Imaging of ONOO− fluctuations during drug-induced liver/kidney injury in vitro and in vivo via a dicyanoisophorone-based NIR fluorescent probe with a large Stokes shift†
Fei
Kong‡
b,
Hengqing
Liu
c,
Jie
Huang
d and
Jingcan
Qin
 *a
*a
aDepartment of Radiology, Changhai Hospital, Naval Medical University, Changhai Road 168, Shanghai 200433, China. E-mail: qinjingcan1988@163.com
bSchool of Chemistry and Chemical Engineering, Shanghai Jiao Tong University, Dongchuan Road 800, Shanghai 200240, China
cSchool of Life Science, Fudan University, Songhu Road 2005, Shanghai 200438, China
dSchool of Biomedical Engineering, Guangzhou Medical University, Guangzhou 511436, China
First published on 28th August 2024
Abstract
Current clinical indicators for assessing liver/kidney injury are functional rather than injury indicators, which may cause some delays in the diagnosis of drug-induced liver injury (DILI) and kidney injury (DIKI). Therefore, the development of noninvasive and real-time methods for the effective diagnosis of DILI/DIKI is of great benefit to their clinical management. Herein, we constructed a dicyanoisophorone-based near-infrared (NIR) fluorescent probe (PNDP). Upon the addition of ONOO−, the probe exhibits 111.4-fold fluorescence enhancement at 665 nm with a large Stokes shift of 175 nm as well as excellent selectivity, strong anti-interference capability, and a low limit of detection (118.9 nmol L−1). More significantly, the PNDP was successfully employed for the sensitive detection of ONOO− in living cells and DILI/DIKI mice models. In vitro and in vivo bioimaging experiments demonstrated that the PNDP has greater versatility and promising potential for use as a diagnostic agent for the diagnosis of drug-induced hepatotoxicity and nephrotoxicity by monitoring ONOO− fluctuations.
1. Introduction
Drug-induced liver injury (DILI) and kidney injury (DIKI) are among the most common clinical issues that can give rise to aberrant liver/kidney function, posing a substantial threat to human health.1,2 Currently, the clinical diagnosis of DILI and DIKI mainly relies on monitoring the changes in physiological and biochemical markers in the blood, such as alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum creatinine and blood urea nitrogen (BUN). However, these markers primarily reflect liver and kidney function rather than injuries, which potentially lead to a certain diagnostic lag in the case of DILI and DIKI. Once diagnostic delay occurs in clinical settings, its potential consequences can be grave, exacerbating a patient's condition and, worse, leading to death.3–5 Therefore, early and accurate diagnosis holds great benefit to the effective treatment of DILI and DIKI. Therefore, the development of noninvasive and real-time methods for the effective diagnosis of DILI and DIKI is essential.Peroxynitrite (ONOO−) is a crucial reactive nitrogen species (RNS) that arises from the reaction between nitric oxide (NO) and superoxide radical anions (O2˙−) within biological environments. ONOO− exhibits strong reactivity towards diverse biomolecules, including proteins, nucleic acids, and lipids, thereby inducing cellular structural and functional damage.6–8 Currently, an increasing number of studies have demonstrated the up-regulation of ONOO− in the liver and kidneys during DILI and DIKI.9,10 Consequently, ONOO− is considered a potential biomarker for the early detection of DILI and DIKI. However, the elusive property of ONOO− under physiological conditions, including its ultra-short half-life (∼20 ms) and high chemical reactivity, presents significant difficulties in its direct monitoring in situ.11–13
Recently, fluorescence probes have emerged as a promising method of increasing interest for tracking ONOO− owing to their simplicity, high sensitivity, exceptional spatial and temporal resolution, non-invasiveness, and real-time visualization in situ.5,12,14–17 A number of fluorescent probes have been developed for ONOO− detection in biological systems.18–23 However, the majority of these probes suffer from the drawbacks of a small Stokes shift that causes potential crosstalk between the excitation and emission spectra and short emission wavelengths that are susceptible to interference in living systems. These will result in a reduction of fluorescence contrast, which compromises the sensitivity and accuracy of in vivo imaging and sensing.24–26 Therefore, it is of crucial importance to develop novel fluorescent probes located in the near-infrared (NIR) region with a large Stokes shift for ONOO− monitoring.
Noting these abovementioned ideas, in this work, we designed and synthesized a dicyanoisophorone-based NIR fluorescent probe (peroxynitrite detection probe, PNDP) for ONOO− detection (Scheme 1A). PNDP itself showed no noticeable fluorescence emission while in the presence of ONOO−, while it exhibited 111.4-fold fluorescence enhancement at 665 nm with a large Stokes shift of 175 nm, as well as excellent selectivity and strong anti-interference capability. Moreover, PNDP possesses a low limit of detection (LOD, 118.9 nmol L−1) and minimal cytotoxicity, and has been successfully employed for real-time in situ monitoring of ONOO− fluctuations in living cells and DILI/DIKI mouse models (Scheme 1B). The in vitro and in vivo bioimaging experiments confirmed that PNDP exhibits promising potential for use as a tool to determine the occurrence of DILI and DIKI, and assess the extent of liver and kidney injuries.
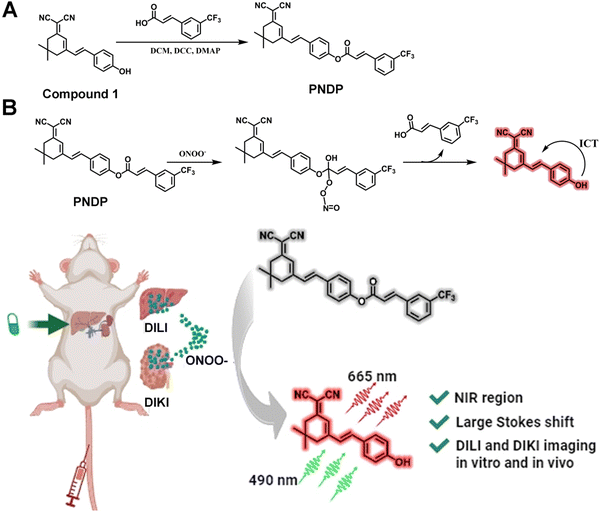 | ||
| Scheme 1 (A) The synthetic of the PNDP. (B) The sensing mechanism of the PNDP toward ONOO− for in vitro and in vivo imaging of DILI and DIKI. | ||
2. Experimental section
2.1. Reagents and methods
Detailed information on the reagents and methods is provided in the ESI.† The synthetic pathway of PNDP is illustrated in Scheme 1A.2.2. Synthesis of compound 127,28
P-Hydroxybenzaldehyde (1.22 g, 10 mmol) and piperidine (0.5 mL) were added to an ethanol solution (25 mL) of dicyanoisophorone (1.86 g, 10 mmol) (Fig. S1, ESI†). Subsequently, the mixture solution was stirred at 80 °C overnight. After the reaction was completed, the solvent was removed under reduced pressure and the residuals were dissolved with CH2Cl2, washed with water (twice), and dried over Na2SO4. Finally, the organic phase was evaporated again to a crude product, which was further purified by silica column chromatography (elution: dichloromethane). Yield: 1.96 g (67.5%) (Fig. S2, ESI†). 1H NMR (500 MHz, CDCl3) δ(ppm): 7.46 (d, J = 8.4 Hz, 2H), 7.02 (m, 1H), 6.90–6.85 (m, 3H), 6.81 (s,1H), 2.61 (s, 2H), 2.48 (s, 2H), 1.07 (s, 6H).2.3. Synthesis of PNDP
Compound 1 (145.5 mg, 0.5 mmol) was added into anhydrous CH2Cl2 (20 mL), followed with the addition of EDC (115 mg, 0.6 mmol), 4-dimethylaminopyridine (DMAP, 6.11 mg, 0.05 mmol) and 3-(trifluoromethyl) cinnamic acid (3-TCA) (108.2 mg, 0.5 mmol). The reaction mixture was stirred at room temperature for 2 h, and the solvent was then removed under reduced pressure. The product was purified by silica gel column chromatography (CH2Cl2/CH3OH = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) to obtain PNDP. Yield: 185.6 mg, (76%) (Fig. S3, ESI†). 1H NMR (500 MHz, DMSO-d6) δ(ppm): 8.23 (s, 1H), 8.16 (d, J = 7.85 Hz, 1H), 8.00 (d, J = 16.05 Hz, 1H), 7.83 (t, J = 5.0, 15.0 Hz, 1H),7.80 (d, J = 8.75 Hz, 2H), 7.70 (t, J = 7.60, 15.5 Hz, 1H), 7.31–7.34 (dd, J =16.25, 39.5 Hz, 2H), 7.30 (d, J = 10.1 Hz, 1H), 7.10 (d, J = 16.2 Hz, 1H), 6.90 (s, 1H), 2.63 (s, 2H), 2.57 (s, 2H), 1.45–1.90 (m, 2H), 1.12–1.36 (m, 2H), 1.04 (s, 6H). 13C NMR (125 MHz, DMSO-d6) (Fig. S4, ESI†). δ(ppm): 170.82, 164.99, 156.17, 151.68, 145.36, 136.94, 135.48, 134.30, 132.70, 130.53, 130.47, 130.22, 129.49, 127.57, 125.96, 125.93, 125.51, 123.41, 123.34, 122.81, 119.76, 114.30, 113.48, 77.01, 42.77, 40.49, 40.33, 40.16, 39.99, 39.83, 39.66, 39.49, 38.64, 32.36, 32.16, 30.85, 27.92, 25.91, 24.82. MS: calcd 488.17, found 487.26 ([M − H]−) (Fig. S5, ESI†).
1, v/v) to obtain PNDP. Yield: 185.6 mg, (76%) (Fig. S3, ESI†). 1H NMR (500 MHz, DMSO-d6) δ(ppm): 8.23 (s, 1H), 8.16 (d, J = 7.85 Hz, 1H), 8.00 (d, J = 16.05 Hz, 1H), 7.83 (t, J = 5.0, 15.0 Hz, 1H),7.80 (d, J = 8.75 Hz, 2H), 7.70 (t, J = 7.60, 15.5 Hz, 1H), 7.31–7.34 (dd, J =16.25, 39.5 Hz, 2H), 7.30 (d, J = 10.1 Hz, 1H), 7.10 (d, J = 16.2 Hz, 1H), 6.90 (s, 1H), 2.63 (s, 2H), 2.57 (s, 2H), 1.45–1.90 (m, 2H), 1.12–1.36 (m, 2H), 1.04 (s, 6H). 13C NMR (125 MHz, DMSO-d6) (Fig. S4, ESI†). δ(ppm): 170.82, 164.99, 156.17, 151.68, 145.36, 136.94, 135.48, 134.30, 132.70, 130.53, 130.47, 130.22, 129.49, 127.57, 125.96, 125.93, 125.51, 123.41, 123.34, 122.81, 119.76, 114.30, 113.48, 77.01, 42.77, 40.49, 40.33, 40.16, 39.99, 39.83, 39.66, 39.49, 38.64, 32.36, 32.16, 30.85, 27.92, 25.91, 24.82. MS: calcd 488.17, found 487.26 ([M − H]−) (Fig. S5, ESI†).
3. Results and discussion
3.1. Design and synthesis of PNDP
Fluorescent probes for ONOO− bioimaging in sophisticated biological systems have recently attracted considerable attention (Table S1, ESI†). However, although they showed higher LODs towards ONOO−, most of the reported probes suffer from short emission wavelengths (less than 650 nm) or a short Stokes shift, which may not be favorable for in vivo imaging applications. Therefore, the development of a NIR probe with a larger emission wavelength and higher sensitivity is of great significance for ONOO− detection under different conditions. In this work, we designed and synthesized a new ONOO−-responsive NIR fluorescent probe comprising a dicyanoisophorone derivative as the fluorophore and 3-TCA as the recognition group (Scheme 1A). Dicyanoisophorone-based fluorophores possess many attractive properties, such as a good fluorescence quantum yield, large Stokes shift, as well as long emission wavelengths in the NIR region.29 The free PNDP without ONOO− showed no obvious fluorescence emission (Φ < 0.01). Upon the addition of ONOO−, it exhibited a remarkable fluorescence enhancement at 665 nm with a large Stokes shift of 175 nm (Φ = 0.0126).30,31 In addition, the chemical structure of PNDP was fully confirmed by spectroscopic data, including 1H NMR, 13C NMR and MS (Fig. S1–S5, ESI†).3.2. Spectral response of PNDP to ONOO−
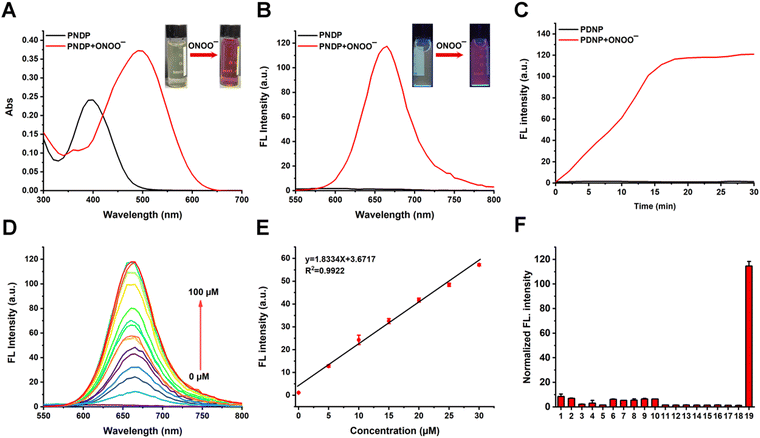
The optical properties of PNDP (20 μM) were investigated in a PBS-DMSO solution mixture (pH = 7.4, 10 mM, v/v, 10% DMSO) both in the absence and presence of ONOO− (100 μM). As shown in Fig. 1A, PNDP displayed a maximal absorbance at 400 nm in the absence of ONOO−. However, upon the addition of ONOO−, the peak absorbance of PNDP at 400 nm vanished, while a distinct absorption band emerged at 490 nm. Furthermore, the colors of the PNDP solution were changed to red from light yellow, which was observable by the naked eye. These phenomena suggested that the addition of ONOO− could trigger a structural change in PNDP, which was further confirmed by LC-MS (Fig. S6, ESI†). As shown in Fig. 1B, the free PNDP did not display obvious fluorescence under 490 nm excitation. However, once exposed to ONOO−, the fluorescence emission of PNDP at 665 nm exhibited a 111.4-fold enhancement with a Stokes shift of 175 nm. This indicated that PNDP can be used as an efficient fluorescent probe for ONOO− detection.Subsequently, the time-dependent fluorescence spectra of PNDP (20 μM) induced by ONOO− (100 μM) were measured to evaluate the response rate of PNDP toward ONOO−. As shown in Fig. 1C, with the passage of time, the fluorescence intensity of PNDP rapidly increased and reached saturation after 20 min, demonstrating the feasibility of rapid detection for ONOO−. In addition, titration experiments were conducted to investigate the relationship between the fluorescence intensity of PNDP (20 μM) and the concentration range of ONOO− (0–100 μM), as shown in Fig. 1D. The results revealed a gradual increase in fluorescence intensity concomitant with increasing ONOO− concentration, establishing a linear equation y = 1.8334x + 3.6717 (R2 = 0.9922). Given this, the LOD for ONOO− (Fig. 1E) was calculated as 118.9 nM, according to the equation: 3σ/s (σ, standard deviation; s, the slope of the linear equation).32,33 Such a low LOD of PNDP toward ONOO− is beneficial for tracking ONOO− fluctuations both in vitro and in vivo due to the low concentration in biological systems.
Selectivity and anti-interference ability are two crucial parameters for assessing the quality of a fluorescence probe. To evaluate the selectivity of PNDP (20 μM) towards ONOO− (100 μM) and its resistance to interference from other ions, a series of related substances (including H2O2, ClO−, ˙OH, 1O2, O2˙−, NO2−, H2S, GSH, Cys, Hcy, NO2−, CO32−, HCO3−, SO42−, Na+, Mg2+, Cu2+, and K+) were co-incubated with PNDP to monitor the fluorescence behavior. As shown in Fig. 1F, the enhancement fluorescence signal of PNDP was only generated in the presence of ONOO−, while no significant fluorescence change was observed with other analytes. Furthermore, considering the physiological pH value of approximately 7.4, we investigated the impact of pH on the fluorescence response of PNDP towards ONOO−. As shown in Fig. S6 (ESI†), PNDP still exhibited remarkable fluorescence emission in the presence of ONOO− at physiological pH (6.5–8.5). These results indicated that PNDP had excellent selectivity toward ONOO−, which holds great prospects for ONOO− detection in a physiological environment.
3.3. The sensing mechanism of PNDP toward ONOO−
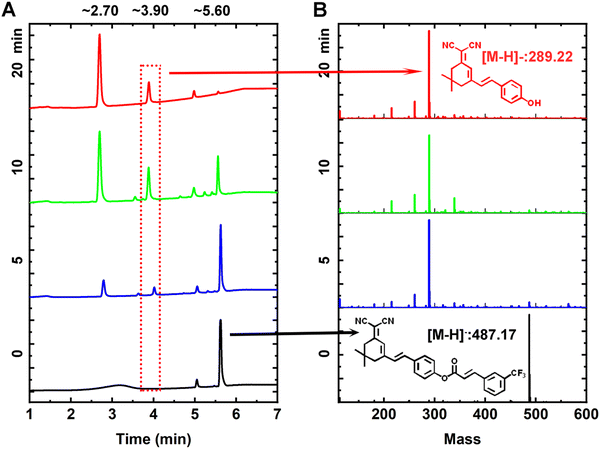
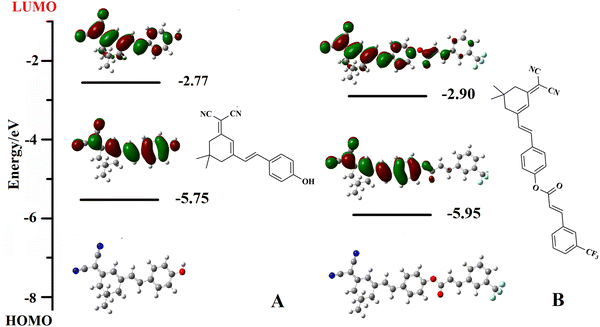
The sensing mechanism of PNDP toward ONOO− is shown in Scheme 1. Initially, PNDP exhibited no obvious fluorescence emission at 665 nm, owing to the effect of the 3-TCA blocking ICT process. The addition of ONOO− triggered the removal of 3-TCA from PNDP and the subsequent exposure of a hydroxyl group, which facilitated the ICT process. Consequently, a significant fluorescence enhancement was observed at 665 nm.34–38 Moreover, the dynamic reaction process of PNDP with ONOO− was characterized by LC-MS and further confirmed the hypothesis. As shown in Fig. 2, the UV absorption band of PNDP exhibited a gradual decrease, while the UV absorption band of Compound 1 demonstrated a significant increase with increasing incubation time. More importantly, the fragment peaks of Compound 1 ([M − H]−, 289.22), 3-TCA ([M − H]−, 215.13) and the intermediate product formed by ONOO− ([M − H]−, 262.80) could be detected in the mass spectrum (ESI−) (Fig. S7, ESI†). These demonstrated that Compound 1 and 3-TCA were produced in the reaction process of PNDP with ONOO−. In other words, the addition of ONOO− could trigger the cleavage of 3-TCA from PNDP, and then release the hydroxyl group of PNDP (Scheme 1B).To further explain the sensing mechanism of PNDP toward ONOO−, the energy changes of PNDP before and after reaction with ONOO− were simulated using density functional theory calculation (DFT). As shown in Fig. 3B, the HOMO of PNDP covered the entire dicyanoisophorone-based fluorophore, while the introduction of the 3-TCA group strengthened the distribution of LUMO on the 3-TCA group (acceptor), which attenuated the ICT process. Upon the addition of ONOO−, 3-TCA was removed from PNDP, thereby releasing compound 1. The HOMO and LUMO of compound 1 covered the entire molecular skeleton. However, its HOMO tended to be distributed on phenol (donor), and its LUMO tended to localize on the dicyanoisophorone moiety (acceptor), which facilitated the ICT process. In addition, the bandgaps (HOMO–LUMO) of PNDP and compound 1 were 3.05 eV and 2.98 eV, respectively. This indicated that the electron transitions of Compound 1 occurred more easily than that of PNDP, and matched the red-shifted absorption in the UV-vis spectra.39–42
3.4. Fluorescence imaging of ONOO− in living cells
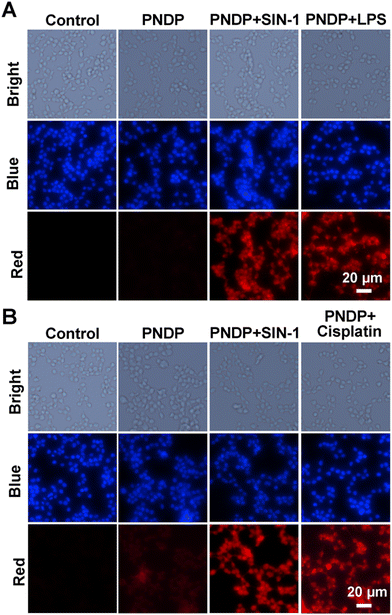
To further investigate the potential application of PNDP for the detection of ONOO− in living cells, the fluorescence imaging of PNDP for ONOO− was performed in both HepG2 cells and HK-2 cells. Prior to the bioimaging, the cytotoxicity of PNDP was tested using HepG2 cells and HK-2 cells by standard CCK-8 assays. As shown in Fig. S8a and b (ESI†), after treatment with various concentrations of PNDP (5–50 μM) for 24 h, more than 85% viability of HepG2 and HK-2 cells was retained, indicating the low cytotoxicity of PNDP. Then, PNDP was utilized to image intracellular ONOO−. As shown in Fig. 4, there was little apparent fluorescence observed in HepG2/HK-2 cells incubated only with PNDP. In contrast, bright red fluorescent signals were detectable when HepG2/HK-2 cells were sequentially pretreated with SIN-1 (a ONOO− donor, 100 μM, 1 h) and PNDP (20 μM). Considering that some hepato- and nephrotoxic drugs induce an increase in intracellular ONOO−, herein, LPS (1 μg mL−1, 10 h) and cisplatin (30 μM, 24 h) were employed as agents to stimulate the upregulation of endogenous ONOO− levels in HepG2 and HK-2 cells. As shown in Fig. 4, co-incubation of the cells pretreated with LPS (or cisplatin) with PNDP also resulted in strong emitted fluorescence signals. These findings indicated that PNDP could serve as an effective tool for the real-time monitoring of exogenous and endogenous ONOO− in living cells, laying a foundation for the utilization of PNDP to diagnose DILI and DIKI in vivo.3.5. In vivo imaging of ONOO− in DILI and DIKI mice models
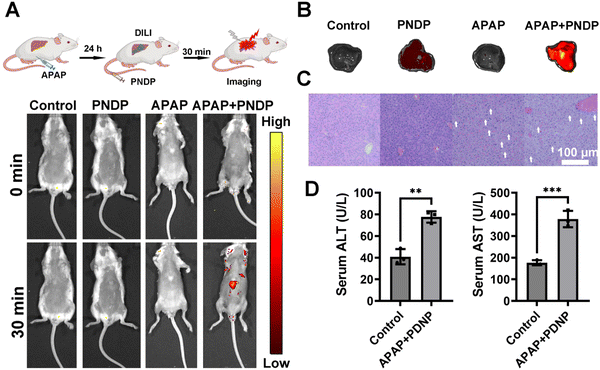
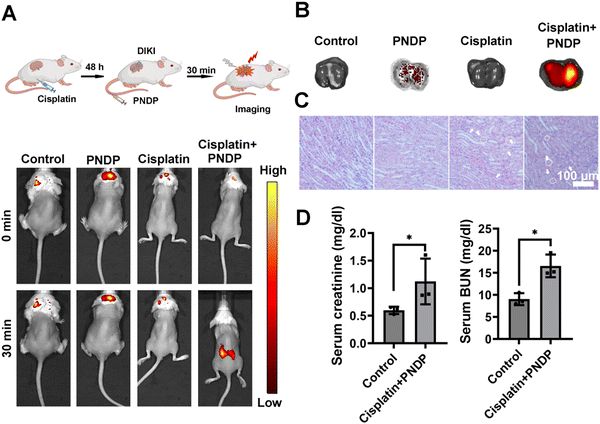
Encouraged by the above results, we anticipated that the PNDP could in vivo respond to ONOO− in DILI and DIKI mice. Firstly, hemolytic tests conducted using mouse red blood cells demonstrated a significantly low hemolysis rate of PNDP at different concentrations (Fig. S9 and S10, ESI†), thereby indicating the suitability of PNDP for intravenous administration. Subsequently, PNDP was applied to monitor the upregulation of ONOO− in the APAP-induced DILI mouse model. As shown in Fig. 5A, no significant fluorescence signal was observed in both DILI model mice and control mice injected with PBS, and no fluorescent signal was detected in the control mice injected with PNDP. However, bright fluorescent signals were observed in the DILI model mice injected with PNDP. The livers were then dissected and imaged. As shown in Fig. 5B, obvious fluorescent signals were observed in the livers of the APAP-induced DILI model mice injected with PNDP, and almost no fluorescent signals were generated in the livers of unprobed mice. These might be attributed to the up-regulation of ONOO− in mice induced by the excess of APAP. Meanwhile, histopathological analyses of the livers of mice in each group were performed. As shown in Fig. 5C, in normal liver tissues, the hepatocytes were neatly arranged in a radial pattern. Furthermore, the structure of the hepatic lobules and hepatic sinusoids were clear without obvious pathological damage. However, in APAP-induced damaged liver tissues, hepatocyte cytoplasmic looseness and vacuoles, as well as hepatocyte necrosis (white arrows) were observed. Meanwhile, the serum ALT and AST levels of the mice showed a significant increase in the APAP-treated group of mice (Fig. 5D). All the data of the physiologic and biochemical markers further confirmed the accuracy of PNDP diagnosis of DILI.Furthermore, we investigated the in vivo imaging of cisplatin-induced DIKI in mice models using PNDP. Mice were first induced with cisplatin (20 mg kg−1, 48 h), then PNDP was injected i.v., and fluorescence imaging was performed 30 min later. As shown in Fig. 6A, the control and DIKI model group mice injected with PBS and the control mice injected with PNDP had almost no fluorescence in the renal region, whereas the DIKI model mice emitted strong red fluorescence after PNDP treatment. Fluorescence imaging of isolated kidneys further showed that the kidneys of the DIKI model mice injected with PNDP exhibited significant fluorescence (Fig. 6B). Simultaneously, H&E staining analysis of renal sections showed that the cisplatin-treated kidneys exhibited significant inflammation and injury with tubular cell necrosis (white arrows), tubular injury (cytoplasmic vacuoles, circles), and elevated serum Creatinine and BUN contents, confirming that the cisplatin-induced AKI model was successfully established. Taken together, these results confirmed that PNDP could be used for the in vivo imaging of DILI and DIKI by monitoring ONOO−.
4. Conclusion
In summary, we constructed a novel dicyanoisophorone-based NIR fluorescent probe named PNDP for specific response toward ONOO−. The probe exhibited excellent performance, including a large Stokes shift (175 nm), outstanding fluorescence response (111.4-fold), excellent selectivity, strong anti-interference capability, and low LOD (118.9 nmol L−1). Furthermore, PNDP has been successfully utilized in both in vitro and in vivo imaging of the drug-induced, up-regulated changes of ONOO− in living cell lines and DILI/DIKI mice. All these results suggest that PNDP has broader versatility, and can be a promising tool for diagnosing DILI/DIKI by monitoring ONOO− fluctuations. We anticipate that PNDP can find potential applications for the early diagnosis of drug-induced ALI and AKI, and the screening of effective therapeutic candidates.Data availability
All relevant data are present within the manuscript and its additional files.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was supported by The Foundation for Basic Medical Research Project of Naval Medical University (2022MS023) and China Postdoctoral Science Foundation (2020M671151).References
- R. Allison, A. Guraka, I. T. Shawa, G. Tripathi, W. Moritz and A. Kermanizadeh, Drug, J. Toxicol. Environ. Health B Crit. Rev., 2023, 26, 442–467 CrossRef CAS PubMed.
- J. A. Kellum and J. R. Prowle, Nat. Rev. Microbiol., 2018, 14, 217–230 Search PubMed.
- W. B. Qu, C. H. Niu, X. Y. Zhang, W. Chen, F. B. Yu, H. Liu, X. H. Zhang and S. F. Wang, Talanta, 2019, 197, 431–435 CrossRef CAS PubMed.
- G. F. Sueta, N. Campolo, M. Trujillo, S. Bartesaghi, S. Carballal, N. Romero, B. Alvarez and R. Radi, Chem. Rev., 2018, 118, 1338–1408 CrossRef.
- R. Radi, Peroxynitrite, J. Biol. Chem., 2013, 288, 26464–26472 CrossRef CAS.
- N. Wang, X. Lu, J. Wang, H. Wang, B. Zhang, W. Zhao and J. Zhang, Anal. Chem., 2023, 95, 5967–5975 CrossRef CAS.
- Y. J. Geng, Z. Wang, J. Y. Zhou, M. G. Zhu, J. Liu and T. D. James, Chem. Soc. Rev., 2023, 52, 3873–3926 RSC.
- X. Wang, X. Wang, Z. Bai, K. Du, J. Zhang and Q. Han, Talanta, 2024, 270, 125581 CrossRef CAS.
- J. T. Hou, K. K. Yu, K. Sunwoo, W. Y. Kim, S. Koo, J. Wang, W. X. Ren, S. Wang, X. Q. Yu and J. S. Kim, Chem., 2020, 6, 832–866 CAS.
- M. Yan, Y. Huo, S. Yin and H. Hu, Redox Biol., 2018, 17, 274–283 CrossRef CAS.
- G. F. Sueta and R. Radi, ACS Chem. Biol., 2009, 4, 161–177 CrossRef.
- G. Liu, X. Xie, Y. Li, J. Zhang, X. Jiao, X. Dou, X. Wang and B. Tang, Trends Anal. Chem., 2023, 169, 117371 CrossRef CAS.
- A. J. Shuhendler, K. Pu, L. Cui, J. P. Uetrecht and J. Rao, Nat. Biotechnol., 2014, 32, 373–380 CrossRef CAS.
- M. Li, P. Lei, S. Shuang, C. Dong and L. Zhang, Spectrochim. Acta A., 2023, 303, 123179 CrossRef CAS PubMed.
- B. J. Bezner, L. S. Ryan and A. R. Lippert, Anal. Chem., 2019, 92, 309–326 CrossRef.
- J. Chen, D. Huang, M. She, Z. Wang, X. Chen, P. Liu, S. Zhang and J. Li, ACS Sens., 2021, 6, 628–640 CrossRef CAS PubMed.
- W. L. Jiang, Y. Li, W. X. Wang, Y. T. Zhao, J. Fei and C. Y. Li, Chem. Commun., 2019, 55, 14307–14310 RSC.
- N. Wang, H. Wang, J. Zhang, X. Ji, H. Su, J. Liu, J. Wang and W. Zhao, Chin. Chem. Lett., 2022, 33, 1584–1588 CrossRef CAS.
- W. Xu, Q. Yang, J. Zeng, L. Tan, L. Zhou, L. Peng, Y. Zhou, C. Xie, K. Luo and Z. Zhang, Sensor Actuators, B, 2022, 359, 131565 CrossRef CAS.
- C. Wang, W. Shu, Q. Chen, C. Yang, S. Su, M. Gao, R. Zhang, J. Jing and X. Zhang, Spectrochim. Acta, Part A, 2021, 260, 119990 CrossRef CAS PubMed.
- J. Liu, M. Zhao, F. Zhao, X. Song and Y. Ye, Spectrochim. Acta, Part B, 2023, 387, 133131 Search PubMed.
- X. Z. Zhu, Z. Zhang, Q.-Q. Xu, R. Dong, Y. Chen, Y. Y. Wu and C. Y. Wang, Tetrahedron, 2023, 149, 133686 CrossRef CAS.
- K. Dębowska, D. Dębski, B. Michałowski, A. D. Defratyka, M. Jakubowska, A. Selmi, R. Smulik, Ł. Piotrowski, J. Adamus, A. Marcinek, S. Chlopicki and A. Sikora, Chem. Res. Toxicol., 2016, 29, 735–746 Search PubMed.
- X. Zeng, X. Chen, J. Chen, M. Ma, H. Jin, S. Yu and Z. Liu, Dyes Pigments., 2023, 210, 110993 CrossRef CAS.
- F. Wang, B. Guo, X. Guo, B. Gu, Y. Shen and J. Long, J. Photoch. Photobio. A, 2022, 427, 113796 CrossRef CAS.
- Y. Deng, X. Shi, X. Hu, L. Xu, X. Liu, G. Gao, R. Wang and G. Liang, Anal. Chem., 2023, 95, 6496–6500 CrossRef CAS PubMed.
- J. C. Qin, Z. W. Li, Z. H. Fu and Z. H. Zhang, Spectrochim. Acta, Part B, 2022, 357, 131430 CAS.
- L. D. Amico, G. Rassu, V. Zambrano, A. Sartori, C. Curti, L. Battistini, G. Pelosi, G. Casiraghi and F. Zanardi, J. Am. Chem. Soc., 2014, 36, 11107–11114 Search PubMed.
- L. X. Dai, Q. Zhang, Q. Q. Ma and W. Y. Lin, Coord. Chem. Rev., 2023, 489, 215193 CrossRef CAS.
- J. X. Hong, Q. F. Xia, W. Y. Feng and G. Q. Feng, Dyes Pigments, 2018, 159, 604–609 CrossRef CAS.
- X. S. He, Q. Yan, W. J. Zhao, D. Yi, Y. Z. Yang, B. He, M. H. Lv, X. Y. Hu, S. C. Liang and X. L. Zhong, Dyes Pigments, 2024, 225, 112078 CrossRef CAS.
- H. Lv, X. F. Yang, Y. Zhong, Y. Guo, Z. Li and H. Li, Anal. Chem., 2014, 86, 1800–1807 CrossRef CAS PubMed.
- J. C. Qin, Z. H. Fu, L. M. Tian and Z. Y. Yang, Spectrochim. Acta, Part A, 2020, 229, 117868 CrossRef CAS.
- J. Cui, S. Zang, H. Nie, T. Shen, S. Su, J. Jing and X. Zhang, Spectrochim. Acta, Part B, 2021, 328, 129069 CAS.
- S. Y. Gong, J. H. Hong, E. B. Zhou and G. Q. Feng, Talanta, 2019, 201, 40–44 CrossRef CAS.
- F. J. Huo, Y. Q. Zhang, P. Ning, X. M. Meng and C. X. Yin, J. Mater. Chem. B, 2017, 5, 2798–2803 RSC.
- X. Y. Liu, X. Q. Li, P. Dong, Z. M. Wu, J. W. Gao and Q. M. Wang, Microchim. Acta, 2020, 187, 313 CrossRef CAS PubMed.
- W. Shu, S. P. Zang, C. Wang, M. X. Gao, J. Jing and X. L. Zhang, Anal. Chem., 2020, 92, 9982–9988 CrossRef CAS PubMed.
- W. Chen, M. Yang, N. Luo, F. L. Wang, R. Q. Yu and J. H. Jiang, Analyst, 2019, 144, 6922–6927 RSC.
- Y. L. Cui, G. Z. Hu, T. Wu, J. L. Yang, Y. M. Nie and Y. M. Zhou, Bioorg. Chem., 2022, 129, 106107 CrossRef CAS PubMed.
- S. Ding, A. X. Xu, A. K. Sun, Y. Xia and Y. J. Liu, ACS Omega, 2020, 5, 19695–19701 CrossRef CAS PubMed.
- S. X. An, Y. F. Lin, T. Q. Ye, T. W. Bai, D. Y. He, L. H. Guo, Z. S. Qian, L. Li, H. Y. Liu and J. B. Wang, Talanta, 2024, 267, 125247 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01446d |
| ‡ Dongchuan Road 880, Shanghai 200240, China. |
| This journal is © The Royal Society of Chemistry 2024 |