOF2, resulting in a high phase transition temperature.21 2D NbOX2 materials possess suitable conduction band minimums (CBMs) and valence band maximums (VBMs) for photocatalytic water splitting, which can be further tuned by stacking.22 Furthermore, the proper built-in electric field, high carrier mobility, and suitable band edges in 2D NbOXY make it a potential candidate for overall water splitting.23 2D MoOCl2 has hyperbolic optical properties with two wide hyperbolic windows from 0.41 to 2.90 eV and from 3.63 to 5.54 eV.24
Compared with bulk counterparts, 2D materials can be modified by various methods, such as surface engineering,25,26 defect engineering,27 strain engineering,28 heterostructure engineering,29,30 and composition engineering,31 to achieve ideal properties for targeted applications. Although numerous investigations have been carried out on 2D metal oxide dihalides,22,32–34 studies on the ferroelectricity of 2D metal sulfide dihalides are limited. In this work, we present a systematic study of 2D MSX2 based on first-principles calculations. We reveal the intrinsic ferroelectricity in MSX2 and piezoelectric effects vary with tension.
2. Methods
All calculations were performed by the Vienna ab initio simulation package (VASP) with the projector augmented wave (PAW) potential and Perdraw–Burke–Ernzerhof (PBE) energy functional.35–39 A vacuum slab of 15 Å along the c-axis was used to avoid interactions between neighboring layers, which has been tested (Fig. S1, ESI†). The cut-off energy for the plane-wave basis set was 550 eV, and the convergence threshold and residual forces were 1 × 10−7 eV and 1 × 10−3 eV Å−1, respectively.36,40,41 A 2 × 2 × 1 supercell was used to study magnetic properties. The Γ-centered k-meshes for sampling the Brillouin zone (BZ) were 12 × 12 × 1, 6 × 6 × 1, and 3 × 3 × 1 for structural relaxation, electronic structures, and phonon calculations, respectively.42 The Γ-only grid was used for the AIMD simulation. The Heyd–Scuseria–Ernzerhof hybrid functional (HSE06) was used to calculate band structures accurately.43 The climbing image nudged elastic band (CI-NEB)44 method was implemented to determine the energy barrier of the ferroelastic phase transition. To describe the Coulomb interaction among partially occupied d-electrons of transition metals, the GGA+U45 method was adopted in our calculations. The various values of 1, 2, and 3 eV elucidate the effects of Hubbard U on structural and electronic properties.
The density-functional perturbation theory46 (DFPT) was used to calculate the phonon dispersion with the Phonopy47 code; 3 × 3 × 1 supercells were used for the phonon calculations as well as AIMD simulations. The Γ-centered k-meshes were 18 × 18 × 1 for the calculations of piezoelectric parameters via the DFPT. To study the thermodynamic stability, the ab initio molecular dynamic (AIMD) simulation48 was performed. The simulated temperature, time step, and time period in AIMD were 300 K, 1 fs, and 8 ps, respectively. VASPKIT49 was used as the post-processing tool for data analysis.
To determine Néel temperatures (TN), Monte Carlo (MC) simulations50 were performed based on the Wolff algorithm of the classical Heisenberg model using the MCSOLVER code.51,52 In the MC simulations, a 100 × 100 square supercell containing 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 local magnetic moments was adopted; 40
000 local magnetic moments was adopted; 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MC steps were performed at each temperature, and the calculations lasted for 6.4 × 109 loops at each temperature.
000 MC steps were performed at each temperature, and the calculations lasted for 6.4 × 109 loops at each temperature.
3. Results and discussion
3.1 Structural properties
MSX2 has four atoms in a unit cell, including one metal atom (M = Zr and Nb), one sulfur atom and two halogen atoms (X = Cl, Br, and I). Similar to 2D TiOX2, VOX2, and VOXY,4,53 in-plane off-center displacement of metal atoms leads to multiferroic behavior. The FE phases with the space group of Pmm2 (No. 25) and PE phases with the space group of Pmmm (No. 47) were investigated. The FE structures have two kinds of M–S bond lengths (dM–S2 and dM–S1) and two kinds of S–M–X bond angles (Fig. 1). The differences between θ1 and θ2 decrease as X changes from Cl to I. In contrast, the lengths of M–S bonds are the same in the PE phases, and the bond angles of S–M–X remain 90° (Table S1, ESI†). Moreover, two kinds of X–M–X angles are found in the structures. The polar displacement (P.D.) leads to in-plane ferroelectricity, which can be defined as follows:53| | | P.D. = |dM–S2 − dM–S1|/2, | (1) |
where dM–S2 indicates the long bond length between metal and sulfur atoms, and dM–S1 is the short one.
 |
| | Fig. 1 (a) Top and (b) and (c) side views of a 2 × 2 × 1 supercell of FE MSX2 and (d) top and (e) side views of PE MSX2. M–S2 indicates the long bond length between metal and sulfur atoms, M–S1 is the short counterpart, and the black arrow indicates the direction of FE polarization. | |
The P.D. decreases dramatically with the increase in U in ZrSX2. In contrast, the P.D. varies slightly with U in NbSX2 (Fig. S2 and Table S2, ESI†). There are imaginary optical phonon modes at the Γ point for PE ZrSX2 and little imaginary frequency for FE counterparts, suggesting spontaneous symmetry breaking and off-center displacements of metal atoms4,54 (Fig. S3 and S4, ESI†). The negligible imaginary dispersions of the FE phases may be induced by the calculation accuracy and lattice instabilities related to long wave undulations, which have been reported in other 2D materials.55,56 Correspondingly, the lattice parameter b in the FE phase is larger than that in the PE phase because of the off-center displacements of metal atoms in MSX2 (Table S1, ESI†). Furthermore, PE ZrSX2 materials with U = 3 eV are dynamically stable with negligible imaginary frequencies (Fig. S4, ESI†). NbSX2 materials are dynamically unstable without U, while exhibiting similar FE phenomena with U = 3 eV (Fig. 2a and Fig. S5, S6, ESI†). NbSCl2 shows a weak tolerance to tension (Fig. S6, ESI†). All of the ZrSX2 materials exhibit dynamically stable FE phases without U and PE phases with U = 3 eV. MSCl2 and MSBr2 are unstable with U = 1 eV and 2 eV, but have stable FE phases with U = 3 eV (Fig. S3–S8, ESI†). Previous reports indicate that NbSX2 and VOX2 exhibited varied polarizations with U.4,57 Therefore, ZrSX2 without and with U (3 eV) and NbSX2 with U = 3 eV were investigated.
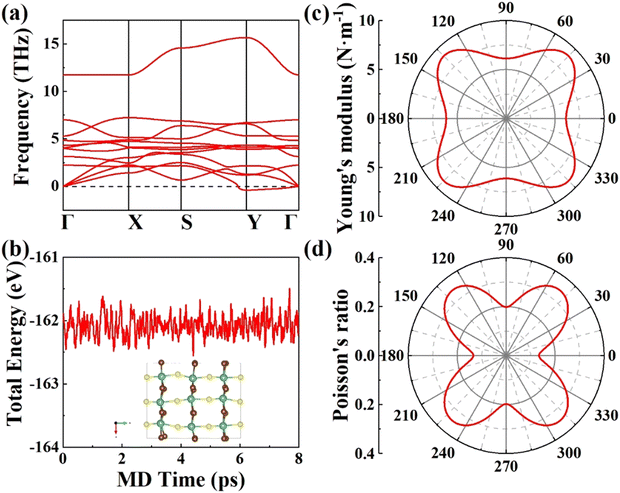 |
| | Fig. 2 (a) Phonon spectrum, (b) AIMD simulation, (c) Young's moduli, and (d) Poisson's ratio of FE NbSBr2 with U = 3 eV. | |
3.2 Structural stability
To ensure thermodynamic and mechanical stabilities, we carried out AIMD simulations and calculated mechanical parameters. The AIMD simulations show that the total energies of the structures have little fluctuations over time, and the mean squared displacement (MSD) was defined as| |  | (2) |
where x(t) and x(0) is the position of atom i at a time of t and 0, respectively, and N stands for the total number of atoms.
The MSDs are less than 0.6 Å2, indicating thermodynamic stability (Fig. 2b and Fig. S9 and S10, ESI†).58 Stable FE phases are supposed to retain positive elastic constants restricted by the Born–Huang criteria:59
We see that FE ZrSX
2, ZrSX
2 with
U = 3 eV, and FE NbSX
2 with
U = 3 eV are mechanically stable as obtained elastic constants satisfy the Born–Huang criteria. Their Young's moduli (
Ex and
Ey), Poisson's ratios (
νx and
νy) and shear moduli (
G) are also obtained (
Table 1). Thus, we confirm that MSX
2 materials are dynamically, thermodynamically, and mechanically stable. In addition, the distorted shapes of Young's moduli and Poisson's ratios reflect their anisotropic nature (
Fig. 2c and d and Fig. S11–S15, ESI
†). Previous studies have indicated that the mechanical and piezoelectric properties may be enhanced under tension along polarization.
60,61 Thus, we also obtained the corresponding parameters at a tension of 5% along
b-axes. With the tension along the
b-axis,
C22 drops significantly, leading to a lower
Ey and a higher
νx. Conversely,
C22 soars with a larger
U in ZrSX
2, resulting in higher
Ey and smaller Poisson's ratios.
Table 1 Mechanical parameters of the structures
| MSX2 |
Tension (%) |
U (eV) |
Stiffness tensor (GPa) |
Young's modulus E (GPa) |
Poisson's ratio |
Shear modulus G (GPa) |
|
C
11
|
C
12
|
C
22
|
C
66
|
C
11
C
22 − C122 (±) |
X
|
y
|
X
|
y
|
G
|
| ZrSCl2 |
0 |
0 |
18.569 |
3.606 |
23.747 |
4.577 |
+ |
18.021 |
23.047 |
0.152 |
0.194 |
7.482 |
| ZrSCl2 |
5 |
0 |
17.766 |
2.839 |
4.957 |
3.582 |
+ |
16.140 |
4.503 |
0.573 |
0.160 |
7.464 |
| ZrSCl2 |
0 |
3 |
18.877 |
0.959 |
57.459 |
4.926 |
+ |
18.861 |
57.410 |
0.017 |
0.051 |
8.959 |
| ZrSBr2 |
0 |
0 |
16.083 |
3.192 |
24.629 |
4.485 |
+ |
15.669 |
23.995 |
0.130 |
0.198 |
6.446 |
| ZrSBr2 |
5 |
0 |
15.621 |
2.633 |
4.936 |
3.577 |
+ |
14.216 |
4.492 |
0.533 |
0.169 |
6.494 |
| ZrSBr2 |
0 |
3 |
16.102 |
0.926 |
57.017 |
4.762 |
+ |
16.087 |
56.964 |
0.016 |
0.058 |
7.588 |
| ZrSI2 |
0 |
0 |
13.550 |
2.554 |
27.386 |
4.361 |
+ |
13.312 |
26.905 |
0.093 |
0.188 |
5.498 |
| ZrSI2 |
5 |
0 |
13.424 |
2.196 |
5.582 |
3.524 |
+ |
12.560 |
5.223 |
0.393 |
0.164 |
5.614 |
| ZrSI2 |
0 |
3 |
13.479 |
0.895 |
56.740 |
4.491 |
+ |
13.465 |
56.681 |
0.016 |
0.066 |
6.292 |
| NbSCl2 |
0 |
3 |
18.943 |
4.931 |
15.986 |
6.013 |
+ |
17.422 |
14.702 |
0.308 |
0.260 |
7.006 |
| NbSCl2 |
5 |
3 |
20.242 |
1.481 |
3.439 |
4.898 |
+ |
19.604 |
3.331 |
0.431 |
0.073 |
9.381 |
| NbSBr2 |
0 |
3 |
17.985 |
3.562 |
26.608 |
6.122 |
+ |
17.508 |
25.903 |
0.134 |
0.198 |
7.212 |
| NbSBr2 |
5 |
3 |
17.967 |
3.455 |
2.784 |
5.000 |
+ |
13.679 |
2.120 |
1.241 |
0.192 |
7.256 |
| NbSI2 |
0 |
3 |
16.385 |
3.088 |
31.860 |
6.024 |
+ |
16.086 |
31.278 |
0.097 |
0.188 |
6.649 |
| NbSI2 |
5 |
3 |
16.659 |
3.050 |
3.551 |
5.027 |
+ |
14.039 |
2.993 |
0.859 |
0.183 |
6.805 |
The formation energies were also obtained to demonstrate their possibility for experimental realization:62
| | | Ef = (EMSX2 − EM − ES − 2EX)/4, | (5) |
where
EMSX2 is the ground state energy of MSX
2 per chemical formula, and
EM,
ES, and
EX are the chemical potentials of metal, sulfur and halogen atoms calculated from their elemental forms, respectively. The calculated formation energies are negative (Table S3, ESI
†), indicating that they are possible to be achieved experimentally. Moreover, other phases, including MS and X
2, as well as MX
2 and S, were considered to calculate the formation energy of MSX
2. Their formation energies are obtained as follows:
| | | Ef1 = (EMSX2 − EMS − 2EX) | (6) |
| | | Ef2 = (EMSX2 − ES − EMX2), | (7) |
where
EMS and
EMX2 are the energies of bulk MS and MX
2, respectively. The results indicate that MSI
2 may decompose into MS and I
2 and others may not (Table S4, ESI
†). We find that energy barriers between FE and AFE structures are 19, 17, 8, 60, 52, and 38 meV per unit cell for ZrSCl
2, ZrSBr
2, ZrSI
2, NbSCl
2, NbSBr
2, and NbSI
2, respectively (Fig. S16, ESI
†), which are much lower than those of the VOCl
2 monolayer
19 and LiNbO
3 perovskite.
63 FE polarizations are switchable, and the energy barriers for FE-AFE switches in NbSX
2 are higher than the thermal energy at room temperature (∼26 meV), indicating stable FE states (Fig. S16, ESI
†).
3.3 Magnetic properties
FE ZrSX2 materials do not show magnetism as the energies do not change with spin (Table S5, ESI†). In contrast, the FE NbSX2 materials demonstrate magnetic behaviors. To determine the magnetic ground states of NbSX2, one FM state and three AFM states were studied in a 2 × 2 × 1 supercell (Fig. S17, ESI†). The exchange energy per unit cell is the energy difference between stable AFM and FM states:62,64where EAFM is the energy of the most stable AFM state, EFM is the energy of the FM state, and N is the number of unit cells inside a supercell (N = 4). The exchange energies are −226, −36, and −17 meV per unit cell for NbSCl2, NbSBr2, and NbSI2, respectively, illustrating the AFM3 ground state (Table 2 and Table S6, ESI†). The exchange energy of NbSCl2 is much higher than that of VTe2.64 Compared with FM-stabilized materials, AFM-stabilized ones do not have redundant magnetic fields and are significantly insensitive to external magnetic fields due to their zero total magnetization. Moreover, 2D AFM structures have fast spin dynamics and high packing density, making them potential candidates for fostering innovation in spin-based devices.65–68
Table 2 Exchange energies (Eex), magnetic moments, exchange coupling parameters, and Néel temperatures
| NbSX2 |
U (eV) |
E
ex (meV per unitcell) |
Nb (μB) |
S (μB) |
X (μB) |
J
a
(meV) |
J
b
(meV) |
J
ab
(meV) |
T
N (K) |
| NbSCl2 |
3 |
−226 |
±0.863 |
±0.072 |
0 |
−28.593 |
0.328 |
0.218 |
25 |
| NbSBr2 |
3 |
−36 |
±0.870 |
±0.069 |
0 |
−4.618 |
0.099 |
0.116 |
4.5 |
| NbSI2 |
3 |
−17 |
±0.868 |
±0.063 |
0 |
−2.356 |
0.203 |
0.194 |
2 |
Magnetic anisotropy describes the preferred orientations of magnetic moment vectors.69 The magnetic anisotropy energies (MAEs) in xOz, yOz, and xOy planes for NbSCl2, NbSBr2 and NbSI2, respectively, are investigated by including the spin–orbital coupling (SOC) effect, which is defined as70–72
where
E‖ and
E⊥ are energies per chemical formula with the in-plane and out-of-plane spins with respect to the easy axes, respectively.
The angular dependent results show that easy magnetization axes are [100], [001], and [010], and the MAEs are 0.073, 0.050 and 0.036 meV per unit for NbSCl2, NbSBr2 and NbSI2, respectively (Fig. S18, ESI†), which are much higher than those of cubic Fe73 and Ni.74 As X changes from Cl to I, the easy magnetization axes are altered, and the MAEs decrease. Specifically, the total energy varies significantly with the magnetization direction in the xOy and yOz planes. The significant the MAEs of NbSX2 make them potential candidates for nanoscale devices such as magnetic recording media.75
The magnetic moments of niobium atoms are close to 1μB per Nb. The nearest–neighbor (NN) and next–nearest–neighbor (NNN) exchange interactions are considered to determine the exchange parameters of NbSX2.19,54 The NN exchange coupling parameters along the a- and b-directions are Ja and Jb, and the NNN exchange coupling parameter is Jab (Fig. S17, ESI†).76,77 The Hamiltonian is written as
| |  | (10) |
where
H0 is the nonmagnetic Hamiltonian,
Mi is the net magnetic moment at site
i, 〈
ij〉
a is nearest Nb atoms along the
a-direction, and 〈〈
kl〉〉 stands for NNN Nb atoms. The NN and NNN exchange coupling parameters can be solved by the energies of one FM state and three AFM states. The total energies of the four magnetic states are calculated as
19| | | EFM = E0 − 4M2 (Ja + Jb + 2Jab) | (11) |
| | | EAFM1 = E0 − 4M2 (−Ja + Jb − 2Jab) | (12) |
| | | EAFM2 = E0 − 4M2 (Ja − Jb − 2Jab) | (13) |
| | | EAFM3 = E0 − 4M2 (−Ja − Jb − 2Jab) | (14) |
After obtaining the MAEs and exchange parameters, the
TN can be determined by the MCSOLVER code. The
TN of NbSCl
2 is higher than that of VOF
278 and VOI
279 (
Table 3 and Fig. S19, ESI
†).
Table 3 Piezoelectric parameters of the structures
| MSX2 |
Tension (%) |
U (eV) |
e
21 (10−10 C m−1) |
e
22 (10−10 C m−1) |
d
21 (pm V−1) |
d
22 (pm V−1) |
| ZrSCl2 |
0 |
0 |
1.754 |
−19.836 |
0.264 |
−0.875 |
| ZrSCl2 |
5 |
0 |
0.530 |
−11.489 |
0.441 |
−2.570 |
| ZrSBr2 |
0 |
0 |
1.476 |
−19.387 |
0.255 |
−0.820 |
| ZrSBr2 |
5 |
0 |
0.401 |
−10.965 |
0.440 |
−2.456 |
| ZrSI2 |
0 |
0 |
1.236 |
−21.474 |
0.243 |
−0.807 |
| ZrSI2 |
5 |
0 |
0.234 |
−10.551 |
0.349 |
−2.027 |
| NbSCl2 |
0 |
3 |
4.379 |
−37.886 |
0.922 |
−2.654 |
| NbSBr2 |
0 |
3 |
0.537 |
−13.883 |
0.137 |
−0.540 |
| NbSBr2 |
5 |
3 |
0.159 |
−9.832 |
0.904 |
−4.653 |
| NbSI2 |
0 |
3 |
0.356 |
−13.069 |
0.101 |
−0.420 |
| NbSI2 |
5 |
3 |
0.000 |
−8.951 |
0.548 |
−2.991 |
3.4 Electronic properties
The band structures and densities of states (DOSs) were calculated based on the magnetic ground states to uncover the mechanism of FE switching and magnetism. FE ZrSX2 are direct bandgap semiconductors with flat bands around the CBMs (Fig. S20 and Table S7, ESI†). FE NbSX2 are indirect bandgap semiconductors (Fig. 3, Fig. S21 and Table S7, ESI†). PE ZrSX2, PE NbSCl2, and PE NbSBr2 have small direct bandgaps with the CBM and VBM at Γ points (Fig. 3, Fig. S21 and Table S7, ESI†). Moreover, the FE ZrSX2 materials are direct bandgap semiconductors according to the HSE06 method, whose CBMs and VBMs meet the requirements of photocatalysts (Fig. S22 and Table S7, ESI†).
 |
| | Fig. 3 Band structures of (a) FE and (b) PE NbSBr2, PDOSs of (c) FE and (d) PE NbSBr2, PDOSs of Nb atoms in (e) FE and (f) PE NbSBr2, and PDOSs of S atoms in (g) FE and (h) PE NbSBr2 with U = 3 eV. | |
The partial density of states (PDOSs) illustrate the hybridization between metal and non-metal atoms (Fig. S23–S27, ESI†). Around the CBMs, the spin-polarizations dominantly originate from the d-orbitals of metal atoms, while the p-electrons of non-metal atoms contribute mostly to the VBMs of ZrSX2 (Fig. S23 and S24, ESI†). Differently, the d-electrons of niobium atoms dominate the states near the VBMs of NbSCl2 and NbSBr2. Furthermore, the spin-polarizations near the CBMs in ZrSX2 mainly originate from the dz2 orbital. In contrast, significant spin-splitting around the VBMs induced by the dz2 orbitals and CBMs by the dxy and dyz orbitals leads to magnetic moments of 0.863, 0.870, and 0.868μB per Nb for NbSCl2, NbSBr2, and NbSI2, respectively. The charge distribution reflects the bonding among atoms. The ELF contour around the center of M–X bonds is near 0, revealing ionic interaction (Fig. S25 and S26, ESI†). According to Goodenough–Kanamori–Anderson rules,80–83 the super-exchange usually leads to AFM characteristics in FE NbSX2 when the average angles of cation–anion–cation bond angles are around 90°. The average angles of θ1 and θ2 as well as α1 and α2 are 90° (Fig. 1 and Table S1, S8, ESI†), confirming the AFM nature. Therefore, the super-exchange is responsible for the AFM state observed in NbSX2 because of their semiconducting nature.84–87
Similar to metal oxide dihalides, the off-center displacements of metal atoms give rise to multiferroic characters. The bandgaps of the FE phases are much larger than their PE counterparts (Table S7, ESI†), and a similar trend has been reported in 2D VOCl2.19 We find that the energy levels of the dxz orbitals are much lower in the FE phases than those in the PE phases, leading to d electrons with low energies (Fig. 3e, f and Fig. S27, S29, and S30, ESI†). The energies of FE phases are lower than those of the PE phases, which can be elucidated by the PJTE mechanism.88 According to the PJTE mechanism, similar to Ti4+, Zr4+ exhibits a d0 configuration. In contrast, Nb4+ demonstrates a d1 configuration like Ti3+ and V4+.89 Zr4+ leads to a zero magnetic moment, whereas Nb4+ is endowed with magnetism, leading to multiferroic characteristics in NbSX2. Unpaired electrons are found in Nb4+, whereas all the electrons are paired in Zr4+, causing the spin-splitting in Nb4+. There is spin-splitting in the t1u orbitals of S atoms in FE NbSX2, leading to magnetic moments of over 0.06μB per sulfur. However, the magnetic moments are 0 in ZrSX2 and PE NbSX2.
3.5 Piezoelectric properties
To further investigate the FE switching in ZrSX2, the piezoelectric parameters were obtained. The piezoelectric strain coefficients are defined as53,90| | | d21 = (e21C22 − e22C12)/(C11C22 − C122) | (15) |
| | | d22 = (e22C11 − e21C12)/(C11C22 − C122), | (16) |
where eij is piezoelectric stress coefficients and Cij is stiffness tensors. Due to smaller C22 and larger off-center displacement of metal atoms, the strain coefficients become significant under tension along the direction of FE switching (Table 3). The piezoelectric stress coefficients are much higher than those of some 2D Janus transition metal dichalcogenides,91 as well as the h-BN monolayer.92 At a tension of 5%, the Poisson's ratios along the [010] axes are much lower than those along the [100] axes. Consequently, negative Poisson's ratios are found in NbSX2 (Fig. S14, ESI†). Moreover, the variations of X–M–X angles are more significant than those of S–M–X angles at a tension (Table S8, ESI†), leading to higher Poisson's ratios along the [100] axes rather than the [010] axes. Similar trends are found in penta-BCP because of the major fluctuation of bond angles.90 By considering FE switching stability, piezoelectric stress and strain coefficients, we see that NbSX2 can be applied for sensors and energy conversion.93,94
4. Conclusions
In summary, we propose a 2D family MSX2 (M = Zr and Nb; X = Cl, Br, and I) and investigate their stability, magnetic, electronic, and piezoelectric properties on the basis of the first-principles calculations for multiferroicity. We find that FE ZrSX2, PE ZrSX2, and FE NbSX2 are stable, and the FE switching of NbSX2 is stable at room temperature. The off-center displacements of M ions induce differences between the electronic structures of the FE and PE phases. It is expected that designed structures can be applied to nanoscale spintronic devices. This work may provide guidance on the design of 2D materials with promising multiferroicity.
Conflicts of interest
There is no conflict to declare.
Acknowledgements
This work was supported by the Science and Technology Development Fund (FDCT) from Macau SAR (0050/2023/RIB2, 0023/2023/AFJ, 006/2022/ALC, and 0111/2022/A2), Multi-Year Research Grants (MYRG-GRG2023-00010-IAPME and MYRG2022-00026-IAPME) from the Research & Development Office at the University of Macau, and the Shenzhen-Hong Kong-Macao Science and Technology Research Programme (Type C) (SGDX20210823103803017) from Shenzhen.
References
- D. Sando, Y. Yang, E. Bousquet, C. Carretero, V. Garcia, S. Fusil, D. Dolfi, A. Barthelemy, P. Ghosez, L. Bellaiche and M. Bibes, Nat. Commun., 2016, 7, 10718 CrossRef CAS PubMed.
- Y. Zhai, A. Fan, K. Zhong, D. V. Karpinsky, Q. Gao, J. Yi and L. Liu, J. Eur. Ceram. Soc., 2024, 44, 224–232 CrossRef CAS.
- R. Fei, W. Kang and L. Yang, Phys. Rev. Lett., 2016, 117, 097601 CrossRef PubMed.
- A. Alam, M. Noor and M. Nolan, Nanoscale, 2022, 14, 11676–11683 RSC.
- A. Bhatnagar, Y. Kim, D. Hesse and M. Alexe, Nano Lett., 2014, 14, 5224–5228 CrossRef CAS PubMed.
- G.-L. Yuan and J. Wang, Appl. Phys. Lett., 2009, 95, 252904 CrossRef.
- K. M. Batoo, M. F. Ijaz, A. Imran and S. Pandiaraj, Chemosphere, 2024, 346, 140410 CrossRef CAS PubMed.
- Q. Sun, Y. Fang, S. Wang, W. Ma, Q. Zhang, J. Wang, Y. Wang, J. Lang, J. Zhang, Y. Liu and J. Yang, Mater. Sci. Eng. B, 2024, 299, 116921 CrossRef CAS.
- N. Zhang, J. Ding, Y. Wang, X. Liu, Y. Li, M. Liu, Z. Fu, Y. Yang, J. Su, G. Song, F. Yang, Y. Guo and J. Liu, J. Phys.: Condens. Matter, 2021, 33, 135803 CrossRef CAS PubMed.
- H. Yamada, V. Garcia, S. Fusil, S. Boyn, M. Marinova, A. Gloter, S. Xavier, J. Grollier, E. Jacquet, C. Carretero, C. Deranlot, M. Bibes and A. Barthelemy, ACS Nano, 2013, 7, 5385–5390 CrossRef CAS PubMed.
- L. Bocher, A. Gloter, A. Crassous, V. Garcia, K. March, A. Zobelli, S. Valencia, S. Enouz-Vedrenne, X. Moya, N. D. Mathur, C. Deranlot, S. Fusil, K. Bouzehouane, M. Bibes, A. Barthelemy, C. Colliex and O. Stephan, Nano Lett., 2012, 12, 376–382 CrossRef CAS PubMed.
- J. Y. Son, J. H. Lee, S. Song, Y. H. Shin and H. M. Jang, ACS Nano, 2013, 7, 5522–5529 CrossRef CAS PubMed.
- Z. Yu, B. Li, H. Bai and H. Pan, J. Mater. Chem. A, 2023, 11, 19074–19082 RSC.
- J. Chu, Y. Wang, X. Wang, K. Hu, G. Rao, C. Gong, C. Wu, H. Hong, X. Wang, K. Liu, C. Gao and J. Xiong, Adv. Mater., 2021, 33, e2004469 CrossRef PubMed.
- T. Song, X. Cai, M. W. Tu, X. Zhang, B. Huang, N. P. Wilson, K. L. Seyler, L. Zhu, T. Taniguchi, K. Watanabe, M. A. McGuire, D. H. Cobden, D. Xiao, W. Yao and X. Xu, Science, 2018, 360, 1214–1218 CrossRef CAS PubMed.
- H. Bai, D. Liu, P. Zhou, J. Feng, X. Sui, Y. Lu, H. Liu and H. Pan, J. Mater. Chem. A, 2022, 10, 25262–25271 RSC.
- W. Song, R. Fei and L. Yang, Phys. Rev. B, 2017, 96, 235420 CrossRef.
- H. Li, S. Ruan and Y. J. Zeng, Adv. Mater., 2019, 31, e1900065 CrossRef PubMed.
- H. Ai, X. Song, S. Qi, W. Li and M. Zhao, Nanoscale, 2019, 11, 1103–1110 RSC.
- G. Song, C. Zhang, T. Xie, Q. Wu, B. Zhang, X. Huang, Z. Li, G. Li and B. Gao, Phys. Chem. Chem. Phys., 2022, 24, 20530–20537 RSC.
- X. Gu, X. Li, Y. Diao, Z. Lu, Z. Dong, H. Fu and C. Zhong, Comput. Mater. Sci., 2023, 228, 112311 CrossRef CAS.
- B. Mortazavi, M. Shahrokhi, B. Javvaji, A. V. Shapeev and X. Zhuang, Nanotechnology, 2022, 33, 275701 CrossRef CAS PubMed.
- T. Su, C. H. Lee, S.-D. Guo, G. Wang, W.-L. Ong, L. Cao, W. Zhao, S. A. Yang and Y. S. Ang, Mater. Today Phys., 2023, 31, 101001 CrossRef CAS.
- J. Zhao, W. Wu, J. Zhu, Y. Lu, B. Xiang and S. A. Yang, Phys. Rev. B, 2020, 102, 245419 CrossRef CAS.
- F. Zhou, S. Yan, F. Yang, X. Liao, S. Lin, M. Liao, S. Lou, Y. Liu, Z. Gong, K. B. Tom, Y. Deng, X. Wang, Y. Huang, J. Zhu and J. Yao, ACS Nano, 2020, 14, 15544–15551 CrossRef CAS PubMed.
- J. Huang, M. Li, J. Chen, Y. Cheng, Z. Lai, J. Hu, F. Zhou, N. Qu, Y. Liu and J. Zhu, Surf. Interfaces, 2023, 38, 102825 CrossRef CAS.
- J. Huang, J. Xue, M. Li, J. Chen, Y. Cheng, Z. Lai, J. Hu, F. Zhou, N. Qu, Y. Liu and J. Zhu, Appl. Surf. Sci., 2023, 635, 157757 CrossRef CAS.
- F. Li, H. Ai, D. Liu, K. H. Lo and H. Pan, J. Mater. Chem. A, 2021, 9, 17749–17759 RSC.
- H. Bai, D. Liu and H. Pan, Mater. Horiz., 2023, 10, 5126–5132 RSC.
- J. Xue, J. Huang, M. Li, J. Chen, Z. Wei, Y. Cheng, Z. Lai, N. Qu, Y. Liu and J. Zhu, Metals, 2023, 13, 1690 CrossRef CAS.
- N. Qu, M. Chen, M. Liao, Y. Cheng, Z. Lai, F. Zhou, J. Zhu, Y. Liu and L. Zhang, Materials, 2023, 16, 2633 CrossRef CAS PubMed.
- J. Zhao, W. Wu, J. Zhu, Y. Lu, B. Xiang and S. A. Yang, Phys. Rev. B, 2020, 102, 245419 CrossRef CAS.
- Y. Jia, M. Zhao, G. Gou, X. C. Zeng and J. Li, Nanoscale Horiz., 2019, 4, 1113–1123 RSC.
- C. Liu, X. Zhang, X. Wang, Z. Wang, I. Abdelwahab, I. Verzhbitskiy, Y. Shao, G. Eda, W. Sun, L. Shen and K. P. Loh, ACS Nano, 2023, 17, 7170–7179 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- G. Kresse and J. Furthmuller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 49, 14251–14269 CrossRef CAS PubMed.
- P. Hohenberg and W. Kohn, Phys. Rev., 1964, 136, B864–B871 CrossRef.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- P. E. Blochl, Phys. Rev. B: Condens. Matter Mater. Phys., 1994, 50, 17953–17979 CrossRef PubMed.
- G. Kresse and D. Joubert, Phys. Rev. B: Condens. Matter Mater. Phys., 1999, 59, 1758–1775 CrossRef CAS.
- H. J. Monkhorst and J. D. Pack, Phys. Rev. B: Solid State, 1976, 13, 5188–5192 CrossRef.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- G. Henkelman, B. P. Uberuaga and H. Jónsson, J. Chem. Phys., 2000, 113, 9901–9904 CrossRef CAS.
- V. I. Anisimov, J. Zaanen and O. K. Andersen, Phys. Rev. B: Condens. Matter Mater. Phys., 1991, 44, 943–954 CrossRef CAS PubMed.
- S. Baroni, S. d Gironcoli and A. D. Corso, Rev. Mod. Phys., 2001, 73, 515–562 CrossRef CAS.
- A. Togo, F. Oba and I. Tanaka, Phys. Rev. B: Solid State, 2008, 78, 134106 CrossRef.
- G. Kresse and J. Hafner, Phys. Rev. B: Condens. Matter Mater. Phys., 1993, 47, 558–561 CrossRef CAS PubMed.
- V. Wang, N. Xu, J. C. Liu, G. Tang and W. T. Geng, Comput. Phys. Commun., 2021, 267, 108033 CrossRef CAS.
- N. Metropolis, A. W. Rosenbluth, M. N. Rosenbluth, A. H. Teller and E. Teller, J. Chem. Phys., 1953, 21, 1087–1092 CrossRef CAS.
- L. Liu, S. Chen, Z. Lin and X. Zhang, J. Phys. Chem. Lett., 2020, 11, 7893–7900 CrossRef CAS PubMed.
- L. Liu, X. Ren, J. Xie, B. Cheng, W. Liu, T. An, H. Qin and J. Hu, Appl. Surf. Sci., 2019, 480, 300–307 CrossRef CAS.
- A. Mahajan and S. Bhowmick, J. Phys. Chem. C, 2023, 127, 11407–11418 CrossRef CAS.
- H. Ai, F. Li, H. Bai, D. Liu, K. H. Lo, S. A. Yang, Y. Kawazoe and H. Pan, npj Comput. Mater., 2022, 8, 60 CrossRef CAS.
- S. Cahangirov, M. Topsakal, E. Akturk, H. Sahin and S. Ciraci, Phys. Rev. Lett., 2009, 102, 236804 CrossRef CAS PubMed.
- H. Şahin, S. Cahangirov, M. Topsakal, E. Bekaroglu, E. Akturk, R. T. Senger and S. Ciraci, Phys. Rev. B: Condens. Matter Mater. Phys., 2009, 80, 155453 CrossRef.
- H. P. You, N. Ding, J. Chen and S. Dong, Phys. Chem. Chem. Phys., 2020, 22, 24109–24115 RSC.
- Z. Gao, M. Wang, H. Zhang, S. Chen, C. Wu, I. D. Gates, W. Yang, X. Ding and J. Yao, Sol. Energy Mater. Sol. Cells, 2021, 233, 111401 CrossRef CAS.
- B. Li, J. Geng, H. Ai, Y. Kong, H. Bai, K. H. Lo, K. W. Ng, Y. Kawazoe and H. Pan, Nanoscale, 2021, 13, 8038–8048 RSC.
- Y. Chen, X. Pu, X. Xu, M. Shi, H. J. Li and D. Wang, Sensors, 2022, 23, 91 CrossRef PubMed.
- X. Liang and C. Wang, Acta Mater., 2022, 241, 118406 CrossRef CAS.
- J. Geng, I. N. Chan, H. Ai, K. H. Lo, Y. Kawazoe, K. W. Ng and H. Pan, Phys. Chem. Chem. Phys., 2020, 22, 17632–17638 RSC.
- M. Ye and D. Vanderbilt, Phys. Rev. B, 2016, 93, 134303 CrossRef.
- H. Pan, J. Phys. Chem. C, 2014, 118, 13248–13253 CrossRef CAS.
- X. Lan, Y. Gao, Y. Ge, W. Wan and Y. Liu, AIP Adv., 2023, 13, 125014 CrossRef CAS.
- M. Fiebig, N. P. Duong, T. Satoh, B. B. Van. Aken, K. Miyano, Y. Tomioka and Y. Tokura, J. Phys. D: Appl. Phys., 2008, 41, 164005 CrossRef.
- S. Rahman, J. F. Torres, A. R. Khan and Y. Lu, ACS Nano, 2021, 15, 17175–17213 CrossRef CAS PubMed.
- T. Jungwirth, X. Marti, P. Wadley and J. Wunderlich, Nat. Nanotechnol., 2016, 11, 231–241 CrossRef CAS PubMed.
- D. Yi, C. L. Flint, P. P. Balakrishnan, K. Mahalingam, B. Urwin, A. Vailionis, A. T. N'Diaye, P. Shafer, E. Arenholz, Y. Choi, K. H. Stone, J. H. Chu, B. M. Howe, J. Liu, I. R. Fisher and Y. Suzuki, Phys. Rev. Lett., 2017, 119, 077201 CrossRef PubMed.
- G. H. O. Daalderop, P. J. Kelly and M. F. H. Schuurmans, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 11919–11937 CrossRef CAS PubMed.
- X. Wang, D. S. Wang, R. Wu and A. J. Freeman, J. Magn. Magn. Mater., 1996, 159, 337–341 CrossRef CAS.
- H. S. Sarmah and S. Ghosh, J. Phys. D: Appl. Phys., 2023, 56, 345002 CrossRef.
- Y. Sun, Z. Zhuo, X. Wu and J. Yang, Nano Lett., 2017, 17, 2771–2777 CrossRef CAS PubMed.
- G. H. Daalderop, P. J. Kelly and M. F. Schuurmans, Phys. Rev. B: Condens. Matter Mater. Phys., 1990, 41, 11919–11937 CrossRef CAS PubMed.
- H. L. Zhuang, P. R. C. Kent and R. G. Hennig, Phys. Rev. B, 2016, 93, 134407 CrossRef.
- C. Ederer and N. A. Spaldin, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 74, 024102 CrossRef.
- J. He, P. Lyu, L. Z. Sun, Á. M. Garcíaa and P. Nachtigall, J. Mater. Chem. C, 2016, 4, 6500–6509 RSC.
- H. You, N. Ding, J. Chen and S. Dong, Phys. Chem. Chem. Phys., 2020, 22, 24109–24115 RSC.
- N. Ding, J. Chen, S. Dong and A. Stroppa, Phys. Rev. B, 2020, 102, 165129 CrossRef CAS.
- Y. Guo, Y. H. Zhang, S. J. Yuan, B. Wang and J. L. Wang, Nanoscale, 2018, 10, 18036–18042 RSC.
- J. B. Goodenough and A. L. Loeb, Phys. Rev., 1955, 98, 391–408 CrossRef CAS.
- P. W. Anderson, Phys. Rev., 1959, 115, 2–13 CrossRef CAS.
- J. Kanamori, Prog. Theor. Phys., 1963, 30, 275–289 CrossRef CAS.
- H. Pan, J. Phys. Chem. C, 2014, 118, 13248–13253 CrossRef CAS.
- C. Song, K. W. Geng, F. Zeng, X. B. Wang, Y. X. Shen, F. Pan, Y. N. Xie, T. Liu, H. T. Zhou and Z. Fan, Phys. Rev. B: Condens. Matter Mater. Phys., 2006, 73, 024405 CrossRef.
- H. Pan, Sci. Rep., 2014, 4, 7524 CrossRef CAS PubMed.
- R. A. De. Groot and F. M. Mueller, Phys. Rev. Lett., 1983, 50, 2024–2027 CrossRef CAS.
- I. B. Bersuker, Phys. Rev. Lett., 2012, 108, 137202 CrossRef PubMed.
- Y. Zhang, L. Lin, A. Moreo, G. Alvarez and E. Dagotto, Phys. Rev. B: Condens. Matter Mater. Phys., 2021, 103, L121114 CrossRef CAS.
- C. Hou, Y. Shen, W. Sun, Y. Chen, D. Ni and Q. Wang, J. Mater. Chem. C, 2022, 10, 10302–10309 RSC.
- W. Chen, X. Hou, X. Shi and H. Pan, ACS Appl. Mater. Interfaces, 2018, 10, 35289–35295 CrossRef CAS PubMed.
- K.-A. N. Duerloo, M. T. Ong and E. J. Reed, J. Phys. Chem. Lett., 2012, 3, 2871–2876 CrossRef CAS.
- W. Wu, L. Wang, Y. Li, F. Zhang, L. Lin, S. Niu, D. Chenet, X. Zhang, Y. Hao, T. Heinz, J. Hone and Z. Wang, Nature, 2014, 514, 470–474 CrossRef CAS PubMed.
- H. Zhu, Y. Wang, J. Xiao, M. Liu, S. Xiong, Z. Wong, Z. Ye, Y. Ye, X. Yin and X. Zhang, Nat. Nanotechnol., 2015, 10, 151–155 CrossRef CAS PubMed.
|
| This journal is © The Royal Society of Chemistry 2024 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article b,
Zhichao
Yu
b,
Chi Tat
Kwok
*ab and
Hui
Pan
b,
Zhichao
Yu
b,
Chi Tat
Kwok
*ab and
Hui
Pan
 *bc
*bc
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 local magnetic moments was adopted; 40
000 local magnetic moments was adopted; 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 MC steps were performed at each temperature, and the calculations lasted for 6.4 × 109 loops at each temperature.
000 MC steps were performed at each temperature, and the calculations lasted for 6.4 × 109 loops at each temperature.




