Oxygen-defective ruthenium oxide as an efficient and durable electrocatalyst for acidic oxygen evolution reaction†
Jingwei
Wang‡
ab,
Lejuan
Cai‡
 a,
Zhipeng
Yu
a,
Zhipeng
Yu
 *a,
Hao
Tan
a,
Xinyi
Xiang
a,
Kaiyang
Xu
a,
Yang
Chao
a,
Sitaramanjaneya Mouli
Thalluri
c,
Fei
Lin
a,
Haoliang
Huang
a,
Chenyue
Zhang
a,
Yang
Zhao
a,
Wenlong
Wang
*a,
Hao
Tan
a,
Xinyi
Xiang
a,
Kaiyang
Xu
a,
Yang
Chao
a,
Sitaramanjaneya Mouli
Thalluri
c,
Fei
Lin
a,
Haoliang
Huang
a,
Chenyue
Zhang
a,
Yang
Zhao
a,
Wenlong
Wang
 *ab and
Lifeng
Liu
*ab and
Lifeng
Liu
 *a
*a
aSongshan Lake Materials Laboratory (SLAB), Dongguan 523808, P. R. China. E-mail: zhipeng.yu@inl.int; liu.lifeng@sslab.org.cn
bInstitute of Physics, Chinese Academy of Sciences, Beijing 100090, P. R. China. E-mail: wwl@iphy.ac.cn
cInternational Iberian Nanotechnology Laboratory (INL), 4715-330 Braga, Portugal
First published on 12th November 2024
Abstract
Proton exchange membrane water electrolysis (PEMWE) is considered a promising technology for green hydrogen production in combination with renewable energy. However, the high cost and, particularly, the scarcity of iridium (Ir) for use as oxygen evolution reaction (OER) catalysts in the anode severely impede large-scale deployment of PEMWE. Herein, we report the synthesis of oxygen-defective ruthenium oxide (HP-RuOx), which can serve as a cost-effective alternative to Ir-based catalysts, showing outstanding electrocatalytic performance for acidic OER. HP-RuOx was obtained through a sol–gel process using hexamethylenetetramine (HMTA) and polyvinylpyrrolidone (PVP) as co-surfactants. The defect-rich nature of HP-RuOx proves to be effective in enhancing the catalytic activity toward acidic OER. Specifically, HP-RuOx exhibits an overpotential of only 237 mV at a current density of 10 mA cm−2 in 0.05 M H2SO4, outperforming commercial RuO2 and other RuOx control catalysts. Both in situ differential electrochemical mass spectrometry (DEMS) studies and theoretical calculations reveal that the OER occurring on HP-RuOx proceeds predominantly through the adsorbate evolution mechanism (AEM) and the lattice oxygen barely participates in the OER. Consequently, the defect-rich HP-RuOx demonstrates good electrocatalytic stability in 0.05 M H2SO4, with only a 90 mV potential increase after 140 hours of OER at 100 mA cm−2. Furthermore, the performance of HP-RuOx is also evaluated in membrane electrode assemblies (MEAs), which can reach 1 A cm−2 at 1.60 V at 60 °C and stably operate at 0.5 A cm−2 for 230 hours with minimal degradation, showing substantial potential for use as an efficient and durable OER catalyst in PEMWE.
1. Introduction
The ever-increasing environmental pollution and concerns about the depletion of fossil fuels are driving the energy transition toward renewable and sustainable sources, and green hydrogen (H2) has now been broadly accepted to be an important energy vector in this transition, which will significantly contribute to carbon neutrality.1 A promising approach to green H2 production is through water electrolysis powered by renewable energy such as solar and wind energies. Among different water electrolysis technologies currently developed, proton exchange membrane water electrolysis (PEMWE) stands out as the most favorable one because it has a fast response, wide dynamic operating range, large operating current densities, and low gas crossover, which make it a good fit to the fluctuating renewable power.2–4 Notwithstanding many advantages, large-scale deployment of PEM water electrolyzers faces substantial challenges. Operating in harsh acidic environments, electrocatalytically active and acid-stable materials are critically needed to drive the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) taking place at the cathode and anode of PEM electrolyzers. So far, platinum group metals (PGMs), such as platinum (Pt), iridium (Ir) and ruthenium (Ru), are still the state-of-the-art catalysts used for PEMWE.5–8 In particular, Ir-based materials are indispensable to catalyze the anodic OER in acidic electrolytes with long-term stability. However, PGMs are not only expensive but also scarce in the Earth's crust, which raises serious concerns about their availability to satisfy the need for the foreseen installation of hundreds of gigawatt-level PEM electrolyzers in the coming decades. In fact, a recent techno-economic analysis has pointed out that the supply of Ir may become a bottleneck for large-scale deployment of PEM electrolyzers.9In this context, many research efforts have recently been dedicated to lowering Ir utilization in electrocatalysts10–14 or searching for alternatives to Ir-based OER catalysts.15–17 Among various candidates, RuO2 has been revitalized as a promising one because of its outstanding catalytic activity for acidic OER and its higher abundance and accordingly much lower price than that of Ir.18–20 Nonetheless, to enable the usage of RuO2 catalysts for practical PEMWE, their poor catalytic stability must be overcome.21–23 When catalyzing the OER at a high voltage or a large current density, the valence state of Ru is inevitably increased and meanwhile the lattice-oxygen oxidation mechanism (LOM) is activated, resulting in the formation of dissoluble [RuO4] species, which deteriorate the structural integrity of RuO2 and eventually lead to rapid performance decay.24–28 Therefore, the key to enhancing catalytic stability of RuO2 is to suppress the LOM pathway and enable the OER to proceed through the adsorbate evolution mechanism (AEM).25,29 To this end, various strategies including cation doping,30–32 defect engineering,33–36 nanostructuring,37,38 and electronic structure regulation,40,41 have been proposed. These methods can effectively enrich electrons around Ru active sites and make them less soluble during the OER.39–41 For example, Sun's group reported that high-valent niobium (Nb) doping enhanced the electron density of Ru and the strength of Ru–O bonds, which inhibited the dissolution of Ru in the RuO2 lattice and ultimately improved the catalytic stability of the Nb-doped RuO2 for acidic OER.31 Zhang and co-workers synthesized sodium-decorated amorphous/crystalline RuO2 with abundant oxygen vacancies, which served as an efficient OER electrocatalyst under pH-universal conditions and exhibited outstanding electrocatalytic stability, given the good resistance to acid corrosion and oxidation of such materials.36 Moreover, Wang et al. constructed defect-rich MnOx/RuO2 catalysts that showed good catalytic properties thanks to the electronic structure modulation of Ru through oxygen vacancies and heterogeneous interfaces.42
Overall, engineering crystal defects such as vacancies, interstitial atoms, and amorphous/crystalline interfaces, has been demonstrated to be an effective strategy for promoting the catalytic performance of RuO2 toward acidic OER. The presence of defects may lower the O 2p band and Fermi level, increase the covalency of metal–oxygen bonds, and reduce the adsorption of oxygen-containing intermediates on RuO2 surfaces, thereby reducing the reaction energy barriers and improving catalytic performance.43–45 Besides, it was also reported that randomly distributed amorphous domains in catalysts could help to accommodate possible structural changes during electrocatalysis through self-regulation, which is expected to effectively increase the catalytic stability in long-term operation.36,46 Notwithstanding some progress, many defect engineering strategies involve complicated materials synthesis procedures, and the catalytic stability enhancement of RuO2-based materials was mostly demonstrated only at small current densities (e.g., 10 mA cm−2) in an aqueous model system. Hence, there is a pressing need for a simple and cost-effective method to introduce defects in RuO2 and validate the stability improvement at relatively low Ru loadings and high current densities in a practically useful device.
In this work, using hexamethylenetetramine (HMTA) and polyvinylpyrrolidone (PVP) as co-surfactants, we prepared an oxygen-defective RuOx catalyst (hereafter denoted as HP-RuOx) that shows substantially improved OER performance in acidic media. The use of HMTA and PVP co-surfactants led to the generation of abundant crystal boundaries and oxygen vacancies in the resulting RuOx, demonstrating outstanding electrocatalytic activity in 0.05 M H2SO4 with an overpotential (η10) of 237 mV to reach a current density of 10 mA cm−2. In situ differential electrochemical mass spectrometry (DEMS) measurements revealed that the OER taking place on HP-RuOx catalysts is inclined to proceed in the AEM pathway, thereby showing markedly enhanced catalytic stability in acidic media at a high current density of 100 mA cm−2. The HP-RuOx can continuously catalyze the OER in 0.05 M H2SO4 at this current density for 140 h without notable degradation. Density functional theory (DFT) calculations reveal that the presence of abundant oxygen vacancies results in an optimized electronic structure, so that the OER preferentially proceeds through the AEM pathway, enhancing the stability of subsurface oxygen in HP-RuOx. When HP-RuOx was used as the anode catalyst in a membrane electrode assembly (MEA), the cell merely required voltages of 1.42, 1.52 and 1.60 V to reach current densities of 100, 500, and 1000 mA cm−2 at 60 °C. Besides, the cell was able to operate at 500 mA cm−2 for 230 h with only minimal decay.
2. Materials and methods
2.1. Chemicals and materials
Commercial ruthenium(IV) oxide (commercial RuO2, ≥99.9%), ruthenium(III) chloride hydrate (RuCl3·xH2O), and Nafion® perfluorinated resin solution (5 wt%) were purchased from Alfa Aesar. Polyvinylpyrrolidone (PVP K30, MW = 58![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), hexamethylenetetramine (HMTA, C6H12N4, ≥99.0%), and isopropyl alcohol (C3H8O, IPA, ≥99.5%) were obtained from Maclin. Ethanol (C2H6O, ≥99.7%) and sulfuric acid (H2SO4, 95.0–98.0%) were bought from Xilong Scientific. 18OH2 (99.0%) was purchased from Energy Chemical. All reagents and materials were used as received without further purification. Deionized (DI) water with a resistivity value of 18.2 MΩ cm−1 was employed to prepare all solutions and electrolytes.
000), hexamethylenetetramine (HMTA, C6H12N4, ≥99.0%), and isopropyl alcohol (C3H8O, IPA, ≥99.5%) were obtained from Maclin. Ethanol (C2H6O, ≥99.7%) and sulfuric acid (H2SO4, 95.0–98.0%) were bought from Xilong Scientific. 18OH2 (99.0%) was purchased from Energy Chemical. All reagents and materials were used as received without further purification. Deionized (DI) water with a resistivity value of 18.2 MΩ cm−1 was employed to prepare all solutions and electrolytes.
2.2. Synthesis of RuOx catalysts
The HP-RuOx catalyst was prepared by a sol–gel method followed by the pyrolysis process. In a typical synthesis, 64 mg of RuCl3·xH2O were dissolved in 10 mL of ethanol in a 100 mL vial and the mixture was sonicated for 10 min to obtain a homogeneous reddish-brown solution. Afterward, 70 mg of HMTA and 60 mg of PVP were added to the vial and a clear suspension was obtained. Next, the vial was capped and placed in an oil bath, which was maintained at 80 °C for 6 h, resulting in a dark green solution. Subsequently, the solution was transferred to a Petri dish to allow the solvent to evaporate at 70 °C in a fume hood, yielding a gel. Finally, the gel was placed in a muffle furnace and annealed at 400 °C in air for 3 h at a ramping rate of 5 °C min−1. After cooling the sample down to room temperature, black HP-RuOx catalyst powder was obtained. Likewise, P-RuOx and H-RuOx catalysts were prepared using the same procedure but in the presence of only PVP or only HMTA, respectively.2.3. Materials characterization
X-ray diffraction (XRD) was performed on a Panalytical Aeris diffractometer with Cu Kα radiation (λ = 0.15406 nm, current: 7.5 mA, voltage: 40 kV) at a scan rate of 10° min−1 in the 2θ range of 10–90°. Raman spectroscopy measurements were carried out on a confocal Raman spectrometer (Horiba/LabRam HR Evolution). X-ray photoelectron spectroscopy (XPS) was conducted on a Thermo Fisher Scientific K-Alpha spectrometer with Al-Kα radiation (1486.6 eV). Transmission electron microscopy (TEM) and high-angle annular dark-field scanning TEM (HAADF-STEM) were performed on a JEOL JEM F200 microscope equipped with an energy-dispersive X-ray spectrometer (EDS), at an acceleration voltage of 200 kV. The specific surface areas of samples were measured using the N2 adsorption/desorption isotherms and calculated based on the Brunauer–Emmett–Teller (BET) method at relative pressures (P/P0) ranging from 0.0 to 1.0. The pore size and pore volume of the samples were analyzed by the Barrett–Joyner–Halenda (BJH) method based on the N2 desorption isotherms. X-ray absorption near-edge structure (XANES) and extended X-ray absorption fine structure (EXAFS) spectra were acquired at the Balder beamline, MAX IV Synchrotron (Sweden). The Ru dissolution during electrocatalysis was detected using an inductively coupled plasma-mass spectrometer (ICP-MS, Agilent 7800).2.4. Electrochemical measurements
Electrocatalytic tests were performed in a three-electrode configuration using a Biologic electrochemical workstation (VMP3e). A rotating disk electrode was employed to assess the OER activity, with a glassy carbon electrode (GC, diameter: 4 mm) as the working electrode, a Pt wire as the counter electrode, and a Hg/HgSO4 electrode as the reference electrode. To prepare the catalyst ink, 5 mg of the as-prepared catalyst, 750 μL of IPA, 230 μL of H2O, and 20 μL of 5 wt% Nafion® solution were mixed and sonicated for 30 min. Then, 25 μL of the ink was drop-cast onto the GC electrode that was finely polished with 0.5 μm and 0.05 μm Al2O3 slurries, resulting in a catalyst loading of about 1.0 mgcat cm−2 (Ru loading: 0.76 mgRu cm−2). Linear sweep voltammetry (LSV) curves were obtained in O2-saturated 0.05 M H2SO4 at a scan rate of 2 mV s−1 and a rotation speed of 1600 rpm, and all LSV curves were 90% iR-corrected. The electrochemical impedance spectroscopy (EIS) measurements were performed at 1.467 V vs. the reversible hydrogen electrode (RHE) for the OER in the frequency range of 105 to 0.01 Hz with a 10 mV sinusoidal perturbation. To assess the long-term stability, the catalysts were loaded on gold-coated titanium felt (Ti@Au, 1 cm × 1 cm) with different loadings (0.5, 1.0 and 2.0 mgcat cm−2), and chronopotentiometry was performed at 100 mA cm−2. Tafel analyses were conducted in the potential range of 0.2–1.0 V vs. RHE based on the acquired LSV curves, according to the following equation:η = a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j j | (1) |
| ECSA = Cdl/Cs | (2) |
| TOF (s−1) = (j × A)/(4 × F × n) | (3) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 C mol−1 is the Faraday constant, and n (mol) stands for the molar number of Ru on the electrode. All Ru cations in the catalysts were assumed to be catalytically active, so the calculated TOF values represent the lower limits of the actual turnover frequency. Besides, the stability number (i.e., Snumber) was calculated based on the following equation:50,51
500 C mol−1 is the Faraday constant, and n (mol) stands for the molar number of Ru on the electrode. All Ru cations in the catalysts were assumed to be catalytically active, so the calculated TOF values represent the lower limits of the actual turnover frequency. Besides, the stability number (i.e., Snumber) was calculated based on the following equation:50,51| Snumber = nO2/nRu(dissolved) | (4) |
2.5. In situ DEMS measurements
DMES measurements were performed on a Hiden HPR-40 electrochemical differential mass spectrometer equipped with a CHI-660e electrochemical workstation. A catalyst-loaded porous carbon paper, an Ag/AgCl electrode and a Pt wire were used as the working, reference and counter electrodes, respectively. The working electrode was first conditioned by performing 8 CV cycles at a scan rate of 5 mV s−1 in a 0.05 M H2SO4 solution containing 18OH2, to make the catalyst 18O-labeled. To obtain similar current densities, the CV potential ranges for HP-RuOx and commercial RuO2 were set to 1.1–1.6 V and 1.1–1.9 V vs. RHE, respectively. After that, the interior of the cell was rinsed with 0.05 M H2SO4 solution containing 16OH2 eight times to ensure complete removal of any residual 18OH2. Finally, the cell was filled with 0.05 M H2SO4 containing 16OH2, and CV was performed under the same conditions as described above. A porous polytetrafluoroethylene (PTFE) membrane was used at the bottom of the cell as an interface between the solution and the vacuum for gas–liquid separation. The O2 produced during the electrochemical tests was monitored synchronously by mass spectrometry.2.6. DFT calculations
DFT calculations were carried out using the VASP code and the Perdew–Burke–Ernzerhof (PBE) functional to model the exchange–correlation energy.52–54 A 9 × 9 × 9 k-point mesh was selected to sample the Brillouin zone of RuO2 cells. The RuO2 (110) surface was modeled with a (4 × 2) supercell consisting of three Ru–O layers. The thickness of the vacuum layer in all slabs was set to 20 Å to eliminate the interactions between the layers caused by the periodic boundary condition. To investigate the impact of oxygen vacancies on the electronic structure and local charge distribution of RuO2, two scenarios were analyzed: the RuO2 (110) facet with a single oxygen vacancy (1Ov-RuOx) and the RuO2 (110) facet with dual oxygen vacancies (2Ov-RuOx). All possible configurations of 1Ov-RuOx and 2Ov-RuOx models were considered, and the thermodynamically most stable structures were selected for subsequent calculations. The cut-off energy was set to 520 eV, with the structural relaxation criteria of 10−4 eV per atom for energy convergence and 0.01 eV Å−1 for force convergence. The vdW-DF2 method was applied to describe the long-range van der Waals (vdW) interaction in all the structures.55,56 All free energies were corrected by considering the zero-point energy and entropy corrections under the standard conditions (i.e., P0 = 1 bar and T0 = 298.15 K).The adsorption energy values of H2O and O* on pristine RuO2 (110), 1Ov-RuOx and 2Ov-RuOx, were calculated, and the adsorption energy (ΔGads) was computed as follows:
| ΔGads = Gtotal − Esurf − GH2O | (5) |
| Ea = Etotal − Esurf − EO | (6) |
| H2O* → OH* + H+ + e− | (7) |
| OH* → O* + H+ + e− | (8) |
| O* + H2O → O*(Ov-H2O) | (9) |
| O*(Ov-H2O) + H2O → OOH*(Ov-H2O) + H+ + e− | (10) |
| OOH*(Ov-H2O) → H2O* + O2 + H+ + e− | (11) |
Thus, the Gibbs energy changes of the above steps (ΔGn) can be calculated via the following equations:
| ΔG1 = GOH* + GH − GH2O* | (12) |
| ΔG2 = GO* + GH − GOH* | (13) |
| ΔG3 = GO*(Ov-H2O) − GO* − GH2O | (14) |
| ΔG4 = GOOH*(Ov-H2O) + GH − GO*(Ov-H2O) − GH2O | (15) |
| ΔG5 = GH2O* +GO2 + GH − GOOH*(Ov-H2O) | (16) |
2.7. Electrochemical tests in a MEA
The MEA was prepared through the catalyst-coated membrane (CCM) method. Specifically, the HP-RuOx catalyst and Nafion® solution (mass ratio − mcat![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) mNafion = 3
mNafion = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) were dispersed in isopropanol. After ultrasonication for 30 min, a homogeneous ink was obtained, which was then sprayed onto a piece of Nafion® NR212 membrane at 80 °C as the anode catalyst layer. Another ink, consisting of commercial Pt/C (TKK, 40 wt%) catalysts, was prepared in a similar way and then sprayed onto a piece of PTFE membrane. The Pt/C thin film was subsequently transferred to the other side of the HP-RuOx coated Nafion® NR212 membrane as the cathode catalyst layer. The catalyst coverage area was 1 cm × 1 cm for both catalyst layers, with the anode and cathode catalyst loadings being 2.0 mgcat cm−2 and 0.5 mgPt cm−2, respectively. Besides, porous Ti@Au felt and a carbon paper were used as the porous transport layers (PTLs) for the anode and cathode, respectively. A single-cell PEM electrolyzer was finally obtained by assembling the CCM, PTLs and two Ti@Au end plates with flow fields altogether. The steady-state polarization curve was collected in the galvanostatic mode from 0 to 3 A cm−2, and the stability was assessed at a constant current density of 500 mA cm−2. The cell temperature was maintained at 60 °C during the tests, and all electrochemical data are presented without iR correction.
1) were dispersed in isopropanol. After ultrasonication for 30 min, a homogeneous ink was obtained, which was then sprayed onto a piece of Nafion® NR212 membrane at 80 °C as the anode catalyst layer. Another ink, consisting of commercial Pt/C (TKK, 40 wt%) catalysts, was prepared in a similar way and then sprayed onto a piece of PTFE membrane. The Pt/C thin film was subsequently transferred to the other side of the HP-RuOx coated Nafion® NR212 membrane as the cathode catalyst layer. The catalyst coverage area was 1 cm × 1 cm for both catalyst layers, with the anode and cathode catalyst loadings being 2.0 mgcat cm−2 and 0.5 mgPt cm−2, respectively. Besides, porous Ti@Au felt and a carbon paper were used as the porous transport layers (PTLs) for the anode and cathode, respectively. A single-cell PEM electrolyzer was finally obtained by assembling the CCM, PTLs and two Ti@Au end plates with flow fields altogether. The steady-state polarization curve was collected in the galvanostatic mode from 0 to 3 A cm−2, and the stability was assessed at a constant current density of 500 mA cm−2. The cell temperature was maintained at 60 °C during the tests, and all electrochemical data are presented without iR correction.
3. Results and discussion
Fig. 1a schematically illustrates the synthesis process of HP-RuOx. First, Ru complexes were formed by mixing RuCl3 with HMTA and PVP co-surfactants in an ethanol solution at 80 °C. Afterward, ethanol was evaporated, and the obtained gel was then calcined at 400 °C in air for 3 h, resulting in the formation of HP-RuOx powders. For comparison, two control RuOx catalysts using only HMTA or PVP as the surfactant were also synthesized under the same conditions (hereafter denoted as H-RuOx and P-RuOx, respectively). The crystal structure of the samples was first examined by XRD (Fig. S1†), and the peaks of all samples are consistent with those of typical rutile RuO2 (JCPDF no. 40-1290), proving that phase-pure rutile ruthenium oxide was obtained. When compared to commercial RuO2 (Alfa Aesar), the full width at half maximum (FWHM) of major diffraction peaks, such as (110), (101) and (211), becomes wider for all three homemade RuOx catalysts. For example, the FWHM of the (110) peak is 0.965°, 0.751°, and 0.751° for HP-RuOx, P-RuOx, and H-RuOx (Fig. S1†), respectively, much larger than that of commercial RuO2 (0.429°), indicating the lower degree of crystallinity of the homemade catalysts. Moreover, the major diffractions of these homemade RuOx catalysts were found to shift a bit toward lower angles, which implies the existence of tensile stress in the lattice. Additionally, Raman spectroscopy was further employed to characterize the structure of these catalysts (Fig. S2†). The three homemade RuOx samples exhibit characteristic peaks similar to those of commercial RuO2, confirming the formation of a rutile structure. Moreover, the FWHM of HP-RuOx is found to be broader than that of other controls, indicating its relatively low crystallinity.The microstructure of homemade RuOx catalysts was further examined by TEM. All samples showed nanoparticulate morphology (Fig. 1b, S3 and S4†). Statistical analyses (Fig. 1b inset and S5†) revealed that the average particle size of HP-RuOx is 11.0 ± 2.5 nm, which is smaller than that of P-RuOx (14.8 ± 7.4 nm) and H-RuOx (14.3 ± 5.1 nm). Moreover, the HP-RuOx nanoparticles (NPs) exhibited a better Gaussian distribution in size in a narrower range, relative to that of P-RuOx and H-RuOx. The selected-area electron diffraction (SAED) image of HP-RuOx displayed a ring pattern (Fig. 1c), characteristic of a polycrystalline structure, which can be indexed to the diffractions from (110), (101), (200) and (211) crystal planes of rutile RuO2 (JCPDF no. 40-1290), consistent with the XRD results. The EDS elemental mapping in the HAADF-STEM mode revealed a uniform distribution of both Ru and O elements over individual RuOx NPs (Fig. 1d, S3c and S4c†), indicating that no segregation occurred during the thermal treatment. High-resolution TEM (HRTEM) imaging was further carried out to examine the atomic structure of HP-RuOx. Twin structures were often observed under a HRTEM (Fig. 1e), the presence of which is expected to be able to tune the electronic structure of electrocatalysts, enhancing catalytic activity.58,59 Misalignment of the lattice was also seen in HP-RuOx (Fig. 1f), as evidenced by two sets of neighboring diffraction spots along the same direction shown in the fast Fourier transform (FFT) pattern (inset, Fig. 1f) and the lattice distortion in the inverse fast Fourier transform (IFFT) pattern (Fig. S6†). The lattice spacings in crystalline regions are measured to be 0.32, 0.27, and 0.23 nm, corresponding to the distances of (110), (101), and (200) crystal planes of rutile RuO2, respectively. Besides crystallites, an amorphous phase was also found in HP-RuOx, P-RuOx, and H-RuOx NPs (Fig. 1g, S3b and S4b†), mainly located at nanoparticle edges and grain boundaries that do not notably influence the bulk crystallinity. Previous reports suggest that defects and lattice distortions may regulate the electronic structure of electrocatalysts, helping to improve the catalytic activity by changing the strength of adsorption of reaction intermediates on the catalyst surface.34,37,60 Moreover, the presence of amorphous regions and grain boundaries enables the exposure of more catalytically active sites,61,62 boosting electrocatalytic performance.
The OER activity of the HP-RuOx catalyst was first evaluated in a three-electrode cell in O2-saturated 0.05 M H2SO4 using a rotating disk electrode (RDE) to precisely control the loading area and to conveniently compare with the activity of RuO2-based catalysts reported by other groups. To make a just comparison, P-RuOx, H-RuOx and commercial RuO2 were also tested under the same conditions with the same catalyst loading density. In this work, 0.05 M H2SO4 (pH = 1.2), rather than 0.5 M H2SO4 as commonly reported in the literature, was employed in electrochemical tests, considering that the pH value in the vicinity of the PEM under operational conditions was virtually close to 1.51,63–65 The mass loading of HP-RuOx catalysts was first optimized in the range of 0.5–2.0 mgcat cm−2 (0.38–1.52 mgRu cm−2). The OER activity was dependent on the mass loading, as expected, though it does not vary significantly (Fig. S7a†). Moreover, the Tafel slope measured at different loadings was similar (Fig. S7b†). Although the best performance was achieved with a loading of 1.5 mgcat cm−2, to verify if the HP-RuOx catalyst can meet the low PGM utilization target proposed by the Fuel Cell & Hydrogen Joint Undertaking (FCH JU2) of the European Union,66 the catalytic performance was mainly assessed at a catalyst loading of 1.0 mgcat cm−2, given that the activity difference under these two loadings was insignificant (Fig. S7†).
Fig. 2a shows linear sweep voltammograms (LSVs) of HP-RuOx and other control catalysts. The HP-RuOx catalyst requires an overpotential (η10) of 237 mV to reach 10 mA cm−2, which is lower than that of P-RuOx (η10 = 246 mV), H-RuOx (η10 = 259 mV), and commercial RuO2 (η10 = 347 mV). Since the glassy carbon electrode in the RDE is not suitable for long-term stability tests under harsh acidic conditions due to carbon corrosion, gold-coated porous titanium felt (Ti@Au felt) was used as the current collector to assess the long-term stability of all catalysts. Accordingly, the LSV curves of different catalysts loaded on the Ti@Au felt were also acquired under the same testing conditions (Fig. S8†). The η10 values of all catalysts tested on the Ti@Au felt are only slightly higher than those tested using the RDE (η10 = 243, 258, 291 and 352 mV for HP-RuOx, P-RuOx, H-RuOx and commercial RuO2).
Tafel analysis was carried out to investigate the OER kinetics. The Tafel slope of HP-RuOx is 58.7 mV dec−1 (Fig. 2b), smaller than that of P-RuOx (61.9 mV dec−1), H-RuOx (67.1 mV dec−1), and commercial RuO2 (98.8 mV dec−1), suggesting that the OER taking place on HP-RuOx is faster. Furthermore, the electrochemical double layer capacitance (Cdl) of the catalysts was measured by performing cyclic voltammetry (CV) in the non-faradaic potential region at various scan rates (Fig. S9†). HP-RuOx shows the highest Cdl value of 37.2 mF cm−2 (Fig. 2c), which can be translated to an electrochemically active surface area (ECSA) of 620 cm2 (Fig. S10a†), significantly higher than that of P-RuOx (543 cm2), H-RuOx (328 cm2), and commercial RuO2 (102 cm2). Moreover, N2 adsorption/desorption isotherms were acquired for all samples (Fig. S11†). The BET surface area of HP-RuOx was determined to be 61.9 m2 g−1, slightly higher than that of P-RuOx (57.0 m2 g−1) but markedly larger than that of H-RuOx (25.1 m2 g−1) and commercial RuO2 (18.4 m2 g−1), consistent with the trend in the ECSA estimated from Cdl values. HP-RuOx exhibits the largest pore size and pore volume (Fig. S12†), in agreement with the BET surface area data. It is believed that the large pore size and pore volume would facilitate the transport of reactant/product molecules and promote the OER kinetics. Furthermore, the turnover frequency as an intrinsic metric of OER activity was calculated for all catalysts (Fig. S10b†). Interestingly, although HP-RuOx shows the highest TOF values under various overpotentials, it does not exhibit the best specific activity (Fig. S10c†). This means that the specific surface area does not directly correlate with the number of electrocatalytically active sites, and it is not a major contributor to the OER performance, either. It is believed that the oxygen vacancies and surface defects that are associated with the catalyst's electronic structure, along with the pore size and pore volume that facilitate mass transport, play a more important role in governing OER performance, as will be elaborated in the following section.
Besides, electrochemical impendence spectroscopy (EIS) measurements were carried out, revealing that all homemade RuOx catalysts exhibit much smaller charge transfer resistance (Rct) than commercial RuO2, suggesting favorable charge transfer in RuOx (Fig. S13 and Table S1†).
The long-term stability of all catalysts was further assessed using chronopotentiometry (CP) at a constant current density of 100 mA cm−2 (Fig. 2d). To do so, the catalyst ink was sprayed on the Ti@Au felt. Previous studies reported the usage of carbon-based current collectors for acidic OER,67–69 which are prone to be oxidized and corroded over a long term in harsh acidic environments, especially at a high potential or current density. According to our observation, a carbon paper loaded with commercial RuO2 catalysts became rather fragile after the OER stability test at 100 mA cm−2 only for 4 h (Fig. S14†); in contrast, the Ti@Au felt electrode loaded with the same amount of commercial RuO2 and assessed under identical conditions was still mechanically robust and electrically conductive after the stability test. This suggests that carbon paper current collectors are not of favorable choice for the OER in acidic media (Fig. S15†). The CP tests in the aqueous model system revealed that HP-RuOx catalysts exhibited outstanding catalytic stability, able to continuously catalyze the OER at 100 mA cm−2 for 140 h, with a potential increase of only 90 mV. In stark contrast, the potential needed to maintain 100 mA cm−2 increased by more than 300 mV after 120 h for P-RuOx and 60 h for H-RuOx (Fig. 2d). In particular, for commercial RuO2 catalysts, the electrode became deactivated within only 4 h, likely originating from the formation of soluble RuO4 species that led to the structural collapse of RuO2, considering that no physical detachment of RuO2 catalysts was observed during the experiment. The Ru dissolution of HP-RuOx catalysts during the CP test was monitored by ICP-MS. After 125 h of testing, the dissolved Ru in the electrolyte was found to be about 3.1% of the total Ru loaded on the electrode. The Snumber was further calculated to be 3.8 × 105, which favorably compares to that of the state-of-the-art RuO2-based catalysts51,70 and even IrO2 catalysts50,71 reported in the literature, demonstrating the outstanding stability of HP-RuOx. Furthermore, how the mass loading of HP-RuOx influences the stability was also investigated. Increasing the loading to 2.0 mgcat cm−2 could substantially enhance the stability, enabling the electrode to operate at 100 mA cm−2 up to 600 h without significant degradation (only a 60 mV potential increase). By contrast, lowering the loading to 0.5 mg cm−2 accelerated the degradation, leading to a potential increase of 190 mV within 100 h (Fig. S16†). In addition, HP-RuOx was also found to favorably compare to many Ru-based OER catalysts reported recently (Fig. 2e and Table S2†), such as a/c-RuO2 (η10 = 220 mV, 60 h @ 10 mA cm−2),36 Ru@MoO(S)3 (η10 = 292 mV, 24 h @ 10 mA cm−2),72 and Y2MnRuO7 (η10 = 270 mV, 45 h @ 10 mA cm−2),63 although it was slightly inferior to SnRuOx catalysts (η10 = 194 mV, 250 h @ 100 mA cm−2).70
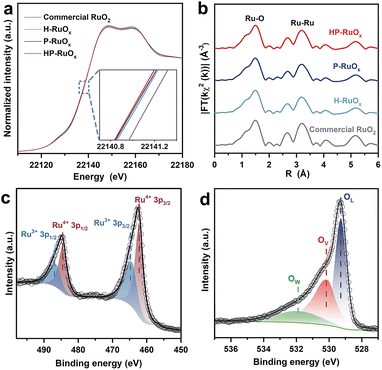
To elucidate the outstanding OER performance of HP-RuOx relative to that of other controls, synchrotron X-ray absorption spectroscopy (XAS) was performed to examine the electronic and ligand structures of the catalysts. Fig. 3a shows the Ru K-edge XANES spectra of all catalysts, where the three homemade RuOx catalysts exhibit an absorption edge at the energy lower than that of commercial RuO2, suggesting that the oxidation state of Ru in RuOx is less than +4. Furthermore, HP-RuOx shows the lowest K-edge energy, indicating that more Ru3+ species likely exist in HP-RuOx. Fig. 3b displays k2-weighted EXAFS spectra of all catalysts, which are similar in feature. The dominant peak at 1.50 Å arises from Ru–O bonding, while the relatively weak peaks at 2.67 and 3.19 Å are attributed to the Ru–Ru scattering paths.73,74 Furthermore, the first-shell coordination was quantitatively analyzed by fitting the EXAFS spectra (Fig. S17†). According to the fitted ligand structure parameters (Table S3†), the average Ru–O coordination number (CN) decreases in the following order: commercial RuO2 > H-RuOx > P-RuOx > HP-RuOx, suggesting that there are more oxygen vacancies in HP-RuOx.
Besides, XPS was conducted to probe the surface chemical state of catalysts. The survey spectra of all catalysts show very similar features (Fig. S18†), verifying the good compositional purity of the homemade RuOx catalysts. Fig. 3c shows the high-resolution Ru 3p XPS spectrum of HP-RuOx, where the binding energy (BE) peaks appearing at 462.3 and 484.5 eV correspond to Ru4+ 3p3/2 and Ru4+ 3p1/2 components, and the ones centered at 464.3 and 486.6 eV correspond to Ru3+ 3p3/2 and Ru3+ 3p1/2 components.34,75 The presence of Ru3+ components implies the non-stoichiometry of the homemade ruthenium oxide catalysts and the presence of oxygen vacancies. As for the O 1s spectrum, three components appear at 529.3, 530.1, and 532.0 eV, which are assigned to lattice oxygen (OL), surface adsorbed oxygenated species (O−, O22−, OH−, etc.), and oxygen from adsorbed water (OW),76,77 respectively. It is worth noting that the oxygenated species are usually adsorbed on vacant lattice oxygen sites (Ov), and therefore the XPS signal of these species can indirectly reflect the content of Ov in the catalysts.76,78 The BE peak positions of Ru 3p and O 1s XPS spectra for P-RuOx, H-RuOx, and commercial RuO2 are essentially the same as those for HP-RuOx (Fig. S19†), but the contents of Ru3+ and Ov differ from each other (Table S4†), following the order of HP-RuOx (41.0%) > P-RuOx (39.3%) > H-RuOx (34.6%) > commercial RuO2 (30.5%) for the Ov content. This indicates that there are more oxygen vacancies existing on and near the surface of HP-RuOx, lowering the coordination number of Ru, which agrees well with the above XAS analysis. These oxygen vacancies are expected to not only effectively modify the electronic structure of RuO2, but also regulate the catalytic behavior of active sites, as demonstrated by our DFT calculations that will be discussed in detail below.
In order to elucidate the catalytic mechanism, we conducted isotope 18O-labeled in situ DEMS experiments to assess the involvement of lattice oxygen in the OER process.51,70 The CV tests were performed on as-prepared HP-RuOx and commercial RuO2 catalysts in 0.05 M H2SO4 containing 18OH2 and also on 18O isotope-labeled catalysts (see Section 2.5) in 0.05 M H2SO4 containing 16OH2, respectively. During the electrochemical processes, the produced oxygen was detected by mass spectrometry, and signals of m/z = 32 (16O16O), 34 (18O16O) and 36 (18O18O) were synchronously monitored (Fig. S20†). The 34O2 signals result from direct coupling of 16O in the lattice of ruthenium oxide catalysts with 18O in the electrolyte (as shown in the tests in 0.05 M H2SO4 containing 18OH2) and the 18O in the lattice with 16O in the electrolyte (as shown in the tests for 18O-labeled catalysts in 0.05 M H2SO4 prepared with 16OH2), and the intensity of 34O signals reflects the degree of involvement of the lattice oxygen mechanism (i.e., LOM).79 According to the DEMS results, the majority of oxygen produced by HP-RuOx and commercial RuO2 catalysts was 36O2 in 0.05 M H2SO4 containing 18OH2 or 32O2 in 0.05 M H2SO4 prepared using regular water (i.e., 16OH2), demonstrating that lattice oxygen did not contribute substantially toward the OER, and the dominant reaction pathway is through the adsorbate evolution mechanism (AEM). Upon peak-area integration and background correction as reported in the literature,80 the ratio (R) of 34O2 to (34O2 + 36O2) produced by HP-RuOx was calculated to be 6.1% for the test carried out in 0.05 M H2SO4 containing 18OH2 (Fig. 4a), which is much lower than that (20.4%) produced by commercial RuO2 tested under the same conditions (Fig. 4b). Similarly, the ratio of 34O2 produced by 18O-labeled HP-RuOx (4.8%, Fig. 4c) was also markedly smaller compared to that generated by 18O-labeled commercial RuO2 (7.4%, Fig. 4d) for the OER taking place in 0.05 M H2SO4 prepared with regular water. The DEMS experiments unambiguously demonstrate that the LOM pathway is substantially suppressed in the HP-RuOx catalyst, which effectively enhances the long-term catalytic stability of HP-RuOx, consistent with the results shown in Fig. 2d.
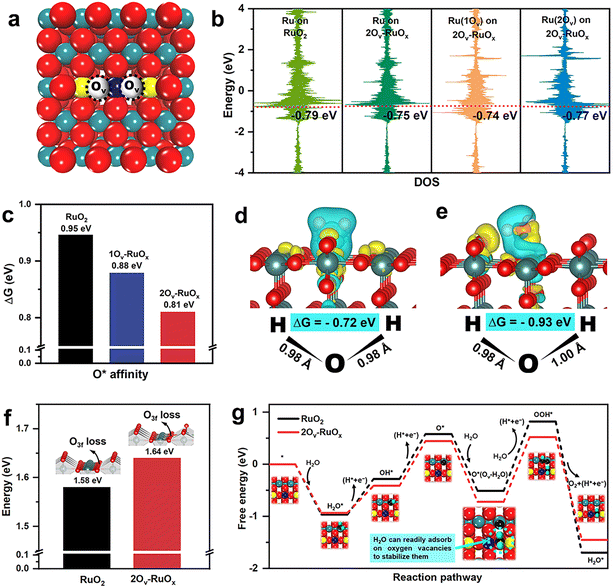
To gain insight into the exceptional OER activity and stability of the oxygen-defective HP-RuOx, DFT calculations were conducted. Defect-free RuO2 (110) and its variants, including RuO2 (110) with one oxygen vacancy (denoted as 1Ov-RuOx) and RuO2 (110) with two oxygen vacancies (denoted as 2Ov-RuOx), were chosen as model catalysts for the simulations. Two types of O atoms exist on the RuO2 (110) surface (Fig. S21†), i.e., bridging O atoms (Obr) and threefold O atoms (O3f).81 Among all possible configurations of oxygen vacancies in 1Ov-RuOx and 2Ov-RuOx, we selected the thermodynamically most stable configurations for subsequent calculations (Fig. 5a, S22 and S23†). Different from the O3f vacancies caused by heteroatom doping, the intrinsic oxygen vacancies of RuO2 (110) tend to be generated on Obr.82,83 Our calculation results show that the formation energies of Obr vacancies are much lower than those of O3f vacancies in both 1Ov-RuOx and 2Ov-RuOx (Fig. S23†). Furthermore, Bader charge analysis was carried out to investigate the regulatory effect of oxygen vacancies on the valence electrons of Ru sites in RuOx models (Fig. S24†). The results reveal that the presence of oxygen vacancies can lead to partial reduction of the Ru atoms around the vacancies. For example, the valence state of Ru(1Ov) decreases from +1.7e to +1.6e. It is noteworthy that dual oxygen vacancies exert larger regulation on the Ru sites around them, as shown in the two-dimensional valence charge density distribution contours (Fig. S25†), where the valence state of Ru(2Ov) sites is further reduced to +1.4e. This suggests that dual oxygen vacancies intensify the non-uniform charge distribution on the RuO2 (110) surface, favorable for the accumulation of negative charges on Ru sites for efficient OER.
Additionally, the projected density of states (PDOS) analysis was performed. It shows that in 2Ov-RuOx, the Ru 3d orbital is closer to the Fermi level, leading to an increased density of states near the Fermi level that facilitates electron delocalization during oxygen adsorption (Fig. 5b and S26†). Besides, the affinity of the oxygen atom (*O), one of the key parameters governing the adsorption/desorption of intermediates during the OER, was also compared for pristine RuO2, 1Ov-RuOx and 2Ov-RuOx (Fig. 5c). 2Ov-RuOx shows an *O affinity energy of 0.81 eV, lower than that of 1Ov-RuOx (0.88 eV) and pristine RuO2 (0.95 eV), indicating that the oxygenated intermediates are more easily adsorbed on the surface of RuOx containing more oxygen vacancies.
Considering that the adsorption of H2O molecules on catalytically active sites is the prerequisite for water dissociation and subsequent O2 evolution,84,85 the H2O adsorption behaviors were further investigated. The results show that in terms of thermodynamics, H2O can spontaneously adsorb on both Ov and Ru sites of defect-rich 2Ov-RuOx (Fig. S27 and Table S5†), with adsorption energies of −0.72 eV and −0.93 eV, respectively. Interestingly, the manner of H2O adsorption on the Ov and Ru sites is largely different. Specifically, the H2O molecule adsorbed on the Ov site exhibits a symmetric configuration, with the length of both O–H bonds being 0.98 Å (Fig. 5d). In contrast, the H2O molecule adsorbed on the Ru site displays an asymmetric configuration with the O–H bond lengths being 0.98 Å and 1.00 Å (Fig. 5e), respectively, thereby facilitating the water activation and dissociation. Furthermore, the adsorbed H2O shows a higher degree of asymmetry on the Ru sites of defect-rich 2Ov-RuOx, compared to that on the Ru sites of defect-free RuO2 (Fig. S28†), reaffirming that the presence of oxygen vacancies is more conducive to efficient H2O activation and dissociation of H2O. Therefore, it is believed that the oxygen vacancies in defective RuOx (e.g., 2Ov-RuOx) do not directly participate in the OER process as active sites, but they tend to stabilize themselves by adsorbing H2O to regulate localized electrons at catalytically active Ru sites, where H2O activation and dissociation can be accomplished more easily. Moreover, the adsorbed H2O molecules can act as stabilizers to prevent the intermediate species from recombination with oxygen vacancies, contributing to better catalytic stability.
To further verify whether the OER process follows the AEM or the LOM, the lattice oxygen stability of both 2Ov-RuOx and pristine RuO2 surfaces was compared. The enthalpy change arising from the subsurface oxygen loss is calculated to be 1.58 eV for defect-free pristine RuO2 and 1.64 eV for defect-rich 2Ov-RuOx (Fig. 5f), demonstrating that the existence of abundant oxygen vacancies can effectively stabilize the lattice oxygen, which is in good agreement with our in situ DEMS results. Furthermore, the free energy diagrams for the OER via the AEM pathway on 2Ov-RuOx and RuO2 are plotted. The conventional AEM pathway typically follows the evolution of H2O* → OH* → O* → OOH* → O2 in acidic media.18,58,84 However, for defective RuOx, since H2O adsorption is spontaneous with a free energy of −1.17 eV, a H2O molecule would be first adsorbed on 2Ov-RuOx to stabilize oxygen vacancies before the second H2O molecule participates in the subsequent O–O coupling process. The Gibbs free energy step diagrams (Fig. 5g) indicate that the potential determining step (PDS) in the OER occurs with a considerably lower energy barrier for 2Ov-RuOx (1.24 eV), compared to that for pristine RuO2 (1.33 eV) and 1Ov-RuOx (1.25 eV, Fig. S29†), highlighting favorable OER thermodynamics on 2Ov-RuOx. Furthermore, during H2O dissociation steps, 2Ov-RuOx also exhibits better performance than 1Ov-RuOx and defect-free RuO2 (Table S6†).
Overall, our DFT calculations align with experimental findings and underscore the pivotal role of oxygen vacancies in boosting OER activity through improving H2O activation while reducing the energy barrier of the PDS on Ru sites. Additionally, the proactive adsorption of H2O molecules on oxygen vacancies bypasses the LOM and drives the OER to take place via the AEM pathway, enhancing the stability of RuOx electrocatalysts. These results are consistent with previous studies.33,83,86 highlighting the positive impact of oxygen vacancies on catalytic performance toward the OER.
Given the excellent OER performance in the half-cell tests, we further evaluated the potential of HP-RuOx for practical use in PEMWE. To do so, we constructed an MEA consisting of a Nafion 212 membrane sandwiched between a HP-RuOx anode catalyst layer and a Pt/C (40 wt% Pt, TKK) cathode catalyst layer. Fig. 6a schematically illustrates the components of a PEMWE single cell (see the photograph of the cell in Fig. S30†). For comparison, an MEA comprising commercial RuO2 as the anode catalyst was also constructed and tested under the same conditions. According to the recorded polarization curve (Fig. 6b), when operated at 60 °C, the cell comprising HP-RuOx‖40 wt% Pt/C catalysts required voltages of 1.42, 1.52 and 1.60 V to reach current densities of 100, 500 and 1000 mA cm−2, which are much lower than those of the cell containing commercial RuO2‖40 wt% Pt/C catalysts (1.57, 1.67 and 1.75 V at 100, 500 and 1000 mA cm−2). Moreover, the electrolyzer using HP-RuOx as the anode catalyst was able to operate at 500 mA cm−2 for 230 h with merely an insignificant voltage increase, whereas the use of commercial RuO2 led to the activity loss after only 24 h of operation (Fig. 6c).
 | ||
| Fig. 6 (a) Schematic illustration of a single-cell PEM electrolyzer. (b) Polarization curves of the cell measured at 60 °C. (c) Stability test of the PEM electrolyzers operated at 500 mA cm−2. | ||
To further investigate the stability of the HP-RuOx catalyst, we compared the crystal structure and electronic structure of HP-RuOx before and after subjecting to a 150 h stability test at 500 mA cm−2. The XRD pattern of the HP-RuOx after the stability test virtually does not change relative to the initial one taken before the test (Fig. S31†), indicating no crystal reconstruction and/or lattice collapse occurred. Besides, XPS examination also revealed that there is no notable difference in the Ru 3p spectrum before and after the stability test (Fig. S32†). The comparison of O 1s spectra is tricky, given that the Nafion introduced on the catalyst surface, which is difficult to remove completely, interferes with the analysis. Nonetheless, the post-mortem examination of HP-RuOx catalysts unambiguously verified that the structural reconstruction and surface chemistry change, if any, is minimal, demonstrating the outstanding stability of the HP-RuOx catalyst.
4. Conclusions
In conclusion, oxygen-defective HP-RuOx catalysts have been successfully prepared by a facile sol–gel method using HMTA and PVP as co-surfactants, followed by post-calcination in air. The as-obtained HP-RuOx catalysts possess abundant oxygen vacancies, as demonstrated by comprehensive physico-chemical characterization, which endow it with outstanding electrocatalytic performance toward the OER under acidic conditions. In particular, HP-RuOx exhibits much improved catalytic stability. DEMS measurements confirm that the OER catalyzed by HP-RuOx proceeds predominantly through the AEM pathway, while the lattice oxygen only makes a minor contribution. Theoretical calculations reveal that oxygen vacancies can regulate the electronic structure of ruthenium oxide, resulting in non-uniform charge distribution and improved H2O activation, which is favorable for the OER and reduces the energy barrier of the potential-determining step. More interestingly, H2O molecules can preferentially adsorb on oxygen vacancies and serve as stabilizers, preventing these vacancies from recombining with oxygenated species during the OER and thereby markedly enhancing catalytic stability. These theoretical simulations rationally validate our experimental results. Furthermore, the electrocatalytic performance of HP-RuOx has also been investigated in MEAs, which can operate at 500 mA cm−2 for 230 h with negligible performance degradation, showing substantial promise for use as an alternative cost-effective OER catalyst in PEM electrolyzers.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Jingwei Wang: conceptualization, methodology, investigation, experiment, writing – original draft. Lejuan Cai: methodology, investigation, experiment, funding acquisition. Zhipeng Yu: conceptualization, methodology, investigation, supervision. Hao Tan: formal analysis. Xinyi Xiang: formal analysis. Kaiyang Xu: formal analysis. Yang Chao: investigation, methodology. Sitaramanjaneya Mouli Thalluri: investigation. Fei Lin: methodology. Haoliang Huang: formal analysis. Chenyue Zhang: formal analysis. Yang Zhao: formal analysis. Wenlong Wang: supervision, resources, project administration. Lifeng Liu: writing – review & editing, supervision, resources, project administration, funding acquisition, formal analysis, conceptualization. All authors commented on the final manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
L. Liu acknowledges the financial support from the Ministry of Science and Technology of China through the Talent Recruitment Programme (grant no. 22J4021Z311) and the startup grant from Songshan Lake Materials Laboratory (grant no. Y2D1051Z311). L. Cai acknowledges the financial support from the National Natural Science Foundation of China (grant no. 22109108). The authors thank Dr Stuart Ansell for his assistance in the XAS experiments performed at the Balder Beamline, MAX IV, Sweden.Notes and references
- R. Chaubey, S. Sahu, O. O. James and S. Maity, Renewable Sustainable Energy Rev., 2013, 23, 443–462 CrossRef CAS.
- M. Carmo, D. L. Fritz, J. Merge and D. Stolten, Int. J. Hydrogen Energy, 2013, 38, 4901–4934 CrossRef CAS.
- L. F. Liu, Curr. Opin. Chem. Eng., 2021, 34, 100743 CrossRef.
- W. J. Zhu, Z. H. Huang, M. T. Zhao, R. P. Huang, Z. C. Wang and H. F. Liang, Environ. Chem. Lett., 2022, 20, 3429–3452 CrossRef CAS.
- Z. C. Chen, L. Guo, L. Pan, T. Q. Yan, Z. X. He, Y. Li, C. X. Shi, Z. F. Huang, X. W. Zhang and J. J. Zou, Adv. Energy Mater., 2022, 12, 2103670 CrossRef CAS.
- S. Cherevko, S. Geiger, O. Kasian, N. Kulyk, J. P. Grote, A. Savan, B. R. Shrestha, S. Merzlikin, B. Breitbach, A. Ludwig and K. J. J. Mayrhofer, Catal. Today, 2016, 262, 170–180 CrossRef CAS.
- C. L. Rong, K. Dastafkan, Y. Wang and C. Zhao, Adv. Mater., 2023, 35, 2211884 CrossRef CAS.
- Q. L. Wang, Y. Q. Cheng, H. B. Tao, Y. H. Liu, X. H. Ma, D. S. Li, H. B. Yang and B. Liu, Angew. Chem., Int. Ed., 2023, 62, e202216645 CrossRef CAS PubMed.
- C. Minke, M. Suermann, B. Bensmann and R. Hanke-Rauschenbach, Int. J. Hydrogen Energy, 2021, 46, 23581–23590 CrossRef CAS.
- J. R. Esquius, A. P. LaGrow, H. Y. Jin, Z. P. Yu, A. Araujo, R. Marques, A. Mendes and L. F. Liu, Mater. Futures, 2024, 3, 015102 CrossRef CAS.
- Y. N. Wang, M. C. Zhang, Z. Y. Kang, L. Shi, Y. C. Shen, B. Y. Tian, Y. C. Zou, H. Chen and X. X. Zou, Nat. Commun., 2023, 14, 5119 CrossRef CAS PubMed.
- X. X. Xiong, J. L. Tang, Y. Ji, W. Q. Xue, H. Y. Wang, C. X. Liu, H. L. Zeng, Y. Z. Dai, H. J. Peng, T. T. Zheng, C. Xia, X. Y. Liu and Q. Jiang, Adv. Energy Mater., 2024, 14, 2304479 CrossRef CAS.
- J. Y. Xu, J. J. Li, Z. Lian, A. Araujo, Y. Li, B. Wei, Z. P. Yu, O. Bondarchuk, I. Amorim, V. Tileli, B. Li and L. F. Liu, ACS Catal., 2021, 11, 3402–3413 CrossRef CAS.
- J. Y. Xu, Z. Lian, B. Wei, Y. Li, O. Bondarchuk, N. Zhang, Z. P. Yu, A. Araujo, I. Amorim, Z. C. Wang, B. Li and L. F. Liu, ACS Catal., 2020, 10, 3571–3579 CrossRef CAS.
- S. Y. Hao, M. Liu, J. J. Pan, X. N. Liu, X. L. Tan, N. Xu, Y. He, L. C. Lei and X. W. Zhang, Nat. Commun., 2020, 11, 5368 CrossRef CAS PubMed.
- J. Y. Liu, T. Y. Wang, Z. J. Lin, M. Y. Liao, S. X. Liu, S. Y. Wang, Z. Cai, H. Sun, Y. Shen, Y. H. Huang and Q. Li, Energy Environ. Sci., 2024, 17, 3088–3098 RSC.
- L. S. Qiu, G. K. Zheng, Y. He, L. C. Lei and X. W. Zhang, Chem. Eng. J., 2021, 409, 128155 CrossRef CAS.
- X. J. Cui, P. J. Ren, C. Ma, J. Zhao, R. X. Chen, S. M. Chen, N. P. Rajan, H. B. Li, L. Yu, Z. Q. Tian and D. H. Deng, Adv. Mater., 2020, 32, 1908126 CrossRef CAS PubMed.
- Y. C. Lin, Z. Q. Tian, L. J. Zhang, J. Y. Ma, Z. Jiang, B. J. Deibert, R. X. Ge and L. Chen, Nat. Commun., 2019, 10, 162 CrossRef PubMed.
- H. Yu and J. J. Ge, Curr. Opin. Electrochem., 2023, 39, 101296 CrossRef CAS.
- K. Klyukin, A. Zagalskaya and V. Alexandrov, J. Phys. Chem. C, 2019, 123, 22151–22157 CrossRef CAS.
- C. Roy, R. R. Rao, K. A. Stoerzinger, J. Hwang, J. Rossmeisl, I. Chorkendorff, Y. Shao-Horn and I. E. L. Stephens, ACS Energy Lett., 2018, 3, 2045–2051 CrossRef CAS.
- C. Spöri, J. T. H. Kwan, A. Bonakdarpour, D. P. Wilkinson and P. Strasser, Angew. Chem., Int. Ed., 2017, 56, 5994–6021 CrossRef.
- H. Jin, S. Choi, G. J. Bang, T. Kwon, H. S. Kim, S. J. Lee, Y. Hong, D. W. Lee, H. S. Park, H. Baik, Y. Jung, S. J. Yoo and K. Lee, Energy Environ. Sci., 2022, 15, 1119–1130 RSC.
- M. Sohail, W. Q. Lv and Z. W. Mei, ACS Sustain. Chem. Eng., 2023, 11, 17564–17594 CrossRef CAS.
- N. Yao, H. N. Jia, J. Zhu, Z. P. Shi, H. J. Cong, J. J. Ge and W. Luo, Chem, 2023, 9, 1882–1896 CAS.
- Y. C. Lin, Y. Dong, X. Z. Wang and L. Chen, Adv. Mater., 2023, 35, 2210565 CrossRef CAS.
- Q. L. Wu, K. Jiang, J. H. Han, D. C. Chen, M. Luo, J. Lan, M. Peng and Y. W. Tan, Sci. China Mater., 2022, 65, 1262–1268 CrossRef CAS.
- Y. C. Yao, S. L. Hu, W. X. Chen, Z. Q. Huang, W. C. Wei, T. Yao, R. R. Liu, K. T. Zang, X. Q. Wang, G. Wu, W. J. Yuan, T. W. Yuan, B. Q. Zhu, W. Liu, Z. J. Li, D. S. He, Z. G. Xue, Y. Wang, X. S. Zheng, J. C. Dong, C. R. Chang, Y. X. Chen, X. Hong, J. Luo, S. Q. Wei, W. X. Li, P. Strasser, Y. E. Wu and Y. D. Li, Nat. Catal., 2019, 2, 304–313 CrossRef CAS.
- L. G. Li, L. Z. Bu, B. L. Huang, P. T. Wang, C. Q. Shen, S. X. Bai, T. S. Chan, Q. Shao, Z. W. Hu and X. Q. Huang, Adv. Mater., 2021, 33, 2105308 CrossRef CAS PubMed.
- H. Liu, Z. Zhang, J. J. Fang, M. X. Li, M. G. Sendeku, X. Wang, H. Y. Wu, Y. P. Li, J. J. Ge, Z. B. Zhuang, D. J. Zhou, Y. Kuang and X. M. Sun, Joule, 2023, 7, 558–573 CrossRef CAS.
- J. W. Su, R. X. Ge, K. M. Jiang, Y. Dong, F. Hao, Z. Q. Tian, G. X. Chen and L. Chen, Adv. Mater., 2018, 30, 1801351 CrossRef.
- T. L. Feng, J. K. Yu, D. Yue, H. Q. Song, S. Y. Tao, G. I. N. Waterhouse, S. Y. Lu and B. Yang, Appl. Catal., B, 2023, 328, 122546 CrossRef CAS.
- R. X. Ge, L. Li, J. W. Su, Y. C. Lin, Z. Q. Tian and L. Chen, Adv. Energy Mater., 2019, 9, 1901313 CrossRef.
- Z. X. Wu, Y. L. Wang, D. Z. Liu, B. W. Zhou, P. F. Yang, R. Z. Liu, W. P. Xiao, T. Y. Ma, J. S. Wang and L. Wang, Adv. Funct. Mater., 2023, 33, 2307010 CrossRef CAS.
- L. J. Zhang, H. Jang, H. H. Liu, M. G. Kim, D. J. Yang, S. G. Liu, X. E. Liu and J. Cho, Angew. Chem., Int. Ed., 2021, 60, 18821–18829 CrossRef CAS.
- S. H. Liu, H. Tan, Y. C. Huang, Q. B. Zhang, H. P. Lin, L. Li, Z. W. Hu, W. H. Huang, C. W. Pao, J. F. Lee, Q. Y. Kong, Q. Shao, Y. Xu and X. Q. Huang, Adv. Mater., 2023, 35, 2305659 CrossRef CAS.
- Z. L. Zhao, Q. Wang, X. Huang, Q. Feng, S. Gu, Z. Zhang, H. Xu, L. Zeng, M. Gu and H. Li, Energy Environ. Sci., 2020, 13, 5143–5151 RSC.
- S. Kaushik, X. Xiao and Q. Xu, Energychem, 2023, 5, 100104 CrossRef CAS.
- Q. Qin, T. T. Wang, Z. J. Li, G. L. Zhang, H. Jang, L. Q. Hou, Y. Wang, M. G. Kim, S. G. Liu and X. Liu, J. Energy Chem., 2024, 88, 94–102 CrossRef CAS.
- X. F. Wang, H. Jang, S. G. Liu, Z. J. Li, X. H. Zhao, Y. F. Chen, M. G. Kim, Q. Qin and X. Liu, Adv. Energy Mater., 2023, 13, 2301673 CrossRef CAS.
- Z. X. Wu, Y. L. Wang, D. Z. Liu, B. W. Zhou, P. F. Yang, R. Z. Liu, W. P. Xiao, T. Y. Ma, J. S. Wang and L. Wang, Adv. Funct. Mater., 2023, 33, 2307010 CrossRef CAS.
- C. F. Dickens and J. K. Norskov, J. Phys. Chem. C, 2017, 121, 18516–18524 CrossRef CAS.
- P. Gayen, S. Saha, K. Bhattacharyya and V. K. Ramani, ACS Catal., 2020, 10, 7734–7746 CrossRef CAS.
- Y. Qin, T. T. Yu, S. H. Deng, X. Y. Zhou, D. M. Lin, Q. Zhang, Z. Y. Jin, D. F. Zhang, Y. B. He, H. J. Qiu, L. H. He, F. Y. Kang, K. K. Li and T. Y. Zhang, Nat. Commun., 2022, 13, 3784 CrossRef CAS.
- S. Anantharaj and S. Noda, Small, 2020, 16, 1905779 CrossRef CAS.
- C. Y. Shang, C. Cao, D. Y. Yu, Y. Yan, Y. T. Lin, H. L. Li, T. T. Zheng, X. P. Yan, W. C. Yu, S. M. Zhou and J. Zeng, Adv. Mater., 2019, 31, 1805104 CrossRef PubMed.
- Z. P. Yu, Y. F. Li, A. Torres-Pinto, A. P. LaGrow, V. M. Diaconescu, L. Simonelli, M. J. Sampaio, O. Bondarchuk, I. Amorim, A. Araujo, A. M. T. Silva, C. G. Silva, J. L. Faria and L. F. Liu, Appl. Catal., B, 2022, 310, 121318 CrossRef CAS.
- Z. P. Yu, C. W. Si, F. J. Escobar-Bedia, A. P. LaGrow, J. Y. Xu, M. J. Sabater, I. Amorim, A. Araujo, J. P. S. Sousa, L. J. Meng, J. L. Faria, P. Concepcion, B. Li and L. F. Liu, Inorg. Chem. Front., 2022, 9, 4142–4150 RSC.
- S. Geiger, O. Kasian, M. Ledendecker, E. Pizzutilo, A. M. Mingers, W. T. Fu, O. Diaz-Morales, Z. Z. Li, T. Oellers, L. Fruchter, A. Ludwig, K. J. J. Mayrhofer, M. T. M. Koper and S. Cherevko, Nat. Catal., 2018, 1, 508–515 CrossRef CAS.
- Z. Y. Wu, F. Y. Chen, B. Lie, S. W. Yu, Y. Z. Finfrock, D. M. Meira, Q. Q. Yan, P. Zhu, M. X. Chen, T. W. Song, Z. Yin, H. W. Liang, S. Zhang, G. Wang and H. Wang, Nat. Mater., 2023, 22, 100–108 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Phys. Rev. B, 1996, 54, 11169 CrossRef CAS.
- G. Kresse and D. Joubert, Phys. Rev. B, 1999, 59, 1758 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865 CrossRef CAS PubMed.
- M. Dion, H. Rydberg, E. Schröder, D. C. Langreth and B. I. Lundqvist, Phys. Rev. Lett., 2004, 92, 246401 CrossRef CAS.
- K. Lee, É. D. Murray, L. Z. Kong, B. I. Lundqvist and D. C. Langreth, Phys. Rev. B, 2010, 82, 081101 CrossRef.
- J. K. Nørskov, J. Rossmeisl, A. Logadottir, L. Lindqvist, J. R. Kitchin, T. Bligaard and H. Jónsson, J. Phys. Chem. B, 2004, 108, 17886 CrossRef.
- D. Chen, R. H. Yu, K. S. Yu, R. H. Lu, H. Y. Zhao, J. X. Jiao, Y. T. Yao, J. W. Zhu, J. S. Wu and S. C. Mu, Nat. Commun., 2024, 15, 3928 CrossRef CAS PubMed.
- S. L. Zhao, M. Li, M. Han, D. D. Xu, J. Yang, Y. Lin, N. E. Shi, Y. N. Lu, R. Yang, B. T. Liu, Z. H. Dai and J. C. Bao, Adv. Funct. Mater., 2018, 28, 1706018 CrossRef.
- X. Han, M. Y. Jin, T. T. Chen, T. Chou, J. D. Chen, S. Wang, Y. Yang, J. Wang and H. L. Jin, ACS Mater. Lett., 2024, 6, 748–755 CrossRef CAS.
- X. S. Qiao, H. J. Kang, Y. Li, K. Cui, X. Jia, X. H. Wu and W. Qin, Appl. Catal., B, 2022, 305, 121034 CrossRef CAS.
- B. B. Yang, J. Y. Xu, D. Bin, J. Wang, J. Z. Zhao, Y. X. Liu, B. X. Li, X. N. Fang, Y. Liu, L. Qiao, L. F. Liu and B. H. Liu, Appl. Catal., B, 2021, 283, 119583 CrossRef CAS.
- D. Galyamin, J. Torrero, I. Rodríguez, M. J. Kolb, P. Ferrer, L. Pascual, M. A. Salam, D. Gianolio, V. Celorrio, M. Mokhtar, D. G. Sanchez, A. S. Gago, K. A. Friedrich, M. A. Peña, J. A. Alonso, F. Calle-Vallejo, M. Retuerto and S. Rojas, Nat. Commun., 2023, 14, 2010 CrossRef CAS PubMed.
- J. Q. Shan, C. X. Guo, Y. H. Zhu, S. M. Chen, L. Song, M. Jaroniec, Y. Zheng and S. Z. Qiao, Chem, 2019, 5, 445–459 CAS.
- Y. N. Wang, M. C. Zhang, Z. Y. Kang, L. Shi, Y. C. Shen, B. Y. Tian, Y. C. Zou, H. Chen and X. X. Zou, Nat. Commun., 2023, 14, 5119 CrossRef CAS.
- https://www.clean-hydrogen.europa.eu/knowledge-management/strategy-map-and-key-performance-indicators/fch-2022-ju-mawp-key-performance-indicators-kpis_en .
- J. C. Fan, Y. J. Mu, X. Ge, L. Zhang, W. W. Li, H. L. Dong, D. W. Wang, W. Zhang, J. Y. Ma, W. T. Zheng and X. Q. Cui, ACS Catal., 2023, 13, 4120–4126 CrossRef CAS.
- Y. Z. Wen, C. Liu, R. Huang, H. Zhang, X. B. Li, F. P. G. de Arquer, Z. Liu, Y. Y. Li and B. Zhang, Nat. Commun., 2022, 13, 4871 CrossRef CAS.
- X. B. Zheng, J. R. Yang, P. Li, Q. S. Wang, J. B. Wu, E. Zhang, S. H. Chen, Z. C. Zhuang, W. H. Lai, S. X. Dou, W. P. Sun, D. S. Wang and Y. D. Li, Sci. Adv., 2023, 9, eadi8025 CrossRef CAS PubMed.
- Z. P. Shi, J. Li, Y. B. Wang, S. W. Liu, J. B. Zhu, J. H. Yang, X. Wang, J. Ni, Z. Jiang, L. J. Zhang, Y. Wang, C. P. Liu, W. Xing and J. J. Ge, Nat. Commun., 2023, 14, 843 CrossRef CAS.
- Y. B. Chen, H. Y. Li, J. X. Wang, Y. H. Du, S. B. Xi, Y. M. Sun, M. Sherburne, J. W. Ager, A. C. Fisher and Z. C. J. Xu, Nat. Commun., 2019, 10, 572 CrossRef CAS PubMed.
- D. Chen, R. H. Yu, D. L. Wu, H. Y. Zhao, P. Y. Wang, J. W. Zhu, P. X. Ji, Z. H. Pu, L. Chen, J. Yu and S. C. Mu, Nano Energy, 2022, 100, 107445 CrossRef CAS.
- S. Chen, H. Huang, P. Jiang, K. Yang, J. F. Diao, S. P. Gong, S. Liu, M. X. Huang, H. Wang and Q. W. Chen, ACS Catal., 2020, 10, 1152–1160 CrossRef CAS.
- J. Q. Wang, C. Xi, M. Wang, L. Shang, J. Mao, C. K. Dong, H. Liu, S. A. Kulinich and X. W. Du, ACS Catal., 2020, 10, 12575–12581 CrossRef CAS.
- D. J. Morgan, Surf. Interface Anal., 2015, 47, 1072–1079 CrossRef CAS.
- E. Ciftyurek, Z. S. Li and K. Schierbaum, Sensors, 2023, 23, 29 CrossRef CAS.
- K. X. Wang, Y. L. Wang, B. Yang, Z. J. Li, X. T. Qin, Q. H. Zhang, L. C. Lei, M. Qiu, G. Wu and Y. Hou, Energy Environ. Sci., 2022, 15, 2356–2365 RSC.
- G. Q. Zhao, W. Guo, M. M. Shan, Y. Y. Fang, G. M. Wang, M. X. Gao, Y. F. Liu, H. G. Pan and W. P. Sun, Adv. Mater., 2024, 36, 2404213 CrossRef CAS.
- A. Grimaud, O. Diaz-Morales, B. H. Han, W. T. Hong, Y. L. Lee, L. Giordano, K. A. Stoerzinger, M. T. M. Koper and Y. Shao-Horn, Nat. Chem., 2017, 9, 457–465 CrossRef CAS.
- H. L. Huang, Y. C. Chang, Y. C. Huang, L. L. Li, A. C. Komarek, L. H. Tjeng, Y. Orikasa, C. W. Pao, T. S. Chan, J. M. Chen, S. C. Haw, J. Zhou, Y. F. Wang, H. J. Lin, C. T. Chen, C. L. Dong, C. Y. Kuo, J. Q. Wang, Z. W. Hu and L. J. Zhang, Nat. Commun., 2023, 14, 2112 CrossRef CAS PubMed.
- T. L. Feng, Y. Wang, A. Herklotz, M. F. Chisholm, T. Z. Ward, P. C. Snijders and S. T. Pantelides, Phys. Rev. B, 2021, 103, 035409 CrossRef CAS.
- Y. Wang, X. Lei, B. Zhang, B. Bai, P. Das, T. Azam, J. P. Xiao and Z. S. Wu, Angew. Chem., Int. Ed., 2024, 63, e202316903 CrossRef CAS.
- D. F. Zhang, M. N. Li, X. Yong, H. Q. Song, G. I. N. Waterhouse, Y. F. Yi, B. J. Xue, D. L. Zhang, B. Z. Liu and S. Y. Lu, Nat. Commun., 2023, 14, 2517 CrossRef CAS PubMed.
- Y. M. Xu, Z. X. Mao, J. F. Zhang, J. P. Ji, Y. Zou, M. Y. Dong, B. Fu, M. Q. Hu, K. D. Zhang, Z. Y. Chen, S. Chen, H. J. Yin, P. R. Liu and H. J. Zhao, Angew. Chem., Int. Ed., 2024, 63, e202316029 CrossRef CAS PubMed.
- Q. Yao, B. L. Huang, Y. Xu, L. G. Li, Q. Shao and X. Q. Huang, Nano Energy, 2021, 84, 105909 CrossRef CAS.
- Z. P. Yu, J. Y. Xu, Y. F. Li, B. Wei, N. Zhang, Y. Li, O. Bondarchuk, H. W. Miao, A. Araujo, Z. C. Wang, J. L. Faria, Y. Y. Liu and L. F. Liu, J. Mater. Chem. A, 2020, 8, 24743–24751 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: Fig. S1–S32, including supplementary Raman spectra, XRD patterns, TEM and HRTEM images, IFFT patterns, size distribution histograms, LSV and CV curves, Tafel slopes, EIS analysis, CP plots, EXAFS fitting data, XPS spectra, BET curves, DMES measurement results, computation models and analysis of different catalysts, digital photograph of a single-cell electrolyzer, and XRD and XPS of HP-RuOx after the OER; Tables S1–S6. See DOI: https://doi.org/10.1039/d4ta06592a |
| ‡ J. W. W. and L. J. C. contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |