Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Controlling CO2 flux in a CO2-permeable membrane with a H2O driving force†
Jacqueline A.
Penn
 ,
Wenting
Hu
,
Wenting
Hu
 ,
Ian S.
Metcalfe
,
Ian S.
Metcalfe
 and
Greg A.
Mutch
and
Greg A.
Mutch
 *
*
Materials, Concepts & Reaction Engineering (MatCoRE) Group, School of Engineering, Newcastle University, Newcastle upon Tyne, NE1 7RU, UK. E-mail: greg.mutch@newcastle.ac.uk
First published on 14th October 2024
Abstract
Gas separation membranes hold significant promise for carbon capture and storage (CCS) as they offer high modularity in combination with technical simplicity. It is routinely expected that a difference in the partial pressure of CO2 (i.e., a CO2 driving force) across a CO2-permeable membrane dictates CO2 flux. Here, however, we show that in a molten-salt membrane fabricated using molten hydroxides, a H2O driving force in the opposite direction to CO2 permeation exerts control. We demonstrate this by using the opposing H2O driving force to operate the membrane in ways that challenge the conventional understanding of CO2-permeable membranes. For example, increasing the CO2 flux whilst decreasing the CO2 driving force. Throughout, we employ a model membrane support to facilitate recovery (and subsequent characterisation) of the molten salt, showing that membranes fabricated using molten hydroxides transform into majority molten carbonate membranes during CO2 separation. The carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydroxide ratio is shown to be a function of time, temperature, and gas-phase composition, and high carbonate
hydroxide ratio is shown to be a function of time, temperature, and gas-phase composition, and high carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydroxide ratios are correlated with high CO2 fluxes. Overall, our work demonstrates that molten-salt membranes evolve, and that a H2O driving force can be used to control CO2 flux.
hydroxide ratios are correlated with high CO2 fluxes. Overall, our work demonstrates that molten-salt membranes evolve, and that a H2O driving force can be used to control CO2 flux.
1. Introduction
To limit global warming to less than 1.5 °C above pre-industrial levels by 2100, global CO2 emissions must reach net-zero by 2050. Carbon capture and storage (CCS) is expected to play a critical role in enabling the transition to net-zero.1 CCS is an attractive option as it can often be deployed without significant process changes, and because it is currently a lower-cost option than many electrification approaches for emission-intensive industries (e.g., cement, iron, steel, refineries, chemicals etc.).2 In addition, it can offer the potential for negative emissions in certain configurations, i.e., in direct air capture,3 and bioenergy with CCS processes.4There are clear opportunities for membranes in CCS processes, particularly in situations where there are high concentrations of CO2 in the feed gas (e.g., cement, iron, steel, refineries, chemicals etc.), where lower capture rates are tolerable, and at smaller scales. For example, with a feed gas containing 10% CO2, the energy requirement for a two-stage polymeric membrane process with a capture rate of 60% and >99% CO2 product is the same as an amine-based solvent process with a capture rate of 90% and >99% CO2 product. However, if the feed gas contains 30% CO2, the membrane process requires less than half the energy of the solvent process.5 Similarly, a comparative techno-economic analysis of post-combustion CO2 capture (12% CO2 feed gas; 96% CO2 product) using a modern amine solvent, solid adsorbent, and a polymeric membrane found that the adsorbent and membrane processes are more cost-competitive with the solvent process when low capture rates are tolerable and at small scales (<100 t CO2 per day).6
Whilst various polymeric membrane materials and process configurations have been investigated for carbon capture, few have made it beyond pilot-plant scale (TRL 5–6).1,7 Inorganic membranes have been investigated for carbon capture even less, due largely to disadvantages (relative to polymeric membranes) in terms of processability, brittleness, and cost. Nonetheless, at the lab scale (TRL 3–4), they appear to offer good chemical and thermal stability, and in certain configurations, very high CO2 perm-selectivity (a key performance metric for application in CCS). For example, supported molten-salt membranes (porous inorganic support, infiltrated with a molten salt), have been shown to provide exceptionally high CO2 perm-selectivity when they are fabricated using molten carbonates (CO2 permeability of 10−12 to 10−10 mol m−1 s−1 Pa−1 and CO2/N2 selectivity up to 1000).8 For comparison, state-of-the-art polymeric membranes (TRL 3–4) offer CO2 permeability one order of magnitude lower (10−13 mol m−1 s−1 Pa−1) and CO2/N2 selectivity several orders of magnitude lower (10),9 whilst for post-combustion CCS in general, CO2 permeability on the order of 10−13–10−12 mol m−1 s−1 Pa−1 in combination with CO2/N2 selectivity of 50–100 is required.10 Thus, supported molten-salt membranes appear to offer sector-leading CO2 perm-selectivity, at or beyond the level required for application.
The wide range of observed CO2 perm-selectivity in supported molten-salt membranes is due, at least in part, to the use of a very wide array of solid support materials and membrane geometries. Support materials have included nominally inert ceramics (e.g., Al2O3),11–13 oxide-ion conducting ceramics (e.g., doped CeO2),11,14 mixed electron and oxide-ion conducting ceramics (e.g., La0.6Sr0.4Co0.8Fe0.2O3−δ),15,16 and metals (e.g., Ag).17–19 Support geometries have included pellets,11,12,14–19 hollow fibres,13,20 and tubes.19,21–23 As pore volume and tortuosity is expected to impact CO2 flux,24–26 these are introduced and/or controlled using a variety of methods, most often by partial sintering of ceramic powders,11,12,14–17,19 but also by electrochemical dealloying,18 phase inversion,13 and tape-casting and freeze-drying.27 To produce pores with very low tortuosity, advanced manufacturing techniques have also been employed, including laser drilling,19,23 and laser-directed solidification.28
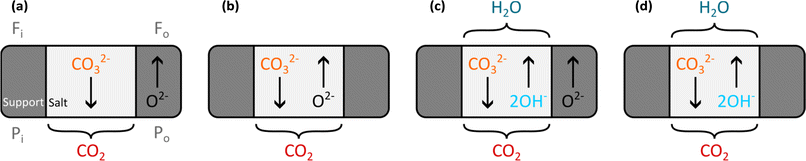
Such a wide array of chemical and physical properties can make detailed comparisons of membrane performance and permeation mechanism difficult, particularly in the absence of thorough characterisation, which is often the case. Nonetheless, various permeation mechanisms relying on the counter-diffusion of ionic species have been proposed to explain experimental observations (Fig. 1). For example, in the case of oxide-ion conducting supports, the counter-diffusion of oxide ions in the solid support, and carbonate ions in the molten carbonates is proposed (Fig. 1a).29 Due to the lower ionic conductivity of solid supports compared to molten carbonates, increasing the solid fraction of the membrane can lead to higher CO2 fluxes.29‡ In the case of inert solid supports, as there is no route for the transport of ionic species in the solid, CO2 permeation is expected to be facilitated by the molten carbonate alone. Low CO2 fluxes observed for membranes with inert supports have been justified based on the low concentration of oxide ions in molten carbonates, compared to the majority ions (i.e., alkali metals and carbonate) (Fig. 1b).
The composition of molten carbonates has also been discussed in the context of H2O-containing sweep gases. For example, in CeO2,14 BaZr0.8Y0.2O3−δ,30 and Sc0.1Ce0.01Zr0.89O1.95,31 supported molten-salt membranes, CO2 flux increases of 30 to 300% were observed for H2O-containing sweep gases, compared to ‘dry’ sweep gases.§ It was suggested that hydroxide ions in the molten carbonate, formed via reaction with H2O, contributed to an increased flux. In only one study to date, the molten carbonates normally used during membrane fabrication were substituted with molten hydroxides (we note here that this membrane was tested with a H2O-containing sweep gas also, and employed a CeO2 support).32 With H2O-containing sweep gases, it is proposed that counter-diffusion of hydroxide and carbonate ions occurs in the molten salt, in addition to the counter-diffusion of oxide ions in the solid support and carbonate ions in the molten salt (Fig. 1c), leading to high CO2 flux.
In the case of the molten-salt membrane fabricated using molten hydroxides, the authors made the interesting suggestion that the molten-salt composition changes in response to the partial pressure of CO2 in the feed gas. For example, a surprising increase in CO2 permeability observed with a decrease in the feed-gas CO2 partial pressure was attributed to the lower CO2 partial pressure leading to the molten salt having a lower carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydroxide ratio (and therefore a higher overall ionic conductivity, as molten hydroxides have a higher ionic conductivity than molten carbonates).32 This suggestion that the composition of molten salts may change in response to gas phase conditions is particularly interesting as it suggests that the starting composition of a molten-salt membrane may be relatively unimportant. A deeper understanding of membrane performance therefore requires detailed characterisation of the molten salt as a function of operating conditions.
hydroxide ratio (and therefore a higher overall ionic conductivity, as molten hydroxides have a higher ionic conductivity than molten carbonates).32 This suggestion that the composition of molten salts may change in response to gas phase conditions is particularly interesting as it suggests that the starting composition of a molten-salt membrane may be relatively unimportant. A deeper understanding of membrane performance therefore requires detailed characterisation of the molten salt as a function of operating conditions.
A final complication for all the work on supported molten-salt membranes is in understanding the exact nature of the CO2 flux-driving force relationship. Recent work studied the partial pressure dependence of CO2 flux in a membrane with an oxide-ion conducting support and molten carbonates under ‘dry’ conditions (Fig. 1a).§ This was achieved by varying the porosity of the support and therefore solid fraction of the membrane.26 Whilst the authors noted that CO2 flux generally increased with CO2 partial pressure in the feed gas, there were important differences noted. When CO2 flux was controlled by carbonate-ion conduction in the molten salt (low porosity support/high solid fraction), CO2 flux showed a logarithmic dependence on CO2 partial pressure (eqn (1)). When CO2 flux was controlled by oxide-ion conduction in the solid phase (high porosity support/low solid fraction), a power-law dependence on CO2 partial pressure was shown (eqn (2)),
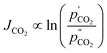 | (1) |
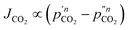 | (2) |
 and
and 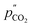 are the partial pressures of CO2 at the feed and permeate sides of the membrane, respectively. Thus, one might expect CO2 flux in molten-salt membranes fabricated using molten carbonates and inert supports to follow a logarithmic relationship (as there is no route for transport in the solid support, and therefore carbonate-ion conduction must control flux). Indeed, this relationship was observed in our recent work under dry sweep-gas conditions (<100 ppm H2O).33 However, with H2O present in the sweep gas, the CO2 driving force no longer had a strong effect on CO2 flux, and instead, a H2O driving force appeared to exert control (eqn (3)),
are the partial pressures of CO2 at the feed and permeate sides of the membrane, respectively. Thus, one might expect CO2 flux in molten-salt membranes fabricated using molten carbonates and inert supports to follow a logarithmic relationship (as there is no route for transport in the solid support, and therefore carbonate-ion conduction must control flux). Indeed, this relationship was observed in our recent work under dry sweep-gas conditions (<100 ppm H2O).33 However, with H2O present in the sweep gas, the CO2 driving force no longer had a strong effect on CO2 flux, and instead, a H2O driving force appeared to exert control (eqn (3)), | (3) |
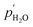 and
and  are the partial pressures of H2O at the feed and permeate sides of the membrane, respectively. Note that as H2O was fed to the permeate side of the membrane (in the sweep gas), the driving force for H2O (and therefore, H2O permeation) was in the opposite direction to the CO2 driving force (and therefore, CO2 permeation). In the same work we showed that carbonate-like ions in molten carbonates can act as a carrier for both CO2 and H2O, with their transport occurring in opposite directions.33 The mechanism involved several energetically-viable transport routes, with complex, inter-connected equilibria and different carrier forms, so the reader is referred to that work for further detail. Overall, however, H2O at the CO2 permeate-side, gas-membrane interface drove the release of CO2 from that interface and CO2 at the CO2 feed-side, gas-membrane interface drove the release of H2O at that interface, from a shared carbonate-like carrier.
are the partial pressures of H2O at the feed and permeate sides of the membrane, respectively. Note that as H2O was fed to the permeate side of the membrane (in the sweep gas), the driving force for H2O (and therefore, H2O permeation) was in the opposite direction to the CO2 driving force (and therefore, CO2 permeation). In the same work we showed that carbonate-like ions in molten carbonates can act as a carrier for both CO2 and H2O, with their transport occurring in opposite directions.33 The mechanism involved several energetically-viable transport routes, with complex, inter-connected equilibria and different carrier forms, so the reader is referred to that work for further detail. Overall, however, H2O at the CO2 permeate-side, gas-membrane interface drove the release of CO2 from that interface and CO2 at the CO2 feed-side, gas-membrane interface drove the release of H2O at that interface, from a shared carbonate-like carrier.
Based on our recent work and the discussion above, we were motivated to investigate the potential for changes to molten-salt composition with operating conditions e.g., time, temperature, and gas-phase composition, and to understand the role of the H2O driving force on CO2 flux in membranes fabricated using molten hydroxides. To limit permeation to the molten salt alone, and to attempt to simplify mechanism (Fig. 1d), we prepared a model, inert support with pores of very low tortuosity (laser-drilled alumina) (Fig. 2), which was infiltrated with molten hydroxides. A series of permeation experiments and corresponding characterisations of recovered molten salts were performed to understand the role of H2O in H2O-containing feed and sweep gases on CO2 flux, and on molten-salt composition. Importantly, we monitored the H2O concentration at both sides of the membrane continuously (whilst also monitoring the permeate-side outlet for CO2). This is unusual, as it has been common practice to only measure the permeate-side outlet (to measure CO2 flux) in experiments with supported molten-salt membranes. This allowed us to experimentally demonstrate the counter-permeation of CO2 and H2O in a membrane fabricated using molten hydroxides for the first time. Moreover, there was a very clear influence of both the molten-salt composition (carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydroxide ratio) and particularly the H2O driving force (from CO2 permeate side to CO2 feed side) on CO2 flux (from CO2 feed side to CO2 permeate side).
hydroxide ratio) and particularly the H2O driving force (from CO2 permeate side to CO2 feed side) on CO2 flux (from CO2 feed side to CO2 permeate side).
2. Experimental
2.1 Materials
Lithium hydroxide (powder, reagent grade, ≥98%), sodium hydroxide (powder, reagent grade, 97%), potassium hydroxide (powder, for synthesis), hydrochloric acid solution (0.1 mol l−1 reagent grade) and phenolphthalein solution (0.5% w/w in ethanol![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (1
water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
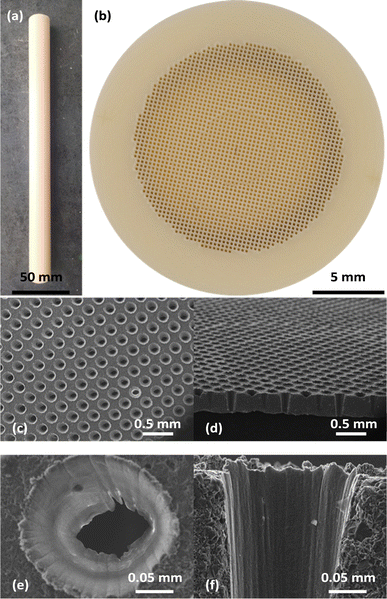
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) were purchased from Sigma-Aldrich. Lithium carbonate (powder, 99%), sodium carbonate (powder, 98%) and potassium carbonate (granular, ≥99%) were purchased from Alfa Aesar. Bromocresol green (sodium salt, 0.04% w/v aqueous solution) was purchased from Thermo Fisher Scientific. Fully dense alumina tubes with one closed end were purchased from Precision Ceramics (length 235 ± 1.6 mm, outer diameter (OD) 19.05 mm, inner diameter (ID) 17.78 mm, OD/ID ± 0.635 mm, and closed-end thickness 0.46 mm) (Fig. 2a). The closed ends of the tubes were laser drilled by Laser Micromachining Ltd to form an array of ∼2000 truncated conical holes (∼150 μm entrance, ∼50 μm exit) on a square pitch of ∼250 μm (Fig. 2b–f). A perfluoropolyether based, chemically inert, high-temperature vacuum grease (PFPE 501) was purchased from Apiezon. All gases were supplied by BOC.
1)) were purchased from Sigma-Aldrich. Lithium carbonate (powder, 99%), sodium carbonate (powder, 98%) and potassium carbonate (granular, ≥99%) were purchased from Alfa Aesar. Bromocresol green (sodium salt, 0.04% w/v aqueous solution) was purchased from Thermo Fisher Scientific. Fully dense alumina tubes with one closed end were purchased from Precision Ceramics (length 235 ± 1.6 mm, outer diameter (OD) 19.05 mm, inner diameter (ID) 17.78 mm, OD/ID ± 0.635 mm, and closed-end thickness 0.46 mm) (Fig. 2a). The closed ends of the tubes were laser drilled by Laser Micromachining Ltd to form an array of ∼2000 truncated conical holes (∼150 μm entrance, ∼50 μm exit) on a square pitch of ∼250 μm (Fig. 2b–f). A perfluoropolyether based, chemically inert, high-temperature vacuum grease (PFPE 501) was purchased from Apiezon. All gases were supplied by BOC.
2.2 Membrane preparation, membrane reactor and flow system
A mixture containing 43.5 mol% lithium hydroxide, 31.5 mol% sodium hydroxide, and 25 mol% potassium hydroxide was prepared, and thoroughly mixed in a Fluxana MUK mixer, inside a nitrogen glove box (to avoid deliquescence and limit reaction with atmospheric CO2). The composition of the hydroxide mixture was chosen as upon full conversion to carbonate, the carbonate ternary eutectic would form. The hydroxide mixture was stored in the glove box, with portions weighed out and removed periodically to make pellets for permeation experiments. These pellets were prepared by pressing the amount of hydroxide mixture required to fill the volume of the laser-drilled holes (upon melting) at 3 tons in an Atlas Power T25 hydraulic press, with the pellets having the diameter required to cover the laser-drilled area.The laser-drilled, closed-end alumina tube was housed in a custom-made membrane reactor,19,23,33 with gas inlets/outlets and a thermocouple port positioned near the closed end of the alumina tube (Fig. S1a†). The tube (outer diameter ∼19 mm) was placed inside the stainless-steel base of the membrane reactor (inner diameter ∼20 mm), and vacuum grease was applied around this connection to form the seal. The pellet of hydroxide mixture was placed on the laser-drilled, closed end of the tube. A quartz cover was then sealed to the base of the membrane reactor using a rubber O-ring under compression. This arrangement creates two chambers: a CO2 feed-side chamber inside the alumina tube, and a CO2 permeate-side chamber between the alumina tube and quartz cover, with both chambers having a gas inlet and outlet (Fig. S1a†). Hereafter, these are frequently referred to simply as feed-side chamber and permeate-side chamber. Based upon the residence time distribution response for both chambers (Fig. S2†), the membrane was considered to be exposed to the outlet conditions of each chamber.
The constructed membrane reactor was located within a vertical split-tube furnace (Vecstar) and connected to an automated flow system containing mass flow controllers (Brooks) and 4-way electronic valves (VICI), which allowed for the delivery of feed- and sweep-gas streams (Fig. S1b and c†). All flow rates, for all experiments and gas mixtures, were set to 50 ml min−1 (NTP), by measuring the appropriate gas-stream outlet using an electronic flow meter (GFM Pro, Thermo Scientific).
The flow system permitted the independent humidification of the feed- and sweep-gas streams, due to the presence of two in-line, water-filled permeation tubes. These consist of two water-filled reservoirs, separated from the gas streams by two water-permeable membranes, contained within two furnaces. The quantity of water transferred across the water-permeable membranes, and therefore the resulting H2O concentration in the gas streams, was controlled by varying the temperature of the furnaces (Fig. S3†).
2.3 Permeation experiments and measurement of CO2 and H2O flux
To melt the hydroxide mixture, and infiltrate the laser-drilled holes, the membrane reactor was heated to the experimental temperature at 2 °C min−1. During heating, ∼1% H2O in Ar was supplied to both the feed-side and permeate-side chamber inlets, to limit decomposition of the hydroxide mixture.34 At the experimental temperature, the feed-side chamber inlet was switched to a CO2 in N2 mixture, whilst the permeate-side chamber inlet remained as Ar (throughout the experimental campaign, the feed- and permeate-side gas streams were humidified to different extents). The presence of different inert gases in the feed- and permeate-side streams (N2 and Ar) permitted the detection of transmembrane leaks. Experiments were stopped if N2 was detected significantly above background levels in the permeate-side outlet, or if N2 levels were seen to be increasing (see below).The CO2 and/or H2O mole fraction in the feed- and permeate-side outlet streams were monitored by two, in-line infrared analysers (LI-COR, LI-850 CO2/H2O). The mole fraction of N2 in the permeate-side outlet stream was also monitored by a quadrupole mass spectrometer (Hiden, QGA), connected to the outlet of the corresponding infrared analyser. The infrared analysers and mass spectrometer were calibrated to account for systematic error, and any air ingress into the membrane reactor, flow system, or analytical equipment. This was performed by flowing Ar (99.999%, total impurities ≤10 ppm maximum, typical impurity concentrations: 3 ppm H2O, 6 ppm N2, 2 ppm O2) through the flow system, membrane reactor, infrared analysers, and mass spectrometer to obtain a background. The background measured before an experiment confirmed the absence of significant leaks into the experimental apparatus, whilst the background measured after an experiment was subtracted from the collected experimental data. Typically, the background levels were ∼200 ppm of N2 and ∼10 ppm of CO2. Gases of certificated mole fraction (369 ppm CO2 in Ar, 400 ppm N2 in Ar, and 903 ppm H2O in Ar) were used to calibrate the infrared analysers and mass spectrometer. Instrumental drift in the mass spectrometer was also accounted for by normalising CO2, H2O, and N2 signals against Ar (majority species).
The mole fraction of CO2 and/or H2O in the permeate-side outlet was converted to flux (mol s−1 m−2) using eqn (4),
 | (4) |
2.4 Membrane characterisation
Following permeation experiments, the molten salt component of the membrane was recovered by sonicating the laser-drilled, closed-end of the cooled membrane in ∼60 ml of deionised water for 30 minutes at 35 °C. Subsequently, 8 ml samples were titrated against 0.1 mol l−1 HCl, using the two indicators separately (phenolphthalein and bromocresol green). Phenolphthalein indicates (v1) upon complete conversion of CO32− to HCO3−, and bromocresol green indicates (v2) upon complete conversion of CO32− to H2CO3. The titration was performed in triplicate, with the mean volume of HCl consumed used in calculations; (v2 − v1) for CO32−, and (2(v1) − v2) for OH−.32,35,36 The reliability of the titration was tested both for accuracy compared to known mixtures of hydroxides and carbonates, and for any effect that cooling procedures (rate and atmosphere) had on composition. We note here that the titration was shown to accurately identify the composition of seven different known mixtures, and that the largest difference between actual and measured composition was <4% (Table S1†). Moreover, the rate of cooling and cooling atmosphere did not appear to have a significant effect (Table S2†), and therefore all membranes were cooled in dry Ar (Ar with ≤3 ppm H2O) at 5 °C min−1.Samples of the laser-drilled, closed-end alumina tubes, both prior to permeation experiments and following several, extended permeation experiments lasting a total of ∼800 h (∼500 h at 400–700 °C, and ∼300 h of heating/cooling), were prepared by cutting with a diamond saw (Top Tech Precision, CL40). A first cut removed the closed end of the tubes, to create a pellet shape containing the laser-drilled holes. A second cut split the pellet shape in two, creating semi-circular pieces and revealing the cross section. The prepared samples were rinsed with deionised water, and sonicated in deionised water for 30 minutes at 35 °C. For SEM imaging and EDX analysis, the semi-circular samples were carbon coated (EMScope TB500) before analysis with a JSM-IT510 InTouchScope™ Scanning Electron Microscope with integrated EDX. Images and EDX spectra were taken under high vacuum with secondary electron detection using an accelerator voltage of 20 kV and a working distance of 10 mm. Images and EDX spectra were collected from various sample orientations to access different surfaces, and within the laser-drilled holes (i.e., the cross-section view). Samples without carbon coating were analysed by Raman spectroscopy (Horiba LabRAM HR Evolution) using a 455 mW, 532 nm Nd:YAG laser (Laser Quantum), a non-dispersive filter to reduce the power at the sample to between ∼50 and 100% of the laser power, a 600 g mm−1 grating and a 20× objective (Olympus LMPLFLN20X). Each Raman spectrum was obtained as 10–15 accumulations of 10–15 s acquisitions.
3. Results and discussion
3.1 CO2 flux with an alternating wet and dry sweep gas
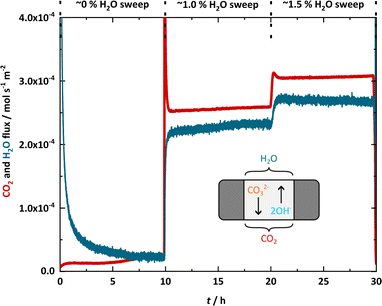
A hydroxide-infiltrated membrane was heated to 600 °C with ∼1% H2O in Ar supplied to both the feed- and permeate-side chamber inlets, before the feed-side chamber inlet was switched to 50% CO2 in N2 (the permeate-side chamber inlet remained as ∼1% H2O in Ar). CO2 flux was measured, before cycling between a dry (Ar with ≤3 ppm H2O) and wet (∼1% H2O in Ar) sweep gas (Fig. 3). Despite there being a CO2 partial pressure difference across the membrane (i.e., a CO2 driving force), initially there was no significant CO2 flux. At ∼2.5 h, CO2 flux ‘breakthrough’ occurred, before the CO2 flux reached ∼2.5 × 10−4 mol s−1 m−2. Upon switching to the dry sweep gas at 3 h, there was a decrease in CO2 flux, reaching ∼1 × 10−5 mol s−1 m−2 at 6 h. Note that hereafter, we frequently refer to fluxes on the order of 10−4 mol s−1 m−2 as ‘high’, and fluxes on the order of 10−5 mol s−1 m−2 as ‘low’, for simplicity, and due to the order-of-magnitude difference. | ||
| Fig. 3 CO2 flux with an alternating wet and dry sweep gas. Feed-side chamber inlet: 50% CO2 in N2. Permeate-side chamber inlet: dry (Ar with ≤3 ppm H2O) and wet (∼1% H2O in Ar). T = 600 °C. | ||
Further cycling between the wet and dry sweep gas resulted in a similar response from the membrane, i.e., abrupt, order-of-magnitude changes between high and low CO2 flux, and a clear link between the presence of H2O in the sweep gas and high CO2 flux. It was also notable that the CO2 flux in the wet cycles increased across the cycles. First, the high flux achieved at the end of the previous wet cycle was recovered quickly before the flux continued to increase slowly. As the conditions (temperature, CO2 concentration in the feed gas, H2O concentration in the sweep gas etc.) of the wet cycles remained constant, this implied that the membrane composition might be changing in time. We note here that characterisation of the membrane support (SEM-EDX and Raman as described in Section 2.4) showed that even after ∼800 h (>1 month) of membrane operation, there were no significant changes to the physical properties of the membrane support (Fig. S4†), and that there were only minor changes to the support chemistry (e.g., limited formation of LiAlO2) (Table S3 and Fig. S5†). This suggested that the order-of-magnitude changes in CO2 flux occurring on much shorter timescales (between wet and dry cycles of 3 h) were more likely arising due to the presence of H2O in the sweep gas, and that changes occurring on even shorter timescales (breakthrough and increasing flux within wet cycles) may be due to an evolving molten-salt composition.
3.2 CO2 flux as a function of time and H2O concentration in the feed and sweep gases
Considering the potential for changes in the molten salt with time, and a clear link between CO2 flux and the presence of H2O in the sweep gas (Fig. 3), two series of experiments were conducted. In the first series, CO2 flux and molten-salt composition (titrations with the recovered salt as described in Section 2.4), were measured as a function of time with fixed feed- and sweep-gas compositions (Fig. 4a). In the second series, the H2O concentration in the feed and sweep gas was varied, with the CO2 flux and membrane composition measured after a fixed amount of time (Fig. 4b). All experiments were conducted at 600 °C. Thus, the first series investigated the effect of time in the presence of a H2O-containing sweep gas, and the second series investigated the effect of H2O concentration in the H2O-containing sweep gas (and in one case, the feed gas). To attempt to avoid any influence of membrane ‘history’, all experiments were carried out separately, i.e., following recovery of the salt from the membrane support by washing (as described in Section 2.4), the membrane support was re-infiltrated with fresh molten hydroxides and tested at a different condition (recalling that no significant changes were noted in the membrane support following >1 month of operation). Examples of the full experimental traces (i.e., mole fractions of CO2, N2 and H2O on the permeate side) from the permeation experiments and titration data are provided in Fig. S6 and Table S4† for the first series (Fig. 4a), and in Fig. S7 and Table S5† for the second series (Fig. 4b).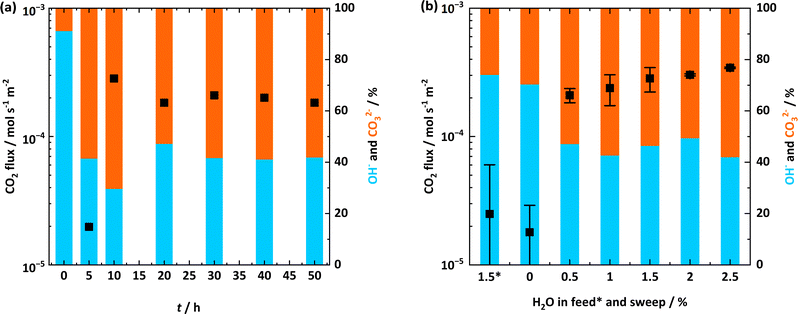 | ||
| Fig. 4 CO2 flux as a function of time and H2O concentration in feed and sweep gases. In both (a) and (b) points are CO2 flux, and coloured bars are triplicate titrations of recovered salts. (a) CO2 flux and titrations of recovered salts after separate experiments lasting for 0, 5, 10, 20, 30, 40, and 50 h. Feed-side chamber inlet: 50% CO2 in N2. Permeate-side chamber inlet: ∼0.5% H2O in Ar. T = 600 °C. (b) Mean CO2 flux with standard deviation (n = 2–4) and titrations of recovered salts after separate 20 h experiments with different H2O concentrations in feed (marked with an *) and sweep gas. Feed-side chamber inlet: 50% CO2 in N2 (with 1.5% H2O for *). Permeate-side chamber inlet: dry (Ar with ≤3 ppm H2O) for * and 0, and ∼0.5, 1, 1.5, 2, and 2.5% H2O in Ar. T = 600 °C. Full experimental traces and data for (a) are provided in Fig. S6, and Table S4†. Example experimental traces for (b) are provided in Fig. S7,† and the repeats used to calculate mean and error bars in (b) are provided in Table S5†. | ||
In the first series (effect of time), hydroxide-infiltrated membranes were heated to 600 °C with ∼1% H2O in Ar supplied to both the feed- and permeate-side chamber inlets, before the feed-side chamber inlet was switched to 50% CO2 in N2 and the permeate-side chamber inlet was switched to ∼0.5% H2O in Ar. After 0, 5, 10, 20, 30, 40, and 50 h of operation following the gas switches (in separate experiments), CO2 flux was recorded (points in Fig. 4a) and triplicate titrations were carried out to characterise the recovered molten salt (bars in Fig. 4a).
First, it was noted again that there was a breakthrough period in all the experiments, after which CO2 flux increased significantly in the absence of any other changes (Fig. S6†). The breakthrough period was of approximately the same duration in each separate experiment (∼5 h). For this reason, the CO2 flux reported at 5 h in Fig. 4a is low as it is taken from transient data where CO2 breakthrough is beginning (Fig. S6a†). Nonetheless, this makes for an interesting comparison, as it is notable that after 5 h, the molten-salt composition was relatively stable at ∼60% carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∼40% hydroxide (bars), whilst the CO2 flux (points) was also high (on the order of 10−4 mol s−1 m−2). Although there are small differences in the CO2 flux and composition, we must recall here that these results are taken from separate permeation experiments and their corresponding triplicate titrations, and that we are discussing an order-of-magnitude difference in CO2 flux before and after breakthrough. We also note here that the 0 h membrane (heated to 600 °C with ∼1% H2O in Ar supplied to both the feed- and permeate-side chamber inlets but cooled in dry Ar without being exposed to the 50% CO2 in N2 feed gas) had a recovered salt composition of ∼10% carbonate
∼40% hydroxide (bars), whilst the CO2 flux (points) was also high (on the order of 10−4 mol s−1 m−2). Although there are small differences in the CO2 flux and composition, we must recall here that these results are taken from separate permeation experiments and their corresponding triplicate titrations, and that we are discussing an order-of-magnitude difference in CO2 flux before and after breakthrough. We also note here that the 0 h membrane (heated to 600 °C with ∼1% H2O in Ar supplied to both the feed- and permeate-side chamber inlets but cooled in dry Ar without being exposed to the 50% CO2 in N2 feed gas) had a recovered salt composition of ∼10% carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∼90% hydroxide. This is expected, as despite handling and preparing the hydroxide mixture under an inert gas in a glove box, atmospheric CO2 will react with the hydroxide pellet during the amount of time required to prepare the membrane (between the hydroxides leaving the glove box and the permeation experiment starting), and during the time required to recover the hydroxides from the membrane afterwards (between the membrane being removed from the reactor and the titration being performed).
∼90% hydroxide. This is expected, as despite handling and preparing the hydroxide mixture under an inert gas in a glove box, atmospheric CO2 will react with the hydroxide pellet during the amount of time required to prepare the membrane (between the hydroxides leaving the glove box and the permeation experiment starting), and during the time required to recover the hydroxides from the membrane afterwards (between the membrane being removed from the reactor and the titration being performed).
In the second series (effect of H2O concentration), hydroxide-infiltrated membranes were heated to 600 °C with ∼1% H2O in Ar supplied to both the feed- and permeate-side chamber inlets, before the feed-side chamber inlet was switched to 50% CO2 in N2, as above. However, here the permeate-side chamber inlet was switched to ∼0 (dry, Ar with ≤3 ppm H2O), 0.5, 1.0, 1.5, 2.0, and 2.5% H2O in Ar in separate experiments. Additionally, in a further separate experiment, ∼1.5% H2O was added to the 50% CO2 in N2 feed gas (with the sweep gas being dry Ar). As discussed in more detail below, the permeation experiments were repeated several times to assess experimental uncertainty (Table S5†). Following ∼20 h at each condition, CO2 flux was recorded (points in Fig. 4b are the mean and error bars are standard deviation from the repeated permeation experiments), and corresponding triplicate titrations were carried out (bars in Fig. 4b), before the membrane was reinfiltrated and tested at the next condition. Before discussing the results, we note again that there was a breakthrough period in every experiment of ∼5 h, and that 20 h was chosen based on the results in Fig. 4a (i.e., no significant change in molten-salt composition or CO2 flux was expected).
First, it was apparent that under a dry sweep gas (Ar with ≤3 ppm H2O), CO2 flux was low and on the same order of magnitude (10−5 mol s−1 m−2) as in the dry cycles of Fig. 3. Second, whilst adding ∼1.5% H2O to the feed gas did not result in any significant increase in CO2 flux, there was also no deleterious effect on CO2 flux. This is important, as there are significant costs associated with drying feed gases to combat deleterious effects in other classes of membranes, e.g., polymeric membranes.6 When ∼0.5% H2O was added to the sweep gas, however, the CO2 flux increased, again by an order of magnitude to 10−4 mol s−1 m−2. Moreover, the CO2 flux appeared to increase further as the H2O concentration in the sweep gas increased (Fig. 4b).
Considering that all the discussion so far has related to order-of-magnitude arguments, here we assessed experimental uncertainty by repeating CO2 flux measurements for each condition up to four times (Table S5†). This repetition was to check whether any differences in CO2 flux were beyond experimental uncertainty. Indeed, the CO2 flux did increase with increasing H2O concentration in the sweep gas. Interestingly, the molten-salt composition showed a corresponding trend (bars in Fig. 4b). Under dry sweep-gas conditions and with H2O in the feed gas (when CO2 flux was low, on the order of 10−5 mol s−1 m−2), the recovered salts were ∼25% carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∼75% hydroxide. Conversely, under wet sweep-gas conditions (when CO2 flux was high, on the order of 10−4 mol s−1 m−2), the recovered salts were ∼60% carbonate
∼75% hydroxide. Conversely, under wet sweep-gas conditions (when CO2 flux was high, on the order of 10−4 mol s−1 m−2), the recovered salts were ∼60% carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∼40% hydroxide. We note that this composition is the same as that following breakthrough (at and after 5 h) in Fig. 4a.
∼40% hydroxide. We note that this composition is the same as that following breakthrough (at and after 5 h) in Fig. 4a.
Here it is important to highlight that the CO2 flux in Fig. 4b is increasing as the H2O driving force in the opposite direction is increasing, as in our recent work with a molten-salt membrane fabricated using molten carbonates we showed that CO2 flux can depend on the H2O driving force in the opposite direction (eqn (3)).33 Considering the results in Fig. 4b, it seemed reasonable to suspect that the H2O driving force may be influencing CO2 flux here also, but this time in a membrane fabricated using molten hydroxides. To test this hypothesis, the measurement of both permeate-side and feed-side outlet composition is required to see if CO2 and H2O are counter-permeating, and to test the extent of the influence that the H2O driving force (from CO2 permeate-side to CO2 feed-side) has over CO2 flux (from CO2 feed-side to CO2 permeate-side).
3.3 Measuring the counter-permeation of CO2 and H2O and evidencing the role of the H2O driving force on CO2 flux
To investigate the counter-permeation of CO2 and H2O, a hydroxide-infiltrated membrane was heated to 600 °C with ∼1% H2O in Ar supplied to both the feed- and permeate-side chamber inlets, before the feed-side chamber inlet was switched to 50% CO2 in N2 and the permeate-side chamber inlet was switched to ∼0 (dry, Ar with ≤3 ppm H2O), 1, and 1.5% H2O in Ar, in a single, continuous experiment (Fig. 5). The CO2 flux was calculated from the permeate-side outlet composition, and the H2O flux was calculated from the feed-side outlet composition. There was clear evidence for the counter-permeation of H2O and CO2, i.e., with increases in the concentration of H2O in the sweep gas, the fluxes of H2O and CO2 (in opposite directions) both increased. Moreover, it appeared that the magnitude of the CO2 and H2O fluxes were similar, suggesting that their permeation was linked (we note that this was also the case in our prior work with a membrane fabricated using molten carbonates).33With the understanding that CO2 and H2O counter-permeate, and that their permeation may be linked, it is therefore important to consider the role of the H2O driving force on CO2 flux. Thus, in a final series of permeation experiments, the CO2 driving force was increased whilst the H2O driving force in the opposite direction was held constant, and conversely, the CO2 driving force was held constant whilst the H2O driving force in the opposite direction was increased. This was achieved by heating hydroxide-infiltrated membranes to 600 °C with ∼1% H2O in Ar supplied to both the feed- and permeate-side chamber inlets, before the feed-gas chamber inlet was switched to 1.5, 10 or 50% CO2 in N2, whilst the permeate-side chamber inlet was switched to ∼1, 1.5, or 2.0% H2O in Ar (Fig. 6). The results shown in Fig. 6 are from three separate experiments using the three different feed gases (1.5, 10, and 50% CO2 in N2). During the experiments with the 1.5 and 10% CO2 in N2 feed gases, the permeate-side chamber inlet was increased from ∼1, to 1.5 and 2.0% H2O in Ar after 10 h at each condition. The CO2 flux (points) is an average of 5 h of stable CO2 flux, 5 h after the change in the permeate-side chamber inlet condition. During this 5 h period, the CO2 flux was measured every 5 s, and the maximum variation in CO2 flux was 6%.
With a ∼3000% increase in feed-gas CO2 concentration (from 1.5 to 50%), CO2 flux increased by ∼15% (which based on the results in Fig. 4b is within our experimental uncertainty). Whilst initially the lack of a significant increase in CO2 flux with a significant increase in the CO2 driving force might be surprising, this is due to the assumption that CO2 flux should be linked only to the CO2 driving force. If instead the H2O driving force is exerting control over the CO2 flux, this result can be explained based on the ∼1% H2O supplied for both feed-gas conditions, i.e., the CO2 driving force may not have a strong influence in the presence of H2O in the sweep gas.
We note that these results in Fig. 6 are consistent with the only previous article that studied a membrane fabricated using molten hydroxides.32 In that work, the authors reported an unusual apparent CO2 permeability increase as the feed-gas CO2 concentration was decreased from 50 to 20% CO2, whilst the sweep gas contained 4.5% H2O. Based on their method to determine permeability (which assumed eqn (2) with n = 1), were the fluxes with the 50 and 20% CO2 feed gases similar, the apparent permeability would be expected to increase by a factor of ∼2.5 (i.e., 50/20), which was indeed the case (from ∼6.5 to ∼16 × 10−11 mol m−1 Pa−1 s−1).32 Were we to use the same approach here, we would report a ∼30-fold increase in apparent permeability (i.e., 50/1.5), for CO2 fluxes that are within our experimental uncertainty. Moreover, the authors suggested that the apparent increase in CO2 permeability with a decrease in CO2 driving force was likely due to the lower feed-gas CO2 concentration resulting in a lowering of the carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydroxide ratio in the membrane during operation.32 They suggested this as molten hydroxides have a higher ionic conductivity than molten carbonates, however, no direct experimental evidence was provided to support this assertion. Contrasting with this prior suggestion, our results from triplicate titrations carried out following further, separate experiments with 10 and 50% CO2 feed gases, suggested that there was no significant difference in the carbonate
hydroxide ratio in the membrane during operation.32 They suggested this as molten hydroxides have a higher ionic conductivity than molten carbonates, however, no direct experimental evidence was provided to support this assertion. Contrasting with this prior suggestion, our results from triplicate titrations carried out following further, separate experiments with 10 and 50% CO2 feed gases, suggested that there was no significant difference in the carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydroxide ratio (Table S6†). It is also worth noting that these titrations were performed on salts recovered from permeation experiments with 10 and 50% CO2 feed gases (and a ∼1% H2O in Ar sweep gas) where the temperature was increased from 500, to 600, and 700 °C. The CO2 fluxes at 700 °C for both feed gas conditions were the same at ∼7 × 10−4 mol s−1 m−2, the highest reported in our work (Fig. S8†). Interestingly, the carbonate
hydroxide ratio (Table S6†). It is also worth noting that these titrations were performed on salts recovered from permeation experiments with 10 and 50% CO2 feed gases (and a ∼1% H2O in Ar sweep gas) where the temperature was increased from 500, to 600, and 700 °C. The CO2 fluxes at 700 °C for both feed gas conditions were the same at ∼7 × 10−4 mol s−1 m−2, the highest reported in our work (Fig. S8†). Interestingly, the carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydroxide ratio was also the highest reported at ∼80% carbonate
hydroxide ratio was also the highest reported at ∼80% carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∼20% hydroxide (Table S6†). We also wish to highlight here again the results in Fig. 4b, where CO2 flux increased with an increasing concentration of H2O in the sweep gas, but the composition of the membrane remained relatively constant at ∼60% carbonate
∼20% hydroxide (Table S6†). We also wish to highlight here again the results in Fig. 4b, where CO2 flux increased with an increasing concentration of H2O in the sweep gas, but the composition of the membrane remained relatively constant at ∼60% carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ∼40% hydroxide. Thus, overall, it seems likely that a lowering of the carbonate
∼40% hydroxide. Thus, overall, it seems likely that a lowering of the carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydroxide ratio may not explain increases in CO2 flux, as across several of our experiments, high CO2 fluxes are correlated with high carbonate
hydroxide ratio may not explain increases in CO2 flux, as across several of our experiments, high CO2 fluxes are correlated with high carbonate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hydroxide ratios. Moreover, although CO2 permeability can be made to increase with a reduction in the CO2 feed-gas concentration, this is simply due to the use of a linear CO2 driving force (assuming eqn (2) with n = 1) in the calculation of CO2 permeability when the CO2 flux is not changing significantly (due to the influence of the opposing H2O driving force).
hydroxide ratios. Moreover, although CO2 permeability can be made to increase with a reduction in the CO2 feed-gas concentration, this is simply due to the use of a linear CO2 driving force (assuming eqn (2) with n = 1) in the calculation of CO2 permeability when the CO2 flux is not changing significantly (due to the influence of the opposing H2O driving force).
Finally, and to demonstrate very clearly the importance of controlling the opposing H2O driving force, the H2O concentration in the sweep gas was increased whilst the feed-gas chamber inlet was held constant at 1.5 or 10% CO2 in N2. Despite 1.5% CO2 being the lowest feed-gas CO2 concentration employed in our work (and therefore the lowest CO2 driving force), the CO2 flux was at the highest level we report at 600 °C (>4.5 × 10−4 mol s−1 m−2). This was achieved by increasing the H2O concentration in the sweep gas from ∼1, to 1.5, and 2% (Fig. 6), which increased the opposing H2O driving force (Fig. 1d). This ∼100% increase in the opposing H2O driving force had a much more significant effect on CO2 flux (∼75% increase) than that of a ∼3000% increase in CO2 driving force, which resulted in a ∼15% increase in CO2 flux. Moreover, the ∼75% increase in CO2 flux is occurring without an increase in CO2 driving force. Thus, whereas one might expect the CO2 driving force, not the opposing H2O driving force, to exert more significant control on CO2 flux, it appears that both the CO2 and H2O driving forces impact CO2 flux, but that the opposing H2O driving force has a much more significant effect (compare arrows in Fig. 6).
We note here that our discussion of e.g., a ∼100% increase in the opposing H2O driving force, and a ∼3000% increase in CO2 driving force (both also labelled on Fig. 6) is based on assuming linear driving force models (for simplicity of discussion). However, it is also apparent that none of the previously proposed CO2 flux-driving force relationships (eqn (1)–(3)), including the linear model (eqn (2) with n = 1), can describe the results in Fig. 6 adequately, and that a much more complex relationship exists between the measured CO2 flux and the partial pressures of the gases present. To develop such relationships, considering the four partial pressures involved (CO2 on the feed and permeate side, and H2O on the feed and permeate side), we note that this becomes a very large, multi-dimensional kinetic space to sample.
4. Conclusions
Detailed understanding of promising CO2-permeable membranes is important for the future CCS sector. In this work, we have carefully interrogated a model molten-salt membrane to understand the role of H2O on CO2 flux. Whilst it had been shown previously that CO2 flux increases when H2O is introduced to the sweep gas of molten-salt membranes fabricated using molten carbonates and molten hydroxides, prior attempts at explaining this phenomenon invoked arguments based on a higher ionic conductivity of molten hydroxides compared to molten carbonates. Instead, in this work, we have shown that a molten-salt membrane fabricated using molten hydroxides becomes majority molten carbonate (∼60%) following breakthrough of CO2, which then remains majority molten carbonate under conditions that provide high CO2 fluxes (i.e., with H2O in the sweep gas). Conversely, the membrane remains majority molten hydroxide (∼75%) under conditions that provide low CO2 fluxes (under a dry sweep gas and when H2O is present only in the feed gas). Increasing the H2O concentration in the sweep gas did not appear to modify the molten-salt composition further, but the CO2 flux did increase further. Thus, both molten-salt composition and H2O driving force exert control over CO2 flux in molten-salt membranes fabricated using molten hydroxides.To demonstrate the importance of the H2O driving force, several examples were provided where the careful application of the H2O driving force (in the opposite direction to the CO2 driving force and CO2 flux) was used to challenge our routine understanding of CO2-permeable membranes. This included driving CO2 flux from 1.5 and 10% CO2 feed gases to levels higher than that achieved with a 50% CO2 feed gas. Interestingly, the highest CO2 fluxes (∼7 × 10−4 mol s−1 m−2 at 700 °C for both the 10 and 50% CO2 feed gases with a ∼1% H2O in Ar sweep gas) were correlated with the highest carbonate content in the molten salt (∼80%).
Overall, our results suggest that the composition of the molten salt in a molten-salt membrane is a function of time, temperature, and gas-phase composition, which is why we have been careful throughout to refer to different molten-salt membranes as being fabricated using a given molten salt. Moreover, controlling the H2O driving force in the opposite direction to CO2 permeation is required to exploit the potential of molten-salt membranes, and calculations intended to communicate this performance clearly (e.g., CO2 permeability) must be considered in this context. For example, calculating a CO2 permeability using a linear CO2 driving force may be somewhat meaningless when there is an opposing H2O driving force, as one can arrive at very different apparent CO2 permeabilities when the CO2 flux has not changed (and vice versa). For this reason, as is common practice with e.g., temperature and CO2 feed-gas concentration, measuring and reporting the sweep-gas H2O concentration alongside CO2 permeability should now be required.
Data availability
The data presented in this work is available at https://data.ncl.ac.uk/, DOI: https://doi.org/10.25405/data.ncl.26335333.Author contributions
JAP (investigation); JAP and WH (formal analysis, validation); JAP and GAM (visualization, writing – original draft, data curation); JAP, ISM and GAM (conceptualization, methodology); JAP, WH, ISM and GAM (writing – review & editing); ISM and GAM (supervision, project administration, funding acquisition, resources).Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Engineering & Physical Sciences Research Council [grant numbers EP/V047078/1, EP/W03395X/1, EP/Y034961/1]. ISM acknowledges funding from the Royal Academy of Engineering through a Chair in Emerging Technologies Award entitled “Engineering Chemical Reactor Technologies for a Low-Carbon Energy Future” (Grant CiET1819/2/57). GAM was supported by the Royal Academy of Engineering under the Research Fellowship scheme. The authors are grateful to Dr Ingham of IGIsystems for helpful discussions during the design and operation of the flow system, and to the Electron Microscopy and Analysis Unit, SAgE Analytical, Newcastle University for assistance with the SEM-EDX measurements.Notes and references
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. MacDowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- D. Danaci, M. Bui, C. Petit and N. Mac Dowell, Environ. Sci. Technol., 2021, 55, 10619–10632 CrossRef CAS PubMed.
- M. Erans, E. S. Sanz-Pérez, D. P. Hanak, Z. Clulow, D. M. Reiner and G. A. Mutch, Energy Environ. Sci., 2022, 15, 1360–1405 RSC.
- M. Fajardy and N. Mac Dowell, Energy Environ. Sci., 2017, 10, 1389–1426 RSC.
- R. W. Baker, B. Freeman, J. Kniep, Y. I. Huang and T. C. Merkel, Ind. Eng. Chem. Res., 2018, 57, 15963–15970 CrossRef CAS.
- S. E. Zanco, J.-F. Pérez-Calvo, A. Gasós, B. Cordiano, V. Becattini and M. Mazzotti, ACS Eng. Au, 2021, 1, 50–72 CrossRef CAS.
- R. Hou, C. Fong, B. D. Freeman, M. R. Hill and Z. Xie, Sep. Purif. Technol., 2022, 300, 121863 CrossRef CAS.
- G. A. Mutch, L. Qu, G. Triantafyllou, W. Xing, M. L. Fontaine and I. S. Metcalfe, J. Mater. Chem. A, 2019, 7, 12951–12973 RSC.
- B. Comesana-Gandara, J. Chen, C. G. Bezzu, M. Carta, I. Rose, M.-C. Ferrari, E. Esposito, A. Fuoco, N. B. McKeown and J. C. Jansen, Energy Environ. Sci., 2019, 12, 2733–2740 RSC.
- T. C. Merkel, H. Lin, X. Wei and R. Baker, J. Membr. Sci., 2010, 359, 126–139 CrossRef CAS.
- J. L. Wade, C. Lee, A. C. West and K. S. Lackner, J. Membr. Sci., 2011, 369, 20–29 CrossRef CAS.
- M. Kazakli, G. A. Mutch, L. Qu, G. Triantafyllou and I. S. Metcalfe, J. Membr. Sci., 2020, 600, 117855 CrossRef CAS.
- M. Kazakli, G. A. Mutch, G. Triantafyllou, A. G. Gil, T. Li, B. Wang, J. J. Bailey, D. J. L. Brett, P. R. Shearing, K. Li and I. Metcalfe, J. Membr. Sci., 2021, 617, 118640 CrossRef CAS.
- W. Xing, T. Peters, M. L. Fontaine, A. Evans, P. P. Henriksen, T. Norby and R. Bredesen, J. Membr. Sci., 2015, 482, 115–119 CrossRef CAS.
- M. Anderson and Y. S. Lin, J. Membr. Sci., 2010, 357, 122–129 CrossRef CAS.
- E. I. Papaioannou, H. Qi and I. S. Metcalfe, J. Membr. Sci., 2015, 485, 87–93 CrossRef CAS.
- N. Xu, X. Li, M. A. Franks, H. Zhao and K. Huang, J. Membr. Sci., 2012, 401–402, 190–194 CrossRef CAS.
- J. Fang, N. Xu, T. Yang, P. Zhang, J. Tong and K. Huang, J. Membr. Sci., 2017, 523, 439–445 CrossRef CAS.
- L. A. McNeil, G. A. Mutch, F. Iacoviello, J. J. Bailey, G. Triantafyllou, D. Neagu, T. S. Miller, E. I. Papaioannou, W. Hu, D. J. L. Brett, P. R. Shearing and I. S. Metcalfe, Energy Environ. Sci., 2020, 13, 1766–1775 RSC.
- Z. Xu, Q. Zheng, S. Wang, Z. Zhang, Z. Liu, G. Zhang and W. Jin, J. Membr. Sci., 2021, 635, 119506 CrossRef CAS.
- X. Dong, J. Ortiz Landeros and Y. S. Lin, Chem. Commun., 2013, 49, 9654–9656 RSC.
- X. Dong, H.-C. Wu and Y. S. Lin, J. Membr. Sci., 2018, 564, 73–81 CrossRef CAS.
- S. Tsochataridou, G. A. Mutch, D. Neagu, E. I. Papaioannou, M. L. Sanjuán, B. Ray, R. I. Merino, V. M. Orera and I. S. Metcalfe, ACS Appl. Mater. Interfaces, 2020, 12, 16436–16441 CrossRef CAS PubMed.
- Z. Rui, M. Anderson, Y. S. Lin and Y. Li, J. Membr. Sci., 2009, 345, 110–118 CrossRef CAS.
- J. Ortiz-Landeros, T. Norton and Y. S. Lin, Chem. Eng. Sci., 2013, 104, 891–898 CrossRef CAS.
- J. Y. S. Lin and O. Ovalle-Encinia, J. Membr. Sci. Lett., 2023, 3, 100041 CrossRef.
- S. G. M. Carvalho, E. N. S. Muccillo, F. C. Fonseca, M. Müller, F. Schulze-Küppers, S. Baumann, W. A. Meulenberg, O. Guillon and R. Muccillo, J. Membr. Sci., 2022, 648, 120355 CrossRef CAS.
- L. Grima, G. A. Mutch, P. B. Oliete, W. Bucheli, R. I. Merino, E. I. Papaioannou, J. J. Bailey, M. D. Kok, D. J. L. Brett, P. R. Shearing, I. S. Metcalfe and M. L. Sanjuán, J. Membr. Sci., 2021, 630, 119057 CrossRef CAS.
- F. M. B. Marques, S. G. Patrício, E. Muccillo and R. Muccillo, Electrochim. Acta, 2016, 210, 87–95 CrossRef CAS.
- K. Zhang, S. Sun, N. Xu and K. Huang, J. Membr. Sci., 2022, 650, 120421 CrossRef CAS.
- S. Sun, Y. Wen and K. Huang, ACS Sustainable Chem. Eng., 2021, 9, 5454–5460 CrossRef CAS.
- M. R. Cerón, L. S. Lai, A. Amiri, M. Monte, S. Katta, J. C. Kelly, M. A. Worsley, M. D. Merrill, S. Kim and P. G. Campbell, J. Membr. Sci., 2018, 567, 191–198 CrossRef.
- I. S. Metcalfe, G. A. Mutch, E. I. Papaioannou, S. Tsochataridou, D. Neagu, D. J. L. Brett, F. Iacoviello, T. S. Miller, P. R. Shearing and P. A. Hunt, Nat. Energy, 2024, 9, 1074–1083 CAS.
- T. Terai, H. Mohri and Y. Takahashi, J. Nucl. Mater., 1991, 179–181, 808–811 CrossRef CAS.
- E. P. Partridge and W. C. Schroeder, Ind. Eng. Chem., Anal. Ed., 1932, 4, 271–273 CrossRef CAS.
- A. A. Benedetti-Pichler, M. Cefola and B. Waldman, Ind. Eng. Chem., Anal. Ed., 1939, 11, 327–332 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta05021e |
| ‡ Assuming due consideration is given to percolation of the molten salt at the highest solid support fractions and noting that the condition of maximum ambipolar diffusion will be different at different temperatures, and for different support materials. |
| § Apostrophes around dry (i.e., ‘dry’) denotes that the precise H2O concentration in the sweep gases were not measured/provided. |
| This journal is © The Royal Society of Chemistry 2024 |