Open Access Article
Open Access ArticleFunctional hemostatic hydrogels: design based on procoagulant principles
Boxiang
Zhang†
a,
Min
Wang†
a,
Heng
Tian
b,
Hang
Cai
c,
Siyu
Wu
b,
Simin
Jiao
d,
Jie
Zhao
 e,
Yan
Li
fg,
Huidong
Zhou
b,
Wenlai
Guo
e,
Yan
Li
fg,
Huidong
Zhou
b,
Wenlai
Guo
 *b and
Wenrui
Qu
b
*b and
Wenrui
Qu
b
aDepartment of Colorectal & Anal Surgery, The Second Hospital of Jilin University, Changchun 130000, Jilin Province, China
bDepartment of Hand Surgery, The Second Hospital of Jilin University, 218 Ziqiang Street, Changchun, 130041, P. R. China. E-mail: guowl19@jlu.edu.cn
cDepartment of Pharmacy, The Second Hospital of Jilin University, Changchun, 130041, P. R. China
dDepartment of Gastrointestinal Nutrition and Hernia Surgery, The Second Hospital of Jilin University, 218 Ziqiang Street, Changchun, 130041, P. R. China
eKey Laboratory of Bionic Engineering, Ministry of Education, Jilin University, Changchun, 130022, P. R. China
fTrauma and Reparative Medicine, Karolinska University Hospital, Stockholm, Sweden
gThe Division of Orthopedics and Biotechnology, Department of Clinical Science, Intervention and Technology (CLINTEC), Karolinska Institutet, Stockholm, Sweden
First published on 6th January 2024
Abstract
Uncontrolled hemorrhage results in various complications and is currently the leading cause of death in the general population. Traditional hemostatic methods have drawbacks that may lead to ineffective hemostasis and even the risk of secondary injury. Therefore, there is an urgent need for more effective hemostatic techniques. Polymeric hemostatic materials, particularly hydrogels, are ideal due to their biocompatibility, flexibility, absorption, and versatility. Functional hemostatic hydrogels can enhance hemostasis by creating physical circumstances conducive to hemostasis or by directly interfering with the physiological processes of hemostasis. The procoagulant principles include increasing the concentration of localized hemostatic substances or establishing a physical barrier at the physical level and intervention in blood cells or the coagulation cascade at the physiological level. Moreover, synergistic hemostasis can combine these functions. However, some hydrogels are ineffective in promoting hemostasis or have a limited application scope. These defects have impeded the advancement of hemostatic hydrogels. To provide inspiration and resources for new designs, this review provides an overview of the procoagulant principles of hemostatic hydrogels. We also discuss the challenges in developing effective hemostatic hydrogels and provide viewpoints.
1. Introduction
The weight of blood constitutes 7–8% of a healthy adult's body weight and bleeding more than 20% of one's total blood volume at once might result in hemorrhagic shock, organ failure, and even death. Accidental uncontrolled hemorrhage is common in high-risk environments such as battlefields and disasters.1 Furthermore, heart, liver, spine, and joint surgeries may also result in significant blood loss. Therefore, hemorrhage management in the operating room is critical.2 Hemorrhage is the leading cause of preventable deaths resulting from trauma.3 Uncontrolled bleeding caused by trauma accounts for approximately one-third of pre-hospital deaths, and it remains the second leading cause of death among civilians aged 5–44.4 Moreover, on the first day of a traumatic accident, approximately 40% of patients die from bleeding.5,6 Prompt and adequate hemostasis can significantly reduce mortality due to trauma.7 Conventional methods to stop bleeding include compression, cautery, and surgery, and traditional hemostatic materials, like hemostatic gauze and bandages, have been extensively utilized.8 However, gauze or bandages must be removed entirely after hemostasis because they do not degrade, which can lead to secondary damage, slower healing, and more discomfort.9 Recently, polymeric hemostatic materials have provided viable solutions to clinical hemostatic issues. Continuous research has been conducted on enhanced hemostatic materials, including biodegradable hydrogels, sponges, and powders. Hydrogels exhibit good adaptability to irregular wounds.10 However, some hydrogels are questioned because of their poor biocompatibility or mechanical properties.11 In addition, hydrogels can realize morphological transformation with sponges, and are endowed with additional shape memory ability.12 The high water absorption and swelling properties of sponges can endow them with great hemostatic potential,13 and the most common mechanism by which hemostatic sponges promote coagulation is by absorbing water. In addition, some sponges can also interfere with the physiological mechanism of hemostasis.14,15 But hemostatic sponges are easily infected by bacteria.13 Hemostatic powder can promote hemostasis by absorbing water, sealing wounds, and loading drugs to activate the coagulation cascade. It is worth noting that some hemostatic powders can transform into hydrogels, which improves the portability of hemostatic materials.16–19 The advantages of powder include convenience, time efficiency, diverse application methods, and suitability for large-area wounds. However, hemostatic powder cannot be applied to noncompressible torso hemostasis.20Ideal hemostatic materials should (1) stop arterial and venous bleeding in large vessels within 2 min; (2) require no prior preparation; (3) be easy to use; (4) be lightweight and durable; (5) be strong enough; (6) be safe; and (7) be inexpensive.21 Polymeric materials, particularly hydrogels, have shown great potential and garnered interest in hemostasis due to their biocompatibility, flexibility, water absorption, porosity, and multi-functionality.22–24 However, there are currently only a few hemostatic hydrogels on the market (Table 1) and the extensive data (randomized prospective clinical trials) supporting the efficacy of authorized hemostatic dressings has numerous gaps. Moreover, unclear procoagulant mechanisms may limit the further development of hemostatic hydrogels. For example, patients with coagulopathy cannot be treated with hydrogels that rely solely on the coagulation system to promote hemostasis.25,26 Thus, this review investigates hydrogel procoagulant mechanisms. References and design ideas are also offered.
| Materials | Components | Indications | Procoagulant mechanism | Advantages | Limitation |
|---|---|---|---|---|---|
| Tegaderm™ alginate dressings | Alginic acid | Cavity wounds | Concentration; | Multifunction | Cannot be surgically implanted; |
| Ca2+ ion | Diabetic ulcers | Ca2+ ion | Cannot be used on exudating wounds; | ||
| Donor sites | |||||
| Leg ulcers | |||||
| Pressure ulcers | |||||
| HemCon GuardaGel™ | Pectin | Minor bleeding from minor topical | Intervention with coagulation cascade | Flexible | Insufficient tissue; |
| Oxidized cellulose | Cuts and lacerations | Adhesion | |||
| Potassium sorbate | |||||
| Glycerol | |||||
| Ethanol | |||||
| Bondiloxs topical hemostatic dressing | Chitosan fibres | Minor external bleeding | Physical barrier | — | For trauma use only |
| Lactic acid | Exudate from sutures | ||||
| Controlling moderate to severe bleeding | |||||
| BloodSTOP iX Battle Matrix | Regenerated cellulose | External temporary control of minor to moderate bleeding of traumatic wounds. | Physical barrier – adhesion; | Completely soluble in water; | Potential risk of embolism |
| Intervention with coagulation cascade | Removable | ||||
| WoundClot | Modified cellulose | Minor skin surface bleeding wounds such as minor cuts and minor abrasions | Physical barrier – sealing, adhesion | Completely soluble in water; | Non-degradable |
| Removable | |||||
| Gel-e Flex | Palmitoyl-N-acetylglucomasine (chitosan) | Local management of bleeding such as lacerations and minor bleeding | Physical barrier | — | The service life is only 28 days |
| gel-e Flex+ gel OTC | Palmitoyl-N-acetylglucomasine (chitosan) | Local management of bleeding wounds such as minor cuts, minor lacerations and minor abrasions | Physical barrier | Service life up to two years | Non-degradable |
The procoagulant principles of hydrogels will be reviewed in terms of physical and physiological aspects. The former means that through physical features like tissue adhesion,27 surface charge,28 and water absorption,29 hydrogels create an optimal environment for hemostasis. Specific methods include concentrating hemostatic substances30 and creating physical barriers to seal wounds.31 The latter means that hydrogel accelerates hemostasis by intervening directly in its physiological processes. Specific methods include intervening blood cells,28,32 adding coagulation factors33 and procoagulant substances,34 and providing additional attachment sites for blood cells.35 Based on these cells, proteins, and factors mentioned above, functional hydrogels can cater to different hemostatic needs. In addition, numerous studies have been conducted to enhance hemostasis by combining physical and physiological dimensions.36
This review sums up the principles of functional hemostatic hydrogels that promote hemostasis along with the features, functional groups, and other components necessary for hydrogels to achieve these principles. Based on the material requirements of the body's coagulation system, we provide inspiration and references for the design of functional hemostatic hydrogels. Furthermore, we discuss the shortcomings or gaps in the current methods of hemostatic function hydrogels for promoting coagulation. Finally, suggestions for possible future directions of functional hemostatic hydrogels are given.
2. Physical hemostasis
Hydrogels promote hemostasis by altering the physical forms of the blood or tissue, referred to as physical hemostasis. The physical forms mainly include localized hemostatic substances’ concentration, wound shapes, and the adhesion location of procoagulant substances. Most hemostatic hydrogels have a physical hemostasis function, and these studies highlight properties like high swelling capacity,37 high tissue adhesion,38 and vast surface area.39 Physical hemostasis does not directly intervene in the hemostatic process but rather prepares the way for primary hemostasis.2.1. Increasing the concentration of localized hemostatic substances
Increasing the concentration of localized hemostatic substances can promote hemostasis by increasing the contact between various hemostatic substances. By absorbing wound exudate, hydrogels can increase hemostatic substances’ concentration. In this review, the two main approaches for designing hydrogels for this function will be discussed: incorporating a porous structure and enhancing hydrophilicity.Hyaluronic acid (HA) is a component of extracellular matrix (ECM) and has high biocompatibility. Zhu et al.29 introduced additional methoxy polyethylene glycol (mPEG) to create a hemostatic synthetic polymer hydrogel to facilitate the control of the structure and material composition of the hydrogel. The results showed that the hydrogel had a porous microstructure and absorbed 1235.27% of its weight of PBS when it reached swelling equilibrium. Moreover, the hydrogel group had lower blood-clotting index (BCI) values than the gauze group, and it controlled hepatic bleeding in mice within 120 s. (Fig. 1) The authors concluded that the porous structure induced by HA and mPEG led to the excellent absorption capacity of the hydrogel and further promoted hemostasis by concentrating blood.
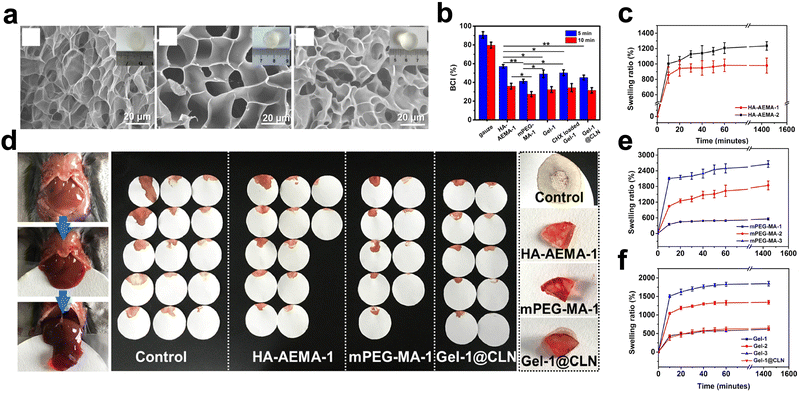 | ||
| Fig. 1 Application of microporous structured hydrogels based on hyaluronic acid and polyethene glycol in hemostasis. (a) SEM images of hydrogels. (b) blood-clotting index of hydrogels. (c, e and f) The swelling ratio of hydrogels. (d) Application in the mouse liver injury model. Reprinted from ref. 15 with permission.29 Copyright 2023 American Chemical Society. | ||
Chitosan, a polysaccharide derived from marine biological extracts,46,47 is thought to create porous microstructures by cross-linking. Bal-Ozturk et al.48 generated sponge-like nanostructured hydrogels with interlinked pore structures by cross-linking chitosan, alginic acid (AA), and the antimicrobial component ZnO. Chitosan is assumed to be primarily responsible for the pore structure. Notably, an acidic environment induces mutual repulsion among the protonated amino groups on chitosan, speeding up the hydrogel's expansion. However, ZnO induces increased intermolecular hydrogen bonding,49 which compresses the chitosan chains and reduces the material's porosity. Additionally, the increased electron bonding due to ZnO reduces the hydrogel's swelling capacity. Therefore, the authors investigated the optimal content of ZnO for hemostasis. The results of in vivo experiments showed that for the rabbit ear peripheral capillary hemorrhage model, the hemostatic time in the experimental group with the minimum amount of ZnO was 93 ± 10.41 s, and the total blood loss was 263 ± 168.62 mg.
Gelatin is an affordable, abundant, and immunogenic alternative to collagen. Additionally, it is frequently utilized to make porous microstructures.50,51 However, uneven cross-linking can result in fragility for gelatin-based hydrogels created through covalent cross-linking. Physical entanglement enables natural polymers to overcome this flaw. Zainab et al.52 prepared a novel hydrogel gelatin-TA (GelTA) using tannic acid (TA), a plant polyphenol, and gelatin. It is believed that the porous microstructure is one of the causes of the high swelling performance. At a TA concentration of 0.3 g ml−1, the porosity of the hydrogel was 72.1 ± 6.3%. Excessive TA concentration or an acidic environment would reduce the porosity and affect water absorption. Moreover, several research studies incorporated nanoparticles with an anisotropic surface charge into the gelatin mixture. For instance, LAPONITE® nano clays improve mechanical characteristics by interacting electrostatically with gelatin. The results showed that these hydrogels with a large pore size exhibited good hemostatic efficiency.53,54
Silk protein fibre is a cheap, biocompatible, natural protein. Huang et al.55 utilized a cross-linking network of silk fibroin and polyurethane to enhance the porous microstructures. The results showed that the hydrogel's water absorption was increased up to 4.3 times the original value. However, the slow nucleation rate prolonged the preparation time of SF-based hydrogels. Bian et al.56 used biocompatible ethyl lauroyl arginine hydrochloride (LAE) as a surfactant to trigger the gelation of silk proteins. The hydrogel prepared by this method has a hierarchical porous structure, which is believed to contribute to enhanced fluid absorption, and subsequent experimental results showed that this hydrogel absorbed up to 2,898% of its weight of blood in 100 s and possessed good hemostatic capacity.
γ-Poly(glutamic acid) (γ-PGA) is a natural homopolymer based on mammalian abundant glutamic acid.57,58 Chen et al.59 made a hemostatic hydrogel containing γ-PGA-DA, which could absorb water. However, like other in situ cross-linked hydrogels, the swelling capacity of γ-PGA-DA was affected by the concentration of the substance or the H2O2 level in the reaction environment. Furthermore, an excessive cross-link density would reduce the swelling capacity by reducing the hydrogel's pore size. Then, SEM images also confirmed this conclusion. In vivo experiments demonstrated that the porous structure of γ-PGA-DA accelerated hemostasis.
The swelling potential of cellulose is a result of its thick reticulated structure. Chen et al.60 produced an innovative hemostatic hydrogel by combining cellulose and pectin in an ionic liquid. The liver hemorrhage model's positive hemostatic impact was due to its unique physical structure.
Chondroitin sulfate (CS) is a glycosaminoglycan commonly sulfated in the ECM of human tissues. Zhang et al.61 synthesized a hemostatic hydrogel from CS modified with 5-hydroxytryptamine (5-HT) and serotonin. Serotonin is a procoagulant substance released from activated platelets. The results showed that the hydrogel had an irregular porous structure. The equilibrium swelling of the hydrogel was 68% in 12 h when the polymer concentration was 2 wt%. The hydrogel stopped bleeding the mouse liver hemorrhage model in about 30 s.
Arista is an absorbable hemostatic particle with a microporous structure originating from inert plants, which has a wide range of applications in the field of surgery.62 Inspired by Arista, Cui et al.63 developed a new hydrogel with a swelling rate higher than 2000% based on carboxy starch carboxylic starch. In another study, the hydrogels formed by grafting acrylic acid (AAc) and acrylamide (AAm) onto starch achieved swelling rates of up to 18000%, as reported in the results.64
Metal–organic gels (MOGs) are novel metal–organic materials with porous microstructures. Inspired by MOGs, Yang et al.45 created a multifunctional hydrogel with hemostatic properties by combining Zn2+ and 4,5-imidazole dicarboxylic acid. The SEM pictures revealed that this hydrogel has linked porous microstructures, which were believed to be responsible for swelling. Unfortunately, the results of the swelling test were not included in this study.
The hydrogel's porous microstructure aids in fluid absorption to promote hemostasis. However, for hydrogels formed by covalent bonding, excessive cross-linking or uneven microstructure distribution will reduce the porosity of the hydrogel, which will further reduce the hemostatic substances’ concentration ability.48 Second, the multifunctional hydrogels' porosity may be influenced by various materials. Thus, the appropriate ratio of raw materials is also worth considering. Finally, it is crucial to evaluate whether a significant increase in hemostatic substances’ concentration can lead to either wound dryness or excessive blood loss.
Carboxyl is a common hydrophilic group. γ-PGA has carboxyl groups in its side chain, allowing it to be grafted with various functional groups.67,71 Moreover, γ-PGA is suitable for manufacturing hemostatic materials due to its hydrophilicity. In a study on γ-PGA and poly(lysine),66 the positively charged amino in the system imparted good hydrophilicity to the hydrogel, and this new hydrogel achieved excellent concentration properties for hemostatic substances.
A superabsorbent polymer was synthesized by grafting a hydrophilic carboxymethyl group and acrylic acid onto chitosan.43,72,73 The water absorption capacity of the modified hydrogel was improved, and the swelling ratio of this hydrogel in distilled water could exceed 900. Besides, a novel hydrogel was synthesized from carboxymethyl chitosan and kappa-carrageenan, a highly hydrophilic sulfated polysaccharide polymer derived from seaweed,74,75 which is believed to promote hemostasis by concentrating blood. However, swelling test results for this hydrogel were not reported.76
A commonly used component for enhancing hydrophilicity is the hydroxyl group. Some hydrogels have been treated using hydroxyl-rich compounds to increase their water absorption. Luo et al.77 synthesized a gelatin-based hemostatic hydrogel. The hydrogel was modified using dopamine (DA), N-hydroxy succinimide (NHS), and HA. HA is hygroscopic,78 DA possesses a polyphenolic structure, and NHS is hydrophilic. The results showed that the water contact angle of the hydrogels decreased from the initial 102.5 ± 3.5° to 61.2 ± 2.6°, (Fig. 2) which proved that the hydrogel achieved better hydrophilicity. Polar groups like hydroxyl, carboxyl, and amino79 are thought to increase the hydrogels' hydrophilicity.
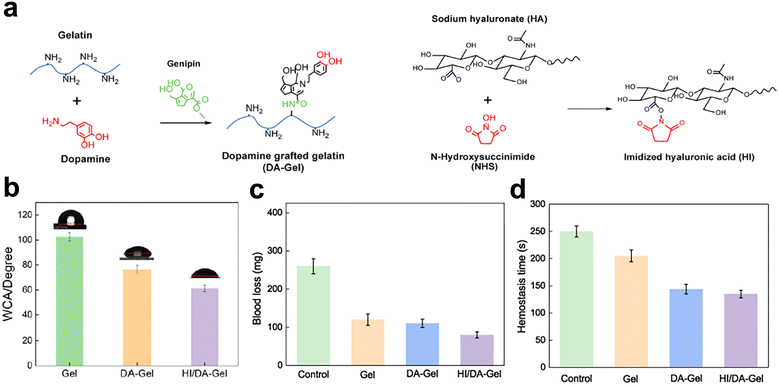 | ||
| Fig. 2 Gelatin enhanced hydrophilic and hemostatic effects by grafting hydrophilic substances. (a) and (b) Dopamine, N-hydroxy succinimide, and HA were grafted onto gelatin. (c) The water contact angle of hydrogels. (d) and (e) Blood loss and hemostasis time of hydrogels. Reprinted from ref. 63 with permission.77 Copyright 2022 Elsevier. | ||
Besides introducing hydrophilic functional groups, substances with excellent hydrophilicity have been extensively used to improve the water absorption of hemostatic hydrogels. Bovine serum albumin (BSA), a biocompatible natural protein, has great hemostatic potential due to its hydrophilicity, but its weak mechanical qualities restrict its applicability. Wang et al.80 added the inorganic salt NaCl to BSA to facilitate the gelation process by shielding the electrostatic repulsion between BSA molecules, resulting in the better water-holding capacity of the hydrogel. This BSA-based hydrogel's equilibrium swelling water content reached 76%. Hydroxyethyl cellulose (HEC), a non-ionic natural cellulose ether, exhibits good hydrophilic characteristics due to the presence of abundant hydroxyl groups.68 Wang et al.81 introduced mesoporous silica (with a porous structure and high specific surface area) based on quaternized HEC69 to synthesize the quaternized HEC/mesocellular silica foam (MCF) hydrogel sponge (QHM). The highest swelling ratio of this material reached 7013.4 ± 310.4%.
In a study examining hemostatic hydrogels focusing on water absorption and mechanical properties,82 the double bond of polybutadiene is grafted with poly(acrylic acid) (PAA) through free-radical polymerization to increase the water absorption capacity of the hydrogel. The results indicate that glassy polystyrene imparts strength to the hydrogel, while polybutadiene imparts rubbery ductility. This nanophase-separated structure enables the hydrogel to swell an order of magnitude faster than other hydrogels. Poly(ethylene glycol) (PEG) is a non-toxic, non-immunogenic material widely used in tissue engineering scaffolds.83 Moreover, PEG is considered an excellent hemostatic material due to its porous structure and hydrophilic nature, which can absorb large amounts of water from serum.
Furthermore, the design of a 4-arm PEG hydrogel, based on PEG, is a new research direction in recent years.84 The 4-arm PEG-based hydrogel achieved a swelling rate of up to 1790%. In vivo, experiments showed that bleeding from a 3 cm diameter and 1 cm depth porcine skin laceration model could be stopped within 30 s. One of the reasons for hemostasis was thought to be the high swelling rate.85 Poly(vinyl alcohol) (PVA) hydrogels have good hydrophilicity, biocompatibility, and degradability86 and have been used to manufacture hemostatic hydrogels due to their hydrophilicity.87
The major components of kaolinite are mineral kaolinite and aluminum silicate,70 and the well-known trauma dressing QuikClot Combat Gauze (QCCG) contains kaolinite. Inspired by QCCG, Tamer et al.88 cross-linked PVA with kaolin, and the physical structure of the hydrogel was thought to improve the poor absorption of QCCG for osmosis. The results showed that this hydrogel absorbed 365 ± 15% water after 1 h. Zeolite, an aluminosilicate mineral with tunable hydrophilicity,89 can rapidly concentrate blood from the site of injury. Besides, some studies posit that because zeolite has coagulation factor V and coagulation factor X on its surface, which are considered precursors for the synthesis of thrombin, zeolite can accelerate hemostasis by accelerating the physiological mechanism of hemostasis.90 However, the exothermic reaction of zeolite in use limits its application.91 Recently, the heat-release phenomenon that occurs when zeolite promotes hemostasis has been solved.92 Fathi et al.93 combined zeolite with chitosan and alginate. The objective was to exploit chitosan's biocompatibility to compensate for zeolite's drawbacks. At 1 h, the group without zeolite absorbed more PBS solution than those with zeolite. However, adding zeolite significantly increased the material's absorption capacity at 5 or 10 min, which was considered more practical. Furthermore, the zeolite group could clot whole sheep blood within 15 s, while the alginate group took 5.5 min, and the chitosan-loaded alginate group took about 2 min.
Hydrogels are modified to enhance their hydrophilicity, facilitating their ability to increase hemostatic substances’ concentration and thus promoting hemostasis. However, some studies suggest that the removal of hydrophilic hemostatic dressings after achieving hemostasis may pose difficulties, such as a risk of secondary injury like more bleeding.94,95 Besides, it remains to be studied whether excessive striving for hydrophilicity can lead to excessive blood loss.
2.2. Establishing a physical barrier
Hydrogel promotes hemostasis by adhering or sealing bleeding wounds, which is called the physical barrier of hemostasis. Traditional emergency hemostatic modalities like electrocautery can harm tissue.96 Moreover, traditional hemostatic methods have limited effectiveness on irregular or deep wounds.97 It may be more beneficial to use hydrogels to overcome these difficulties. Hydrogel adhesion to a bleeding wound is the most common way to achieve it.98,99 Many materials improve adhesion.32,100–102 Tissue sealing requires the hydrogel to have the ability to adapt to different shapes. This review will describe these hydrogels according to tissue adhesion and tissue sealing. More information on physical barriers can be found in Table 2.| Hydrogels | Material composition | Chemical modification/addition | Characterize | Characterize realization mechanism | Animal model | Ref. | |
|---|---|---|---|---|---|---|---|
| Increasing localized hemostatic substances’ concentration | Multifunctional CODM hydrogel | Polyvinylpyrrolidone | Oxidized alginate-modified | Swelling ratio: 481.2 ± 8.6% | Montmorillonite–NH2 improves water absorption | Rat femoral artery bleeding model | 237 |
| Dopamine | Carboxymethyl-modified | ||||||
| Chitosan | –NH2 | ||||||
| Montmorillonite | |||||||
| PEG–CMC–THB–PRTM hydrogel | Chitosan | Carboxymethyl-modified aldehyde group | Swelling ratio >10 times | Protamine increases crosslinking density | Rat liver hemorrhage model | 238 | |
| Polyethylene glycol | 2,3,4-Trihydroxy | ||||||
| Phenyl aldehyde | |||||||
| Protamine | |||||||
| CAO/ATR hydrogel | Chitosan | Carboxyethyl-modified | High porosity and swelling ratio (2078% ± 244%) | –OH groups on TA increased affinity for water adsorption | - | 239 | |
| Sodium alginate (OSA) | Silver | Physical interactions between polymer chains and silver-tannic acid nanoparticles (Ag–TA NPs) | |||||
| Tannic acid | |||||||
| PCbM wound dressing | Polyvinyl alcohol | Ag ion | Swelling rate is about 60% | AgCu metal–organic frameworks reduces the crosslinking density | Rat liver and tail hemorrhage model | 240 | |
| Chitosan | Cu ion | Water contact angle: 18° ± 3° | |||||
| COP hydrogel | Chitosan | Carboxymethyl-modified | Swelling ratio: 41.09 ± 2.68 g g−1 | γ-Glutamic acid has high absorbency | Rat tail hemorrhage model | 241 | |
| Dextran | Oxidized | ||||||
| Poly-γ-glutamic acid | |||||||
| HG-CB@R hydrogel | Hyaluronic acid | β-Cyclodextrin-grafted | Swelling ratio: 230.8% | Iron-affinitive BP groups enhance swelling performance | Rat liver bleeding, heart puncture, tail vein bleeding model and femoral artery bleeding model | 242 | |
| Bisphosphonate groups | |||||||
| Physical barrier | G-P15/HA-P1/G-Q1/HA-NB0.4 | Hyaluronic acid | PNIPAM-grafted gelatin (G-P) | Maximum burst pressure of 282 ± 4.1 mmHg | Abundant polar functional groups(–OH, –NH2, and –COOH) form intensive hydrogen bonds with the polar groups on the tissue surface. | Mouse liver puncture model | 243 |
| Tissue | Gelatin | The quaternary ammonium modified gelatin (G-Q) | The –CHO forms a dynamic Schiff base with the primary –NH2 on gelatin and the tissue surface | ||||
| Adhesion | The PNIPAM-grafted HA (HA-P) | The β-sheet structures of gelatin is helpful to exposure of –OH, –NH2, | |||||
| The o-nitrobenzyl (NB) modified HA (HA-NB). | |||||||
| CMCS/ODex/γ-PGA (COP) hydrogel | Chitosan | Carboxymethyl-modified | The complete gelation time is 18.49 ± 2.77 s | Triple-network structure: | Rat liver hemorrhage model | 244 | |
| Dextran | Oxidized | The intramolecular amide bonds | |||||
| γ-Polyglutamic acid | The intermolecular amide bonds | ||||||
| The Schiff base bonds | |||||||
| FCMCS/rGO/PDA multi-component system | Chitosan | Carboxymethyl-modified | Adhesive strength is about 34 kPa | The structure of PDA is similar to mussel-adhesive proteins and adheres to many organic and inorganic surfaces | — | 245 | |
| Polydopamine (PDA) | |||||||
| Graphene oxide (GO) | |||||||
| DMHA hydrogel | Maleic hyaluronic acid | Catechol-modified | Maximum adhesive force of ∼55 N | Hydrogen bonds formed between catechol dihydroxy and amines or hydroxyl groups | Rat liver injury model | 246 | |
| Periodate-oxidized | UV photopolymerization between acrylate and thiol groups | Rabbit femoral artery bleeding model | |||||
| CMCS/PD hydrogel | Chitosan | Carboxymethyl-modified | Burst pressure: 327.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ± ± ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9.3 mmHg 9.3 mmHg |
A large number of aldehyde groups on the hydrogel are anchored to amino groups on the tissue | Rat liver hemorrhage model | 247 | |
| Polyaldehyde dextran | rat tail severance model | ||||||
| rabbit liver and cardiac hemorrhage models | |||||||
| levan-catechol hydrogel | Levan | Catechol-modified | Adhesive strength: 42.17 ± 0.24 kPa | Covalent and non-covalent bonds between nucleophiles group on tissue, and hydroxyl with catechol groups, oxidized o-quinone groups, hydroxyl groups | rat liver perforation wound models | 248 | |
| Tetra-PEG-NHS hydrogel | Gelatin | Amino-modified | Ruptured pressure: 55 kPa | Succinimidyl-active ester moieties tightly bound to amino groups on the tissue surface | Rabbit arterial, hepatic, and cardiac hemorrhagic models | 249 | |
| PEG | Tetra-armed | ||||||
| H(C-A/OD/TA/HNT2) hydrogel | Chitosan | carboxymethyl-modified | |||||
| Dextran | Oxidized | ||||||
| tannic acid | Halloysite nanotubes | ||||||
| Physical barrier | GelMA-SN-ZF nanocomposite sealants | Gelatin | Methacryloyl-modified | Injectable | Shape adaptability | Rat liver and artery bleeding models | 250 |
| Tissue sealing | Zinc ferrite | Shorter gelation time | ZF and SN mediate the generation of hydrogen bonds and electrostatic | ||||
| Silicate nanoplatelets | Burst pressure is about 30 kPa | Interactions | |||||
| CGD hydrogel | Chitosan | Dihydrocaffeic acid (DHCA) | Injectable | Arboxylate and hydroxyl groups of DHCA and β-glycerophosphate enhance chitosan thermogelation | Rat hepatic hemorrhage and tail amputation models | 251 | |
| β-Glycerophosphate | |||||||
| GelDA/DACNC/Ca2+/Fe3+ hydrogel | Gelatin | Catechol-conjugated | Injectable | Double dynamic bonds in the hydrogel network | Rat tail amputation, liver bleeding | 252 | |
| Cellulose | Dialdehyde-modified Ca2+ | Self-adaptability | Rabbit ear artery, liver bleeding models | ||||
| Fe3+ | |||||||
| CQCS@gel | Chitosan | Catechol-functionalized quaternized | Injectable | Catechol groups bond with moieties (e.g., amines, thiols) in tissue surfaces via Schiff base reaction and Michael addition | Rat-tail amputation, liver hemorrhage and carotid bleeding models | 253 | |
| Poly(ethylene glycol) | Dibenzaldehyde-terminated | Self-healing | Dynamical reversible Schiff-base linkages could re-form at the broken sites | ||||
| Adhesive strength: 33.5 ± 7.8 kPa | |||||||
| Burst pressure: 146 mm Hg | |||||||
| T-STH hemostats | Poly(N-isopropyl acrylamide) | Silicate nanoplatelets | Injectable | Shear-thinning properties | Rat liver bleeding model | 254 | |
| Tri-BA@PVA/G | Poly(vinyl alcohol) | Guanosine | Injectable | A large number of dynamic chemical bonds inside the hydrogel network | Rat liver hemorrhage model | 255 | |
| Boronic acid | K+ ion | Shape adaptation ability | |||||
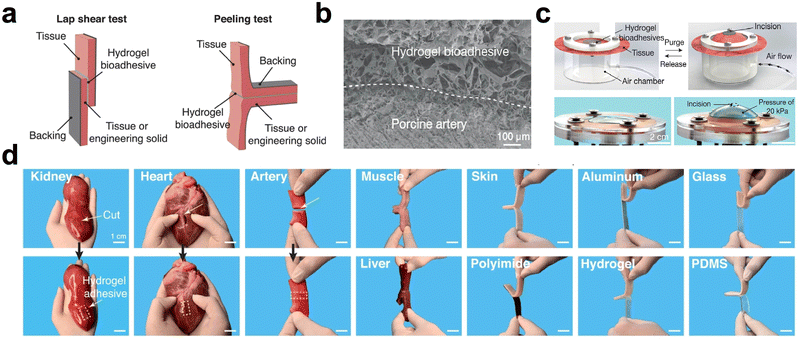 | ||
| Fig. 3 Adhesion performance of the hydrogel. (a) Schematic of hydrogel adhesion verified by lap shear and 180° peeling tests. (b) SEM images of hydrogel-adhered porcine arterial tissue (scale bar: 100 μm). (c) Photograph of a hydrogel undergoing a bursting pressure test (scale bar: 2 cm). (d) Photographs of hydrogels adhering to a variety of surfaces. Reprinted from ref. 88 with permission.107 Copyright 2021 Wiley. | ||
Hydrogels can achieve adhesion function by using the phenolic moiety, which has a strong binding affinity for nucleophiles in human tissues108 and can form covalent connections with amino and sulfhydryl groups in tissues. The phenolic moiety is dispersed on polydopamine,109 TA,110 gallic acid (GA).111 The number of phenolic groups on the molecular backbone of TA is high.112 Increasing the TA proportion in the hydrogel system can significantly improve tissue adhesion.113 For example, a TA-based hydrogel showed a maximum adhesion strength of 68.2 kPa to porcine skin and a good hemostasis effect in a liver hemostasis model with a blood loss of only 0.07 g.114 To overcome the challenge of creating hydrogels from pure polyphenolic chemicals, Shao et al.115 produced a hemostatic hydrogel using thioctic acid as a macromolecular spacer and TA of phytic acid. Results from shear-tension experiments show that this hydrogel can adhere to various material surfaces. However, the oxidation of phenolic hydroxyl groups leads to reduced adhesion. Therefore, it is necessary to control the phenolic hydroxyl group oxidation.99
Hydrogel adhesion is improved by catechol, a benzene derivative with two adjacent phenolic groups. Metal ions bind with catechol, improving mechanical characteristics. Wang et al.116 added Fe3+ to catechol-functionalized chitosan (CCS) to induce catechol–Fe3+ chelation. This double cross-linking mechanism increased the possibility of linking the material with amino, thiol, and imidazole covalent groups. The lap shear strength between the hydrogel and the porcine skin was 18.1 kPa. Polyphenols can also create dynamic covalent connections with boron (B3+) and ferric ions (Fe3+).117 Self-polymerizing dopamine yields polydopamine (PDA).118 Phenyl boric acid engages in an electrostatic interaction with the amino group on chitosan. A boronic ester bond links the catechol and boronic acid groups.119 These two reasons together improve the mechanical properties of the hydrogel.42 Based on these parameters, a chitosan-based hydrogel was created, exhibiting an adhesion strength of 27.6 kPa to porcine skin120 and demonstrating excellent hemostasis in a liver hemorrhage model. Zhong et al.121 used catechol to modify the four-arm PEG with many sulfhydryl groups, significantly improving wet tissue adhesion and mechanical strength by inducing the Michael addition reaction. Furthermore, in a study of CCS with PEG,122 the hydrogels could withstand burst pressures over 120 mmHg. Catechol and aldehyde groups are believed to work together to provide excellent tissue adhesion.
Besides catechol, substances containing catechol structures can also enhance adhesion. The bioactive substance 3,4-dihydroxy-l-phenylalanine (DOPA) with catechol found in mussel secretions is a good choice for improving adhesion.123 Xie et al.124 grafted DOPA onto gelatin methacryloyl, which significantly improved the adhesion of the hydrogel to the surface of various wet tissues (e.g., porcine skin, heart, and lungs). The in vivo experiments showed that the hydrogel facilitated rapid wound closure. Moreover, Yan et al.125 modified poly(L-glutamic acid) with DOPA to improve the mechanical properties of hydrogels. The results showed that the adhesion strength was 28.3 ± 3.1 kPa. Hydrogel can be a physical barrier to stop bleeding due to its increased catechol content, improving wet tissue adhesion. To address the limitation of poor tissue adhesion of pure HA hydrogels, grafting DOPA is a common approach,126,127 and the modified hydrogels have a good barrier effect on arterial, venous, and irregular wound bleeding.
Adhesive hydrogels can quickly create Schiff base bonds with tissue amino groups.128 Dodecyl aldehyde is a substance commonly used to enhance adhesion. Moreover, after modification with this substance, the hydrophobic aliphatic side chains of the hydrogel can insert into the cell membranes of porcine skin,129 a process known as hydrophobic interactions. Hydrophobic and aldehyde groups promoted adhesion,130 for example, in a study with a chitosan-based hydrogel. The results showed a maximum adhesion strength of 20.43 ± 0.71 kPa.131 Besides chitosan, aldehyde groups are also used to enhance the adhesion of HA-based hydrogels.132 Furthermore, in a study of oxidized carboxy-methyl cellulose (OCMC) and ECM, OCMC could form imine bonds with the intrinsic amines of ECM, and the number of aldehyde groups in the hydrogel backbone was considered critical for adhesion properties.133 This OCMC-based hydrogel exhibited a maximum adhesion strength of 185 ± 10 kPa and a burst pressure of 14.58 ± 0.32 kPa.
Hydroxyl groups can interact chemically with amino groups or amide bonds in tissues and enhance the adhesion of hydrogels.99 The hydroxyl groups in starch, cellulose, and HA are abundant. Two phenolic acids that are abundant in nature are protocatechuic acid (PA) and glyoxylic acid (GA), with GA having one more hydroxyl group than PA in its structure.134 In one study, GA and PA were grafted onto gelatin to form two hydrogels: GEG (GA-engineered gelatin) and PEG (PA-engineered gelatin).135 The adhesion strengths of the two hydrogels were 35.1 and 28.3 kPa, respectively. Specifically, the additional hydroxyl groups promoted the formation of more Schiff bases between gelatin and histone proteins, which could be the reason for improved adhesion.
Urea groups can promote hydrogen bond formation and thus enhance the hydrogels' tissue adhesion. Zhang et al.136 synthesized IEM-Gln using L-glutamine and 2-isocyanate ethyl methacrylate (IEM). Ordered hydrogen bonds are formed in the presence of urea groups. Acrylamide (AM) and IEM-Gln undergo polymerization through various hydrogen bonds, resulting in the formation of a new polymer hydrogel. The results show that this hydrogel adheres to numerous material surfaces, with an average adhesion strength of 6.7 kPa to pig skin. Multiple hydrogen bonds and electrostatic interactions contribute to good adhesion. This hydrogel also promoted hemostasis in a rat liver damage model with 91 mg blood loss by acting as a physical barrier (Fig. 4).
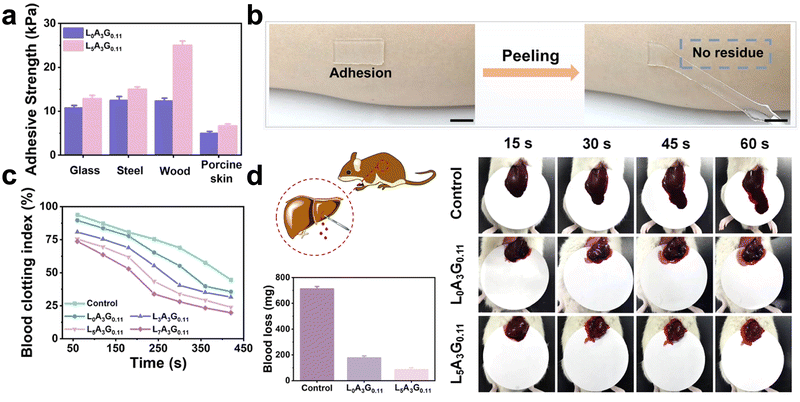 | ||
| Fig. 4 A hemostatic hydrogel that exerts an adhesion effect. (a) The adhesion strength of hydrogels to different materials. (b) Photograph of hydrogel, scale: 10 mm. (c) Dynamic whole-blood-clotting evaluation of hydrogels. (d) In vivo hemostatic properties of hydrogels, including schematic diagrams of rat liver injury models, photographs of liver blood loss, and comparisons of blood loss. Reprinted from ref. 117 with permission.136 Copyright 2021 American Chemical Society. | ||
Hydrogels can enhance tissue adhesion by grafting a variety of functional groups. However, these functional groups must remain stable to prevent any performance failures. Moreover, there is a need to explore whether super adhesion makes the removal of hydrogels difficult. Finally, hydrogels that achieve a physical barrier function by adhering to tissue should have enhanced mechanical properties.
Swellable hydrogels can fill the wound to block blood flow. One study added flaxseed gum to a cellulose system to synthesize a cellulose/flaxseed gum composite hydrogel with over 200% moisture expansion.139 Another study synthesized pectin/cellulose hydrogels. In vivo, experiments demonstrated the outstanding hemostatic properties of the two hydrogels discussed above. It was believed that the creation of a physical barrier contributed to the promotion of hemostasis. Furthermore, starch has several applications in hemostasis. Zeinab et al.141 synthesized a hydrogel with high swelling properties by grafting AAc and AAm on starch. The in vivo experiments showed that this hydrogel swells rapidly and blocks blood flow in the rat femoral artery by forming aggregates.
In situ formed hydrogels have the inherent advantage of acting as a physical barrier. However, they exhibit limitations in terms of mechanical strength and suitability for emergency use. Yu et al.142 intended to improve these defects by applying more hydrogen bonds. The hydrogel stopped bleeding within 8 s in a liver bleeding model, reducing bleeding by 92.56%. Additionally, aldehyde hydroxyethyl starch (AHES) and amino carboxymethyl chitosan (ACC) combined to form an in situ AHES/ACC hydrogel with hemostatic function through a Schiff base reaction. The in vivo hemostatic experiment exhibited that the AHES/ACC hydrogel formed a physical barrier on the wound through its three-dimensional network structure. This in situ hemostatic mechanism controls bleeding and blood loss effectively.143 Luo et al.140 also created an in situ formed HA/gelatin hydrogel. Burst pressure testing results determined the hermeticity of the hydrogel, and the burst pressure was measured to be 24.71 ± 11.58 kPa. Moreover, the blood loss in the rat liver hemorrhage model using the HA/G hydrogel was 120.4 ± 149.5 mg, approximately 50% less than the negative control group.
Injectable hydrogels are more adaptable to irregular wounds, and the four-armed PEG has better mechanical strength than linear PEG.144 Huang et al.121 produced a hydrogel utilizing benzaldehyde-terminated four-arm poly(ethylene glycol) grafted with carboxymethyl chitosan. This hydrogel was believed to be synthesized and sealed at the wound site. In the hemostasis test, the hydrogels flowed into the wound and could seal it with a hemostasis time of 120 ± 10 s and a blood loss of 0.29 ± 0.11 g. The wound's movement may lead to the tearing of the hemostatic dressing. A hemostatic injectable hydrogel was synthesized using N-carboxyethyl chitosan and oxidized sodium alginate. This hydrogel can self-heal due to the formation of Schiff base bonds by the abundant amino and aldehyde groups. In the rat hepatic hemorrhage model, the hydrogel was significantly successful at stopping bleeding, and the bleeding volume was approximately one-third that of the control group (Fig. 5). The physical barrier effect was responsible for the exceptional hemostatic effect.145
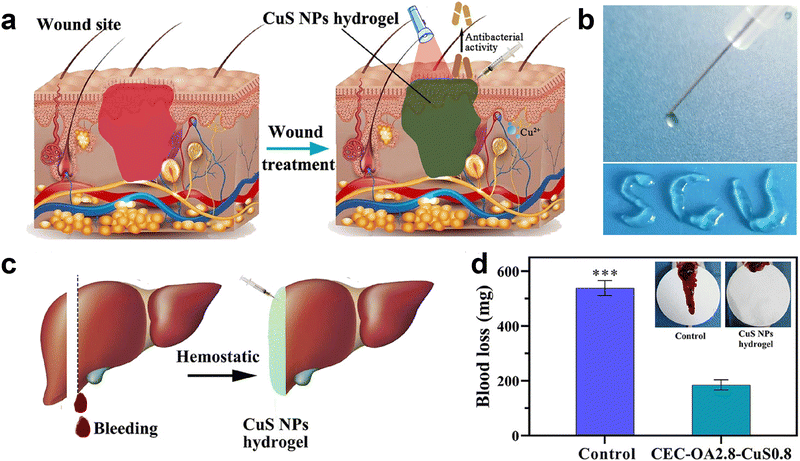 | ||
| Fig. 5 Hydrogel promotes hemostasis by sealing wounds. (a) Schematic diagram of hydrogel sealing a bleeding wound. (b) Photograph of the injectable hydrogel. (c) and (d) Schematic diagram of the injectable hydrogel applied to hemostasis in a liver injury model and comparison of blood loss. Reprinted from ref. 126 with permission.145 Copyright 2021 American Chemical Society. | ||
Hydrogels can block blood flow by sealing a wound, which requires the hydrogel to be swellable or flowable. Notably, in situ-formed hydrogels are more difficult to manipulate during the application,146 which may not meet the requirements for emergency hemostasis. Furthermore, it is yet to be investigated whether hydrogels that fill the tissue by swelling can cause wound damage or discomfort. Finally, a hemostatic hydrogel with self-healing qualities helps reduce dressing tears caused by transportation or activity, making it more appropriate for use in emergencies.
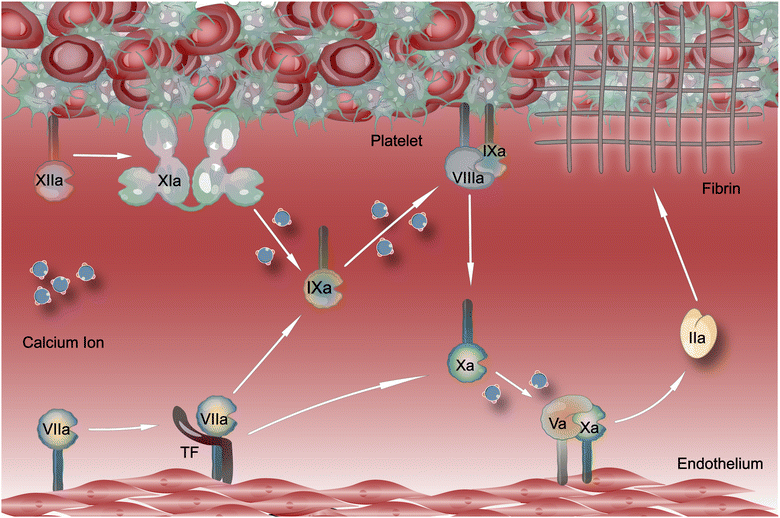
3. Physiological hemostasis
The hemostatic process was roughly divided into three stages: vasoconstriction, primary, and secondary hemostasis.147 When a blood vessel ruptures, mediators such as endothelin and platelet-derived thromboxane A2 (TXA2)148,149 and the neurogenic reflex mechanisms of the blood vessels themselves, induce myogenic contraction of the blood vessel wall, resulting in a reduction in the local blood flow rate and bleeding volume. The primary hemostasis occurs in the first 3 to 7 min after vessel rupture,150 producing platelet thrombi.151 Tissue factors (TF) released from the damaged vessel may also initiate secondary hemostasis; thus, secondary and primary hemostasis may start simultaneously.152,153 Principal components of secondary hemostasis include the successive activation of coagulation factors, production of thrombin, and conversion of fibrinogen to fibrin.154 A fibrin clot reinforces the platelet thrombus formed during primary hemostasis.153 Thrombin is an allosteric serine protease with coagulation activity. It is an essential component of the hemostasis system.155 Fibrin develops from insoluble fibrin monomers. The latter is produced when thrombin converts soluble fibrinogen into fibrin.156The physiological process of hemostasis involves interactions between various extracellular ligands, soluble proteins, platelet receptors, coagulation factors, and fibrin.157–159 Hydrogels that can activate the hemostatic system directly have been designed based on these substances. Reducing the elapsed time for the aggregation of coagulation substances is the core problem that hemostatic materials must solve. The complex mechanism of the coagulation physiological process and numerous hemostatic substances provide numerous targets for hydrogels to promote hemostasis. Therefore, there are many types of hydrogel materials with this function.160–162 This review classifies the available hydrogel hemostatic interventions into the following categories: the intervention of platelets, RBCs, and the initiation of the intrinsic pathway.
3.1. Intervention with platelets
Primary hemostasis relies on platelets. After vascular rupture, exposed subcutaneous collagen adheres to and activates some platelets via the large transmembrane protein integrin on the platelet surface.163,164 Moreover, the damaged vascular endothelium exposes a soluble plasma glycoprotein, von Willebrand factor,165,166 which can lead to a small amount of platelet adhesion.167 The plasma membrane in the region near the binding site responds to the shear forces of blood flow, leading to the activation of these platelets.159,168,169 Activated platelets extend many pseudopodia and release the contents of internal granules, mainly 5-HT, intrinsic adenosine diphosphate (ADP), and TXA2.170–172 The above vasoactive chemicals activate other platelets, which repeat the same process to increase platelet aggregation. Finally, platelets generate a thrombus at the vascular injury site to complete primary hemostasis.Direct intervention in the physiological state of platelets can promote hemostasis more quickly and directly. It is a method by which hydrogel intervenes in primary hemostasis. Hydrogels are designed to attract and activate platelets through surface charge, increased platelet adhesion sites, and the provision of bioactive substances. This review classifies platelet-interfering hydrogels based on electrostatic interactions, attachment site provision, and the addition of bioactive substances.
Amino groups are commonly positively charged groups. There is an electrostatic interaction between the negative charge on the surface of the blood cells and the positive surface charge of the hemostatic material. Liu et al.173 introduced aminated silver nanoparticles to a gelatin system, and the hydrogel's hemostatic properties were enhanced by the protonated amino group. The amino group on the backbone of chitosan is among the fundamental reasons for its inherent hemostatic ability.174 Zheng et al.175 proposed a hydrogel containing quaternary ammonium chitosan that reduced blood loss by 80% in a liver injury model. SEM images showed aggregated platelets on the hydrogel's surface. In another study on chitosan, in vitro experiments measured a minimum BCI of 82.19 ± 1.19%. This hydrogel was thought to have better hemostatic properties. However, the results of in vivo experiments were not reported.176
Synthetic silica nanoplatelets exhibit a favorable charge distribution with positive charge at the surface edges and negative charge at the top and bottom.177 Combining gelatin with this nanomaterial, Gaharwar et al.178 produced a hydrogel. Platelet aggregation was observed on the surface of the hybrid hydrogel, which was not observed on gelatin alone. Electrostatic or hydrophobic interactions have been proposed to explain this phenomenon.179In vivo, hemostasis tests showed that this hydrogel significantly reduced bleeding. Moreover, LAPONITE® (LP), a common nano silicate, has been widely used to boost physiological hemostasis. For example, in a study of polyglutamic acid, a moderate amount of LP could reduce blood loss from 872 to 88 mg in a rat liver hemorrhage model, significantly improving the hemostatic performance.180
The positively charged surface of certain antimicrobial peptides (AMPs) confers antibacterial action. Additionally, they increase platelet adhesion. Atefyekta et al.181 synthesized a hydrogel using AMPs and performed an in vitro coagulation test using whole human blood. Results showed that AMPs induced significant platelet agglutination.
Substances with a positive surface charge are frequently employed to stimulate the physiological changes in platelets. This is a frequent and efficient method.
Hydrogels with high porosity can facilitate platelet adhesion. The scanning electron microscopy images (SEMs) of these hydrogels show porous microstructures and numerous platelet adhesion sites (Fig. 6). Since most hydrogels inherently possess a networked structure, numerous materials can provide platelet adhesion sites. For instance, gelatin-based hydrogels have been developed to increase surface porosity and homogeneity with smaller hole sizes, resulting in superior platelet adhesion.44 Moreover, chitosan is utilized more frequently due to its inherent porosity.183,184 The morphological characterization of these hydrogels is confirmed by SEM images. Zhang et al.183 synthesized a hydrogel using sericin, chitosan, and PVA as the backbone, and TEM images showed a perforated structure on the surface of the hydrogel. The in vitro coagulation experiments showed that the blood co-incubated with this hydrogel had the lowest absorbance, indicating the best coagulation properties of the material. Numerous aggregated activated platelets were visible on the hydrogel surface in SEM images. Furthermore, molecular cross-linking agents, like cysteamine-modified chitosan, can improve the three-dimensional structure of the hydrogel.185
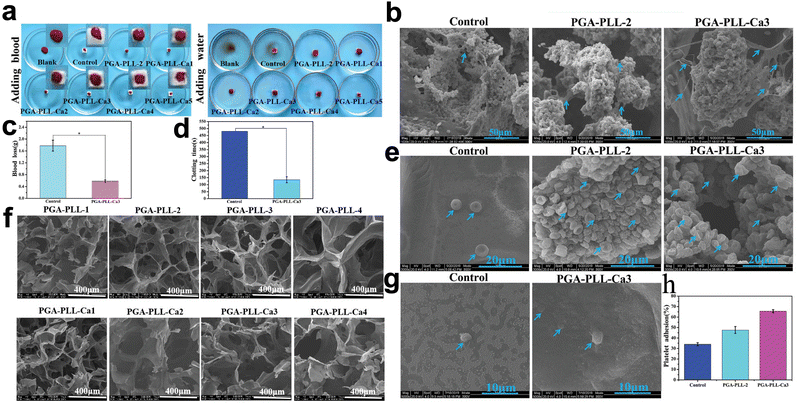 | ||
| Fig. 6 Application of poly(glutamic acid), poly(lysine), and calcium ion-based multifunctional PGA-PLL-Ca composite hydrogels in hemostasis. (a) Photograph of the hydrogel after adding 100 μl of whole blood dropwise and adding 20 ml of deionized water dropwise and incubating for 5 minutes. (b) SEM images of hydrogels after absorption of whole blood. (c) and (d) Blood loss and clotting time in rat liver injury models. (e) and (g) SEM images of erythrocytes and platelets attached to the hydrogel surface. (f) SEM images of lyophilized PGA-PLL hydrogels with different cross-linker concentrations. (h) Platelet adhesion quantification. Reprinted from ref. 52 with permission.66 Copyright 2021 Wiley. | ||
Receptors on the platelet cell membrane or membrane surface can also anchor platelets. In a study involving a cell adhesive peptide conjugate (Pept-1),182 hemostasis was accelerated through the binding of platelet surface a8bb3 receptors to RGD moieties on Pept-1. Chen et al.186 also attached blood cells by complexing carboxyl groups with Fe3+ in the blood cells. In vitro, coagulation assays revealed low BCI values, and scanning electron micrographs revealed that this hydrogel had a strong ability to adsorb blood cells.
Hydrogels are also capable of attaching and reacting with platelets. For instance, undecanal-modified chitosan, a hydrophobically modified chitosan, has hydrophobic fatty side chains that bind to the hydrophobic interior of platelets, generating a robust hemagglutination reaction.174 Chen et al.187 also prepared hemostatic hydrogels using similar logic, and dodecyl-modified chitosan contributed to an excellent anchoring effect on platelets.
Hydrogels can offer a greater surface area, receptors, and interactions to support platelet attachment compared to wounds. Further research is needed to determine whether thrombus loosening and further bleeding occur upon the removal of platelet-loaded hemostatic hydrogels.
Keratin, a natural structural protein, possesses intrinsic hemostatic properties, and keratin is believed to mediate platelet adhesion through integrins and has found wide application in the field of functional hemostatic hydrogels.188,189 In a study involving keratin-based hydrogels, the in vitro hemostatic time of the hydrogels was reduced to 12 min compared to 15 min in the control group. The in vivo experimental results showed a 56.8 and 60.7% reduction in blood loss in the experimental group at 30 and 120 s, respectively. The authors attribute this to keratin's inherent characteristics.190 Moreover, Burnett et al.35 synthesized a hemostatic keratin hydrogel. Notably, the active portion of integrins was partially blocked by antibodies, which significantly decreased the platelet adsorption capacity of the hydrogels, providing further evidence of integrin-mediated platelet adhesion. However, keratin can be used not only as a substrate but also as an additive product to promote hemostasis. Sun et al.191 incorporated keratin into fibrin-based hydrogels. The hemostasis effect of the hydrogel was twice that of fibrin.
ECM, composed of collagen, glycoproteins, and glycosaminoglycan, is a biologically active substance capable of directly activating platelets.192 Ventura et al.193 prepared ECM hydrogels by a lyophilization method. The highest ECM group had the lowest BCI and maximum platelet adhesion. The authors determined that this substance may be the finest gelatin substrate. Human-like collagen (HLC), derived from collagen, is a material with excellent biocompatibility and low immunogenicity.194 In one study, chemically modified HLC was added to an HA-based hydrogel. The results of the hemostasis test showed that the hydrogel effectively stopped acute hemorrhage in the liver, reducing the amount of bleeding from approximately 750 to 15 mg.195
Platelet-dense granules contain natural platelet-activating ADP. Liu et al.174 created a hydrogel using ADP, HA, and chitosan. ADP-modified HA activated platelets through P2Y12 receptors. In particular, the experimental group with added ADP showed the best coagulation effect on platelet-rich plasma (PRP).
There are numerous activation mechanisms for platelets, and most bioactive chemicals that promote physiological changes in platelets are endogenous. Platelet particles contain numerous platelet-activating substances that can be used in hemostatic hydrogels.
3.2. Intervention with RBCs
As the most abundant blood cells, RBCs do not play a significant role in the traditional physiology of hemostasis. However, there is growing body of evidence that RBCs play a role in hemostasis and thrombosis.196 Hematocrit and blood flow conditions are physical manifestations of the procoagulant effect of RBCs, and a low hematocrit may result in reduced significant thrombosis.197 Moreover, RBCs are associated with many cells or molecules that perform hemostatic functions. For example, RBCs adhere on the endothelium of the vessel wall under certain conditions,198 direct cellular contact of RBCs with platelets and promotion of thrombosis,199 a fibrin-related aggregation of RBCs,200,201 and the regulation of formed clots and thrombi in vivo by RBCs.202 More detailed mechanisms have been revealed in the following reviews.203,204Hydrogels interfere with red blood cells in three ways: electrostatic interactions,43 including bioactive chemicals, and supplying attachment sites.205 We have listed some studies that can interfere with red blood cells in a table (Table 3).
| Materials | Mechanisms | Functional substance | Verification Methods | Ref. |
|---|---|---|---|---|
| (BCI: blood-clotting index; SEM: scanning electron microscope) | ||||
| Silk fibroin/chitosan | Electrostatic interaction | The amino groups of chitosan | BCI | 256 |
| Catechol-modified oxidized hyaluronic acid/aminated gelatin/Fe3+ | Electrostatic interaction | The amino group in aminated gelatin | — | 257 |
| N-Citraconyl-chitosan/acrylates/arginine | Electrostatic interaction | Chitosan/arginine | — | 258 |
| Chitosan/gelatin/polyvinyl alcohol | Electrostatic interaction | Chitosan | BCI | 259 |
| Gelatin/dextran/ethylenediamine | Electrostatic interaction | Ethylenediamine | BCI/SEM | 260 |
| 3-Carboxy-phenylboronic acid/gelatin/poly(vinyl alcohol) | Electrostatic interaction | 3-Carboxy-phenylboronic acid | BCI/SEM | 261 |
| Sodium alginate/hemoglobin/carbon quantum dots | Addition of bioactive substances | Sodium lginate/hemoglobin | BCI/SEM | 34 |
| Hydroxybutyl chitosan/chitosan/dopamine | Addition of bioactive substances | Dopamine | SEM | 262 |
| Dopamine/modified poly(l-glutamate) | Addition of bioactive substances | Dopamine | Red blood cell attachment/BCI | 263 |
| Porcine acellular dermal matrix | Addition of bioactive substances | Extracellular matrix | BCI | 264 |
| Gelatin methacrylate/adenine acrylate/CuCl2 | Addition of bioactive substances | Gelatin | — | 265 |
| Carboxymethyl cellulose/dopamine | Provision of attachment sites | Dopamine | BCI/SEM | 186 |
| Four-armed benzaldehyde-terminated polyethylene glycol/dodecyl-modified chitosan/vascular endothelial growth factor | Provision of attachment sites | Dodecyl-modified chitosan | — | 187 |
| Short peptide RG-5/halloysite nano-tubes/Alginate/gelatin | Provision of attachment sites | Halloysite nano-tubes | — | 266 |
| Poly(vinyl formal) | Provision of attachment sites | Poly(vinyl formal) | — | 267 |
| Catechol derivatives/gelatin derivatives/ureido-pyrimidinone | Provision of attachment sites | Ureido-pyrimidinone | BCI | 268 |
3.3. Intervention with the coagulation cascade
The coagulation cascade reaction is the main element of secondary hemostasis in the coagulation mechanism, and this reaction is a critical event in the massive production of thrombin (Fig. 7). Hydrogels can interfere with the physiological processes of the coagulation cascade by affecting the levels of coagulation factors or other substances in the wound environment. The number of hemostatic hydrogels able to intervene in the coagulation cascade has expanded steadily in recent years. These investigations have heavily relied on the precise chemicals loaded for their intended purpose. This review describes these hydrogels according to three perspectives: initiation of the intrinsic pathway,206 addition of coagulation factors, and addition of bioactive substances.Substances of biological origin can activate intrinsic pathways via FXII. Silk proteins were thought to be capable of initiating the endogenous coagulation pathway by activating FXII.213 Bai et al.108 combined TA with silk protein, and this hydrogel can stop the bleeding in a rat liver hemorrhage model with a blood loss of only 35.2 ± 8.6 mg. HLC is also similar to collagen, an artificial substance synthesized from human collagen cDNA fragments.214 Shang et al.215 synthesized a hemostatic hydrogel using HLC and investigated the HLC effect on the initiation of the coagulation cascade. The kit measured the amount of FXII, and the results showed that the FXII secretion was significantly increased in the liver wounds of the hemorrhage model in the experimental group. This hydrogel design strategy is thought to promote hemostasis by increasing FXII.
Some studies have also initiated intrinsic pathways through some negatively charged substances. Kappa carrageenan was used as a coating for the starch/cellulose nanofiber system, and the results of in vitro coagulation experiments showed that this strategy achieved lower BCI values. The authors concluded that the negative charge on the hydrogel surface promoted the FXII activation, further enhancing the hydrogel's coagulation function. Nonetheless, a more conclusive investigation of the source of the negative charge on the hydrogel surface must be conducted.216 Besides, synthetic silicate nanoplatelets have also been used to initiate coagulation cascades due to their negatively charged surfaces.136,217 Gaharwar et al.178 used gelatin as a substrate to piggyback synthetic silicate nanoplatelets. The results of in vitro coagulation experiments showed that the clotting time of this hydrogel was reduced by up to 77% compared to the control group. Furthermore, mesocellular silica was also used in the study due to its similar properties.81,206 Notably, the FXII-mediated coagulation cascade is not triggered at low MCF concentrations. Besides, kaolin (the main component is aluminum silicate) can also initiate the coagulation reaction by activating FXII and has been used to make hemostatic hydrogels.218,219
Hydrogels can promote hemostasis by activating the intrinsic pathway of the coagulation cascade through FXII. However, some studies only intervene in the coagulation cascade and do not activate platelets. The coagulation cascade is a series of protein hydrolysis reactions on the activated platelets' surface.220 It must be determined whether hydrogels that merely intervene in the coagulation cascade will result in insufficiently activated platelets, diminishing their ability to promote hemostasis.
In research on hemostatic hydrogels with added CaCl2, the absorbance of the hydrogels in the experimental group increased and then declined with increasing Ca2+ concentration, indicating that the hydrogels' hemostatic capacity increased and then reduced.66 The authors concluded that the reason for this phenomenon is that the formation of blood clots increases with increasing Ca2+ concentration, which hinders the Ca2+ release within the system. Therefore, hydrogels with this hemostatic function must be used with attention to uniform Ca2+ release. Cheng et al.222 added nanoscale Ca (OH)2 to carboxymethyl chitosan, a sodium alginate-based system formed through ion cross-linking, aiming to solve the contradiction between the stable preservation and rapid release of Ca2+. The results show that Ca(OH)2 formed under alkaline conditions can stably store more Ca2+. The authors concluded that the acidic blood flowing from the wound further promoted the rapid release of Ca2+ from Ca(OH)2.
Current methods of hydrogel intervention in the coagulation cascade must be significantly improved. The coagulation cascade is a complicated reaction involving dozens of components223 and offers various hydrogel intervention sites. However, current studies are limited to initiating the reaction or adding a single coagulation factor, which may be limited by technical conditions. It should be noted that more than just relying on Ca2+ alone may be required to accelerate the coagulation cascade. The addition of more clotting factors may be a more efficient approach.
PolyP is a macromolecule composed of repeating phosphate units.224,225 Human platelets contain this bioactive substance, and PolyP is associated with FXI activation. The polymerization degree also influences the physiological effects of PolyP.226–228 PolyP can be added by coupling to a hydrogel backbone without a substrate. Chen et al.229 combined polyP with poly(aspartic hydrazide) (PAH) to form a novel hydrogel that reduced blood loss in rabbit ear arteries by 71.2% and hemostasis time by 68.6%. A hydrogel loaded with PolyP was synthesized,230 and the results of in vivo testing showed a 69.96% reduction in hemostasis and a 74.79% reduction in hemostasis time in the treated group. Moreover, in a study on HA-based hemostatic hydrogels,231 the PolyP introduction increased the clotting rate, an indicator of clot mechanical strength, by 1.5-fold. The authors concluded that PolyP accelerates fibrin clot formation. The fibrin clot is among the end products of the coagulation cascade and is indirect evidence of the physiological effects of PolyP. Cao et al.232 synthesized a PolyP/TA hydrogel, and the BCI values of all the hydrogels in the experimental group containing PolyP were less than 20%. Blood loss in the experimental group's mouse hepatic hemorrhage model was just 10% of the blank groups. The above results demonstrated the procoagulant properties of PolyP.
DNA is also used in hydrogels to promote hemostasis. For example, TA was used as a substrate, and DNA was added to synthesize a hemostatic hydrogel.112 The in vivo hemostasis test showed that this hydrogel could stop bleeding in the mouse liver in approximately 53 s, while the blank group required about 133 s. However, a fully DNA-based hydrogel lacked procoagulant action, indicating that the idea that DNA promotes hemostasis requires further exploration.233
In recent years, several new materials have evolved, including short peptides. Some short peptides have hemostatic action. For example, the ideal amphipathic peptide (IAP), a combination of leucine and lysine residues, has been used to prepare functional hemostatic hydrogels. Charbonneau et al.234 synthesized a novel hydrogel using IAP and quantified FIX, FX, and thrombin using ELISA kits. Specifically, IAP promoted the activity of these essential molecules in the coagulation cascade. The in vivo results showed that this hydrogel was effective in hemostasis in various hemorrhage models. A modified chitosan backbone was used to carry I3QGK, an ultrashort peptide with hemostatic activity, to form a hydrogel.184 Interestingly, the hydrogel could cause a rapid loss of fresh whole blood fluidity when the concentration of I3QGK was maintained above at least 5.03 mg ml−1, demonstrating the extreme procoagulant ability of the hydrogel.
As a cascade reaction, the coagulation cascade comprises multiple components and is simple to initiate. The addition of bioactive chemicals makes hydrogels a prospective option for interfering with the coagulation cascade.
4. Synergistic hemostasis
The effect of promoting hemostasis by a single procoagulant mechanism may be limited. In recent years, some high-level studies have promoted hemostasis in physical and physiological ways, which is a better solution.81,103,235,236Hydrogels can have several procoagulant characteristics and induce hemostasis through a synergistic action. Typically, these hemostatic hydrogels exhibit significant swelling or tissue adherence. Besides, they can also interfere with blood cells and coagulation cascades. (Fig. 8) For example, in a hemostatic study, a hydrogel with synergistic hemostatic function can increase hemostatic substances’ concentration, adsorb, and activate platelets, attract RBCs, and activate coagulation factor FXII, which all contribute to the excellent hemostatic effect. In addition, we have listed some studies (Table 4). It is worth mentioning that promoting hemostasis by exerting synergistic effects may be the future development direction of hemostatic hydrogels.
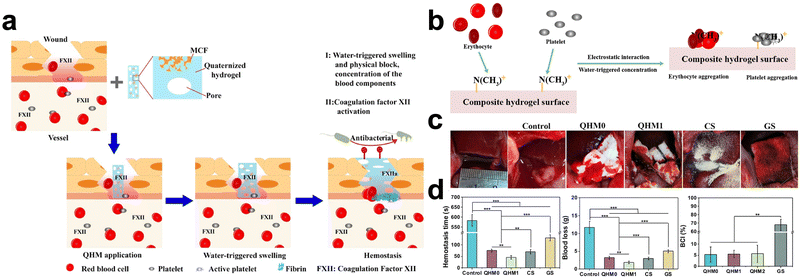 | ||
| Fig. 8 Quaternized hydroxyethyl cellulose and mesocellular silica foam-based hydrogels for hemostasis. (a) Schematic diagram of a hydrogel that promotes hemostasis by concentrating blood, attracting red blood cells and platelets and activating coagulation factor FXII. (b) Schematic diagram of hydrogel attracting red blood cells and platelets. (c) Hemostatic behavior of hydrogels in a rabbit liver injury model. (d) Hemostasis time and blood loss in rabbit liver injury models, blood clotting indexes (BCI) of various hydrogels in vitro experiments. Reprinted from ref. 67 with permission.81 Copyright 2019 American Chemical Society. | ||
| Materials | Procoagulant mechanisms | Animal models | Hemostasis time and blood loss | Ref. |
|---|---|---|---|---|
| Chitosan | Physical barrier; provide attachment sites; intervention with RBCs; intervention with coagulation cascade | The mouse liver hemorrhage model | <50 s (liver) | 269 |
| Alginate | Concentration; physical barrier; intervention with blood cells; intervention with coagulation cascade | The rabbit-ear artery model; the rabbit-liver bleeding model; the rat-tail amputation model; the rat-liver injury model | 97 ± 14 s, 215 ± 19 mg (ear); 81 ± 16 s (liver); 60 ± 11 s (tail) | 222 |
| Bletilla striata polysaccharide | Concentration of blood; physical barrier | The mouse-tail amputation model; the mice-liver injury model | 81.0 ± 8.8 s, 59.92 ± 10.9 mg (tail); 60.8 ± 4.2 s, 84.68 ± 10.9 mg (liver) | 270 |
| Cellulose | Concentration of blood; intervention with blood cells; intervention with coagulation cascade | The rat-tail amputation model; the rabbit-liver lethal defect model | 45 ± 8 s (liver) | 81 |
| Gelatin | Concentration of blood; intervention with blood cells; intervention with coagulation cascade | The mice-liver trauma model; the mice-tail amputation model | 19.6 mg, 56 s (liver); 26.8 mg, 65 s (tail) | 232 |
| Polyacrylamide | Concentration of blood; physical barrier; Intervention with platelets | The rat liver puncture model | Reduced by 1 minute on average (rat liver); reduced by 12 minutes on average (ovine liver) | 271 |
| The rat tail amputation model; the ovine liver laceration model | ||||
| Polyethylene glycol | Physical barrier; intervention with blood cells | The rat-tail amputation model | The blood loss decreased 10.3 times, the hemostasis time decreased 4.2 times | 272 |
| Chitosan | Intervention with palates; physical barrier | The mouse liver trauma model; the mouse liver incision model; the mouse-tail amputation model | 143.9 ± 60.3 mg (liver trauma); 207 ± 19.3 mg (liver incision); 30 ± 4.0 mg (tail amputation) | 38 |
| Poly(ethylene glycol) | Physical barrier; intervention with RBCs | The mouse hemorrhaging liver model; the mouse heart bleeding model | 0.037 mg (liver); greatly decreased blood loss compared to the blank group (1.08 g) | 273 |
| Poly(ethylene glycol) | Physical barrier; concentration of blood | The mouse liver bleeding model | 41.1 ± 15.2 mg | 274 |
5. Conclusion and outlooks
Stopping uncontrollable bleeding is challenging in high-risk environments like battlefields, disasters, and operating rooms. Traditional means of hemostasis are difficult to meet the need for hemostasis due to several drawbacks. Therefore, there is a need to develop materials that promote hemostasis. Recently, polymeric materials, particularly hydrogels, have shown great potential for hemostasis. However, commercially available hemostatic hydrogels are insufficient in stopping bleeding quickly and have a limited range of application. Therefore, these materials cannot satisfy emergency situations' hemostatic needs and unique groups' needs. Numerous functional hemostatic hydrogels capable of promoting hemostasis through several procoagulant processes have been produced by researchers. This review describes several procoagulant mechanisms of hydrogels: First, hydrogels can change the physical forms of the blood or tissue near the wound to create conditions for hemostasis. The physical forms include hemostatic substances’ concentration, wound shapes, and the adhesion location of procoagulant substances. To achieve these functions, hydrogels with a porous structure, hydrophilicity, swelling, mobility, or tissue adhesion are required. Second, hydrogels can directly promote hemostasis by intervening in the physiological mechanisms of hemostasis. RBCs, platelets, and the coagulation cascade are crucial for the physiological processes of hemostasis. Achieving these functions requires hydrogels with surface charge, large surface area, or the ability to load multiple bioactive substances. Finally, hydrogels can exert physical and physiological procoagulant functions through synergistic effects. In conclusion, the existing hydrogels are comprehensive in their procoagulant mechanisms and exhibit excellent hemostatic ability.However, there are still areas for improvement in the design and development of hemostatic hydrogels. First, the high increase in hemostatic substances’ concentration may result in wound drying or excessive blood loss, rendering the hydrogel inappropriate for use in young children with low blood volume. It is crucial to select a more effective physiological procoagulant mechanism to develop a hydrogel. Second, some hydrogels are difficult to remove, leading to secondary injury or bleeding. Therefore, selecting more appropriate interactions between tissue and hydrogel is necessary when enhancing tissue adhesion. Third, some hydrogels compress the wound causing discomfort, so it is necessary to improve the shape adaptability of the hydrogel. Fourth, hydrogels that solely intervene in the physiological process of hemostasis may face challenges in stopping bleeding in coagulation-problem patients successfully. Therefore, synergistic hemostasis may represent the future of hemostatic hydrogel development.
In conclusion, elucidating the procoagulant mechanism may provide ideas for improving functional hemostatic hydrogels. Furthermore, more interventions in the physiological process of hemostasis may provide additional opportunities for the development of functional hemostatic hydrogels.
Author contributions
Boxiang Zhang: writing – original draft; writing – review and editing; conceptualization; software; and revision of the manuscript; Min Wang: writing – original draft; writing – review and editing; conceptualization; and revision of the manuscript; Heng Tian: revision of the manuscript; Hang Cai: revision of the manuscript; Siyu Wu: revision of the manuscript; Simin Jiao: revision of the manuscript; Huidong Zhou: revision of the manuscript; Jie Zhao: revision of the manuscript; Yan Li: revision of the manuscript; Wenlai Guo: revision of the manuscript; and Wenrui Qu: revision of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financed by the Jilin Science and Technology Agency funds in China [grant numbers: 20210101282JC, 20210402001GH, YDZJ202201ZYTS019, YDZJ202201ZYTS572, YDZJ202201ZYTS071 and YDZJ202301ZYTS499], the Jilin Provincial Development and Reform Commission [grant number: 2023C040-2], and the Norman Bethune Project of Jilin University (grant number: 2022B44). We thank Home for Researchers editorial team (https://www.home-for-researchers.com) for language editing service.References
- B. J. Eastridge, R. L. Mabry, P. Seguin, J. Cantrell, T. Tops, P. Uribe, O. Mallett, T. Zubko, L. Oetjen-Gerdes, T. E. Rasmussen, F. K. Butler, R. S. Kotwal, J. B. Holcomb, C. Wade, H. Champion, M. Lawnick, L. Moores and L. H. Blackbourne, J. Trauma Acute Care Surg., 2012, 73, S431–S437 CrossRef PubMed.
- P. M. Mannucci and M. Levi, N. Engl. J. Med., 2007, 356, 2301–2311 CrossRef CAS PubMed.
- S. A. D. Kyle, J. Kalkwarf, Y. Yang, C. Thetford, L. Myers, M. Brock, D. A. Wolf, D. Persse, C. E. Wade and J. B. Holcomb, J. Trauma Acute Care Surg., 2020, 89, 716–722 CrossRef PubMed.
- B. J. Eastridge, R. L. Mabry, P. Seguin, J. Cantrell, T. Tops, P. Uribe, O. Mallett, T. Zubko, L. Oetjen-Gerdes, T. E. Rasmussen, F. K. Butler, R. S. Kotwal, J. B. Holcomb, C. Wade, H. Champion, M. Lawnick, L. Moores and L. H. Blackbourne, J. Trauma Acute Care Surg., 2012, 73, S431–S437 CrossRef PubMed.
- F. U. R. A. Malik, K. U. Shah, S. S. Naz and S. Qaisar, J. Biomed. Mater. Res., Part B, 2021, 109, 1465–1477 CrossRef PubMed.
- P. T. Y. C. H. Tien and F. Brenneman, Curr. Orthop., 2004, 18, 304–310 CrossRef.
- M. A. Schreiber and B. Tieu, Surgery, 2007, 142, S61–S66 CrossRef PubMed.
- B. L. Bennett, Wilderness Environ. Med., 2017, 28, S39–S49 CrossRef PubMed.
- M. Hoekstra, M. Hermans, C. Richters and R. Dutrieux, J. Wound Care, 2002, 11, 113–117 CrossRef CAS PubMed.
- M. Xie, Y. Zeng, H. Wu, S. Wang and J. Zhao, Int. J. Biol. Macromol., 2022, 219, 1337–1350 CrossRef CAS PubMed.
- Z. Li and Z. Lin, Aggregate, 2021, 2, 62–87 Search PubMed.
- W. Zheng, Z. Zhang, Y. Li, L. Wang, F. Fu, H. Diao and X. Liu, Chem. Eng. J., 2022, 447, 137482 CrossRef CAS.
- Y.-B. Zhang, H.-J. Wang, A. Raza, C. Liu, J. Yu and J.-Y. Wang, Int. J. Biol. Macromol., 2022, 205, 110–117 CrossRef CAS PubMed.
- J. Tang, W. Yi, J. Yan, Z. Chen, H. Fan, D. Zaldivar-Silva, L. Agüero and S. Wang, Int. J. Biol. Macromol., 2023, 247, 125754 CrossRef CAS PubMed.
- J. Lee, H. N. Choi, H. J. Cha and Y. J. Yang, Biomacromolecules, 2023, 24, 1763–1773 CrossRef CAS PubMed.
- Z. Wang, T. Lyu, Q. Xie, Y. Zhang, H. Sun, Y. Wan and Y. Tian, Appl. Mater. Today, 2023, 35, 101948 CrossRef.
- J. Wang, C. Li, W. Zhang, W. Huang, Z. Liu, R. Shi, S. Wang, S. Liu, W. Shi and Y. Li, Biomater. Sci., 2023, 11, 3616–3628 RSC.
- Z. Tan, X. Li, C. Yu, M. Yao, Z. Zhao, B. Guo, L. Liang, Y. Wei, F. Yao and H. Zhang, Int. J. Biol. Macromol., 2023, 232, 123449 CrossRef CAS PubMed.
- Y. Fang, L. Zhang, Y. Chen, S. Wu, Y. Weng and H. Liu, Carbohydr. Polym., 2023, 312, 120819 CrossRef CAS PubMed.
- L. Zheng, Q. Wang, Y. S. Zhang, H. Zhang, Y. Tang, Y. Zhang, W. Zhang and X. Zhang, Chem. Eng. J., 2021, 416, 129136 CrossRef CAS.
- A. E. Pusateri, J. B. Holcomb, B. S. Kheirabadi, H. B. Alam, C. E. Wade and K. L. Ryan, J. Trauma Acute Care Surg., 2006, 60, 674–682 CrossRef PubMed.
- M. Bahram, N. Mohseni and M. Moghtader, Emerging concepts in analysis and applications of hydrogels, IntechOpen, 2016 Search PubMed.
- S. Aswathy, U. Narendrakumar and I. Manjubala, Heliyon, 2020, 6, e03719 CrossRef CAS PubMed.
- E. M. Ahmed, J. Adv. Res., 2015, 6, 105–121 CrossRef CAS PubMed.
- X. Liu, M. Hou, X. Luo, M. Zheng, X. Wang, H. Zhang and J. Guo, Biomacromolecules, 2021, 22, 319–329 CrossRef CAS PubMed.
- C. You, Q. Li, X. Wang, P. Wu, J. K. Ho, R. Jin, L. Zhang, H. Shao and C. Han, Sci. Rep., 2017, 7, 10489 CrossRef PubMed.
- Y. Liang, Z. Li, Y. Huang, R. Yu and B. Guo, ACS Nano, 2021, 15, 7078–7093 CrossRef CAS PubMed.
- Q. Tang, C. Chen, Y. Jiang, J. Huang, Y. Liu, P. M. Nthumba, G. Gu, X. Wu, Y. Zhao and J. Ren, J. Mater. Chem. B, 2020, 8, 5756–5764 RSC.
- J. Zhu, F. Li, X. Wang, J. Yu and D. Wu, ACS Appl. Mater. Interfaces, 2018, 10, 13304–13316 CrossRef CAS PubMed.
- B. J. D. Barba, C. T. Aranilla, L. S. Relleve, V. R. C. Cruz, J. R. Vista and L. V. Abad, Radiat. Phys. Chem., 2018, 144, 180–188 CrossRef CAS.
- F. Zhou, Y. Yang, W. Zhang, S. Liu, A. B. Shaikh, L. Yang, Y. Lai, H. Ouyang and W. Zhu, Appl. Mater. Today, 2022, 26, 101290 CrossRef.
- L. Teng, Z. Shao, Q. Bai, X. Zhang, Y. S. He, J. Lu, D. Zou, C. Feng and C. M. Dong, Adv. Funct. Mater., 2021, 31, 2105628 CrossRef CAS.
- C. Y. Zou, X. X. Lei, J. J. Hu, Y. L. Jiang, Q. J. Li, Y. T. Song, Q. Y. Zhang, J. Li-Ling and H. Q. Xie, Bioact. Mater., 2022, 16, 388–402 CAS.
- Q. Zhang, Z. Li, M. Zhang, W. Wang, J. Shen, Z. Ye and N. Zhou, Langmuir, 2020, 36, 13263–13273 CrossRef CAS PubMed.
- L. R. Burnett, M. B. Rahmany, J. R. Richter, T. A. Aboushwareb, D. Eberli, C. L. Ward, G. Orlando, R. R. Hantgan and M. E. Van Dyke, Biomaterials, 2013, 34, 2632–2640 CrossRef CAS PubMed.
- L. Wei, J. Tan, L. Li, H. Wang, S. Liu, J. Chen, Y. Weng and T. Liu, Int. J. Mol. Sci., 2022, 23, 1249 CrossRef CAS PubMed.
- I. Koumentakou, Z. Terzopoulou, A. Michopoulou, I. Kalafatakis, K. Theodorakis, D. Tzetzis and D. Bikiaris, Int. J. Biol. Macromol., 2020, 162, 693–703 CrossRef CAS PubMed.
- J. He, M. Shi, Y. Liang and B. Guo, Chem. Eng. J., 2020, 394, 124888 CrossRef CAS.
- H. Li, F. Cheng, X. Wei, X. Yi, S. Tang, Z. Wang, Y. S. Zhang, J. He and Y. Huang, Mater. Sci. Eng., C, 2021, 118, 111324 CrossRef CAS PubMed.
- M. Suneetha, K. M. Rao and S. S. Han, ACS Omega, 2019, 4, 12647–12656 CrossRef CAS PubMed.
- R. Hajosch, M. Suckfuell, S. Oesser, M. Ahlers, K. Flechsenhar and B. Schlosshauer, J. Biomed. Mater. Res., Part B, 2010, 94, 372–379 CrossRef PubMed.
- K. Han, Q. Bai, W. Wu, N. Sun, N. Cui and T. Lu, Int. J. Biol. Macromol., 2021, 183, 2142–2151 CrossRef CAS PubMed.
- Y. Chen, Y. Zhang, F. Wang, W. Meng, X. Yang, P. Li, J. Jiang, H. Tan and Y. Zheng, Mater. Sci. Eng., C, 2016, 63, 18–29 CrossRef CAS PubMed.
- L. S. Gomez-Aparicio, J. Bernaldez-Sarabia, T. A. Camacho-Villegas, P. H. Lugo-Fabres, N. E. Diaz-Martinez, E. Padilla-Camberos, A. Licea-Navarro and A. B. Castro-Cesena, Biomater. Sci., 2021, 9, 726–744 RSC.
- Z. Yang, X. Fu, L. Zhou, J. Yang, T. Deng, J. Chen, Y. Wen, X. Fu, D. Shen, Z. Yuan, Z. Du, S. Luo and C. Yu, Chem. Eng. J., 2021, 423, 130202 CrossRef CAS.
- V. Dodane and V. D. Vilivalam, Pharm. Sci. Technolo. Today, 1998, 1, 246–253 CrossRef CAS.
- Z. Hu, D.-Y. Zhang, S.-T. Lu, P.-W. Li and S.-D. Li, Mar. Drugs, 2018, 16, 273 CrossRef PubMed.
- A. Bal-Ozturk, O. Karal-Yilmaz, Z. P. Akguner, S. Aksu, A. Tas and H. Olmez, J. Appl. Polym. Sci., 2019, 136, 47522 CrossRef.
- S. Baghaie, M. T. Khorasani, A. Zarrabi and J. Moshtaghian, J. Biomater. Sci., Polym. Ed., 2017, 28, 2220–2241 CrossRef CAS PubMed.
- D. S. Yoon, Y. Lee, H. A. Ryu, Y. Jang, K. M. Lee, Y. Choi, W. J. Choi, M. Lee, K. M. Park, K. D. Park and J. W. Lee, Acta Biomater., 2016, 38, 59–68 CrossRef CAS PubMed.
- H. J. Jang, Y. M. Kim, B. Y. Yoo and Y. K. Seo, J. Biomater. Appl., 2018, 32, 716–724 CrossRef CAS PubMed.
- Z. Ahmadian, A. Correia, M. Hasany, P. Figueiredo, F. Dobakhti, M. R. Eskandari, S. H. Hosseini, R. Abiri, S. Khorshid, J. Hirvonen, H. A. Santos and M. A. Shahbazi, Adv. Healthcare Mater., 2021, 10, e2001122 CrossRef PubMed.
- L. Teng, K. Xia, T. Qian, Z. Hu, L. Hong, Y. Liao, G. Peng, Z. Yuan, Y. Chen and Z. Zeng, ACS Biomater. Sci. Eng., 2022, 8, 2076–2087 CrossRef CAS PubMed.
- C. Xue, H. Xie, J. Eichenbaum, Y. Chen, Y. Wang, F. W. van den Dolder, J. Lee, K. Lee, S. Zhang, W. Sun, A. Sheikhi, S. Ahadian, N. Ashammakhi, M. R. Dokmeci, H. J. Kim and A. Khademhosseini, Biotechnol. J., 2020, 15, e1900456 CrossRef PubMed.
- Y. Huang, B. Zhang, G. Xu and W. Hao, Compos. Sci. Technol., 2013, 84, 15–22 CrossRef CAS.
- X. Bian, C. Cui, Y. Qi, Y. Sun, Z. Zhang and W. Liu, Adv. Funct. Mater., 2022, 32, 2207349 CrossRef CAS.
- M. Obst and A. Steinbüchel, Biomacromolecules, 2004, 5, 1166–1176 CrossRef CAS PubMed.
- I. Bajaj and R. Singhal, Bioresour. Technol., 2011, 102, 5551–5561 CrossRef CAS PubMed.
- W. Chen, R. Wang, T. Xu, X. Ma, Z. Yao, B. Chi and H. Xu, J. Mater. Chem. B, 2017, 5, 5668–5678 RSC.
- W. Chen, S. Yuan, J. Shen, Y. Chen and Y. Xiao, Front. Bioeng. Biotechnol., 2020, 8, 627351 CrossRef PubMed.
- X. Zhang, Z. Ma, Y. Ke, Y. Xia, X. Xu, J. Liu, Y. Gong, Q. Shi and J. Yin, Mater. Adv., 2021, 2, 5150–5159 RSC.
- B. A. Bruckner, L. N. Blau, L. Rodriguez, E. E. Suarez, U. Q. Ngo, M. J. Reardon and M. Loebe, J. Cardiothorac. Surg., 2014, 9, 134 CrossRef PubMed.
- R. Cui, F. Chen, Y. Zhao, W. Huang and C. Liu, J. Mater. Chem. B, 2020, 8, 8282–8293 RSC.
- Z. Mirzakhanian, K. Faghihi, A. Barati and H. R. Momeni, Int. J. Polym. Mater. Polym. Biomater., 2016, 65, 779–788 CrossRef CAS.
- J. Drelich, E. Chibowski, D. D. Meng and K. Terpilowski, Soft Matter, 2011, 7, 9804–9828 RSC.
- R. Wei, T. Chen, Y. Wang, Q. Xu, B. Feng, J. Weng, W. Peng and J. Wang, Macromol. Biosci., 2021, 21, e2000367 CrossRef PubMed.
- C. Gentilini, Y. Dong, J. R. May, S. Goldoni, D. E. Clarke, B. H. Lee, E. T. Pashuck and M. M. Stevens, Adv. Healthcare Mater., 2012, 1, 308–315 CrossRef CAS PubMed.
- S. Gorgieva and V. Kokol, Carbohydr. Polym., 2011, 85, 664–673 CrossRef CAS.
- C. Dai, Y. Yuan, C. Liu, J. Wei, H. Hong, X. Li and X. Pan, Biomaterials, 2009, 30, 5364–5375 CrossRef CAS PubMed.
- G. Varga, Epitoanyag, 2007, 59, 6–9 CrossRef.
- M. Matsusaki, T. Serizawa, A. Kishida and M. Akashi, Biomacromolecules, 2005, 6, 400–407 CrossRef CAS PubMed.
- T.-Y. Liu, S.-Y. Chen, Y.-L. Lin and D.-M. Liu, Langmuir, 2006, 22, 9740–9745 CrossRef CAS PubMed.
- L. Shen, Y. Zhang, W. Yu, R. Li, M. Wang, Q. Gao, J. Li and H. Lin, J. Colloid Interface Sci., 2019, 543, 64–75 CrossRef CAS PubMed.
- T. Coviello, P. Matricardi, C. Marianecci and F. Alhaique, J. Controlled Release, 2007, 119, 5–24 CrossRef CAS PubMed.
- J. Alam, M. Alhoshan, A. K. Shukla, A. Aldalbahi and F. A. A. Ali, Eur. Polym. J., 2019, 120, 109219 CrossRef CAS.
- B. J. D. Barba, C. Tranquilan-Aranilla and L. V. Abad, Radiat. Phys. Chem., 2016, 118, 111–113 CrossRef CAS.
- X. Luo, F. Ao, Q. Huo, Y. Liu, X. Wang, H. Zhang, M. Yang, Y. Ma and X. Liu, Biomater. Adv., 2022, 139, 212983 CrossRef CAS PubMed.
- H. F. Liu, J. S. Mao, K. D. Yao, G. H. Yang, L. Cui and Y. L. Cao, J. Biomater. Sci., Polym. Ed., 2004, 15, 25–40 CrossRef CAS PubMed.
- A. Z. Bazmandeh, E. Mirzaei, M. Fadaie, S. Shirian and Y. Ghasemi, Int. J. Biol. Macromol., 2020, 162, 359–373 CrossRef CAS PubMed.
- Y. Wang, R. Fu, X. Ma, X. Li and D. Fan, Macromol. Biosci., 2021, 21, e2000396 CrossRef PubMed.
- C. Wang, H. Niu, X. Ma, H. Hong, Y. Yuan and C. Liu, ACS Appl. Mater. Interfaces, 2019, 11, 34595–34608 CrossRef CAS PubMed.
- E. D. Bain, T. R. Long, F. L. Beyer, A. M. Savage, M. D. Dadmun, H. Martin, J. L. Lenhart and R. A. Mrozek, Macromolecules, 2018, 51, 4705–4717 CrossRef CAS.
- Y. Liu, H. Meng, S. Konst, R. Sarmiento, R. Rajachar and B. P. Lee, ACS Appl. Mater. Interfaces, 2014, 6, 16982–16992 CrossRef CAS PubMed.
- T. Sakai, T. Matsunaga, Y. Yamamoto, C. Ito, R. Yoshida, S. Suzuki, N. Sasaki, M. Shibayama and U.-I. Chung, Macromolecules, 2008, 41, 5379–5384 CrossRef CAS.
- Y. Bu, L. Zhang, J. Liu, L. Zhang, T. Li, H. Shen, X. Wang, F. Yang, P. Tang and D. Wu, ACS Appl. Mater. Interfaces, 2016, 8, 12674–12683 CrossRef CAS PubMed.
- H. Yuk, T. Zhang, G. A. Parada, X. Liu and X. Zhao, Nat. Commun., 2016, 7, 12028 CrossRef PubMed.
- H. Pan, D. Fan, W. Cao, C. Zhu, Z. Duan, R. Fu, X. Li and X. Ma, Polymers, 2017, 9, 727 CrossRef PubMed.
- T. M. Tamer, M. M. Sabet, A. M. Omer, E. Abbas, A. I. Eid, M. S. Mohy-Eldin and M. A. Hassan, Sci. Rep., 2021, 11, 3428 CrossRef CAS PubMed.
- C. Wang, H. Guo, S. Leng, J. Yu, K. Feng, L. Cao and J. Huang, Crit. Rev. Solid State Mater. Sci., 2021, 46, 330–348 CrossRef CAS.
- X. Shang, H. Chen, V. Castagnola, K. Liu, L. Boselli, V. Petseva, L. Yu, L. Xiao, M. He and F. Wang, Nat. Catal., 2021, 4, 607–614 CrossRef CAS.
- F. Arnaud, T. Tomori, W. Carr, A. McKeague, K. Teranishi, K. Prusaczyk and R. McCarron, Ann. Biomed. Eng., 2008, 36, 1708–1713 CrossRef PubMed.
- L. Yu, X. Shang, H. Chen, L. Xiao, Y. Zhu and J. Fan, Nat. commun., 2019, 10, 1932 CrossRef PubMed.
- P. Fathi, M. Sikorski, K. Christodoulides, K. Langan, Y. S. Choi, M. Titcomb, A. Ghodasara, O. Wonodi, H. Thaker, M. Vural, A. Behrens and P. Kofinas, J. Biomed. Mater. Res., Part B, 2018, 106, 1662–1671 CrossRef CAS PubMed.
- T. Zhu, J. Wu, N. Zhao, C. Cai, Z. Qian, F. Si, H. Luo, J. Guo, X. Lai, L. Shao and J. Xu, Adv. Healthcare Mater., 2018, 7, e1701086 CrossRef PubMed.
- Z. Li, A. Milionis, Y. Zheng, M. Yee, L. Codispoti, F. Tan, D. Poulikakos and C. H. Yap, Nat. Commun., 2019, 10, 5562 CrossRef CAS PubMed.
- J. C. Lantis, F. M. Durville, R. Connolly and S. D. Schwaitzberg, J. Laparoendosc. Adv. Surg. Tech., Part A, 1998, 8, 381–394 CrossRef PubMed.
- L. Li, B. Yan, J. Yang, L. Chen and H. Zeng, Adv. Mater., 2015, 27, 1294–1299 CrossRef CAS PubMed.
- W. Han and S. Wang, Gels, 2022, 9, 2 CrossRef PubMed.
- X. B. Zhang, L. X. Shi, W. Y. Xiao, Z. Wang and S. T. Wang, Adv. Nanobiomed. Res., 2023, 3, 2200115 CrossRef CAS.
- X. Xia, X. Xu, B. Wang, D. Zhou, W. Zhang, X. Xie, H. Lai, J. Xue, A. Rai, Z. Li, X. Peng, P. Zhao, L. Bian and P. W. Y. Chiu, Adv. Funct. Mater., 2022, 32, 2109332 CrossRef CAS.
- S. Komatsu, Y. Nagai, K. Naruse and Y. Kimata, PLoS One, 2014, 9, e102778 CrossRef PubMed.
- C. Ding, M. Tian, R. Feng, Y. Dang and M. Zhang, ACS Biomater. Sci. Eng., 2020, 6, 3855–3867 CrossRef CAS PubMed.
- H. Li, X. Zhou, L. Luo, Q. Ding and S. Tang, Carbohydr. Polym., 2022, 281, 119039 CrossRef CAS PubMed.
- P. Ni, S. Ye, S. Xiong, M. Zhong, J. Shan, T. Yuan, J. Liang, Y. Fan and X. Zhang, J. Mater. Chem. B, 2023, 11, 5207–5222 RSC.
- X.-X. Lei, J.-J. Hu, C.-Y. Zou, Y.-L. Jiang, L.-M. Zhao, X.-Z. Zhang, Y.-X. Li, A.-N. Peng, Y.-T. Song and L.-P. Huang, Bioact. Mater., 2023, 27, 461–473 CAS.
- M. F.-H. Lee and A. Ananda, Auris Nasus Larynx, 2023, 50, 365–373 CrossRef PubMed.
- K. Zhang, X. Chen, Y. Xue, J. Lin, X. Liang, J. Zhang, J. Zhang, G. Chen, C. Cai and J. Liu, Adv. Funct. Mater., 2021, 32, 2111465 CrossRef.
- S. Bai, X. Zhang, P. Cai, X. Huang, Y. Huang, R. Liu, M. Zhang, J. Song, X. Chen and H. Yang, Nanoscale Horiz., 2019, 4, 1333–1341 RSC.
- R. Mrówczyński, R. Markiewicz and J. Liebscher, Polym. Int., 2016, 65, 1288–1299 CrossRef.
- Z. Guo, W. Xie, J. Lu, X. Guo, J. Xu, W. Xu, Y. Chi, N. Takuya, H. Wu and L. Zhao, J. Mater. Chem. B, 2021, 9, 4098–4110 RSC.
- Y.-G. Jang, E.-B. Ko and K.-C. Choi, J. Nutr. Biochem., 2020, 84, 108444 CrossRef CAS PubMed.
- M. Shin, J. H. Ryu, J. P. Park, K. Kim, J. W. Yang and H. Lee, Adv. Funct. Mater., 2015, 25, 1270–1278 CrossRef CAS.
- Y. N. Chen, L. Peng, T. Liu, Y. Wang, S. Shi and H. Wang, ACS Appl. Mater. Interfaces, 2016, 8, 27199–27206 CrossRef CAS PubMed.
- Y. Yang, X. Zhao, J. Yu, X. Chen, R. Wang, M. Zhang, Q. Zhang, Y. Zhang, S. Wang and Y. Cheng, Bioact. Mater., 2021, 6, 3962–3975 CAS.
- X. H. Shao, X. Yang, Y. Zhou, Q. C. Xia, Y. P. Lu, X. Yan, C. Chen, T. T. Zheng, L. L. Zhang, Y. N. Ma, Y. X. Ma and S. Z. Gao, Soft Matter, 2022, 18, 2814–2828 RSC.
- L. Wang, X. Zhang, K. Yang, Y. V. Fu, T. Xu, S. Li, D. Zhang, L. N. Wang and C. S. Lee, Adv. Funct. Mater., 2019, 30, 1904156 CrossRef.
- J. Guo, H. Sun, K. Alt, B. L. Tardy, J. J. Richardson, T. Suma, H. Ejima, J. Cui, C. E. Hagemeyer and F. Caruso, Adv. Healthcare Mater., 2015, 4, 1796–1801 CrossRef CAS PubMed.
- S. M. Kang, N. S. Hwang, J. Yeom, S. Y. Park, P. B. Messersmith, I. S. Choi, R. Langer, D. G. Anderson and H. Lee, Adv. Funct. Mater., 2012, 22, 2949–2955 CrossRef CAS PubMed.
- S. Lamping, T. Otremba and B. J. Ravoo, Angew. Chem., Int. Ed., 2018, 57, 2474–2478 CrossRef CAS PubMed.
- P. Deng, X. Liang, F. Chen, Y. Chen and J. Zhou, Appl. Mater. Today, 2022, 26, 101362 CrossRef.
- W. Huang, Y. Wang, Y. Chen, Y. Zhao, Q. Zhang, X. Zheng, L. Chen and L. Zhang, Adv. Healthcare Mater., 2016, 5, 2813–2822 CrossRef CAS PubMed.
- W. Fang, L. Yang, L. Hong and Q. Hu, Carbohydr. Polym., 2021, 271, 118428 CrossRef CAS PubMed.
- J. Saiz-Poseu, J. Mancebo-Aracil, F. Nador, F. Busqué and D. Ruiz-Molina, Angew. Chem., Int. Ed., 2019, 58, 696–714 CrossRef CAS PubMed.
- M. Xie, Z. Zheng, S. Pu, Y. G. Jia, L. Wang and Y. Chen, Macromol. Biosci., 2022, 22, e2100491 CrossRef PubMed.
- S. Yan, W. Wang, X. Li, J. Ren, W. Yun, K. Zhang, G. Li and J. Yin, J. Mater. Chem. B, 2018, 6, 6377–6390 RSC.
- Y. Liang, X. Zhao, T. Hu, B. Chen, Z. Yin, P. X. Ma and B. Guo, Small, 2019, 15, e1900046 CrossRef PubMed.
- D. Wang, P. Xu, S. Wang, W. Li and W. Liu, Eur. Polym. J., 2020, 134, 109763 CrossRef CAS.
- J. Hoque, R. G. Prakash, K. Paramanandham, B. R. Shome and J. Haldar, Mol. Pharmaceutics, 2017, 14, 1218–1230 CrossRef CAS PubMed.
- M. B. Dowling, R. Kumar, M. A. Keibler, J. R. Hess, G. V. Bochicchio and S. R. Raghavan, Biomaterials, 2011, 32, 3351–3357 CrossRef CAS PubMed.
- J. Qu, X. Zhao, Y. Liang, T. Zhang, P. X. Ma and B. Guo, Biomaterials, 2018, 183, 185–199 CrossRef CAS PubMed.
- X. Wei, C. Cui, C. Fan, T. Wu, Y. Li, X. Zhang, K. Wang, Y. Pang, P. Yao and J. Yang, Int. J. Biol. Macromol., 2022, 199, 401–412 CrossRef CAS PubMed.
- Y. Xiong, L. Chen, P. Liu, T. Yu, C. Lin, C. Yan, Y. Hu, W. Zhou, Y. Sun, A. C. Panayi, F. Cao, H. Xue, L. Hu, Z. Lin, X. Xie, X. Xiao, Q. Feng, B. Mi and G. Liu, Small, 2022, 18, e2104229 CrossRef PubMed.
- H. Zhu, X. Mei, Y. He, H. Mao, W. Tang, R. Liu, J. Yang, K. Luo, Z. Gu and L. Zhou, ACS Appl. Mater. Interfaces, 2020, 12, 4241–4253 CrossRef CAS PubMed.
- D. S. Hwang, H. Zeng, Q. Lu, J. Israelachvili and J. H. Waite, Soft Matter, 2012, 8, 5640–5648 RSC.
- T. Thiruselvi, S. Thirupathi Kumara Raja, R. Aravindhan, S. K. Shanuja and A. Gnanamani, RSC Adv., 2016, 6, 19973–19981 RSC.
- M. Zhang, J. Yu, K. Shen, R. Wang, J. Du, X. Zhao, Y. Yang, K. Xu, Q. Zhang, Y. Zhang and Y. Cheng, Chem. Mater., 2021, 33, 6453–6463 CrossRef CAS.
- H. Weng, W. Jia, M. Li and Z. Chen, Carbohydr. Polym., 2022, 294, 119767 CrossRef CAS PubMed.
- W. Qiu, H. Han, M. Li, N. Li, Q. Wang, X. Qin, X. Wang, J. Yu, Y. Li and F. Li, J. Colloid Interface Sci., 2021, 596, 312–323 CrossRef CAS PubMed.
- Y. Deng, J. Chen, J. Huang, X. Yang, X. Zhang, S. Yuan and W. Liao, Cellulose, 2020, 27, 3971–3988 CrossRef CAS.
- J. W. Luo, C. Liu, J. H. Wu, L. X. Lin, H. M. Fan, D. H. Zhao, Y. Q. Zhuang and Y. L. Sun, Mater. Sci. Eng., C, 2019, 98, 628–634 CrossRef CAS PubMed.
- Z. Mirzakhanian, K. Faghihi, A. Barati and H. R. Momeni, J. Biomater. Sci., Polym. Ed., 2015, 26, 1439–1451 CrossRef CAS PubMed.
- J. Yu, X. Li, N. Chen, S. Xue, J. Zhao, S. Li, X. Hou and X. Yuan, Colloids Surf., B, 2022, 215, 112508 CrossRef CAS PubMed.
- J. Liu, J. Li, F. Yu, Y. X. Zhao, X. M. Mo and J. F. Pan, Int. J. Biol. Macromol., 2020, 147, 653–666 CrossRef CAS PubMed.
- K. Fujii, H. Asai, T. Ueki, T. Sakai, S. Imaizumi, U.-I. Chung, M. Watanabe and M. Shibayama, Soft Matter, 2012, 8, 1756–1759 RSC.
- Y. Kong, Z. Hou, L. Zhou, P. Zhang, Y. Ouyang, P. Wang, Y. Chen and X. Luo, ACS Biomater. Sci. Eng., 2021, 7, 335–349 CrossRef CAS PubMed.
- A. Ovsianikov, M. Malinauskas, S. Schlie, B. Chichkov, S. Gittard, R. Narayan, M. Löbler, K. Sternberg, K.-P. Schmitz and A. Haverich, Acta Biomater., 2011, 7, 967–974 CrossRef CAS PubMed.
- M. Rodrigues, N. Kosaric, C. A. Bonham and G. C. Gurtner, Physiol. Rev., 2019, 99, 665–706 CrossRef CAS PubMed.
- D. A. Triplett, Clin. Chem., 2000, 46, 1260–1269 CrossRef CAS.
- S. Palta, R. Saroa and A. Palta, Indian J. Anaesth., 2014, 58, 515–523 CrossRef CAS PubMed.
- M. McMichael, J. Vet. Emerg. Crit. Care, 2005, 15, 1–8 CrossRef.
- K. Broos, H. B. Feys, S. F. De Meyer, K. Vanhoorelbeke and H. Deckmyn, Blood Rev., 2011, 25, 155–167 CrossRef CAS PubMed.
- H. H. Versteeg, J. W. Heemskerk, M. Levi and P. H. Reitsma, Physiol. Rev., 2013, 93, 327–358 CrossRef CAS PubMed.
- J. M. Stassen, J. Arnout and H. Deckmyn, Curr. Med. Chem., 2004, 11, 2245–2260 CrossRef CAS PubMed.
- J. W. Weisel, Adv. Protein Chem., 2005, 70, 247–299 CrossRef CAS PubMed.
- E. Di Cera, Mol. Aspects Med., 2008, 29, 203–254 CrossRef PubMed.
- J. W. Weisel and R. I. Litvinov, Fibrous Proteins, 2017, 405–456 CAS.
- D. Lasne, B. Jude and S. Susen, Can. J. Anaesth., 2006, 53, S2–S11 CrossRef PubMed.
- A. T. Franco, A. Corken and J. Ware, Blood, 2015, 126, 582–588 CrossRef CAS PubMed.
- B. Estevez and X. Du, Physiology, 2017, 32, 162–177 CrossRef CAS PubMed.
- H. Zheng, S. Wang, F. Cheng, X. He, Z. Liu, W. Wang, L. Zhou and Q. Zhang, Chem. Eng. J., 2021, 424, 130148 CrossRef CAS.
- D. Zhang, Z. Xu, H. Li, C. Fan, C. Cui, T. Wu, M. Xiao, Y. Yang, J. Yang and W. Liu, Biomater. Sci., 2020, 8, 1455–1463 RSC.
- N. Zandi, B. Dolatyar, R. Lotfi, Y. Shallageh, M. A. Shokrgozar, E. Tamjid, N. Annabi and A. Simchi, Acta Biomater., 2021, 124, 191–204 CrossRef CAS PubMed.
- S. A. Santoro, Cell, 1986, 46, 913–920 CrossRef CAS PubMed.
- I. D. Campbell and M. J. Humphries, Cold Spring Harbor Perspect. Biol., 2011, 3, a004994 Search PubMed.
- J.-f Dong, J. L. Moake, L. Nolasco, A. Bernardo, W. Arceneaux, C. N. Shrimpton, A. J. Schade, L. V. McIntire, K. Fujikawa and J. A. López, Blood, J. Am. Soc. Hematol., 2002, 100, 4033–4039 CAS.
- T. A. Springer, Blood, 2014, 124, 1412–1425 CrossRef CAS PubMed.
- R. K. Andrews and M. C. Berndt, Thromb. Res., 2004, 114, 447–453 CrossRef CAS PubMed.
- W. Zhang, W. Deng, L. Zhou, Y. Xu, W. Yang, X. Liang, Y. Wang, J. D. Kulman, X. F. Zhang and R. Li, Blood, J. Am. Soc. Hematol., 2015, 125, 562–569 CAS.
- Z. M. Ruggeri, Blood, J. Am. Soc. Hematol., 2015, 125, 423–424 CAS.
- E. M. Golebiewska and A. W. Poole, Blood Rev., 2015, 29, 153–162 CrossRef CAS PubMed.
- M. D. Linden, Methods Mol. Biol., 2013, 992, 13–30 CrossRef CAS PubMed.
- T. Gremmel, A. L. Frelinger, 3rd and A. D. Michelson, Semin. Thromb. Haemostasis, 2016, 42, 191–204 CrossRef CAS PubMed.
- R. Liu, L. Dai, C. Si and Z. Zeng, Carbohydr. Polym., 2018, 195, 63–70 CrossRef CAS PubMed.
- Y. Liu, H. Niu, C. Wang, X. Yang, W. Li, Y. Zhang, X. Ma, Y. Xu, P. Zheng, J. Wang and K. Dai, Bioact. Mater., 2022, 17, 162–177 CAS.
- K. Zheng, Y. Tong, S. Zhang, R. He, L. Xiao, Z. Iqbal, Y. Zhang, J. Gao, L. Zhang, L. Jiang and Y. Li, Adv. Funct. Mater., 2021, 31, 2102599 CrossRef CAS.
- N. D. Sanandiya, S. Lee, S. Rho, H. Lee, I. S. Kim and D. S. Hwang, Carbohydr. Polym., 2019, 208, 77–85 CrossRef CAS PubMed.
- A. Radomski, P. Jurasz, D. Alonso-Escolano, M. Drews, M. Morandi, T. Malinski and M. W. Radomski, Br. J. Pharmacol., 2005, 146, 882–893 CrossRef CAS PubMed.
- A. K. Gaharwar, R. K. Avery, A. Assmann, A. Paul, G. H. McKinley, A. Khademhosseini and B. D. Olsen, ACS Nano, 2014, 8, 9833–9842 CrossRef CAS PubMed.
- P. Roach, D. Farrar and C. C. Perry, J. Am. Chem. Soc., 2005, 127, 8168–8173 CrossRef CAS PubMed.
- M. H. Kim, J. Lee, J. N. Lee, H. Lee and W. H. Park, Acta Biomater., 2021, 123, 254–262 CrossRef CAS PubMed.
- S. Atefyekta, E. Blomstrand, A. K. Rajasekharan, S. Svensson, M. Trobos, J. Hong, T. J. Webster, P. Thomsen and M. Andersson, ACS Biomater. Sci. Eng., 2021, 7, 1693–1702 CrossRef CAS PubMed.
- Z. Zhai, K. Xu, L. Mei, C. Wu, J. Liu, Z. Liu, L. Wan and W. Zhong, Soft Matter, 2019, 15, 8603–8610 RSC.
- M. Zhang, D. Wang, N. Ji, S. Lee, G. Wang, Y. Zheng, X. Zhang, L. Yang, Z. Qin and Y. Yang, Polymers, 2021, 13, 199–209 CrossRef PubMed.
- R. Hao, X. Peng, Y. Zhang, J. Chen, T. Wang, W. Wang, Y. Zhao, X. Fan, C. Chen and H. Xu, ACS Appl. Mater. Interfaces, 2020, 12, 55574–55583 CrossRef CAS PubMed.
- S. Wang, X. Ji, S. Chen, C. Zhang, Y. Wang, H. Lin and L. Zhao, Eur. Polym. J., 2022, 162, 110875 CrossRef CAS.
- X. Chen, C. Cui, Y. Liu, C. Fan, M. Xiao, D. Zhang, Z. Xu, Y. Li, J. Yang and W. Liu, Biomater. Sci., 2020, 8, 3760–3771 RSC.
- G. Chen, Y. Yu, X. Wu, G. Wang, J. Ren and Y. Zhao, Adv. Funct. Mater., 2018, 28, 1801386 CrossRef.
- M. B. Rahmany, R. R. Hantgan and M. Van Dyke, Biomaterials, 2013, 34, 2492–2500 CrossRef CAS PubMed.
- D. Wang, W. Li, Y. Wang, H. Yin, Y. Ding, J. Ji, B. Wang and S. Hao, Colloids Surf., B, 2019, 182, 110367 CrossRef CAS PubMed.
- A. Tang, Y. Li, Y. Yao, X. Yang, Z. Cao, H. Nie and G. Yang, Biomater. Sci., 2021, 9, 4169–4177 RSC.
- Z. Sun, X. Chen, X. Ma, X. Cui, Z. Yi and X. Li, J. Mater. Chem. B, 2018, 6, 6133–6141 RSC.
- E. J. Kim, J. S. Choi, J. S. Kim, Y. C. Choi and Y. W. Cho, Biomacromolecules, 2016, 17, 4–11 CrossRef CAS PubMed.
- R. D. Ventura, A. R. Padalhin and B. T. Lee, Mater. Lett., 2018, 232, 130–133 CrossRef CAS.
- S. Shen, D. Fan, Y. Yuan, X. Ma, J. Zhao and J. Yang, Chem. Eng. J., 2021, 426, 130610 CrossRef CAS.
- Y. Yuan, D. Fan, S. Shen and X. Ma, Chem. Eng. J., 2022, 433, 133859 CrossRef CAS.
- J. W. Weisel and R. I. Litvinov, J. Thromb. Haemostasis, 2019, 17, 271–282 CrossRef CAS PubMed.
- R. Marchioli, G. Finazzi, G. Specchia, R. Cacciola, R. Cavazzina, D. Cilloni, V. De Stefano, E. Elli, A. Iurlo and R. Latagliata, N. Engl. J. Med., 2013, 368, 22–33 CrossRef CAS PubMed.
- V. X. Du, D. Huskens, C. Maas, R. Al Dieri, P. G. de Groot and B. de Laat, Semin. Thromb. Hemostasis, 2014, 40, 72–80 CAS.
- C. Klatt, I. Krüger, S. Zey, K.-J. Krott, M. Spelleken, N. S. Gowert, A. Oberhuber, L. Pfaff, W. Lückstädt and K. Jurk, J. Clin. Invest., 2018, 128, 3906–3925 CrossRef PubMed.
- M. Spengler, M. Svetaz, M. Leroux, S. Bertoluzzo, P. Carrara, F. Van Isseldyk, D. Petrelli, F. Parente and P. Bosch, Clin. Hemorheol. Microcirc., 2011, 47, 279–285 CAS.
- E. B. Assayag, N. Bornstein, I. Shapira, T. Mardi, Y. Goldin, T. Tolshinski, Y. Vered, V. Zakuth, M. Burke and S. Berliner, Int. J. Cardiol., 2005, 98, 271–276 CrossRef PubMed.
- K. C. Gersh, C. Nagaswami and J. W. Weisel, J. Thromb. Haemostasis, 2009, 102, 1169–1175 CrossRef CAS PubMed.
- J. Weisel and R. Litvinov, J. Thromb. Haemostasis, 2019, 17, 271–282 CrossRef CAS PubMed.
- G. Barshtein, R. Ben-Ami and S. Yedgar, Expert Rev. Cardiovasc. Ther., 2007, 5, 743–752 CrossRef PubMed.
- M. Wang, J. Hu, Y. Ou, X. He, Y. Wang, C. Zou, Y. Jiang, F. Luo, D. Lu, Z. Li, J. Li and H. Tan, ACS Appl. Mater. Interfaces, 2022, 14, 17093–17108 CrossRef CAS PubMed.
- M. Kedzierska, S. Blilid, K. Milowska, J. Kolodziejczyk-Czepas, N. Katir, M. Lahcini, A. El Kadib and M. Bryszewska, Int. J. Mol. Sci., 2021, 22, 11386 CrossRef CAS PubMed.
- N. Mackman, Anesth. Analg., 2009, 108, 1447 CrossRef CAS PubMed.
- S. P. Grover and N. Mackman, Arterioscler., Thromb., Vasc. Biol., 2019, 39, 331–338 CrossRef CAS PubMed.
- P. E. van der Meijden, I. C. Munnix, J. M. Auger, J. W. Govers-Riemslag, J. M. Cosemans, M. J. Kuijpers, H. M. Spronk, S. P. Watson, T. Renné and J. W. Heemskerk, Blood, J. Am. Soc. Hematol., 2009, 114, 881–890 CAS.
- M. Didiasova, L. Wujak, L. Schaefer and M. Wygrecka, Cell. Signal., 2018, 51, 257–265 CrossRef CAS PubMed.
- S. P. Grover and N. Mackman, Arterioscler., Thromb., Vasc. Biol., 2018, 38, 709–725 CrossRef CAS PubMed.
- N. Mackman, R. E. Tilley and N. S. Key, Arterioscler., Thromb., Vasc. Biol., 2007, 27, 1687–1693 CrossRef CAS PubMed.
- L. Zhang, Y. Zhang, F. Ma, X. Liu, Y. Liu, Y. Cao and R. Pei, J. Mater. Chem. B, 2022, 10, 915–926 RSC.
- Z. Chen, D. Fan and L. Shang, Biomed. Mater., 2020, 16, 012001 CrossRef PubMed.
- S. Gao, L. Qu, C. Zhu, P. Ouyang and D. Fan, Sci. China: Technol. Sci., 2020, 63, 2449–2463 CrossRef CAS.
- S. Tavakoli, M. Kharaziha, S. Nemati and A. Kalateh, Carbohydr. Polym., 2021, 251, 117013 CrossRef CAS PubMed.
- N. Golafshan, R. Rezahasani, M. Tarkesh Esfahani, M. Kharaziha and S. N. Khorasani, Carbohydr. Polym., 2017, 176, 392–401 CrossRef CAS PubMed.
- A. Basu, J. Hong and N. Ferraz, Macromol. Biosci., 2017, 17, 1700236 CrossRef PubMed.
- X. Fan, S. Wang, Y. Fang, P. Li, W. Zhou, Z. Wang, M. Chen and H. Liu, Mater. Sci. Eng., C, 2020, 109, 110649 CrossRef CAS PubMed.
- D. Green, Hemodialysis Int., 2006, 10, S2–S4 Search PubMed.
- T. Subramaniam, M. B. Fauzi, Y. Lokanathan and J. X. Law, Int. J. Mol. Sci., 2021, 22, 6486 CrossRef CAS PubMed.
- H. Cheng, W. Shi, L. Feng, J. Bao, Q. Chen, W. Zhao and C. Zhao, J. Mater. Chem. B, 2021, 9, 6678–6690 RSC.
- F. Risser, I. Urosev, J. Lopez-Morales, Y. Sun and M. A. Nash, Biophys. Rev., 2022, 1–35, DOI:10.1007/s12551-022-00950-w.
- N. J. Mutch, Blood, 2019, 134, SCI-18 CrossRef.
- S. Bru, J. M. Martínez-Laínez, S. Hernández-Ortega, E. Quandt, J. Torres-Torronteras, R. Martí, D. Canadell, J. Ariño, S. Sharma, J. Jiménez and J. Clotet, Mol. Microbiol., 2016, 101, 367–380 CrossRef CAS PubMed.
- S. H. Choi, S. A. Smith and J. H. Morrissey, Blood, 2011, 118, 6963–6970 CrossRef CAS PubMed.
- S. A. Smith, S. H. Choi, R. Davis-Harrison, J. Huyck, J. Boettcher, C. M. Rienstra and J. H. Morrissey, Blood, 2010, 116, 4353–4359 CrossRef CAS PubMed.
- J. L. Mitchell, A. S. Lionikiene, G. Georgiev, A. Klemmer, C. Brain, P. Y. Kim and N. J. Mutch, Blood, 2016, 128, 2834–2845 CrossRef PubMed.
- D. Chen, X. Liu, Y. Qi, X. Ma, Y. Wang, H. Song, Y. Zhao, W. Li and J. Qin, Colloids Surf., B, 2022, 214, 112430 CrossRef CAS PubMed.
- D. Chen, X. Zhou, L. Chang, Y. Wang, W. Li and J. Qin, Biomacromolecules, 2021, 22, 2272–2283 CrossRef CAS PubMed.
- M. Sakoda, M. Kaneko, S. Ohta, P. Qi, S. Ichimura, Y. Yatomi and T. Ito, Biomacromolecules, 2018, 19, 3280–3290 CrossRef CAS PubMed.
- C. Cao, N. Yang, Y. Zhao, D. Yang, Y. Hu, D. Yang, X. Song, W. Wang and X. Dong, Nano Today, 2021, 39, 101165 CrossRef CAS.
- H. Stoll, H. Steinle, K. Stang, S. Kunnakattu, L. Scheideler, B. Neumann, J. Kurz, I. Degenkolbe, N. Perle, C. Schlensak, H. P. Wendel and M. Avci-Adali, Macromol. Biosci., 2017, 17, 1600252 CrossRef PubMed.
- S. Charbonneau, C. A. Lemarie, H. T. Peng, J. G. Ganopolsky, P. N. Shek and M. D. Blostein, J. Trauma Acute Care Surg., 2012, 72, 136–142 CrossRef CAS PubMed.
- D. Zhang, Z. Hu, L. Zhang, S. Lu, F. Liang and S. Li, Materials, 2020, 13, 5038 CrossRef CAS PubMed.
- J. Zhu, H. Han, F. Li, X. Wang, J. Yu, X. Qin and D. Wu, Chem. Mater., 2019, 31, 4436–4450 CrossRef CAS.
- Y. Ouyang, Y. Zhao, X. Zheng, Y. Zhang, J. Zhao, S. Wang and Y. Gu, Int. J. Biol. Macromol., 2023, 242, 124960 CrossRef CAS PubMed.
- Q. Zhou, X. Zhou, Z. Mo, Z. Zeng, Z. Wang, Z. Cai, L. Luo, Q. Ding, H. Li and S. Tang, Int. J. Biol. Macromol., 2023, 224, 370–379 CrossRef CAS PubMed.
- E. Khadem, M. Kharaziha and S. Salehi, Mater. Today Bio., 2023, 20, 100650 CrossRef CAS PubMed.
- N. Zhang, X. Zhang, Y. Zhu, D. Wang, R. Li, S. Li, R. Meng, Z. Liu and D. Chen, Polymers, 2023, 15, 4362 CrossRef CAS PubMed.
- Z. Chen, J. Yao, J. Zhao and S. Wang, Int. J. Biol. Macromol., 2023, 225, 1235–1245 CrossRef CAS PubMed.
- S. Xu, J. You, S. Yan, L. Zhu and X. Wu, J. Mater. Chem. B, 2023, 11, 9950–9960 RSC.
- H. Song, L. Xing, W. Liu, X. Wang, Z. Hou, Y. Wang, Z. Zhang, Y. Li, T. Li, X. Wang, H. Chen, S. Xing and J. Xu, Biomacromolecules, 2023, 24, 3327–3344 CrossRef CAS PubMed.
- Z. Chen, J. Zhao, H. Wu, H. Wang, X. Lu, M.-A. Shahbazi and S. Wang, Carbohydr. Polym., 2023, 303, 120434 CrossRef CAS PubMed.
- M. Suneetha, S. Zo, S. M. Choi and S. S. Han, Int. J. Biol. Macromol., 2023, 241, 124641 CrossRef CAS PubMed.
- P. Fan, Q. Dong, J. Yang, Y. Chen, H. Yang, S. Gu, W. Xu and Y. Zhou, Carbohydr. Polym., 2023, 313, 120854 CrossRef CAS PubMed.
- D. Zhang, L. Mei, Y. Hao, B. Yi, J. Hu, D. Wang, Y. Zhao, Z. Wang, H. Huang and Y. Xu, Biomater. Res., 2023, 27, 1–15 CrossRef PubMed.
- A. Osman, E. Lin and D. S. Hwang, Carbohydr. Polym., 2023, 299, 120172 CrossRef CAS PubMed.
- H. Wang, J. Cheng, F. Sun, X. Dou, J. Liu, Y. Wang, M. Li, J. Gao, X. Liu and X. Wang, Adv. Mater., 2023, 35, 2208622 CrossRef CAS PubMed.
- R. Haghniaz, H. Montazerian, A. Rabbani, A. Baidya, B. Usui, Y. Zhu, M. Tavafoghi, F. Wahid, H. J. Kim and A. Sheikhi, Adv. Healthcare Mater., 2023, 2301551 CrossRef CAS PubMed.
- C. Liu, C. Liu, Z. Liu, Z. Shi, S. Liu, X. Wang, X. Wang and F. Huang, Int. J. Biol. Macromol., 2023, 224, 1091–1100 CrossRef CAS PubMed.
- H. Cheng, Q. Yu, Q. Chen, L. Feng, W. Zhao and C. Zhao, Biomater. Sci., 2023, 11, 931–948 RSC.
- W. Fang, L. Yang, Y. Chen and Q. Hu, Acta Biomater., 2023, 161, 50–66 CrossRef CAS PubMed.
- M. Mecwan, R. Haghniaz, A. H. Najafabadi, K. Mandal, V. Jucaud, J. V. John and A. Khademhosseini, Biomater. Sci., 2023, 11, 949–963 RSC.
- N. Wang, K. Yu, K. Li and X. Yu, J. Mater. Chem. B, 2023, 11, 1232–1239 RSC.
- Z. Xu, T. Chen, K. Q. Zhang, K. Meng and H. Zhao, Polym. Int., 2021, 70, 1741–1751 CrossRef CAS.
- Y. Yuan, S. Shen and D. Fan, Biomaterials, 2021, 276, 120838 CrossRef CAS PubMed.
- I. A. Duceac, L. Verestiuc, C. D. Dimitriu, V. Maier and S. Coseri, Polymers, 2020, 12, 1473 CrossRef CAS PubMed.
- L. Fan, H. Yang, J. Yang, M. Peng and J. Hu, Carbohydr. Polym., 2016, 146, 427–434 CrossRef CAS PubMed.
- C. Guo, Y. Wu, W. Li, Y. Wang and Q. Kong, ACS Appl. Mater. Interfaces, 2022, 14, 30480–30492 CrossRef CAS PubMed.
- Y. Wang, Y. Wu, L. Long, L. Yang, D. Fu, C. Hu, Q. Kong and Y. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 33584–33599 CrossRef CAS PubMed.
- X. Zhang, G. H. Sun, M. P. Tian, Y. N. Wang, C. C. Qu, X. J. Cheng, C. Feng and X. G. Chen, Int. J. Biol. Macromol., 2019, 138, 321–333 CrossRef CAS PubMed.
- Q. Bai, L. Teng, X. Zhang and C. M. Dong, Adv. Healthcare Mater., 2022, 11, e2101809 CrossRef PubMed.
- D. Cai, S. Chen, B. Wu, J. Chen, D. Tao, Z. Li, Q. Dong, Y. Zou, Y. Chen, C. Bi, D. Zu, L. Lu and B. Fang, Mater. Today Bio., 2021, 12, 100127 CrossRef CAS PubMed.
- J. Chen, J. He, Y. Yang, L. Qiao, J. Hu, J. Zhang and B. Guo, Acta Biomater., 2022, 146, 119–130 CrossRef CAS PubMed.
- T. Zhang, J. Zhao, X. Lv, F. Liu, X. Wang, K. Li, Z. Bai, H. Chen and W. Tian, J. Nanopart. Res., 2021, 23, 240 CrossRef CAS.
- O. Goncharuk, O. Korotych, Y. Samchenko, L. Kernosenko, A. Kravchenko, L. Shtanova, C. U. C. O. Tssmall, T. Poltoratska, N. Pasmurtseva, I. Mamyshev, E. Pakhlov and O. Siryk, Mater. Sci. Eng., C, 2021, 129, 112363 CrossRef CAS PubMed.
- X. Zhao, Y. Liang, Y. Huang, J. He, Y. Han and B. Guo, Adv. Funct. Mater., 2020, 30, 1910748 CrossRef CAS.
- F. Song, Y. Kong, C. Shao, Y. Cheng, J. Lu, Y. Tao, J. Du and H. Wang, Acta Biomater., 2021, 136, 170–183 CrossRef CAS PubMed.
- J. Xiang, Y. Wang, L. Yang, X. Zhang, Y. Hong and L. Shen, Int. J. Biol. Macromol., 2022, 196, 1–12 CrossRef CAS PubMed.
- A. M. Behrens, M. J. Sikorski, T. Li, Z. J. Wu, B. P. Griffith and P. Kofinas, Acta Biomater., 2014, 10, 701–708 CrossRef CAS PubMed.
- B. Tao, C. Lin, Z. Yuan, Y. He, M. Chen, K. Li, J. Hu, Y. Yang, Z. Xia and K. Cai, Chem. Eng. J., 2021, 403, 126182 CrossRef CAS.
- Y. Hao, W. Zhao, H. Zhang, W. Zheng and Q. Zhou, Carbohydr. Polym., 2022, 287, 119336 CrossRef CAS PubMed.
- Y. Hao, C. Yuan, J. Deng, W. Zheng, Y. Ji and Q. Zhou, ACS Appl. Mater. Interfaces, 2022, 14, 16006–16017 CrossRef CAS PubMed.
Footnote |
| † Contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |