Open Access Article
Open Access ArticleConcentration-tuned diverse response to selective biogenic amines using a reusable fluorophore: monitoring protein-rich food spoilage†
Madhuparna
Chakraborty
 ,
Pandiyan
Sivasakthi
,
Pandiyan
Sivasakthi
 ,
Pralok K.
Samanta
,
Pralok K.
Samanta
 and
Manab
Chakravarty
and
Manab
Chakravarty
 *
*
Department of Chemistry, Department of Chemistry, Birla Institute of Technology and Science-Pilani, Hyderabad Campus, Hyderabad-500078, India. E-mail: manab@hyderabad.bits-pilani.ac.in1
First published on 21st February 2024
Abstract
Maintaining the freshness of food is essential for a healthy and quality life. Nevertheless, it remains a global challenge. Hence, an easy detection and monitoring protocol would be highly desirable. A cyanoacrylic acid (CAA)-based fluorophore is manifested as a reusable platform that responds diversely against different concentrations of selective aliphatic biogenic amines (BAs) in both solution and vapor phases. Slow spoilage of the protein-rich food is progressively monitored through emission shifts visible to the naked eye. This fluorophore provides easy and naked-eye detection of the BA vapor through a change in emission, i.e., red → orange → orange-yellow → cyan → green and quantum yield enhancement, which occur in stepwise increments of vapor concentrations. The probe design includes π-conjugated functionalized fluorescent molecules linked to multiple twisting sites, resulting in both solid and solution-state emission. The attached carboxylic acid responds quickly with selective BAs, mainly putrescine (PUT), cadaverine (CAD), and spermidine (SPM), where the concentration-based emission variation has appeared to be distinct and prominent against PUT [sensitivity (μM): 2 (solution); 3.3 (vapour)]. The selectivity towards diamine can be clarified by the formation of carboxylic acid salts and the consequent proton exchanges between free and protonated amines. In addition, –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N···H interaction is likely to develop within this ammonium carboxylate system, providing extra stability. Such ammonium carboxylate salt formation and gradual change in the molecular arrangement, resulting in symmetry development, are validated by FT-IR and wide-angle X-ray diffraction studies. Besides, this fact is supported by DFT studies that validate intramolecular H-atom exchange between free amine and ammonium salt units. A fluorophore-coated coverslip, filter paper, or silica gel-coated Al-plate is fruitfully utilized to detect the freshness of fish and chicken, which reveals the potential of this probe to prevent food waste and control food safety.
N···H interaction is likely to develop within this ammonium carboxylate system, providing extra stability. Such ammonium carboxylate salt formation and gradual change in the molecular arrangement, resulting in symmetry development, are validated by FT-IR and wide-angle X-ray diffraction studies. Besides, this fact is supported by DFT studies that validate intramolecular H-atom exchange between free amine and ammonium salt units. A fluorophore-coated coverslip, filter paper, or silica gel-coated Al-plate is fruitfully utilized to detect the freshness of fish and chicken, which reveals the potential of this probe to prevent food waste and control food safety.
Introduction
The entwined relationship between societal development and nutrition will collapse if food safety and food waste are poorly monitored. Hence, measuring and tracking the freshness of food from producers to consumers has become a looming challenge globally.1–3 Among many other parameters, biogenic amines (BAs) are essential biomarkers to detect the freshness of protein-rich food.4–7 The BAs are important metabolites for cell growth and neural transmission but also cause acute toxicity after reaching a certain level (>20 mg kg−1).8 By considering different classifications of BAs, aliphatic ones such as putrescine (PUT), cadaverine (CAD), and spermidine (SPM) offer a significant presence to flora and fauna due to an easy enzymatic and thermal decarboxylation of the corresponding amino acids.9,10 Both CAD and PUT are foul-smelling compounds found in decaying animals and cause food poisoning, gastrointestinal damage, tachycardia, asthma, and abnormal blood pressure. Further, it affects the mammalian brain by influencing depression by augmenting the toxic effect of histamine and is responsible for bad breath and the foul smell of decaying corpses.11,12 CAD and PUT have been found in nature, emanating from flowers in the forest area.13 The enzymatic conversion of these BAs shows a significant demand for rapid and ultrasensitive detection to maintain food freshness. The conventional approaches to detecting BAs include expensive analytical techniques like HPLC, GC, capillary electrophoresis, and electrochemical and enzymatic methods.14–16 However, the detection involves time-consuming, complex, and costly procedures and skilled professionals.17,18 Therefore, developing a handy and portable sensor for rapid on-site BA detection is essential. In response to these challenges, various cost-effective optical-based sensors have emerged as sensitive protocols;19–22 however, the pitfalls of these probes include (i) non-reusability and poor color-contrast, (ii) scarcity of dyes for concentration-tuned detection of BAs (an urgent need to avoid food wastage) (iii) multistep synthesis of the probe using a high energy-consumption protocol (iv) restriction to solution state emission only (v) lack of sensitivity and selectivity (vi) most importantly, lack of vapor-responsive dyes, and (vii) practical applicability for effective and quick testing of chicken or fish.23–25 Moreover, a typical fluorophore shows its response only by fluorescence quenching mechanism via a chemical reaction of an amine with an electrophilic center (emission quenching is not preferred due to misguided promises).26–28 In this context, AIE-switching was realized to detect BAs with a dye that was synthesized using harsh reaction conditions, and detection in the solid state could have been more impressive and recyclable.29,30 Fluorophores were established to recognize PUT,31,32 but fluorophore-based special recognition for selective PUT is sporadic. Apart from a report on the polymer-appended system,18 small molecules, A-B (Fig. 1), were identified earlier for selective PUT detection with significant drawbacks.33,34This work focuses on the design of new small fluorophores (mainly TCNA, Fig. 1) that reversibly and quickly detect PUT selectively with higher sensitivity, along with an indication for the presence of two other vital BAs [cadaverine (CAD) and spermidine (SPM)] in both solution and vapor phases. However, the response difference between CAD/SPM and PUT solutions can be readily envisioned through the naked eye. Excitingly, this probe exhibits a concentration-based rapid detection of PUT vapor [0.292 mg L−1 (3.3 μM)], and the solid-state emission variation is very much detectable (red to green for PUT; red to yellow for CAD/SPM) through naked-eye under a 365 nm UV lamp irradiation. Although these probes exhibit a dual-state emissive nature,35,36 we highlight the solid-state emission switching by exposing BA vapors due to their practical desirability and handling suitability. A detailed inspection shows a change in the wide-angle X-ray diffraction (WAXRD) pattern of the emissive solid exposed to diverse PUT vapor concentrations. Experimental outcomes confirm amino-carboxylate salt formation with BAs, keeping a free –NH2 group intact. The selectivity with biogenic amines corresponds to the cooperativity effect to produce an intermolecular –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interaction (a rare event) with the appropriate cyano-functionality. A gradual enhancement in BA concentrations and modulation of supramolecular interactions will lead to the stepwise formation of salt and intermolecular H-bonding. The variation in crystallinity (as shown in WAXS) and FT-IR data validate the role of intermolecular –C
N⋯H interaction (a rare event) with the appropriate cyano-functionality. A gradual enhancement in BA concentrations and modulation of supramolecular interactions will lead to the stepwise formation of salt and intermolecular H-bonding. The variation in crystallinity (as shown in WAXS) and FT-IR data validate the role of intermolecular –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions. Such an approach is inimitable in this valuable research field, facilitating easy freshness detection for protein-rich food. The concentration-based variation in emission is also commendable and suitable for intelligent food packaging.1 Further, a new inexpensive thin film-based platform is invented to detect spoiled fish and chicken by observing the gradual emission change through the naked eye. This strategy also enables the platform to be reusable more than six times. The molecular packing and available void space were also considered for effective interactions of specific BAs with admirable sensitivity.
N⋯H interactions. Such an approach is inimitable in this valuable research field, facilitating easy freshness detection for protein-rich food. The concentration-based variation in emission is also commendable and suitable for intelligent food packaging.1 Further, a new inexpensive thin film-based platform is invented to detect spoiled fish and chicken by observing the gradual emission change through the naked eye. This strategy also enables the platform to be reusable more than six times. The molecular packing and available void space were also considered for effective interactions of specific BAs with admirable sensitivity.
Results and discussion
Design and synthesis of simple and suitable molecular probes
While designing a suitable molecule (Fig. 1), the following points are considered: (a) strong emission in solution: extensive π-conjugations with a prominent fluorophore, i.e. anthracene core (b) emissive in solid: introduction of a tiny flanking donor,37,38 thiophene, and another substituted vinyl stator, which offers conformational twists and avoid strong intermolecular π⋯π stacking and restricted motion in the solid state, resulting in radiative decay profiles (emissive solid), (c) site for amine attack as primarily employed in the literature39–41 through (i) Michael addition where amine acts as a substantial Michael donor, and therefore Michael acceptor site was generated in this probe by adding acrylic dicyano (TCN) or cyano-acid (TCNA) functionality, (ii) –COOH group was connected to rapidly produce the amine carboxylate salt and change the probe's emission profile (d) acrylic cyano group can facilitate inter/intramolecular interactions between cyano and –NH2 groups of BAs.42,43 Moreover, this whole strategy can adjust its reversibility by neutralizing the salt using acid vapor and making this probe recyclable.The designed molecule was conveniently synthesized without expensive metal/ligand-catalyzed reactions, as shown in Scheme 1. Thiophene-linked anthracenyl phosphonate was introduced earlier using a simple electrophilic aromatic substitution reaction.37,38 The same phosphonate was utilized in a partial olefination reaction with a controlled concentration of terephthaldehyde to produce TAA in 50% yield in pure form (unreacted phosphonate was recovered). As a suitable substrate for the Knoevenagel reaction, TAA was converted to our target molecules TCN and TCNA, which are ideal for Michael reactions and salt formation. All the synthesized compounds are well characterized by FT-IR, multinuclear-NMR, Mass, and single-crystal X-ray diffraction studies (only TCNA).
Photophysical studies
Photophysical features were examined at the outset for all three compounds: TAA, TCN, and TCNA. The maximum absorption at λmax ∼ 397–405 nm (for TAA and TCNA) and λmax ∼ 422–440 nm (for TCN) appeared in the absorption spectra by changing different polar solvents (Fig. S1, ESI†). The emission profiles demonstrate a nearly no/feeble emission at λmax ∼ 500 nm for TAA in the solution state, possibly because of multiple rotors against the olefinic stator. Even solvent polarity variation did not exhibit visually detectable variation in the emission (Fig. S2a, ESI†). However, TCNA and TCN display superior emission profiles in different solvents [relative Φf (%) = 9 and 11, respectively, in 1,4-dioxane; (Fig. S2b and S3, ESI†)] as compared to TAA. Both TCN and TCNA exhibited a bathochromic shift and weaker emission in relatively more polar solvents such as MeCN. The yellow emission of TCNA at λmax ∼ 560 nm in dioxane was chosen for subsequent studies to avoid solubility and safety issues. The optical purity of the sample is further validated with almost similar absorption and excitation spectra (Fig S3a, ESI†).Aggregation-induced emission (AIE)-feature
Twisted rotors across the olefinic stator can restrict intramolecular motion and enhance the fluorescence (FL) in the aggregate state.44,45 With this thought, AIE studies were performed for all these compounds. Interestingly, TAA and TCN showed an interesting AIE feature with an intensity enhancement upon gradually adding water [fraction of water fw (v/v)] in 10 μM acetonitrile solution. The FL intensity was boosted 10–14 times with a typical redshift [Fig. S4, S5 for spectra, Fig. S7a, b for I/I0vs. fw plot (I0: FL intensity before adding water and I: FL intensity after the addition of water), ESI†] for both TAA and TCN, which turned into yellow and red emissive molecules in the aggregate state, respectively. However, TCNA was not emissive in the aggregate form; instead, it shows FL quenching under this acetonitrile/water binary solvent system (see Fig. S6 and S7c, ESI†). As TCNA differs only with a –CO2H functionality, its dimerization tendency in the aggregate state might favor emission quenching. Comparative detailed studies to decipher the dissimilar AIE features have recently been explored.38a However, the responses of amines in the solution and aggregated state are herein investigated.Fluorescence response influenced by amines in solution/aggregated states
Indeed, all these suitably functionalized fluorophores deserve responses against amines. The aldehyde group of TAA can spontaneously react with amine, and the resulting emission may differ.46a Further, TCN holds an interactive site for the amine, an influential Michael donor.46b Therefore, we have initially explored all these probes with various amines, including BAs. Before any spectroscopic investigations, fluorophores TAA and TCN (10 μM) in 1,4-dioxane solution were screened with a wide range of amines such as aniline, triphenylamine, diphenylamine, triethylamine, 2-phenylethylamine, tryptamine, N-ethyldiisopropylamine, spermine, spermidine (SPM), 1,3-diamino propane (1,3-DAP), putrescine (PUT), cadaverine (CAD) and 1,6-diaminohexane. However, no significant color change was visualized (Fig. S8a and b, ESI†) for weakly emissive TCN and TAA solutions. Further, decently emissive AIE states of TAA and TCN molecules were treated with the amine solution (1 mM in DMAc). Unfortunately, the emission from the aggregated dye for TCN and TAA remained unchanged (Fig. S9 and S10, ESI†). It indicates that these amines do not respond against aldehyde or towards Michael addition reactions under ambient conditions. A strong electron-rich environment of such fluorophores might hinder the approach of amines towards reactive centers, and extensive electronic conjugations reduce the nucleophilicity of –CHO functionality.Next, numerous amines (1 mM in DMAc) were added to the yellow emissive TCNA solution in dioxane (10 μM). The optical density is somewhat reduced, but there is no appreciable change in the absorption λmax. However, a blue-shifted emission from yellow to cyan or green was visualized in the naked eye only for three vital BAs (PUT, CAD, and SPM), which are realized to be responsible for protein-rich food spoilage. Moreover, the maximum shift of 32 nm, resulting in bluish cyan, is notable upon adding PUT solution (see Fig. 2). SPM displays cyano emission with a 23 nm shift, but CAD shows a green emission with only a 15 nm shift. These results designate the specificity towards BAs in the solution state, and that too with slightly longer chain lengths because 1,3-DAP and ethylene diamine did not display any emission change (Fig. 2).
This observation has motivated us to focus on these three BAs individually. Upon treating these individual amines with TCNA solution, a superior sensitivity was noted with PUT compared to CAD and SPM, as presented in Fig. 3 (see Table S1 for the gradual shifts, ESI†). A clean emission shift was detectable with the lowest concentration of PUT as 1–5 μM and CAD/SPM as 4–12 μM, much lower than the required freshness detection limit (in the range of mM).8b
The absorption spectra of TCNA did not change upon gradually adding PUT, CAD, and SPM (Fig. S11, ESI†). It indicates no significant interactions between TCNA and these BAs in the solution state under ambient light. However, the observed emission changes upon the gradual addition of BAs were notable. It is pertinent to specify that the acidity of the dye and basicity of BAs will increase in the excited state, promoting amine-acid interactions.47,48 The newly formed ammonium carboxylate anion will diminish electron flow from the aromatic rings to the electron-deficient cyanocarboxylate part, resulting in a blue-shifted emission. Also, lifetime studies (Fig. S12 and Table S2, ESI†) indicate a diminution in the excited state lifetime on gradual enhancement in amine concentration, demonstrating a dynamic quenching behavior.
Photophysical features in the solid state and response to BA vapors
While exposing to various amine vapors on the thin film of TCNA using 30 μL of amine liquid in a 200 mL closed jar under identical conditions for 15–20 min, we recognized a blue-shifted emission upon exposure to most of the amines (Fig. 4) except ammonia and spermine (see Fig. 4b: images 2, &12). Like the solution state, blue-shifted emission in a solid state is due to the lack of electronic conjugation after the ammonium carboxylate salt formation (discussed later). After exposing the amine fumes for half an hour, we recorded the emission spectrum and absolute Φf (%) for all these films (see Table S3, ESI†). These important BAs display the maximum blue-shifted emission and enhanced Φf (%), as presented in Fig. 4c. Notably, the emission shift (91 nm) with PUT vapor was the longest (Fig. 4a–c) and could be easily discriminated from the changes created by other amines (including other BAs).With the estimated vapor pressure (mm Hg) at 25 °C, [Put 2.3, Cad 0.97, SPM 0.027], PUT can better flow in the air freely, at least with a half-life of 6 h.13 Therefore, we emphasized the detection of BA vapors using the probe's solid-state emission because it will offer a handy approach for practical and on-site applications. We proceeded with TCNA as it was selectively and rapidly reactive with three essential BAs. Although the compound TCNA was AIE-inactive, it displays perceptible red emission (λmax = 604 nm, absolute Φf = 2%) in the crystalline state. Multiple twisted sites in the probe and numerous supramolecular interactions offer only weak intermolecular π⋯π interactions, leading to its behavior as a solid-state emitter (detailed crystal structure/packing: vide infra). Strong hydrogen bonding and multiple intermolecular interactions in the crystal lattice classically generate the red-shifted emission with reduced quantum yield. To expose amine vapor to these solid-emitting probes, a thin film on a glass platform would be the most convenient to handle. Therefore, a thin film was prepared by dropping the TCNA solution (1 mM in 1,4-dioxane) on a glass coverslip and subsequent evaporation to dryness at 25 °C. The red emissive thin film was exposed separately to these three BA vapors (Fig. 4c). A unique and quick blueshift of 91 nm (red to green) was observed after exposure to PUT vapor, whereas 48 and 54 nm blueshift (red to yellow) were observed in the thin film emission with CAD and SPM vapor, respectively (Fig. 4c). Interestingly, the solid-state fluorescence intensity [absolute Φf (%)] was significantly improved in all cases after the amine exposures (Fig. 4c). This result looks unique for PUT with an appreciable blueshift compared to the other two BAs, and the reasons are elucidated with evidence.
Concentration-variation emission studies
The above outcomes on abrupt emission shift motivated us to investigate concentration-based emission variation under the vapor phase. At the outset, the probe (1 mM) was drop cast on a silica gel-coated Al-plate and fixed at the wall of the 200 mL glass jar [Fig. 5a (under ambient light)], closed tightly with a rubber septum, and properly enclosed with Teflon tape (Fig. 5a). Six such jars were made ready for the screening and PUT liquid was added through the septum in different μL volumes (5, 7, 12, 30, and 40, Fig. 5b) using a Hamiltonian microsyringe, and the jar was kept at 25 °C. While monitoring the color change using a 365 nm UV lamp, emission changes from red to (i) orange (for 5 μL), (ii) orange-yellow (for 7 μL), (iii) yellow (for 12 μL), and finally (iv) cyan (for both 30 and 40 μL) were identified.As stated before, the vapor pressure (at 25 °C) of PUT and CAD are different, i.e., 2.3 mm Hg and 0.921 mm Hg, respectively. Also, the boiling point of PUT is lower (159 °C) than CAD (179 °C); thus, the evaporation process of PUT is much more favorable than CAD. Again, inside the closed jar, PUT vapor touches the fluorescent dye and reacts to form salt. Therefore, PUT vapor will continue reacting with the dye until it reaches the vapor–liquid equilibrium in the closed jar. During this process, the degree of various interactions between the fluorophore and PUT molecules will be altered, resulting in emission variation. Hence, after reaching equilibrium at a specific concentration, emission remains unchanged even after increasing the amount of PUT.
Such diverse emissions at different concentrations were further investigated spectroscopically; therefore, we repeated the same experiment with a clean TCNA-drop-casted glass coverslip (1 mM in 1,4-dioxane). The red-emissive coverslip was affixed inside the wall of the glass jar (volume of 200 mL). The glass jar was sealed with a septum and Teflon tape (Fig. 5a and b). The emission color change of the fixed coverslip was monitored using a 365 nm UV lamp. Different volumes of PUT were added to the bottom of the sealed chamber at 25 °C. Although the emission color change was rapid and visually detectable, the emission spectra (Fig. 6a) were recorded after 10 min. The observed emission shift slightly differs between silica gel-coated Al-plates and glass surfaces, being a chemically different entity.
 | ||
| Fig. 6 (a) Emission spectra for all the coverslips at different PUT concentrations. (b) a plot of emission wavelength (nm) and absolute Φf (%) with [PUT] (mg L−1). | ||
The PUT liquid [1 μL (4.43 mg L−1)] was added precisely and found an instant 28 nm blue-shifted emission from red (λmax= 604 nm) to yellowish orange (λmax= 576 nm) along with an enhancement in Φf (%) [see Table S4 and Fig. 5c]. Gradually, the experiment was performed with different amine concentrations by increasing the amount of PUT. The red emissive film became yellow with a blueshift of 52 nm with 1.7 times enhancement in Φf (%). All these emission variations are rapid (within 10 s), and even the shifts remain intact till the next PUT addition. When the volume of PUT was increased to 15 μL, there was a 73 nm blueshift with a greenish emission with almost unaffected Φf (%). Still, on further increasing of PUT amount [20, 25 or 30 μL], an overall 91 nm blue-shifted emission [513 nm, green] was noticed along with impressive heightening in the Φf (%) from ∼3 to ∼10. Further, the sensitivity of TCNA was tested by repeating the same experiment with 2 μL of PUT in a 6 L closed jar, and a 28 nm blue-shifted emission was detected [red to yellowish orange; Fig. S13A, ESI†], which further validates the PUT vapor detection at a much lower concentration as low as 3.3 μM. Hence, wavelength shift and quantum yield with PUT concentration are summarized in Fig. 6b. While performing the same concentration-tuned emission studies with SPM and CAD, the red emissive film was converted to yellow [only 54 nm and 49 nm blueshift for SPM and CAD, respectively, Fig. S13B (ESI†); for other related data, spectra, and plots, see Fig. S14 and Tables S5, S6, ESI†]. Unlike PUT, we hardly find any additional variations from the yellow emissive state for SPM and CAD, even after adding excess (30 μL) amines. This result also dictates that SPM is considerably less responsive than CAD because of the much lower vapor pressure for SPM. Notably, Φf (%) was significantly higher (14.4) for SPM than CAD (7.4). The green coloration results from the direct touch of PUT liquid and vapor, while CAD and SPM demonstrate only yellow emission. Thus, this probe is unique in recognizing PUT compared to other common BAs such as SPM and CAD.
Mechanistic insight for the concentration-guided emission
We attempted to find the mechanistic viewpoint on how the probe, TCNA, interacts with such specific BAs. It assists in interpreting selective and sensitive concentration-based detection of PUT. Although CAD and SPM responded significantly, the concentration-based variation was prominent only with PUT due to its slightly higher basicity and decent vapor pressure than CAD and SPM.13,49 However, the carboxylic acid functionality would immediately form an ammonium carboxylate salt, changing the overall molecular electronic distribution within TCNA. A rapid formation of ammonium salt, i.e., TCNASA [no intramolecular proton exchange (IPE)] or TCNASB [with IPE], consisting of supramolecular interactions, is anticipated at 25 °C (Fig. 7).Experimental and theoretical studies were conducted to support this TCNAS formation. Firstly, ammonium carboxylate salt formation is recognized upon slow exposure of PUT vapor as obtained from FT-IR studies (Fig. S15a, ESI†) that depict a broad envelope at 3750–2750 cm−1 with the disappearance of the O–H stretching (cm−1) at ∼3641 & 3067–2855 for pure TCNA. Subsequently, the appearance of a broad peak at 3413 cm−1 and merged stretching (cm−1) at ∼1600 and 1500 (NH bending vibration) designate the presence of an –NH3+ unit.50a Moreover, the vanishing of intense C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching (cm−1) at 1700, followed by the arrival of two bands at 1630–1567, further confirms the formation of a negatively charged carboxylate ion (Fig. S15a, ESI†).50b Next, we attempted to locate the –C
O stretching (cm−1) at 1700, followed by the arrival of two bands at 1630–1567, further confirms the formation of a negatively charged carboxylate ion (Fig. S15a, ESI†).50b Next, we attempted to locate the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching to validate the presence of –C
N stretching to validate the presence of –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions. However, strong-electronic conjugations of TCNA offer a very weak –C
N⋯H interactions. However, strong-electronic conjugations of TCNA offer a very weak –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N stretching at 2223 cm−1,51 gradually weakening with a 12 cm−1 shift due to the lowering of force constant. Lastly, we could notice a broad signature at ∼2142 cm−1 for TCNAG (TCNA-Green form), which could be attributed to the presence of the –C
N stretching at 2223 cm−1,51 gradually weakening with a 12 cm−1 shift due to the lowering of force constant. Lastly, we could notice a broad signature at ∼2142 cm−1 for TCNAG (TCNA-Green form), which could be attributed to the presence of the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H motif and other merged combination bands. The electron-withdrawing properties of the carboxylic acid group will be lost after turning into an ammonium carboxylate. Thus, the π-electron delocalization will end mainly in the cyano group, developing intermolecular –C
N⋯H motif and other merged combination bands. The electron-withdrawing properties of the carboxylic acid group will be lost after turning into an ammonium carboxylate. Thus, the π-electron delocalization will end mainly in the cyano group, developing intermolecular –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions. Notably, accumulated negative charge on nitrogen will enable the resonance-affected H-bonding, as reported after analyzing several amino and cyano-based systems.52 The linear cyano group can also act as an H-bond acceptor by simultaneously interacting with two amine groups.53 However, such possibilities may not effectively operate in this system as the dicyano compound TCN was inactive in recognizing the diamines. Next, 1H-NMR-spectrum reveals the disappearance of carboxyl proton from TCNA (Fig. S16A-a, ESI†) after titrating with PUT, further justifying the salt formation. There is a slight upfield shift of ∼δ 0.1 in trans-olefinic protons (J = 16.0 Hz) of TCNA, the closest part of the reactive center (Fig. S16A-b, ESI†), demonstrating the marginal enhancement of electron density around those protons after the amine treatment. Moreover, one proton will undergo intramolecular exchange to form a stable cyclic complex TCNASB, which is still eligible for –C
N⋯H interactions. Notably, accumulated negative charge on nitrogen will enable the resonance-affected H-bonding, as reported after analyzing several amino and cyano-based systems.52 The linear cyano group can also act as an H-bond acceptor by simultaneously interacting with two amine groups.53 However, such possibilities may not effectively operate in this system as the dicyano compound TCN was inactive in recognizing the diamines. Next, 1H-NMR-spectrum reveals the disappearance of carboxyl proton from TCNA (Fig. S16A-a, ESI†) after titrating with PUT, further justifying the salt formation. There is a slight upfield shift of ∼δ 0.1 in trans-olefinic protons (J = 16.0 Hz) of TCNA, the closest part of the reactive center (Fig. S16A-b, ESI†), demonstrating the marginal enhancement of electron density around those protons after the amine treatment. Moreover, one proton will undergo intramolecular exchange to form a stable cyclic complex TCNASB, which is still eligible for –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions. However, we attempted to locate the change in the 13C-NMR signal for –C
N⋯H interactions. However, we attempted to locate the change in the 13C-NMR signal for –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N to recognize –C
N to recognize –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions, but the lack of solubility became detrimental in identifying the difference. However, no product from Michael-type addition was indicated in the 1H NMR spectrum.
N⋯H interactions, but the lack of solubility became detrimental in identifying the difference. However, no product from Michael-type addition was indicated in the 1H NMR spectrum.
Nevertheless, gradual salt formation followed by the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions would stabilize the overall entities. Therefore, we were interested in finding the stepwise changes in each wide-angle X-ray scattering (WAXS) pattern (Fig. 8).
N⋯H interactions would stabilize the overall entities. Therefore, we were interested in finding the stepwise changes in each wide-angle X-ray scattering (WAXS) pattern (Fig. 8).
 | ||
| Fig. 8 Changes in the WAXS pattern at different emissions (images are taken under a 365 nm UV-lamp). | ||
Few distinct diffractions of the probe TCNA could be identified before exposing PUT. A slight exposure demonstrates changes in the WAXS pattern where a few diffractions appeared and a few disappeared. However, the intensity of the diffracted signals is enhanced in the TCNAO (orange) sample with slightly higher interplanar distances. On further concentration-enhancement of the PUT vapor, the yellow emitting solid TCNAY displayed only a few diffractions (mostly broad). Notably, the sample appeared as a slightly gummy solid (different texture in SEM) at this stage, which could be the origin of such a broader diffraction pattern. However, the green emitting TCNAG shows a well-diffracted WAXS pattern, indicating a higher crystallinity and symmetry development in the sample due to the salt formation and generation of the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H complex. Thus, the molecule tends stepwise to transform to a more crystalline phase upon diamine fuming. The amine vapor instantly reacts with the acid probe, and liquid–vapor equilibrium is accelerated by enhancing the concentration of amine vapor. All these experimental findings confirm the requirement of diamines and address how concentration variation gradually changes the molecular arrangement, contributing to emission switching. To get better insight into the morphological transitions of all the forms of TCNA after exposing PUT vapor, scanning electron microscopic (SEM) images were captured (Fig. S15b, ESI†). It clearly specifies the morphological differences in various emissive forms. TCNA mainly shows an aggregated semi-crystalline state for the probe, which is gradually distributed in TCNAY, and finally, there is a development in crystallinity with TCNAG. This result is in agreement with the WAXS pattern discussed above.
N⋯H complex. Thus, the molecule tends stepwise to transform to a more crystalline phase upon diamine fuming. The amine vapor instantly reacts with the acid probe, and liquid–vapor equilibrium is accelerated by enhancing the concentration of amine vapor. All these experimental findings confirm the requirement of diamines and address how concentration variation gradually changes the molecular arrangement, contributing to emission switching. To get better insight into the morphological transitions of all the forms of TCNA after exposing PUT vapor, scanning electron microscopic (SEM) images were captured (Fig. S15b, ESI†). It clearly specifies the morphological differences in various emissive forms. TCNA mainly shows an aggregated semi-crystalline state for the probe, which is gradually distributed in TCNAY, and finally, there is a development in crystallinity with TCNAG. This result is in agreement with the WAXS pattern discussed above.
All these deviations in IR/NMR, SEM images, and WAXS patterns of TCNA after exposing PUT vapor are primarily accountable for the observed outcomes. Moreover, all these variations display a variance in the ground state, showing a difference in the absorption spectra (Fig. S17, ESI†). The UV absorption of TCNA solid indicates a red dye (λmax = 532 nm), which shifted to 438 nm (yellow solid, 94 nm blueshift) for TCNAY after exposure to PUT vapor. Finally, TCNAG displayed the absorption λmax at 420 nm (yellowish green, overall 112 nm blueshift in solid state absorption spectra). It designates that the changes in ambient light are also visually promising and easily detectable. Further, LC-MS studies (in the negative ion mode, Fig. S18, ESI†) prove the existence of TCNASB, which appeared with a peak at 543 [M–2H]− along with the molecular ion peak at 456 [M–H]−.
In addition, a few more control experiments were performed to establish the mechanistic insights. A few molecules, such as 4-aminobutanol, N,N-dimethylbutane-1,4-diamine and lysine (acting as bases and H-bond acceptors), were treated with the TCNA solution. In the solution state, no significant change in the emission has been observed (Fig. S19, ESI†) because 4-aminobutanol would possibly prefer forming intra/intermolecular H-bonding, and the interactions between these amine analytes and TCNA solution are not impressive. Further, the solid probe was treated with these liquid amines to investigate the response in the vapor phase. Although these liquids have much higher boiling points (°C) [206 for 4-aminobutanol and 181 for N,N-dimethylbutane-1,4-diamine] and lower vapor pressure (at 25 °C) than PUT, we attempted with these liquid amines (30 μL) inside a closed 200 mL jar. Both 4-aminobutanol and N,N-dimethylbutane-1,4-diamine responded in the vapor phase (Fig. S20, ESI†), resulting in a yellow emissive solid. Notably, diamine showed a better response with a 44 nm shift. Thus, vapor can interact better after getting trapped inside the layer of a solid-emissive probe. Still, unlike other amines, PUT exhibits the unique green emission. Thus, these molecules are interesting to investigate regarding the emission features, particularly the vapor concentration-based emission change. The difference in vapor pressure, basicity, and boiling point of these amines compared to PUT creates the interaction variation.
To further validate the proposed H-bonding between –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H, the emission shift was studied by experimenting with an H-bonding competing solvent, such as MeOH (Fig. S21, ESI†). A 32 nm blue-shifted emission was noticed in a 1,4-dioxane solution of TCNA after adding PUT. However, the shift was reduced to only 23 nm in the presence of 10% MeOH in 1,4-dioxane (Fig S21, ESI†). In addition, the polarity enhancement reduced the emission intensity, as expected. We speculate that the presence of MeOH restricts the –C
N⋯H, the emission shift was studied by experimenting with an H-bonding competing solvent, such as MeOH (Fig. S21, ESI†). A 32 nm blue-shifted emission was noticed in a 1,4-dioxane solution of TCNA after adding PUT. However, the shift was reduced to only 23 nm in the presence of 10% MeOH in 1,4-dioxane (Fig S21, ESI†). In addition, the polarity enhancement reduced the emission intensity, as expected. We speculate that the presence of MeOH restricts the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions within the dye and PUT unit in the solution state and, therefore, makes a barrier to bring a green emission.
N⋯H interactions within the dye and PUT unit in the solution state and, therefore, makes a barrier to bring a green emission.
Explanation with single crystal X-ray structure/molecular packing
To decipher a detailed view of the crystal lattice, we determined the crystal structure and molecular packing of the compound TCNA, driven by various intermolecular interactions listed in ESI† (Fig. 9b and Fig. S22, S23, Tables S7, S8, ESI†). Two symmetrically independent molecules are in an asymmetric unit with multiple twists (Fig. 9a). Such a twisted structure prevents strong π⋯π interactions [the centroid distances (Å) are within the range of 4.77, 8.54, and 11.53, Fig. S22b, ESI†], resulting in an emissive solid. In addition, multiple supramolecular interactions such as acid dimerization and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions holding diverse directionalities could be responsible for reducing the overall quantum yield. A strong intermolecular H-bonding appeared with a distance of 1.75–1.79 Å and a bond angle of ∼176° (Fig. 9b). The –C
N⋯H interactions holding diverse directionalities could be responsible for reducing the overall quantum yield. A strong intermolecular H-bonding appeared with a distance of 1.75–1.79 Å and a bond angle of ∼176° (Fig. 9b). The –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N distance of 1.150 Å specifies the presence of conjugations.50 However, weaker –C
N distance of 1.150 Å specifies the presence of conjugations.50 However, weaker –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions are noticed in the crystal within the distance range of 2.6–2.7 Å, and nonlinear bond angles of –C
N⋯H interactions are noticed in the crystal within the distance range of 2.6–2.7 Å, and nonlinear bond angles of –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H make these existing interactions much more vulnerable (Fig. 9b and Fig. S22a, ESI†). Even after several attempts, a suitable single crystal of TCNA-PUT complex could not be generated after the PUT exposure, possibly because of PUT's instability (hygroscopic and tendency to react with O2, CO2) in the air.
N⋯H make these existing interactions much more vulnerable (Fig. 9b and Fig. S22a, ESI†). Even after several attempts, a suitable single crystal of TCNA-PUT complex could not be generated after the PUT exposure, possibly because of PUT's instability (hygroscopic and tendency to react with O2, CO2) in the air.
 | ||
| Fig. 9 (a) Single-crystal X-ray structures with few selected bond angles (°), (b) partial molecular packing of TCNA with few significant noncovalent interaction distances (Å) and angles (°) (further details: see Fig. S22a and S23a, ESI†). | ||
The blue-shifted emission can be described as the relative reduction of conjugation in TCNAS. Moreover, the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N unit prefers a slightly tilted conformation rather than a linear site.53,54 After the generation of the –C
N unit prefers a slightly tilted conformation rather than a linear site.53,54 After the generation of the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H system, the overall conjugation in the system is significantly reduced, and therefore, a 91 nm blueshift in emission appeared. Thus, the system becomes more twisted, which prevents π⋯π stackings and leads to a radiative decay. The enhancement in the quantum yield can be attributed to the well-known crystallization-induced emission enhancement effect (CIEE).55–57 Upon PUT fuming, the molecule experiences a few weaker supramolecular interactions, enabling the rigidification of the molecular structure and blocking the non-radiative decay.
N⋯H system, the overall conjugation in the system is significantly reduced, and therefore, a 91 nm blueshift in emission appeared. Thus, the system becomes more twisted, which prevents π⋯π stackings and leads to a radiative decay. The enhancement in the quantum yield can be attributed to the well-known crystallization-induced emission enhancement effect (CIEE).55–57 Upon PUT fuming, the molecule experiences a few weaker supramolecular interactions, enabling the rigidification of the molecular structure and blocking the non-radiative decay.
Further, the selective and sensitive gradual detection of PUT can be supported with the void space available (367 Å3 obtained from Mercury; Fig. S23b, ESI†) in the crystal lattice of TCNA. The PUT vapor can be easily trapped in the space available and produce the ammonium carboxylate salt that offers successive intramolecular proton exchange/–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interactions. Close contact in a confined system would desire gradual and effective interactions. Thus, the available void space and the carboxylic acid group can help interactions for most of the amines exposed in the solid state and display emission shift. However, monoamine would not have any scope for further proton exchange and, therefore, can be differentiated. Unlike solution states, ethylene–diamine and 1,3-DAP can interact similarly to other BAs. However, the emission shifts and quantum yield enhancements were not impressive.
N⋯H interactions. Close contact in a confined system would desire gradual and effective interactions. Thus, the available void space and the carboxylic acid group can help interactions for most of the amines exposed in the solid state and display emission shift. However, monoamine would not have any scope for further proton exchange and, therefore, can be differentiated. Unlike solution states, ethylene–diamine and 1,3-DAP can interact similarly to other BAs. However, the emission shifts and quantum yield enhancements were not impressive.
Reversibility nature
The reusable fluorophores to detect BAs are hardly established in this field because the fluorophores mostly react with the BAs, and therefore, returning to the original state is problematic. After realizing the possible perception, we expected the reversibility nature of the probe in the presence of acid. The acid (HCl) fumes can destroy the salt and other interactions, enabling it to return to the initial stage for further use. We have conducted the reversibility experiment and identified the visually detected color changes for the 6th time with intact emission quantum yield, as presented in Fig. S24 (ESI†). Thus, we have established this probe as a reusable platform for practical applications.Theoretical investigation
To attain further rationalization on our thought of TCNA-PUT interactions, optimized molecular structures of TCNA and TCNA-PUT were determined using DFT (density functional theory)/TD (time-dependent)-DFT studies using CAM-B3LYP58 functional and 6-31+G(d) basis set. The calculated absorption and emission λmax match well with the experimentally observed results. The theoretically optimized structures (Fig. 10 and Table S9, ESI†) of individual species displayed a significant difference in the bond lengths (Å)/angles (°) compared to TCNA crystal, as expected. TCNA shows a linear –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N functionality with a bond length of 1.164 with a –C–C
N functionality with a bond length of 1.164 with a –C–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N angle of 177 (almost linear, Fig. S25A, ESI†). Nevertheless, the TCNA-PUT complex offers the transfer of the acidic proton of amine nitrogen (bond length 1.753 Å), and a subsequent intramolecular proton exchange between two amine functionalities via forming a stable cyclic complex TCNASB (Fig. 10) was noticed, as discussed earlier in Fig. 7.
N angle of 177 (almost linear, Fig. S25A, ESI†). Nevertheless, the TCNA-PUT complex offers the transfer of the acidic proton of amine nitrogen (bond length 1.753 Å), and a subsequent intramolecular proton exchange between two amine functionalities via forming a stable cyclic complex TCNASB (Fig. 10) was noticed, as discussed earlier in Fig. 7.
 | ||
| Fig. 10 Optimized molecular structure (gaseous state) of (a) TCNASB: TCNA-PUT and (b) TCNA-1,3-DAP with selected angles (°) and interatomic distances/bond length (Å). (Partial structure is shown here for clarity; For further details, see Fig. S22B, ESI†). | ||
The related bond/interatomic distances and angles (Fig. 10) would support this intramolecular proton exchange. The enhancement in the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N bond length (1.168 Å) was noted due to –C
N bond length (1.168 Å) was noted due to –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interaction (2.373 Å). It is pertinent to mention that the –C
N⋯H interaction (2.373 Å). It is pertinent to mention that the –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interaction in the molecular crystal packing of TCNA lies between 2.583–2.741 Å, representing much stronger interactions between the amine NH and –C
N⋯H interaction in the molecular crystal packing of TCNA lies between 2.583–2.741 Å, representing much stronger interactions between the amine NH and –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N functionality in the gaseous state. These calculations validate our mechanistic assumption on the selectivity of diamines. The nitrile group also became nonlinear after the interactions with diamines, and the slight change in the dihedral angle also reduced the electronic π-conjugation, responsible for the blueshift.
N functionality in the gaseous state. These calculations validate our mechanistic assumption on the selectivity of diamines. The nitrile group also became nonlinear after the interactions with diamines, and the slight change in the dihedral angle also reduced the electronic π-conjugation, responsible for the blueshift.
Further, to understand the deviation with smaller amines such as 1,3-DAP, we focused on the TCNA-1,3-DAP interactions, and therefore, the molecular geometry was optimized (Fig. 10b). It primarily disclosed two notable differences that include a strong salt formation with 1,3-DAP (being a relatively stronger base) and weak hydrogen exchange between the two amine groups because of the non-preferable ∠NH⋯N 136° angle and longer N–H⋯N interatomic distance 1.961 Å compared to TCNA-PUT system (Fig. 10a). Moreover, –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H interaction is weaker in 1,3-DAP, as validated with a relatively larger interatomic distance of 2.392 Å (2.373 Å for TCNA-PUT). Thus, the other –NH2 functionality of DAP was relatively more accessible, which could be the reason for being nonresponsive in solution state emission. However, these groups will be confined to the solid state and display an emission shift.
N⋯H interaction is weaker in 1,3-DAP, as validated with a relatively larger interatomic distance of 2.392 Å (2.373 Å for TCNA-PUT). Thus, the other –NH2 functionality of DAP was relatively more accessible, which could be the reason for being nonresponsive in solution state emission. However, these groups will be confined to the solid state and display an emission shift.
Further, the highest-occupied molecular orbital (HOMO) of TCNA is mainly built up with an anthracene core. At the same time, the lowest unoccupied molecular orbital (LUMO) is occupied by the cyano/acid attached ring (Fig. S26, ESI†). The PUT-TCNA complexes do not change the HOMO significantly, but the LUMO formation differs due to the carboxylate formation, which enhances electron density. Thus, LUMO is destabilized by ∼0.9 eV, leading to the blueshift (Table S9, ESI†).
Detection of the freshness of fish and chicken
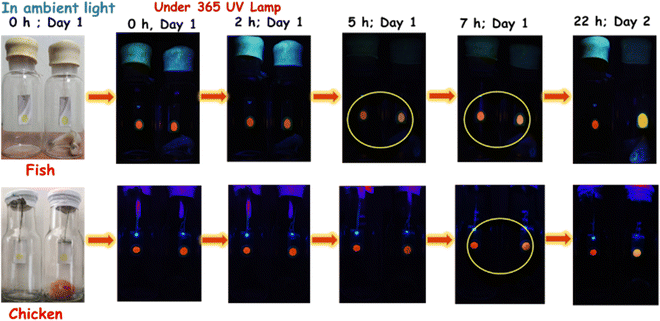
The remarkable blue-shifted emission and sensitivity, specifically towards PUT (a vital marker for detection of food's freshness) in the vapor phase, have encouraged us to monitor the freshness of market-available fresh fish and chicken samples (on a small scale). The probe was drop cast on a Whatman filter paper (cheapest and easiest to introduce), fixed inside the glass jar containing the fish/chicken sample, and sealed with a rubber septum, and an empty jar was used as a control to monitor the changes (see Fig. 11). The emission change was surveyed under a 365 nm UV lamp with different time intervals. A slight emission shift was observed after 5 h for fish; however, the difference was intensified after 7 h for chicken. The change would be more promising if a large sample scale is tested within a small period.The orange emissive filter paper became yellow after 22 h, whereas the control spot remained unchanged. The same experiment was also performed with a piece of fish pellet, and a similar change was noticed after 5 h (Fig. 11). In the case of the fresh chicken sample, the emission started changing within 7 h (a little slower in forming BAs vapor). After 22 h, the change was detectable. The direct touch of spoiled chicken could also be identified with its rapid emission change from red to green. Thus, the quick and distinct emission color change using TCNA has been successfully established to detect fish/chicken freshness using an inexpensive strategy.
Experimental
Materials and general conditions
All the chemicals were purchased from Merck, and the solvents for column chromatography were purchased from Finar. Spectroscopic grade solvents used for UV-Vis and fluorescence spectroscopy were purchased from Finar. All the photophysical studies were performed at room temperature, 298 ± 2 K. 1H, and 13C NMR spectra were recorded on Bruker 400 MHz spectrometers with operating frequencies of 101 MHz for 13C. Chemical shifts (δ) are reported in ppm relative to the residual solvent signal (δ 2.50, δ 7.26 for 1H NMR and δ 39.5, δ 77.0 for 13C NMR).Synthesis and characterizations
1H NMR (400 MHz, CDCl3) δ 8.41 (d, J = 8.9 Hz, 2H), 7.89 (d, J = 8.8 Hz, 2H), 7.63–7.54 (m, 3H), 7.42 (dd, J = 8.7, 6.5 Hz, 2H), 7.31 (dd, J = 5.2, 3.4 Hz, 1H), 7.19 (m, 1H), 4.29 (d, J = 22.5 Hz, 2H), 4.08–3.83 (m, 4H), 1.17 (t, J = 7.1 Hz, 6H).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at room temperature. The reaction mixture was heated to reflux for 7 hours at 80 °C. The reaction was monitored by TLC. Upon completion, the reaction mixture was allowed to cool to room temperature. The resulting precipitate was washed with cold ethanol and dried under a high vacuum to obtain an orange solid.
1) at room temperature. The reaction mixture was heated to reflux for 7 hours at 80 °C. The reaction was monitored by TLC. Upon completion, the reaction mixture was allowed to cool to room temperature. The resulting precipitate was washed with cold ethanol and dried under a high vacuum to obtain an orange solid.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
Single crystal measurement
All measurements were carried out using a Rigaku XtaLAB P200 diffractometer using multi-layer mirror monochromated Cu-Kα radiation (λ = 1.54184 Å). The measurements were taken at a temperature of −173 ± 1 1 °C up to a maximum 2θ value of 149.81°. CrysAlisPro was used to collect and process data (Rigaku Oxford Diffraction). Cu-Kα radiation has a linear absorption coefficient, μ, of 18.391 cm−1. The transmission factors ranged from 0.227 to 0.593 when an empirical absorption correction was implemented. The data were corrected for the Lorentz and polarization effects. The structure was solved using direct methods (SIR2011)31 and expanded using Fourier techniques. The non-hydrogen atoms were refined anisotropically. Hydrogen atoms were purified using the riding model. All calculations were performed using the Olex2 crystallographic software package except for the refinement, which was performed using SHELXL Version 2014/7.WAXS measurement
WAXS measurements were carried out using a Xenocs Nano-inXider SW-L SAXS/WAXS system with a dual detector with Cu Kα micro focus within the range of 5° to 60° at a scanning speed of 2° min−1. The sample was placed and spread over a Kapton tape, and data were recorded in transmission geometry. The results are presented after background subtraction using xenoc XSACT software for all the samples.Methods and measurements
All the fluorophores were synthesized using a method reported earlier. The electronic absorption spectra were recorded with UV 3600 Plus (Shimadzu). The FL spectra were recorded using a Hitachi spectrofluorometer (F7000) using a 1 cm path-length quartz cuvette. Origin Pro 8.5 software is used to plot the obtained data.Preparation of solutions
The stock solution of 10−3 M TCNA was prepared in 1,4-dioxane. The 10−3 M stock solution for all the amines was prepared in N,N-dimethylacetamide (DMAc).Absorption and FL studies
Absorption studies were carried out with the sample (2 mL, 10 μM) in a quartz cuvette (1 cm × 1 cm). The wavelength range was kept within 550 to 200 nm. Emission spectra of the same sample are instantaneously recorded with the range from 415 to 800 nm, with a PMT voltage of 400 eV and excitation slit/emission slit 5.0.Absolute quantum yield measurement
The solid-state absolute quantum yield measurement used a calibrated integrating sphere method with an absolute error of ±2%.Time-resolved decay measurement
Detection limit calculation
A series of fluorometric titrations were conducted to detect the amount of PUT with a 10 μM solution of TCNA.Study of TCNA coated thin films for visual detection of putrescine
For the thin film study, a 10−3 M solution of TCNA was drop-cast on thin glass coverslips and dried at room temperature. The dried glass coverslips were affixed on the wall of a 200 mL glass bottle sealed with a septum. Different volumes of putrescine were added, and the observed fluorescence color switching was instantaneous. All the images were taken with a Xiaomi Mi. A3 (f/1.8, 1/3, ISO250) after 15 min. Other information is articulated in detail in the ESI.†Paper strip sensing study
For the solid-state sensing study, a solid TCNA sample was dispersed on strips of Whatman filter paper (15 cm × 4.5 cm) and dropped with different concentrations of putrescine (2 μL) solution in DMAc. The image was taken with a Xiaomi Mi. A3 (f/1.8, 1/3, ISO250) after 1 min. However, the blue-shifted emission color change was instantaneous. Other information is articulated in detail in the ESI.†Theoretical details
The ground state (S0) geometry of the molecules was optimized using the density functional theoretical (DFT) method with the CAM-B3LYP56 functional and 6-31+G(d) basis set. After geometry optimization, frequency calculations were done to confirm the absence of any unstable normal modes. The excited state (S1) calculation uses the TD (time-dependent)-DFT method. All the DFT and TD-DFT calculations were performed using the Gaussian 09 package.59 All the MOs are visualized in Avogadro software60 and reported an isosurface value equal to 0.02 a.u.Conclusions
In conclusion, a suitably designed small molecule with a cyanoacrylic acid opening was realized as a solution and solid-state emitter. Comparative studies with analogous probes revealed that the BAs did not respond toward aldehyde functionality or Michael acceptors introduced in this set of analogs. The TCNA probe could selectively recognize PUT, CAD, and SPM in the solution state, with a higher blue shift for PUT in μM level sensitivity. However, this probe shows its uniqueness with its more glowing blue shift in the solid state for all three amines specifically, while PUT shows a maximum 91 nm shift from red to green. This probe detected PUT vapor concentration (as low as 0.292 mg L−1) with a distinct color change at each level. Further, this probe was repeatedly used for the detection of PUT. Our experimental (FT-IR, NMR) outcomes infer that TCNA forms a salt with PUT vapor and gradually turns into a complex through –C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N⋯H bonding and proton exchange behavior with –NH3+ and NH2, which was inefficient for TCNA-DAP entity. The DFT studies also confirm this observation. The emission shifts with intensity enhancement were monitored by WAXS studies that offer a variation in the diffraction pattern with a gradual development of well-diffracted signals. Finally, coverslip/Al-coated silica-gel/paper-based platforms were substantiated to successfully detect the freshness of chicken and fish. The same platform would be perfect for determining the spoiled protein-rich food at different stages of putrefaction, preventing food waste, and enriching the food safety solution.
N⋯H bonding and proton exchange behavior with –NH3+ and NH2, which was inefficient for TCNA-DAP entity. The DFT studies also confirm this observation. The emission shifts with intensity enhancement were monitored by WAXS studies that offer a variation in the diffraction pattern with a gradual development of well-diffracted signals. Finally, coverslip/Al-coated silica-gel/paper-based platforms were substantiated to successfully detect the freshness of chicken and fish. The same platform would be perfect for determining the spoiled protein-rich food at different stages of putrefaction, preventing food waste, and enriching the food safety solution.
Author contributions
Madhuparna performed all the reactions, conducted all the experiments, and presented. The manuscript draft was partially prepared. Manab designed the molecule, analyzed the results, and prepared the final manuscript. PKS and PS performed the DFT/TDDFT studies and generated the relevant figures.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank LSRB [389/FSH&ABB/2021] for financial support. Special thanks: BITS-Pilani Hyderabad Campus X-ray diffraction and NMR facility are acknowledged. Special thanks to Prof. Aravind Raghavan for helping collect and process WAXS data and fruitful discussions. MC thanks BITS-Pilani Hyderabad Campus for MC's fellowship. We thank Dr. Suman for helping us solve the crystal structure.References
- R. Jia, W. Tian, H. Bai, J. Zhang, S. Wang and J. Zhang, Amine-responsive cellulose-based ratiometric fluorescent materials for real-time and visual detection of shrimp and crab freshness, Nat. Commun., 2019, 10, 1–8 CrossRef PubMed.
- S. K. Kannan, B. Ambrose, S. Sudalaimani, M. Pandiaraj, K. Giribabu and M. Kathiresan, A review on chemical and electrochemical methodologies for the sensing of biogenic amines, Anal. Methods, 2020, 12, 3438–3453 RSC.
- R. S. Andre, L. A. Mercante, M. H. M. Facure, R. C. Sanfelice, L. Fugikawa-Santos, T. M. Swager and D. S. Correa, Recent Progress in Amine Gas Sensors for Food Quality Monitoring: Novel Architectures for Sensing Materials and Systems, ACS Sens., 2022, 7, 2104–2131 CrossRef CAS PubMed.
- T. Gao, E. S. Tillman and N. S. Lewis, Detection and Classification of Volatile Organic Amines and Carboxylic Acids Using Arrays of Carbon Black-Dendrimer Composite Vapor Detectors, Chem. Mater., 2005, 17, 2904–2911 CrossRef CAS.
- N. Kaur, S. Chopra, G. Singh, P. Raj, A. Bhasin, S. K. Sahoo, A. Kuwar and N. Singh, Chemosensors for biogenic amines and biothiols, J. Mater. Chem. B, 2018, 6, 4872–4902 RSC.
- M. Gao, S. Li, Y. Lin, Y. Geng, X. Ling, L. Wang, A. Qin and B. Z. Tang, Fluorescent Light-Up Detection of Amine Vapors Based on Aggregation-Induced Emission, ACS Sens., 2016, 2, 179–184 CrossRef.
- H. Ye, S. Koo, B. Zhu, Y. Ke, R. Sheng, T. Duan, L. Zeng and J. S. Kim, Real-Time Fluorescence Screening Platform for Meat Freshness, Anal. Chem., 2022, 94, 15423–15432 CrossRef CAS PubMed.
- (a) M. Schirone, L. Esposito, F. D’Onofrio, P. Visciano, M. Martuscelli, D. Mastrocola and A. Paparella, Biogenic Amines in Meat and Meat Products: A Review of the Science and Future Perspectives, Foods, 2022, 11, 788–812 CrossRef CAS PubMed; (b) B. del Rio, B. Redruello, D. L. M. Linares, V. Ladero, P. R. Madiedo, M. Fernandez, M. C. Martin and M. A. Alvarez, The biogenic amines putrescine and cadaverine show in vitro cytotoxicity at concentrations that can be found in foods, Sci. Rep., 2019, 9, 120 CrossRef PubMed.
- A. I. Danchuk, N. S. Komova, S. N. Mobarez, S. Y. Doronin, N. A. Burmistrova, A. V. Markin and A. Duerkop, Optical sensors for determination of biogenic amines in food, Anal. Bioanal. Chem., 2020, 412, 4023–4036 CrossRef CAS PubMed.
- L. Jiang, H. Ye, D. Ma, J. Rodrigues, R. Sheng and D. Min, A smartphone-adaptable fluorescent sensing tag for non-contact and visual monitoring of the freshness of fish, Analyst, 2022, 147, 923–931 RSC.
- M. Ikeda, T. Yoshii, T. Matsui, T. Tanida, H. Komatsu and I. Hamachi, Montmorillonite− supramolecular hydrogel hybrid for fluorocolorimetric sensing of polyamines, J. Am. Chem. Soc., 2011, 133, 1670–1673 CrossRef CAS PubMed.
- L. Wunderlichová, L. Buňková, M. Koutný, P. Jančová and F. Buňka, Formation, degradation, and detoxification of putrescine by foodborne bacteria: a review, Compr. Rev. Food Sci. Food Saf., 2014, 13, 1012–1030 CrossRef.
- K. Saeki, K. Ikari, H. Yokoi, S.-I. Ohira, H. Okochi and K. Toda, Biogenic Diamines and Their Amide Derivatives Are Present in the Forest Atmosphere and May Play a Role in Particle Formation, ACS Earth Space Chem., 2022, 6, 421–430 CrossRef CAS.
- A. Onal, A review: current analytical methods for the determination of biogenic amines in foods, Food Chem., 2007, 103, 1475–1486 CrossRef.
- M. Papageorgiou, D. Lambropoulou, C. Morrison, E. Kłodzinska, J. Namiesnik and J. Płotka-Wasylka, TrAC, Trends Anal. Chem., 2018, 98, 128–142 CrossRef CAS.
- N. Verma, V. Hooda, A. Gahlaut, A. Gothwal and V. Hooda, Literature update of analytical methods for biogenic amines determination in food and beverages, Crit. Rev. Biotechnol., 2020, 40, 1–14 CrossRef CAS PubMed.
- (a) Z. Song, M. Cai, Y. Zhao, M. Jin and S. Deng, Simultaneous determination of 14 biogenic amines and metabolites in bullfrog blood using UFLC-MS/MS with SPE, Anal. Methods, 2015, 7, 7436–7442 RSC; (b) R. L. Self, W.-H. Wu and H. S. Marks, Simultaneous quantification of eight biogenic amine compounds in tuna by matrix solid-phase dispersion followed by HPLC–orbitrap mass spectrometry, J. Agric. Food Chem., 2011, 59, 5906–5913 CrossRef CAS PubMed.
- G. Munzi, S. Failla and S. D. Bella, Highly selective and sensitive colorimetric/fluorometric dual mode detection of relevant biogenic amines, Analyst, 2021, 146, 2144–2151 RSC.
- B. Prusti, S. Tripathi, A. Jain and M. Chakravarty, Concentration-Guided Visual Detection of Multiphase Aliphatic Biogenic Amines through Amine–Phenol Recognition Using a Dual-State Emitter, ACS Appl. Mater. Interfaces, 2023, 15, 16492–16504 CrossRef CAS PubMed.
- R. Roy, A. Pramanik, T. Dutta, V. Sharma, Kovida and A. L. Koner, Harnessing solution and solid-state emissive materials from aliphatic biogenic amine-induced transient assembly and spontaneous disassembly, Mater. Chem. Front., 2022, 6, 3489–3503 RSC.
- L. Zeng, X. Xiao, H. Ye, D. Ma and J. Zhou, Fast visual monitoring of the freshness of beef using a smart fluorescent sensor, Food Chem., 2022, 394, 133489 CrossRef CAS PubMed.
- B. Zhu, L. Jiang, T. Chen, G. Bao, L. Zeng, X. Hu and H. Yuan, A colorimetric and fluorescence lighting-up probe for the determination of biogenic primary diamine during the spoilage of fish, Dyes Pigm., 2021, 186, 108963 CrossRef CAS.
- S. Sentellas, Ó. Núñez and J. Saurina, Recent Advances in the Determination of Biogenic Amines in Food Samples by (U)HPLC, J. Agric. Food Chem., 2016, 64, 7667–7678 CrossRef CAS PubMed.
- H. Ahangari, S. Kurbanoglu, A. Ehsani and B. Uslu, Latest trends for biogenic amines detection in foods: enzymatic biosensors and nanozymes applications, Trends Food Sci. Technol., 2021, 112, 75–87 CrossRef CAS.
- M. Venkateswarulu, P. Gaur and R. R. Koner, Sensitive molecular optical material for signaling primary amine vapors, Sens. Actuators, B, 2015, 210, 144–148 CrossRef CAS.
- S. Mallick, F. Chandra and A. L. Koner, A ratiometric fluorescent probe for detection of biogenic primary amines with nanomolar sensitivity, Analyst, 2016, 141, 827–831 RSC.
- L. Wang, S. Xin, C. Zhang, X. Ran, H. Tang and D. Cao, Development of a novel chromophore reaction-based fluorescent probe for biogenic amines detection, J. Mater. Chem. B, 2021, 9, 9383–9394 RSC.
- Z. Jiao, Y. Zhang, W. Xu, X. T. Zhang, H. B. Jiang, P. C. Wu, Y. Y. Fu, Q. G. He, H. M. Cao and J. G. Cheng, Highly Efficient Multiple-Anchored Fluorescent Probe for the Detection of Aniline Vapor Based on Synergistic Effect: Chemical Reaction and PET, ACS Sens., 2017, 2, 687–694 CrossRef CAS PubMed.
- P. Alam, N. L. C. Leung, H. Su, Z. Qiu, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, A highly sensitive bimodal detection of amine vapours based on aggregation induced emission of 1,2-dihydroquinoxaline derivatives, Chem. – Eur. J., 2017, 23, 14911–14917 CrossRef CAS PubMed.
- R. Roy, N. R. Sajeev, V. Sharma and A. L. Koner, Aggregation induced emission switching based ultrasensitive ratiometric detection of biogenic diamines using a perylenediimide-based smart fluorophore, ACS Appl. Mater. Interfaces, 2019, 11, 47207–47217 CrossRef CAS PubMed.
- L. Li, W. Li, X. Ran, L. Wang, H. Tang and D. Cao, A highly efficient, colorimetric and fluorescent probe for recognition of aliphatic primary amines based on a unique cascade chromophore reaction, Chem. Commun., 2019, 55, 9789–9792 RSC.
- A. Orouji, F. Ghasemi, A. Bigdeli and M. R. Hormozi-Nezhad, Providing multicolor plasmonic patterns with Au@ Ag core–shell nanostructures for visual discrimination of biogenic amines, ACS Appl. Mater. Interfaces, 2021, 13, 20865–20874 CrossRef CAS PubMed.
- F. Kong, Y. Mu, X. Zhang, Q. Lu, Z. Yang, J. Yao and L. Zhao, A novel fluorescent probe of alkyne compounds for putrescine detection based on click reaction, RSC Adv., 2022, 12, 26630–26638 RSC.
- X. Qi, W.-F. Wang, J. Wang, J.-L. Yang and Y.-P. Shi, Highly selective colorimetric detection of putrescine in fish products using o-phthalaldehyde derivatization reaction, Food Chem., 2018, 259, 245–250 CrossRef CAS PubMed.
- J. L. B. Vázquez, Y. A. A. Sánchez, L. A. R. Cortés and B. R. Molina, Dual-state emission (DSE) in organic fluorophores: design and applications, Chem. Mater., 2021, 33, 7160–7184 CrossRef.
- F. Yu, H. Zhao, Y. Li, G. Xia and H. Wang, D–A-Type fluorophores with efficient dual-state emission for imaging at ultralow concentration, Mater. Chem. Front., 2022, 6, 155–162 RSC.
- M. Chakraborty and M. Chakravarty, Variation in solvato-, AIE-and mechano-fluorochromic behavior for furanyl and thiophenyl-substituted anthranyl π-conjugates: the role of tiny flanking donor groups, Mater. Adv., 2021, 2, 6418–6427 RSC.
- (a) M. Chakraborty and M. Chakravarty, Varied Optical features and mechanofluorochromism in dicyanovinyl vs. cyanoacrylicacid-linked twisted π-conjugates: a potential reusable platform for security applications, Mater. Today Chem., 2024, 35, 101836–101845 CrossRef CAS; (b) M. Chakraborty and M. Chakravarty, Flanking-donor-controlled diversity in mechanical-force-induced reversible fluorochromism and enhanced emission for carboxylic acid and ester linked solid-state emitters, CrystEngComm, 2023, 25, 1126–1135 RSC.
- L. Wang, X. Ran, H. Tang and D. Cao, Recent advances on reaction-based amine fluorescent probes, Dyes Pigm., 2021, 194, 109634 CrossRef CAS.
- A. R. Longstreet, M. Jo, R. R. Chandler, K. Hanson, N. Zhan, J. J. Hrudka, H. Mattoussi, M. Shatruk and D. T. McQuade, Ylidenemalononitrile enamines as fluorescent “turn-on” indicators for primary amines, J. Am. Chem. Soc., 2014, 136, 15493–15496 CrossRef CAS PubMed.
- Y. Hu, T. Han, N. Yan, J. Liu, X. Liu, W.-X. Wang, J. W. Y. Lam and B. Z. Tang, Visualization of Biogenic Amines and In Vivo Ratiometric Mapping of Intestinal pH by AIE-Active Polyheterocycles Synthesized by Metal-Free Multicomponent Polymerizations, Adv. Funct. Mater., 2019, 29, 1902240 CrossRef.
- H. Ye, Y. Ke, W. Li, B. Zhu, L. Jiang, X. Hu and L. Zeng, Molecular engineering of fluorescence probe for real-time non-destructive visual screening of meat freshness, Anal. Chim. Acta, 2023, 1254, 341125 CrossRef CAS PubMed.
- D. R. Turner, A. J. Edwards and R. O. Piltz, Nitrile groups as hydrogen-bond acceptors in a donor-rich hydrogen-bonding network, CrystEngComm, 2012, 14, 6447–6464 RSC.
- P. Zhou, P. Li, Y. Zhao and K. Han, Restriction of flip-flop motion as a mechanism for aggregation-induced emission, J. Phys. Chem. Lett., 2019, 10, 6929–6935 CrossRef CAS PubMed.
- C. Zhu, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-induced emission: a trailblazing journey to the field of biomedicine, ACS Appl. Bio Mater., 2018, 1, 1768–1786 CrossRef CAS PubMed.
- (a) J. Kumpf, J. Freudenberg, K. Fletcher, A. Dreuw and U. H. F. Bunz, Detection of amines with extended distyrylbenzenes by strip assays, J. Org. Chem., 2014, 79, 6634–6645 CrossRef CAS PubMed; (b) G. Zhang, A. S. Loch, J. C. M. Kistemaker, P. L. Burn and P. E. Shaw, Dicyanovinyl-based fluorescent sensors for dual mechanism amine sensing, J. Mater. Chem. C, 2020, 8, 13723–13732 RSC.
- Z. Wen, L. J. Karas, C.-H. Wu, I. Judy and C. Wu, How does excited-state antiaromaticity affect the acidity strengths of photoacids?, Chem. Commun., 2020, 56, 8380–8383 RSC.
- K. J. Nelson, P. J. Brown, H. E. Rudel and K. Takematsu, Divergent excited state proton transfer reactions of bifunctional photoacids 1-ammonium-2-naphthol and 3-ammonium-2-naphthol in water and methanol, Phys. Chem. Chem. Phys., 2019, 21, 24383–24392 RSC.
- L. Wang, S. Xin, C. Zhang, X. Ran, H. Tang and D. Cao, Development of a novel chromophore reaction-based fluorescent probe for biogenic amines detection, J. Mater. Chem. B, 2021, 9, 9383–9394 RSC.
- (a) R. M. Silverstein, F. X. Webster and D. J. Kiemle, Spectrometric Identification of Organic compounds, John Wiley & Sons, 7th edn, 2005, pp. 102–103 Search PubMed; (b) B. C. Smith, Spectroscopy, 2018, 33(1), 14–20 CAS.
- B. C. Smith, Spectroscopy, 2019, 34(7), 18–21 Search PubMed.
- N. Ziao, J. Graton, C. Laurence and J.-Y. Le Questel, Amino and cyano N atoms in competitive situations: which is the best hydrogen-bond acceptor? A crystallographic database investigation, Acta Cryst., 2001, B57, 850–858 CAS.
- D. R. Turner, A. J. Edwards and R. O. Piltz, Nitrile groups as hydrogen-bond acceptors in a donor-rich hydrogen-bonding network, CrystEngComm, 2012, 14, 6447–6451 RSC.
- J.-Y. Le Questel, M. Berthelot and C. Laurence, Hydrogen-bond acceptor properties of nitriles: a combined crystallographic and ab initio theoretical investigation, J. Phys. Org. Chem., 2000, 13, 347–358 CrossRef CAS.
- S. Yang, S. Zhou, H. Li, Y. Nie, H. Xu, W. Liu, J. Miao, Y. Li, G. Gao, J. You and X. Jiang, Multistimuli-responsive squaraine dyad exhibiting concentration-controlled vapochromic luminescence, ACS Appl. Mater. Interfaces, 2022, 14, 16611–16620 CrossRef CAS PubMed.
- Y. Q. Dong, C. Li, W. Zhao, Y. Dong and B. Z. Tang, Stimulus responsive luminescent materials: crystallization-induced emission enhancement, J. Mol. Eng. Mater., 2013, 01, 1340010–1340013 CrossRef.
- G. Liao, J. Zhang, X. Zheng, X. Jia, J. Xu, F. Zhao, N. Wang, K. Liu, P. Chen and X. Yin, Crystallization-induced emission enhancement of highly electron-deficient dicyanomethylene-bridged triarylboranes, Chem. Commun., 2021, 57, 7926–7929 RSC.
- Takeshi Yanai, D. P. Tew and N. C. Handy, A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP), Chem. Phys. Lett., 2004, 393, 51–57 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel and G. E. Scuseriaet al., Gaussian 09, Revision D.02, Gaussian, Inc., Wallingford CT, 2009 Search PubMed.
- M. D. Hanwell, D. E. Curtis, D. C. Lonie, T. Vandermeerschd, E. Zurek and G. R. Hutchison, Avogadro: an advanced semantic chemical editor, visualization, and analysis platform, J. Cheminf., 2012, 4, 1–17 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2262179. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d3tb02569a |
| This journal is © The Royal Society of Chemistry 2024 |