Constructing sodiophilic interconnected ion-transport channels towards a stable Na-metal anode†
Yi
Ding
,
Min
Guo
*,
Yawei
Zhang
,
Song
Lu
 *,
Jiadi
Ying
,
Yeqing
Wang
,
Tiancun
Liu
*,
Jiadi
Ying
,
Yeqing
Wang
,
Tiancun
Liu
 and
Zhixin
Yu
and
Zhixin
Yu
 *
*
Institute of New Energy, School of Chemistry and Chemical Engineering, Shaoxing University, Shaoxing 312000, China. E-mail: gm@usx.edu.cn; lusong1993@gmail.com; zhixin.yu@uis.no
First published on 28th June 2024
Abstract
Electrochemical cells utilizing metals (e.g., Li, Na, K) as anodes have sparked significant interest in both academia and industry. However, the rapid growth of sodium dendrites and irregular deformation are limiting the usage of sodium metal anodes. Fast ion transport is crucial for metal deposition and stripping. Herein, we fabricated a hybrid metal anode by physically mixing superionic conductor Na3V2(PO4)3 particles with sodium metal using a facile and scalable rolling and folding method. The superionic conductor particles with high affinity for Na+ are beneficial for charged Na-ion channels formation. These channels serve as reservoirs to continuously release and deliver sodium ions, compensating for the ionic flux of the electrolyte. Moreover, the interconnected Na+-conducting channels also reduce the diffusion barrier and accelerate Na+ migration, thereby homogenizing the local Na-ion flux and steering uniform Na deposition. These characteristics collectively contribute to dendrite-free Na electrodeposition and long cycle life (over 1000 h for 2 mA h cm−2 at 0.5 mA cm−2) in symmetric cells. Remarkably, when paired with Na3V2(PO4)3 cathodes, the full cell achieves high capacity retention (87.8% after 1000 cycles at a current density of 5C) and excellent rate performance (57.3 mA h g−1 at 50C).
Introduction
In recent years, lithium-ion batteries (LIBs) have been widely utilized in transportation vehicles and energy storage devices. However, the ever-increasing market demand for efficient battery technologies is outpacing the supply of LIBs, resulting from the geographical disparity of lithium resources and cost fluctuations of the relevant raw material.1–3 Other alkali metal-ion batteries with large theoretical capacity and abundant natural reserves have aroused intensive research interest, including lithium-, sodium-, and potassium-ion batteries.4,5 Due to the similar physical and chemical properties of sodium (Na) to those of lithium (Li) as well as its abundant resources, sodium-ion batteries (SIBs) have become an appealing supplement to LIBs, especially in low-speed electric vehicles and green energy storage systems.6–8 The sodium metal anode (SMA) has a large theoretical capacity (1166 mA h g−1) and low redox potential (−2.714 V vs. the standard hydrogen electrode).9,10 However, several intrinsic problems hinder the development of sodium metal batteries (SMBs), including detrimental dendrite growth, aggressive side reactions and poor processability.11,12Ion transfer and migration kinetics profoundly affect the plating and stripping processes of metal batteries (e.g., Li, Na).13,14 Rational regulation of ion transport behavior to realize even ion flux and specific deposition morphology can enable uniform ion nucleation and reduced polarization, which are favorable for improving the cycle life and fast charging capabilities. Undoubtedly, numerous strategies have been devoted to addressing the critical barriers associated with the ion transport kinetics of metal anodes.15 Huang's group constructed a multifunctional protective interphase with a high sodium ion affinity and low diffusion barrier.16 The vanadium oxide (Na3VO4 or K3VO4) ion conductive layer modulates the uniformity of surface ions (Na+, K+) and guides a homogeneous deposition. Xu et al. designed a zincophilic array as a Zn host, which is conducive to lowering the nucleation barrier and boosting the ion diffusion kinetics of Zn deposition throughout the composite metal matrix.17 For soft metal materials such as sodium and lithium, an ideal metal matrix should also synergistically possess high intrinsic sodiophilicity/lithiophilicity and fast ionic mobility. Duan et al. fabricated a lithium–graphite (Li–C) composite anode with significantly improved wettability for ceramic-based solid-state electrolytes.18 LiC6 particles are tightly mixed in a Li metal matrix, and the hybrid anode exhibits flexibility and twistability, which are advantageous for the electrode manufacturing process. Zheng et al. exploited the spontaneous reaction between Na and SnO2 to construct a dual ion/electron-conductive matrix.19 The interconnected dual-conductive skeleton with high sodiophilicity exhibited enhanced surface chemistry and prolonged cycling lifespan. Zhao et al. engineered a Na hybrid electrode with sodiophilic Na3Bi-penetration.20 The optimized anode ensured uniform sodium ion nucleation and rapid ion migration within the bulk metal, contributing to a dendrite-free Na morphology.
Some reported electrode materials, including graphite,21 activated carbon,22 carbon nanotubes (CNTs),23 hard carbon,24 MoS2 (ref. 25) and montmorillonite,26 have been confirmed to be beneficial for restraining the dendrite growth of the metal anode. These materials have high affinity for metal ions and can guide the initial nucleation behavior. However, these electrode materials commonly act as heterogeneous nucleation sites for metal plating or as a protective interface, making it difficult to obtain superior internal ion transport for fast ion deposition and stripping operation. Therefore, developing dendrite-free sodium metal anodes with high sodiophilicity and fast bulk ion diffusion capability remains a significant challenge for fast-charging Na metal batteries.
As a typical natrium superionic conductor (NASICON) material, Na3V2(PO4)3 (NVP) is renowned for its superior ion diffusion capability and excellent thermal stability, which stem from its innate three-dimensional framework structure for Na+ insertion/extraction and the strong covalent bonds of P–O in PO43− groups.27,28 The sodiation characteristic of NVP (Na3V2(PO4)3 + Na+ + e− ↔ Na3+xV2(PO4)3) during the initial deposition process (below 1.0 V) could increase Na+ affinity for the metal matrix, causing the ingress of sodium ions into the Na bulk.29 Simultaneously, adding inorganic particles into the metal bulk phase is widely adopted to improve the mechanical durability of the metal and enable it to withstand the structural stress during volume changes. The solid particles dispersed in the metal matrix can prevent grain boundary sliding and reduce the deformation of the composite material under an external force.30,31
Herein, we designed a composite electrode by integrating NVP inorganic particles with metallic Na through a facile and scalable physical mixing method. The resulting composite effectively minimizes the charging/discharging overpotential and enhances ion transfer dynamics during the deposition/dissolution steps of the Na anode. The superionic conductor attracts Na+ to form charged Na-ion transfer channels due to their high affinity for Na+. These channels act as reservoirs to sustain the flow of Na+ ions into or out of the electrode, similar to an expressway for high-speed ion transport.32,33 Consequently, the interconnected ion transport channels ensure a homogeneous ion distribution. This design also reduces the diffusion energy barrier, accelerates Na+ ion migration, and inhibits the formation of sodium dendrites.34 The excellent properties of NVP endow the fabricated NVP-Na anode with a homogeneous and dense Na evolution morphology. Specifically, the NNVP20‖NNVP20 symmetric cell demonstrated prolonged cycling capability and a significantly reduced polarization overpotential, maintaining a restricted overpotential of 134.2 mV at 0.5 mA cm−2 for 2 mA h cm−2 over 1000 hours. Furthermore, when paired with an NVP cathode, the full cell delivered superior electrochemical performance with a high capacity retention of 87.8% after 1000 cycles at 5C. Our work underscores the pivotal role of the superionic conductor network in enabling the reversible plating/stripping of Na metal.
Experimental section
Preparation of the NNVP composite anode
The modified Na composite foil was prepared via a facile mechanical rolling–stacking operation in a glovebox filled with Ar. The fresh metal Na is preserved in kerosene, and the surface oxide should be removed before use. Na metal was first rolled into a thin sheet with a stainless-steel roller on a tetrafluoroethylene base. Thereafter, the NVP powder (Canrd Co. Ltd) was sprinkled evenly over the surface of Na metal. After repeating the folding–rolling processes 10 times, a compact hybrid foil with NVP nanoparticles homogeneously embedded in the metallic Na block was formed with a thickness of less than 100 μm. By controlling the NVP content, composite foils with 5, 10, 20, and 50 wt% NVP powder were prepared and denoted as NNVP05, NNVP10, NNVP20 and NNVP50, respectively. Bare Na without adding NVP materials was also prepared for comparison.Materials characterization
The morphology of NVP particles was recorded using a scanning electron microscope (SEM, JSM-6360LV). X-ray diffraction (XRD, Empyrean) was employed to investigate the structure of the NVP sample in the 2θ range of 10–70°. Raman spectroscopy and Fourier transform infrared spectrophotometry (FTIR) were conducted on an American Thermo Fisher Scientific DXR3 micro and NICOLET6700 instrument. Nitrogen physisorption was performed with a Micromeritics ASAP2020Plus to characterize the specific surface area of the samples.Electrochemical measurement
All batteries were assembled in an argon glovebox with water and oxygen content below 0.2 ppm. CR2032-type cells were used to assemble symmetric cells using an electrolyte composed of 1.0 M NaClO4 in propylene carbonate (PC) with 5 vol% fluoroethylene carbonate (FEC) as an additive. Glass fiber was used as a separator. For NVP cathode preparation, NVP, carbon nanotubes (CNTs), and poly(vinyl difluoride) (PVDF) were mixed at a weight ratio of 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The formed slurry was coated uniformly on an Al foil, then the foil was dried at 90 °C under vacuum for 12 h. The mass loading of NVP is around 2.5 mg cm−2. The NVP cathode and composite anode were used to assemble half-cells with CR2025-type coin cells and tested between 2.3 and 4.0 V. The bare Na electrodes were used as the control group.
10. The formed slurry was coated uniformly on an Al foil, then the foil was dried at 90 °C under vacuum for 12 h. The mass loading of NVP is around 2.5 mg cm−2. The NVP cathode and composite anode were used to assemble half-cells with CR2025-type coin cells and tested between 2.3 and 4.0 V. The bare Na electrodes were used as the control group.
A Swagelok cell was used for optical observation. The electrodes were cut into a diameter of 12 mm for assembling the symmetric cell. The Na–Na symmetric cells were cycled at 0.5 mA cm−2 for 0.5 mA h cm−2. That is, galvanostatic charging for 1 hour and then discharging for another hour represents one cycle. In the first half-cycle (charging for 1 hour, Fig. S1†), the bulk Na on the cathode dissolves and deposits on the counter electrode (anode side). Correspondingly, in the subsequent half cycle (discharging for 1 hour, Fig. S1†), the sodium on the anode dissolves and deposits on the cathode. 2 hours' testing time represents one cycle (a complete stripping and plating process). 4 hours represents two cycles.
Results and discussion
Due to the non-uniformity of the metal electrode surface and high reactivity of Na with organic electrolyte, pure metal Na is prone to forming an unstable solid electrolyte interphase (SEI) and Na dendrites. As depicted in Scheme 1a, the low specific surface area of the pure metallic sodium foil will result in local charge accumulation and side reactions will occur on the interphase of the pure sodium anode. The uneven interphase of the bare Na anode can gradually induce the growth of Na-dendrite during the plating/stripping process, eventually leading to undesirable structures and short lifespans. | ||
| Scheme 1 (a) Plating process of the bare Na electrode. (b) Preparation of the NVP-Na hybrid electrode. (c) Plating and stripping process of the NVP-Na hybrid electrode. | ||
We designed an NVP-Na composite electrode with numerous fast ion-conducting channels, which are beneficial for rapid ion migration and low surface transfer resistance. The hybrid NVP-Na composite electrodes were prepared via a facile physical mixing of Na3V2(PO4)3 particles and sodium metal with repeated rolling and folding (Scheme 1b). Pure metallic Na tends to be soft and sticky due to the weak metal bonding force. After adding NVP particles and repeatedly rolling, the NVP-Na composite electrode gains higher hardness and mechanical strength. The final fabricated NVP-Na composite displays a blackened surface after multiple cycles of rolling due to the carbon coated NVP structure, while the pure Na metal appears to have shiny metallic luster, as shown in Fig. S2.†
During the plating process, sodium ions near the surface are captured by fast ion conductor channels and continuously transported into the interior of the electrode, preventing the accumulation of Na+ ions at the surface and reducing the electrostatic interaction between Na+ and surface irregularities (Scheme 1c). For the electrochemical dissolution reaction, the relatively high ion diffusion rate of sodium vanadium phosphate can also accelerate Na+ diffusion from the bulk phase due to the concentration gradient. After multiple cycles, the hybrid anode exhibits suppressed dendrite growth and superior fast charging capabilities. Additionally, the NVP networks within the electrode act as a structural skeleton, restraining irregular volume change and enhancing charging/discharging lifespan.
A series of characterizations were carried out for the pristine NVP sample. A typical NASICON structure of Na3V2(PO4)3 (JCPDS card no. 053-0018) without impurities was observed by X-ray diffraction (Fig. S3†). The morphology of the purchased NVP powder was analyzed by SEM. Fig. S4† reveals that NVP powders have irregular particle morphology with particle size from tens of nanometers to a few microns. N2 physisorption shows the specific surface area of NVP particles is 14.5 m2 g−1 (Fig. S5†). To further confirm the composition of NVP, the FTIR technique was applied (Fig. S6†). The sample displays typical stretching vibrations of the NVP phase, with the vibration of P–O bonds in PO4 tetrahedra at 578 and 1058 cm−1, and the vibration of V3+–O2− bonds in isolated VO6 octahedra at 630 cm−1. In addition, Raman spectra were used to analyze the structure of the surface carbon layer coated on NVP particles. As shown in Fig. S7,† the spectra exhibit two characteristic bands of carbonaceous materials at 1351 and 1583 cm−1, which are the D band (structural disorder) and G band (graphite-like structures). The peak intensity ratio of the D and G bands is 0.93. The existence of a conductive carbon layer indicates that NVP has desirable electronic conductivity.
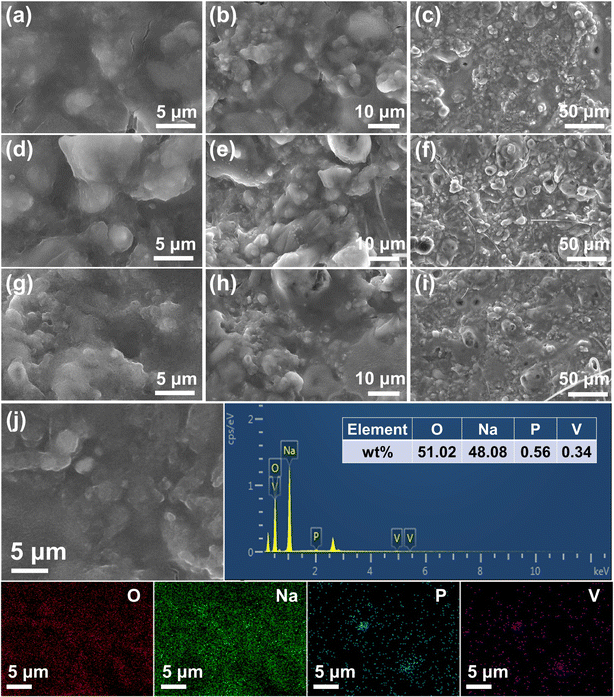
To evaluate the uniform distribution of NVP particles in hybrid electrodes, top-view and cross-sectional SEM images of the hybrid electrode are presented in Fig. S8 and S9.† The NVP/Na electrode shows a flat plane with uniformly distributed NVP particles. As shown in Fig. S9,† nano-sized NVP particles are observed to be homogeneously distributed in the bulk structure. The images of EDS mapping further confirm the uniform distribution of O, Na, P, and V elements, which reveals that NVP has been successfully incorporated into the bulk structure of the hybrid anode.
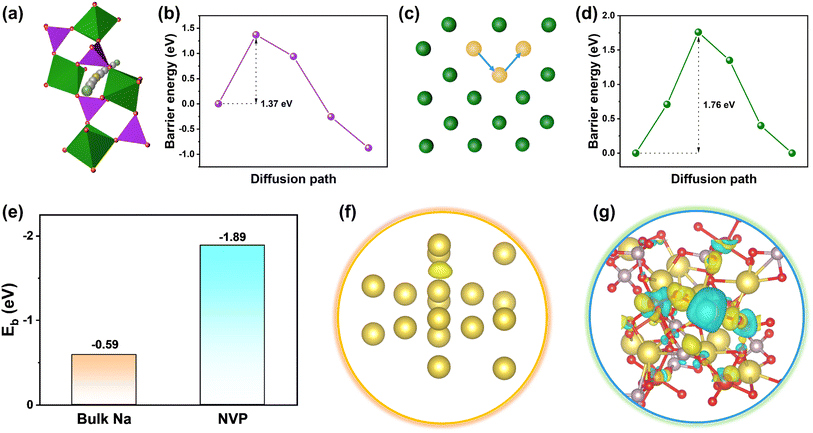
Density functional theory (DFT) calculation was employed to reveal the Na+ diffusion ability on bulk Na and NVP. As shown in Fig. 1a–d, a possible pathway in NVP was indicated based on the O–P–V bonds. It can be found that Na+ should overcome an energy barrier of 1.37 eV during the diffusion process. By contrast, a higher energy barrier of 1.76 eV was obtained in bulk Na, indicating more difficult Na+ transport. The binding energies (Eb) of Na+ on bulk Na and NVP were also compared (Fig. 1e), with −0.59 and −1.89 eV, respectively, implying that NVP has higher sodiophilicity. This was also demonstrated by the charge density difference (Fig. 1f–g), in which a weak electron exchange is indicated between Na+ and bulk Na, while a remarkable electron interaction between the Na+ and O atom of NVP can be found. These results suggest that preferential adsorption of Na+ onto NVP contributes to dispersing sodium ions uniformly at the interface, and further forming charged ion transport channels in the bulk phase.
The ion transport behavior and kinetics are crucial for the morphological evolution of a metal electrode. The cycling process of Na electrodeposition and dissolution at a current density of 0.5 mA cm−2 with capacity of 0.5 mA h cm−2 was monitored using a Swagelok cell with a visualization window to observe the real-time morphology of the Na evolution. As shown in Fig. 2a and the local enlarged images, the limited diffusion surface and sluggish Na transport kinetics of a bare Na‖Na symmetric cell cause the ions to move unevenly and aggregate. The ion aggregation makes Na-ion flux more concentrated on the tip of sodium, resulting in ever-growing dendrites with the consumption of Na metal and electrolyte. As the deposition/solution proceeds, uncontrolled destructive dendrites apparently form on the electrode surface. A large amount of inactive dead Na debris could be observed floating on the electrolyte as the reaction continues, eventually bringing about sharp deterioration of the electrochemical performance. The morphologies of the Na anode before and after cycling were further investigated by SEM characterization. A flat surface of pure Na is apparent in Fig. S10a and b,† while large numbers of Na dendrites and loose dead Na are found on the surface after 2 cycles under Swagelok cell testing conditions at 0.5 mA cm−2 for 0.5 mA h cm−2 (Fig. S10c and d†). The dendrite structures on the surface exhibit an average diameter of a few to several tens of micrometers and lengths of tens to hundreds of micrometers. The filamentary and interwoven dendrites form a loose and porous structure of the Na anode, which in turn leads to large volume changes, severe side reactions, and large interfacial impedance.
 | ||
| Fig. 2 In situ optical observation of symmetric cells with a Swagelok cell at different cycles and the representing schematic diagrams. (a) Na‖Na symmetric cell, (b) NNVP20‖NNVP20 symmetric cell. | ||
In comparison, the NNVP20‖NNVP20 symmetric cell cycled under the same conditions maintains a stable structure and flat surface without dendrites after multiple cycles, indicating salient dendrite suppression ability (Fig. 2b and the enlarged images). In addition, some pores are observed within the bulk of the NNVP20 electrode during the cycling process, which might be related to the rapid ion transport properties of NVP conductive channels. During the plating/stripping process, the deposition/dissolution process of Na+ ions is not restrained on the two-dimensional surface of the electrode, but ions within the bulk can also undergo rapid movement due to the large number of fast ionic conducting channels in the bulk electrode. Sodium ions preferentially transport through the sodiated Na3+xV2(PO4)3 network with a low overpotential rather than breaking the highly resistive SEI interphase on the surface of Na metal. These interconnected channels provide abundant routes for ions to enter and exit the electrode, homogenizing the ion flux and enhancing the ion transport kinetics. After multiple cycles, the pores become larger and interconnected, endowing fast and continuous plating/stripping ability in three-dimensional space. That is, Na+ can be deposited into the pores rather than only on the surface of the electrode. The composite electrode with holes can also act as a skeleton and restrain the irregular volume change, which helps in reducing the volume fluctuation of the electrode. As expected, the hybrid anode exhibits suppressed dendrite growth and flat surface evolution.
To further validate the effect of ionic transport channels, optical real-time observation of hybrid electrodes with different thicknesses (∼80 μm and ∼260 μm) was conducted. As shown in Fig. S11,† both electrodes show fewer dendrites than the bare Na electrode after different cycles. The thin (Fig. S11b†) and thick (Fig. S11c†) hybrid Na electrodes show a stable bulk structure with no whisker-like dendrite formation after 4 hours (2 cycles) and 5 hours (2.5 cycles). Even after cycling for 10 hours (5 cycles), the integrity of electrodes remains intact, accompanied by smaller volume changes and a small amount of dendrite generation. In sharp contrast, the bare Na‖Na symmetric cell presents a significant amount of filamentary Na dendrites as well as large amounts of dead Na floating above the solution. There is almost no dead Na floating above the electrolyte for the hybrid electrode. These results indicate that the dendrite behavior cannot been completely avoided, but can be significantly suppressed for the modified Na anode due to the fast ion diffusion kinetics of the NVP matrix and high affinity between NVP and Na.
SEM was further applied to analyze the Na electrodeposition morphology. As shown in Fig. 3a–c, the surface of the NNVP20 electrode remains relatively flat without obvious whiskers or dendrites after 50 cycles at 0.5 mA cm−2 for 1 mA h cm−2. Some NVP particles can be observed to distribute uniformly on the surface of the Na electrode. The element mapping of the NNVP20 electrode after 50 cycles (Fig. 3j) shows a homogeneous distribution of Na, V and P elements, confirming the existence of NVP in the anode and that the NVP skeleton won't collapse during the cycles. After 100 and 200 cycles, a relatively flat and smooth external surface and dendrite-free morphology can still be observed in Fig. 3d–i. Only a few bumps and pores appear due to the plating and stripping cycles. These results demonstrate that the high sodiophilicity and fast Na+ transport kinetics enabled by the superionic conductor facilitate sodium ion ingress and uniform dispersion in the anode, mitigating the volume fluctuation and Na-dendrite formation.
Consequently, both optical microscopy and SEM characterization confirmed that constructing sodiophilic interconnected ion-transport pathways can suppress the occurrence of sodium dendrites.
To measure the cycling stability of Na metal electrodes, galvanostatic charging/discharging tests of symmetric sodium cells were conducted at various current densities and area capacities. Fig. 4a and c compare the electrochemical performance of Na and NVP-Na cells at current densities of 0.5 and 1.0 mA cm−2 with capacity limitations of 0.5 and 1.0 mA h cm−2. The cycling stability of the composite electrodes with 5, 10, 20, and 50 wt% NVP was compared to illustrate the effect of NVP content. As shown in Fig. 4a–d, the symmetric cells of the NVP-Na composite display a progressively prolonged cycle life and gradually decreased polarization with increasing NVP content. This can be explained by saying that a larger amount of NVP could contribute more to the transport pathway, resulting in lower transport energy barriers and less polarization at the surface and within the bulk. However, excessive inorganic particles imply decreased substrate viscosity and weakened structural stability. It is worth noting that the NNVP50 anode exhibits very limited reduction in voltage hysteresis compared with that of the NNVP20‖NNVP20 electrode. Therefore, there is a possibly of an optimum amount of superionic conductor addition. Besides, excessive NVP particles will make the structure of the metal anode less stable, leading to a short lifespan in the plating/stripping cycles. In contrast, the bare Na‖Na cell shows large voltage hysteresis and is shorted after dozens of cycles, while the voltage hysteresis of NVP-Na symmetric cells is smaller and remains almost unchanged for more than 500 hours, indicating improved deposition/dissolution reversibility (Fig. 4a–d). The low overpotential and smooth voltage profiles could be ascribed to the deposition affinity of Na+ ions and fast ion transport kinetics. The long cycle life further confirms that the suppressed dendrite growth enhances the reversibility of plating and stripping for the Na anode.
When applying a longer galvanostatic time of 4 hours at 0.5 mA cm−2 (Fig. 4e and f), the lifespan of Na stripping/plating in the NNVP20‖NNVP20 cell increases to 1000 hours with increased overpotential from 40.4 to 134.2 mV, which may be caused by the unstable and progressively thickened Na/electrolyte interphase layer due to the direct contact of metallic sodium and electrolyte.35,36 The bare Na‖Na cell short-circuits quickly under the same test conditions. The enlarged curves for both cells also reveal a low nucleation barrier and overpotential (Fig. 4f). At a higher current density of 2 mA cm−2, the NNVP20‖NNVP20 symmetric cell still maintains a long cycle lifespan (Fig. 4g), indicating the excellent fast charging ability.
To reveal the interfacial stability in the symmetrical cell, electrochemical impedance spectroscopy (EIS) was also performed to further elucidate the enhanced ion transport kinetics of the composite electrode. Fig. 4h and i present the Nyquist plots of bare Na and NNVP20 symmetric cells at selected cycles. Before cycling, both Na and NNVP20 electrodes show large resistance due to the spontaneously generated thick SEI layer. After 10 and 50 cycles, the semi-circle for the bare Na electrode was bigger than that of the NNVP20 composite electrode, implying that the interfacial charge transfer impedance of the bare Na is larger than that of the NNVP20 electrode. The large impedance of the bare Na anode suggests non-uniform sodium deposition and the formation of dead Na. The semi-circle of the NNVP20 electrode after 10 cycles was smaller than that after 50 cycles, while the opposite was observed for the bare Na electrode. This indicates that the NNVP20 electrode has a shorter activation process than the bare Na electrode. The noticeably lower impedance of the NNVP20 composite electrode indicates that Na can form a relatively stable interphase and deposit uniformly.
Furthermore, when the NNVP20 symmetrical cell was cycled stepwise from 0.5 to 8.0 mA cm−2 with a constant capacity of 1.0 mA h cm−2, the electrode exhibits stable voltage curves with low overpotentials. Thus, the interfacial stability of NNVP20 could be well maintained. By contrast, the Na‖Na cell has a significantly increased polarization overpotential with an obvious nucleation and electro-dissolution barrier (Fig. 4j). The Na+ transport in three-dimensional space of the composite electrode is beneficial for continuous plating/stripping while avoiding Na+ aggregation on the surface. The stable deposition behavior with high reversibility endows the sodium anode with exceptional fast charge ability and long cycling lifespan.
The phase structure and composition of the hybrid anode before and after cycles were characterized by XRD and SEM, respectively. As shown in Fig. S12,† the diffraction peaks at 28.7°, 32.1° and 52.8° correspond to the NASICON structured NVP. The phase structure of NVP remains intact when rolled into the Na bulk and after 25 cycles. Moreover, the uniform distribution of P and V elements is observed in the EDS mapping both before and after cycling. The element ratio of V and P remains unchanged (pre-cycle: 0.62, Fig. S13; † post-cycle: 0.61, Fig. 3j). These results reveal that the structure and composition of NVP particles are not changed after cycling.
To gain insight into the beneficial effect of the bulk ion transfer pathways, we measured voltage–time curves of the composite electrodes with surface exposed NVP and embedded NVP. As depicted in Fig. 5, we fabricated a Na electrode with NVP spreading on the Na metal surface (SNVP). Next, galvanostatic plating/stripping tests of three symmetric cells with bare Na, SNVP and NNVP20 anodes were carried out under 1 mA h cm−2 at 0.5 mA cm−2. As shown in Fig. 5e, the surface-dispersed ion diffusion channels could facilitate uniform ion distribution and accelerate interfacial Na+ ion transport. As expected, the SNVP‖SNVP symmetric cell shows a lower overpotential than that of the bare Na‖Na cell, indicating some beneficial effect of the NVP powder on the surface. Moreover, the NNVP20‖NNVP20 symmetric cell with interconnected ion transport channels presents the lowest voltage hysteresis and flat plating/stripping profiles (Fig. 5b and c), demonstrating stable Na+ ion removal and re-entry processes. For the bare Na electrode, Na+ needs to break the resistive SEI layer and strips at a vulnerable place during deposition and dissolution, which requires a high pit-formation energy. The limited diffusion surface and sluggish Na transport kinetics cause sodium ions to move unevenly and aggregate at hump-sites, eventually leading to dendrites and dead Na (Fig. 5d). In contrast, the NNVP20 composite electrode promotes fast interfacial and internal mass transfer, enabling low interfacial impedance and fast internal ion transport kinetics (Fig. 5d). Naturally, the NNVP20 composite could achieve uniform Na-ion deposition and excellent electrochemical performances.
The size effect of NVP particles on electrochemical performances was further investigated. A micron-sized NVP cathode was synthesized via a solution method.37 The crystal structure of the synthesized micron-sized NVP is shown in Fig. S14,† which exhibits high crystallinity that can be ascribed to the typical NASICON structure (JCPDS card no. 053-0018). The morphology of NVP particles is presented in Fig. S15,† and irregular micron-sized grains with abundant porous structures are observed. As shown in Fig. S16,† the polarization of the micron-sized NVP-Na symmetric cell is larger than that of the nano-sized NVP-Na electrode, indicating that the particle size of NVP has an effect on the electrochemical performance of the symmetric cell. As expected, large NVP particles are not as uniformly distributed in the bulk sodium as nanoparticles, which affects the continuity of the ion conducting networks.
To illustrate Na-ion transport kinetics, linear sweep voltammetry (LSV) and galvanostatic intermittent titration technique (GITT) measurements were conducted. Fig. 6a and b show that the GITT curves of the NNVP20 symmetric cell have obviously lower overpotential compared with those of the bare Na symmetric cell, demonstrating fast mass transfer in the composite electrode. The large spikes at the beginning of each cycle for the Na‖Na cell imply a prominent Na nucleation overpotential, and the increased polarization after a temporary platform is ascribed to the electro-dissolution of bulk Na during stripping. In contrast, the NNVP20 symmetric cell shows almost no nucleation and bulk dissolution overpotential (Fig. 6b). The stable stripping and plating curves indicate a low energy barrier for both nucleation and plating/stripping of the composite electrode. The Tafel plots and the corresponding exchange current density are exhibited in Fig. 6c. The exchange current density of the composite electrode (0.078 mA cm−2) was clearly higher than that of the bare Na electrode (0.009 mA cm−2), again indicating its fast mass transfer and Na+ deposition/dissolution kinetics (Fig. 6d).
The NNVP20 anode was paired with an NVP cathode to construct a half-cell to further explore the plating/stripping performance of the composite anode (Fig. 7a). As shown in Fig. 7c, the half-cells with NNVP20 and bare Na foil as anodes and NVP as cathodes display a similar discharge capacity at the low current density of 0.2C (98.4 mA h g−1). However, at increasing current densities, the cell with the NNVP20 anode displays superior electrochemical stability and high rate capability. It shows a specific capacity of 83.4 mA h g−1 when the current density increases to 5C and a high capacity retention of 87.8% with an average coulombic efficiency (CE) of 99.8% after 1000 cycles (Fig. 7b), showing excellent long-term cycling performance and fast charging capability. In contrast, the half-cell with bare Na foil as an anode only shows a capacity of 76.7 mA h g−1 at 5C due to the unstable Na metal anode. In addition, the specific capacity was 57.3 mA h g−1 even at a high current rate of 50C for the cell with the NNVP20 anode, much higher than that of the cell with the bare Na anode (3.5 mA h g−1). At 30C, the capacity of the half-cell with bare Na as the anode deteriorates severely, while the half-cell with the NVP/Na anode retains a specific capacity of 72.5 mA h g−1 with almost no capacity degradation after 100 cycles (Fig. S17a†). At a higher current rate of 50C, the cell with the NVP/Na anode still exhibits stable capacity retention over 100 cycles (Fig. S17b†), while the specific capacity of the bare Na cell is almost 0 mA h g−1 due to the large polarization at 50C (Fig. 7c). The charging/discharging curves at a current density of 10C are compared in Fig. 7d. A low polarization (140.6 mV) is observed for the NVP/NNVP20 cell, while the NVP/Na cell shows a much higher potential difference of 243.9 mV. The high specific capacity and outstanding rate behavior of the cell with the NNVP20 composite anode further demonstrate the positive influence by constructing fast ion transport channels in the electrode.
Conclusion
We have fabricated a composite NVP-Na metal anode with interconnected ion conductive networks via a facile and scalable rolling and folding method. By controlling the mixing ratios of NVP, the NNVP20 composite shows the lowest overpotential and longest cycle lifespan. Fast sodium deposition/solution kinetics are enabled by the homogenized interfacial ion distribution and fast internal Na+ ion diffusion. This composite anode displays a stable plating morphology and low transport barriers. Consequently, the NVP-Na composite anode exhibits long cycle lifespan (over 1000 h at 0.5 mA cm−2, 2 mA h cm−2) and superior cycle stability with low overpotential (62.8 mV at 0.5 mA cm−2, 1 mA h cm−2). Full cells with the NVP-Na composite anode also show an improved rate performance (57.3 mA h g−1 at 50C) and excellent capacity retention (87.8%) after 1000 cycles, surpassing those of the bare Na anode. The facile fabrication process in this work is easily applicable for the modification of other metal anodes, enabling its potential application in high specific energy batteries.Data availability
Data are available from the authors upon reasonable request.Author contributions
G. M. proposed the idea. G. M. and D. Y. conceived and designed the experiments. L. S. conducted theoretical calculations to explain the fast ion diffusion ability. D. Y., Z.-Y. W., L.-T. C, Y.-J. D., and W.-Y. Q. carried out the experiments. All authors participated in the analysis of the experimental results and discussion of the manuscript. D. Y., G. M., L. S. and Y.-Z. X. participated in preparing the paper.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LQ23B030005) and National Natural Science Foundation of China (No. 22208220).References
- B. Dunn, H. Kamath and J. M. Tarascon, Science, 2011, 334, 928–935 CrossRef CAS.
- H.-W. Lee, R. Y. Wang, M. Pasta, S. Woo Lee, N. Liu and Y. Cui, Nat. Commun., 2014, 5, 5280 CrossRef CAS PubMed.
- R. Carter, L. Oakes, A. Douglas, N. Muralidharan, A. P. Cohn and C. L. Pint, Nano Lett., 2017, 17, 1863–1869 CrossRef CAS PubMed.
- J. Sun, H. W. Lee, M. Pasta, H. Yuan, G. Zheng, Y. Sun, Y. Li and Y. Cui, Nat. Nanotechnol., 2015, 10, 980–985 CrossRef CAS.
- J. Wang, G. Li, Q. Wang, L. Huang, X. Gan, M. Li and Z. Song, Energy Storage Mater., 2023, 63, 102956 CrossRef.
- S. Li, W. He, B. Liu, J. Cui, X. Wang, D.-L. Peng, B. Liu and B. Qu, Energy Storage Mater., 2020, 25, 636–643 CrossRef.
- B. Sun, P. Li, J. Zhang, D. Wang, P. Munroe, C. Wang, P. H. L. Notten and G. Wang, Adv. Mater., 2018, 30, e1801334 CrossRef PubMed.
- B. Sayahpour, W. Li, S. Bai, B. Lu, B. Han, Y.-T. Chen, G. Deysher, S. Parab, P. Ridley, G. Raghavendran, L. H. B. Nguyen, M. Zhang and Y. S. Meng, Energy Environ. Sci., 2024, 17, 1216–1228 RSC.
- S. S. Chi, X. G. Qi, Y. S. Hu and L. Z. Fan, Adv. Energy Mater., 2018, 8, 1702764 CrossRef.
- B. Lee, E. Paek, D. Mitlin and S. W. Lee, Chem. Rev., 2019, 119, 5416–5460 CrossRef CAS PubMed.
- Q. Lu, A. Omar, L. Ding, S. Oswald, M. Hantusch, L. Giebeler, K. Nielsch and D. Mikhailova, J. Mater. Chem. A, 2021, 9, 9038–9047 RSC.
- P. Xu, X. Li, M.-Y. Yan, H.-B. Ni, H.-H. Huang, X.-D. Lin, X.-Y. Liu, J.-M. Fan, M.-S. Zheng, R.-M. Yuan and Q.-F. Dong, J. Mater. Chem. A, 2021, 9, 22892–22900 RSC.
- C. Song, J. Zhao, S. Ma and G. Li, ACS Energy Lett., 2023, 8, 3404–3411 CrossRef CAS.
- L. Li, M. Zhu, G. Wang, F. Yu, L. Wen, H.-K. Liu, S.-X. Dou and C. Wu, J. Energy Chem., 2022, 71, 595–603 CrossRef CAS.
- X.-B. Cheng, C. Yan, X.-Q. Zhang, H. Liu and Q. Zhang, ACS Energy Lett., 2018, 3, 1564–1570 CrossRef CAS.
- H. Yu, D. Jiang, X. Cheng, P. Lu, S. Li, H. Zhang, Y. Jiang and F. Huang, Adv. Funct. Mater., 2023, 33, 2307628 CrossRef CAS.
- X. Xu, S. Li, Z. Cao, S. Yang and B. Li, Adv. Energy Mater., 2024, 14, 2303971 CrossRef CAS.
- J. Duan, W. Wu, A. M. Nolan, T. Wang, J. Wen, C. Hu, Y. Mo, W. Luo and Y. Huang, Adv. Mater., 2019, 31, e1807243 CrossRef PubMed.
- X. Zheng, W. Yang, Z. Wang, L. Huang, S. Geng, J. Wen, W. Luo and Y. Huang, Nano Energy, 2020, 69, 104387 CrossRef CAS.
- W. Zhao, M. Guo, Z. Zuo, X. Zhao, H. Dou, Y. Zhang, S. Li, Z. Wu, Y. Shi, Z. Ma and X. Yang, Engineering, 2022, 11, 87–94 CrossRef CAS.
- Z. Sun, Y. Ye, J. Zhu, E. Zhou, J. Xu, M. Liu, X. Kong, S. Jin and H. Ji, Small, 2022, 18, e2107199 CrossRef PubMed.
- Y. Liu, X. Liu, G. Zhang, X. Shi, P. Zhang, Y. Fan, Y. Huang and R. Zhang, ACS Appl. Mater. Interfaces, 2023, 15, 26691–26699 CrossRef CAS.
- Y.-J. Kim, J. Lee, S. Yuk, H. Noh, H. Chu, H. Kwack, S. Kim, M.-H. Ryou and H.-T. Kim, J. Power Sources, 2019, 438, 227005 CrossRef CAS.
- J. Liang, W. Wu, L. Xu and X. Wu, Carbon, 2021, 176, 219–227 CrossRef CAS.
- D. Zhang, B. Li, S. Wang and S. Yang, ACS Appl. Mater. Interfaces, 2017, 9, 40265–40272 CrossRef CAS.
- C. Luo, H. Wang, Y. Qian, X. Shi, Z. Mao, G. Li, C. Yang, Y. Gong, A. Tang and H. Yang, J. Power Sources, 2022, 548, 232038 CrossRef CAS.
- H. Ma, B. Zhao, J. Bai, P. Wang, W. Li, Y. Mao, X. Zhu, Z. Sheng, X. Zhu and Y. Sun, Adv. Sci., 2023, 10, e2203552 CrossRef.
- W. J. Li, C. Han, W. Wang, F. Gebert, S. L. Chou, H. K. Liu, X. Zhang and S. X. Dou, Adv. Energy Mater., 2017, 7, 1700274 CrossRef.
- M. Guo, H. Dou, W. Zhao, X. Zhao, B. Wan, J. Wang, Y. Yan, X. Wang, Z.-F. Ma and X. Yang, Nano Energy, 2020, 70, 104479 CrossRef CAS.
- L. Fu, X. Wang, B. Zhang, Z. Chen, Y. Li and Y. Sun, Nano Res., 2024, 17, 4031–4038 CrossRef CAS.
- B. Xu and F. Guo, Int. J. Solids Struct., 2021, 216, 222–230 CrossRef CAS.
- T. Ortmann, S. Burkhardt, J. K. Eckhardt, T. Fuchs, Z. Ding, J. Sann, M. Rohnke, Q. Ma, F. Tietz, D. Fattakhova-Rohlfing, C. Kübel, O. Guillon, C. Heiliger and J. Janek, Adv. Energy Mater., 2022, 13, 2202712 CrossRef.
- H. Duan, Y. You, G. Wang, X. Ou, J. Wen, Q. Huang, P. Lyu, Y. Liang, Q. Li, J. Huang, Y. X. Wang, H. K. Liu, S. X. Dou and W. H. Lai, Nano–Micro Lett., 2024, 16, 78 CrossRef CAS.
- Z. Wang, Z. Mu, T. Ma, W. Yan, D. Wu, Y. Li, M. Yang, J. Peng, Y. Xia, S. Shi, L. Chen, H. Li and F. Wu, Adv. Energy Mater., 2024, 14, 2400003 CrossRef CAS.
- Y. Wang, H. Dong, N. Katyal, H. Hao, P. Liu, H. Celio, G. Henkelman, J. Watt and D. Mitlin, Adv. Mater., 2022, 34, e2106005 CrossRef.
- Y. Ye, Y. Zhao, T. Zhao, S. Xu, Z. Xu, J. Qian, L. Wang, Y. Xing, L. Wei, Y. Li, J. Wang, L. Li, F. Wu and R. Chen, Adv. Mater., 2021, 33, e2105029 CrossRef PubMed.
- M. Guo, Y. Zhang, S. Qi, T. Liu, J. Ying, Y. Wang, Y. Chai, G. Gao and Z. Yu, Energy Fuel., 2023, 37, 17575–17584 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta03489a |
| This journal is © The Royal Society of Chemistry 2024 |