Open Access Article
Open Access ArticleCationic-motif-modified exosomes for mRNA delivery to retinal photoreceptors†
Héctor A.
Millán Cotto
 a,
Tanvi Vinod
Pathrikar
a,
Bill
Hakim
a,
Helna M.
Baby
a,
Hengli
Zhang
a,
Peng
Zhao
b,
Ronak
Ansaripour
a,
Rouzbeh
Amini
a,
Tanvi Vinod
Pathrikar
a,
Bill
Hakim
a,
Helna M.
Baby
a,
Hengli
Zhang
a,
Peng
Zhao
b,
Ronak
Ansaripour
a,
Rouzbeh
Amini
 ac,
Rebecca L.
Carrier
ab and
Ambika G.
Bajpayee
ac,
Rebecca L.
Carrier
ab and
Ambika G.
Bajpayee
 *abc
*abc
aDepartment of Bioengineering, Northeastern University, Boston, MA 02115, USA. E-mail: millancotto.h@northeastern.edu; pathrikar.t@northeastern.edu; hakim.bi@northeastern.edu; baby.h@northeastern.edu; zhang.hengli@northeastern.edu; ansaripour.r@northeastern.edu; r.amini@northeastern.edu; rebecca@coe.northeastern.edu; a.bajpayee@northeastern.edu; Tel: +1 617-373-7018
bDepartment of Chemical Engineering, Northeastern University, Boston, MA 02115, USA. E-mail: pe.zhao@northeastern.edu
cDepartment of Mechanical and Industrial Engineering, Northeastern University, Boston, MA 02115, USA
First published on 1st July 2024
Abstract
Topical treatment of vitreoretinal diseases remains a challenge due to slow corneal uptake and systemic clearance. Exosomes are emerging nanocarriers for drug delivery due to biocompatibility and cellular targeting properties. To apply them for retinal targeting via the topical route, exosomes must traverse various ocular barriers including the cornea, lens, vitreous humor (VH), and the retina itself. Here we engineered high-purity milk-derived exosomes by anchoring arginine-rich cationic motifs via PEG2000 lipid insertion on their surface. Modification enabled exosomes to use weak-reversible electrostatic interactions with anionic glycosaminoglycan (GAG) and water content of the tissue to enhance their transport rate and retention. Addition of cationic motifs neutralized the anionic surface charge of exosomes (−24 to −2 mV) without impacting size or morphology. Cationic-motif-modified exosomes exhibited two-fold faster steady state diffusivity through bovine corneas compared to unmodified exosomes. Fluorescence recovery after photobleaching confirmed that cationic-motif-modified exosomes can diffuse through VH without steric hindrance. In healthy VH, cationic-motif-modified exosomes demonstrated stronger binding resulting in three-fold lower average diffusivity that enhanced by six-fold in 50% GAG-depleted VH recapitulating advanced liquefaction. Cationic-motif-modified exosomes penetrated through the full-thickness of porcine retinal explants resulting in ten-fold higher uptake in photoreceptors and three-fold greater transfection with encapsulated eGFP mRNA compared to unmodified exosomes. Cationic-motif-modified exosomes are safe to use as they did not adversely affect the mechanical swelling properties of the cornea or lens nor impact retinal cell viability. Cationic-motif-modified exosomes, therefore, offer themselves as a cell-free nanocarrier platform for gene delivery to retinal photoreceptors potentially via the topical route.
1 Introduction
Vitreoretinal diseases remain a challenge due to a lack of reliable retinal-targeting treatment options.1,2 Invasive methods, like injections and ocular implants, can only be used sparingly because they incur increasing risks of retinal detachment, cataracts, and intraocular pressure destabilization when used repeatedly.2–6 Topical treatment would prevent these risks but is currently not feasible due to slow corneal uptake, which results in ∼90% loss of the therapeutic.7–12 Both delivery modes suffer from fast clearance and low retinal targeting. Over the last decade, there has been a growing interest in developing polymeric and liposomal based synthetic nano-carriers to surmount these challenges, but their translatability has been limited by issues like high cytotoxicity, immunogenicity, lipophilicity-induced aggregation leading to vision occlusion, and a lack of retinal targeting.2,7Exosomes are cell-derived extracellular vesicles that play an important role in intercellular communication via genetic material and growth factors acquired from the host cells.13 They have been applied in targeted drug delivery to a variety of tissues owing to their non-immunogenicity, stability, and cellular targeting properties.13–20 In recent work, cell derived exosomes have been used as nanocarriers to deliver small molecule drugs and gene-based therapeutics to the retina.16,21–23 However, to be used for retinal cell-targeting via the topical route, exosomes must be able to navigate through various ocular tissue barriers including the cornea, lens, vitreous humor (VH), and the retina itself (Fig. 1). Their intrinsic physicochemical properties facilitate overcoming only some of these challenges. For instance, their lipid bilayer allows them to avoid the tight junction of the corneal epithelial layer by penetrating intracellularly.24,25 The size of exosomes ranges between 30 to 250 nm, which allows them to circumnavigate the lens capsule that has a pore size smaller than 20 nm and is known to trap small drugs.26–29 Moreover, exosomes are small enough to diffuse through the 500 nm pore size of VH without steric hindrance.30–32
To enable their anterior-to-posterior transport needed for topical delivery, exosomes can be modified to take advantage of the anionic glycosaminoglycan (GAG) and water content of the tissue. The GAG content in the cornea gives it a negative fixed charge density (FCD) of −50 mM;33,34 thus, introducing cationic motifs onto the anionic lipid bilayer of exosomes allows them to cling onto the corneal surface thereby minimizing getting flushed out into the systemic flow by the tear film.24,33 Moreover, the cornea is 78% water, most of which is in the stroma, implying that imparting greater hydrophilicity to exosome surface could enhance their transport through the stroma.25 Similarly, hydrophilic carriers have shown rapid diffusion through the VH, which is 98% water.30,31 The solid matrix of the VH is composed of about 70% collagen and 30% GAGs; the GAGs are comprised of 96% hyaluronic acid and about 4% of uniformly distributed chondroitin and heparin sulfate GAGs, giving the VH a low negative FCD of −0.5 mM.32 In older patients, vitreous liquefaction is associated with up to a 50% loss of collagen and GAG content, and aggregation of collagen fibrils combined with increased water content.32,35 Regardless of age, interactions with the GAG and water content within these tissues will dictate exosome transport across the eye. Therefore, we hypothesize that intra-ocular transport properties of exosomes can be enhanced by modifying their anionic surface to introduce cationic charges and increase hydrophilicity. These modifications would allow modified exosomes to effectively diffuse from the front to the back of the eye by taking advantage of weak-reversible charge-based binding interactions to transport across the cornea, both healthy and degenerated vitreous, and penetrate deep into the retina. Our group has established a low-cost method for harvesting a high yield and high purity bovine-milk derived exosomes by combining casein chelation with differential ultracentrifugation followed by size exclusion chromatography.17 We have also developed methods for surface modification of exosomes via lipid insertion and click chemistry.17,36 We have employed these techniques to conjugate hydrophilic and zwitterionic motifs onto milk-derived exosomes to improve their intestinal mucin transport for applications in oral gene delivery.17,36 We recently designed charge-reversed exosomes for delivering drugs to chondrocytes residing in deep layers of avascular, anionic and aggrecan rich cartilage tissue.37
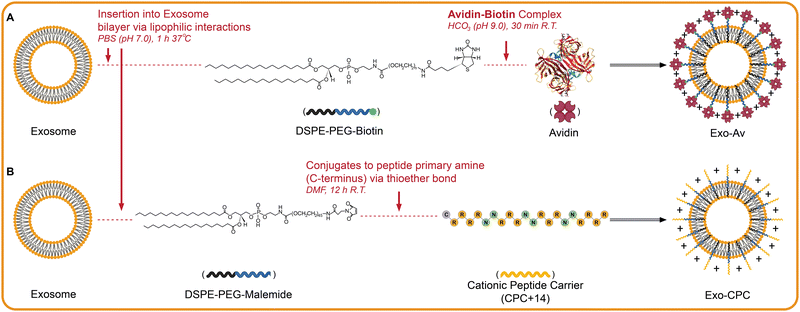
The goal of this study was to engineer retinal targeting milk-derived exosomes for mRNA delivery to retinal cells via the topical route. To that end, exosomes were surface modified via anchoring of (i) cationic motifs to neutralize their net negative charge and (ii) PEG motifs to enhance their surface hydrophilicity. A 66 kDa basic glycoprotein, Avidin (Av) and a short 20 AA long arginine and polar asparagine rich cationic peptide carrier (CPC, RRRR(NNRRR)3R) of net +14 charge conjugated to PEG2000 were used as surface anchoring motifs to synthesize two cationic-motif-modified exosome formulations, Exo-Av and Exo-CPC. These modifications neutralized their anionic surface charge without significantly impacting morphology or size. Our non-equilibrium intra-tissue transport characterization revealed that cationic-motif-modified exosomes diffused faster than unmodified exosomes across the cornea and in GAG-depleted VH but were slower in the healthy VH owing to charge-based binding with negatively charged GAG binding sites. Exosomes were found to be safe and did not impact the electro-mechano-chemical properties of the tissue. Using a porcine retina explant culture model, our results show that Exo-CPC transported across the entire retina, from the inner limiting membrane (ILM) through the outer neuronal layer (ONL, Fig. 1), and effectively transfected the photoreceptors residing in the retinal ONL with the encapsulated eGFP mRNA. Native unmodified exosomes were ineffective in targeting the deep retinal layers. These findings, for the first time, demonstrate the potential of milk-derived cationic-motif-modified exosomes for mRNA delivery to photoreceptors in the retina via the topical route.
2 Materials and Methods
2.1 Materials
Cationic peptide carriers of +14 net charge (CPC), sequence: RRRR(NNRRR)3R, were synthesized by MIT Biopolymers and Proteomics (Cambridge, MA). 1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[maleimide(polyethyleneglycol)-2000] (ammonium salt) (DSPE-PEG-malemide) was purchased from Avanti Polar Lipids (Alabaster, AL). Phosphate-buffered saline (PBS), trypsin-EDTA, dimethylformamide (DMF), fluorescein isothiocyanate (FITC), 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI), 3 and 100 kDa molecular weight cut-off Amicon Ultra Centrifugal Filters, and Lipofectamine 2000 reagent were obtained from Sigma-Aldrich (St. Louis, MO). Dimethyl sulfoxide (DMSO), triethylamine (TEA), and Paraformaldehyde (PFA) were obtained from Fisher BioReagents (Pittsburgh, PA). Ethylenediaminetetraacetic acid (EDTA) was procured from Quality Biological (Gaithersburg, MD). Protease inhibitor tablets, Dulbecco's Modified Eagle Medium (DMEM), Ethylenediamine tetraacetic acid (EDTA), and Pierce bicinchoninic acid (BCA) Protein Assay Kit were purchased from Thermo Fisher Pierce (Rockford, IL). qEV10 35 nm SEC columns were purchased from Izon Science (Medford, MA). CD63 Exo-Flow Capture Kit was purchased from System Bioscience (Palo Alto, CA). Optimal cutting temperature (OCT) Compound and enhanced green fluorescent protein (eGFP) mRNA were procured from Fisher Scientific. Cyanine5 (Cy5) NHS-ester was acquired from Lumiprobe (Cockeysville, MD). Zombie Red staining was obtained from BioLegend Way (San Diego, CA). Lastly, high-glucose Dulbecco's Modified Eagle's Medium (DMEM) without phenol red, penicillin–streptomycin antibiotic–antimycotic (PSA), and Opti-MEM were purchased from Gibco (Grand Island, NY).2.2 Exosome harvest, modification, and characterization
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700, and 19
700, and 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 400 rpm; only the supernatant was transferred to subsequent runs. Exo were pelleted with a final run at 23
400 rpm; only the supernatant was transferred to subsequent runs. Exo were pelleted with a final run at 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 rpm for two hours and resuspended in 600 μL of PBS (pH 7.0). The resuspended Exo were then run through a 35 nm size exclusion chromatography (SEC) column and collected into ten fractions (5 mL each) for protein quantification via the bicinchoninic acid (BCA) assay. Fractions with the highest protein content were aliquoted with 5 × 1011 particles per tube in PBS and stored at −80 °C for future use.
200 rpm for two hours and resuspended in 600 μL of PBS (pH 7.0). The resuspended Exo were then run through a 35 nm size exclusion chromatography (SEC) column and collected into ten fractions (5 mL each) for protein quantification via the bicinchoninic acid (BCA) assay. Fractions with the highest protein content were aliquoted with 5 × 1011 particles per tube in PBS and stored at −80 °C for future use.
For modification with CPC, 20 μg of DSPE-PEG-maleimide (5.88 nmol, 1.0 equiv.) was first mixed with 20 μg of Cy5-labeled CPC (5.55 nmol, 0.9 equiv.) in 100 μL of DMF overnight at RT. This reaction linked DSPE-PEG-maleimide with the cysteine on the C-terminus of CPC via click chemistry (Fig. 2(B)). Lyophilized DSPE-PEG-CPC was resuspended in 40 μL of DMSO and added dropwise to 1 tube of Exo (5 × 1011 particles) in 1 mL PBS while stirring continuously at 37 °C for 1 h. Unbound DSPE-PEG-CPC was filtered out using a 35 nm pore-sized SEC column and modified Exo were collected into twelve 500 μL fractions. Fractions were analyzed for Cy5 absorbance (617/685 Ex/Em) and protein content (BCA assay) to isolate the fraction with highest concentration of CPC-modified Exo. These are referred to as Exo-CPC.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 events were measured for each measurement. As additional confirmation, magnetic beads with captured Exo were imaged under 20× and 40× magnification using a ZEISS LSM 880 inverted confocal microscope (Carl Zeiss NTS Ltd, Oberkochen, Germany). Beads were imaged using FITC, Texas Red, and Cy5 channels as appropriate to confirm presence of modifications on Exo. Finally, unlabeled Exo were imaged using negative staining TEM to evaluate their morphology. Alcian blue was used for the mesh and uranyl acetate for Exo. Specifically, a grid was coated with 1% Alcian Blue to produce a positively charged surface and then Exo was added for adsorption. Finally, the grid was incubated with heavy metal salt uranyl acetate to negatively stain Exo and imaged.
000 events were measured for each measurement. As additional confirmation, magnetic beads with captured Exo were imaged under 20× and 40× magnification using a ZEISS LSM 880 inverted confocal microscope (Carl Zeiss NTS Ltd, Oberkochen, Germany). Beads were imaged using FITC, Texas Red, and Cy5 channels as appropriate to confirm presence of modifications on Exo. Finally, unlabeled Exo were imaged using negative staining TEM to evaluate their morphology. Alcian blue was used for the mesh and uranyl acetate for Exo. Specifically, a grid was coated with 1% Alcian Blue to produce a positively charged surface and then Exo was added for adsorption. Finally, the grid was incubated with heavy metal salt uranyl acetate to negatively stain Exo and imaged.
2.3 Ocular tissue harvest and preparation
2.4 Transport of Exo through cornea
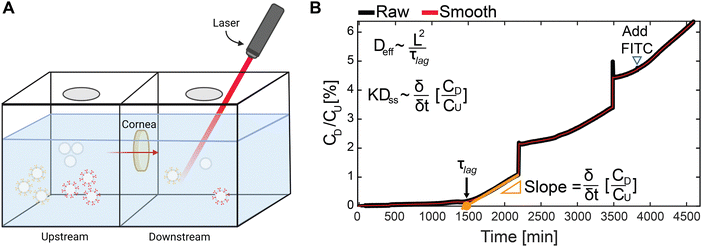
A clear poly(methyl methacrylate) chamber with two compartments (Fig. 4(A)) was used to measure the real-time one-dimensional transport of Exo as previously described.43–45 In brief, the chamber compartments were first washed with 0.5% non-fat dried bovine milk to prevent non-specific binding. 6 mm diameter bovine corneal explant (∼1.2 mm thick) was held between the chambers. 2 mL of PBS supplemented with protease inhibitors (PBS-PI) was added to both compartments and kept at stirring to prevent any stagnation effects in the chamber. Once started (t = 0), 80 μg of FITC-labeled unmodified or cationic-motif-modified Exo (1 mg mL−1) was injected in the upstream compartment. The Exo concentration in the downstream chamber was monitored continuously with a spectrofluorometer. To calibrate the downstream concentration, 20 μL from the upstream chamber was added to the downstream once the Exo concentration profile became linear. From the concentration profiles, effective diffusivity (Deff) was estimated as: | (1) |
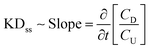 | (2) |
2.5 Mechanical assessment of corneal explants and lenses
The bulk modulus (Eeq) and swelling pressure (Δβ) of corneal explants and lenses were computed from load and displacement measurements collected with a TA ElectroForce 5500 Series dynamic mechanical analysis apparatus (TA Instruments, New Castle, DE) using a modified protocol from Warren et al.48 Uniaxial, unconfined, stress relaxation testing was performed to remove transverse heterogeneity by allowing for radial transport of Exo and minimize loss from non-specific binding of cationic-motif-modified Exo to the porous membrane needed for confined testing. Bovine corneal explants (8 mm diameter, ∼1.2 mm thick) were placed in a 15 mm diameter stainless steel well and compressed with a 10 mm diameter probe. Similarly, lenses (15 mm diameter, ∼7 mm thickness) were placed in a 2 mL bath within a 22 mm diameter aluminum chamber with an accompanying 18 mm diameter plate probe. The loading regime consisted of at least three intermittent strain ramps and dwelling periods that applied stress on the tissue and then allowed it to relax.48 Corneal explants were imposed a 0.08 N preload and equilibrated for 1100 s before its first stress-relaxation cycle. The first ramp applied 8% strain and all subsequent ramps applied 2% strain. While compressive stresses are represented by negative values, here we report the absolute value of the compressive strain. All dwelling periods held the strain for 1100 s. Cumulative strain did not exceed 16% to remain within the linear response of the cornea.49,50 Lens tissues underwent the same protocol with a 0.05 N preload and 1000 s dwelling periods.The stress and strain curves at each relaxation interval were fit to the standard linear solid model48 to calculate the equilibrium stress (σeq) and characteristic time using non-linear least squares regression:
 | (3) |
σeq = (Eeq(![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) ) × ε0) + β( ) × ε0) + β(![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) ) ) | (4) |
![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) ).48,51 Here the bulk modulus is a representation of the measurable tissue stiffness in triphasic framework.49,51–53 Therefore, it is composed of a non-electrostatic (ENEeq, arising from support by the solid macromolecular ECM) and electrostatic (EEeq, arising from like-charge repulsions within the tissue) component:48
).48,51 Here the bulk modulus is a representation of the measurable tissue stiffness in triphasic framework.49,51–53 Therefore, it is composed of a non-electrostatic (ENEeq, arising from support by the solid macromolecular ECM) and electrostatic (EEeq, arising from like-charge repulsions within the tissue) component:48| Eeq = ENEeq + EEeq | (5) |
To assess the impact of cationic-motif-modified-Exo on the bulk modulus and change in swelling pressures of the cornea and lens, tissues were incubated with unmodified Exo, Exo-Av or Exo-CPC (1.67 μg mL−1) for one hour at RT.
Impact of charge shielding due to the uptake of modified Exo on the bulk moduli of cornea and lens was estimated using a charge shielding test as described previously.48 Tissues were first equilibrated in a hypotonic bath solution (0.02 M NaCl) for 40 min at RT and then subjected to five stress-relaxation cycles. In between each cycle, the salinity of the bath was increased, and the tissue was allowed to equilibrate for the duration of a dwelling period. Then a reverse stress-relaxation cycle was performed (−6% and −2% strain for cornea and lens, respectively) to collect additional datapoints without exceeding the 16% strain limit. The tested concentrations were as follows: 0.05, 0.10, 0.19, 0.6, and 1.40 M NaCl. The bulk modulus measured at the highest bath salinity (1.40 M) can be attributed to non-electrostatic component (ENEeq) arising from the solid macromolecular ECM since all electrostatic interactions would be shielded at this high salinity. Since this non-electrostatic component of the bulk modulus, ENEeq, would not change with bath salinity and can be assumed to be constant, the electrostatic component (EEeq) that arises from like-charge repulsions within the tissue was estimated at each salinity by subtracting ENEeq from the total bulk modulus, Eeq. A theoretical estimation of the electrostatic component of the bulk modulus was computed using an ideal Donnan model previously described by our group,51
 | (6) |
 | (7) |
Evaluating the swelling pressure at multiple strains (here 0.1 and 0.2) eqn (6) was used to theoretically predict the electrostatic component of the bulk modulus as:
 | (8) |
Bulk modulus predictions were computed using the estimated electrostatic component (eqn (8)) and the measured non-electrostatic component (Table 3).
Δβ = β(![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) 0.15M) − β( 0.15M) − β(![[C with combining macron]](https://www.rsc.org/images/entities/i_char_0043_0304.gif) 1.4M) 1.4M) | (9) |
2.6 Transport studies through the vitreous humor
 | (10) |
 | (11) |
 | (12) |
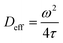 | (13) |
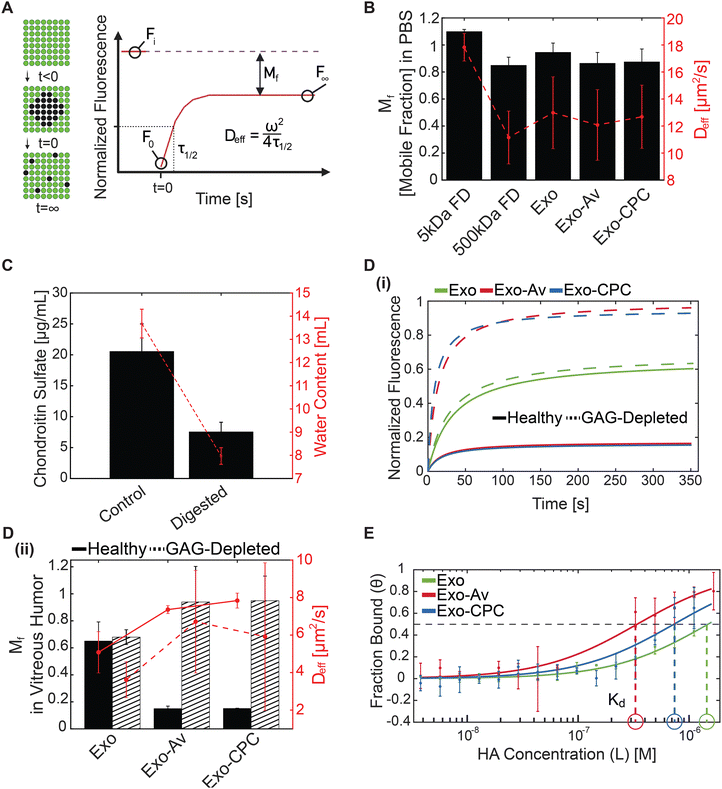
Lastly, the particle population flux was approximated as the average diffusivity (PA), to better understand how the entire particle population travels through the VH.36 FRAP only considers mobile particles when computing the diffusivity coefficient so by ignoring the immobilized particles it assumes that they will remain trapped indefinitely. Therefore, PA provides an approximation of the diffusion of the entire population through the tissue by averaging the diffusivity of mobile and immobile particles, and is defined as:
| PA = Mf × Deff | (14) |
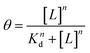 | (15) |
2.7 Delivery of mRNA to the retina
2.8 Statistical analysis
The data are reported as mean ± standard deviation. All characterization and transport experiments used N = 4–6 independent repeats. All mechanical analysis and retinal explant experiments used N = 3–4 repeats. Experimental groups were compared using one-way analysis of variance (ANOVA) and post hoc Tukey's honestly significant difference (Tukey's HSD) test. Statistical significance was defined as p values below 0.05.3 Results
3.1 Exosome harvest, modification, and characterization
Fractions 5–7 from the Exo purification were expected to contain high purity Exo, 3 × 109 particles per μg, within the desired size range, 60–200 nm, according to previous work by our group.17,36 Fraction 6 was selected for further experiments because it measured the highest protein content (450 ± 67 μg mL−1), indicating the highest content of Exo (Fig. 3(B)). Fraction 10 of the SEC-separated Exo modified with Avidin-Texas Red (Exo-Av) showed the highest Texas Red and protein content, demonstrating presence of Exo-Av (Fig. 3(C)-(i)). Similarly, fraction 10 of the SEC-separated Exo modified with CPC (Exo-CPC) presented the highest Cy5 signal and protein content (Fig. 3(C)-(ii)). The molar equivalent ratio of Avidin and CPC per Exo was estimated to be 600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 450
1 and 450![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, respectively, based on the absorbance and protein measurements from fraction 10 of each group (Table 1). The use of Avidin motifs increased the mean ζ-potential values of Exo from −22.3 mV to −6.6 mV and to −2.0 mV for Exo-CPC (Table 1). These increments represent a near complete shielding of the anionic surface charge of Exo.
1, respectively, based on the absorbance and protein measurements from fraction 10 of each group (Table 1). The use of Avidin motifs increased the mean ζ-potential values of Exo from −22.3 mV to −6.6 mV and to −2.0 mV for Exo-CPC (Table 1). These increments represent a near complete shielding of the anionic surface charge of Exo.
| Ratio Exo to Motif loading | Motif configuration (molecular weight [kDa])/net charge | Size [nm] | PDI [%] | Surface charge [mV] | |
|---|---|---|---|---|---|
| Exo | — | — | 192 ± 38 | 21.6 ± 7.7 | −22.3 ± 2.8 |
| Exo-Av | 1:600 | Four identical subunits (66)/+6 to +20 | 257 ± 36 | 23.3 ± 8.6 | −6.6 ± 4.3 |
| Exo-CPC | 1:450 | RRRR(NNRRR)3R (2.99)/+14 | 237 ± 39 | 22.5 ± 7.9 | −2.0 ± 0.8 |
The strong FITC signal from unmodified Exo formulations from the flow cytometry data indicated successful binding of Exo to the CD63 antibodies on magnetic beads (green, Fig. 3(D)). Exo-Av (red, Fig. 3(D)-(i)) demonstrated both Texas Red of Avidin and FITC signal of unmodified Exo, and Exo-CPC (purple, Fig. 3(D)-(ii)) exhibited both Cy5 of CPC and FITC of unmodified Exo confirming successful surface modifications. Confocal images of the magnetic beads displayed dual fluorescence of both the Exo and the surface anchoring further corroborating the findings of the flow cytometric analysis (Fig. 3(E)). Finally, TEM imaging revealed that Exo morphology was not affected post modification (Fig. 3(F)).
3.2 Transport of Exo through cornea
Unmodified Exo demonstrated 2.8-fold and 4.4-fold higher effective diffusivity (Deff) than that of Exo-Av and Exo-CPC, respectively, due to a lack of electrostatic binding interactions with the anionic constituents of the tissue (Fig. 4 and Table 2). Once steady state was achieved, Exo-CPC displayed the highest steady state diffusivity (KDss), about 1.6-fold faster than unmodified Exo and 2.3-fold faster than Exo-Av. A contributing factor to this trend is the coulombic attractive forces between the anchored CPC motifs and the negative FCD of the cornea that owing to charge-based Donnan effects46,48 can enhance both the concentration and the rate of transport of cationic solutes into the anionic tissue.46,47 The Donnan partitioning factor, K, was defined as 1 for unmodified Exo as they have no attractive coulombic interactions within the matrix of the cornea; assuming that the steady state diffusivity, Dss is constant for all Exo formulations due to their similar sizes (Table 1), K for cationic-motif-modified Exo can be estimated by taking ratios of their respective KDss (Table 2). K for Exo-CPC measured greater than 1 (K ∼ 1.6) implying that its concentration at the tissue interface would partition up by a factor of 1.6, which would enhance its flux into the tissue compared to unmodified Exo. Similarly, K for Exo-Av was measured to be less than 1 (K ∼ 0.7) implying that its uptake could not achieve equilibrium potentially owing to aggregation issues (observed experimentally) that could hinder its intra-tissue transport.56 Free FITC diffused as expected given its small size and hydrophilicity.43,57,58| D eff [× 10−7 cm2 s−1] | KDss [× 10−4 cm2 s−1] | K | |
|---|---|---|---|
| FITC | 1.00 ± 0.13 | 0.39 ± 0.16 | — |
| Exo | 0.31 ± 0.11 | 0.99 ± 0.23 | 1 |
| Exo-Av | 0.19 ± 0.10 | 0.70 ± 0.24 | 0.7 |
| Exo-CPC | 0.07 ± 0.01 | 1.63 ± 0.16 | 1.63 |
3.3 Electro-mechano-chemical effects of modified Exo on corneal explants and lenses
Exposure to cationic-motif-modified Exo was found to minimally impact the total bulk modulus (Eeq) of both tissues. There were no significant changes in stiffness of either tissue after one hour incubation with any of the Exo formulations (Fig. 5(A)-(i), (ii)). Increasing bath salinities reduced the bulk moduli of both tissues (Fig. 5(C)). The lens response followed the ideal Donnan Model prediction (black line, R2 = 0.91, Fig. 5(C)-(ii)) closely, but the cornea displayed a weak fit (R2 = 0.42, Fig. 5(C)-(i)) which could be attributed to differences in their composition. Corneal stroma is two orders of magnitude thicker than the lens capsule (500 vs. 3.5 μm) and nearly 30% of its dry weight is composed of GAGs, which plays a key role in maintaining the laminar organization of the stroma.33,59–62 The corneal stroma, therefore, is more sensitive to counterion condensation and the subsequent changes to collagen fibril organization explaining the poor fit with the ideal Donnan Model values (ESI,† Fig. S1).48,63,64 At physiological salinity (∼0.15 M), the electrostatic component accounted for 68% and 52% of the bulk modulus in cornea and lens, respectively (Table 3).| Saline bath [M] | Cornea | Lens | ||
|---|---|---|---|---|
| E NEeq [kPa] | E Eeq [kPa] | E NEeq [kPa] | E Eeq [kPa] | |
| 0.05 | 3.19 ± 0.27 | 1.97 ± 0.39 | ||
| 0.10 | 2.35 ± 0.62 | 1.21 ± 0.17 | ||
| 0.15 | 1.08 ± 0.45 | 1.93 ± 0.81 | 1.14 ± 0.23 | 0.46 ± 0.23 |
| 0.60 | 1.32 ± 0.47 | 0.08 ± 0.07 | ||
| 1.4 | 0 | 0 | ||
The change in swelling pressure showed no significant difference between experimental and control groups for either tissue (Fig. 5(D)-(i), (ii) and Table 3). Corneal explants incubated in Exo-Av showed a drop of 150 Pa in swelling pressure compared to the PBS control while the Exo-CPC-incubated lenses showed a drop of 100 Pa. This close to 30% reduction in corneal swelling pressure is within the normal physiological range and may result in ∼10% decrease in stromal thickness.65,66 The corneal endothelial layer can counteract this tissue deswelling by modulating metabolic activity to allow for more water to transport into the tissue.67,68
3.4 Transport studies through vitreous humor
| PBS | Healthy VH | GAG-depleted VH | |||||||
|---|---|---|---|---|---|---|---|---|---|
| D eff [μm2 s−1] | M f | P A [μm2 s−1] | D eff [μm2 s−1] | M f | P A [μm2 s−1] | D eff [μm2 s−1] | M f | P A [μm2 s−1] | |
| 5 kDa FD | 17.8 ± 1.0 | 1.0 | 17.8 | — | — | — | — | — | — |
| 500 kDa FD | 11.2 ± 2.0 | 0.85 | 9.5 | — | — | — | — | — | — |
| Exo | 13.0 ± 2.7 | 0.95 | 12.3 | 5.1 ± 1.1 | 0.65 | 3.3 | 3.6 ± 1.0 | 0.68 | 2.5 |
| Exo-Av | 12.1 ± 2.6 | 0.86 | 10.4 | 7.4 ± 0.2 | 0.15 | 1.1 | 6.7 ± 2.7 | 0.94 | 6.3 |
| Exo-CPC | 12.7 ± 2.3 | 0.88 | 11.1 | 7.8 ± 0.4 | 0.15 | 1.2 | 5.9 ± 3.9 | 0.94 | 5.6 |
In healthy VH, cationic-motif-modified Exo exhibited 1.5-fold faster mean Deff than that of unmodified Exo potentially owing to the presence of transport enhancing hydrophilic PEGs (Fig. 6(D)-(i), (ii) and Table 4). Cationic-motif-modified Exo exhibited 4-fold lower mean Mf owing to charge-based binding with the VH GAGs (Fig. 6(D)-(i) and Table 4). It should be noted that FRAP only considers mobile particles when computing the effective diffusivity coefficients. To estimate the transport flux of the entire particle population, we introduced an average diffusivity parameter (PA = Mf × Deff), which averages the diffusivity of both mobile and immobile particles.36 While the Deff of unmodified Exo measured slower than that of cationic-motif-modified Exo, PA indicated that unmodified Exo would diffuse 3-fold faster in VH owing to its larger mobile fraction (Mf, Exo: 0.65; Exo-Av: 0.15; Exo-CPC: 0.15). These data highlight the competing effects of charge-induced hindrance and hydrophilic PEG-induced enhancement on particle transport. In younger patient population, with little to no vitreous liquefaction, the slower moving cationic-motif-modified Exo can be used to create intravitreal drug depots and provide sustained drug release.
In GAG-depleted VH, all Exo formulations exhibited lower Deff compared to that in the healthy VH (0.70×, 0.91×, and 0.76× for Exo, Exo-Av, and Exo-CPC, respectively). The reduction was likely due to the loss of water content following GAG depletion which, in ex vivo tissue, reduces the distance between collagen fibrils thereby decreasing the tissue mesh size.30,72In vivo, the volume of the VH remains relatively constant despite collagen and GAG degradation, which would instead increase the tissue mesh size.32,72 Cationic-motif-modified Exo presented a 1.75-fold faster Deff than the unmodified Exo and close to 100% mobility in GAG-depleted VH (Fig. 6(D)-(ii) and Table 4). Reinforcing these trends, PA indicated cationic-motif-modified Exo had a 2.3-fold higher diffusivity than unmodified Exo (Table 4). The enhanced mobility and transport rate of cationic-motif-modified Exo in GAG-depleted VH can be attributed to the reduced charge-based binding interactions owing to depletion of GAGs as well as the presence of hydrophilic PEGs that are known to enhance macromolecule transport in the VH.30,31 Therefore, in older patients with severe vitreous degeneration, the cationic-motif-modified Exo would transport rapidly across the vitreous to reach and target the retinal cells.
| K d [μM] | Hill coefficient | |
|---|---|---|
| Exo | 1.48 ± 0.83 | 1.04 |
| Exo-Av | 0.34 ± 0.14 | 1.92 |
| Exo-CPC | 0.73 ± 0.15 | 0.90 |
3.5 Retinal transport, viability, and transfection
4 Discussion
In this study we established the potential of cationic-motif-modified milk-derived exosomes for targeted delivery of mRNA to retinal photoreceptors via the topical route. We designed two exosome formulations (Exo-Av and Exo-CPC) by anchoring a basic glycoprotein Avidin and an arginine-rich cationic peptide carrier with a net charge of +14 via PEG lipid insertion onto milk-derived exosomes without affecting their size or morphology (Fig. 2, 3(F) and Table 1). Our lab has previously designed Avidin and CPC-based nanocarriers for targeted drug delivery to other negatively charged tissues including cartilage,39,54,74 intervertebral discs85 and mucin,17,36 and for diagnostic imaging applications.86 Here, cationic and PEG motifs were anchored in sufficient density to enable a near complete shielding of the anionic surface charge of exosomes while making them hydrophilic (Table 1). This allowed them to take advantage of weak-reversible ionic binding interactions with the anionic GAGs and water content in various ocular tissue compartments including the cornea, vitreous humor (VH), and the retina that enhanced their intra-tissue transport and binding properties. Our work shows that owing to the presence of cationic motifs on exosomes, their steady state diffusivity through the cornea was enhanced by 2-fold compared to unmodified exosomes (Table 2). Our FRAP results confirm that milk exosomes with a size range of 60–200 nm can transport through the VH without steric hindrance (Fig. 6(D)-(ii)). In the retina, cationic-motif-modified exosome, Exo-CPC penetrated through the full thickness of the tissue, from ganglion cell layer (GCL) to the outer nuclear layer (ONL), by taking advantage of charge-based binding interactions with the tissue GAGs that resulted in a 20- and a 10-fold higher uptake in GCL and the deep ONL layers, respectively, compared to unmodified exosomes (Fig. 7(A)). Cationic motifs also increased the contact time of Exo-CPC with the anionic cell lipid bilayer enhancing their mRNA transfection efficiency by 3-fold compared to unmodified exosomes (Fig. 7(B)-(i), (ii)). Finally, cationic-motif-modified exosomes did not adversely impact the electro-mechano-chemical homeostasis in cornea or the lens and elicited no cytotoxic effect on retinal cells (Fig. 7(C)-(i), (ii)). Cationic-motif-modified exosomes, therefore, offer themselves as a nanocarrier platform system for drug and gene delivery to retinal photoreceptors potentially via the topical route.The eye presents several pharmacokinetic barriers for the treatment of vitreoretinal diseases via the topical delivery (Fig. 1). While invasive methods like intraocular injections can bypass some of these barriers, they are associated with patient discomfort and increasing risks of errors and infections when used repeatedly. Topical delivery to target the back of the eye is desirable but currently not feasible since 95–99% of drugs lose their bioavailability at the cornea following topical application as they get flushed into the circulation by the tear film. Therapeutics must penetrate the 20 nm intercellular junction of the corneal epithelium or move intracellularly quickly. We show that when Exo-CPC contacts the corneal surface, the cationic motifs facilitate electrostatic binding interactions at a microscopic scale with anionic GAGs in the corneal stroma resulting in partitioning up of Exo-CPC concentration by a Donnan factor, K of 1.6 that enhances its initial rate of transport and its steady state diffusivity by 2-fold compared to unmodified exosomes. Previous studies using rabbits have shown that topically applied cationic lipids loaded with acyclovir result in 2–4-fold higher bioavailability in cornea compared to free drug or anionic lipids despite 2–4-fold slower permeability through the cornea.24 This charge-based surface binding combined with enhanced flux can help exosomes to cling onto the corneal surface which would prevent their clearance and facilitate efficient transport through the cornea for topical applications.24 Additionally, the presence of PEGs eases Exo-CPC diffusion through the stroma by minimizing hydrophobic trapping within collagen pockets.25 Exo-Av suffered from aggregation issues resulting in lower partitioning of K = 0.7; further optimization of the formulation by changing the extent of avidin functionalization and use of surfactants like Tween 20 can reduce ionic crosslinking induced aggregation issues.17 Our SEC-purified exosomes have a controlled size range with low polydispersity index (Table 1) and thus can also circumvent getting trapped within the lens capsule with a 20 nm pore size.26
The next significant ocular barrier is the VH wherein the solute diffusion is primarily governed by hydrophobic interactions with collagen fibers and electrostatic binding interactions with its uniformly distributed GAGs.31 Its pore size is in the 500 nm range31 that imposes negligible steric hindrance on exosome diffusion. Intravitreal injections of exosomes in mice have demonstrated their ability to transport through the VH and reach the retina.22,23,87 Similarly, 200 nm-sized PEG-functionalized polystyrene nanoparticles exhibited 2× faster diffusivity in VH than their non-PEGylated counterparts.31 When functionalized with amines to induce cationic surface charge between +7 and +39 mV, a majority of these particles became immobile and were trapped within the VH.30,31 In healthy VH, the cationic-motif-modified exosomes demonstrated stronger binding interactions with HA resulting in a 3-fold slower average diffusivity (PA) compared to unmodified exosomes. This was corroborated by microscale thermophoresis data that measured significantly stronger binding affinity of cationic-motif-modified exosomes when compared to that of unmodified exosomes (Kd, Exo-Av: 0.34 μM, Exo-CPC: 0.73 μM vs. Exo: 1.48 μM). It is important to note that despite their slowed transport, the cationic-motif-modified exosomes can transport across the VH compartment as their binding to HA is weak and reversible in nature. They also do not experience steric hindrance owing to their smaller size than the VH pores. Recent work has shown that extracellular vesicles are capable of deforming and thus can penetrate through the decellularized scaffolds of pore size smaller than their diameter.88 The slowed transport through the VH offers an opportunity for applications in forming intravitreal drug depots for sustained delivery of encapsulated drugs while also targeting the retina. Average diffusivities of cationic-motif-modified exosomes, as expected, increased by 6 times in GAG-depleted VH recapitulating advanced vitreous liquefaction observed in an 80-year-old patient, and by 2 times compared to unmodified exosomes owing to the diminished level of charge-based binding and the presence of hydrophilic PEGs that reduced binding within hydrophobic pockets of collagen fibrils (Fig. 6(D)-(i), (ii) and Table 4). As such, exosomes have the potential to rapidly transport through an aged VH to reach the retina for applications in delivery of small molecule and gene-based therapeutics. There are little to no enzymes or immune cells in the VH due to the acellular and avascular nature of the tissue that can cause exosome degradation;89 however, their stability in vivo should be evaluated in future studies.
The final ocular barrier is the retina itself. Exo-CPC showed a 20-fold stronger presence in the GCL and 10-fold higher uptake in the ONL of porcine retinas compared to unmodified exosomes (Fig. 7(A)). This increased penetration depth and uptake can be attributed to the increasing concentration of chondroitin sulfate GAGs from GCL to the ONL (Fig. 1). Exo-CPC also resulted in 3× higher transfection efficiency compared to unmodified exosomes likely due to the increased contact time with the anionic cell layer which induced endocytosis and micropinocytosis pathways.74–77,79 Moreover, the higher GAG content in the ONL potentially contributed to the preferential transfection of photoreceptors by Exo-CPC (Fig. 7(B)-(i)). Only Exo-CPC was evaluated here as Exo-Av formulation suffered from ionic crosslinking-induced aggregation.
Porcine retinas were used in this study because of their similarity in organization and thickness to that of humans. Introducing a high density of cationic charges comes at the risk of charge shielding within the negatively charged cornea and lens that can cause deswelling,34,49,90 which may ultimately result in blurry vision.61,91 Extensive published work has established milk-exosomes as safe, non-immunogenic and stable carriers, and our group has demonstrated the safety of CPCs in high doses in negatively charged tissues like cartilage.37,43,74 Similarly, in our current work, the size and morphology of exosomes is not significantly affected post modification (Fig. 3 and Table 1). Furthermore, cationic-motif-modified exosomes do not induce significant corneal or lens deswelling. Additionally, Exo-CPC was safe to use as it did not impact retinal explant viability.
A limitation of this study is the use of ex vivo corneal explants. The corneal epithelial cell layer is the limiting step for drug transport and the endothelial cell layer is responsible for regulating the water content within the tissue; both die once the tissue is harvested.67 Additionally, the lack of a viable corneal endothelial cell layer may have had an impact on the swelling pressure measurements that could not be accounted for. Also, it is important that the rate of transport of exosomes across cornea is faster than its clearance rate following topical administration. Thus, the impact of dynamic barriers, like tear film and aqueous humor, on transport and transfection efficacy of cationic-motif-modified exosomes should be evaluated in vivo in future studies. Moreover, the tolerance of each ocular tissue to higher doses exosomes doses should be assessed.
5 Conclusions
In summary, these findings, for the first time, demonstrate the potential of milk-derived cationic-motif-modified exosomes for mRNA delivery to photoreceptors in the retina via the topical route. Exo-CPC has the potential for targeting several ocular tissues via the topical route by taking advantage of GAG and water content for delivery of anti-inflammatory small molecule drugs and gene-based therapeutics and can be applied in the treatment of diabetic retinopathy, age-related macular degeneration, or glaucoma.Author contributions
Héctor Millán Cotto designed and executed all the experiments and wrote the manuscript. Tanvi Pathrikar and Helna Mary supported milk exosome harvest, surface modification, and mRNA loading. Bill Hakim supported one-dimensional corneal transport, mechanical testing, and microscale thermophoresis measurements. Hengli Zhang supported fluorescent recovery after photobleaching measurements and performed flow cytometry measurements and transmission electron microscopy imaging. Rebecca Carrier, Peng Zhao, and Ronak Ansaripour supported retinal explant model design, harvesting, and cryosectioning. Rouzbeh Amini supported cornea, lens, and vitreous humor harvesting. Ambika Bajpayee conceived the idea, procured funding, designed experiments, oversaw data analysis and writing of the manuscript. All authors were involved in writing, reviewing, and approving the final version of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Institute for Chemical Imaging of Living Systems at Northeastern University for consultation and imaging support. This study was supported by the National Science Foundation (NSF) CAREER Award 2141841 and by the National Institute of Health (NIH) Trailblazer R21 grant EB028385.References
- GBD 2019 Blindness and Vision Impairment Collaborators, Lancet Glob. Health, 2021, 9, e144–e160 CrossRef.
- M. Tawfik, F. Chen, J. L. Goldberg and B. A. Sabel, Naunyn-Schmiedebergs Arch. Pharmakol., 2022, 395, 1477–1507 CrossRef CAS.
- G. A. Peyman, E. M. Lad and D. M. Moshfeghi, Retina, 2009, 29, 875–912 CrossRef.
- R. Amini, V. H. Barocas, H. P. Kavehpour and J. P. Hubschman, Retina, 2011, 31, 1656–1663 CrossRef PubMed.
- L. Gsellman and R. Amini, Invest. Ophthalmol. Vis. Sci., 2016, 57, 3340 CrossRef CAS.
- N. Rashidi, V. S. Thomas and R. Amini, Transl. Vis. Sci. Technol., 2019, 8, 4 CrossRef.
- D. Khiev, Z. A. Mohamed, R. Vichare, R. Paulson, S. Bhatia, S. Mohapatra, G. P. Lobo, M. Valapala, N. Kerur, C. L. Passaglia, S. S. Mohapatra and M. R. Biswal, Nanomaterials, 2021, 11, 173 CrossRef CAS.
- D. A. Lee and E. J. Higginbotham, Am. J. Health-Syst. Pharm., 2005, 62, 691–699 CrossRef PubMed.
- J. Lusthaus and I. Goldberg, Med. J. Aust., 2019, 210, 180–187 CrossRef.
- W. Zhang, M. R. Prausnitz and A. Edwards, J. Controlled Release, 2004, 99, 241–258 CrossRef CAS PubMed.
- F. T. Fraunfelder and S. M. Meyer, J. Ocul. Pharmacol. Ther., 1987, 3, 177–184 CrossRef CAS.
- M. Löscher, C. Seiz, J. Hurst and S. Schnichels, Pharmaceutics, 2022, 14, 134 CrossRef.
- D. M. Pegtel and S. J. Gould, Annu. Rev. Biochem., 2019, 88, 487–514 CrossRef CAS.
- S. Samanta, S. Rajasingh, N. Drosos, Z. Zhou, B. Dawn and J. Rajasingh, Acta Pharmacol. Sin., 2018, 39, 501–513 CrossRef CAS.
- S. E. L. Andaloussi, S. Lakhal, I. Mäger and M. J. A. Wood, Adv. Drug Delivery Rev., 2013, 65, 391–397 CrossRef.
- D. Pollalis, D. Kim, G. K. G. Nair, C. Kang, A. V. Nanda and S. Y. Lee, Cells, 2022, 11, 2573 CrossRef CAS PubMed.
- M. R. Warren, C. Zhang, A. Vedadghavami, K. Bokvist, P. K. Dhal and A. G. Bajpayee, Biomater. Sci., 2021, 9, 4260–4277 RSC.
- D. Pan, X. Chang, M. Xu, M. Zhang, S. Zhang, Y. Wang, X. Luo, J. Xu, X. Yang and X. Sun, J. Chem. Neuroanat., 2019, 96, 134–139 CrossRef CAS PubMed.
- R. Kar, R. Dhar, S. Mukherjee, S. Nag, S. Gorai, N. Mukerjee, D. Mukherjee, R. Vatsa, M. Chandrakanth Jadhav, A. Ghosh, A. Devi, A. Krishnan and N. D. Thorat, ACS Biomater. Sci. Eng., 2023, 9, 577–594 CrossRef CAS.
- K. D. Popowski, B. López de Juan Abad, A. George, D. Silkstone, E. Belcher, J. Chung, A. Ghodsi, H. Lutz, J. Davenport, M. Flanagan, J. Piedrahita, P.-U. C. Dinh and K. Cheng, J. Extracell. Vesicles, 2022, 1, 100002 CrossRef.
- S. J. Wassmer, L. S. Carvalho, B. György, L. H. Vandenberghe and C. A. Maguire, Sci. Rep., 2017, 7, 45329 CrossRef CAS PubMed.
- A. R. Hajrasouliha, G. Jiang, Q. Lu, H. Lu, H. J. Kaplan, H.-G. Zhang and H. Shao, J. Biol. Chem., 2013, 288, 28058–28067 CrossRef CAS.
- B. Mead and S. Tomarev, Stem Cells Transl. Med., 2017, 6, 1273–1285 CrossRef CAS PubMed.
- S. L. Law, K. J. Huang and C. H. Chiang, J. Controlled Release, 2000, 63, 135–140 CrossRef CAS PubMed.
- S. Kakkar, M. Singh, S. Mohan Karuppayil, J. S. Raut, F. Giansanti, L. Papucci, N. Schiavone, T. C. Nag, N. Gao, F.-S. X. Yu, M. Ramzan and I. P. Kaur, J. Drug Targeting, 2021, 29, 631–650 CrossRef CAS PubMed.
- B. P. Danysh and M. K. Duncan, Exp. Eye Res., 2009, 88, 151–164 CrossRef CAS PubMed.
- J. F. Hejtmancik and A. Shiels, Prog. Mol. Biol. Transl. Sci., 2015, 134, 119–127 Search PubMed.
- J. R. Sabah, H. Davidson, E. N. McConkey and L. Takemoto, Mol. Vision, 2004, 10, 254–259 CAS.
- J. S. Friedenwald, Arch. Ophthalmol., 1930, 3, 182 CrossRef.
- B. T. Käsdorf, F. Arends and O. Lieleg, Biophys. J., 2015, 109, 2171–2181 CrossRef.
- Q. Xu, N. J. Boylan, J. S. Suk, Y.-Y. Wang, E. A. Nance, J.-C. Yang, P. J. McDonnell, R. A. Cone, E. J. Duh and J. Hanes, J. Controlled Release, 2013, 167, 76–84 CrossRef CAS.
- M. M. Le Goff and P. N. Bishop, Eye, 2008, 22, 1214–1222 CrossRef CAS.
- E. Pacella, F. Pacella, G. De Paolis, F. R. Parisella, P. Turchetti, G. Anello and C. Cavallotti, Ophthalmol. Eye Dis., 2015, 7, OED.S17204 CrossRef.
- S. R. Eisenberg and A. J. Grodzinsky, J. Orthop. Res., 1985, 3, 148–159 CrossRef CAS.
- P. N. Bishop, Encyclopedia of the Eye, Elsevier, 2010, pp. 37–43 Search PubMed.
- C. Zhang, H. Zhang, H. A. Millán Cotto, T. L. Boyer, M. R. Warren, C.-M. Wang, J. Luchan, P. K. Dhal, R. L. Carrier and A. G. Bajpayee, Biomater. Sci., 2024, 12, 634–649 RSC.
- C. Zhang, T. V. Pathrikar, H. M. Baby, J. Li, H. Zhang, A. Selvadoss, A. Ovchinnikova, A. Ionescu, S. Chubinskaya, R. E. Miller and A. G. Bajpayee, Small Methods, 2024, e2301443 CrossRef PubMed.
- C. Zhang, T. He, A. Vedadghavami and A. G. Bajpayee, MethodsX, 2020, 7, 100882 CrossRef CAS.
- T. He, C. Zhang, T. Colombani, S. A. Bencherif, R. M. Porter and A. G. Bajpayee, Osteoarthr. Cartilage, 2023, 31, 187–198 CrossRef CAS PubMed.
- J. Li, S. Shi, X. Zhang, S. Ni, Y. Wang, C. A. Curcio and W. Chen, Invest. Ophthalmol. Vis. Sci., 2012, 53, 5675 CrossRef CAS.
- S. di Lauro, D. Rodriguez-Crespo, M. J. Gayoso, M. T. Garcia-Gutierrez, J. C. Pastor, G. K. Srivastava and I. Fernandez-Bueno, Mol Vis, 2016, 22, 243 CAS.
- V. Alarautalahti, S. Ragauskas, J. J. Hakkarainen, H. Uusitalo-Järvinen, H. Uusitalo, J. Hyttinen, G. Kalesnykas and S. Nymark, Invest. Ophthalmol. Visual Sci., 2019, 60, 1914–1927 CrossRef CAS.
- A. Vedadghavami, E. K. Wagner, S. Mehta, T. He, C. Zhang and A. G. Bajpayee, Acta Biomater., 2019, 93, 258–269 CrossRef CAS.
- A. G. Bajpayee, C. R. Wong, M. G. Bawendi, E. H. Frank and A. J. Grodzinsky, Biomaterials, 2014, 35, 538–549 CrossRef CAS.
- A. Vedadghavami, S. Mehta and A. G. Bajpayee, J. Visualized Exp., 2020 DOI:10.3791/61340-v.
- A. G. Bajpayee and A. J. Grodzinsky, Nat. Rev. Rheumatol., 2017, 13, 183–193 CrossRef CAS PubMed.
- A. Vedadghavami, C. Zhang and A. G. Bajpayee, Nano Today, 2020, 34, 100898 CrossRef CAS PubMed.
- M. R. Warren, A. Vedadghavami, S. Bhagavatula and A. G. Bajpayee, Biophys. J., 2022, 121, 3542–3561 CrossRef CAS.
- S. R. Eisenberg and A. J. Grodzinsky, J. Biomech. Eng., 1987, 109, 79–89 CrossRef CAS.
- S. Krag, T. Olsen and T. T. Andreasse, Invest. Ophthalmol. Visual Sci., 1997, 38, 357–563 CAS.
- M. R. Warren and A. G. Bajpayee, Bioelectricity, 2022, 4, 248–258 CrossRef.
- E. Han, S. S. Chen, S. M. Klisch and R. L. Sah, Biophys. J., 2011, 101, 916–924 CrossRef CAS.
- X. Lux Lu, C. Miller, F. H. Chen, X. Edward Guo and V. C. Mow, J. Biomech., 2007, 40, 2434–2441 CrossRef.
- S. Mehta, T. L. Boyer, S. Akhtar, T. He, C. Zhang, A. Vedadghavami and A. G. Bajpayee, Osteoarthr. Cartilage, 2023, 31, 780–792 CrossRef CAS PubMed.
- A. Vedadghavami, T. He, C. Zhang, S. M. Amiji, B. Hakim and A. G. Bajpayee, Acta Biomater., 2022, 151, 278–289 CrossRef CAS PubMed.
- C. D. DiDomenico, M. Lintz and L. J. Bonassar, Nat. Rev. Rheumatol., 2018, 14, 393–403 CrossRef CAS PubMed.
- R. Gaudana, H. K. Ananthula, A. Parenky and A. K. Mitra, AAPS J., 2010, 12, 348–360 CrossRef CAS PubMed.
- K. M. Hämäläinen, K. Kananen, S. Auriola, K. Kontturi and A. Urtti, Invest. Ophthalmol. Visual Sci., 1997, 38, 627–634 Search PubMed.
- E. M. Espana and D. E. Birk, Exp. Eye Res., 2020, 198, 108137 CrossRef CAS PubMed.
- M. Sridhar, Indian J. Ophthalmol., 2018, 66, 190 CrossRef PubMed.
- X. Ruan, Z. Liu, L. Luo and Y. Liu, BMJ Open Ophthalmol., 2020, 5, e000459 CrossRef PubMed.
- Y. Komai and T. Ushiki, Invest. Ophthalmol. Vis. Sci., 1991, 32, 2244–2258 CAS.
- G. S. Manning, J. Chem. Phys., 1969, 51, 924–933 CrossRef CAS.
- G. S. Manning, J. Phys. Chem. B, 2007, 111, 8554–8559 CrossRef CAS.
- C. H. Dohlman, B. O. Hedbys and S. Mishima, Invest. Ophthalmol. Visual Sci., 1962, 1, 158–162 CAS.
- B. O. Hedbys and C. H. Dohlman, Exp. Eye Res., 1963, 2, 122–129 CrossRef CAS PubMed.
- A. O. Eghrari, S. A. Riazuddin and J. D. Gottsch, in Progress in Molecular Biology and Translational Science, eds. J. F. Hejtmancik and J. M. Nickerson, Academic Press, 2015, pp.7–23 Search PubMed.
- S. J. Tuft and D. J. Coster, Eye, 1990, 4, 389–424 CrossRef PubMed.
- L. Peeters, N. N. Sanders, K. Braeckmans, K. Boussery, J. Van de Voorde, S. C. De Smedt and J. Demeester, Invest. Ophthalmol. Vis. Sci., 2005, 46, 3553 CrossRef PubMed.
- K. Braeckmans, L. Peeters, N. N. Sanders, S. C. De Smedt and J. Demeester, Biophys. J., 2003, 85, 2240–2252 CrossRef CAS PubMed.
- R. Y. Foos and N. C. Wheeler, Ophthalmology, 1982, 89, 1502–1512 CrossRef CAS PubMed.
- J. Sebag, Encyclopedia of the Eye, Elsevier Science and Technology, St. Louis, MO, 1st edn, 2010, pp.307–315 Search PubMed.
- M. Inatani and H. Tanihara, Prog. Retinal Eye Res., 2002, 21, 429–447 CrossRef CAS PubMed.
- A. Vedadghavami, B. Hakim, T. He and A. G. Bajpayee, Arthritis Res. Ther., 2022, 24, 172 CrossRef CAS.
- C. C. Young, A. Vedadghavami and A. G. Bajpayee, Bioelectricity, 2020, 2, 68–81 CrossRef PubMed.
- J. F. Liang and V. C. Yang, Biochem. Biophys. Res. Commun., 2005, 335, 734–738 CrossRef CAS PubMed.
- C. Foged and H. M. Nielsen, Expert Opin. Drug Delivery, 2008, 5, 105–117 CrossRef CAS PubMed.
- C. Foerg, U. Ziegler, J. Fernandez-Carneado, E. Giralt and H. P. Merkle, Pharm. Res., 2007, 24, 628–642 CrossRef CAS PubMed.
- I. Nakase, A. Tadokoro, N. Kawabata, T. Takeuchi, H. Katoh, K. Hiramoto, M. Negishi, M. Nomizu, Y. Sugiura and S. Futaki, Biochemistry, 2007, 46, 492–501 CrossRef CAS PubMed.
- X. Luan, K. Sansanaphongpricha, I. Myers, H. Chen, H. Yuan and D. Sun, Acta Pharmacol. Sin., 2017, 38, 754–763 CrossRef CAS PubMed.
- H. Kalra, C. G. Adda, M. Liem, C.-S. Ang, A. Mechler, R. J. Simpson, M. D. Hulett and S. Mathivanan, Proteomics, 2013, 13, 3354–3364 CrossRef CAS PubMed.
- X.-C. Jiang and J.-Q. Gao, Int. J. Pharm., 2017, 521, 167–175 CrossRef CAS PubMed.
- T. He, C. Zhang, A. Vedadghavami, S. Mehta, H. A. Clark, R. M. Porter and A. G. Bajpayee, J. Controlled Release, 2020, 318, 109–123 CrossRef CAS PubMed.
- T. He, I. Shaw, A. Vedadghavami and A. G. Bajpayee, Cartilage, 2022, 13, 194760352210930 CrossRef PubMed.
- E. K. Wagner, A. Vedadghavami, T. D. Jacobsen, S. A. Goel, N. O. Chahine and A. G. Bajpayee, Sci. Rep., 2020, 10, 12017 CrossRef CAS PubMed.
- C. Zhang, A. Vedadghavami, T. He, J. F. Charles and A. G. Bajpayee, ACS Nano, 2023, 17, 6649–6663 CrossRef CAS PubMed.
- M. Ma, B. Li, M. Zhang, L. Zhou, F. Yang, F. Ma, H. Shao, Q. Li, X. Li and X. Zhang, Exp. Eye Res., 2020, 191, 107899 CrossRef CAS PubMed.
- S. Lenzini, R. Bargi, G. Chung and J.-W. Shin, Nat. Nanotechnol., 2020, 15, 217–223 CrossRef CAS PubMed.
- H. Lund-Andersen, J. Sebag, B. Sander and M. La Cour, Adv. Organ Biol., 2005, 181–194 Search PubMed.
- E. Martinez-Enriquez, A. de Castro, A. Mohamed, N. G. Sravani, M. Ruggeri, F. Manns and S. Marcos, Invest. Ophthalmol. Vis. Sci., 2020, 61, 11 CrossRef PubMed.
- Y. S. Rabinowitz, Surv. Ophthalmol., 1998, 42, 297–319 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tb00849a |
| This journal is © The Royal Society of Chemistry 2024 |