Open Access Article
Open Access ArticleMercaptoimidazole-capped gold nanoparticles as a potent agent against plant pathogenic fungi†
Tang
Xu‡
abd,
Wenshuai
Hao‡
cg,
Ran
Du‡
b,
Dai
Dai
e,
Cuixia
Wang
a,
Suhua
Li
b,
Carol Sze Ki
Lin
 f,
Ruitao
Cha
f,
Ruitao
Cha
 *ch,
Jianbin
Yan
*b and
Chong
Li
*ch,
Jianbin
Yan
*b and
Chong
Li
 *a
*a
aKunpeng Institute of Modern Agriculture at Foshan, Shenzhen Branch, Guangdong Laboratory of Lingnan Modern Agriculture, Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences, Shenzhen, China. E-mail: lichonglx@caas.cn
bShenzhen Branch, Guangdong Laboratory for Lingnan Modern Agriculture, Shenzhen Key Laboratory of Agricultural Synthetic Biology, Genome Analysis Laboratory of the Ministry of Agriculture and Rural Affairs, Agricultural Genomics Institute at Shenzhen, Chinese Academy of Agricultural Sciences, Shenzhen, China. E-mail: jianbinlab@caas.cn
cLaboratory of Theoretical and Computational Nanoscience, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology, Beijing 100190, China. E-mail: chart@nanoctr.cn
dSchool of Life Sciences, Henan University, Kaifeng 475004, China
eDepartment of Environmental Systems Science, ETH Zürich, Zurich 8092, Switzerland
fSchool of Energy and Environment, City University of Hong Kong, Hong Kong, China
gBiomanufacturing Center, Department of Mechanical Engineering, Tsinghua University, Beijing 100084, China
hNMPA Key Laboratory for Quality Research and Evaluation of Pharmaceutical Excipients, National Institutes for Food and Drug Control, 2 Tiantan Xi Li, Beijing, 100050, China
First published on 30th September 2024
Abstract
Plant pathogenic fungi pose a substantial challenge to agricultural production, but the conventional fungicide-based approaches are losing importance. As agents with broad-spectrum antibacterial effects, gold nanoparticles (Au NPs) are found to have antifungal effects; however, no study has examined their application in agriculture as fungicides. Accordingly, this study investigates the activity of 2-mercaptoimidazole-capped Au NPs (MI-Au NPs) against the ‘top’ plant pathogenic fungi, finding that they could inhibit Magnaporthe oryzae, Botrytis cinerea, Fusarium pseudograminearum and Colletotrichum destructivum by inducing cytoplasmic leakage. Moreover, MI-Au NPs are found to protect plants from infection by B. cinerea. Specifically, pot experiments demonstrate that MI-Au NPs decrease the incidence rate of B. cinerea infection in Arabidopsis thaliana from 74.6% to 6.2% and in Solanum lycopersicum from 100% to 10.9%, outperforming those achieved by imazalil. Furthermore, the biosafety assays reveal that MI-Au NPs cannot penetrate the cuticle of plant cells or negatively influence plant growth, and it is safe to mammalian cells. In summary, the findings of this study will support the development of NP-based antifungal agents for use in agriculture.
1 Introduction
Plant pathogenic fungi pose a substantial challenge to global agricultural production, causing an annual crop loss of 10–20% that is equal to US$100–$200 billion.1 Plant pathogenic fungi that destroy the most crops are Magnaporthe oryzae, Botrytis cinerea, Fusarium graminearum, Fusarium oxysporum, Colletotrichum spp. and so forth.2 In particular, M. oryzae is a devastating cereal pathogen that causes neck rot and panicle blast, which can cause up to 80% rice loss during severe outbreaks. B. cinerea primarily affects the horticultural crops, leading to annual losses of US$10–$100 billion.3 Currently, broad-spectrum fungicides such as mancozeb and chlorothalonil are employed as control strategies to mitigate fungal damage to crops.4,5 However, over the past 50 years, there has been a concerning increase in the number of plant pathogenic fungi that have developed resistance to fungicides.6 For example, B. cinerea is considered to have a high risk of rapidly developing resistance to fungicides due to the mutations that modify the target gene of fungicides and overexpression of drug efflux transporters.7 Resistance of B. cinerea to new fungicides, such as benzimidazole, dicarboximide, and diethofencarb, has been observed only a few years after their application.8 This has led to the emergence of nanoparticles (NPs) as a promising alternative.Studies have demonstrated that various types of NPs can impede bacterial growth by preventing or overcoming biofilm formation. Silver-based NPs (Ag NPs) and gold-based NPs (Au NPs) have displayed efficacy in this regard by disrupting bacterial cell membranes, generating reactive oxygen species, penetrating bacterial cell membranes, or inducing intracellular antibacterial effects.9 Their diminutive sizes endow them with unique characteristics (e.g., enhanced cellular interaction), and potential for versatile and controllable applications.10 When combined with antibacterial agents such as organic compounds or antibiotics, they exhibit a synergistic effect against pathogenic bacteria.11 Furthermore, Ag NPs have demonstrated activity against a diverse array of plant pathogens, such as B. cinerea, F. oxysporum and Cladosporium cucumerinum.12 The antifungal efficacy of Ag NPs could be even maximized by reducing their size or increasing the concentration.13 Nevertheless, akin to chemical pesticides, the repeated use of Ag NPs can result in microbial resistance.14
Au NPs have gained increasing interest due to their good biocompatibility and ability to effectively exert antimicrobial activity.15,16 Au NPs can be coated with various ligands such as a non-ionic chemical polyethylene glycol, or conjugated with small molecules such as antibiotics. These modifications enhance Au NPs’ stabilities and antimicrobial activities.17 For instance, the combination of N-heterocyclic prodrugs (e.g., chloroauric acid, HAuCl4) with Au NPs can endow the NPs with optimal antibacterial activities.18 4-Amino-2-pyrimidinethiol-capped Au NPs, 2,4-diamino-6-pyrimidinethiol-capped Au NPs, and negatively charged 4,6-dihydroxyl-2-pyrimidinethiol-capped Au NPs could inhibit the growth of clinically isolated multidrug-resistant Escherichia coli and Pseudomonas aeruginosa.19 Functional Au NPs are potent agents against multi-drug-resistant bacteria.20 Studies reported that bacteria take a longer timeframe to develop resistance to Au NPs than to Ag NPs, indicating that resistance to the former involves more complicated mechanisms. Even if the resistance is acquired, the antibacterial activities of Au NPs can be effectively restored by tuning the sizes or functional groups on the surface.16,20 Currently, Au NPs have been applied to combat pathogenic fungi in medical research, finding that they could significantly inhibit the growth of Candida albicans21,22 and Aspergillus spp.,21–23in vitro. However, to the best of our knowledge, no study has ever examined the activity of Au NPs against plant pathogenic fungi or reported their applications in agriculture.
Following the previous studies,18,19 the objectives of this study were to investigate whether the functionalized Au NPs, with effective antibacterial efficacy, were active against plant pathogenic fungi and to evaluate their applications in agriculture. First, the effects of 2-mercaptoimidazole (MI)-capped Au NPs (MI-Au NPs) and 4,6-diamino-2-pyrimidinethiol (DAPT)-capped Au NPs (D-Au NPs) on selected plant pathogenic fungi including B. cinerea, M. oryzae, F. pseudograminearum, C. destructivum, F. oxysporum, and F. graminearum were investigated in disk diffusion tests, with ‘naked’ Au NPs (N-Au NPs) and imazalil being used as the controls. Next, scanning electron microscopy (SEM), transmission electron microscopy (TEM), and laser scanning confocal microscopy (LSCM) were applied to explore the mechanisms of Au NPs’ antifungal action. Subsequently, detached leaf and pot experiments were carried out to evaluate the ability of MI-Au NPs to protect plants from infection with B. cinerea. Finally, the biological safety of MI-Au NPs was evaluated by examining their effect on plant growth and development, and on human umbilical vein endothelial cells (HUVECs) (Fig. 1).
2 Experimental
2.1 Materials
Chloroauric acid trihydrate (HAuCl4·3H2O) and sodium borohydride (NaBH4) were purchased from Merck (Shanghai, China). 4,6-Diamino-2-pyrimidinethiol (DAPT) and 2-mercaptoimidazole (MI) were purchased from Aladdin (Shanghai, China). Imazalil was purchased from Glpbio (USA). Oatmeal agar (OA) medium, potato dextrose agar (PDA) medium, and potato dextrose broth (PDB) medium were purchased from Coolaber (Beijing, China). Solid Murashige and Skoog (MS) medium was purchased from Sigma-Aldrich (USA). Propidium iodide (PI), disodium ethylenediaminetetraacetic acid (Na2-EDTA), and nitroblue tetrazolium (NBT) were purchased from Coolaber (Beijing, China). Phosphate buffered saline (PBS), glutaraldehyde, osmium tetroxide (OsO4), and fetal bovine serum (FBS) were purchased from Sigma-Aldrich (USA). Dulbecco's modified Eagle's medium (DMEM) was purchased from VivaCell (Shanghai, China). Penicillin–streptomycin solution was purchased from Biological Industries (Israel).S. lycopersicum ‘Ailsa Craig’ (AC) seeds, A. thaliana (Columbia-0) seeds, and B. cinerea were kept at the Agricultural Genomics Institute, Chinese Academy of Agricultural Sciences (Shenzhen, China). M. oryzae was kindly provided by Dr Qun Liu of the China National Rice Research Institute (Hangzhou, China). F. pseudograminearum, C. destructivum, F. oxysporum, and F. graminearum were kindly provided by Dr Chengqi Zhang of Anhui Agricultural University (Hefei, China).
2.2 Preparation and characterization of NPs
MI-Au NPs, D-Au NPs, and N-Au NPs were prepared according to the literature.18,19 Specifically, MI (0.1 mmol, dissolved in 10 mL of methanol, 200 μL of absolute acetic acid and 40 mg of Tween 80) and HAuCl4·3H2O (0.1 mmol, dissolved in 20 mL of methanol) were mixed and stirred in an ice-water bath for 10 mins. NaBH4 (0.3 mmol, dissolved in 5 mL of methanol) was then added dropwise with vigorous stirring. When the solution turned deep red, we decreased the stirring speed but kept stirring for another hour in the ice-water bath. Subsequently, we removed methanol in a vacuum at 40 °C, and an appropriate volume of deionized water was added to the residue. The solution was dialyzed (14 kDa molecular-weight cutoff, Millipore) for 48 h with deionized water, diluted with water and sterilized through a 0.22 μm filter (Millipore). The filtrate was stored at 4 °C until required. D-Au NPs and N-Au NPs were prepared in an analogous fashion; a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio of DAPT to HAuCl4·3H2O was used for the preparation of D-Au NPs, whereas N-Au NPs did not contain ligands.
1 molar ratio of DAPT to HAuCl4·3H2O was used for the preparation of D-Au NPs, whereas N-Au NPs did not contain ligands.
The Au concentrations of all NPs were measured and determined by inductively coupled plasma-optical emission spectrometry (ICP-OES, Optima 5300DV, Perkin-Elmer, USA). First, the gold standard solution (1000 μg mL−1, GSB 04-1715-2004, Guobiao Testing & Certification Co., Ltd, Beijing) was stepwise diluted to obtain solutions with selected concentrations (0.1, 1, 5, 10, and 25 μg mL−1). The solution samples were then introduced to the plasma via a standard cross-flow nebulizer and Scott spray chamber (RF generator has a forward power of 1300 W with plasma, auxiliary and nebulizer gases set at 15, 0.5 and 0.8 L min−1, respectively). A 0.1 s integration time with a fixed read time of 20 s and 5 points per peak provides consistent counting statistics (emission line at 242.80 nm). A standard curve describing the relationship between Au concentration and signal intensity was drawn with an R square greater than 0.996. Afterwards, Au NP samples were dissolved in freshly prepared aqua regia, diluted to 10 mL with ultrapure water and measured by ICP-OES under the same operation conditions. The concentrations of Au NPs were calculated according to the standard curve. The hydrodynamic sizes, size distributions, and zeta potentials of NPs were determined using a Zetasizer Nano ZS (Malvern, UK). The morphologies of NPs were obtained by TEM (HT-7700, Hitachi, Japan) according to the literature.24 All measurements were performed three times.
2.3 Preparation of fungal spores and plants
Several of the reported top fungal pathogens in molecular plant pathology2 were selected in this study. M. oryzae was cultivated on OA medium at 28 °C in darkness for 3 days, grown upright at 25 °C in light for 4 days, and then incubated at 28 °C in darkness for 10–12 days. B. cinerea was cultured on PDA medium at 23 °C in darkness for 10–12 days. F. pseudograminearum, F. oxysporum, and F. graminearum were cultured on PDA at 28 °C in darkness for 5–7 days, whereas C. destructivum was cultured on PDA at 28 °C in darkness for 12–14 days. All fungi were cultured statically until sufficient spores could be collected.As one of the most commonly used model plants, A. thaliana (Columbia-0) was selected as a research object here. As one of the most popular vegetables,25S. lycopersicum was also chosen as the research object. A. thaliana seeds were soaked in 70% ethanol for 10 minutes and 5% sodium hypochlorite for 5 minutes and washed several times in sterile water. The seeds were then placed onto solid MS medium (containing 1% sucrose) and kept at 4 °C in darkness for 2 days for devernalization. Afterwards, the seeds were moved to an illumination incubator and cultivated at 21–23 °C under a 16 h light/8 h dark photoperiod for approximately 7 days. After germination, seedlings were transplanted into pots containing a mixture of vermiculite and peat soil (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) and cultivated for 5 weeks before use. Similar procedures but without devernalization were carried out for S. lycopersicum cultivation.
2) and cultivated for 5 weeks before use. Similar procedures but without devernalization were carried out for S. lycopersicum cultivation.
2.4 Disk diffusion tests
Disk diffusion tests were performed according to the standard procedures published by the Clinical and Laboratory Standards Institute.26,27 Briefly, approximately five colonies of a fungus that were at least 1 mm in diameter were collected and then suspended in sterile distilled water. The turbidity of the resulting suspension was then adjusted to 0.5 McFarland standard at a wavelength of 530 nm, and diluted tenfold with sterile distilled water to obtain the spore suspension with a concentration of 5 × 106 spores per mL. Being dipped in the spore suspension for 5 s, a cotton swab was streaked three times across the PDA or OA medium of plates. About 10 minutes later, paper disks with Au NPs, imazalil or sterile water were placed on the surface of the media, and the plates were incubated at 28 °C for 6–7 days. Finally, the plates with the inhibition zone were photographed, while the zone areas were measured using ImageJ software. Three replicates were performed for each treatment.2.5 Observations of fungi
A PI staining experiment was conducted to explore the effect of MI-Au NPs on the integrity of the cell membrane of B. cinerea. First, B. cinerea spore suspensions with a concentration of 1 × 107 spores per mL were cultured in PDB medium mixed with MI-Au NPs (0–200 μg mL−1) at 28 °C and 220 rpm for 12 h. The solutions were then centrifuged at 8000 rpm for 3 mins and washed with 10 mmol L−1 PBS twice. We incubated the cell suspensions with an equal volume of the PI solution (3 μM in PBS) at 28 °C in darkness for 30 minutes and washed them with PBS twice, and 20 μL of samples were placed on a glass slide with a glass coverslip. The samples were observed using a LSCM (Leica Stellaris 8, Germany) in the fluorescence and differential interference contrast (DIC) mode, with excitation at 535 nm and emissions at 605–700 nm.19,28 The cells penetrated by MI-Au NPs were stained with PI.The influence of MI-Au NPs on the fungal morphology and ultrastructure was examined by TEM, according to the literature.19 After co-cultivation with 100 μg mL−1 MI-Au NPs for 6 days, B. cinerea samples from the edge of the inhibition zone were collected and fixed with 2.5% glutaraldehyde for 3–4 h, washed twice with PBS, and further fixed with 1% OsO4 for 2 h. We then washed the samples three times with PBS, dehydrated them by successive washing with 30%, 50%, 70%, 90%, 95%, and 100% in ethanol (v/v, in water), and 50% ethanol in acetone for 10 minutes each time, and finally dehydrated them with pure acetone twice for 10 minutes. The dehydrated samples were embedded in SPI-Pon 812 resin (Sigma-Aldrich, USA) and polymerized at 60 °C for 24 h. The embedded samples were then cut into super-thin slices, placed on Formvar-coated grids and observed by TEM at an accelerating voltage of 80 kV and using EDS (Tecnai G2 F20 U-TWIN, FEI, USA).
2.6 Fungal infection assay
Detached leaf experiments and pot experiments were performed to test the ability of MI-Au NPs to protect plants (i.e., A. thaliana and S. lycopersicum) against fungal infection. Among the fungi susceptible to MI-Au NP treatment, B. cinerea can infect more than 200 kinds of plants and cause an annual loss of US$10–$100 billion,29,30 thus making it the second fungal pathogen in molecular plant pathology.2 Meanwhile, B. cinerea can infect the plants used in this study with obvious pathogenic characteristics (i.e., leaf lesion) and high morbidity stability. The methods for its infection analysis have been established, so it is selected for the plant infection study. Data obtained from plant experiments were derived from at least ten biologically independent groups.In the detached leaf experiment, 5-week-old plants were first sprayed with 10 mL of MI-Au NPs (100 μg mL−1), imazalil (100 μg mL−1) or mock solution (0.05% Tween 20). Two days later, the 7th–9th rosette leaves of A. thaliana were collected and placed on 0.8% agar plates. Afterwards, the leaves were inoculated with 10 μL of B. cinerea spore suspension (1.0 × 105 spores mL−1) or PDB, cultured at 60% humidity in darkness for 3–4 days, and photographed. Lesion areas caused by B. cinerea were measured using ImageJ software. The experimental processes for S. lycopersicum were similar, except that leaves were collected from the terminal positions of the 5-week-old lateral branches.29,31,32
In the pot experiments, 5-week-old plants were first sprayed with 10 mL of MI-Au NPs (100 μg mL−1), imazalil (100 μg mL−1) or mock solution (0.05% Tween 20). Two days later, they were sprayed with 10 mL of B. cinerea spore suspension (0.5–1.0 × 106 spores per mL) or PDB, and then cultivated in a growth chamber at 21–23 °C (60% humidity) with a 10 h light/14 h dark cycle for 3–5 days. Finally, the phenotypes of plants were photographed, and disease incidence was calculated as the number of infected leaves per plant divided by the total number of leaves per plant.29,33
2.7 Preliminary safety assessment of MI-Au NPs
Five days after the spraying of MI-Au NPs (100 μg mL−1), leaves of the 5-week-old A. thaliana were collected, dried and fixed onto aluminum stubs using high-vacuum carbon adhesive. Next, they were coated with a platinum film34 and examined by SEM equipped with EDS (S-4800, Hitachi, Japan). Leaves to be examined by TEM were cleaned, dried, and sectioned into approximately 1 mm2 fragments.35 These fragments were immersed in PBS containing 2.5% glutaraldehyde (pH = 7 and 0.1 mol L−1) for 3.5 h and in 1% OsO4 for 2 h, dehydrated by successive washing with 50%, 70%, 80%, 90%, 95%, and 100% ethanol (v/v, in water) and 100% acetone for 10 minutes each time. Then, they were embedded in epoxy resin, ultra-sectioned to 60–80 nm using an ultramicrotome (Leica UC7, Germany), and strained with uranyl acetate and lead citrate for 15 minutes. The stained sections were fixed onto nickel grids for TEM analysis.32,34,36S. lycopersicum seeds were washed twice with ultrapure water, disinfected in 20% sodium hypochlorite for 30 minutes, and rinsed successively with an appropriate volume of deionized Na2-EDTA (20 mmol mL−1) and ultrapure water. The seeds were then immersed in MI-Au NP solutions (0, 10, 50, 100, and 200 μg mL−1) for 4 hours, and placed onto the filter papers with corresponding concentrations of MI-Au NPs. Afterwards, they were cultivated at 25 °C (80% humidity) in darkness, and the germination rate was calculated 7 days later.37
When having two true leaves, S. lycopersicum seedlings were transplanted into nursery pots. Seven days later, the seedlings were sprayed with MI-Au NPs (0–200 μg mL−1), twice every day for 15 days. Subsequently, the chlorophylls and carotenoid contents of the seedlings were determined using an 80% acetone method, whereas the superoxide dismutase (SOD) activity was measured using the NBT method.38
Cell viability was determined using Cell Counting Kit-8 (CCK-8), according to the manufacturer's protocol (Beyotime, Shanghai, China). Specifically, cryopreserved HUVECs were activated and cultured in the cell complete medium (CCM) that is composed of 89% DMEM, 10% FBS, and 1% penicillin–streptomycin solution at 37 °C in 5% CO2 for 24 h. Subsequently, the HUVECs were harvested, resuspended in CCM, and transferred to a 96-well plate to give a concentration of 1 × 104 cells per well. The cells were incubated at 37 °C in 5% CO2 overnight. Afterwards, the CCM in the wells was replaced by MI-Au NPs with selected concentrations (12.5 to 200 μg mL−1), and the HUVECs were further cultured statically for 24 h. Finally, the MI-Au NP solutions were removed and replaced by CCK-8 solution (10% in CCM), and the cells were incubated at 37 °C in 5% CO2 for 2 h before microscopic observation. The morphology of HUVECs was observed using a fully automated inverted optical microscope (IX83P2ZF, Olympus, Japan). Except that the HUVECs were not treated by MI-Au NPs, operations to the control groups were the same as the experimental groups. Wells with only CCK-8 solution were used as the blank. These experiments were performed six times.39
2.8 Statistical analysis
The percentage of penetrated cells was determined as the mean of PI-stained spores under three microscopic vision fields of two independent experiments (each field contained 30–100 cells), as described in Eqn (1): | (1) |
The viability of HUVECs was determined from the measurement of their OD450 values, as described below:
 | (2) |
In this study, comparisons among multiple experimental groups were conducted using a one-way ANOVA with Tukey's multiple range test or Student's t test. Data were shown in the form of mean ± standard deviation.
3 Results and discussion
3.1 Synthesis and characterization of Au NPs
Au NPs were characterized by TEM, ICP-OES, and dynamic light scattering and zeta potential measurements. The TEM images showed that MI-Au NPs, D-Au NPs, and N-Au NPs were morphologically regular and formed uniform dispersions (Fig. 2a–c). The concentrations of MI-Au NPs, D-Au NPs, and N-Au NPs were 232.5, 770.5, and 762.3 μg mL−1, respectively. Their average hydrodynamic sizes were 8.04 ± 0.06, 11.08 ± 0.08, and 6.10 ± 0.13 nm, respectively (Fig. 2d–f). The zeta potentials of MI-Au NPs, D-Au NPs, and N-Au NPs were 14.8, 17.8, and −17.6 mV, demonstrating that the surfaces of these small molecule ligand-modified Au NPs were positively charged (Fig. 2g–i).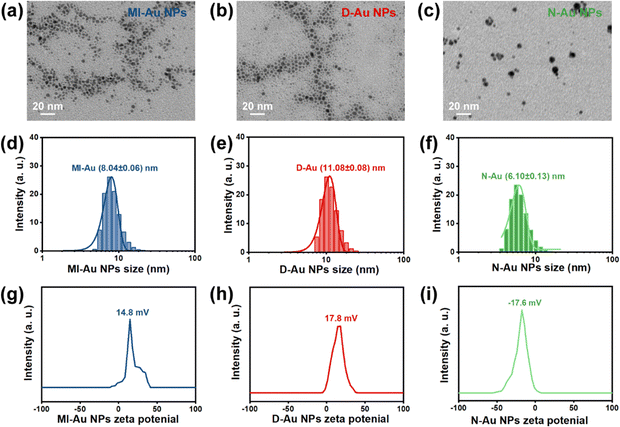 | ||
| Fig. 2 The characterization of Au NPs. (a)–(c) The TEM images of Au NPs. (d)–(f) The hydrodynamic sizes of Au NPs. (g)–(i) The zeta potentials of Au NPs. | ||
3.2 Inhibitory effect of Au NPs on plant pathogenic fungi
The influence of MI-Au NPs, D-Au NPs, and N-Au NPs on Magnaporthe oryzae, Botrytis cinerea, Colletotrichum destructivum, Fusarium oxysporum, Fusarium pseudograminearum, and Fusarium graminearum were performed by disk diffusion tests, investigating the antifungal activity of Au NPs with different functional ligands. According to Fig. 3, all fungi grew well on the PDA plates with paper disks (containing N-Au NPs), suggesting that the fungi were not affected by the NPs. Similar to N-Au NPs, D-Au NPs did not inhibit the growth of any fungi, even at a concentration of 50 μg mL−1. In contrast, the hyphal growth of M. oryzae, B. cinerea, F. pseudograminearum and C. destructivum was inhibited when MI-Au NPs was applied, even at a low concentration of 5 μg mL−1 (middle panel of Fig. 3 and Fig. S1, ESI†). This indicates that Au NPs capped with 2-mercaptoimidazole (MI) have stronger antifungal activity than that capped with 4,6-diamino-2-pyrimidinethiol (DAPT), which agrees with the research that groups on the surface can affect the function of Au NPs directly.16,20 MI is reported to be a potential lead molecule for the synthesis of compounds with good antifungal activity.40–43 Meanwhile, the smaller particle size and positive charge of MI-Au NPs can also contribute to its greater antifungal activity than the other two.44,45 | ||
| Fig. 3 Inhibitory effects of Au NPs on selected plant pathogenic fungi (C0 = 0 μg mL−1, C5 = 5 μg mL−1, C10 = 10 μg mL−1, C25 = 25 μg mL−1, and C50 = 50 μg mL−1). | ||
Next, we compared the activities of MI-Au NPs and a traditional chemical fungicide (i.e., imazalil) against the four selected plant pathogenic fungi. In line with the results of the previous experiment, MI-Au NPs stably inhibited the hyphal growth of the fungi at all concentrations (Fig. 4). In particular, the inhibition zones of F. pseudograminearum and C. destructivum were the largest when 100 μg mL−1 MI-Au NPs was used (Fig. S2, ESI†). In comparison, imazalil did not inhibit the growth of the fungi at the commonly used concentration of 50 μg mL−1.46 In fact, the inhibition of M. oryzae and B. cinerea by imazalil was only observed when its concentration reached 200 μg mL−1, which is the highest concentration ever recommended in research.47 Nevertheless, the application of imazail at this concentration did not impede the growth of F. pseudograminearum and C. destructivum.
 | ||
| Fig. 4 Inhibitory effects of MI-Au NPs and imazalil on selected plant pathogenic fungi (C0 = 0 μg mL−1, C10 = 10 μg mL−1, C50 = 50 μg mL−1, C100 = 100 μg mL−1, and C200 = 200 μg mL−1). | ||
3.3 Antifungal mechanisms of MI-Au NPs
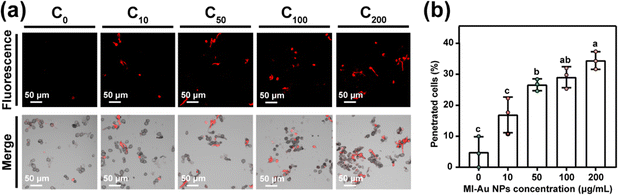
In order to elucidate how the MI-Au NPs inhibited the fungi, the effect of MI-Au NPs on the integrity of B. cinerea was probed by staining cells with propidium iodide (PI) and then examined by LSCM.28,48 As shown in Fig. 5a, when observed in the fluorescence mode, the amount of red stained B. cinerea cells (mainly spores) and their debris increased as the concentration of MI-Au NPs used increased from 10 μg mL−1 to 200 μg mL−1, whereas there was almost no red color observed in the group without MI-Au NPs. Pictures in the merge mode (overlay of fluorescence and DIC images) supported this observation. As PI can stain nucleic acids but cannot penetrate the membrane of viable cells,28 the above-described results indicated that the red-colored cells were not intact, confirming that MI-Au NPs disrupted the cell membrane of B. cinerea. Importantly, with an increase in the concentration of NPs from 10 μg mL−1 to 200 μg mL−1, the proportion of penetrated spores increased from 16.9% to 34.4% (p < 0.001) (Fig. 5b). This suggests that MI-Au NPs alter the membrane integrity in a dose-dependent manner.Next, TEM was used to directly visualize the change in the morphology of the B. cinerea membrane and the leakage of nucleic acids upon treatment with MI-Au NPs. Untreated cells had normal morphologies with an orderly and intact plasma membrane (Fig. 6a and b). However, cells treated with 100 μg mL−1 MI-Au NPs had been destroyed, and some spores had abnormal morphologies (Fig. 6c), which resulted in cytoplasmic leakage (Fig. 6d). This is consistent with the results of the PI staining experiment. Moreover, the mixtures of the leaked cytoplasm and MI-Au NPs appeared as a clustered dot structure, indicating that MI-Au NPs may have interacted with nucleic acids.19,49 The distribution of Au within the spores of B. cinerea was confirmed by energy dispersive spectrometry (EDS) (Fig. 6e and f), indicating that MI-Au NPs can pass through the cell wall and membrane of B. cinerea. The damage to B. cinerea caused by MI-Au NPs is similar to that caused by cationic antibiotics such as polymyxin and gentamicin,50,51 suggesting that MI-Au NPs may have a broad-spectrum antifungal activity.
3.4 Application of MI-Au NPs as antifungal agents in plants
Detached leaves that were pretreated with mock solution, MI-Au NPs and imazalil, but not inoculated with B. cinerea spores were green and healthy (left panel of Fig. 7a), showing that the leaves were not negatively affected by these pretreatments. In comparison, leaves inoculated with B. cinerea spores turned yellow (right panel of Fig. 7a) and had rather large lesions. A 100% incidence rate of B. cinerea to the leaves was observed, demonstrating the strong pathogenicity of B. cinerea to A. thaliana. However, leaves that had been pretreated with MI-Au NPs prior to inoculation with B. cinerea spores remained generally green, and the incidence rate was approximately 70% less than that of the mock group. Similarly, the average lesion area on the MI-Au NP-pretreated leaves (0.062 cm2) was 85.4% less than that on the mock-solution-pretreated leaves (0.425 cm2, Fig. 7b; p < 0.001), demonstrating that MI-Au NPs could protect A. thaliana from B. cinerea infection. Imazalil could also protect leaves from B. cinerea infection, and the average lesion area of imazalil-pretreated leaves was 99.3% less than that of mock-solution-pretreated leaves (Fig. 7b).
Consistent with the A. thaliana experiment results, the treatment of the agents did not have any negative effects on the leaves of S. lycopersicum (left panel of Fig. 7c). Moreover, among the leaves being infected with B. cinerea, the average lesion area on the mock solution-pretreated leaves was much larger than that on A. thaliana leaves (Fig. 7b and d). Furthermore, lesions appeared on all leaves 3 days after inoculation with B. cinerea, irrespective of whether the leaves had been pretreated with the antifungal agents (right panel of Fig. 7c), indicating that B. cinerea was more pathogenic to S. lycopersicum than to A. thaliana. Nevertheless, the average lesion areas on the MI-Au NP- and imazalil-pretreated leaves were much smaller than the mock-solution-pretreated leaves (both p < 0.001, Fig. 7d).
With regard to S. lycopersicum (left panel of Fig. 8c), B. cinerea infection caused more serious disease than that in A. thaliana (Fig. 8a and c). Most of the S. lycopersicum leaves were wilted with large grey lesions on them, and the disease incidence rate was almost 100% (Fig. 8b and d). This is consistent with the detached leaf experiment results and confirms the strong pathogenicity of B. cinerea towards S. lycopersicum plants. Pretreatment of S. lycopersicum plants with imazalil reduced necrosis of the leaves caused by B. cinerea infection, and the disease incidence of 25.5% further indicated the effective protection of imazalil for S. lycopersicum plants. More importantly, the pretreatment of S. lycopersicum plants with MI-Au NPs significantly reduced the necrosis of leaves caused by B. cinerea infection. The leaves remained lush and green, and the disease incidence rate was only 10.9% (p < 0.001), which was 10.9% and 42.7% of that in the mock- and imazalil-pretreated plants, respectively (Fig. 8d).
In general, MI-Au NPs were demonstrated to be effective in protecting plants from B. cinerea infection. The protection effect was especially obvious on S. lycopersicum, even compared with the results achieved by imazalil. This may owe to the differences between the leaf surfaces of the two plants: the trichomes on the surface of S. lycopersicum leaves are denser and longer than that of A. thaliana leaves.52–54 Therefore, S. lycopersicum leaves are more hydrophobic than A. thaliana leaves, which could impede the effective contact of imazalil solution to S. lycopersicum leaves and reduce the residence time on them. However, NPs could effectively come into contact with the hydrophobic leaf surfaces.55
3.5 Biotoxicity of MI-Au NPs
The biocompatibility of Au NPs should be confirmed to support their application in agriculture. Thus, we explored whether MI-Au NPs affected the growth or development of plants and HUVECs, respectively.As one of the indicators to evaluate the phytotoxicity of chemical substances,35,37,57 the influence of MI-Au NPs on plant germination was evaluated. The results showed that the germination of S. lycopersicum seeds was not inhibited by MI-Au NPs: all seeds germinated within 7 days, regardless of the concentration of MI-Au NPs applied (Fig. 9c and Fig. S3, ESI†). In contrast, NPs were previously reported to increase the germination and growth of wheat.58
As indicators to evaluate the plant's response to biotic and abiotic stresses, the effect of MI-Au NP treatment on the accumulation of chlorophylls and carotenoids, and the superoxide dismutase (SOD) activity of plants was measured.37 As shown in Fig. 9d and e, MI-Au NP-treatment caused changes in the content of chlorophylls from 1.5 mg g−1 to 2.0 mg g−1, whereas the contents of carotenoids stabilized at approximately 0.4 mg g−1. However, the contents were not significantly different from those observed in the control groups. As shown in Fig. 9f, plants treated with 50–100 μg mL−1 MI-Au NPs exhibited a 38.4% increase in SOD activity.
4 Conclusions
This study presents the first evidence that Au NPs could be a viable alternative for the management of plant pathogenic fungi, as they could specifically inhibit the activity of fungi by destroying their morphology without damage to the plants. MI-Au NPs exhibited a strong inhibitory effect against the selected ‘top’ plant pathogenic fungi and effectively prevented the infection of B. cinerea to the plants of A. thaliana and S. lycopersicum. The good biocompatibility of MI-Au NPs further demonstrated their safety for use as antifungal agents in labs. In general, this study has important implications for the development of nanoparticle-based antifungal agents to solve the drug-resistant problem of fungi and may promote green and high-quality agricultural development.Author contributions
Tang Xu and Wenshuai Hao: conceptualization, validation, writing – original draft, writing – review & editing, and formal analysis. Ran Du: methodology, visualization, and writing – review & editing. Dai Dai, Cuixia Wang and Carol Sze Ki Lin: writing – review & editing, and validation. Suhua Li: methodology, investigation, and validation. Chong Li, Jianbin Yan and Ruitao Cha: conceptualization, data curation, writing – review & editing, formal analysis, project administration, resources, and supervision.Data availability
The data that support the findings of this study are available from the corresponding author (C. Li) upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge funds from the National Key R&D Program of China (2023ZD04076), the Science and Technology Innovation Project of Foshan (2320001006040), and the Scientific Research Foundation for Principal Investigator (KIMA-QD2022005) of Kunpeng Institute of Modern Agriculture at Foshan, Chinese Academy of Agricultural Sciences. Many thanks to Prof. Qun Liu of the China National Rice Research Institute and Dr Chengqi Zhang from Anhui Agricultural University for providing fungi used in this study.References
- U.S. Department of Agriculture, https://www.ars.usda.gov/oc/dof/food-security-how-do-crop-plants-combat-pathogens/, (accessed 25 April 2024).
- R. Dean, J. a L. Van Kan, Z. A. Pretorius, K. E. Hammond-Kosack, A. Di Pietro, P. D. Spanu, J. J. Rudd, M. Dickman, R. Kahmann, J. Ellis and G. D. Foster, Mol. Plant Pathol., 2012, 13, 414–430 CrossRef PubMed.
- L. Boddy, Pathogens of Autotrophs, in The Fungi, ed. S. C. Watkinson, L. Boddy and N. P. Money, Academic Press, Boston, 2016, pp. 245–292 Search PubMed.
- M. L. Gullino, F. Tinivella, A. Garibaldi, G. M. Kemmitt, L. Bacci and B. Sheppard, Plant Dis., 2010, 94, 1076–1087 CrossRef CAS PubMed.
- S. K. Chen, C. A. Edwards and S. Subler, Soil Biol. Biochem., 2001, 33, 1971–1980 CrossRef CAS.
- D. W. Hollomon, Plant Prot. Sci., 2015, 51, 170–176 CrossRef CAS.
- M. Hahn, J. Chem. Biol., 2014, 7, 133–141 CrossRef PubMed.
- H. J. Rosslenbroich and D. Stuebler, Crop Prot., 2000, 19, 557–561 CrossRef CAS.
- L. Wang, C. Hu and L. Shao, Int. J. Nanomed., 2017, 12, 1227–1249 CrossRef CAS PubMed.
- A. J. Huh and Y. J. Kwon, J. Controlled Release, 2011, 156, 128–145 CrossRef CAS PubMed.
- T. Bruna, F. Maldonado-Bravo, P. Jara and N. Caro, Int. J. Mol. Sci., 2021, 22, 7202 CrossRef CAS PubMed.
- S. W. Kim, J. H. Jung, K. Lamsal, Y. S. Kim, J. S. Min and Y. S. Lee, Mycobiology, 2012, 40, 53–58 CrossRef CAS PubMed.
- I. Akpinar, M. Unal and T. Sar, SN Appl. Sci., 2021, 3, 506 CrossRef CAS.
- A. Panáček, L. Kvítek, M. Smékalová, R. Večeřová, M. Kolář, M. Röderová, F. Dyčka, M. Šebela, R. Prucek, O. Tomanec and R. Zbořil, Nat. Nanotechnol., 2018, 13, 65–71 CrossRef PubMed.
- X. Gu, Z. Xu, L. Gu, H. Xu, F. Han, B. Chen and X. Pan, Environ. Chem. Lett., 2021, 19, 167–187 CrossRef CAS.
- W. Zheng, Y. Jia, Y. Zhao, J. Zhang, Y. Xie, L. Wang, X. Zhao, X. Liu, R. Tang, W. Chen and X. Jiang, Nano Lett., 2021, 21, 1992–2000 CrossRef CAS PubMed.
- C. Tao, Lett. Appl. Microbiol., 2018, 67, 537–543 CrossRef CAS PubMed.
- Y. Feng, W. Chen, Y. Jia, Y. Tian, Y. Zhao, F. Long, Y. Rui and X. Jiang, Nanoscale, 2016, 8, 13223–13227 RSC.
- Y. Zhao, Y. Tian, Y. Cui, W. Liu, W. Ma and X. Jiang, J. Am. Chem. Soc., 2010, 132, 12349–12356 CrossRef CAS PubMed.
- X. Li, S. M. Robinson, A. Gupta, K. Saha, Z. Jiang, D. F. Moyano, A. Sahar, M. A. Riley and V. M. Rotello, ACS Nano, 2014, 8, 10682–10686 CrossRef CAS PubMed.
- R. Krishnamoorthi, S. Bharathakumar, B. Malaikozhundan and P. U. Mahalingam, Biocatal. Agric. Biotechnol., 2021, 35, 102107 CrossRef CAS.
- A. Almansob, A. H. Bahkali and F. Ameen, Nanomaterials, 2022, 12, 814 Search PubMed.
- R. Kalaivani, M. Maruthupandy, T. Muneeswaran, M. Singh, S. Sureshkumar, M. Anand, C. M. Ramakritinan, F. Quero and A. K. Kumaraguru, Int. J. Biol. Macromol., 2020, 146, 560–568 CrossRef CAS PubMed.
- R. A. D. Arancon, S. H. T. Lin, G. Chen, C. S. K. Lin, J. Lai, G. Xu and R. Luque, RSC Adv., 2014, 4, 17114–17119 Search PubMed.
- J. Zhang, S. Liu, X. Zhu, Y. Chang, C. Wang, N. Ma, J. Wang, X. Zhang, J. Lyu and J. Xie, Plants, 2023, 12, 2947 CrossRef CAS PubMed.
- R. M. Humphries, J. Ambler, S. L. Mitchell, M. Castanheira, T. Dingle, J. A. Hindler, L. Koeth and K. Sei, J. Clin. Microbiol., 2018, 56, e01934-17 CrossRef PubMed.
- E. L. Berkow, S. R. Lockhart and L. Ostrosky-Zeichner, Clin. Microbiol. Rev., 2020, 33, e00069-19 CrossRef PubMed.
- W. Yuan, P. Tian, A. Lyu, Y. Lyu, W. Zhang, S. Wei and Y. Hu, Grain Oil Sci. Technol., 2020, 3, 1–8 CrossRef.
- Y. Li, S. Li, R. Du, J. Wang, H. Li, D. Xie and J. Yan, Front. Plant Sci., 2021, 12, 628328 CrossRef PubMed.
- S. AbuQamar, K. Moustafa and L. S. Tran, Crit. Rev. Biotechnol., 2017, 37, 262–274 CrossRef CAS PubMed.
- S. Song, H. Huang, H. Gao, J. Wang, D. Wu, X. Liu, S. Yang, Q. Zhai, C. Li, T. Qi and D. Xie, Plant Cell, 2014, 26, 263–279 CrossRef CAS PubMed.
- M. El-Shetehy, A. Moradi, M. Maceroni, D. Reinhardt, A. Petri-Fink, B. Rothen-Rutishauser, F. Mauch and F. Schwab, Nat. Nanotechnol., 2021, 16, 344–353 CrossRef CAS PubMed.
- X. Cao, C. Wang, X. Luo, L. Yue, J. C. White, W. Elmer, O. P. Dhankher, Z. Wang and B. Xing, ACS Nano, 2021, 15, 11817–11827 CrossRef CAS PubMed.
- J. He, L. Zhang, S. Y. He, E. T. Ryser, H. Li and W. Zhang, Environ. Pollut., 2022, 292, 118448 CrossRef CAS PubMed.
- X. Wang, X. Liu, X. Yang, L. Wang, J. Yang, X. Yan, T. Liang, H. Chr Bruun Hansen, B. Yousaf, S. M. Shaheen, N. Bolan and J. Rinklebe, Ecotoxicol. Environ. Saf., 2022, 242, 113939 CrossRef CAS PubMed.
- M. Hassanisaadi, A. H. S. Bonjar, A. Rahdar, R. S. Varma, N. Ajalli and S. Pandey, Mater. Today Commun., 2022, 33, 104183 CrossRef CAS.
- X. Zhao, W. Zhang, Y. He, L. Wang, W. Li, L. Yang and G. Xing, Chemosphere, 2021, 263, 127943 CrossRef CAS PubMed.
- J. F. Gao, Experimental Guidance for Plant Physiology, Higher Education Press, Beijing, China, 2006 Search PubMed.
- K. Cheng and M. Hao, Int. J. Mol. Sci., 2016, 17, 2000 CrossRef PubMed.
- N. Rani and R. Dahiya, Curr. Comput.-Aided Drug Des., 2017, 13, 48–56 CrossRef CAS PubMed (9).
- N. Rani and R. Singh, Curr. Comput.-Aided Drug Des., 2019, 15, 409–420 CrossRef CAS PubMed.
- N. Rani, P. Kumar and R. Singh, Comb. Chem. High Throughput Screening, 2019, 22, 89–96 CrossRef CAS PubMed.
- N. Rani and R. Singh, Lett. Drug Des. Discovery, 2019, 16, 512–521 CrossRef CAS.
- P. Rajiv, S. Rajeshwari and R. Venckatesh, Spectrochim. Acta, Part A, 2013, 112, 384–387 CrossRef CAS PubMed.
- A. R. Cruz-Luna, H. Cruz-Martínez, A. Vásquez-López and D. I. Medina, J. Fungi, 2021, 7, 1033 CrossRef CAS PubMed.
- H. Dong, Y. He, C. Fan, Z. Zhu, C. Zhang, X. Liu, K. Qian and T. Tang, Nanomaterials, 2022, 12, 3879 CrossRef CAS PubMed.
- R. Villalta, M. Sample, D. Shields and M. Guzmán Quesada, Fitopatología, 2006, 32, 17–33 Search PubMed.
- L. Xu, N. Tao, W. Yang and G. Jing, Ind. Crops Prod., 2018, 112, 427–433 CrossRef CAS.
- L. Wang, W. Hu, J. Deng, X. Liu, J. Zhou and X. Li, RSC Adv., 2019, 9, 28987–28995 RSC.
- M. Vaara and T. Vaara, Antimicrob. Agents Chemother., 1983, 24, 114–122 CrossRef CAS PubMed.
- J. L. Kadurugamuwa, A. J. Clarke and T. J. Beveridge, J. Bacteriol., 1993, 175, 5798–5805 CrossRef CAS PubMed.
- E. Vendemiatti, A. Zsögön, G. F. F. E. Silva, F. A. de Jesus, L. Cutri, C. R. F. Figueiredo, F. A. O. Tanaka, F. T. S. Nogueira and L. E. P. Peres, Plant Sci., 2017, 259, 35–47 CrossRef CAS PubMed.
- J.-H. Kang, F. Shi, A. D. Jones, M. D. Marks and G. A. Howe, J. Exp. Bot., 2010, 61, 1053–1064 CrossRef CAS PubMed.
- M. D. Marks, E. Gilding and J. P. Wenger, Plant J., 2007, 52, 352–361 CrossRef CAS PubMed.
- A. Avellan, J. Yun, B. P. Morais, E. T. Clement, S. M. Rodrigues and G. V. Lowry, Environ. Sci. Technol., 2021, 55, 13417–13431 CrossRef CAS PubMed.
- F. Schwab, G. Zhai, M. Kern, A. Turner, J. L. Schnoor and M. R. Wiesner, Nanotoxicology, 2016, 10, 257–278 CrossRef CAS PubMed.
- D. Duan, J. Tong, Q. Xu, L. Dai, J. Ye, H. Wu, C. Xu and J. Shi, Environ. Pollut., 2020, 267, 115546 CrossRef CAS PubMed.
- A. Joshi, H. Nayyar, K. Dharamvir and G. Verma, AIP Conf. Proc., 2018, 1953, 030058 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb01032a |
| ‡ These authors made equal contributions. |
| This journal is © The Royal Society of Chemistry 2024 |