 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Peripheral halogen atoms in multi-resonant thermally activated delayed fluorescence emitters: the role of heavy atoms in intermolecular interactions and spin orbit coupling†‡
Hector
Miranda-Salinas§
 a,
Jingxiang
Wang§
b,
Andrew
Danos
a,
Jingxiang
Wang§
b,
Andrew
Danos
 a,
Tomas
Matulaitis
b,
Kleitos
Stavrou
a,
Tomas
Matulaitis
b,
Kleitos
Stavrou
 *a,
Andrew P.
Monkman
*a,
Andrew P.
Monkman
 *a and
Eli
Zysman-Colman
*a and
Eli
Zysman-Colman
 *b
*b
aDepartment of Physics, Durham University, Durham, UK DH1 3LE. E-mail: a.p.monkman@durham.ac.uk; kleitos.stavrou@durham.ac.uk
bOrganic Semiconductor Centre, EaStCHEMSchool of Chemistry, University of St Andrews, St Andrews, UK KY16 9ST. E-mail: eli.zysman-colman@st-andrews.ac.uk
First published on 20th December 2023
Abstract
Multi-resonant thermally activated delayed fluorescence materials (MR-TADF) can show narrow-band emission with high photoluminescence quantum efficiency, desirable for applications in organic light emitting diodes (OLEDS). However, they frequently suffer from slow reverse intersystem crossing (RISC) compared to established donor–acceptor TADF emitters, leading to severe device efficiency roll-off at high exciton densities. Introducing heavy atom effects (HAE) by core-substitution has been previously shown to enhance spin orbit coupling and thus RISC in MR-TADF emitters, frequently with oxygen atoms replaced by isoelectronic sulfur or selenium. Here, we explore an alternate HAE strategy using peripheral halogenation of the MR-TADF DiKTa core, comparing tBr-DiKTa and dBr-tBu-DiKTa with non-halogenated Mes3-DiKTa. The two brominated emitters demonstrate improved kRISC because of the HAE, while the rate appears to improve by an additional order of magnitude in the mCP host, because of intermolecular (guest–host) interactions. Despite the beneficial hetero-intermolecular interactions, strong homo-intermolecular interactions result in enhanced non-radiative pathways and lower photoluminescence quantum yields. OLEDs of dBr-tBu-DiKTa hence showed comparable EQEmax with Mes3-DiKTa (21%) and improved efficiency roll-off until 500 cd m−2, although with accelerated roll-off beyond a critical current density. Together with comparisons in less heavily doped devices, these results show that the HAE provided by peripheral halogens improves the device performance up to 500 cd m−2, but also supports detrimental intermolecular interactions that dominate at higher device currents.
Introduction
Organic light-emitting diodes (OLEDs) have emerged as an important alternative to traditional light-emitting devices such as LCDs.1,2 Amongst their advantages, OLEDs are self-emissive and addressable on the scale of single pixels, and so do not require a backlighting panel. As a result, they are energy efficient, and can produce pure black with high contrast. Furthermore, they are also able to be fabricated on a multitude of diverse substrates opening the door to very thin, foldable/rollable and even semi-transparent displays.3The first-generation of OLEDs used fluorescent emitters and thus were limited to a maximum internal quantum efficiency (IQE) of 25%, as only the singlet excitons could produce light. The two main classes of compounds used to raise this efficiency ceiling to 100% IQE are organometallic phosphorescent (Ph) complexes (used in most current commercialized OLEDs) and more recent thermally activated delayed fluorescence (TADF) emitters. In PhOLEDs singlet excitons are converted to triplet excitons, all of which phosphoresce with the assistance of the heavy metal ion, which promotes strong spin–orbit coupling (SOC) facilitating both intersystem crossing (ISC) and phosphorescence emission.4,5 In TADF OLEDs, triplet excitons are up-converted to singlet excitons by reverse intersystem crossing (RISC), all of which may then fluoresce.6,7 TADF is possible when there is both a sufficiently small singlet–triplet energy gap (ΔEST) and non-zero SOC between the relevant singlet and triplet states of different orbital types.
One of the key metrics of TADF materials that affects device performance is the rate of RISC. Rapid RISC (large kRISC) implies the efficient harvesting of triplet excitons and their conversion to singlets, outcompeting both intrinsic non-radiative pathways (increasing an OLED's IQE and stability) as well as multi-excitonic quenching pathways such as triplet–triplet annihilation, and thereby alleviating the efficiency roll-off of the external quantum efficiency (EQE) at higher driving current (where triplet exciton density is the highest). According to Fermi's Golden rule, the rate of RISC can be expressed according to eqn (1):8
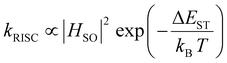 | (1) |
Many strategies have been developed to increase the kRISC.9 For example, extending the π-skeleton and charge delocalization in MR-TADF emitters can reduce the ΔEST of MR-TADF emitters.10–12 Introducing a long-range charge transfer character into the emissive excited state by decorating the MR-TADF core with peripheral donors has also been shown to reduce the ΔEST and enhance SOC.13–16 As SOC is approximately proportional to the fourth power of the nuclear charge of the atoms involved in the emissive transition, another strategy that has been effectively used to enhance kRISC in TADF molecules is to introduce heavy atoms to enhance the SOC.17 Halogenation of donor–acceptor (D–A) TADF molecules (e.g. with Cl, Br, and I) is a common strategy to enhance SOC. Bunz et al. first reported the exploitation of the internal heavy atom effect (HAE) in TADF compounds by decorating halogen atoms onto carbazole donors in multi-carbazole TADF emitters. With increasing numbers of bromine and iodine substituents, the excited-state lifetimes were found to be considerably shortened. Octaiodo derivatives 3f and 5f exhibited the shortest lifetimes of 1.2 and 0.4 μs respectively (Fig. 1). Although the photoluminescence quantum yields (ΦPL) were slightly decreased, halogen substituents were effective at accelerating both ISC and RISC.18 Kim et al. carried out both theoretical and experimental studies of halogenated analogues of 4CzIPN. 4CzIPN-Cl, 4CzIPN-Br, and 4CzIPN-I showed increased calculated SOC matrix elements (SOCME) between S1 and T1, of 0.24, 0.51, and 1.09 cm−1, respectively compared to 4CzIPN (0.22 cm−1). Consequently, both kISC and kRISC increased, with 4CzIPN-I having the fastest kISC and kRISC of 1.10 × 109 and 9.05 × 107 s−1, respectively. However, the OLEDs based on these materials showed lower maximum EQE (EQEmax) and more severe efficiency roll-off with the emitters bearing Br and I substituents, demonstrating that the incorporation of heavy elements does not always uniformly translate into an improved device performance. It is reasonable to speculate that weak C–X halogen bonds could lead to electrical instability in the devices. Additionally, faster kISC (increased in tandem with kRISC) can also compete with radiative singlet decay (ksr), resulting in multiple spin–flip cycles between S1 and T1 rather than direct emission following RISC.19,20 Further examples of HAE investigations of D–A TADF materials are summarised in the (ESI†).21–31
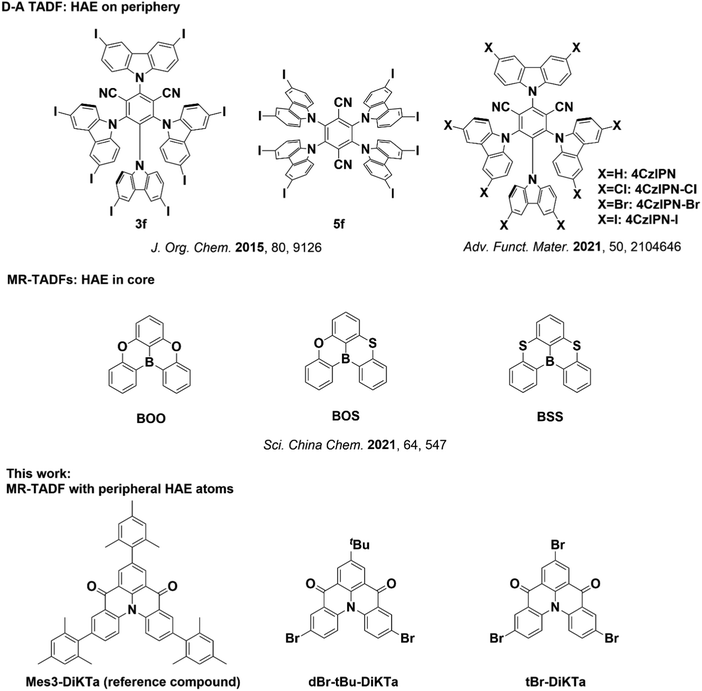 | ||
| Fig. 1 Structures of selected reported TADF materials with HAE, and the structures investigated in this work (other reported MR-TADF materials with HAE are summarized in the ESI,† p. S24). | ||
In contrast to D–A TADF materials, multi-resonant TADF emitters (MR-TADF) are rigid polycyclic aromatic compounds containing a combination of p- and n-dopants, which usually show larger ΔEST (>0.10 eV) and slower kRISC (∼104 s−1).32 RISC is typically enabled through an upper-state crossing mechanism rather than the vibronic coupling of the lower energy triplet states in D–A TADF emitters.33,34 Introducing heavy atoms into MR-TADF emitters is therefore a promising strategy to address their slower kRISC. Indeed, Chen et al. reported the boron/sulfur-based MR-TADF emitter BSS (Fig. 1),35 which possesses a large SOCME of 0.77 cm−1 between the S1 and T1 states and a faster kRISC of 1.18 × 105 s−1 compared to oxygen-containing analogues BOS (SOCME = 0.62 cm−1 and kRISC = 6.1 × 104 s−1) and BOO (SOCME = 0.01 cm−1 and kRISC = 1.1 × 104 s−1). Additional examples of HAE investigations for MR-TADF emitters are included in the ESI,† although we note that in all cases these involve heavy atom substitution directly within the MR-TADF core.35–47
We previously reported the MR-TADF emitter DiKTa and its mesitylated congener, Mes3-DiKTa (Fig. 1), which showed reduced aggregation-caused quenching and aggregate formation due to the presence of the bulky mesityl groups.48 Similar to most MR-TADF emitters, Mes3-DiKTa showed only moderate kRISC and large ΔEST in 3.5 wt% doped mCP films. Here, we demonstrate how replacing the three mesityl groups with bromines (tBr-DiKTa) leads to both faster kRISC and smaller ΔEST but stronger intramolecular interactions in the zeonex host. To minimize intermolecular interactions and suppress concentration quenching while still taking advantage of the peripheral halogen HAE, a dibrominated derivative (dBr-tBu-DiKTa) was also investigated. Although the heavy atom effect is clear in inert matrices, in the mCP host a competing exciplex formation channel (guest–host interaction) provides an alternate exciton harvesting channel. The improved kRISC and stronger hetero-intermolecular interactions evident in optical measurements of dBr-tBu-DiKTa resulted in an EQEmax of 21% in OLEDs, similar to the previously reported device with Mes3-DiKTa, but with an improved efficiency roll-off until 500 cd m−2. The presence of homo-intermolecular interactions (likely induced by the halogen atoms) and the weaker C–Br bonds led to more severe device roll-off at these higher current densities, counterbalancing the higher performance they can unlock at a lower driving current. These results therefore demonstrate both the advantageous and detrimental features of peripheral halogen decoration on the performance of MR-TADF OLEDs.
Results & discussion
Synthesis
The synthesis of dBr-tBu-DiKTa is outlined in Fig. S1 (ESI†). Intermediate 1 was prepared through high-temperature Ullmann coupling. This was then brominated in the presence of NBS and hydrolyzed to the diacid intermediate, 3. Finally, dBr-tBu-DiKTa was obtained following a Friedel–Crafts acylation in an overall yield of 32%. tBr-DiKTa and Mes3-DiKTa were obtained following the protocols in our previous work.48 The identity and purity of dBr-tBu-DiKTa were confirmed using a combination of NMR, HRMS, EA, HPLC and Mp analyses. Specific synthetic procedures and chemical characterization are provided in the ESI.†Theoretical calculations
The optimized geometries of the ground and excited states, and the electronic structures of dBr-tBu-DiKTa, tBr-DiKTa, and Mes3-DiKTa were first calculated using density functional theory (DFT) and time-dependent DFT (TD-DFT) within the Tamm–Dancoff approximation (TDA) at the PBE0/6-31G(d,p) level of theory in the gas phase.49,50 As shown in Fig. S13 (ESI†), the LUMO of these materials is located on the DiKTa core. The HOMOs are likewise distributed over the DiKTa core, and with contributions from the bromine substituents in dBr-tBu-DiKTa and tBr-DiKTa. There are four low-lying triplet states near or below S1, which are implicated in facilitating RISC.33,36,39,51,52 Thus, we calculated the SOC matrix elements (SOCME) for S1–T1, S1–T2, S1–T3, and S1–T4 transitions based on the optimized T1 geometries. With increasing bromine content, SOCME increased from 0.28 to 0.30 and 0.46 cm−1 for S1–T1, from 5.71 to 13.23 and 22.81 cm−1 for S1–T2, from 5.59 to 8.45 and 23.23 cm−1 for S1–T3, and from 8.14 to 26.37 and 33.95 cm−1 for S1–T4 for Mes3-DiKTa, dBr-tBu-DiKTa, and tBr-DiKTa, all respectively. The large enhancement of the SOCME values can thus be directly attributed to the HAE.State energies and difference densities were also calculated using Spin-Component Scaling second-order algebraic diagrammatic construction (SCS-ADC2)/(cc-pVDZ) to provide accurate predictions of the ΔEST values (Fig. 2).53,54 All compounds show similar difference density patterns for both S1 and T1, localized on the DiKTa core and associated with states of short-range charge transfer (SRCT) character. The corresponding ΔEST values are calculated to be 0.29, 0.27, and 0.26 eV for dBr-tBu-DiKTa, tBr-DiKTa, and Mes3-DiKTa, respectively, which largely reproduce the previously reported values.48,55
Electrochemistry
The electrochemical properties of Mes3-DiKTa, dBr-tBu-DiKTa, and tBr-DiKTa were investigated using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) in dichloromethane (DCM). As shown in Fig. S14 (ESI†), due to the inductively electron-withdrawing character of bromine tBr-DiKTa and dBr-tBu-DiKTa show more anodically shifted oxidation and reduction potentials, and thus more stabilized HOMO and LUMO levels compared to Mes3-DiKTa. The oxidation potentials (Eox) and reduction potentials (Ered) versus SCE, taken from the peak values of the DPVs, are 1.66, 1.79, 1.81 eV, and −1.39, −1.27, −1.15 eV for Mes3-DiKTa, dBr-tBu-DiKTa, and tBr-DiKTa, respectively. Both dBr-tBu-DiKTa and tBr-DiKTa also show anodically shifted potentials compared to parent DiKTa (Eox at 1.78 V and Ered at −1.34 V), again the result of the inductively electron-withdrawing bromine substituents.55 The corresponding HOMO and LUMO energies are calculated to be −6.00/−2.95, −6.13/−3.07, and −6.15/−3.19 eV, respectively, where the trend matches well with the DFT calculations (Fig. S13, ESI†). The data are summarized in Table S1 (ESI†).Optical properties
Steady-state absorption and photoluminescence (PL) spectra of dBr-tBu-DiKTa and tBr-DiKTa in different solvents are shown in Fig. S15 (ESI†). Both absorption and PL spectra undergo a small bathochromic shift (120–150 meV) with increasing solvent polarity, a phenomenon consistent with the short–range charge transfer (SRCT) character of the emissive excited states in MR-TADF materials.48,56,57 In toluene (PhMe) solution, the lowest energy absorption maximum of dBr-tBu-DiKTa is centred at 445 nm and the main PL peak, λPL, is at 464 nm. Similar absorption and PL behaviour, at slightly higher wavelengths of 455 and 477 nm, respectively, is observed for tBr-DiKTa (Fig. S15b, ESI†).Comparison of the photophysical properties of dBr-tBu-DiKTa, tBr-DiKTa, and Mes3-DiKTa in the same environment is crucial to understand the effect of the bromine substituents on their ground- and excited-state properties. Fig. 3(a) illustrates this comparison in PhMe solution. The absorption spectral shape is similar in all cases, having a 10 nm peak difference across dBr-tBu-DiKTa (445 nm), Mes3-DiKTa (449 nm), and tBr-DiKTa (455 nm). The molar extinction coefficient, ε, of this lowest energy band is around 5.5 × 104 M−1 cm−1 in the first two compounds, with tBr-DiKTa having a lower value of 3.5 × 104 M−1 cm−1. The latter can be explained by the lower oscillator strength of S1 (0.21 for tBr-DiKTa compared to 0.22 for dBr-tBu-DiKTa and 0.23 for Mes3-DiKTa, Fig. S13, ESI†), as well as the poor solubility of tBr-DiKTa leading to ground-state dimer formation even in solution, which reduces the extinction coefficient value. Following the energy trends of the absorption spectra, the PL spectrum of dBr-tBu-DiKTa is the bluest with a λPL of 464 nm, followed by Mes3-DiKTa and tBr-DiKTa at 472 and 476 nm, respectively. The energetic order of the PL spectra is in reasonably good agreement with the SCS-ADC2 calculations.
Steady-state PL measurements of solution-cast films are shown in Fig. 3(b). At 1 wt% in mCP, Mes3-DiKTa and dBr-tBu-DiKTa have near-identical PL spectra with λPL at 483 nm, while tBr-DiKTa has a broader PL with λPL at 488 nm and an additional redshifted feature at 530 nm. A key difference between solution-state and solid-state PL (i.e. in mCP) is that intermolecular (homo- or hetero-molecular) interactions are stronger in the solid state and contribute to the observed emission broadening.34 Without the steric shielding of the mesityl groups, it is unsurprising that dBr-tBu-DiKTa interact more compared to Mes3-DiKTa, resulting in a slightly broader and red-shifted emission. tBr-DiKTa, which does not contain any sterically bulky groups, appears to interact the most, obvious from the strongest impacts on its PL spectrum.
Doped films at 0.1 wt% of the emitters in zeonex were studied in order to mitigate intermolecular interactions in the solid state (no hetero- and reduced homo-molecular interactions).34 The PL spectra of these films show similar trends to those observed in solution, with dBr-tBu-DiKTa having a λPL at 456 nm, followed by Mes3-DiKTa at λPL of 464 nm and tBr-DiKTa at λPL of 470 nm (Fig. 3(b)). With reduced scope for intermolecular interactions in these dilute films, these trends and absence of significant PL broadening help to verify that intermolecular interactions are the main source of spectral broadening in the 1 wt% mCP films. Lower loading in mCP films could not be pursued though, as the thin films with 1 wt% doping were already approaching the lower limits of emission signal sensitivity, in contrast to the much thicker polymeric zeonex films.
Time-resolved PL studies
Time-resolved PL measurements were performed on the same films to investigate the TADF properties of the materials at both 300 K (room temperature) and at 80 K (LT). Previously reported Mes3-DiKTa48 was used as the benchmark reference material, and diluted in 0.1 wt% zeonex films to allow us to study the HAEs without significant additional complexity due to intermolecular interactions. The time-resolved decays of the zeonex films are shown in Fig. 4(a). With the introduction of bromine atoms, the prompt fluorescence (PF) and delayed fluorescence (DF) lifetimes become shorter, while the DF contribution to the overall emission increases (Table 1). Thus, tBr-DiKTa shows the best TADF response followed by dBr-tBu-DiKTa and Mes3-DiKTa – a clear indicator that the peripheral HAE assists in augmenting SOC. Analysis of the decays with fitted exponential lifetimes58 verifies these trends, with kRISC being higher when more Br atoms are introduced within the emitter (Table 1). Two different methods6,58 were compared for the decay analysis to explore if the enhanced aggregation (leading to complex decays) affects the calculated rate constants, and the results are broadly similar (Table S2, ESI†).| Molecule | Host | SS PLa | LT PH b | ΔESTc | τ PF | τ DF | k F | k ISC | k RISC | Φ PL | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [nm]/[eV] | [eV] | [meV] | [ns] | [ns] | [μs] | [μs] | [× 108 s−1] | [× 108 s−1] | [× 103 s−1] | N2 | ||
| a Steady state emission peak. b Onset energy of the time resolved phosphorescence emission, after 20 ms at 80 K, λexc = 355 nm. c ΔEST estimated from the SS PL and LT PH onset energy. d From mono- or bi-exponential fitting of PF and DF regions. | ||||||||||||
| tBr-DiKTa | zeonex | 470/2.64 | 2.61 | 140 | 1.40 | 6.35 | — | 755.4 | 3.86 | 2.26 | 4.53 | 0.23 |
| mCP | 488/2.54 | 2.52 | 150 | 2.99 | — | 7.01 | 108.5 | 3.34 | 3.53 | 197 | 0.61 | |
| dBr-tBu-DiKTa | zeonex | 456/2.72 | 2.57 | 300 | 2.10 | 7.23 | — | 851.3 | 2.48 | 1.48 | 3.63 | 0.26 |
| mCP | 483/2.57 | 2.53 | 210 | 4.01 | — | 17.4 | 310.2 | 2.49 | 2.76 | 19.3 | 0.82 | |
| Mes3-DiKTa | zeonex | 464/2.67 | 2.55 | 230 | 6.79 | — | — | 912.3 | 1.47 | 0.822 | 1.44 | 0.53 |
| mCP | 483/2.57 | 2.51 | 210 | 4.55 | — | 26.68 | 565.2 | 2.20 | 1.35 | 3.1 | 0.90 | |
Although the time-resolved PL decays establish that the presence of the peripheral bromines enhance the TADF performance (through kISC and kRISC), the greater planarity and electron density of the brominated molecules simultaneously permits an unignorable contribution from aggregates, even at 0.1 wt% concentration in the zeonex matrix. Examining the individual time-resolved PL spectra, those of Mes3-DiKTa show no aggregation contribution at RT, with only a minor contribution at LT manifesting as a broadening of the full width half maximum (FWHM) at the late prompt regime (∼50 ns, Fig. S16 and S17, ESI†). The time-resolved PL spectra of dBr-tBu-DiKTa instead reveal a stronger aggregation contribution, which appears as a broad, red-shifted emission band from 30–100 ns delay time (Fig. 4(c) and Fig. S18, S19, ESI†). The separate monomer and aggregate emission is well-resolved at lower temperatures, where the monomer-like TADF contribution is suppressed. Similar behaviour with even stronger aggregate emission is observed in tBr-DiKTa, with the broad time-resolved spectrum dominant in the PF regime (Fig. 4(c) and Fig. S20, S21, ESI†).
To assess the performance of the emitters in a device-compatible host (and at device-relevant concentrations), time-resolved measurements were also performed on 1 wt% mCP films. Mes3-DiKTa at RT in mCP has similar behaviour as in zeonex, indicating that intermolecular interactions remain mostly suppressed. The singlet energy (PL onset) is lower compared to zeonex films (2.75 and 2.82 eV), likely because of the difference in environment (Fig. S22, ESI†).59,60 Both PF and DF emission lifetimes are shorter compared to those in zeonex films, consistent with the smaller experimental ΔEST (230 and 210 meV) and the rigid matrix effect,61–63 resulting in a two-fold faster kRISC (Table 1). An extra minor decay component in the DF regime is also observed with a lifetime of 26 μs, likely originating from mixed intermolecular and monomer emission (Fig. S22 and S23, ESI†).64
Compared to the PL in zeonex, the PL of dBr-tBu-DiKTa in mCP changes similarly to that of Mes3-DiKTa, because of the differences in the host environment. An enhanced contribution from a new species appears in the late PF regime (Fig. 5, ∼30 ns), observed as a broadened emission that lasts until early μs and is most clearly visible in the contour plot of the normalised time-resolved spectra (Fig. S24, ESI†). Residual host emission from mCP (<400 nm) is also visible in these contour plots during the early PF region, but its contribution is not considered in the time-resolved decay. In the subsequent DF region, the emission becomes narrow again, reflecting a return to pure monomer emission. The intermolecular species contribution dominates at low temperature (where the TADF is completely suppressed) and is coincidentally isoenergetic with the phosphorescence emission. When the room temperature measurement is fitted with exponential lifetimes, the PF has a multi-exponential decay with resolvable components ascribed to both monomer and intermolecular species emission, while the DF lifetime is almost three times shorter compared to zeonex films. As a result, the calculated kRISC becomes over five times faster in mCP (Table 1). The effect of intermolecular interactions is even stronger for tBr-DiKTa in mCP (Fig. 6 and Fig. S25, ESI†). Like dBr-tBu-DiKTa, tBr-DiKTa PF comprises a mixed monomer and intermolecular species emission, with the latter lasting until early μs. The DF lifetime in mCP becomes seven times shorter than in zeonex, and thus the calculated kRISC appears to be over forty times faster (Table 1).
 | ||
| Fig. 5 Time-resolved emission decays and spectra at different time delays, for dBr-tBu-DiKTa in a mCP matrix at 1 wt% concentration at (a) 300 K and (b) 80 K. λexc = 355 nm. | ||
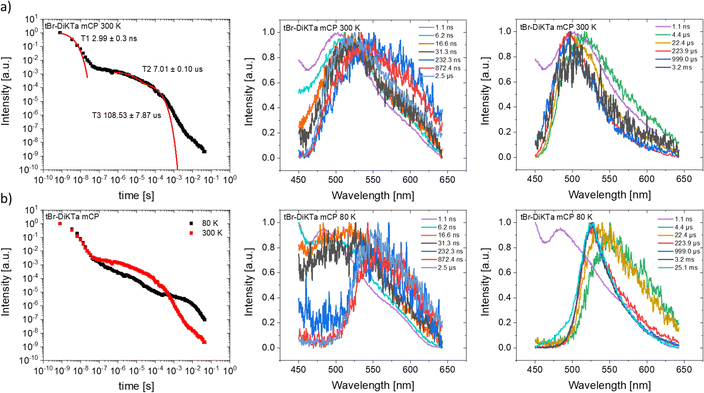 | ||
| Fig. 6 Time-resolved emission decays and spectra at different time ranges, for tBr-DiKTa in a mCP matrix at 1 wt% concentration at (a) 300 K and (b) 80 K. λexc = 355 nm. | ||
These big differences in the calculated kRISC for the brominated emitters in the two different hosts at first seem unreasonable, while a second species is clearly involved in the mCP films which complicates the decay kinetics and fitting. The higher emitter concentration in the mCP films also necessarily promotes stronger intermolecular interactions between the guest molecules. In applications, this could either promote a secondary triplet harvesting pathway, or generate a poorly emissive species that quenches the monomer emission.34,65 To address the latter, different concentrations of dBr-tBu-DiKTa (0.1, 1, 4 wt%) in the inert zeonex host (a host with no electronic interaction with the guest molecules)59 were measured. From the steady-state measurements, a bathochromic shift of the PL onset is observed along with a broadening of the FWHM with increasing concentration (Fig. S26, ESI†). The red shift is assigned to self-absorption while the increased PL contribution at 480 nm is assigned to aggregate emission. Interestingly, the PL spectrum of the 4 wt% doped film in zeonex is like the one of the 1 wt% doped film in mCP, indicating that the degree of aggregation formation/contribution to the PL spectrum is different in the two hosts. From the time-resolved decays, the monomer prompt lifetime decreases with increasing concentration from 2.1 to 1.5 ns at 0.1 and 4 wt%, respectively, indicating parallel energy transfer from the high-energy monomer to the lower-energy aggregate state. The aggregate prompt lifetime, identified from the different time-resolved PL spectra, increases both in length and contribution (Fig. S19 and S27, ESI†). The delayed emission lifetime also appears to decrease with increasing concentration, from 851 to 274 μs for 1 and 4 wt% doped films of dBr-tBu-DiKTa, respectively (Fig. S27a, ESI†). This decrease in prompt and delayed fluorescence lifetimes indicates that the higher emitter concentration leads to stronger intermolecular interactions between the dBr-tBu-DiKTa molecules, thus generating poorly emissive aggregate species, considering the low transient signal this species produces. This is also confirmed by the decrease of the ΦPL with increasing dBr-tBu-DiKTa concentration (26 and 20% for 0.1 and 1 wt% doped zeonex films, respectively). Thus, the unshielded brominated DiKTa molecules have dominant intermolecular interactions at high concentrations, which are at least as impactful as any HAE on the overall photophysics. Nevertheless, at 0.1 wt% with minimal aggregation effects, comparing the three emitters in zeonex hosts reveals the impacts of HAEs on improving the SOC and enhancing the ISC/RISC (Table 1).
While the ΦPL of 1 wt% zeonex films of dBr-tBu-DiKTa, tBr-DiKTa, and Mes3-DiKTa are 20, 15, and 47% respectively, these values change dramatically in mCP (1 wt%) to 82, 61, and 90% (Table S2, ESI†). This big difference in ΦPL in the two hosts cannot be explained by guest–guest interactions at such low film doping concentrations. Focusing on dBr-tBu-DiKTa, this emitter has a good ΦPL in mCP (promising for devices) but is also sensitive to intermolecular interaction because of its reduced peripheral shielding groups. We observe that the ΦPL for dBr-tBu-DiKTa increases to 45% in the UGH-3 host, but this remains far below the 82% measured in mCP. This indicates that the transition to an inert small molecule host (UGH-3) potentially suppresses vibrational motions that may be more active in the more fluid zeonex host (ΦPL 20%). Still, the large mCP ΦPLs cannot be fully explained, and we suggest that it originates from intermolecular interaction between the guest and host molecules, forming an exciplex species.66 Heteromolecular interactions are possible based on the measured HOMO/LUMO values of the three emitters (−6.13/−3.07, −6.15/−3.19, and −6.00/−2.95 eV for dBr-tBu-DiKTa, tBr-DiKTa, and Mes3-DiKTa respectively) and the mCP host (−5.9/−2.4 eV).67 This energetic difference and non-nested HOMO/LUMOs favour the formation of an exciplex between the two molecules but unfortunately this kind of species has been difficult to observe experimentally because of the parallel presence of aggregates. The presence of an exciplex would also explain the big difference between the PL spectra of the three molecules in mCP (Fig. 3(b)), which have different PL energetic order (Mes3-DiKTa → dBr-tBu-DiKTa → tBr-DiKTa) compared to solution samples and zeonex films (dBr-tBu-DiKTa → Mes3-DiKTa → tBr-DiKTa). Furthermore, the absence of the short DF component in zeonex, compared to mCP, is explained by zeonex not containing any electron-donating fragments (such as carbazole in mCP) that could establish an exciplex state with the DiKTa core.
The complicated co-existence of monomer, aggregate, and exciplex emission in the mCP host makes it difficult to distinguish the spectrum of each individual species. This nuanced situation makes it impossible to calculate the real kinetics of these systems. Thus, the calculated kinetics values do not represent the truth, but an estimation of the systems modelled as single species environments.
Devices
OLEDs using the reference material Mes3-DiKTa have been reported to have an EQEmax of 21.1%, with λEL = 480 nm and a FWHM of 36 nm.48 Similar to many MR-TADF devices, these showed a severe efficiency roll-off with an EQE1000 of 4.5%, likely due to the slow emitter kRISC. Herein, devices using a stack of ITO (anode)|NPB (HTL, 40 nm)|mCP (EBL, 10 nm)|emitter:mCP 3.5 wt% (EML, 30 nm)|T2T (HBL, 10 nm)|T2T:LiQ 45% (ETL, 35 nm)|LiQ (1 nm)|Al (cathode, 100 nm) were fabricated using Mes3-DiKTa or dBr-tBu-DiKTa as the emitter, with a representative performance shown in Fig. 7; devices with tBr-DiKTa were not investigated due to the significantly lower ΦPL of the emitter and its strong aggregation formation.The devices show good efficiency with EQEmax of 21.2 and 21.6% for the OLEDs with dBr-tBu-DiKTa and Mes3-DiKTa, respectively. The device with dBr-tBu-DiKTa has a slightly broader EL spectrum (FWHM 54 nm, Fig. 7(c)) compared to the Mes3-DiKTa device, following the PL of the mCP films (Fig. 3(b)), and indicating increased EL contribution from the intermolecular species (Fig. 7(c)). Considering that aggregate formation acts as a quenching contribution to dBr-tBu-DiKTa's ΦPL, we assign the high EQE value and broader EL spectrum to its exciplex contribution with the mCP host. The dBr-tBu-DiKTa OLEDs show higher luminance up to a specific voltage (7 V), which correlates with improved EQE roll-off behaviour up to this specific region. This improved performance is attributed to the HAE that enhances SOC and thus kRISC, enabling more efficient triplet harvesting. Any additional triplet harvesting channels from the exciplex species, that could also improve the device roll-off but were not clearly visible in the photophysical measurements (because monomer, aggregate and exciplex species emission complicate the results), must be acknowledged. At a luminance of 500 cd m−2 (current density of 4 mA cm−2) there is a critical point at which the efficiency roll-off of the OLED with dBr-tBu-DiKTa becomes much worse than the device with Mes3-DiKTa (EQE500 = 6% in both cases, at the crossover point). This effect is possibly a combination of detrimental effects such as weaker C–Br bonds leading to degradation, or increased activity of the aggregate species that sterically shielded Mes3-DiKTa which is more resistant towards forming.
To explore the effect of emitter aggregation on the device efficiency roll-off, additional devices with dBr-tBu-DiKTa at 1 wt% loading were fabricated, and the results are shown in Fig. S28 (ESI†). There is a minor hypsochromic shift of the EL spectrum, accompanied by a narrower FWHM indicating that the intermolecular species EL contribution is reduced. However, intermolecular interactions remain as evidenced by comparison of the EL spectrum to the 0.1 wt% zeonex film PL spectra (Fig. 3(b), and Fig. S28a, ESI†). The electrical response of the device is worse at lower concentrations (Fig. S28b, ESI†), resulting in a decreased EQEmax of 17.9% and increased efficiency roll-off up to 4 mA cm−2. Comparing the two dBr-tBu-DiKTa devices above 4 mA cm−2 (Fig. S28e, ESI†), the efficiency roll-off appears stronger in the 3.5 wt% device while the efficiency roll-off profile of the 1 wt% resembles more closely that of the Mes3DiKTa OLED. The latter observation indicates that among other detrimental factors, guest–guest aggregation quenching plays an important role at higher current densities.
Conclusions
From these results it is possible to understand the competing effects imparted by peripheral bromine substitution on the emission energy and decay lifetimes of the DiKTa derivatives (Table 1 and Fig. 4). Mes3-DiKTa has the slowest PF and DF emission in both investigated hosts. The kinetics of both PF and DF emission are significantly enhanced by SOC associated with the increasing bromine content in dBr-tBu-DiKTa and tBr-DiKTa. The peripheral mesityl decoration in Mes3-DiKTa, however, suppresses intermolecular interactions that are prevalent in the bromine-substituted emitters, resulting in significant aggregate emission in the latter and likely lowering the ΦPL values in the zeonex host. The complexity of the intermolecular interactions and the resulting photophysics is further increased in the mCP host, where an extra exciplex species appears to enhance the ΦPL compared to the inert zeonex host.The low ΦPL, along with the strong intermolecular interactions, even at 0.1 wt% loading, are prohibitive factors for the use of tBr-DiKTa in OLEDs. Comparing the devices with dBr-tBu-DiKTa and Mes3-DiKTa reveals the impact of bromine substitution on the improved kRISC and the lower efficiency roll-off. Despite some indications of EL from aggregates, the device EQEs are nearly identical at low current densities, and the EQE roll-off (up to 4 mA cm−2) was reduced in the OLED with dBr-tBu-DiKTa, indicating improved triplet harvesting up to this critical current density, being in good agreement with the estimated kRISC calculation. Beyond that critical current density, we suggest that SOC-assisted ISC for dBr-tBu-DiKTa leads to a build-up of the triplet exciton population in the EML, resulting in more severe roll-off compared to the device with Mes3-DiKTa. A comparison of 1 and 3.5 wt% dBr-tBu-DiKTa devices shows that the impact of aggregation is mitigated to some extent, although other detrimental parameters contribute to the overall efficiency roll-off in these devices (Table S1, ESI†) when the emitter loading is too low. It appears, therefore, that the effect of peripheral heavy halogen atoms on the performance of MR-TADF OLEDs – both directly in terms of affecting kRISC by enhancing SOC through the HAE, and indirectly through intermolecular interactions – can be both positive or negative depending on the exciton density.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This project has received funding from the European Union's Horizon 2020 research and innovation programme under the Marie Skłodowska Curie grant agreement no 812872 and 101073045 (TADFlife and TADFsolutions). J. W. thanks the China Scholarship Council (202006250026).References
- F. B. Dias, K. N. Bourdakos, V. Jankus, K. C. Moss, K. T. Kamtekar, V. Bhalla, J. Santos, M. R. Bryce and A. P. Monkman, Adv. Mater., 2013, 25, 3707–3714 CrossRef CAS PubMed
.
- S. Y. Lee, T. Yasuda, Y. S. Yang, Q. Zhang and C. Adachi, Angew. Chem., Int. Ed., 2014, 53, 6402–6406 CrossRef CAS PubMed
.
- G. Hong, X. Gan, C. Leonhardt, Z. Zhang, J. Seibert, J. M. Busch and S. Brase, Adv. Mater., 2021, 33, 2005630 CrossRef CAS PubMed
.
- M. A. Baldo, D. F. O'Brien, Y. You, A. Shoustikov, S. Sibley, M. E. Thompson and S. R. Forrest, Nature, 1998, 395, 151–154 CrossRef CAS
.
- X. Xu, X. Yang, J. Zhao, G. Zhou and W. Y. Wong, Asian J. Org. Chem., 2015, 4, 394–429 CrossRef CAS
.
- F. B. Dias, T. J. Penfold and A. P. Monkman, Methods Appl. Fluoresc., 2017, 5, 012001 CrossRef PubMed
.
- J. Gibson, A. P. Monkman and T. J. Penfold, ChemPhysChem, 2016, 17, 2956–2961 CrossRef CAS PubMed
.
- P. K. Samanta, D. Kim, V. Coropceanu and J.-L. Brédas, J. Am. Chem. Soc., 2017, 139, 4042–4051 CrossRef CAS PubMed
.
- K. R. Naveen, P. Palanisamy, M. Y. Chae and J. H. Kwon, Chem. Commun., 2023, 59, 3685–3702 RSC
.
- Y. Kondo, K. Yoshiura, S. Kitera, H. Nishi, S. Oda, H. Gotoh, Y. Sasada, M. Yanai and T. Hatakeyama, Nat. Photonics, 2019, 13, 678–682 CrossRef CAS
.
- S. Oda, B. Kawakami, Y. Yamasaki, R. Matsumoto, M. Yoshioka, D. Fukushima, S. Nakatsuka and T. Hatakeyama, J. Am. Chem. Soc., 2022, 144, 106–112 CrossRef CAS PubMed
.
- X. Lv, J. Miao, M. Liu, Q. Peng, C. Zhong, Y. Hu, X. Cao, H. Wu, Y. Yang, C. Zhou, J. Ma, Y. Zou and C. Yang, Angew. Chem., Int. Ed., 2022, 61, e202201588 CrossRef CAS PubMed
.
- X. Song, S. Shen, S. Zou, F. Guo, Y. Wang, S. Gao and Y. Zhang, Chem. Eng. J., 2023, 467, 143557 CrossRef CAS
.
- Y. Qi, W. Ning, Y. Zou, X. Cao, S. Gong and C. Yang, Adv. Funct. Mater., 2021, 31, 2102017 CrossRef CAS
.
- Y. Xu, C. Li, Z. Li, Q. Wang, X. Cai, J. Wei and Y. Wang, Angew. Chem., Int. Ed., 2020, 59, 17442–17446 CrossRef CAS PubMed
.
- M. Yang, I. S. Park and T. Yasuda, J. Am. Chem. Soc., 2020, 142, 19468–19472 CrossRef CAS PubMed
.
- M. A. Baldo, S. Lamansky, P. E. Burrows, M. E. Thompson and S. R. Forrest, Appl. Phys. Lett., 1999, 75, 4–6 CrossRef CAS
.
- A. Kretzschmar, C. Patze, S. T. Schwaebel and U. H. F. Bunz, J. Org. Chem., 2015, 80, 9126–9131 CrossRef CAS PubMed
.
- H. S. Kim, J. Y. Lee, S. Shin, W. Jeong, S. H. Lee, S. Kim, J. Lee, M. C. Suh and S. Yoo, Adv. Funct. Mater., 2021, 31, 2104646 CrossRef CAS
.
- L. G. Franca, Y. Long, C. Li, A. Danos and A. Monkman, J. Phys. Chem. Lett., 2021, 12, 1490–1500 CrossRef CAS PubMed
.
- J. Xu, X. Zhu, J. Guo, J. Fan, J. Zeng, S. Chen, Z. Zhao and B. Z. Tang, ACS Mater. Lett., 2019, 1, 613–619 CrossRef CAS
.
- Y. Liu, L. Hua, Z. Zhao, S. Ying, Z. Ren and S. Yan, Adv. Sci., 2021, 8, 2101326 CrossRef CAS PubMed
.
- D. de Sa Pereira, D. R. Lee, N. A. Kukhta, K. H. Lee, C. L. Kim, A. S. Batsanov, J. Y. Lee and A. P. Monkman, J. Mater. Chem. C, 2019, 7, 10481–10490 RSC
.
- S. Schott, E. R. McNellis, C. B. Nielsen, H.-Y. Chen, S. Watanabe, H. Tanaka, I. McCulloch, K. Takimiya, J. Sinova and H. Sirringhaus, Nat. Commun., 2017, 8, 15200 CrossRef PubMed
.
- B. H. Drummond, G. C. Hoover, A. J. Gillett, N. Aizawa, W. K. Myers, B. T. McAllister, S. T. E. Jones, Y.-J. Pu, D. Credgington and D. S. Seferos, J. Phys. Chem. C, 2020, 124, 6364–6370 CrossRef CAS
.
- X. Han, X. Wang, Y. Wu, J. Zhao, Y. Liu, H. Shu, X. Wu, H. Tong and L. Wang, J. Mater. Chem. C, 2022, 10, 7437–7442 RSC
.
- Y. Ren, Y. Wada, K. Suzuki, Y. Kusakabe, J. Geldsetzer and H. Kaji, Appl. Phys. Express, 2021, 14, 071003 CrossRef CAS
.
- N. Kanno, Y. Ren, Y. Kusakabe, K. Suzuki, K. Shizu, H. Tanaka, Y. Wada, H. Nakagawa, J. Geldsetzer and H. Kaji, Appl. Phys. Express, 2023, 16, 011006 CrossRef
.
- K. Shizu, Y. Ren and H. Kaji, J. Phys. Chem. A, 2023, 127, 439–449 CrossRef CAS PubMed
.
- D. Zhang, C. Jiang, Z. Wen, X. Feng and K. Li, Chem. – Eur. J., 2022, 28, e202202305 CrossRef CAS PubMed
.
- Y. Wang, W. Ning, W. Yang, L. Li, N. Li, T. Liu, S. Gong, X. Gao and C. Yang, Dyes Pigm., 2023, 214, 111225 CrossRef CAS
.
- S. Madayanad Suresh, D. Hall, D. Beljonne, Y. Olivier and E. Zysman-Colman, Adv. Funct. Mater., 2020, 30, 1908677 CrossRef CAS
.
- M. K. Etherington, J. Gibson, H. F. Higginbotham, T. J. Penfold and A. P. Monkman, Nat. Commun., 2016, 7, 13680 CrossRef CAS PubMed
.
- K. Stavrou, A. Danos, T. Hama, T. Hatakeyama and A. Monkman, ACS Appl. Mater. Interfaces, 2021, 13, 8643–8655 CrossRef CAS PubMed
.
- F. Chen, L. Zhao, X. Wang, Q. Yang, W. Li, H. Tian, S. Shao, L. Wang, X. Jing and F. Wang, Sci. China: Chem., 2021, 64, 547–551 CrossRef CAS
.
- I. S. Park, H. Min and T. Yasuda, Angew. Chem., Int. Ed., 2022, 61, e202205684 CrossRef CAS PubMed
.
- Q. Li, Y. Wu, Q. Yang, S. Wang, S. Shao and L. Wang, ACS Appl. Mater. Interfaces, 2022, 14, 49995–50003 CrossRef CAS PubMed
.
- L. Yang, P. Wang, K. Zhang, S. Wang, S. Shao and L. Wang, Dyes Pigm., 2023, 216, 111371 CrossRef CAS
.
- X. Cao, K. Pan, J. Miao, X. Lv, Z. Huang, F. Ni, X. Yin, Y. Wei and C. Yang, J. Am. Chem. Soc., 2022, 144, 22976–22984 CrossRef CAS PubMed
.
- D. Li, M. Li, D. Liu, J. Yang, W. Li, Z. Yang, H. Yuan, S. Jiang, X. Peng and G. X. Yang, Adv. Opt. Mater., 2023, 2301084 CrossRef CAS
.
- T. Hua, L. Zhan, N. Li, Z. Huang, X. Cao, Z. Xiao, S. Gong, C. Zhou, C. Zhong and C. Yang, Chem. Eng. J., 2021, 426, 131169 CrossRef CAS
.
- Y. X. Hu, J. Miao, T. Hua, Z. Huang, Y. Qi, Y. Zou, Y. Qiu, H. Xia, H. Liu, X. Cao and C. Yang, Nat. Photonics, 2022, 16, 803–810 CrossRef CAS
.
- S. M. Pratik, V. Coropceanu and J.-L. Brédas, ACS Mater. Lett., 2022, 4, 440–447 CrossRef CAS
.
- S. M. Pratik, V. Coropceanu and J.-L. Brédas, Chem. Mater., 2022, 34, 8022–8030 CrossRef CAS
.
- Y. Li, W. Li, J. Hu, X. Yao, L. Hua, W. Cai, S. Shi, C. Zhang, Z. Liu and S. Li, Adv. Opt. Mater, 2023, 11, 2300298 CrossRef CAS
.
- F. Huang, Y.-C. Cheng, H. Wu, X. Xiong, J. Yu, X.-C. Fan, K. Wang and X.-H. Zhang, Chem. Eng. J., 2023, 465, 142900 CrossRef CAS
.
- Y. Hu, J. Miao, C. Zhong, Y. Zeng, S. Gong, X. Cao, X. Zhou, Y. Gu and C. Yang, Angew. Chem., Int. Ed., 2023, 62, e202302478 CrossRef CAS PubMed
.
- D. Hall, S. M. Suresh, P. L. dos Santos, E. Duda, S. Bagnich, A. Pershin, P. Rajamalli, D. B. Cordes, A. M. Z. Slawin, D. Beljonne, A. Köhler, I. D. W. Samuel, Y. Olivier and E. Zysman-Colman, Adv. Opt. Mater., 2020, 8, 1901627 CrossRef CAS
.
- C. Adamo and V. Barone, J. Chem. Phys., 1999, 110, 6158–6170 CrossRef CAS
.
- T. H. Dunning, Jr., J. Chem. Phys., 1989, 90, 1007–1023 CrossRef
.
- I. Kim, K. H. Cho, S. O. Jeon, W. J. Son, D. Kim, Y. M. Rhee, I. Jang, H. Choi and D. S. Kim, JACS Au, 2021, 1, 987–997 CrossRef CAS PubMed
.
- J. M. Kaminski, A. Rodriguez-Serrano, F. Dinkelbach, H. Miranda-Salinas, A. P. Monkman and C. M. Marian, Chem. Sci., 2022, 13, 7057–7066 RSC
.
- A. Pershin, D. Hall, V. Lemaur, J. C. Sancho-Garcia, L. Muccioli, E. Zysman-Colman, D. Beljonne and Y. Olivier, Nat. Commun., 2019, 10, 597 CrossRef CAS PubMed
.
- D. Hall, J. C. Sancho-García, A. Pershin, G. Ricci, D. Beljonne, E. Zysman-Colman and Y. Olivier, J. Chem. Theory Comput., 2022, 18, 4903–4918 CrossRef CAS PubMed
.
- S. Wu, W. Li, K. Yoshida, D. Hall, S. Madayanad Suresh, T. Sayner, J. Gong, D. Beljonne, Y. Olivier, I. D. W. Samuel and E. Zysman-Colman, ACS Appl. Mater. Interfaces, 2022, 14, 22341–22352 CrossRef CAS PubMed
.
- D. Sun, S. M. Suresh, D. Hall, M. Zhang, C. Si, D. B. Cordes, A. M. Z. Slawin, Y. Olivier, X. Zhang and E. Zysman-Colman, Mater. Chem. Front., 2020, 4, 2018–2022 RSC
.
- Y. Zhang, D. Zhang, J. Wei, X. Hong, Y. Lu, D. Hu, G. Li, Z. Liu, Y. Chen and L. Duan, Angew. Chem., Int. Ed., 2020, 59, 17499–17503 CrossRef CAS PubMed
.
- Y. Tsuchiya, S. Diesing, F. Bencheikh, Y. Wada, P. L. Dos Santos, H. Kaji, E. Zysman-Colman, I. D. W. Samuel and C. Adachi, J. Phys. Chem. A, 2021, 125, 8074–8089 CrossRef CAS PubMed
.
- K. Stavrou, L. G. Franca and A. P. Monkman, ACS Appl. Electron. Mater., 2020, 2, 2868–2881 CrossRef CAS PubMed
.
- D. K. A. Phan Huu, S. Saseendran, R. Dhali, L. G. Franca, K. Stavrou, A. Monkman and A. Painelli, J. Am. Chem. Soc., 2022, 144, 15211–15222 CrossRef CAS PubMed
.
- H. Miranda-Salinas, Y.-T. Hung, Y.-S. Chen, D. Luo, H.-C. Kao, C.-H. Chang, K.-T. Wong and A. Monkman, J. Mater. Chem. C, 2021, 9, 8819–8833 RSC
.
- P. L. dos Santos, J. S. Ward, M. R. Bryce and A. P. Monkman, J. Phys. Chem. Lett., 2016, 7, 3341–3346 CrossRef CAS PubMed
.
- G. Méhes, K. Goushi, W. J. Potscavage Jr. and C. Adachi, Org. Electron., 2014, 15, 2027–2037 CrossRef
.
- K. Stavrou, S. Madayanad Suresh, D. Hall, A. Danos, N. A. Kukhta, A. M. Z. Slawin, S. Warriner, D. Beljonne, Y. Olivier, A. Monkman and E. Zysman-Colman, Adv. Opt. Mater., 2022, 10, 2200688 CrossRef CAS
.
- M. K. Etherington, N. A. Kukhta, H. F. Higginbotham, A. Danos, A. N. Bismillah, D. R. Graves, P. R. McGonigal, N. Haase, A. Morherr, A. S. Batsanov, C. Pflumm, V. Bhalla, M. R. Bryce and A. P. Monkman, J. Phys. Chem. C, 2019, 123, 11109–11117 CrossRef CAS PubMed
.
- X. Wu, B.-K. Su, D.-G. Chen, D. Liu, C.-C. Wu, Z.-X. Huang, T.-C. Lin, C.-H. Wu, M. Zhu, E. Y. Li, W.-Y. Hung, W. Zhu and P.-T. Chou, Nat. Photonics, 2021, 15, 780–786 CrossRef CAS
.
- L. Zhang, Y.-X. Zhang, Y. Hu, X.-B. Shi, Z.-Q. Jiang, Z.-K. Wang and L.-S. Liao, ACS Appl. Mater. Interfaces, 2016, 8, 16186–16191 CrossRef CAS PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available: NMR, HRMS and HPLC of dBr-tBu-DiKTa. Cyclic voltammetry. Supplementary computational data for dBr-tBu-DiKTa, tBr-DiKTa and Mes3-DiKTa. Extended HAE TADF emitter literature study. Additional photophysical and OLED data. See DOI: https://doi.org/10.1039/d3tc04394k |
| ‡ The research data supporting this publication can be accessed at https://doi.org/10.17630/9bf6dac8-33d7-409e-b0c6-8ee2e5be708e. |
| § These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |




