Open Access Article
Open Access ArticleUnleashing the antibacterial and antibiofilm potential of silica-based nanomaterials functionalized with an organotin(IV) compound†
Victoria
García-Almodóvar
 ab,
Perla del Rosario
Ardiles
ab,
Perla del Rosario
Ardiles
 b,
Sanjiv
Prashar
b,
Sanjiv
Prashar
 a,
Paulina Laura
Páez
a,
Paulina Laura
Páez
 *b and
Santiago
Gómez-Ruiz
*b and
Santiago
Gómez-Ruiz
 *a
*a
aCOMET-NANO Group, Departamento de Biología y Geología, Física y Química Inorgánica, ESCET, Universidad Rey Juan Carlos, Calle Tulipán s/n, E-28933 Móstoles, Madrid, Spain. E-mail: santiago.gomez@urjc.es
bDepartamento de Ciencias Farmacéuticas, Facultad de Ciencias Químicas, Universidad Nacional de Córdoba, Ciudad Universitaria, Haya de la Torre y Medina Allende, X5000HUA Córdoba, Argentina. E-mail: plpaez@unc.edu.ar
First published on 12th August 2024
Abstract
Bacterial diseases caused by superbugs are expected to be the main cause of death worldwide within a decade as a consequence of the resistance they are acquiring to the antibiotics currently in use, therefore, the field of new antibacterial treatments is currently being thoroughly studied. The present work focuses on the synthesis, functionalization, characterization and antibacterial behaviour of different systems based on three different silica-based nanostructured materials (MSN, mesoporous silica nanoparticles, SBA-15 Santa Barbara amorphous-15 and FSP fibrous slica nanoparticles) which serve as scaffolds for the support of different platforms to target and treat bacterial diseases and biofilm formation. Thus, (3-carboxypropyl)triphenylphosphonium bromide (PPh3+) and a cytotoxic organotin(IV) fragment (Sn) have been incorporated in the silica-based materials to study their potential activity in different antibacterial applications. After a complete characterization of the synthesized systems, which confirmed the incorporation of both the targeting and the therapeutic fragments within the nanostructured materials, the antibacterial study of the materials demonstrated bactericidal capacity against Escherichia coli and perturbation of the bacteria metabolism via oxidative stress through an enhanced ROS (reactive oxygen species) production. In addition, biofilm inhibition and eradication tests of bacterial strains were carried out, showing that the activity of the materials in both biofilm inhibition and eradication is dependent on the concentration of the material. Furthemore, the material MSN-AP(1:1)-PPh3+-Sn containing the targeting triphenylphosphonium and a “SnPh3” fragment is capable of inhibiting and eradicating up to 50% of the formation of biofilms, which is outstanding for metallodrug-functionalized silica-based systems compared with other materials based on metal nanoparticles supported on silica. Finally, a hemolysis study was carried out with the nanostructured systems proving to be non-toxic, making them adequate for their subsequent use in preclinical trials through in vivo models.
1. Introduction
The treatment of bacterial diseases has undergone significant transformation since the discovery of penicillin by Alexander Fleming in 1928, with a wide array of antibiotic drugs such as ciprofloxacin and amoxicillin becoming mainstays of medical practice.1 However, the growing issue of antibiotic resistance presents a global public health challenge. This phenomenon intersects with a variety of fields, including food security, climate change, and health infrastructure stability, necessitating a multidisciplinary approach known as the “One Health” perspective.2–4 The spread of resistant bacteria across human, animal, and environmental sectors, as well as the circulation of resistance determinants between these sectors worldwide, poses a major threat to global health. The persistence and spread of resistant microbial species at the human–animal–environment interface can alter microbial genomes, leading to the emergence of resistant superbugs across various ecological niches.5,6Given the significant threat posed by antibiotic-resistant bacteria, there has been an upsurge in research focusing on novel antibacterial treatments.7 Recently, metals such as silver, copper, and tin have emerged as promising candidates for antibacterial treatments, both as nanoparticles and metal complexes.
In this study, we introduce the use of silica-based nanostructured materials as carrier platforms for different agents, aiming to explore their potential as novel antibacterial systems. Silica-based porous materials offer numerous advantages, including a high surface area (>1000 m2 g−1), customizable internal and external surfaces, biocompatibility, varied pore sizes, and a range of functionalization methods for tailored nanosystem design.8 These properties enable the creation of versatile mesoporous silica nanoparticles with varying morphology, pore size, pore distribution, and other textural, physical, and chemical characteristics, especially in a biological context.9
On one hand, there has been an increase in research focused on the treatment of bacterial diseases and the eradication of superbugs. On the other, silicon-based nanomaterials are well-known for their use as drug encapsulating agents. In recent years, this area of research has grown significantly. Nanoparticles are being utilized to encapsulate antimicrobial peptides (AMPs) to enhance their stability and facilitate their application in the body.10 They are also employed to deliver photosensitive compounds to specific treatment areas.11 Some studies have developed hybrid nanomaterials that combine silica-based nanomaterials with polymers such as PVP12 or even with silver nanoparticles, the latter being extensively studied for their antibacterial properties.13 Additionally, silica nanoparticles are used to encapsulate certain FDA-approved drugs, which can lose efficacy over time due to pathogen resistance.14
The silica systems utilized in this work include: (a) mesoporous silica nanoparticles (MSN),15 known for their spherical morphology and hexagonal pore arrangement; (b) Santa Barbara amorphous-15 (SBA-15),16 characterized by their oval morphology and hexagonal pore distribution; and (c) fibrous silica particles (FSP),17 featuring a fibrous, dandelion-shaped morphology with channels within the fibers for internal and external surface functionalization.
These silica nanoparticles can be functionalized with various compounds, and their biological action can occur through classical or non-classical mechanisms. Classical strategies involve surface adsorption with stimuli-responsive release (e.g., pH change, electrochemical impulse, UV light),18 whereas non-classical strategies involve robust covalent bonds for functionalization on both internal and external surfaces, providing biological activity without the need for therapeutic fragment release.19
In our study, the first compound of interest is (3-carboxypropyl)triphenylphosphonium bromide (PPh3+), a mitochondrial-targeting compound repurposed here to test its potential capability in competing against the double membrane of large negative bacteria due to the morphological similarities with mitochondrial membranes.20
In general, thiphenylphosphonium salts help in targeting bacteria,21–24 therefore, we have combined triphenylphosphonium fragments with organotin(IV) agents (Sn) to leverage their potential advantages, including simplifying synthetic procedures and lowering doses for enhanced antibacterial and antibiofilm activity. This strategy aims to maximize the benefits of organotin(IV)25 while minimizing potential drawbacks, as previous studies have shown that metallodrugs are largely effective against bacteria without inducing resistance.26
Our investigation assesses the antibacterial efficacy of the developed silica-based nanomaterials with triphenylphosphonium targeting and “SnPh3” fragments against Staphylococcus aureus and Escherichia coli strains. The results reveal that these systems are capable of inhibiting and eradicating up to 50% of biofilm formation, outperforming other metal nanoparticles supported on silica and revealing as a plausible alternative to current biofilm-targeting approaches.27 Furthermore, hemolysis studies confirm the non-toxic nature of the nanostructured systems, supporting their potential application in preclinical trials and in vivo models.
2. Materials and methods
2.1. Synthesis and functionalization of materials
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mL) along with TEOS (5 mL, 22.39 mmol) was added drop by drop. The reaction mixture was stirred for 45 minutes before being transferred to a hydrothermal reactor, where it was left to react for 5 hours at 120 °C. The same washing and calcining protocol as for MSN and SBA-15 was applied to obtain the final FSP particles, ensuring removal of any residual surfactant or impurities.
3 mL) along with TEOS (5 mL, 22.39 mmol) was added drop by drop. The reaction mixture was stirred for 45 minutes before being transferred to a hydrothermal reactor, where it was left to react for 5 hours at 120 °C. The same washing and calcining protocol as for MSN and SBA-15 was applied to obtain the final FSP particles, ensuring removal of any residual surfactant or impurities.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 ratio for the three materials (MSN, SBA, and FSP). For MSN, an additional fourth material was prepared with an AP ratio of 1
0.1 ratio for the three materials (MSN, SBA, and FSP). For MSN, an additional fourth material was prepared with an AP ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The mixtures were subjected to vigorous magnetic stirring at 110 °C for 48 hours. Following the reaction, the functionalized materials (MSN-AP(10), MSN-AP(1:1), SBA-AP(10), FSP-AP(10)) were collected by centrifugation and washed with toluene (2 × 20 mL) and diethyl ether (2 × 20 mL). The final materials were dried at 80 °C to yield the desired product.
1. The mixtures were subjected to vigorous magnetic stirring at 110 °C for 48 hours. Following the reaction, the functionalized materials (MSN-AP(10), MSN-AP(1:1), SBA-AP(10), FSP-AP(10)) were collected by centrifugation and washed with toluene (2 × 20 mL) and diethyl ether (2 × 20 mL). The final materials were dried at 80 °C to yield the desired product.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio under vigorous magnetic stirring for 24 hours at 80 °C under a nitrogen atmosphere. Following the organotin(IV) compound formation, the materials from the previous section underwent two vacuum/nitrogen cycles and were redispersed in toluene while maintaining an inert atmosphere. The supernatant resulting from the reaction of the organotin(IV) complex with each material, obtained at a material
2 ratio under vigorous magnetic stirring for 24 hours at 80 °C under a nitrogen atmosphere. Following the organotin(IV) compound formation, the materials from the previous section underwent two vacuum/nitrogen cycles and were redispersed in toluene while maintaining an inert atmosphere. The supernatant resulting from the reaction of the organotin(IV) complex with each material, obtained at a material![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Sn ratio of 1
Sn ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, was then filtered and allowed to react under vigorous magnetic stirring at 110 °C for 24 hours. Finally, to obtain the final materials (MSN-AP(10)-PPh3+-Sn; MSN-AP(1:1)-PPh3+-Sn; SBA-AP(10)-PPh3+-Sn; FSP-AP(10)-PPh3+-Sn), the materials were centrifuged and washed with toluene (2 × 20 mL) and ethanol (2 × 20 mL) before being dried at 80 °C.
0.1, was then filtered and allowed to react under vigorous magnetic stirring at 110 °C for 24 hours. Finally, to obtain the final materials (MSN-AP(10)-PPh3+-Sn; MSN-AP(1:1)-PPh3+-Sn; SBA-AP(10)-PPh3+-Sn; FSP-AP(10)-PPh3+-Sn), the materials were centrifuged and washed with toluene (2 × 20 mL) and ethanol (2 × 20 mL) before being dried at 80 °C.
2.2. Studies on the behaviour of materials in biological media
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio respect to the material and incubated for 24 h at 37 °C. After this time the MIC (minimum concentration at bacterial growth was not observed) can be noted by the simple turbidity of the well, but to confirm the MIC value, 3 μL of a solution of resazurin sodium salt (1 mg mL−1 in distilled H2O) was added to each well, leaving it to react for 30 minutes at 37 °C. Thus, it couldbe observed that the wells in all blue colours show bacterial death and the wells in pink colours show bacterial viability.
1 ratio respect to the material and incubated for 24 h at 37 °C. After this time the MIC (minimum concentration at bacterial growth was not observed) can be noted by the simple turbidity of the well, but to confirm the MIC value, 3 μL of a solution of resazurin sodium salt (1 mg mL−1 in distilled H2O) was added to each well, leaving it to react for 30 minutes at 37 °C. Thus, it couldbe observed that the wells in all blue colours show bacterial death and the wells in pink colours show bacterial viability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio at 37 °C for 1, 4 and 24 h. Then, serial dilutions of the mixture were made in a 1
1 ratio at 37 °C for 1, 4 and 24 h. Then, serial dilutions of the mixture were made in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1 ratio. Finally, 20 μL of these dilutions were seeded in TSA Petri dishes and incubated for 24 h at 37 °C in order to count the CFU mL−1.
0.1 ratio. Finally, 20 μL of these dilutions were seeded in TSA Petri dishes and incubated for 24 h at 37 °C in order to count the CFU mL−1.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, at this point it was important to consider a control with inoculum and PBS. The plate was left to incubate for 1, 4 and 24 h at 37 °C. After the incubation times, 20 μL of the probe 2′,7′-dichlorofluorescein diacetate (DCF) 50 μM was added to each well and incubated for 30 minutes at 37 °C. The fluorescence intensity was measured in a spectrofluorometer Biotek Synergy HT with the excitation and emission wavelengths at 480 and 520 nm, respectively.
1 ratio, at this point it was important to consider a control with inoculum and PBS. The plate was left to incubate for 1, 4 and 24 h at 37 °C. After the incubation times, 20 μL of the probe 2′,7′-dichlorofluorescein diacetate (DCF) 50 μM was added to each well and incubated for 30 minutes at 37 °C. The fluorescence intensity was measured in a spectrofluorometer Biotek Synergy HT with the excitation and emission wavelengths at 480 and 520 nm, respectively.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of sulphanilamide (2% in 5% v/v HCl) and N-(1-naphthyl)ethylenediamine dihydrochloride (0.1% in distilled H2O) was added to each well. The plate was then incubated for 15 minutes at 37 °C, after which the absorbance was measured at 540 nm. It is important for this assay to consider a calibration line, in which serial dilutions of sodium nitrite (NaNO2) in the range of 100–0.05 μM were made, to which the same reagents were added to the samples and measured in the same way.
1 mixture of sulphanilamide (2% in 5% v/v HCl) and N-(1-naphthyl)ethylenediamine dihydrochloride (0.1% in distilled H2O) was added to each well. The plate was then incubated for 15 minutes at 37 °C, after which the absorbance was measured at 540 nm. It is important for this assay to consider a calibration line, in which serial dilutions of sodium nitrite (NaNO2) in the range of 100–0.05 μM were made, to which the same reagents were added to the samples and measured in the same way.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) for 1, 4 and 24 h at 37 °C. After this time, the material was centrifuged at 3000 rpm for 10 minutes and the supernatant was read at 540 nm in the plate reader.
1) for 1, 4 and 24 h at 37 °C. After this time, the material was centrifuged at 3000 rpm for 10 minutes and the supernatant was read at 540 nm in the plate reader.
3. Results and discussion
3.1. Results of synthesis and characterization of the silica-based nanostructured materials
The synthesis of different nanostructured materials based on silica such as MSN, SBA and FSP has been carried out by sol–gel method with different surfactants and protocols (MSN and SBA-15), and by hydrothermal method (FSP) observing that the obtained materials had the expected physical and textural properties.30The nanostructured silica-based systems underwent sequential functionalization with various agents. Initially, an amino-containing ligand (AP ligand) was grafted onto the systems at varying proportions following reported procedures,31 to establish binding sites on both the internal and external surfaces. Subsequently, an EDAC-type reaction32 was employed, utilizing (3-carboxypropyl)triphenylphosphonium bromide (PPh3+), to introduce phosphonium groups aimed at targeting bacterial membranes, facilitating the formation of amide bonds. Finally, a potentially therapeutic organotin(IV) compound (Fig. 1) was incorporated via a protonolysis reaction coupled with the elimination of alcohol. Prior to subjecting the materials to bacterial assays, it is crucial to verify the accuracy of both synthesis and subsequent functionalization processes. To ensure this, a range of physico-chemical characterization techniques were employed, as detailed below.
 | ||
| Fig. 1 Scheme of functionalization of the different nanostructured materials proposed for this antibacterial study. | ||
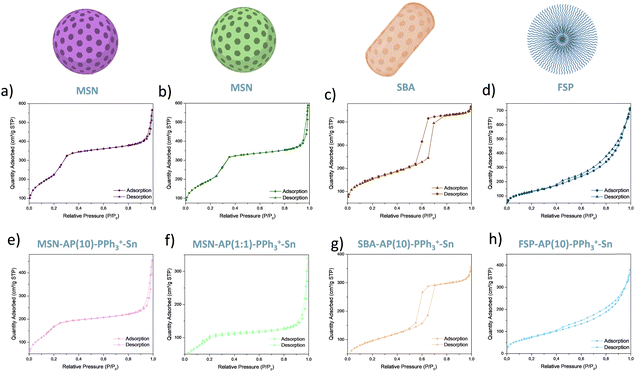
Fig. 2 shows the nitrogen adsorption and desorption isotherms of the initial materials (MSN, SBA and FSP) and the final materials (MSN-AP(10)-PPh3+-Sn; MSN-AP(1:1)-PPh3+-Sn; SBA-AP(10)-PPh3+-Sn; FSP-AP(10)-PPh3+-Sn). For the materials based on MSN nanoplatforms, type IV isotherms are observed. These isotherms are characteristic of mesoporous structures, typical of MSN nanoparticles. However, they could also resemble type VI isotherms, indicative of solid materials with very uniform surfaces. A hysteresis cycle (H2) is present in these materials due to nitrogen condensation by capillarity. This cycle indicates that nitrogen easily enters the pores but exits in a more complex fashion, forming a capillary distribution of nitrogen molecules inside the pores. The SBA-based materials also exhibit clear type IV isotherms, characteristic of their mesoporous structure. The H2 hysteresis cycle is visible here as well, resulting from the interaction between the material and nitrogen. In contrast, FSP particles display type III isotherms. This difference arises from the fibrous morphology of the FSP platform, which increases surface area but alters the interaction with nitrogen. The type III isotherm indicates low interaction between the adsorbate and the adsorbent, with nitrogen entering the fibres easily but experiencing capillary condensation upon exit. These observations underscore the distinct adsorption behaviour and structural characteristics of the different silica-based materials.33–35 Moreover, this technique provides not only isotherm data but also quantitative information on the surface area (m2 g−1), pore volume (cm3 g−1), and pore diameter (nm) of the materials (see Table 1). The starting materials (MSN, SBA, and FSP) exhibit large surface areas, ranging from approximately 822 to 473 m2 g−1. This is significant because a larger surface area facilitates the incorporation of molecules of interest into the nanoplatform's structure.
| Material | BET surface (m2 g−1) | Pore volume (cm3 g−1) | Pore diameter (nm) |
|---|---|---|---|
| MSN | 821.71 | 0.68 | 3.31 |
| MSN-AP(10)-PPh3+-Sn | 646.86 | 0.45 | 2.79 |
| MSN | 721.12 | 0.67 | 3.52 |
| MSN-AP(1:1)-PPh3+-Sn | 384.65 | 0.29 | 3.00 |
| SBA | 551.79 | 0.69 | 4.99 |
| SBA-AP(10)-PPh3+-Sn | 350.72 | 0.50 | 5.96 |
| FSP | 472.80 | 1.01 | 8.54 |
| FSP-AP(10)-PPh3+-Sn | 274.26 | 0.50 | 7.36 |
In the final, functionalized materials (MSN-AP(10)-PPh3+-Sn; MSN-AP(1:1)-PPh3+-Sn; SBA-AP(10)-PPh3+-Sn; FSP-AP(10)-PPh3+-Sn), a noticeable decrease in BET surface area is observed. For instance, the surface area of the highly functionalized (MSN-AP(1:1)-PPh3+-Sn) decreases to 340 m2 g−1. Additionally, pore size and pore volume are significantly reduced post-functionalization, in some cases by up to 50%.
These observations indicate three key points: firstly, the functionalization has been successful, with clear incorporation of the functional groups into the material; secondly, the functionalization seems to occur throughout the whole material, not just on the external surface but also within the internal pores, explaining the reductions in pore size and volume. Finally, materials with a higher amount of AP ligand show a greater decrease in BET surface area, suggesting a higher incorporation of both the ligand and the other functional molecules. These findings underscore the effectiveness of the functionalization process and its impact on the structural properties of the silica-based materials.
The MSN-based materials (namely, MSN, MSN-AP(10)-PPh3+-Sn and MSN-AP(1:1)-PPh3+-Sn) show a quasi-spherical morphology with size between 70–90 nm and an ordered hexagonal distribution of pores, as expected when compared with other similar materials.30 In the case of the SBA systems, materials SBA and SBA-AP(10)-PPh3+-Sn materials show a wide rod-like morphology with an elongated dimension. The materials are larger with a particle size distribution of 903.56 nm in length and 691.26 in width, although with a similar hexagonal pore distribution. Both the morphology, particle size and porous arrangement agree with other published SBA-15 materials.
Finally, for the fibrous particles, i.e., FSP and FSP-AP(10)-PPh3+-Sn, a spherical particle morphology composed of symmetrical fibres is observed. These particles have an intermediate size between MSN and SBA, with average sizes of approximately 500 nm. Quantitative data were obtained using the ImageJ program to generate Gaussian distributions and numerical values of the mean particle size, as shown in Table 2.37 Additionally, scanning electron microscopy (SEM) was used for the SBA-based materials to observe their distribution and morphology in general. The SEM images can be seen in Fig. S1 (ESI†).
| Material | Particle size (nm) |
|---|---|
| MSN | 71.20 ± 16.29 |
| MSN-AP(10)-PPh3+-Sn | 77.01 ± 17.76 |
| MSN | 87.26 ± 18.30 |
| MSN-AP(1:1)-PPh3+-Sn | 79.69 ± 14.87 |
| SBA | 902.71 ± 143.65 |
| SBA-AP(10)-PPh3+-Sn | 904.41 ± 97.02 |
| FSP | 513.08 ± 115.20 |
| FSP-AP(10)-PPh3+-Sn | 466.04 ± 74.49 |
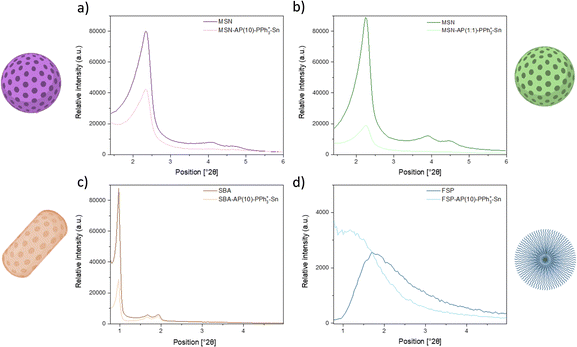
In this regard, silica-based materials displayed a distinct band at approximately 250 nm following functionalization with the AP ligand, attributed to the integration of the organic fragment. Subsequent incorporation of the PPh3+ moiety led to an intensification of this peak, with further enhancement observed upon the introduction of the organotin(IV) complex. Notably, the latter transformation also manifested a characteristic peak around 270 nm, indicative of the presence of the metal-containing fragment.39
| Material | (hkl) | 2θ (°) | d hkl (Å) |
|---|---|---|---|
| MSN | 100 | 2.38 | 37.05 |
| 110 | 4.13 | 21.38 | |
| 200 | 4.75 | 18.60 | |
| MSN-AP(10)-PPh3+-Sn | 100 | 2.37 | 37.20 |
| 110 | 4.13 | 21.38 | |
| 200 | 4.76 | 18.57 | |
| MSN | 100 | 2.28 | 38.35 |
| 110 | 3.92 | 22.53 | |
| 200 | 4.54 | 19.44 | |
| MSN-AP(1:1)-PPh3+-Sn | 100 | 2.28 | 38.66 |
| 200 | 4.48 | 19.73 | |
| SBA | 100 | 0.96 | 92.34 |
| 110 | 1.66 | 53.30 | |
| 200 | 1.92 | 45.92 | |
| SBA-AP(10)-PPh3+-Sn | 100 | 0.96 | 91.67 |
| 110 | 1.67 | 52.97 | |
| 200 | 1.93 | 45.70 | |
| FSP | — | 1.70 | 51.92 |
| FSP-AP(10)-PPh3+-Sn | — | 1.22 | 71.79 |
TG was used to quantify the amount of AP ligand in the MSN-AP(10), MSN-AP(1:1), SBA-AP(10), and FSP-AP(10) materials, and to assess their thermal stability. ICP-MS was employed to accurately quantify the number and quantity of specific elements in the samples. The materials were digested in 1 mg mL−1 KOH (2 M) and agitated for 48 hours before filtration and measurement. Using ICP-MS, we measured the phosphorus content in MSN-AP(10)-PPh3+, MSN-AP(1:1)-PPh3+, SBA-AP(10)-PPh3+ and FSP-AP(10)-PPh3+ to determine the amount of PPh3+ incorporated. Similarly, the tin content in MSN-AP(10)-PPh3+-Sn, MSN-AP(1:1)-PPh3+-Sn, SBA-AP(10)-PPh3+-Sn and FSP-AP(10)-PPh3+-Sn was quantified, which is crucial for evaluating the antibacterial potential, as tin acts as the cytotoxic agent in these nanostructures.
Table 4 shows the TG data for materials functionalized with AP. For a theoretical 10% ligand incorporation, the actual amount ranges between 6% and 7%, with the highest being in the FSP nanostructure. For the material with a theoretical 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ligand ratio, the actual amount is about 17%, roughly three times higher than the previous ones. Although these values are below the theoretical amount, they are consistent with previous results from our group.41 The free amino groups on the surface serve as anchor points for the targeting agent (PPh3+), as confirmed by TG measurements Fig. S4 (ESI†). Column 3 of Table 4 presents the phosphorus measurements by ICP-MS, indicating the actual amount of PPh3+ in each nanomaterial. Although the values are approximately ten times lower than those of AP, they should suffice for functionality, which will be verified in subsequent antibacterial tests. Additionally, materials with more AP ligand (e.g., MSN-AP(1:1)) contain more triphenylphosphonium salt, corroborating that the NH2 groups of the ligand are covalent binding points to the silica. Finally, using ICP-MS, we quantified the tin content in the final materials. The synthesis of the tin complex (detailed in Section 2.1.7) ensures that the metal content is independent of the initial functionalization amount, although highly functionalized silica complicates further incorporation. Functionalization values are close to 2%, with the highly functionalized MSN (MSN-AP(1:1)-PPh3+-Sn) having 0.12% less tin than the less functionalized MSN (MSN-AP(10)-PPh3+-Sn). Notably, the FSP nanoplatform contains the highest amount of metal at ca. 1.91%, likely due to its fibrous pore morphology facilitating molecule incorporation. This comprehensive quantification confirms the effective functionalization of the nanomaterials and sets the stage for evaluating their antibacterial properties.
1 ligand ratio, the actual amount is about 17%, roughly three times higher than the previous ones. Although these values are below the theoretical amount, they are consistent with previous results from our group.41 The free amino groups on the surface serve as anchor points for the targeting agent (PPh3+), as confirmed by TG measurements Fig. S4 (ESI†). Column 3 of Table 4 presents the phosphorus measurements by ICP-MS, indicating the actual amount of PPh3+ in each nanomaterial. Although the values are approximately ten times lower than those of AP, they should suffice for functionality, which will be verified in subsequent antibacterial tests. Additionally, materials with more AP ligand (e.g., MSN-AP(1:1)) contain more triphenylphosphonium salt, corroborating that the NH2 groups of the ligand are covalent binding points to the silica. Finally, using ICP-MS, we quantified the tin content in the final materials. The synthesis of the tin complex (detailed in Section 2.1.7) ensures that the metal content is independent of the initial functionalization amount, although highly functionalized silica complicates further incorporation. Functionalization values are close to 2%, with the highly functionalized MSN (MSN-AP(1:1)-PPh3+-Sn) having 0.12% less tin than the less functionalized MSN (MSN-AP(10)-PPh3+-Sn). Notably, the FSP nanoplatform contains the highest amount of metal at ca. 1.91%, likely due to its fibrous pore morphology facilitating molecule incorporation. This comprehensive quantification confirms the effective functionalization of the nanomaterials and sets the stage for evaluating their antibacterial properties.
| Material | AP weight (%) | AP mmol g−1 | P (%) | Sn (%) |
|---|---|---|---|---|
| MSN-AP(10) | 5.41 | 0.28 | — | — |
| MSN-AP(1:1) | 16.59 | 0.86 | — | — |
| SBA-AP(10) | 6.77 | 0.35 | — | — |
| FSP-AP(10) | 7.00 | 0.36 | — | — |
| MSN-AP(10)-PPh3+ | — | — | 0.045 ± 0.001 | — |
| MSN-AP(1:1)-PPh3+ | — | — | 0.101 ± 0.001 | — |
| SBA-AP(10)-PPh3+ | — | — | 0.047 ± 0.001 | — |
| FSP-AP(10)-PPh3+ | — | — | 0.043 ± 0.001 | — |
| MSN-AP(10)-PPh3+-Sn | — | — | not analyzed | 1.802 ± 0.001 |
| MSN-AP(1:1)-PPh3+-Sn | — | — | not analyzed | 1.681 ± 0.001 |
| SBA-AP(10)-PPh3+-Sn | — | — | not analyzed | 1.672 ± 0.001 |
| FSP-AP(10)-PPh3+-Sn | — | — | not analyzed | 1.905 ± 0.014 |
3.2. Results of biological studies
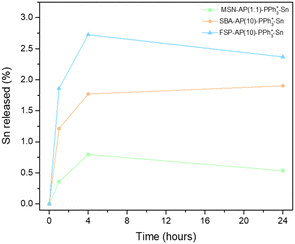
Fig. 5 illustrates the percentage of Sn released by each material over time, relative to the percentage of Sn functionalized in the material (as detailed in Table 4). All materials exhibited a gradual release of tin, reaching a maximum release at 4 hours and stabilizing by 24 hours. The metal release values remained below 3% of the total Sn functionalized in each material, which is in line with similar systems published in our group. This low release rate is attributed to the non-classical functionalization of the nanoplatforms, where all molecules are covalently incorporated. Consequently, the material functions cohesively in the biological environment without needing to release the cytotoxic agent. In particular, the FSP-type silica showed rapid metal release, possibly attributable to its fibrous structure, which may provide a more accessible environment for release compared to materials with hexagonal pores and long channels.
As depicted in Fig. 6 the final materials exhibited diffusion halos in the S. aureus strain, while the starting and intermediate materials did not. This observation underscores the significant role of the cytotoxic agent, specifically the organotin(IV) complex, in combating this bacterial strain. Qualitative data, measured from the inhibition halo of each material (Table 5), revealed similar halos across the materials, resulting in halo values between 18 and 20 mm. Notably, no difference in activity was observed between MSN-AP(10) and MSN-AP(1:1), suggesting that the ligand quantity did not impact material activity in this assay.
| Material | Diffusion (mm) |
|---|---|
| PBS | 8 ± 1 |
| Ciprofloxacin | 29 ± 1 |
| MSN | 8 ± 1 |
| MSN | 8 ± 1 |
| SBA | 8 ± 1 |
| FSP | 8 ± 1 |
| PBS | 8 ± 1 |
| Ciprofloxacin | 30 ± 1 |
| MSN-AP(10) | 8 ± 1 |
| MSN-AP(1:1) | 8 ± 1 |
| SBA-AP(10) | 8 ± 1 |
| FSP-AP(10) | 8 ± 1 |
| PBS | 8 ± 1 |
| Ciprofloxacin | 29 ± 1 |
| MSN-AP(10)-PPh3+ | 8 ± 1 |
| MSN-AP(1:1)-PPh3+ | 8 ± 1 |
| SBA-AP(10)-PPh3+ | 8 ± 1 |
| FSP-AP(10)-PPh3+ | 8 ± 1 |
| PBS | 8 ± 1 |
| Ciprofloxacin | 30 ± 1 |
| MSN-AP(10)-PPh3+-Sn | 18 ± 1 |
| MSN-AP(1:1)-PPh3+-Sn | 18 ± 1 |
| SBA-AP(10)-PPh3+-Sn | 20 ± 1 |
| FSP-AP(10)-PPh3+-Sn | 20 ± 1 |
Conversely, with the E. coli strain (Fig. 7 and Table 6) none of the materials displayed significant inhibition halos. However, this absence does not preclude potential activity of the final materials against this strain.
| Material | Diffusion (mm) |
|---|---|
| PBS | 8 ± 1 |
| Ciprofloxacin | 37 ± 1 |
| MSN | 8 ± 1 |
| MSN | 8 ± 1 |
| SBA | 8 ± 1 |
| FSP | 8 ± 1 |
| PBS | 8 ± 1 |
| Ciprofloxacin | 40 ± 1 |
| MSN-AP(10) | 8 ± 1 |
| MSN-AP(1:1) | 8 ± 1 |
| SBA-AP(10) | 8 ± 1 |
| FSP-AP(10) | 8 ± 1 |
| PBS | 8 ± 1 |
| Ciprofloxacin | 42 ± 1 |
| MSN-AP(10)-PPh3+ | 8 ± 1 |
| MSN-AP(1:1)-PPh3+ | 8 ± 1 |
| SBA-AP(10)-PPh3+ | 8 ± 1 |
| FSP-AP(10)-PPh3+ | 8 ± 1 |
| PBS | 8 ± 1 |
| Ciprofloxacin | 44 ± 1 |
| MSN-AP(10)-PPh3+-Sn | 8 ± 1 |
| MSN-AP(1:1)-PPh3+-Sn | 8 ± 1 |
| SBA-AP(10)-PPh3+-Sn | 8 ± 1 |
| FSP-AP(10)-PPh3+-Sn | 8 ± 1 |
With these results, subsequent bacterial activity tests were conducted exclusively with the final materials, as the starting and intermediate materials demonstrated no activity against either studied strain.
| Material | MIC [Sn] (μg mL−1) | MBC [Sn] (μg mL−1) | Conclusion |
|---|---|---|---|
| S. aureus (ATCC 29213) | |||
| MSN-AP(10)-PPh3+-Sn | 62.50 [1.13] | 125.00 [2.25] | Bactericide |
| MSN-AP(1:1)-PPh3+-Sn | 62.50 [1.01] | 125.00 [2.10] | Bactericide |
| SBA-AP(10)-PPh3+-Sn | 31.25 [0.52] | 250.00 [4.18] | Bacteriostatic |
| FSP-AP(10)-PPh3+-Sn | 15.63 [0.30] | 62.50 [1.22] | Bacteriostatic |
| E. coli (ATCC 25922) | |||
| MSN-AP(10)-PPh3+-Sn | 250.00 [4.51] | 250.00 [4.51] | Bactericide |
| MSN-AP(1:1)-PPh3+-Sn | 250.00 [4.20] | 250.00 [4.20] | Bactericide |
| SBA-AP(10)-PPh3+-Sn | 250.00 [4.18] | 500.00 [8.36] | Bactericide |
| FSP-AP(10)-PPh3+-Sn | 250.00 [4.88] | 250.00 [4.88] | Bactericide |
Table 7 presents the antibacterial activity data for the bacterial strain S. aureus. The MSN-based materials show identical MIC (minimum inhibitory concentration) and MBC (minimum bactericidal concentration) values, at 62.50 and 125.00 μg mL−1, respectively. However, the MSN-AP(1:1)-PPh3+-Sn has approximately 0.1% less metal content compared to MSN-AP(10)-PPh3+-Sn.
For the SBA-based materials, the MIC value is half that of the MSN materials, while the MBC value is double. These values correlate proportionally with the metal content. In the FSP-based nanomaterials, MIC values are four times lower and MBC values are halved compared to the MSN materials, which also aligns with the amount of incorporated metal.
The fourth column of Table 7 shows the relationship between MIC and MBC values. The MSN-based materials exhibit bactericidal activity despite higher MIC and MBC values. In contrast, the SBA and FSP materials display bacteriostatic activity, although their MIC values are somewhat lower.
The second part of Table 7 summarizes the results for the E. coli bacterial strain. All materials exhibit the same MIC value, with the FSP material containing the highest amount of tin, as confirmed by ICP-MS measurements. The MBC values for SBA are twice as high in both material and metal content. Overall, all materials demonstrate bactericidal activity against E. coli.
Comparing the results for both bacterial strains, we observe that the values for E. coli are similar to those for MSN and higher for SBA and FSP. However, all tested materials show bactericidal activity, highlighting their potential effectiveness against both bacterial strains. Given the potential antibacterial treatment implications, bactericidal activity is considered more advantageous. These results suggest promising potential for nanomaterial-based treatments and imply that PPh3+ primarily influences Gram-negative bacteria.
Analyzing the amount of PPh3+ present in each material, one can easily see that there are no significant differences in the results obtained with the MSN material containing 0.045%, compared to that of the systems containing 0.101%. In this context, one can conclude that with an amount of 0.043% of PPh3+ incorporated in the final material FSP-AP(10)-PPh3+-Sn seems to be sufficient to prove its potential targeting by the increase of the activity, especially against the bacterial strain E. coli.
For the well-known silver nanoparticles, commonly used as antibacterial agents, the MIC values are approximately 125 μg mL−1 and the MBC values are 250 μg mL−1. These values are up to 100 times higher, indicating a lower antibacterial activity of silver nanoparticles compared to the Sn-containing materials studied, when considering the amount of metal, which is the cytotoxic agent in each silica-based material.42
Following bacterial incubation with the materials for varying durations, fluorescence data were collected. To accurately quantify ROS production by live bacteria at each incubation point, these fluorescence data were juxtaposed with CFU mL−1 data obtained from the previous death curve test. Fig. 9 illustrates the percentage of ROS at each hour of incubation. Remarkably, a notable percentage of ROS was detected across all materials, with an observable increase at the 4-hour mark of incubation. Quantitatively, it is evident that at 4 hours, the ROS value in the SBA-AP(10)-PPh3+-Sn material increases by 1018% compared to the value obtained at 1 hour, occurring in both bacterial strains. While significant ROS values are observed in all materials across all time points, the highest values at 4 hours are associated with the SBA-based material. Conversely, at 24 hours, the highest ROS values in E. coli are found in the FSP-based material. These findings indicate that the morphology of the starting material significantly impacts both antibacterial activity and metabolism. Notably, the ROS values reported here surpass those found in the literature for similar materials.36
These results are in concordance with those of the death curve test, wherein a noticeable change, particularly in the E. coli strain, was observed at the 4-hour experiment. This synchronous change in ROS production and bacterial death underscores the significance of oxidative stress as a potential mechanism underlying the antibacterial activity of the materials (for an additional scaling of Fig. 9 with respect to the highest ROS concentration, see Fig. S6 of ESI†).
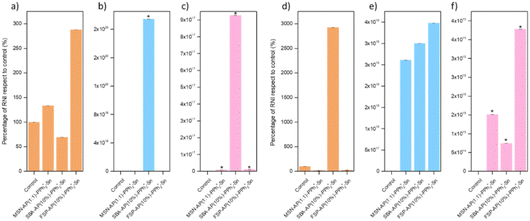
Following incubation of the materials for various durations and subsequent staining with reagents, Fig. 10 presents graphs depicting the percentage of RNI formed. These graphs reveal a trend similar to that observed in the ROS assays, suggesting a direct relationship between metabolic activity, whether oxidative or nitrosative stress, and the death curves obtained, which correspond to colony formation at each time point studied. Consistent with the previous observations, the material with the highest RNI values is SBA-AP(10)-PPh3+-Sn, showing a 1018% increase in metabolic activity at 4 hours (for an additional scaling of Fig. 10 with respect to the highest RNI concentration, see Fig. S7 of ESI†).
Notably, the percentage of RNI follows a trend similar to that of ROS, albeit with slightly lower values. Consequently, it can be inferred that the primary metabolic pathway in both bacterial strains when exposed to MSN-AP(1:1)-PPh3+-Sn, SBA-AP(10)-PPh3+-Sn, and FSP-AP(10)-PPh3+-Sn materials predominantly involves the generation of ROS.
Furthermore, to ascertain the predominant metabolic pathway in each bacterial strain, Table 8 presents the ROS and RNI values obtained in each test, expressed as a percentage, along with the ROS/RNI ratio. Analysis of this ratio reveals that, across both strains and all final materials, the predominant pathway is the generation of reactive oxygen species. These findings are consistent with literature, where ROS generation is commonly observed as the primary route in most cases.44 Additionally, it is notable that in the case of materials MSN-AP(1:1)-PPh3+-Sn and SBA-AP(10)-PPh3+-Sn tested against the E. coli strain, higher values were recorded, indicating a greater generation of ROS in the negative strain. This observation underscores the enhanced ROS production in E. coli compared to S. aureus, further supporting the primary role of ROS in the antibacterial mechanism of these materials. The values obtained in these bacterial metabolism studies cannot be directly compared with those in the literature, as the use of tin-functionalized silicon nanomaterials as antibacterials has not been previously studied. This represents a promising new direction for future research. In the study by Ugalde-Arbizu et al.26 where MSN-type silica materials were examined, the maximum ROS values reported were 60% at 4 hours. In contrast, the significantly higher ROS values obtained in this work highlight the enhanced antibacterial activity of the final materials as plausible coatings.
| Material | Time (h) | ROS (%) | RNI (%) | ROS/RNI |
|---|---|---|---|---|
| S. aureus ATCC 29213 | ||||
| MSN-AP(1:1)-PPh3+-Sn | 1 | 1.76 × 102 | 1.34 × 102 | 1.32 |
| 4 | 1.07 × 102 | 6.89 × 101 | 1.55 | |
| 24 | 5.91 × 102 | 2.88 × 102 | 2.05 | |
| SBA-AP(10)-PPh3+-Sn | 1 | 8.98 × 1013 | 3.02 × 1013 | 2.97 |
| 4 | 1.71 × 1020 | 2.14 × 1020 | 0.80 | |
| 24 | 1.09 × 1015 | 6.67 × 1014 | 1.63 | |
| FSP-AP(10)-PPh3+-Sn | 1 | 5.21 × 1016 | 8.21 × 1015 | 6.35 |
| 4 | 3.28 × 1018 | 9.27 × 1017 | 3.54 | |
| 24 | 3.53 × 1016 | 1.07 × 1016 | 3.29 | |
| E. coli ATCC 25922 | ||||
| MSN-AP(1:1)-PPh3+-Sn | 1 | 9.20 × 101 | 1.80 × 101 | 5.11 |
| 4 | 1.40 × 104 | 2.92 × 103 | 4.78 | |
| 24 | 1.11 × 102 | 2.22 × 101 | 5.01 | |
| SBA-AP(10)-PPh3+-Sn | 1 | 1.16 × 1019 | 2.61 × 1018 | 4.46 |
| 4 | 1.26 × 1019 | 3.00 × 1018 | 4.19 | |
| 24 | 1.15 × 1019 | 3.48 × 1018 | 3.30 | |
| FSP-AP(10)-PPh3+-Sn | 1 | 5.17 × 1015 | 1.51 × 1015 | 3.41 |
| 4 | 1.83 × 1015 | 7.43 × 1014 | 2.46 | |
| 24 | 8.23 × 1015 | 3.78 × 1015 | 2.17 | |
| Material | Time (h) | % of Reduction in ROS levels by GSH |
|---|---|---|
| S. aureus ATCC 29213 | ||
| MSN-AP(1:1)-PPh3+-Sn | 1 | 14.6 |
| 4 | 2.6 | |
| 24 | 18.4 | |
| SBA-AP(10)-PPh3+-Sn | 1 | 44.2 |
| 4 | 60.0 | |
| 24 | 14.0 | |
| FSP-AP(10)-PPh3+-Sn | 1 | 61.1 |
| 4 | 32.6 | |
| 24 | 32.9 | |
| E. coli ATCC 25922 | ||
| MSN-AP(1:1)-PPh3+-Sn | 1 | 12.7 |
| 4 | 42.3 | |
| 24 | 0.0 | |
| SBA-AP(10)-PPh3+-Sn | 1 | 21.4 |
| 4 | 10.3 | |
| 24 | 11.9 | |
| FSP-AP(10)-PPh3+-Sn | 1 | 30.0 |
| 4 | 26.5 | |
| 24 | 26.7 | |
From the present study, one can infer that there was an increase in ROS production after bacterial treatment with the materials, compared to untreated (control) bacteria. In addition, the enhanced ROS generation by the nanomaterials was also confirmed by intracellular ROS levels measurements even in the presence of GSH.
From the obtained results it is clear that GSH protect different cellular components by slightly neutralizing ROS. Table 9 shows that the addition of GSH reduced the ROS levels at all times evaluated, including up to 60% at the maximum ROS in S. aureus and 42% in E. coli. However, there were very interesting results observed for some of the materials, for example the system MSN-AP(1:1)-PPh3+-Sn showed to generate persistent ROS, as the reduction of ROS levels by GSH were between only ca. 3–18% after 1, 4 or 24 hours in the case of S. aureus and even of 0% after 24 hours in the case of E. coli strains.
These results indicate that increased ROS production and the relatively low reduction of the ROS levels by GSH when treating the bacterial strains with MSN-AP(1:1)-PPh3+-Sn greatly contributes to the impact of the nanomaterials on S. aureus and E. coli colonies and their potential viability.
In E. coli, the degree of inhibition correlates closely with material concentration. Conversely, eradicating established biofilms poses a greater challenge, as incubation with the materials occurs after biofilm formation, over a 24-hour period. Despite using lower material concentrations, significant effects of the materials on biofilm eradication are observed. In S. aureus (Fig. 12), while materials exhibit less efficacy in eradicating biofilms compared to inhibiting their formation, there is a trend towards increased eradication with higher material concentrations. In E. coli, eradication percentages increase with material concentration, except for SBA-AP(10)-PPh3+-Sn, which shows no activity.
To the best of our knowledge this is the first organotin(IV)-based nanomaterial which shows a good degree of eradication in both E. coli and S. aureus biofilms, opening up the opportunity to further explore this kind of systems more in detail in preclinical trials in a short-term future.
 | ||
| Fig. 13 Percentages of hemolysis obtained in the assay after incubation of erythrocytes with the final materials at SUBMIC, MIC and SUPRAMIC concentrations (* = p < 0.05). | ||
However, at longer incubation periods, notably 24 hours, the percentage of hemolysis escalates, particularly evident in MSN-AP(10)-PPh3+-Sn material. Consequently, this material is excluded from future bacterial tests due to the considerable increase in hemolysis.
The final materials, SBA-AP(10)-PPh3+-Sn and FSP-AP(10)-PPh3+-Sn, exhibited IC50 values of 48.33 μg mL−1 and 99.95 μg mL−1, respectively, in terms of the final material, and 0.81 μg mL−1 and 1.90 μg mL−1, respectively, in terms of tin concentration (see Fig. S10 of ESI†). When compared to the MTT assay results for the SnPh3Cl prodrug, it is evident that incorporating the tin compound within the nanoparticles significantly reduces its toxicity to healthy cells, increasing cell viability by more than 12 times. Thus, using silica nanostructured materials appears to be advantageous for applying the highly cytotoxic SnPh3 moieties in biological environments (in vitro) and is expected to have a lesser impact on organs such as the kidneys. In this context and considering previous studies by our team, which demonstrated that analogous tin-functionalized silica-based nanomaterials are non-toxic to healthy cells in vitro and that kidney biomarkers in mice remain intact after nanoparticle treatment in vivo,28,37,50 it can be concluded that the materials reported here, with their dual antitumoral and antibacterial (and antibiofilm) activity, warrant additional studies to confirm their potential although additional studies should be undertaken to be applied in vivo.
Conclusions
The synthesis and functionalization of final materials, namely MSN-AP(1:1)-PPh3+-Sn, SBA-AP(10)-PPh3+-Sn, and FSP-AP(10)-PPh3+-Sn, were successfully achieved, showcasing potent antibacterial activity against S. aureus and E. coli strains. Notably, the latter exhibited more promising outcomes, potentially attributed to the PPh3+ fragment's influence. Moreover, it was evident that the organotin(IV) complex serves as an effective treatment, as preceding materials lacked activity.Oxidative stress emerged as a plausible mechanism of action for these materials even in the presence of GSH. Their low hemolysis percentages indicate promising prospects for future in vivo applications. Furthermore, observations from biofilm inhibition and eradication tests underscored a concentration-dependent relationship between material activity and efficacy. Particularly noteworthy is the outstanding biofilm inhibition exhibited by MSN-AP(10)-PPh3+-Sn against the S. aureus strain, hinting at its remarkable potential in combating bacterial biofilms.
In conclusion, these findings highlight the multifaceted antibacterial properties of the synthesized materials, suggesting their viability for diverse biomedical applications. Further exploration and refinement of these materials hold promise for combating bacterial infections and advancing therapeutic interventions.
In view of the outstanding properties of the studied organotin(IV)-based nanomaterial which are the first materials of this type showing a good degree of eradication in both E.coli and S. aureus biofilms, in our ongoing research, we are focusing our efforts in gaining additional insights on the antibacterial effect of this kind of systems to be applied in preclinical trials. Our current studies focus towards incorporating therapeutic agents aimed at enhancing wound healing. We are investigating whether our materials hold promise in the treatment of chronic wounds, which can arise from diverse underlying health conditions and are highly susceptible to infection due to compromised tissue integrity and impaired immune response. These conditions may stem from diseases such as diabetes mellitus, autoimmune disorders, or peripheral neuropathies. The future study of our innovative materials is focused, therefore, on offering a potential solution to address challenges associated with antibiotic resistance or limited efficacy of conventional antibiotics in the treatment of chronic wounds. In addition, although the toxicity studies show promising properties by the reduction of the toxicity of the tin prodrug after incorporation within the nanostructured materials, additional studies in this context should be undertaken to confirm the potential ability of the materials to be applied in vivo.
Data availability
The data supporting this article have been included as part of the ESI† or directly in the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank funding from the research project PID2022-136417NB-I00 financed by Ministerio de Ciencia, Innovación y Universidades of Spain MCIU/AEI/10.13039/501100011033/ and “ERDF A way of making Europe”, and from the Research Thematic Network RED2022-134091-T financed by Ministerio de Ciencia, Innovación y Universidades of Spain MCIU/AEI/10.13039/501100011033. We would also like to thank Universidad Rey Juan Carlos for the financial support of our research team COMET-NANO with the project M3271.References
- J. W. Bennett and K.-T. Chung, Advances in Applied Microbiology, Academic Press, 2001, 49, 163–184 Search PubMed.
- K. Kaur, P. Barathe, S. Reddy, V. Mathur and V. Kumar, Med. Plants Antimicrob. Ther., 2024, 1–21 Search PubMed.
- D. W. Kim and C. J. Cha, Exp. Mol. Med., 2021, 53, 301–309 CrossRef CAS.
- S. A. McEwen and P. J. Collignon, Microbiol. Spectr., 2018, 6 DOI:10.1128/microbiolspec.arba-0009-2017.
- B. Aslam, M. Khurshid, M. I. Arshad, S. Muzammil, M. Rasool, N. Yasmeen, T. Shah, T. H. Chaudhry, M. H. Rasool, A. Shahid, X. Xueshan and Z. Baloch, Front. Cell. Infect. Microbiol., 2021, 11, 771510 CrossRef CAS PubMed.
- S. Hernando-Amado, T. M. Coque, F. Baquero and J. L. Martínez, Nat. Microbiol., 2019, 4, 1432–1442 CrossRef CAS PubMed.
- N. B. Fernandes, Y. Nayak, S. Garg and U. Y. Nayak, Coord. Chem. Rev., 2023, 478, 214977 CrossRef CAS.
- Y. Wang, Q. Zhao, N. Han, L. Bai, J. Li, J. Liu, E. Che, L. Hu, Q. Zhang, T. Jiang and S. Wang, Nanomedicine, 2015, 11, 313–327 CrossRef CAS PubMed.
- Y. Wang, K. Gou, X. Guo, J. Ke, S. Li and H. Li, Acta Biomater., 2021, 123, 72–92 CrossRef CAS PubMed.
- G. Yin, X. Chen, Q. Xu, X. Yang, P. Zhang and H. Wang, Mater. Today Commun., 2024, 38, 108555 CrossRef CAS.
- D. M. Dereje, A. García, C. Pontremoli, B. González, M. Colilla, M. Vallet-Regí, I. Izquierdo-Barba and N. Barbero, Microporous Mesoporous Mater., 2024, 372, 113096 CrossRef CAS.
- A. Bachvarova-Nedelcheva, Y. Kostova, L. Yordanova, E. Nenova, P. Shestakova, I. Ivanova and E. Pavlova, Molecules, 2024, 29, 2675 CrossRef CAS PubMed.
- S. Wang, L. Fang, H. Zhou, M. Wang, H. Zheng, Y. Wang, M. D. Weir, R. Masri, T. W. Oates, L. Cheng, H. H. K. Xu and F. Liu, Dent. Mater., 2024, 40, 179–189 CrossRef CAS.
- I. Otri, S. Medaglia, R. Martínez-Máñez, E. Aznar and F. Sancenón, Nanomaterials, 2024, 14, 228 CrossRef CAS PubMed.
- Y. Zhao, B. G. Trewyn, I. I. Slowing and V. S. Y. Lin, J. Am. Chem. Soc., 2009, 131, 8398–8400 CrossRef CAS.
- D. Zhao, Q. Huo, J. Feng, B. F. Chmelka and G. D. Stucky, J. Am. Chem. Soc., 1998, 120, 6024–6036 CrossRef CAS.
- S. M. Sadeghzadeh, R. Zhiani and S. Emrani, RSC Adv., 2017, 7, 24885–24894 RSC.
- M. J. Mitchell, M. M. Billingsley, R. M. Haley, M. E. Wechsler, N. A. Peppas and R. Langer, Nat. Rev. Drug Discovery, 2021, 20, 101–124 CrossRef CAS PubMed.
- D. Díaz-García, S. Prashar and S. Gómez-Ruiz, Int. J. Mol. Sci., 2023, 24, 2332 CrossRef.
- S. Kim, N. G. Yoon, B. Jana, B. H. Kang and J. H. Ryu, Bull. Korean Chem. Soc., 2022, 43, 391–395 CrossRef CAS.
- M. Millard, D. Pathania, Y. Shabaik, L. Taheri, J. Deng and N. Neamati, PLoS One, 2010, 5, e13131 CrossRef PubMed.
- A. J. Martín-Rodríguez, J. M. F. Babarro, F. Lahoz, M. Sansón, V. S. Martín, M. Norte and J. J. Fernández, PLoS One, 2015, 10, 123652 CrossRef.
- S. Kang, K. Sunwoo, Y. Jung, J. K. Hur, K.-H. Park, J. S. Kim and D. Kim, Antibiotics, 2020, 9, 758 CrossRef CAS PubMed.
- M. K. Ibrahim, A. Haria, N. V. Mehta and M. S. Degani, Future Med. Chem., 2023, 15, 2113–2141 CrossRef CAS PubMed.
- D. Díaz-García, K. Montalbán-Hernández, I. Mena-Palomo, P. Achimas-Cadariu, A. Rodríguez-Diéguez, E. López-Collazo, S. Prashar, K. Ovejero Paredes, M. Filice, E. Fischer-Fodor and S. Gómez-Ruiz, Pharmaceutics, 2020, 12, 512 CrossRef.
- M. Ugalde-Arbizu, J. J. Aguilera-Correa, V. García-Almodóvar, K. Ovejero-Paredes, D. Díaz-García, J. Esteban, P. L. Páez, S. Prashar, E. San Sebastian, M. Filice and S. Gómez-Ruiz, Pharmaceutics, 2023, 15, 560 CrossRef CAS PubMed.
- A. Pompilio, M. Scocchi, M. L. Mangoni, S. Shirooie, A. Serio, Y. Ferreira Garcia da Costa, M. S. Alves, G. Şeker Karatoprak, I. Süntar, H. Khan and G. Di Bonaventura, Crit. Rev. Microbiol., 2023, 49, 117–149 CrossRef CAS PubMed.
- K. Ovejero Paredes, D. Díaz-García, V. García-Almodóvar, L. Lozano Chamizo, M. Marciello, M. Díaz-Sánchez, S. Prashar, S. Gómez-Ruiz and M. Filice, Cancers, 2020, 12, 187 CrossRef.
- CLSI (Clinical and Laboratory Standards Institute), 2018.
- L. Wang, X. Han, J. Li, L. Qin and D. Zheng, Powder Technol., 2012, 231, 63–69 CrossRef CAS.
- D. Díaz-García, E. Fischer-Fodor, C. I. Vlad, J. M. Méndez-Arriaga, S. Prashar and S. Gómez-Ruiz, Microporous Mesoporous Mater., 2021, 323, 111238 CrossRef.
- B. L. Tee and G. Kaletunç, Biotechnol. Prog., 2009, 25, 436–445 CrossRef CAS PubMed.
- T. C. Brown, A. Bagheri and C. M. Fellows, Langmuir, 2023, 39, 1914–1926 CrossRef CAS.
- Z. Zhang and Z. Yang, Chin. J. Catal., 2013, 34, 1797–1810 CrossRef CAS.
- M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol and K. S. W. Sing, Pure Appl. Chem., 2015, 87, 1051–1069 CrossRef CAS.
- D. Díaz-García, P. R. Ardiles, S. Prashar, A. Rodríguez-Diéguez, P. L. Páez and S. Gómez-Ruiz, Pharmaceutics, 2019, 11, 30 CrossRef.
- K. Ovejero-Paredes, D. Díaz-García, I. Mena-Palomo, M. Marciello, L. Lozano-Chamizo, Y. L. Morato, S. Prashar, S. Gómez-Ruiz and M. Filice, Biomater. Adv., 2022, 137, 212823 CrossRef CAS.
- M. O. Guerrero-Pérez and G. S. Patience, Canadian J. Chem. Eng., 2020, 98, 25–33 CrossRef.
- F. S. Rocha, A. J. Gomes, C. N. Lunardi, S. Kaliaguine and G. S. Patience, Canadian J. Chem. Eng., 2018, 96, 2512–2517 CrossRef CAS.
- Y. Ishii, Y. Nishiwaki, A. Al-zubaidi and S. Kawasaki, J. Phys. Chem. C, 2013, 117, 18120–18130 CrossRef CAS.
- J. Karges, D. Díaz-García, S. Prashar, S. Gómez-Ruiz and G. Gasser, ACS Appl. Bio Mater., 2021, 4, 4394–4405 CrossRef CAS PubMed.
- A. Toranzo, P. S. Bustos, M. G. Ortega, P. L. Páez and C. Lucero-Estrada, J. Appl. Microbiol., 2022, 132, 209–220 CrossRef CAS PubMed.
- T. Chautrand, D. Souak, S. Chevalier and C. Duclairoir-Poc, Microorganisms, 2022, 10, 924 CrossRef CAS PubMed.
- S. K. Priyadarshini, M. Murugesan, R. D. Michael, P. Aiya Subramani and P. Rajendran, Fish Shellfish Immunol., 2023, 142, 109141 CrossRef CAS PubMed.
- Y. F. Huang, Y. Li, J. Y. Chen, J.-H. Lin, L. Liu, J.-Z. Ye and Y.-B. Su, Biochem. Biophys. Res. Commun., 2022, 625, 134–139 CrossRef CAS PubMed.
- M. A. Quinteros, C. A. Viviana, R. Onnainty, V. S. Mary, M. G. Theumer, G. E. Granero, M. G. Paraje and P. L. Páez, Int. J. Biochem. Cell Biol., 2018, 104, 87–93 CrossRef CAS PubMed.
- M. Ugalde-Arbizu, J. J. Aguilera-Correa, A. Mediero, J. Esteban, P. L. Páez, E. San Sebastian and S. Gómez-Ruiz, Pharmaceuticals, 2022, 15, 884 CrossRef CAS PubMed.
- I. Park, A. Jailani, J. H. Lee, B. Ahmed and J. Lee, Pharmaceutics, 2023, 15, 1679 CrossRef CAS.
- A. G. Veiko, E. Olchowik-Grabarek, S. Sekowski, A. Roszkowska, E. A. Lapshina, I. Dobrzynska, M. Zamaraeva and I. B. Zavodnik, Molecules, 2023, 28, 1252 CrossRef CAS.
- P. C. Choudante, J. Mamilla, L. Kongari, D. Díaz-García, S. Prashar, S. Gómez-Ruiz and S. Misra, J. Drug Delivery Sci. Technol., 2024, 94, 105502 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01106f |
| This journal is © The Royal Society of Chemistry 2024 |