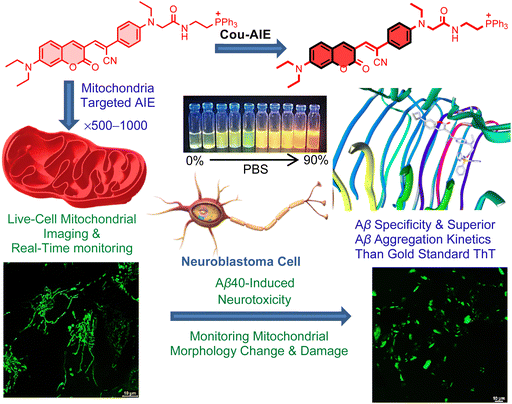
A mitochondria targeting, de novo designed, aggregation-induced emission probe for selective detection of neurotoxic amyloid-β aggregates†
Tapas
Bera
,
Aniruddha
Mondal
,
Samiran
Kar
,
Ayan
Mukherjee
,
Somenath
Banerjee
and
Samit
Guha
 *
*
Department of Chemistry, Organic Chemistry Section, Jadavpur University, Kolkata 700032, India. E-mail: samit.guha@jadavpuruniversity.in; samitfsu@gmail.com
First published on 1st October 2024
Abstract
A striking issue is the scarcity of imaging probes for the early diagnosis of Alzheimer's disease. For the development of Aβ biomarkers, a mitochondria targeting, de novo designed, aggregation-induced emission (AIE) probe Cou-AIE-TPP+ is constructed by engineering the aromatic coumarin framework into the bridge of electron donor–acceptor–donor tethered with a lipophilic cationic triphenylphosphonium (TPP+) group. The synthesized Cou-AIE-TPP+ probe exhibits biocompatibility, noncytotoxicity, and a huge Stokes shift (124 nm in PBS). Cou-AIE-TPP+ has respectable fluorescence augmentation inside the aggregated Aβ40 in comparison with monomeric Aβ40 with a high binding affinity (Kd = 83 nM) to Aβ40 aggregates, is capable of detecting the kinetics of amyloid aggregation, and is superior to the gold standard probe thioflavin T. Fluorescence lifetime and brightness are also augmented when the probe Cou-AIE-TPP+ binds with Aβ aggregates in PBS. Cou-AIE-TPP+ (λem 604 nm) selectively targets and images neuronal cell mitochondria, is useful to monitor mitochondrial morphology alteration and damage during Aβ40-induced neurotoxicity, recognizes neurotoxic Aβ fibrils, and is highly colocalized with thioflavin T, showing a decent Pearson correlation coefficient of 0.91 in the human neuroblastoma SH-SY5Y cell line. These findings indicate that the mitochondria targeting, de novo designed, functional AIE-based solvatofluorochromic Cou-AIE-TPP+ probe is a promising switch on biomarkers for fluorescence imaging of Aβ aggregates and to monitor mitochondrial morphology change and dysfunction during Aβ-induced neurotoxicity, which may offer imperative direction for the advancement of compelling AIE biomarkers for targeted early stage Aβ diagnosis in the future.
Introduction
Alzheimer's disease (AD) is a progressive neurodegenerative disorder, presently one of the top 10 reasons for mortality in the world.1 Almost all brain functions, i.e., memory, verbal, decision, behavioural, activity, cognitive, trouble recognizing friends and family members, and life expectancy, are in due course affected.2,3 The WHO estimates that approximately 10 million individuals globally suffer from AD annually, and that the number will almost double within 2030. Albeit the symptoms of AD are often identified in older age, the neurodegeneration undeniably has a long, silent beginning prior to its clinical diagnosis.4,5 As of right now, there is no effective molecular toolkit for the early diagnosis of AD, which is not curable, or for even preventing the disease from progressing once symptoms start. The aberrant assembly of amyloid-β (Aβ) peptides that promotes Aβ fibrils or plaque deposition is the primary pathological hallmark of AD.6 The Aβ peptides encounter an innate transformation from a soluble monomer, soluble oligomer, or frequently partially folded state to self-assembled insoluble oligomers, protofibrils, and fibrils that are accumulated inside the brain as Aβ plaques.7,8 It is believed that these species cause the neurotoxicity and neurodegeneration connected with the development of AD symptoms.9 Aβ fibrils typically upsurge in the brain as AD progresses.10 There is budding proof showing that elevated Aβ levels cause severe mitochondrial damage and dysfunction, which is one of the most early and notable hallmarks of vulnerable neurons.11–13 This is an appealing hypothesis for the pathogenesis of AD. Moreover, considerable evidence suggests that Aβ progressively accumulates in mitochondria and is responsible for mitochondrial dysfunction and oxidative stress, which are accountable for the progression of AD.14,15CT, PET, SPECT, and MRI imaging techniques are generally utilized to diagnose the advanced stage of AD.16–19 Nevertheless, exposure to high-energy ionizing radiation and radioactive substances, high cost, inaccessibility of real-time detection, lack of target selectivity, and sensitivity of these probes limit their practical application. The burgeoning technology is targeted fluorescence-based detection and imaging of Aβ, which has several advantages such as extraordinary sensitivity at the molecular level, spatiotemporal resolution, low cost, ability to monitor in real time, and no exposure to harmful radiation and radioactive substances, and it has become a smart tool for AD diagnosis.20–22 Thioflavin T (ThT) is a benzothiazole-based fluorescent probe used as a gold standard for Aβ staining.23 Although it is widely used in identifying Aβ fibrils, ThT and its derivatives have certain restrictions, e.g., a minor Stokes shift, high background fluorescence, low penetration depth, enrichment quenching effect on emission after binding with Aβ fibrils, poor sensitivity, incapability to target specific cellular organelles, and unclear cytotoxicity.24 To overcome these pitfalls, several dyes have been reported for the detection of Aβ fibrils.25–27 Nevertheless, the tedious synthesis, inability of mitochondria targeting, and carcinogenicity limit their use as Aβ markers. Furthermore, most of the organic dyes containing aromatic rings exhibit bright fluorescence in dilute organic solutions and often suffer aggregation-caused quenching (ACQ) effects when dispersed into an aqueous system or aggregated inside live cells.28,29 ACQ severely hampers their biological applications and is not suitable for cellular mitochondrial imaging as well as Aβ detection. Tang has made a pioneering contribution to overcome these disadvantages using aggregation-induced emission (AIE), a phenomenon just opposite to that of a traditional ACQ probe.30 AIE probes display no emissions or faint fluorescence in solution due to their excited-state energy dissipation through the intramolecular motions; however, they exhibit boosted emission when aggregated or accumulated inside the target and could overcome the limitations of ACQ probes. There are some reports on AIE probes for the detection and imaging of bioanalytes.31–33 Tetraphenyl ethylene (TPE) is the first AIE probe utilized to detect protein aggregation.34 Next, TPE-TPP is used to analyze early-stage α-syn protein aggregation.35 Tang and associates have reported AIE probes for theranostics of AD.36 Next, they have used an AIE chromophore to detect protein aggregation in living cells.37 Liang and co-workers have reported an AIE luminogen for imaging of AD.38 Hong et al. developed an AIE luminogen to identify human serum albumin and insulin fibrillation.39 Recently, water-soluble AIEgens have been developed by Guo and co-workers to accomplish in vivo mapping of Aβ40 plaques.40 Wang et al. have constructed water-soluble AIE-based wash-free Aβ biomarkers.41 He and Quan have designed an AIE probe for the detection of amyloid fibers and amorphous aggregates.42 Although there has been a paradigm shift in the development of AIE probes for Aβ, none of them have the ability to target the powerhouse of cells, mitochondria, along with neurotoxic Aβ aggregates.43,44 Targeting mitochondria is a difficult task owing to the double membrane system and the extremely negative inner mitochondrial membrane (IMM) potential (ΔΨm −150 to −180 mV).45,46 Mitochondria targeting AIE molecules to monitor mitochondrial morphology change and dysfunction during Aβ-induced neurotoxicity are still in their infancy. There is an unmet need to design superior mitochondria target selective, rapid responsive functional AIE-based fluorescence imaging diagnostic biomarkers for the early detection of Aβ aggregates in AD.
Herein, the de novo designed, mitochondrion targeting and Aβ aggregates detecting functional AIE probe Cou-AIE-TPP+ has six distinctive structural characteristics: (a) an unsymmetrical Cou-AIE-TPP+ probe is engineered by an aromatic coumarin framework into the bridge of an electron donor–acceptor–donor (D–A–D) motif for live cell fluorescence imaging; (b) the intramolecular charge transfer (ICT) among the D and A residues in the D–A–D chromophore is responsible for the solvatofluorochromic effect, with bathochromic shift of the emission upon increase of solvent polarity exhibiting a significant Stokes shift of 124 nm in PBS; (c) the probe exhibits AIE phenomena due to restriction of intramolecular motions such as rotation and vibration upon aggregation and targeted binding; (d) a lipophilic cationic triphenylphosphonium (TPP+) functionality conjugated on the terminal position for targeting living neuronal cell mitochondria by exploiting the highly negative IMM potential;45,46 (e) Cou-AIE-TPP+ (λem = 604 nm) has appropriate lipophilicity, exhibits fluorescence augmentation for aggregated Aβ compared to monomeric Aβ with high specificity and strong binding affinity (Kd = 83 nM), and is superior to the gold standard probe ThT (λem = 480 nm) for detecting the kinetics of amyloid Aβ aggregation; and (f) the AIE probe has light-up characteristics including fluorescence lifetime and brightness augmentation when Cou-AIE-TPP+ binds with Aβ aggregates in PBS (Fig. 1). Selective targeting, 3D cellular imaging, and real-time tracking of human neuroblastoma SH-SY5Y live-cell mitochondria by Cou-AIE-TPP+ are established by confocal laser scanning microscopy (CLSM). After rapid internalization into the neuronal cell membrane, Cou-AIE-TPP+ might aggregate inside the mitochondria with an expectation of 500–1000-fold higher concentrations related to the extracellular matrix (ΔΨplasma![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) membrane = −30 to −60 mV vs. ΔΨm −150 to −180 mV), thus exhibiting the AIE effect inside the mitochondria. Inspection through SH-SY5Y cell staining demonstrates that the synthesized Cou-AIE-TPP+ (λem 604 nm) effectively recognizes neurotoxic exogenous Aβ fibrils and colocalizes with ThT, showing a Pearson correlation coefficient of 0.91. Moreover, Cou-AIE-TPP+ is also able to detect mitochondrial morphology change and dysfunction induced by exogenous Aβ. To the best of our knowledge, this is the first report on a de novo designed, biocompatible, noncytotoxic, mitochondria targeting, functional AIE-based fluorescent probe capable of monitoring amyloid aggregation kinetics, recognizing neurotoxic exogenous Aβ fibrils, and being useful to monitor mitochondrial morphology alteration and dysfunction during Aβ-mediated neurotoxicity. These findings indicate that the mitochondria targeting, de novo designed Cou-AIE-TPP+ probe is a promising biomarker for the fluorescence detection and imaging of Aβ that may offer imperative guidance for the advancement of compelling AIE biomarkers for mitochondria targeted early diagnosis of neurotoxic Aβ in the future.
membrane = −30 to −60 mV vs. ΔΨm −150 to −180 mV), thus exhibiting the AIE effect inside the mitochondria. Inspection through SH-SY5Y cell staining demonstrates that the synthesized Cou-AIE-TPP+ (λem 604 nm) effectively recognizes neurotoxic exogenous Aβ fibrils and colocalizes with ThT, showing a Pearson correlation coefficient of 0.91. Moreover, Cou-AIE-TPP+ is also able to detect mitochondrial morphology change and dysfunction induced by exogenous Aβ. To the best of our knowledge, this is the first report on a de novo designed, biocompatible, noncytotoxic, mitochondria targeting, functional AIE-based fluorescent probe capable of monitoring amyloid aggregation kinetics, recognizing neurotoxic exogenous Aβ fibrils, and being useful to monitor mitochondrial morphology alteration and dysfunction during Aβ-mediated neurotoxicity. These findings indicate that the mitochondria targeting, de novo designed Cou-AIE-TPP+ probe is a promising biomarker for the fluorescence detection and imaging of Aβ that may offer imperative guidance for the advancement of compelling AIE biomarkers for mitochondria targeted early diagnosis of neurotoxic Aβ in the future.
Results and discussion
Molecular design and synthesis
A de novo designed unsymmetrical AIE probe Cou-AIE-TPP+ is constructed by engineering the aromatic 2H-chromen-2-one motif into the bridge of an electron donor (N,N-diethyl amino unit)–acceptor (π-conjugated electron-withdrawing acrylonitrile units)–donor (N,N-dialkylated amino unit) framework tethered with a lipophilic cationic TPP+ group (Fig. 2). 7-(Diethylamino)coumarin (1) is synthesized via a one-pot Knoevenagel reaction between 4-(diethylamino)salicylaldehyde and diethyl malonate (Fig. S1, ESI†). The Vilsmeier–Haack synthesis of 1 produces the intermediate 7-(diethylamino)coumarin-3-carbaldehyde (2) (Fig. S2–S5, ESI†). The condensation of compounds 2 and 5 forms compound 6, which is hydrolyzed to obtain compound 7 (Fig. S10–S17, ESI†). Amidation of compound 7 with (2-aminoethyl)triphenylphosphonium bromide yields the target molecule Cou-AIE-TPP+, which is characterized by 1D (1H, 13C, DEPT-135, 31P) NMR, 2D COSY NMR, and HRMS (Fig. S18–S24, ESI†). Cou-AIE-TPP+ consists of an end-capped lipophilic cationic mitochondria targeting TPP+ functionality and exhibits one 31P NMR peak at 23.9 ppm (Fig. S22, ESI†). The aromatic residues might exhibit π-effects and hydrophobic interactions with suitable groups at the binding site of Aβ aggregates. A control probe, Cou-AIE, lacking the lipophilic cationic TPP+ functionality is constructed and characterized (Fig. 2 and Fig. S6–S9, ESI†). The Aβ40 peptide is synthesized using the microwave-assisted Fmoc-SPPS protocol on Wang resin (Fig. S25, ESI†). The mass of the HPLC-purified Aβ40 peptide is confirmed by MALDI-TOF MS (Fig. S26 and S27, ESI†). | ||
| Fig. 2 Chemical structures of Cou-AIE-TPP+ and the control molecule Cou-AIE lacking the lipophilic cationic TPP+ functionality. | ||
Photophysical properties
We first investigated the photophysical characteristics of Cou-AIE-TPP+; as anticipated, it exhibited excellent optical properties. Cou-AIE-TPP+ displayed unique solvatochromic fluorescence behaviors with an ICT mechanism among the D and A residues in the D–A–D chromophore. The absorption spectra of Cou-AIE-TPP+ display a very small shift amongst solvents with different polarities [(λmax)Toluene = 475 nm and (λmax)PBS = 480 nm] (Fig. S28a, ESI†). In contrast, the fluorescence emission wavelength exhibits a significant bathochromic shift with increasing the dielectric constant (ε) and polarity of the solvent [(λem)Toluene = 517 nm and (λem)PBS = 604 nm] (Fig. 3a and Fig. S28c, Table S1, ESI†).47 The correlation between the λem and the dielectric constant of the solvents is linear (Fig. S28d, ESI†). The maximum Stokes shift is observed at 124 nm in aqueous PBS with a narrow abs/em bandwidth that can evade self-quenching, minimize cross-talk, and significantly enhance the imaging quality (Fig. S28b and Table S1, ESI†). However, this phenomenon is less significant in non-polar solvents, e.g., toluene, CHCl3, etc. The absorbance spectra of Cou-AIE-TPP+ originate from electronic orbital transitions, particularly when π and π* orbital dipole moments align closely, making solvent polarity minimally impactful. In contrast, emission spectra result from charge transitions, and a substantial difference in dipole moments between ground and excited states renders the emission spectra of Cou-AIE-TPP+ sensitive to solvent polarity and hence it exhibits solvatofluorochromism. Moreover, Cou-AIE-TPP+ is well soluble in THF and nonfluorescent but aggregates in H2O or aqueous PBS, and the aggregated form exhibits bright orange emission (Fig. 3b). It clearly shows that Cou-AIE-TPP+ switches the emission from the “OFF” to the “ON” state in a THF/PBS or THF/H2O solvent mixture. We excited the lipophilic cationic Cou-AIE-TPP+ compound at 480 nm in the THF/PBS solvent mixture and observed dual emission bands, one at ca. 600 nm along with a shoulder at 735 nm, which is attributed to aggregates formed. Moreover, the emission intensities at ca. 600 nm and 735 nm progressively increase while the aggregation state increases with the increase in PBS volume percent at a single excitation wavelength (λex = 480 nm) (Fig. 3b and Fig. S28e, ESI†). A 78-fold augmentation from 100% THF to a 10% THF/90% PBS mixture is observed, demonstrating the efficient AIE property of Cou-AIE-TPP+. Cou-AIE-TPP+ is nonfluorescent in a good solvent like THF owing to the energy dispersion in a non-radiative manner instigated by its intramolecular motion, whereas the aggregation might impede the intramolecular motions (rotation and vibration) and result in the energy transition in the radiative pathway, responsible for the AIE effect. In contrast, the control probe Cou-AIE lacking the lipophilic cationic TPP+ functionality exhibits a 56-fold rise in fluorescence from 100% THF to a 10% THF/90% PBS mixture, demonstrating a mild AIE impact in comparison with Cou-AIE-TPP+ (Fig. S29, ESI†). The lipophilic cationic (2-aminoethyl)triphenylphosphonium residue of Cou-AIE-TPP+ has a substantial impact on aggregation and the AIE effect in the THF/PBS mixture. There are some reports in the literature that the AIE molecules, by having their dark state mechanism, can cause their dark state mechanism to be inhibited upon aggregation by restricting intramolecular motion (RIM), leading to the phenomenon of fluorescence emission.48,49Secondary structure determination and morphology of Aβ40
The secondary structural information and the monomer, oligomer, protofibril, and fibril formation of Aβ40 are systematically characterized using FT-IR, CD, X-ray fiber diffraction (XRD), and TEM experiments. Initially, the FT-IR spectrum of Aβ40 exhibits a peak at 1650 cm−1 (amide I region) that signifies a random coil structure. The FT-IR spectrum of Aβ40 fibril displays peaks at 3290 cm−1 (N–H stretching), 1624 cm−1 (amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching), and 1513 cm−1 (N–H bending), signifying the construction of an intermolecular H-bonded β-sheet secondary structure (Fig. S30a and b, ESI†). Initially, the CD spectrum of Aβ40 monomers (10 μM) in DI H2O with 1% TFE shows a negative peak at 198 nm, indicative of a dominating random coil structure (Fig. S30c, ESI†). However, after 5 days, secondary structural transformation from a random coil to a β-sheet is established by CD. A shorter wavelength positive band at 195 nm and a longer wavelength negative peak at 217 nm, consistent with that of Aβ40 fibrils, are observed (Fig. S30c, ESI†). The PXRD pattern of the Aβ40 fibril displays peaks at 4.5 Å (meridional reflection, distance between adjacent β-strands within the β-sheets) and 10.6 Å (equatorial reflection, distances of β-sheets) characteristic of a cross-β-diffraction pattern (Fig. S30d, ESI†). The Aβ40 monomers, oligomers, protofibrils, and mature fibrils acquired at 0 h, 12 h, 24 h, and 5 days, respectively, are imaged using transmission electron microscopy (TEM) to verify their morphology and size (Fig. S31a–d, ESI†). The selected area electron diffraction pattern confirms the cross-β structure (Fig. S31e, ESI†).
O stretching), and 1513 cm−1 (N–H bending), signifying the construction of an intermolecular H-bonded β-sheet secondary structure (Fig. S30a and b, ESI†). Initially, the CD spectrum of Aβ40 monomers (10 μM) in DI H2O with 1% TFE shows a negative peak at 198 nm, indicative of a dominating random coil structure (Fig. S30c, ESI†). However, after 5 days, secondary structural transformation from a random coil to a β-sheet is established by CD. A shorter wavelength positive band at 195 nm and a longer wavelength negative peak at 217 nm, consistent with that of Aβ40 fibrils, are observed (Fig. S30c, ESI†). The PXRD pattern of the Aβ40 fibril displays peaks at 4.5 Å (meridional reflection, distance between adjacent β-strands within the β-sheets) and 10.6 Å (equatorial reflection, distances of β-sheets) characteristic of a cross-β-diffraction pattern (Fig. S30d, ESI†). The Aβ40 monomers, oligomers, protofibrils, and mature fibrils acquired at 0 h, 12 h, 24 h, and 5 days, respectively, are imaged using transmission electron microscopy (TEM) to verify their morphology and size (Fig. S31a–d, ESI†). The selected area electron diffraction pattern confirms the cross-β structure (Fig. S31e, ESI†).
Monitoring of Aβ protein misfolding and aggregation kinetics using a targeted AIE probe Cou-AIE-TPP+
There is an unmet need to develop extrinsic, targeted reporter probes to observe the amyloid aggregation process. We use an ex situ Cou-AIE-TPP+ probe to provide comprehensive information on the Aβ40 protein misfolding and aggregation process as an alternative to ThT, the gold standard for monitoring Aβ fibril formation. The solvatofluorochromic AIE probe Cou-AIE-TPP+ exhibits a remarkable inflection in its photophysics in the presence of Aβ40 aggregates. To investigate the advantages of this de novo designed Cou-AIE-TPP+ probe over conventionally used ThT, the amyloid detection performance and aggregation kinetics are compared. The Cou-AIE-TPP+ probe is capable of monitoring the kinetic progress of the Aβ40 fibrillation process with superior efficiency in contrast to ThT. Cou-AIE-TPP+ displays very high selectivity for the Aβ40 aggregates as compared to the native monomeric form of the protein. The Cou-AIE-TPP+ probe is dormant in the presence of monomeric Aβ40 forms. After binding to Aβ aggregates, a significant fluorescence augmentation of Cou-AIE-TPP+ at 604 nm along with a shoulder at 721 nm in NIR emission wavelength is detected at a single excitation wavelength (λex = 480 nm) (Fig. 4a, b and Fig. S32a, e, ESI†).50,51 Such aggregation-induced emission augmentation at 604 nm and 721 nm at a single excitation wavelength of 480 nm is owing to the RIM while the aggregation state increases. However, owing to the decrease in polarity instigated via Aβ40 aggregation, the solvatochromic fluorescence probe Cou-AIE-TPP+ exhibits a blue shift of 17 nm. We find that Cou-AIE-TPP+ can report the nucleation (lag phase), elongation (growth phase), and saturation stages of Aβ40 fibril formation (Fig. 4b). The lag phase designates Aβ40 monomers and soluble oligomers; the elongation phase denotes early aggregates and late aggregates; and finally, the aggregation progression reaches the saturation stage, where the majority of the Aβ40 peptides are converted to mature fibrils. Fluorescence-based quantitative assay displays that the emission intensity is boosted by 84-fold for Cou-AIE-TPP+ (1 μM) after incubation with Aβ40 mature fibrils (10 μM) in PBS, while only a 6-fold amplification of emission intensity is detected for the gold standard probe ThT (1 μM) under the same conditions (Fig. 4c and Fig. S32b, c, ESI†). The kinetics of aggregation results using Cou-AIE-TPP+ show approximately 1.9, 2.4, and 2.5 times augmented fluorescence intensity compared to ThT in detecting the Aβ early aggregates, late aggregates, and mature fibrils, respectively (Fig. 4b). However, for a control experiment, a negligible fluctuation is detected for Cou-AIE-TPP+ in PBS in the absence of Aβ40 over a 200 min time span. So, a fluorescence kinetics assay based on Cou-AIE-TPP+ can be used to detect multiple states of Aβ protein aggregation and can be used to differentiate monomers, early aggregates, late aggregates, and mature fibrils. Furthermore, when the ThT fluorescence after binding to Aβ40 fibril is saturated, upon further addition of ThT, the fluorescence intensity of the probe sharply decreased due to self-quenching. However, under the same conditions, Cou-AIE-TPP+ displays only negligible fluctuations. The binding affinity (Kd) of Cou-AIE-TPP+ is quantitatively determined by a nonlinear curve fitting using the fluorescence-based saturation binding method, signifying that the synthesized Cou-AIE-TPP+ probe binds tightly to the Aβ fibrils with 10.7 times higher binding affinity (dissociation constant Kd = 83 nM) in comparison with ThT (Kd = 890 nM) (Fig. 4d). The detection selectivity of the control probe Cou-AIE for the Aβ40 fibril is compared to that of Cou-AIE-TPP+. The results show that Cou-AIE-TPP+ yields a 1.5-time amplified signal in detecting Aβ fibrils relative to that of the control probe, Cou-AIE, lacking the lipophilic cationic TPP+ functionality (Fig. 4b). Moreover, Cou-AIE-TPP+ senses the aggregation progression considerably earlier compared to ThT and Cou-AIE under the same conditions, which signifies that Cou-AIE-TPP+ can detect trace aggregated Aβ in the early incubation period (Fig. 4b). Furthermore, the binding affinity of the control molecule Cou-AIE toward the Aβ fibrils is lower (Kd = 413 nM) in comparison with Cou-AIE-TPP+, signifying that the lipophilic cationic TPP+ functionality has a substantial impact on binding to the Aβ40 aggregates (Fig. S33, ESI†). It could be interpreted that Cou-AIE-TPP+ accumulates into the hydrophobic pockets and is bound to the Aβ40 aggregates with the help of binding functionalities, e.g., N,N′-diethylamino, TPP+, and aromatic π skeleton, resulting in the diminution of conformational freedom and restriction of intramolecular motions. Moreover, the fluorescence lifetime and brightness are also augmented when the probe Cou-AIE-TPP+ binds with Aβ oligomers and fibrils in comparison with monomers in PBS (τCou-AIE-TPP+ = 2.168 ns, τCou-AIE-TPP+/Aβ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) fibrils = 5.648 ns) (Fig. 4e and Fig. S32a, ESI,†Table 1). Such an escalation in the fluorescence lifetime is ascribed to the decrease in non-radiative progression in the excited state of Cou-AIE-TPP+ which is apparently a consequence of diminished micropolarity and enhanced microviscosity in the aggregated and fibrillar states of Aβ40. Moreover, absorption change of Cou-AIE-TPP+ after binding with Aβ fibrils is also noticed (Fig. S32d, ESI†). Bright orange-red fluorescence is detected when the Aβ fibril/Cou-AIE-TPP+ complex is exposed to UV light (Fig. S32a, ESI†). The formation of fibrils is further confirmed by CLSM of the Aβ40 fibrils stained with Cou-AIE-TPP+ (Fig. S34, ESI†). Moreover, Cou-AIE-TPP+ exhibits insignificant emission alteration in the presence of various amino acids and native proteins, authorizing its selectivity to Aβ40 (Fig. S39a, ESI†). The specific recognition of Aβ40 fibrils over others indicates the potential utility of Cou-AIE-TPP+ as an Aβ biomarker owing to the critical structural skeleton integrating ICT-induced solvatofluorochromism with AIE phenomena and the right lipophilicity for multiple states of Aβ protein aggregation targeting. Our synthesized probe Cou-AIE-TPP+ is compared with the reported Aβ binding probes in Table S2 (ESI†).52–60
fibrils = 5.648 ns) (Fig. 4e and Fig. S32a, ESI,†Table 1). Such an escalation in the fluorescence lifetime is ascribed to the decrease in non-radiative progression in the excited state of Cou-AIE-TPP+ which is apparently a consequence of diminished micropolarity and enhanced microviscosity in the aggregated and fibrillar states of Aβ40. Moreover, absorption change of Cou-AIE-TPP+ after binding with Aβ fibrils is also noticed (Fig. S32d, ESI†). Bright orange-red fluorescence is detected when the Aβ fibril/Cou-AIE-TPP+ complex is exposed to UV light (Fig. S32a, ESI†). The formation of fibrils is further confirmed by CLSM of the Aβ40 fibrils stained with Cou-AIE-TPP+ (Fig. S34, ESI†). Moreover, Cou-AIE-TPP+ exhibits insignificant emission alteration in the presence of various amino acids and native proteins, authorizing its selectivity to Aβ40 (Fig. S39a, ESI†). The specific recognition of Aβ40 fibrils over others indicates the potential utility of Cou-AIE-TPP+ as an Aβ biomarker owing to the critical structural skeleton integrating ICT-induced solvatofluorochromism with AIE phenomena and the right lipophilicity for multiple states of Aβ protein aggregation targeting. Our synthesized probe Cou-AIE-TPP+ is compared with the reported Aβ binding probes in Table S2 (ESI†).52–60
| Compound | Fluorescence lifetime (τ/ns) | QY |
|---|---|---|
a ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Relative QYs are measured using rhodamine-B as the reference probe.
b Relative QYs are measured using rhodamine-B as the reference probe.
b ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) τ values are determined using the time-correlated single photon counting (TCSPC) technique. τ values are determined using the time-correlated single photon counting (TCSPC) technique.
|
||
| Cou-AIE-TPP+ | 2.168 ± 0.126 | 0.45 |
| Cou-AIE-TPP+ + Aβ40 monomers | 2.253 ± 0.042 | 0.47 |
| Cou-AIE-TPP+ + Aβ40 oligomers | 2.981 ± 0.085 | 0.52 |
| Cou-AIE-TPP+ + Aβ40 fibrils | 5.648 ± 0.057 | 0.70 |
Competitive binding and displacement assay: Cou-AIE-TPP+ binds strongly to Aβ40 aggregates and displaces fibril-bound ThT
To acquire more information regarding the binding sites of Aβ40 aggregates for Cou-AIE-TPP+, we explored a competitive binding strategy with the known amyloid probe ThT. A displacement assay that titrates ThT (400 nM)-bound Aβ40 fibrils (10 μM) with Cou-AIE-TPP+ is used to acquire more insights. When Cou-AIE-TPP+ (0 to 400 nM) is gradually added to the ThT/Aβ40 fibrillar complex, ThT's fluorescence at λem 480 nm progressively decreases while Cou-AIE-TPP+'s red fluorescence intensity at λem 604 nm sharply increases with an increase in Cou-AIE-TPP+ concentration (Fig. 4f). It signifies that Cou-AIE-TPP+ is indeed binding and displacing ThT from the ThT/Aβ40 fibrillar complex. Similar to this, when ThT and Cou-AIE-TPP+ are introduced into the Aβ40 fibril solution concurrently, ThT's fluorescence intensity is attenuated with a less than 2-fold increase while Cou-AIE-TPP+'s fluorescence intensity enhances dramatically. This result indicates that Cou-AIE-TPP+ would enter with ThT in the same binding site and have a stronger affinity for Aβ40 fibrils in comparison with ThT. Due to its great binding affinity and highly specific fluorescence response, Cou-AIE-TPP+ offers promise for the early detection of Aβ40 aggregates.Isothermal titration calorimetry (ITC)
ITC quantifies the heat released or absorbed in a biomolecular binding incident in solution and calculates the binding affinity and thermodynamic parameters of molecular interactions in a single test. The negative heat generation in the titration of Aβ40 with Cou-AIE-TPP+ in ITC designates that the binding is exothermic in aqueous PBS solution and is predominantly enthalpy-driven (ΔH = −4.54 kcal mol−1) (Fig. S35, ESI†). The free energy change (ΔG) is negative (ΔG = −2.3 kcal mol−1), which implies that the interactions between the Aβ40 oligomer and Cou-AIE-TPP+ are spontaneous in nature.Diffusion-ordered spectroscopy (DOSY) and diffusion by NMR
Proton diffusion-ordered NMR spectroscopy (DOSY) is utilized to scrutinize the diffusion of Cou-AIE-TPP+ in solution-state inside the Aβ40 oligomers (Fig. S36 and S37, ESI†). DOSY is a prevailing toolkit for inspecting molecular association via determining the diffusion coefficients of molecules that endorse us to validate the diffusion of Cou-AIE-TPP+ to Aβ40 oligomers. A set of spin echo spectra is recorded with different time intervals, and signal decays are investigated to acquire the diffusion coefficients (D) (Fig. 5). The diffusion coefficient is calculated using the relative intensities ∼7.0–7.5 ppm that correspond to the aromatic hydrogens of the Cou-AIE-TPP+. In comparison with free Cou-AIE-TPP+ (faster diffusing species), which has a diffusion coefficient of 3.11 ± 0.14 × 10−10 m2 s−1, the diffusivity of Cou-AIE-TPP+ in the presence of 10 μM Aβ40 oligomer (slower diffusing complex) is considerably reduced to 2.77 ± 0.03 × 10−10 m2 s−1 in PBS solution. The DOSY experiment signifies that the binding of Cou-AIE-TPP+ to the Aβ40 oligomer reduces the extent of Brownian molecular motion or diffusion owing to the larger construct in comparison with free Cou-AIE-TPP+.Theoretical calculations based on DFT, TDDFT, and molecular docking
The DFT energy minimized structure of Cou-AIE-TPP+ at the B3LYP/6-31G* level is used to investigate the molecular interaction with Aβ40 aggregates. The MO calculation shows that the electron density for the HOMO level is mainly localized around the electron-donating groups N,N-diethyl amino phenyl unit and N,N-dialkylated amino phenyl unit, while the LUMO lobe coefficient is larger on the electron-withdrawing acrylonitrile unit (Fig. S38A, ESI†). The D–A–D structure narrows the bandgap (2.8 eV) of the Cou-AIE-TPP+ molecule due to ICT. Such electron distribution is characteristic of the D–A–D system, which results in solvatofluorochromism. A time-dependent density functional theory (TDDFT) at the B3LYP/6-31+G* level using the Gaussian 09 program is performed to further investigate the luminescence properties of the probe Cou-AIE-TPP+ in the excited state (Table S3, ESI†). The electron distribution in the HOMO, LUMO, LUMO+1, and LUMO+2 levels for Cou-AIE-TPP+ along with the energy is shown in Fig. S38B (ESI†). A molecular docking study is performed to further uncover the possible binding sites and molecular interactions between Cou-AIE-TPP+ and Aβ40 fibrils. Molecular docking of Cou-AIE-TPP+ with Aβ40 fibril (PDB ID: 2LMQ) displays noncovalent interactions such as van der Waals interactions, conventional hydrogen bonding, carbon–hydrogen bonds, σ–π interactions, π–sulfur interactions, π–π interactions, edge-to-face or T-shape π–π interactions, alkyl–π interactions between the Gly33, Ile31, Gly37, Gly38, Val39, Met35, Ala30, Asp23, Lys28, Asn27, Ser26, Phe19, Ile 31, Ile32, and Leu17 amino acid residues of the Aβ40 peptide and TPP+, coumarin and aromatic ring interactions, and amide and lactone C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, N centre of cyanide in Cou-AIE-TPP+ with a docking score of −7.1 kcal mol−1 (Fig. 6). The theoretical modeling suggests the presence of π-aromatic residues in the Cou-AIE-TPP+ molecule, e.g., the π-surface of TPP+, coumarin, and aromatic rings play a pivotal function in the high binding affinity of the exposed hydrophobic patches of Aβ40 fibrils. The Cou-AIE-TPP+ probe is positioned inside the tunnel along the fibril axis and has efficient Aβ40 recognition. Cou-AIE-TPP+ enters the hydrophobic sites and is bound to the Aβ40 fibrils, which restrict its conformational freedom, rotation, and vibration; this results in an augmentation of Cou-AIE-TPP+ fluorescence. It is worth noting that Cou-AIE-TPP+ has substantially higher estimated binding affinities to Aβ40 in comparison to ThT, with much more intermolecular noncovalent interactions.
O, N centre of cyanide in Cou-AIE-TPP+ with a docking score of −7.1 kcal mol−1 (Fig. 6). The theoretical modeling suggests the presence of π-aromatic residues in the Cou-AIE-TPP+ molecule, e.g., the π-surface of TPP+, coumarin, and aromatic rings play a pivotal function in the high binding affinity of the exposed hydrophobic patches of Aβ40 fibrils. The Cou-AIE-TPP+ probe is positioned inside the tunnel along the fibril axis and has efficient Aβ40 recognition. Cou-AIE-TPP+ enters the hydrophobic sites and is bound to the Aβ40 fibrils, which restrict its conformational freedom, rotation, and vibration; this results in an augmentation of Cou-AIE-TPP+ fluorescence. It is worth noting that Cou-AIE-TPP+ has substantially higher estimated binding affinities to Aβ40 in comparison to ThT, with much more intermolecular noncovalent interactions.
Biocompatibility and cytotoxicity study using Cou-AIE-TPP+
The sustained durability of Cou-AIE-TPP+ in the presence of potential interferences is a crucial prerequisite for its high-performance targeted bioimaging applications. No noteworthy fluctuations are noticed in the emission of Cou-AIE-TPP+ at 604 nm in the presence of various cations (Na+, K+, Ca2+) in the physiological environment (pH 7.4, 37 °C) throughout 24 h (Fig. S39a, ESI†). Additionally, Cou-AIE-TPP+ is pretty stable in the presence of small reactive species, e.g., Cys, H2O2, tBuOOH, NO, and ClO− in PBS (pH 7.4, 37 °C) over 24 h (Fig. S39a, ESI†). Furthermore, we have also evaluated the fluorescence variations of Cou-AIE-TPP+ in the presence of a series of different amino acids, e.g., Gly, Leu, Gln, Thr, Tyr, Arg, and Lys. As illustrated in Fig. S39a (ESI†), negligible fluorescence signal fluctuations of Cou-AIE-TPP+ at 604 nm are perceived in the presence of various amino acids in physiological environments (PBS, pH 7.4, 37 °C, 24 h). The specificity of Cou-AIE-TPP+ for Aβ fibrils is evaluated with potentially competitive species, including insulin and lysozyme fibrils. Cou-AIE-TPP+ exhibits a 6.5-fold and 3.8-fold higher binding affinity to Aβ40 fibrils in comparison with insulin and lysozyme fibrils, respectively (Fig. S39a, ESI†). The huge augmentation in the fluorescence signal confirms the effectiveness of Cou-AIE-TPP+ at a higher detection sensitivity for Aβ40 fibrils. The fluorescence deviation of Cou-AIE-TPP+ in the presence of human serum albumin (HSA) protein is tested in PBS (pH 7.4, 37 °C, 24 h). Cou-AIE-TPP+ exhibits a 9-fold higher binding affinity to Aβ40 fibrils in comparison with HSA protein (Fig. S39b, ESI†). Moreover, Aβ40 can effectively be detected by Cou-AIE-TPP+ in the presence of HSA protein (Fig. S39b, ESI†). The absorption and emission signal of Cou-AIE-TPP+ remains steady in fresh PBS, FBS-PBS, RPMI 1640, and DMEM over 24 h at 37 °C, pH 7.4, suggesting that the probe is stable or there is little interaction in various cell culture media. Moreover, Aβ40 can efficiently be sensed by Cou-AIE-TPP+ in the presence of various cell culture media (PBS, FBS-PBS, RPMI 1640, and DMEM) (Fig. S39c and d, ESI†). No noteworthy alterations in absorption and emission of the probe Cou-AIE-TPP+ are detected against factors within biological microenvironments such as temperature (10–60 °C) and pH (4.0–8.0) fluctuations over 24 h (Fig. S40, ESI†). Cell viability studies using a standard XTT assay on SH-SY5Y cells are performed. The outcomes display that there is insignificant cytotoxicity of Cou-AIE-TPP+, even when the concentration is 50 μM over 72 h (Fig. S41, ESI†). Adequate lipophilicity is required to achieve blood–brain barrier (BBB) penetrability as well as substantial uptake inside the mitochondria. The BBB penetration capability is often assessed through the oil–water partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P).61,62 Experimentally, the n-octanol/PBS partition coefficient (log
P).61,62 Experimentally, the n-octanol/PBS partition coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) value of Cou-AIE-TPP+ is determined to be 1.3 by Poctanol/PBS = [C]octanol layer/[C]PBS layer, envisioning more reasonable lipophilicity compared to that of ThT (log
P) value of Cou-AIE-TPP+ is determined to be 1.3 by Poctanol/PBS = [C]octanol layer/[C]PBS layer, envisioning more reasonable lipophilicity compared to that of ThT (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P = 0.16).26 The log
P = 0.16).26 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P value suggests that the lipophilicity of Cou-AIE-TPP+ is appropriate to penetrate the BBB and has great potential to accumulate in the mitochondria. Considering these, we envisage that the multifunctional Cou-AIE-TPP+ probe has the potential to be exploited as a harmless biomarker for targeted imaging of mitochondria and the detection of Aβ aggregates.
P value suggests that the lipophilicity of Cou-AIE-TPP+ is appropriate to penetrate the BBB and has great potential to accumulate in the mitochondria. Considering these, we envisage that the multifunctional Cou-AIE-TPP+ probe has the potential to be exploited as a harmless biomarker for targeted imaging of mitochondria and the detection of Aβ aggregates.
Mitochondria-targeted imaging utilizing Cou-AIE-TPP+ using a confocal laser scanning microscope
The lipophilic cationic TPP+ functionality tethered probe Cou-AIE-TPP+ is an excellent choice for targeted mitochondrial imaging owing to its exceptional biostability, noncytotoxicity, ICT-based solvatofluorochromism, AIE effect, large Stokes shift, and ultrabrightness. The subcellular localization of Cou-AIE-TPP+ in human neuroblastoma SH-SY5Y live-cells is executed using CLSM. After swift internalization through the SH-SY5Y live-cell membrane, it is expected that Cou-AIE-TPP+ would accumulate inside the mitochondria at a 500–1000-fold higher concentration related to the extracellular matrix (ΔΨplasma![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) membrane = −30 to −60 mV vs. ΔΨm −150 to −180 mV) in accordance with the Nernst equation, thus exhibiting the AIE effect inside the mitochondria. So, the AIE-based Cou-AIE-TPP+ probe is anticipated to resolve the fluorescence quenching issue of conventional ACQ-based mitochondria targeting fluorescent biomarkers, which arises at high concentrations in an aggregation state inside the mitochondria. A colocalization experiment is performed in live SH-SY5Y cells with Cou-AIE-TPP+ (500 nM) and the commercially available mitochondria-specific marker Mito-Tracker Deep Red. The CLSM experiment confirms that Cou-AIE-TPP+ localizes inside the SH-SY5Y live-cell mitochondria with a good Pearson correlation coefficient (PCC) of 0.83 (Fig. 7 and Fig. S42, S43, ESI†). Real-time mitochondrial tracking over extended periods (2.13 min) in human neuroblastoma live SH-SY5Y cells stained with the mitochondria targeting Cou-AIE-TPP+ probe is acquired (Fig. S44, S45 and Video S1, ESI†). For live SH-SY5Y cell 3D imaging, CLSM images of live cells stained with the mitochondria-selective Cou-AIE-TPP+ are captured every 0.4 μm on the Z-axis. 32 frames for distinct channels are collected over a 10 min time period to obtain the live SH-SY5Y cell 3D CLSM video (Fig. S46, Video S2, ESI†). It confirms that lipophilic cationic Cou-AIE-TPP+ molecules proficiently and selectively accumulate inside the live SH-SY5Y cell mitochondria and exhibit an AIE effect guided by the highly negative ΔΨm.
membrane = −30 to −60 mV vs. ΔΨm −150 to −180 mV) in accordance with the Nernst equation, thus exhibiting the AIE effect inside the mitochondria. So, the AIE-based Cou-AIE-TPP+ probe is anticipated to resolve the fluorescence quenching issue of conventional ACQ-based mitochondria targeting fluorescent biomarkers, which arises at high concentrations in an aggregation state inside the mitochondria. A colocalization experiment is performed in live SH-SY5Y cells with Cou-AIE-TPP+ (500 nM) and the commercially available mitochondria-specific marker Mito-Tracker Deep Red. The CLSM experiment confirms that Cou-AIE-TPP+ localizes inside the SH-SY5Y live-cell mitochondria with a good Pearson correlation coefficient (PCC) of 0.83 (Fig. 7 and Fig. S42, S43, ESI†). Real-time mitochondrial tracking over extended periods (2.13 min) in human neuroblastoma live SH-SY5Y cells stained with the mitochondria targeting Cou-AIE-TPP+ probe is acquired (Fig. S44, S45 and Video S1, ESI†). For live SH-SY5Y cell 3D imaging, CLSM images of live cells stained with the mitochondria-selective Cou-AIE-TPP+ are captured every 0.4 μm on the Z-axis. 32 frames for distinct channels are collected over a 10 min time period to obtain the live SH-SY5Y cell 3D CLSM video (Fig. S46, Video S2, ESI†). It confirms that lipophilic cationic Cou-AIE-TPP+ molecules proficiently and selectively accumulate inside the live SH-SY5Y cell mitochondria and exhibit an AIE effect guided by the highly negative ΔΨm.
Targeted monitoring of mitochondrial morphology alteration, damage, and dysfunction during exogenous Aβ40-induced neurotoxicity
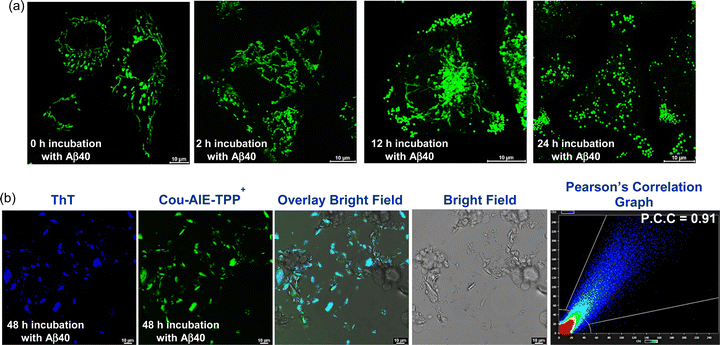
The ability of Cou-AIE-TPP+ to selectively localize in the mitochondria and exhibit the AIE effect may be an imperative tool to monitor mitochondrial morphology alteration, damage, and dysfunction during Aβ-induced neurotoxicity, which is the most early and notable hallmark of vulnerable neurons. We have monitored mitochondrial morphology alteration over time using CLSM in neuroblastoma SH-SY5Y cells stained with mitochondria targeting Cou-AIE-TPP+ (500 nM), followed by incubation with Aβ40 (10 μM). Aβ40 induces a morphological alteration of the cellular mitochondria in a time-dependent manner and is associated with perturbation of mitochondrial function, eventually resulting in neuronal cell death. Initially, elongated rod-shaped mitochondria in SH-SY5Y cells are mostly observed, which are fragmented and transformed to a spherical shape during incubation with Aβ40 over time (0–48 h) monitored through CLSM using Cou-AIE-TPP+ staining (Fig. 8). Mitochondrial fragmentation in SH-SY5Y cells after 2 h of incubation with Aβ40 (10 μM) is noticed, and spherical-shaped morphological transformation along with rod-shaped mitochondria is detected after 12 h of incubation (Fig. 8a). After 24 h of incubation of SH-SY5Y cells with Aβ40, complete transformation to the spherical morphology of mitochondria is noticed (Fig. 8a). After 48 h of incubation of SH-SY5Y cells with Aβ40, we have noticed fatal neurotoxicity, mitochondrial damage, rupture of the cell membrane, as well as cell death and accumulation of aggregated Aβ40 fibrils in the extracellular region of SH-SY5Y cells stained with the Cou-AIE-TPP+ probe, which is colocalized with ThT with a high PCC of 0.91 (Fig. 8b and Fig. S47, ESI†). Mitochondrial morphological changes considering the shape and size have been quantified by Image J software (Fig. S48, ESI†). Different morphologies of mitochondria, e.g., puncta, rod, network, large and round-shaped, are noticed. A statistical distribution of three parameters: number, length, and width/diameter of mitochondria at different time points (0 h, 2 h, 12 h, and 24 h) is calculated from CLSM images through bar diagrams. However, for the control experiment without the treatment with Aβ40, we didn’t observe mitochondrial morphology alteration or damage over time in SH-SY5Y cells stained with Cou-AIE-TPP+.To decipher our hypothesis of mitochondrial membrane depolarization in neuroblastoma SH-SY5Y cells after incubation with Aβ40 for varying times, a JC-1 based mitochondrial membrane potential detection kit on a fluorescence-activated cell sorting (FACS) flow cytometer was used. For healthy mitochondria, the ΔΨm is polarized (high value), JC-1 is incorporated into the mitochondria, and the J-aggregates of JC-1 are perceived. Conversely, when the membrane depolarizes, the J-aggregates of JC-1 seepage are detected from the mitochondria to the cytosol as monomers. A substantial amendment of ΔΨm in SH-SY5Y cells is noticed after treatment with Aβ40 (10 μM) over time (Fig. 9). The percentage of depolarized mitochondria in SH-SY5Y cells increases from 2.1% (Aβ40 untreated control experiment) to 69.5% after treatment with Aβ40 (10 μM) for 24 h, which is the earliest event noticed in mitochondrial dysfunction and damage, a notable hallmark of vulnerable neurons. Both early and late stages of apoptosis for SH-SY5Y cells are observed with varying times of incubation with Aβ40 using the annexin V–FITC/PI apoptosis detection kit via FACS. In the Aβ40 untreated control experiment, 99.9% of SH-SY5Y cells are considered viable [both annexin V and PI −Ve] (Fig. S49a, ESI†). However, with increasing incubation time with Aβ40, there is an increase in SH-SY5Y cells facing early apoptosis (annexin V +Ve and PI −Ve) (Fig. S49b and c, ESI†). annexin V–FITC staining is perceived in a Ca2+-dependent fashion through the recognition of negatively charged exposed phosphatidylserine (PS, typically located in the inner leaflet of the healthy plasma membrane) on the apoptotic cell, which signifies early apoptosis. At this stage, negligible PI staining is detected; moreover, the percentage of viable cells is quite high. After 24 h of incubation with Aβ40, both early (annexin V +Ve and PI −Ve) and late (annexin V +Ve and PI +Ve) stages of apoptosis, along with necrosis (annexin V −Ve and PI +Ve), are detected (Fig. S49d, ESI†). In Aβ40-induced depolarization of ΔΨm, the PS is exposed on the surface of the cell membrane during early apoptosis, and consequently, the mitochondrial transition pores are open, the membrane is ruptured, and mitochondrial caspase activator cytochrome c and other pro-apoptotic proteins are seeped into the cytosol, which triggers mitochondrial dysfunction and cell death via an apoptotic cascade. Furthermore, the noncovalent interaction of the Aβ40 peptide with mitochondrial proteins might also aid in induced mitochondria-targeted morphology alteration, damage, and dysfunction. These findings indicate that the mitochondria targeting, de novo designed, functional AIE-based solvatofluorochromic Cou-AIE-TPP+ probe (λem 604 nm) is a promising switch on biomarkers for monitoring mitochondrial morphology change and damage during exogenous Aβ-induced neurotoxicity, as well as fluorescence imaging and detection of neurotoxic Aβ aggregates, which may offer imperative direction for the advancement of compelling AIE biomarkers for mitochondria targeted early stage Aβ diagnosis in the future.
Conclusions
In conclusion, we establish that the de novo designed Cou-AIE-TPP+ is an excellent ICT-induced solvatofluorochromic functional AIE probe to detect the various stages of Aβ40 aggregation along with the targeted imaging of mitochondria. The probe emits strong fluorescence upon binding to Aβ aggregates with high binding affinity in comparison to the Aβ monomer, is capable of detecting amyloid aggregation kinetics, and is superior to the gold standard probe ThT. The lipophilic cationic Cou-AIE-TPP+ probe selectively accumulates inside the live neuroblastoma cell mitochondria and exhibits the AIE effect inside the mitochondria due to the highly negative ΔΨm. Additionally, the effective live-cell penetration, mitochondrial target selectivity and real-time monitoring ability, adequate lipophilicity, ultrabrightness, biocompatibility, and negligible cytotoxicity of Cou-AIE-TPP+ make it extremely suitable for sensing and imaging of neurotoxic Aβ oligomers in the complicated cellular environment. After 48 h of incubation of neuroblastoma cells with Aβ40, fatal neurotoxicity, mitochondrial damage, and accumulation of aggregated Aβ40 fibrils in the extracellular region of SH-SY5Y cells stained with the Cou-AIE-TPP+ probe are detected. It can be used to study mitochondrial morphology alteration, damage, and dysfunction in Aβ-induced neurotoxicity, recognize and image neurotoxic Aβ fibrils, and may offer imperative guidance for the advancement of compelling AIE biomarkers for mitochondria targeted early diagnosis of Alzheimer's disease in the future.Author contributions
T. B. designed the experiments, developed the methodology, synthesized, purified, and characterized the probes, performed the Aβ aggregation kinetics, spectroscopic assay, and TEM, and analyzed the data. A. M. and S. K. performed the biological assay, including CLSM. A. M. and S. B. synthesized the intermediates and performed the ITC and NMR experiments. S. G. conceptualized and supervised the project as well as wrote and edited the original draft of the manuscript. All authors scrutinized and discussed the data, reviewed, and edited the manuscript and ESI.†Data availability
The data that support the findings of this study are available in the ESI† of this article.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was funded by the SERB, Govt. of India (file no. CRG/2023/000243). We acknowledge the DSTBT, Govt. of West Bengal [file no. STBT-11012(25)/10/2024-ST SEC] for the financial support. T. B. and A. M. acknowledge UGC, and S. K. acknowledges CSIR. We also acknowledge Arun K. Pal and Ayan Datta from IACS and Bholanath Maity from KAUST for valuable discussion.References
- D. S. Knopman, H. Amieva, R. C. Petersen, G. Chételat, D. M. Holtzman, B. T. Hyman, R. A. Nixon and D. T. Jones, Nat. Rev. Dis. Primers, 2021, 7, 33 CrossRef PubMed.
- W. M. van der Flier, M. E. de Vugt, E. M. A. Smets, M. Blom and C. E. Teunissen, Nat. Aging, 2023, 3, 494–505 CrossRef PubMed.
- S. Pospich and S. Raunser, Science, 2017, 358, 45–46 CrossRef CAS PubMed.
- D. J. Selkoe, Nat. Med., 2011, 17, 1060–1065 CrossRef CAS PubMed.
- P. Scheltens, B. D. Strooper, M. Kivipelto, H. Holstege, G. Chételat, C. E. Teunissen, J. Cummings and W. M. van der Flier, Lancet, 2021, 397, 1577–1590 CrossRef CAS PubMed.
- Z. Ismail, B. Creese, D. Aarsland, H. C. Kales, C. G. Lyketsos, R. A. Sweet and C. Ballard, Nat. Rev. Neurol., 2022, 18, 131–144 CrossRef PubMed.
- J. A. Hardy and G. A. Higgins, Science, 1992, 256, 184–185 CrossRef CAS PubMed.
- Q. Xu, Y. Ma, Y. Sun, D. Li, X. Zhang and C. Liu, Aggregate, 2023, 4, e333 CrossRef CAS.
- J. An, K. Kim, H. J. Lim, H. Y. Kim, J. Shin, I. Park, I. Cho, H. Y. Kim, S. Kim, C. McLean, K. Y. Choi, Y. Kim, K. H. Lee and J. S. Kim, Nat. Commun., 2024, 15, 1004 CrossRef CAS PubMed.
- J. Hardy and D. J. Selkoe, Science, 2002, 297, 353–356 CrossRef CAS PubMed.
- M.-Y. Cha, S.-H. Han, S.-M. Son, H.-S. Hong, Y.-J. Choi, J. Byun and I. Mook-Jung, PLoS One, 2012, 7, e34929 CrossRef CAS PubMed.
- J. Ke, Q. Tian, Q. Xu, Z. Fu and Q. Fu, Drug Discovery Today, 2021, 26, 1991–2002 CrossRef CAS PubMed.
- X. Zhua, G. Perryc, M. A. Smitha and X. Wanga, J. Alzheimer's Dis., 2013, 33, S253–S262 Search PubMed.
- M. A. Smith, G. Perry, P. L. Richey, L. M. Sayre, V. E. Anderson, M. F. Beal and N. Kowall, Nature, 1996, 382, 120–121 CrossRef CAS PubMed.
- K. Qian, X. Bao, Y. Li, P. Wang, Q. Guo, P. Yang, S. Xu, F. Yu, R. Meng, Y. Cheng, D. Sheng, J. Cao, M. Xu, J. Wu, T. Wang, Y. Wang, Q. Xie, W. Lu and Q. Zhang, ACS Nano, 2022, 16, 11455–11472 CrossRef CAS PubMed.
- N. S. McKay, B. A. Gordon, R. C. Hornbeck, A. Dincer, S. Flores, S. J. Keefe, N. Joseph-Mathurin, C. R. Jack, R. Koeppe, P. R. Millar, B. M. Ances, C. D. Chen, A. Daniels, D. A. Hobbs, K. Jackson, D. Koudelis, P. Massoumzadeh, A. McCullough, M. L. Nickels, F. Rahmani, L. Swisher, Q. Wang, R. F. Allegri, S. B. Berman, A. M. Brickman, W. S. Brooks, D. M. Cash, J. P. Chhatwal, G. S. Day, M. R. Farlow, C. la Fougère, N. C. Fox, M. Fulham, B. Ghetti, N. Graff-Radford, T. Ikeuchi, W. Klunk, J. H. Lee, J. Levin, R. Martins, C. L. Masters, J. McConathy, H. Mori, J. M. Noble, G. Reischl, C. Rowe, S. Salloway, R. Sanchez-Valle, P. R. Schofield, H. Shimada, M. Shoji, Y. Su, K. Suzuki, J. Vöglein, I. Yakushev, C. Cruchaga, J. Hassenstab, C. Karch, E. McDade, R. J. Perrin, C. Xiong, J. C. Morris, R. J. Bateman and T. L. S. Benzinger, Nat. Neurosci., 2023, 26, 1449–1460 CrossRef CAS PubMed.
- M. Higuchi, N. Iwata, Y. Matsuba, K. Sato, K. Sasamoto and T. C. Saido, Nat. Neurosci., 2005, 8, 527–533 CrossRef CAS PubMed.
- L. Zhu, K. Ploessl and H. F. Kung, Chem. Soc. Rev., 2014, 43, 6683–6691 RSC.
- N. Bandara, A. K. Sharma, S. Krieger, J. W. Schultz, B. H. Han, B. E. Rogers and L. M. Mirica, J. Am. Chem. Soc., 2017, 139, 12550–12558 CrossRef CAS PubMed.
- S. A. Aliyan, N. P. Cook and A. A. Martí, Chem. Rev., 2019, 119, 11819–11856 CrossRef PubMed.
- Y. Venkatesh, N. P. Marotta, V. M.-Y. Lee and E. J. Petersson, Chem. Sci., 2024, 15, 6053–6063 RSC.
- L. Sun, H.-J. Cho, S. Sen, A. S. Arango, T. T. Huynh, Y. Huang, N. Bandara, B. E. Rogers, E. Tajkhorshid and L. M. Mirica, J. Am. Chem. Soc., 2021, 143, 10462–10476 CrossRef CAS PubMed.
- H. Levine, Protein Sci., 1993, 2, 404–410 CrossRef CAS PubMed.
- L.-M. Needham, J. Weber, J. A. Varela, J. W. B. Fyfe, D. T. Do, C. K. Xu, L. Tutton, R. Cliffe, B. Keenlyside, D. Klenerman, C. M. Dobson, C. A. Hunter, K. H. Müller, K. O'Holleran, S. E. Bohndiek, T. N. Snaddon and S. F. Lee, Chem. Sci., 2020, 11, 4578–4583 RSC.
- S. S. Hou, J. Yang, J. H. Lee, Y. Kwon, M. Calvo-Rodriguez, K. Bao, S. Ahn, S. Kashiwagi, A. T. N. Kumar, B. J. Bacskai and H. S. Choi, Nat. Biomed. Eng., 2023, 7, 270–280 CrossRef CAS PubMed.
- W. Fu, C. X. Yan, Z. Q. Guo, J. J. Zhang, H. Y. Zhang, H. Tian and W. H. Zhu, J. Am. Chem. Soc., 2019, 141, 3171–3177 CrossRef CAS PubMed.
- J. Miao, M. Miao, Y. Jiang, M. Zhao, Q. Li, Y. Zhang, Y. An, K. Pu and Q. Miao, Angew. Chem., Int. Ed., 2023, 62, e202216351 CrossRef CAS PubMed.
- X. Ji, Y. Cai, X. Dong, W. Wu and W. Zhao, Nanoscale, 2023, 15, 9290–9296 RSC.
- W. Chen, L. J. Young, M. Lu, A. Zaccone, F. Ströhl, N. Yu, G. S. K. Schierle and C. F. Kaminski, Nano Lett., 2017, 17, 143–149 CrossRef CAS PubMed.
- Y. Duo, G. Luo, W. Zhang, R. Wang, G. G. Xiao, Z. Li, X. Li, M. Chen, J. Yoon and B. Z. Tang, Chem. Soc. Rev., 2023, 52, 1024–1067 RSC.
- X. Chen, Q. Yang, X. Lv, Y. Xiong, B. Z. Tang and X. Huang, Coord. Chem. Rev., 2024, 516, 215970 CrossRef CAS.
- L. Wang, X. Chen, Q. Xia, R. Liu and J. Qu, Ind. Eng. Chem. Res., 2018, 57, 7735–7741 CrossRef CAS.
- X. Zhong, Q. Yang, Y. Chen, Y. Jiang and Z. Dai, J. Mater. Chem. B, 2020, 8, 7375–7381 RSC.
- W. He, R. T. K. Kwok, Z. Qiu, Z. Zhao and B. Z. Tang, J. Am. Chem. Soc., 2024, 146, 5030–5044 CrossRef CAS PubMed.
- M. Kumar, Y. Hong, D. C. Thorn, H. Ecroyd and J. A. Carver, Anal. Chem., 2017, 89, 9322–9329 CrossRef CAS PubMed.
- Y.-L. Wang, C. Fan, B. Xin, J.-P. Zhang, T. Luo, Z.-Q. Chen, Q.-Y. Zhou, Q. Yu, X.-N. Li, Z.-L. Huang, C. Li, M.-Q. Zhu and B. Z. Tang, Mater. Chem. Front., 2018, 2, 1554–1562 RSC.
- H. Wang, Q. Li, P. Alam, H. Bai, V. Bhalla, M. R. Bryce, M. Cao, C. Chen, S. Chen, X. Chen and Y. Chen, et al. , ACS Nano, 2023, 17, 14347–14405 CrossRef CAS PubMed.
- L. Xu, H. Gao, W. Zhan, Y. Deng, X. Liu, Q. Jiang, X. Sun, J.-J. Xu and G. Liang, J. Am. Chem. Soc., 2023, 145, 27748–27756 CrossRef CAS PubMed.
- Y. Hong, L. Meng, S. Chen, C. W. T. Leung, L.-T. Da, M. Faisal, D.-A. Silva, J. Liu, J. W. P. Lam, X. Huang and B. Z. Tang, J. Am. Chem. Soc., 2012, 134, 1680–1689 CrossRef CAS PubMed.
- C. Yan, J. Dai, Y. Yao, W. Fu, H. Tian, W.-H. Zhu and Z. Guo, Nat. Protoc., 2023, 18, 1316–1336 CrossRef CAS PubMed.
- T.-T. Hou, Y.-H. Tang, Z.-Y. Zhang, Z.-J. Li and Y.-L. Wang, J. Innovative Opt. Health Sci., 2023, 16, 2330009 CrossRef CAS.
- W. He, Y. Yang, Y. Qian, Z. Chen, Y. Zheng, W. Zhao, C. Yan, Z. Guo and S. Quan, Aggregate, 2024, 5, e412 CrossRef CAS.
- Q. Hu, M. Gao, G. Feng and B. Liu, Angew. Chem., Int. Ed., 2014, 53, 14225–14229 CrossRef CAS PubMed.
- J. Wang, P. Shangguan, X. Chen, Y. Zhong, M. Lin, M. He, Y. Liu, Y. Zhou, X. Pang, L. Han, M. Lu, X. Wang, Y. Liu, H. Yang, J. Chen, C. Song, J. Zhang, X. Wang, B. Shi and B. Z. Tang, Nat. Commun., 2024, 15, 705 CrossRef CAS PubMed.
- R. S. Das, D. Maiti, S. Kar, T. Bera, A. Mukherjee, P. C. Saha, A. Mondal and S. Guha, J. Am. Chem. Soc., 2023, 145, 20451–20461 CrossRef CAS PubMed.
- J. Zielonka, J. Joseph, A. Sikora, M. Hardy, O. Ouari, J. Vasquez-Vivar, G. Cheng, M. Lopez and B. Kalyanaraman, Chem. Rev., 2017, 117, 10043–10120 CrossRef CAS PubMed.
- W. Wan, L. Zeng, W. Jin, X. Chen, D. Shen, Y. Huang, M. Wang, Y. Bai, H. Lyu, X. Dong, Z. Gao, L. Wang, X. Liu and Y. Liu, Angew. Chem., Int. Ed., 2021, 60, 25865–25871 CrossRef CAS PubMed.
- Q. Peng and Z. Shuai, Aggregate, 2021, 2, e91 CrossRef CAS.
- Y. Tu, J. Liu, H. Zhang, Q. Peng, J. W. Y. Lam and B. Z. Tang, Angew. Chem., Int. Ed., 2019, 58, 14911–14914 CrossRef CAS PubMed.
- X. Liu, N. Zhang, T. Bing and D. Shangguan, Anal. Chem., 2014, 86, 2289–2296 CrossRef CAS PubMed.
- H. Leng, Y. Wang, J. Wang, H. Sun, A. Sun, M. Pistolozzi, L. Zhang and J. Yan, Anal. Chem., 2022, 94, 1999–2006 CrossRef CAS PubMed.
- W. E. Klunk, Y. Wang, G.-F. Huang, M. L. Debnath, D. P. Holt and C. A. Mathis, Life Sci., 2001, 69, 1471–1484 CrossRef CAS PubMed.
- S.-J. Jung, S.-H. Park, E. J. Lee, J. H. Park, Y. B. Kong, J. K. Rho, M. G. Hur, S. D. Yang and Y. D. Park, Arch. Pharmacal Res., 2015, 38, 1992–1998 CrossRef CAS PubMed.
- C. Ran, X. Xu, S. B. Raymond, B. J. Ferrara, K. Neal, B. J. Bacskai, Z. Medarova and A. Moore, J. Am. Chem. Soc., 2009, 131, 15257–15261 CrossRef CAS PubMed.
- M. Ono, H. Watanabe, H. Kimura and H. Saji, ACS Chem. Neurosci., 2012, 3, 319–324 CrossRef CAS PubMed.
- W. Fu, C. Yan, Z. Guo, J. Zhang, H. Zhang, H. Tian and W.-H. Zhu, J. Am. Chem. Soc., 2019, 141, 3171–3177 CrossRef CAS PubMed.
- A. Das, A. Gupta, Y. Hong, J. A. Carver and S. Maiti, Biochemistry, 2020, 59, 1813–1822 CrossRef CAS PubMed.
- J.-W. Yan, J.-Y. Zhu, K.-X. Zhou, J.-S. Wang, H.-Y. Tan, Z.-Y. Xu, S.-B. Chen, Y.-T. Lu, M.-C. Cui and L. Zhang, Chem. Commun., 2017, 53, 9910–9913 RSC.
- L. Sun, H.-J. Cho, S. Sen, A. S. Arango, T. T. Huynh, Y. Huang, N. Bandara, B. E. Rogers, E. Tajkhorshid and L. M. Mirica, J. Am. Chem. Soc., 2021, 143, 10462–10476 CrossRef CAS PubMed.
- T. Zhang, X. Chen, C. Yuan, X. Pang, P. Shangguan, Y. Liu, L. Han, J. Sun, J. W. Y. Lam, Y. Liu, J. Wang, B. Shi and B. Z. Tang, Angew. Chem., Int. Ed., 2023, 62, e202211550 CrossRef CAS PubMed.
- H. Pajouhesh and G. R. Lenz, NeuroRx, 2005, 2, 541–553 CrossRef PubMed.
- S. A. Hitchcock and L. D. Pennington, J. Med. Chem., 2006, 49, 7559–7583 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Materials, methods, syntheses, NMR (1D & 2D), HRMS, UV/vis, fluorescence, FT-IR, TEM, photophysics, ITC, theoretical calculation, XTT assay, confocal microscopy (PDF). Video S1: real-time mitochondrial tracking in live SH-SY5Y cells using Cou-AIE-TPP+ (avi). Video S2: 3D CLSM movie of live SH-SY5Y cells using mitochondria targeting Cou-AIE-TPP+ (avi). See DOI: https://doi.org/10.1039/d4tb01337a |
| This journal is © The Royal Society of Chemistry 2024 |