Preparation of supercapacitor electrode materials from e-waste: eco-friendly Cu recovery from printed circuit board waste using reduced graphene oxide and upcycling to Cu/CuO@C†
Rajendran
Mathaiyan‡
,
Aneesh Anand
Nechikott‡
,
Sajith
Babu M. K.
 ,
Prasant Kumar
Nayak
and
Srinivasarao
Kancharla
,
Prasant Kumar
Nayak
and
Srinivasarao
Kancharla
 *
*
Department of Chemistry, SRM Institute of Science and Technology, Kattankulathur-603203, Tamil Nadu, India. E-mail: srinivak5@srmist.edu.in
First published on 9th September 2024
Abstract
Copper finds its application in nearly all electronic devices owing to its exceptional electrical and thermal characteristics essential for transmitting signals and dispersing heat. As renewable energy technologies like wind turbines and electric vehicles continue gaining prominence, the demand for copper steadily grows yearly. However, as these devices become obsolete after a few years of use, recovering copper from this discarded equipment is imperative, driven by environmental concerns and energy considerations. This research work investigates the utilization of reduced graphene oxide (rGO) in an aqueous medium for the concurrent leaching and sorption processes aimed at recovering copper from printed circuit board flakes. Copper recovery was conducted at a temperature of 25 to 80 °C and a pH of 4 to 10. The optimal conditions for leaching–sorption were identified as a pH of 7 and temperature of 60 °C, yielding a maximum of 82.9 mg g−1 of rGO corresponding to 100% of Cu present in waste printed circuit boards (WPCBs). The recovered solid material serves a dual purpose, (i) for the recovery of copper as copper sulfate using 2 M H2SO4 and (ii) to transform into the Cu/CuO@C material through calcination at 500 °C. The prepared Cu/CuO@C offers a new perspective as a high-performance negative electrode material for supercapacitor applications. Cu/CuO@C exhibits superior performance with a high coulombic efficiency, validated through cyclic voltammetry (CV), galvanostatic charge–discharge (GCD) profiles, and extended cycle testing at 3 A g−1. Cu/CuO@C exhibited a specific capacitance of 432.5 F g−1 at a specific current of 1 A g−1 in the potential range of (−0.8) to 0 V in 1 M KOH. Furthermore, an asymmetric supercapacitor (ASC) is fabricated with activated carbon (AC) as a positive electrode and Cu/CuO@C as a negative electrode, which exhibits a specific capacitance of 88.8 F gcell−1 at a specific current of 1 A g−1 with an operating voltage of 1.2 V and can provide an energy density of 17.8 W h kg−1 in 1 M KOH electrolyte. This work paves the way for the preparation of capacitive electrode materials using e-waste under eco-friendly conditions.
1. Introduction
Electronic waste (e-waste) is the fastest-growing type of solid waste globally due to the increased usage and shortened lifetimes of electronic devices. The generation of electronic garbage increased by over 54.8 million tonnes (MT) globally in 2021, and it is predicted to reach 74.7 MT by 2030.1 Out of which 9.3 MT of officially recorded electronic garbage were collected and recycled in 2019, accounting for 17.4% of all e-waste produced. Since 2014, this amount has increased by 1.8 MT, or about 0.4 MT each year. Nevertheless, at the same time, the total amount of e-waste generated increased by 9.2 MT.2 Copper accounts for approximately 20% of the composition of electronic equipment due to its exceptional thermal conductivity, superb electrical conductivity, impressive ductility and malleability, utility as a corrosion-resistant material, and cost-effectiveness compared to alternative conductive materials.3 Considering the present circumstances, the demand for Cu is projected to surge by 275% to 350% by the year 2050,4 a level that cannot be met solely by primary sources in the long term. Consequently, it is imperative to investigate alternative sources for Cu production. Because of its higher Cu content, e-waste represents a notable secondary source for its extraction.5 Utilizing e-waste as a secondary source for Cu recycling offers environmental and energy-related benefits. Within the realm of e-waste, WPCBs represent the most intricate and potentially hazardous component, constituting roughly 4% of the total e-waste.6 These boards come in different varieties, including single-sided (with a single copper layer), double-sided (featuring two copper layers), and even multi-sided configurations.7 These WPCBs comprise a mixture of metals, organic polymers, and glass fibres.8Cu recovery from its secondary source like e-waste was achieved by various methods, among which hydrometallurgy was identified to have numerous advantages over other methods. Hydrometallurgical recovery involves selective extraction of Cu from acidic leachate solutions9 or selective leaching using liquids or solid materials. Cu leaching from e-waste was performed using mineral acids like H2SO4,10 HNO3,11 and HCl12 usually at higher concentrations. Mineral acid leaching is quantitative, but it suffers from poor selectivity. Alternatively, methods such as ammonia leaching,13 bioleaching,14 and organic acids such as citric acid,15 acetic acid,16etc., have been employed for copper leaching. However, these processes typically operate within a high pH range and yield lower copper recovery of an average of 57–75%. Amino acid glycine was also used to recover Cu by leaching from WPCB board powder.17 Glycine could leach Cu selectively at pH 10 and demonstrated that leaching with coordinating molecules could be a good strategy to recover metals from waste PCBs. Leaching of metal with all these small molecules was performed in an aqueous medium in a dissolved state, which resulted in a metal-dissolved solution as a product. However, leaching using solid materials has obvious advantages in terms of ease of separation, simultaneous leaching–sorption, and multiple-cycle usage of these materials.18 Our group has recently reported selective leaching of Cu from WPCBs using chitosan and phosphorylated cellulose under mild conditions,18 however, only 6–8% of Cu leaching was achieved using these biopolymers. Hydrogen peroxide is often used in combination with the chemical agents to improve Cu leaching yield from e-waste,19 however, it could not be used in combination with these biopolymers since it cleaves the polymer linkages. On the other hand, reduced graphene oxide (rGO) is a carbon material with abundant functional groups (epoxy, hydroxyl, and carboxylic acid), and has high thermal and chemical stability.20 The oxygen groups present on rGO layers can bind the metal ions through coordination21 and enable their recovery, and due to the high chemical stability of rGO, this can be used in combination with oxidizing agents like H2O2. For the first time, we explored rGO for selective leaching of Cu from PCB waste utilizing its exotic properties. Furthermore, the recovered metal sorbed rGO was studied as the negative electrode material for the supercapacitor application.
There has been a surge in interest in energy storage devices as fossil fuel reserves continue to deplete rapidly. The exceptional performance of supercapacitors over the other devices is demonstrated by their high-power density, extended cycle life, high-rate capabilities, swift charging and discharging, wide working temperature range, safety, and lightweight design.22 Researchers are actively exploring electrode materials to advance the development of supercapacitors.23
A collection of materials including metal sulphides like CoS2, NiS,24 various transition metal oxides such as MnO2, NiO,25 RuO2,26 Fe2O3,27 Co3O4,28 SnO2,29 MnO2,30 and CuO,31 and metal hydroxides like Co(OH)2, Ni(OH)2 (ref. 32 and 33) etc. have been investigated as electrode materials for high-power electrochemical pseudo capacitors.34 Among the metal oxides, CuO is one of the most promising pseudo-capacitor electrode materials concerning its environmental compatibility, and cost-effectiveness.35 Because of its low activation energy, CuO with a monoclinic structure is unique among transition metal oxides as it can promote electron transport between cations.36 Most flexible fibre supercapacitors have predominantly utilized carbon-based materials such as activated carbon, carbon nanotubes, or carbon onions as electrode materials. There is an advantage of composites of transition metal oxide and carbon since carbon can help increase the electrical conductivity of metal oxides. Activated carbon (AC)37,38 metal oxide composites,39,40 and rGO composites41,42 possessing specific capacitances in the range of 100–600 F g−1 were explored as negative electrode materials in supercapacitor applications. Developing such promising composite materials from e-waste is advantageous from the perspective of both the environment and the circular economy.
To this end, rGO is employed for the recovery of Cu from printed circuit board e-waste. This extraction process involves simultaneous leaching and sorption within an aqueous medium at pH 7. The Cu(I)/Cu(II)@rGO obtained from the sorption experiment was subsequently treated with a 2 M H2SO4 solution to extract Cu as copper sulfate (CuSO4). Furthermore, Cu(I)/Cu(II)@rGO was also used to prepare Cu(0) and CuO nanoparticle supported carbon (Cu/CuO@C) by calcination at 500 °C. The supercapacitor behaviour of Cu/CuO@C is investigated by electrochemical studies such as CV, and GCD in 1 M KOH as an electrolyte. Interestingly, a specific capacitance of 432.5 F g−1 is achieved for Cu/CuO@C when cycled at 1 A g−1 in 1.0 M KOH electrolyte with 86% capacitance retention after 4000 cycles.
2. Materials and methods
2.1. Materials
Graphite with 99% purity was procured from Research-Lab Fine Chem Industries, Mumbai, India. H2SO4 98% extra pure, was purchased from RANKEM Avantor Performance Materials India Pvt Ltd, Maharashtra, India. KMnO4 99% pure, and HCl 35% extra pure were procured from LOBA Chemie Pvt Ltd, Mumbai, India. NaOH pellets, extra pure AR, 98%, were purchased from SRL, Chennai, India. Printed circuit board waste was collected from local mobile and laptop service centres. Poly(vinylidene fluoride) (PVDF), super-p carbon, and KOH pellets (extra pure AR, 98%) were purchased from SRL, Chennai, India. All the chemicals were used as received.2.2. Experimental section
2.3. Characterization
X-ray diffraction (XRD) analysis was conducted using a Malvern PANalytical X'pert instrument within the 2θ range of 5 to 90°. Fourier-transform infrared (FT-IR) spectra were acquired employing an attenuated total reflection (ATR) technique with a SHIMADZU IRTRACER 100 spectrometer, spanning the wavenumber range from 4000 to 400 cm−1. The quantification of Cu was carried out utilizing an Agilent Cary 60 UV-Vis spectrophotometer. The morphology and electron diffraction patterns of the synthesized material were investigated using a Apreo S model high-resolution scanning electron microscope (SEM) from Thermo Scientific. A Physical Electronics X-ray photoelectron spectroscopy (XPS) instrument was employed to determine the metals present in the material and their oxidation states. The binding energy (EB) was calibrated using the C 1s peak at 284.6 eV, and peak separation was facilitated using CasaXPS software (version 2.3.25 PR 1.0). The quantitative analysis utilized Scofield relative sensitivity factors (RSFs) for correcting the peak areas in CasaXPS software. Thermal stability was assessed via thermogravimetric analysis (TGA) using a Netzsch-STA 2500 Regulus instrument. The electrochemical performance evaluation of Cu/CuO@C was conducted through cyclic voltammetry (CV) and galvanostatic charge–discharge (GCD) tests in a three-electrode cell setup. The working electrode consisted of the active material coated on nickel foam, with Hg/HgO serving as the reference electrode and Pt foil as the counter electrode, employing the ZIVE-SP1 instrument. Aqueous solution of 1 M KOH was utilized as the electrolyte. Raman spectra were recorded using a HORIBA (Japan), LABRAM instrument, employing 633 nm wavelength of light.3. Results and discussion
3.1. Simultaneous leaching and sorption of Cu(II) from waste PCB flakes
The simultaneous leaching and sorption of Cu(II) from printed circuit board flakes was conducted by adding WPCB flakes and rGO in water. Various parameters like rGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WPCB ratio (1
WPCB ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 2
1, 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and 3
1, and 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), pH (4, 7, and 10), H2O2 concentration (1%, 3%, and 5%), temperature (25, 60, and 80 °C), and time are thoroughly studied. The results of parameter studies are presented in Fig. S1a–e.† A good amount of Cu(II) recovery was found under all the conditions, and the suitable set of conditions for 100% Cu recovery are identified as 3
1), pH (4, 7, and 10), H2O2 concentration (1%, 3%, and 5%), temperature (25, 60, and 80 °C), and time are thoroughly studied. The results of parameter studies are presented in Fig. S1a–e.† A good amount of Cu(II) recovery was found under all the conditions, and the suitable set of conditions for 100% Cu recovery are identified as 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 rGO
1 rGO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) WPCB w/w ratio, 60 °C, pH 7, and 48 h. A detailed discussion of the parameter study is provided in the ESI.† The schematic representation of the overall procedure is provided in Fig. 1. The solid materials recovered from the leaching–sorption test were named Cu(I)/Cu(II)@rGO. The Cu(II) leaching was confirmed and quantified by characterizing Cu(I)/Cu(II)@rGO.
WPCB w/w ratio, 60 °C, pH 7, and 48 h. A detailed discussion of the parameter study is provided in the ESI.† The schematic representation of the overall procedure is provided in Fig. 1. The solid materials recovered from the leaching–sorption test were named Cu(I)/Cu(II)@rGO. The Cu(II) leaching was confirmed and quantified by characterizing Cu(I)/Cu(II)@rGO.
The X-ray diffraction (XRD) patterns were collected for graphite and rGO, Cu(I)/Cu(II)@rGO, and Cu/CuO@C and presented in Fig. S2a and b.† The XRD patterns of the pure graphite exhibit a distinct, high-intense (002) reflection at a 2θ of 25.9°, along with three minor peaks at 2θ values of 42°, 44.3°, and 54.1°, which correspond to the (100), (101), and (004) reflections, respectively44 and matches well with JCPDS number 00-012-0212. rGO exhibits reflection at 2θ of 23.6° indicating the distance between graphene layers, and 2θ at 42.8° indicating a short-range order in stacked graphene layers.45
X-ray photoelectron spectroscopy (XPS) spectra were collected for rGO, Cu(I)/Cu(II)@rGO, and Cu/CuO@C and presented in Fig. 2. The C 1s spectra for rGO, Cu(I)/Cu(II)@rGO, and Cu/CuO@C are given as Fig. 2a, d and g. The rGO C 1s spectrum (Fig. 2a) deconvoluted into four peaks with binding energies of EB [C 1s] = 284.6 eV, 285.5 eV, 286.5 eV, and 288.5 eV corresponding to C–C, C–OH (hydroxy), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carbonyl), and OH–C
O (carbonyl), and OH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.46 Similarly, the C 1s spectrum of Cu(I)/Cu(II)@rGO (Fig. 2d) is also deconvoluted into four peaks with binding energies of EB [C 1s] = 284.6 eV, 285.2 eV, 286.4 eV, 288.5 eV which were assigned as C–C, C–OH (hydroxy), C
O, respectively.46 Similarly, the C 1s spectrum of Cu(I)/Cu(II)@rGO (Fig. 2d) is also deconvoluted into four peaks with binding energies of EB [C 1s] = 284.6 eV, 285.2 eV, 286.4 eV, 288.5 eV which were assigned as C–C, C–OH (hydroxy), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (carbonyl), and OH–C
O (carbonyl), and OH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively. The XPS spectra of the O 1s orbital for rGO, Cu(I)/Cu(II)@rGO, and Cu/CuO@C, are shown in Fig. 2b, e and h. There were two distinct components identified in the rGO O 1s spectrum (Fig. 2b). The peak observed at EB [O 1s] = 531.0 eV is assigned to C
O, respectively. The XPS spectra of the O 1s orbital for rGO, Cu(I)/Cu(II)@rGO, and Cu/CuO@C, are shown in Fig. 2b, e and h. There were two distinct components identified in the rGO O 1s spectrum (Fig. 2b). The peak observed at EB [O 1s] = 531.0 eV is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and the peak at EB [O 1s] = 533.0 eV is assigned to C–OH.47 However, in Cu(I)/Cu(II)@rGO, the O 1s spectrum (Fig. 2e) is separated into three peaks. The peaks at EB [O 1s] = 531.6 eV, and 533.0 eV were assigned to C
O, and the peak at EB [O 1s] = 533.0 eV is assigned to C–OH.47 However, in Cu(I)/Cu(II)@rGO, the O 1s spectrum (Fig. 2e) is separated into three peaks. The peaks at EB [O 1s] = 531.6 eV, and 533.0 eV were assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and C–OH, respectively, and a new peak observed at 530.7 eV, is assigned to the oxygen of COOH bonded to Cu(II).48
O, and C–OH, respectively, and a new peak observed at 530.7 eV, is assigned to the oxygen of COOH bonded to Cu(II).48
 | ||
| Fig. 2 XPS spectra of C 1s (a, d and g), O 1s (b, e and h), Cu 2p (c, f and i) for rGO, copper sorbed rGO (Cu(I)/Cu(II)@rGO), material obtained after calcination at 500 °C (Cu/CuO@C). | ||
The XPS spectra of Cu 2p orbital for rGO, Cu(I)/Cu(II)@rGO, and Cu/CuO@C XPS are shown in Fig. 2c, f and i. A comparison of Cu 2p spectra revealed that rGO has no evidence for the presence of Cu (Fig. 2c), while Cu(I)/Cu(II)@rGO (Fig. 2f) and Cu/CuO@C (Fig. 2i) both showed the peaks for Cu 2p orbitals indicating successful leaching of Cu(II) by rGO. Cu 2p spectra for Cu(I)/Cu(II)@rGO (Fig. 2f) showed two major peaks which are deconvoluted to two peaks each. The peaks at EB [Cu 2p1/2] = 952.6 eV, and EB [Cu 2p3/2] = 933.0 eV are assigned to Cu1+, and peaks at EB [Cu 2p1/2] = 954.6 eV, and EB [Cu 2p3/2] = 934.9 eV are assigned to Cu2+ coordinated to the carboxylic acid functional groups of rGO.49 The peaks at EB [Cu 2p] = 962.8, 944.0, and 939.0 eV are assigned as satellite peaks. The “shakeup” process, which occurs when surplus electrons are stimulated to higher energy levels, is typically the cause of these satellite peaks.50,51 Overall, XPS spectra indicated the successful leaching and simultaneous sorption of Cu from e-waste WPCB flakes onto rGO, and Cu is adsorbed to rGO in the form of both Cu2+ and Cu1+ by coordinating with the available oxygen-containing functional groups.52
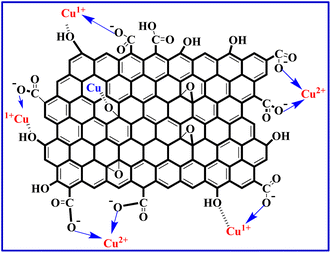
The leaching and sorption of copper ions on reduced graphene oxide (rGO) are primarily driven by coordination with the carboxylic acid functional groups, with additional contributions from hydroxy functional groups. The coordination of copper (Cu) with rGO is detailed in eqn (S1) to (S4) found in the ESI.† The probable chemical structure of the Cu(I)/Cu(II)@rGO complex is illustrated in Fig. 3. It is important to note that during XPS analysis of the material recovered after copper leaching by rGO, the interaction of X-rays with Cu2+ ions could possibly reduce some Cu2+ to Cu+.
Thermogravimetric analysis (TGA) was performed to understand the thermal profile of Cu(I)/Cu(II)@rGO and the result is presented in Fig. S3.† The decrease in weight observed below 100 °C is due to loss of physically adsorbed water. The range between 100 °C and 300 °C shows a loss of 25.1% and a loss of 41.3% between 300 °C and 500 °C. Above 500 °C, the weight of the material remains stable.
 | ||
| Fig. 4 (a) UV-Vis spectra of CuSO4 recovered from the stripping of Cu(I)/Cu(II)@rGO. (b) XRD of recovered CuSO4. (c) FTIR of recovered CuSO4. | ||
The stripped solution was concentrated, and the solids were calcined at 400 °C for 1 hour, to obtain CuSO4 anhydrous salt, and confirmed by XRD, and FTIR presented in Fig. 4b and c. The data obtained agrees with copper sulfate's standard JCPDS file (JCPDS 77-1900).55 Furthermore, SEM-EDAX analysis shown in Fig. S4† suggested high purity of the recovered CuSO4. The stretching vibration of the O–H group was responsible for the band seen at 3055 cm−1 in the recovered copper sulfate sample. We identified the O–H group's bending vibration mode at 1616 cm−1. The S–O group's bending vibration mode was found to be at 675 cm−1, while its stretching vibration mode was found to be at 1041 cm−1. Along with the Cu–O–H vibration, the vibration mode corresponding to the metal ion Cu2+ can be detected and designated as 856 cm−1.55
3.2. Synthesis of Cu/CuO@C from recovered Cu(I)/Cu(II)@rGO
The Cu(I)/Cu(II)@rGO, recovered from the leaching–sorption step was calcined at 500 °C, resulting in Cu(0) and CuO nanoparticle-supported carbon, and was named Cu/CuO@C. The obtained materials were characterized by XPS, XRD, Raman spectroscopy, and SEM. The XRD patterns of the materials are presented in Fig. S2b.† Cu/CuO@C has reflections at 43.0°, 50.1°, and 73.7° corresponding to the Miller indices of (111), (200), and (220), indicating the presence of Cu(0) nanoparticles, which is in good accordance with JCPDS card no.: 85–1326, and a reflection at 25.6° is due to carbon.56 The XRD profile also has two reflections at 36.2°, and 38.3° indicating the presence of CuO. It also showed reflections at 35.2°, 48.5°, 53.1°, 61.1°, 65.8°, 67.7°, and 72.2°. These reflections correspond to the crystallographic planes (110), (−111), (111), (−202), (020), (202), (−113), (−311), (220), and (311) due to the monoclinic structure of CuO. This matches well with the JCPDS no. 5-661.57 XRD suggests that the material is a mixture of Cu(0) and CuO nanoparticles supported on carbon (Cu/CuO@C).The observations in XRD are well complemented by XPS spectra presented in Fig. 2g–i. The C 1s spectrum of Cu/CuO@C (Fig. 2g) was deconvoluted into two peaks with binding energies at EB [C 1s] = 284.6 eV and 283.7 eV which are assigned to C–C and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C respectively.58 It is worth noting that the C 1s corresponding to the COOH group present in rGO and Cu(I)/Cu(II)@rGO disappears after calcination. The O 1s spectra of Cu/CuO@C were also separated into three peaks (Fig. 2h). The peaks with the binding energy at EB [O 1s] = 533.2 eV and EB [O 1s] = 532.0 eV correspond to C–OH and C
C respectively.58 It is worth noting that the C 1s corresponding to the COOH group present in rGO and Cu(I)/Cu(II)@rGO disappears after calcination. The O 1s spectra of Cu/CuO@C were also separated into three peaks (Fig. 2h). The peaks with the binding energy at EB [O 1s] = 533.2 eV and EB [O 1s] = 532.0 eV correspond to C–OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O like rGO. The third peak at EB [O 1s] = 530.7 was assigned for Cu–O.59 Cu 2p spectra of Cu/CuO@rGO (Fig. 2i) also showed two major peaks which are deconvoluted into two peaks each. The peaks at EB [Cu 2p1/2] = 954.2 eV and EB [Cu 2p3/2] = 934.5 eV, are assigned to the presence of CuO. Additionally, two peaks at EB [Cu 2p1/2] = 952.6 eV and EB [Cu 2p3/2] = 932.9 eV are assigned to Cu(0), these assignments are in correlation with the earlier reports.60 The other peaks observed at EB of 962.0 eV, 943.5 eV, and 940.8 eV are identified as satellite peaks. The formation of CuO and Cu(0) nanoparticles is further complemented by the observed XRD patterns as presented in Fig. S2b.†
O like rGO. The third peak at EB [O 1s] = 530.7 was assigned for Cu–O.59 Cu 2p spectra of Cu/CuO@rGO (Fig. 2i) also showed two major peaks which are deconvoluted into two peaks each. The peaks at EB [Cu 2p1/2] = 954.2 eV and EB [Cu 2p3/2] = 934.5 eV, are assigned to the presence of CuO. Additionally, two peaks at EB [Cu 2p1/2] = 952.6 eV and EB [Cu 2p3/2] = 932.9 eV are assigned to Cu(0), these assignments are in correlation with the earlier reports.60 The other peaks observed at EB of 962.0 eV, 943.5 eV, and 940.8 eV are identified as satellite peaks. The formation of CuO and Cu(0) nanoparticles is further complemented by the observed XRD patterns as presented in Fig. S2b.†
The relative concentration of Cu(0) and CuO at the surface of the material was estimated using CasaXPS software using area and a relative sensitive factor (RSF) which was obtained as 57.7% (Cu(0)) and 42.3% (CuO). XPS revealed that the obtained material is a composite comprising Cu(0) and CuO nanoparticles supported on carbon. In Raman spectra, a D band at 1342 cm−1, 1340 cm−1, 1338 cm−1, 1345 cm−1, and a G band 1598 cm−1, 1595 cm−1, 1614 cm−1, 1605 cm−1 were observed for graphite, rGO, Cu(I)/Cu(II)@rGO and Cu/CuO@C (Fig. S5†) showing lattice distortions from the graphene structure. The Raman bands of Cu(I)/Cu(II)@rGO and Cu/CuO@C reveal three more phonon modes at 296, 363, and 670 cm−1 corresponding to Cu–O or CuO61,62 respectively, suggesting the obtained material as a composite of Cu, CuO nanoparticles and carbon. SEM images along with elemental mapping were captured to understand the surface properties of the composite nanoparticles. The SEM images in Fig. 6. 1a, and 2a exhibit the surface morphology of Cu(I)/Cu(II)@rGO, and Cu/CuO@C. The pictures revealed that Cu(0) and CuO spherical nanoparticles are distributed on carbon sheets.
| αhν2 = A(hν − Eg) | (1) |
 | (2) |
The specific capacitance of the Cu/CuO@C electrode was measured from the GCD cycles. Fig. 7b shows the GCD cycles of Cu/CuO@C at a specific current of 1 A g−1 in the potential range of (−0.8 V) to 0 V. The specific capacitance was calculated using eqn (3)
| SC = (I × t)/(m × ΔV) | (3) |
Cu/CuO@C exhibits a specific capacitance of 432.5 F g−1 at a specific current of 1 A g−1. It should be noted that activated carbon can provide 100–200 F g−1 when used as a negative electrode material in supercapacitors.22,78 Zhao et al. had previously synthesized leaf-like CuO on graphite sheets by a hydrothermal method, which exhibited a specific capacitance of 331.9 F g−1 at a specific current of 0.6 A g−1 when used as a positive electrode in 6 M KOH in the potential range of 0 to 0.4 V and displayed a capacitance retention of about 95% after 1000 cycles.79
To examine the rate performance of Cu/CuO@C, GCD was performed at different specific currents varying from 1 A g−1 to 20 A g−1 in the potential range of (−0.8 V) to 0 V in 1 M KOH, which is shown in Fig. 7c. The specific capacitance of Cu/CuO@C decreases with an increase in specific currents due to the decreased utilization of active mass at higher currents. The rate capability of Cu/CuO@C evaluated from GCD is shown in Fig. 7d. Cu/CuO@C exhibits a higher specific capacitance of 432.5 F g−1 at a specific current of 1 A g−1, which decreased to 150 F g−1 at a specific current of 20 A g−1, thus the capacitance retention is about 34.6% of its initial high specific capacitance. The long-term cycling stability of Cu/CuO@C was evaluated by performing galvanostatic cycling at a specific current of 3 A g−1 in the potential range of (−0.8 V) to 0 V for 4000 cycles in 1 M KOH electrolyte. Fig. 7e shows the charge–discharge profile for different cycle numbers of Cu/CuO@C when tested at a specific current of 3 A g−1. An initial Cs of 305 F g−1 is achieved, which gradually decreases to a value of 264 F g−1 after 1000 cycles. Thereafter, the charge discharge profiles merge with each other showing the high cycling stability up to 4000 cycles. Fig. 7f displays the cycling performance of Cu/CuO@C in the potential range of (−0.8 V) to 0 V at a specific current of 3 A g−1 in 1 M KOH. As explained, an initial Cs of 305 F g−1 decreased to 280 F g−1 after 500 cycles, which was then stabilized to a value of 262.8 F g−1 after 4000 cycles. Thus, Cu/CuO@C exhibits excellent cycling stability with a capacitance retention of 86% after 4000 cycles.
For the comparison, the CV and GCD profiles of bare Ni foam are measured under similar experimental conditions and provided in ESI Fig. S7.† Fig S7a† shows the CVs where the current response of bare Ni foam is found to be negligible compared to that of Cu/CuO@C. For further confirmation, we have performed GCD of bare Ni foam (Fig. S7b†) at a specific current of 1 A g−1, where it exhibits a very low specific capacitance (0.7 F g−1).
To observe the morphological changes of Cu/CuO@C electrodes upon cycling, SEM images of both pristine and cycled electrodes have been recorded and compared in Fig. S8.† From the SEM images, no significant changes in the morphology were observed even after cycling for 4000 cycles, indicating the highly stable nature of the prepared Cu/CuO@C electrodes.
| m+/m− = (Cs−ΔE−)/(Cs+ΔE+) | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The cyclic voltammogram of the Cu/CuO@C‖AC asymmetric supercapacitor was analyzed at different scan rates in the voltage of 0–1.2 V in 1 M KOH electrolyte. The CV revealed a pair of oxidation and reduction peaks at 0.48 V and 0.09 V, respectively, indicating the pseudo-capacitive properties of the device. The peak intensity is found to increase with an increase in the scan rates (Fig. 8a).
1. The cyclic voltammogram of the Cu/CuO@C‖AC asymmetric supercapacitor was analyzed at different scan rates in the voltage of 0–1.2 V in 1 M KOH electrolyte. The CV revealed a pair of oxidation and reduction peaks at 0.48 V and 0.09 V, respectively, indicating the pseudo-capacitive properties of the device. The peak intensity is found to increase with an increase in the scan rates (Fig. 8a).
For determining the specific capacitance and rate capability of the Cu/CuO@C‖AC asymmetric supercapacitor, GCD was performed at different specific currents varying from 1 A g−1 to 8 A g−1 in the voltage range of 0 to 1.2 V in 1 M KOH solution (Fig. 8b). The Cu/CuO@C‖AC asymmetric supercapacitor exhibits a specific capacitance of 88.8 F gcell−1 at a specific current of 1 A g−1 in 1 M KOH. Although the specific capacitance of the supercapacitor decreased with an increase in specific current, the Cu/CuO@C‖AC asymmetric supercapacitor can provide a specific capacitance of 42 F gcell−1 at a higher specific current of 8 A g−1, showing its suitability even at high currents. These results clearly signify the high-power characteristics of the Cu/CuO@C‖AC asymmetric supercapacitor in 1 M KOH electrolyte (Fig. 8c).
For evaluating the long-term cycling stability of the Cu/CuO@C‖AC asymmetric supercapacitor, GCD was performed at a specific current of 2 A g−1 in the voltage range of 0–1.2 V; the voltage profile and variation in specific capacitance with cycling are shown in Fig. 8d and e. A specific capacitance of 79 F gcell−1 was obtained during the initial cycles, which decreased to a value of 70 F gcell−1 after continuous 8000 cycles, with a capacitance retention of about 88.6%. The above results signify the superior cycling stability of the Cu/CuO@C‖AC asymmetric supercapacitor in 1 M KOH electrolyte.
 | (5) |
 | (6) |
The Ragone plot for the hybrid supercapacitor is plotted using the values of energy density and power density obtained at different specific currents (Fig. 9). The hybrid Cu/CuO@C‖AC supercapacitor can deliver an energy density of 17.8 W h kg−1 at a specific current of 1 A g−1 in 1 M KOH and possesses a higher power density of 8 kW kg−1. For comparison, we have assembled an AC‖AC symmetric supercapacitor under the same conditions in a coin cell and evaluated its electrochemical performances in a 1 M KOH electrolyte. Fig. S10a.† displays the CV of the AC‖AC symmetric supercapacitor in the voltage range of 0–1.2 V, which is in a rectangular shape. Fig. S10b.† exhibits the GCD of the AC‖AC symmetric supercapacitor, where it was cycled in the voltage range of 0–1.2 V at different specific currents varying from 1 A g−1 to 3 A g−1. The symmetric supercapacitor exhibits a specific capacitance of 11 F gcell−1 at a specific current of 1 A g−1. Based on the rate capability investigations (Fig. S10c†), it is evident that the specific capacitance decreased from 11 F gcell−1 at a specific current of 1 A g−1 to 6.2 F gcell−1 at 3 A g−1. Fig. S10d.† depicts the Ragone plot of the AC‖AC symmetric supercapacitor, where it provides an energy density of only 2.2 W h kg−1 with a power density of 0.6 kW kg−1. These results indicate the superior capacitive performance of Cu/CuO@C‖AC compared to that of the symmetric AC‖AC supercapacitor and that the Cu/CuO@C extracted from e-waste is a useful negative electrode material for hybrid supercapacitor applications.
4. Conclusions
This study highlighted the environmentally conscious process of reclaiming copper from discarded waste printed circuit boards (WPCBs). Reduced graphene oxide material was synthesized and used for the simultaneous leaching and sorption of Cu from WPCB flakes in an aqueous medium. The highest leaching–sorption was achieved at a pH of 7 and a temperature of 60 °C, i.e., 82.9 mg g−1. A specific capacitance of 432.5 F g−1 was obtained for Cu/CuO@C as a negative electrode when cycled at a specific current of 1 A g−1 in the potential range of −(0.8) to 0 V in 1 M KOH electrolyte. Cu/CuO@C exhibits excellent cycling stability with a capacitance retention of 86% after 4000 cycles when cycled at a specific current of 3 A g−1 in 1 M KOH. Interestingly, the Cu/CuO@C‖AC asymmetric supercapacitor exhibits a specific capacitance of 88.8 F g−1 at a specific current of 1 A g−1. Cu/CuO@C‖AC was stable up to 8000 cycles with a capacitance retention of 88.6% upon cycling at a specific current of 2 A g−1 in the voltage range of 0–1.2 V in 1 M KOH, which indicates the remarkable cycling stability of the supercapacitor device. Thus, this study enlightens the application of recovered e-waste for fabricating useful capacitive electrode materials, which will find application in energy storage devices and can be important towards environmental remediation and sustainability.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors would like to thank the Science and Engineering Research Board (SERB), India, for providing financial support through the Start-up Research Grant to S. Kancharla (SRG/2021/001457) and P. K. Nayak (SRG/2022/001178). S. Kancharla would like to thank SRM for providing financial support through Selective Excellence Research Initiative (SERI). RM, AAN and SBMK would like to thank SRM for providing a research scholarship.References
- G. Moyen Massa and V.-M. Archodoulaki, Environments, 2023, 10, 44 CrossRef.
- V. Forti, C. P. Baldé, R. Kuehr and G. Bel, The Global E-Waste Monitor, 2020 Search PubMed.
- H. W. Tan, J. An, C. K. Chua and T. Tran, Adv. Electron. Mater., 2019, 5, 1800831 CrossRef.
- S. Abbas, N. Saqib and U. Shahzad, Geosci. Front., 2023, 15, 101697 CrossRef.
- D. R. Tipre, B. R. Khatri, S. C. Thacker and S. R. Dave, Environ. Sci. Pollut. Res., 2021, 28, 10503–10518 CrossRef CAS PubMed.
- Y. Zhu, B. Li, Y. Wei, S. Zhou and H. Wang, Process Saf. Environ. Prot., 2023, 178, 1083–1093 CrossRef CAS.
- M. Hayati, S. M. S. A. Ganji, S. H. Shahcheraghi and R. R. Khabir, J. Mater. Cycles Waste Manage., 2023, 25, 211–220 CrossRef CAS.
- J. Guo, X. Luo, S. Tan, O. A. Ogunseitan and Z. Xu, J. Hazard. Mater., 2020, 382, 121038 CrossRef CAS PubMed.
- H. Long Le, J. Jeong, J.-C. Lee, B. D. Pandey, J.-M. Yoo and T. H. Huyunh, Miner. Process. Extr. Metall. Rev., 2011, 32, 90–104 CrossRef CAS.
- T. A. G. Martins, M. P. K. Caldas, V. T. de Moraes, J. A. S. Tenório and D. C. R. Espinosa, J. Environ. Chem. Eng., 2021, 9, 106789 CrossRef CAS.
- I. F. F. Neto, C. A. Sousa, M. S. C. A. Brito, A. M. Futuro and H. M. V. M. Soares, Sep. Purif. Technol., 2016, 164, 19–27 CrossRef CAS.
- R. Torres and G. T. Lapidus, Waste Manage., 2016, 57, 131–139 CrossRef CAS PubMed.
- S. C. Pinho, C. A. Ferraz and M. F. Almeida, Waste Biomass Valorization, 2023, 14, 1683–1691 CrossRef CAS.
- M. Madhavan, V. Shetty Kodialbail and M. B. Saidutta, Waste Biomass Valorization, 2024, 15, 1212–1224 Search PubMed.
- N. Nagarajan and P. Panchatcharam, Heliyon, 2023, 9, e13806 CrossRef CAS PubMed.
- C.-H. Wu, C.-Y. Kuo and S.-L. Lo, J. Environ. Sci. Health, Part A, 2004, 39, 2205–2219 CrossRef PubMed.
- Y. Huang, D. Wang, H. Liu, G. Fan, W. Peng and Y. Cao, Sep. Purif. Technol., 2023, 326, 124619 CrossRef CAS.
- M. Manickasundaram, K. Lakshmanan, K. Vediappan and S. Kancharla, Chem. Eng. J., 2024, 484, 149605 CrossRef CAS.
- C. Silva, G. Zeba, C. Rocha, P. Gismonti, J. Afonso, R. Silva, C. Vianna and J. L. Mantovano, Quim. Nova, 2020, 43, 914–922 Search PubMed.
- J. Yan, W. Ye, Z. Jian, J. Xie, K. Zhong, S. Wang, H. Hu, Z. Chen, H. Wen and H. Zhang, Bioresour. Technol., 2018, 266, 447–453 Search PubMed.
- L. Liu, S. Liu, Q. Zhang, C. Li, C. Bao, X. Liu and P. Xiao, J. Chem. Eng. Data, 2013, 58, 209–216 CrossRef CAS.
- A. Shanmugavani and R. K. Selvan, Electrochim. Acta, 2016, 188, 852–862 CrossRef CAS.
- K. P. S. Prasad, D. S. Dhawale, T. Sivakumar, S. S. Aldeyab, J. S. M. Zaidi, K. Ariga and A. Vinu, Sci. Technol. Adv. Mater., 2011, 12, 044602 Search PubMed.
- L. Yu, B. Yang, Q. Liu, J. Liu, X. Wang, D. Song, J. Wang and X. Jing, J. Electroanal. Chem., 2015, 739, 156–163 CrossRef CAS.
- S. J. Zhu, J. Zhang, J. J. Ma, Y. X. Zhang and K. X. Yao, J. Power Sources, 2015, 278, 555–561 CrossRef CAS.
- B. Nisha, Y. Vidyalakshmi and S. Abdul Razack, Adv. Powder Technol., 2020, 31, 1001–1006 CrossRef CAS.
- A. Phakkhawan, P. Suksangrat, P. Srepusharawoot, S. Ruangchai, P. Klangtakai, S. Pimanpang and V. Amornkitbamrung, J. Alloys Compd., 2022, 919, 165702 Search PubMed.
- N. Kandasamy, T. Venugopal and K. Kannan, J. Nanosci. Nanotechnol., 2018, 18, 3960–3968 CrossRef CAS PubMed.
- P. Asen, M. Haghighi, S. Shahrokhian and N. Taghavinia, J. Alloys Compd., 2019, 782, 38–50 Search PubMed.
- Y. Kumar, S. Chopra, A. Gupta, Y. Kumar, S. J. Uke and S. P. Mardikar, Mater. Sci. Energy Technol., 2020, 3, 566–574 CAS.
- K. Krishnamoorthy and S. J. Kim, Mater. Res. Bull., 2013, 48, 3136–3139 CrossRef CAS.
- Y. Tang, Y. Liu, S. Yu, W. Guo, S. Mu, H. Wang, Y. Zhao, L. Hou, Y. Fan and F. Gao, Electrochim. Acta, 2015, 161, 279–289 CrossRef CAS.
- V. Lakshmi, R. Ranjusha, S. Vineeth, S. V. Nair and A. Balakrishnan, Colloids Surf., A, 2014, 457, 462–468 CrossRef CAS.
- T. Xue, C.-L. Xu, D.-D. Zhao, X.-H. Li and H.-L. Li, J. Power Sources, 2007, 164, 953–958 CrossRef CAS.
- J. S. Shaikh, R. C. Pawar, A. V. Moholkar, J. H. Kim and P. S. Patil, Appl. Surf. Sci., 2011, 257, 4389–4397 CrossRef CAS.
- D. R. John, S. Deepapriya, R. M. Cyril, V. P. Annie, M. Jose and D. S. Jerome, AIP Conf. Proc., 2020, 2244, 070018 CrossRef CAS.
- M. S. Rahmanifar, M. Hemmati, A. Noori, M. F. El-Kady, M. F. Mousavi and R. B. Kaner, Mater. Today Energy, 2019, 12, 26–36 CrossRef.
- M. Z. U. Shah, M. Sajjad, H. Hou, S. ur Rahman, A. Mahmood, U. Aziz and A. Shah, J. Energy Storage, 2022, 55, 105492 CrossRef.
- L. Li, P. Gao, S. Gai, F. He, Y. Chen, M. Zhang and P. Yang, Electrochim. Acta, 2016, 190, 566–573 CrossRef CAS.
- X. Li, J. Shao, J. Li, L. Zhang, Q. Qu and H. Zheng, J. Power Sources, 2013, 237, 80–83 CrossRef CAS.
- J. Zhang, Z. Yao, M. Fan, H. Shen and T. Ma, Ionics, 2023, 29, 2043–2052 CrossRef CAS.
- X. Zhang, J. Wang, X. Ji, Y. Sui, F. Wei, J. Qi, Q. Meng, Y. Ren, Y. He and D. Zhuang, J. Mater. Sci.: Mater. Electron., 2020, 31, 16260–16268 CrossRef CAS.
- J. Chen, B. Yao, C. Li and G. Shi, Carbon, 2013, 64, 225–229 CrossRef CAS.
- D. Van Thanh, L.-J. Li, C.-W. Chu, P.-J. Yen and K.-H. Wei, RSC Adv., 2014, 4, 6946 RSC.
- L. Stobinski, B. Lesiak, A. Malolepszy, M. Mazurkiewicz, B. Mierzwa, J. Zemek, P. Jiricek and I. Bieloshapka, J. Electron Spectrosc. Relat. Phenom., 2014, 195, 145–154 CrossRef CAS.
- F. T. Johra and W.-G. Jung, Appl. Surf. Sci., 2015, 357, 1911–1914 CrossRef CAS.
- V. V. T. Padi and M. Cernik, Int. J. Nanomed., 2013, 8, 889–898 Search PubMed.
- C. Shen, I. Cebula, C. Brown, J. Zhao, M. Zharnikov and M. Buck, Chem. Sci., 2012, 3, 1858 RSC.
- J. S. Chen, Y. Gui and D. J. Blackwood, J. Power Sources, 2016, 325, 575–583 CrossRef CAS.
- T. M. Ivanova, K. I. Maslakov, A. A. Sidorov, M. A. Kiskin, R. V. Linko, S. V. Savilov, V. V. Lunin and I. L. Eremenko, J. Electron Spectrosc. Relat. Phenom., 2020, 238, 146878 Search PubMed.
- L. He, J. Liu, L. Yang, Y. Song, M. Wang, D. Peng, Z. Zhang and S. Fang, Electrochim. Acta, 2018, 275, 133–144 Search PubMed.
- W. Wu, Y. Yang, H. Zhou, T. Ye, Z. Huang, R. Liu and Y. Kuang, Water, Air, Soil Pollut., 2013, 224, 1372 CrossRef.
- F. Inoue, H. Philipsen, A. Radisic, S. Armini, Y. Civale, S. Shingubara and P. Leunissen, J. Electrochem. Soc., 2012, 159, D437–D441 CrossRef CAS.
- S. B. Rahardjo, T. E. Saraswati, A. Masykur, N. N. F. Finantrena and H. Syaima, IOP Conf. Ser.: Mater. Sci. Eng., 2018, 349, 012056 Search PubMed.
- H. Wu, V. W. Or, S. Gonzalez-Calzada and V. H. Grassian, Nanoscale, 2020, 12, 19350–19358 RSC.
- R. Seif El-Nasr, S. M. Abdelbasir, A. H. Kamel and S. S. M. Hassan, Sep. Purif. Technol., 2020, 230, 115860 CrossRef CAS.
- R. Raghav, P. Aggarwal and S. Srivastava, AIP Conf. Proc., 2016, 1724, 020078 CrossRef.
- X. Chen, X. Wang and D. Fang, Fullerenes, Nanotubes Carbon Nanostruct., 2020, 1048–1058 CrossRef CAS.
- M. A. Badillo-Ávila, R. Castanedo-Pérez, J. Márquez-Marín, D. E. Guzmán-Caballero and G. Torres-Delgado, Mater. Chem. Phys., 2019, 236, 121759 CrossRef.
- X. Zhan, M. Li, X. Zhao, Y. Wang, S. Li, W. Wang, J. Lin, Z. Nan, J. Yan, Z. Sun, H. Liu, F. Wang, J. Wan, J. Liu, Q. Zhang and L. Zhang, Nat. Commun., 2024, 15, 1056 CrossRef CAS PubMed.
- S. Dutta, K. Das, K. Chakrabarti, D. Jana, S. K. De and S. De, J. Phys. D Appl. Phys., 2016, 49, 315107 CrossRef.
- A. Dandia, S. Bansal, R. Sharma, K. S. Rathore and V. Parewa, RSC Adv., 2018, 8, 30280–30288 RSC.
- S. P. Ashokkumar, H. Vijeth, L. Yesappa, M. Niranjana, M. Vandana and H. Devendrappa, Inorg. Chem. Commun., 2020, 115, 107865 CrossRef CAS.
- J. Lillo-Ramiro, J. M. Guerrero-Villalba, M. L. de Mota-González, F. S. A. Tostado, G. Gutiérrez-Heredia, I. Mejía-Silva and A. C. Castillo, Optik, 2021, 229, 166238 CrossRef CAS.
- S. Sihag, R. Dahiya, S. Rani, Anushree, A. Kumar and V. Kumar, Indian J. Chem. Technol., 2022, 29, 578–582 CAS.
- A. M. Luís, M. C. Neves, M. H. Mendonça and O. C. Monteiro, Mater. Chem. Phys., 2011, 125, 20–25 CrossRef.
- S. Azhar, K. S. Ahmad, I. Abrahams, T. Ingsel, R. K. Gupta and A. El-marghany, Chem. Pap., 2024, 78, 1647–1658 CrossRef CAS.
- A. M. Alturki, Biomass Convers. Biorefin., 2022, 1–12 CAS.
- S. Yadav, N. Rani and K. Saini, Environ. Sci. Pollut. Res., 2022, 30, 71984–72008 CrossRef PubMed.
- D. Majumdar and S. Ghosh, J. Energy Storage, 2021, 34, 101995 CrossRef.
- R. Kumar, P. Rai and A. Sharma, RSC Adv., 2016, 6, 3815–3822 RSC.
- B. K. Singh, A. Shaikh, R. O. Dusane and S. Parida, J. Energy Storage, 2020, 31, 101631 CrossRef.
- G. Wang, J. Huang, S. Chen, Y. Gao and D. Cao, J. Power Sources, 2011, 196, 5756–5760 CrossRef CAS.
- S. K. Shinde, D. P. Dubal, G. S. Ghodake and V. J. Fulari, RSC Adv., 2015, 5, 4443–4447 RSC.
- S. K. Shinde, D.-Y. Kim, G. S. Ghodake, N. C. Maile, A. A. Kadam, D. S. Lee, M. C. Rath and V. J. Fulari, Ultrason. Sonochem., 2018, 40, 314–322 CrossRef CAS PubMed.
- M. Parthibavarman, V. Sharmila, P. Sathishkumar and S. A. Gaikwad, J. Electron. Mater., 2018, 47, 5443–5451 CrossRef CAS.
- J. Wang, Q. Zhang, X. Li, B. Zhang, L. Mai and K. Zhang, Nano Energy, 2015, 12, 437–446 CrossRef CAS.
- C. Dong, Y. Wang, J. Xu, G. Cheng, W. Yang, T. Kou, Z. Zhang and Y. Ding, J. Mater. Chem. A, 2014, 2, 18229–18235 RSC.
- B. Zhao, P. Liu, H. Zhuang, Z. Jiao, T. Fang, W. Xu, B. Lu and Y. Jiang, J. Mater. Chem. A, 2013, 1, 367–373 RSC.
- S. E. Moosavifard, M. F. El-Kady, M. S. Rahmanifar, R. B. Kaner and M. F. Mousavi, ACS Appl. Mater. Interfaces, 2015, 7, 4851–4860 CrossRef CAS PubMed.
- B. Saravanakumar, C. Radhakrishnan, M. Ramasamy, R. Kaliaperumal, A. J. Britten and M. Mkandawire, J. Electroanal. Chem., 2019, 852, 113504 CrossRef CAS.
- H. Sun, L. Xu, J. Li, Y. Li, T. Wu, F. Yu, X. Guo and H. Zhang, Ceram. Int., 2020, 46, 17461–17468 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta04107k |
| ‡ Both the authors have contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |