Iron single-atom anchored N-doped carbon: an efficient catalyst for one-pot, scaled-up pentazolate synthesis†
Pengbo
Wang
,
Xiaopeng
Zhang
,
Shuaijie
Jiang
 ,
Zheting
Dong
,
Ruyi
Lu
,
Yuangang
Xu
,
Zheting
Dong
,
Ruyi
Lu
,
Yuangang
Xu
 ,
Pengcheng
Wang
,
Pengcheng
Wang
 * and
Guo-Ping
Lu
* and
Guo-Ping
Lu
 *
*
School of Chemistry and Chemical Engineering, Nanjing University of Science & Technology, Xiaolingwei 200, Nanjing 210094, P. R. China. E-mail: glu@njust.edu.cn; alexwpch@njust.edu.cn
First published on 16th June 2024
Abstract
The discovery of ambient stable pentazolate salts has paved way for a new frontier in the chemistry of pentazoles, which represent a distinctive category of energetic materials. One challenge impeding their practical applications lies in achieving the scale-up of pentazolate synthesis via a facile and low-cost route. An efficient and recyclable iron single-atom catalyst with FeN4 sites (Fe1@NC-700) has been first developed using a pyrolysis-milling strategy for scaled-up pentazolate synthesis and achieves significant yields (38.4 g, 15% yield for NaN5; 77.6 g, 26% yield for CoN5) via a three-step, one-pot process with a low iron dosage (0.67 mol%). According to experimental and theoretical calculation results, the exceptional catalytic performance of Fe1@NC-700 can be attributed to its stable FeN4 enzyme-like catalytic active sites, which facilitate the formation of a Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate, and it maintains its catalytic activity even under weakly acidic conditions and after five cycles.
O intermediate, and it maintains its catalytic activity even under weakly acidic conditions and after five cycles.
Introduction
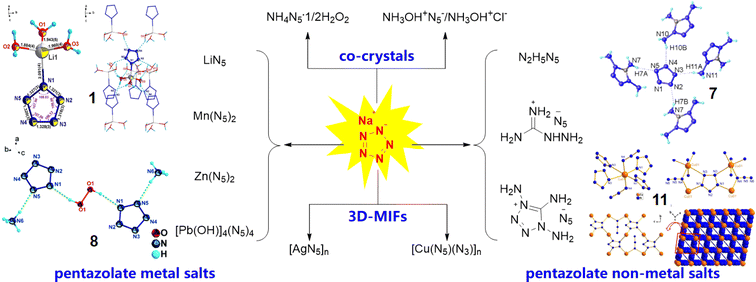
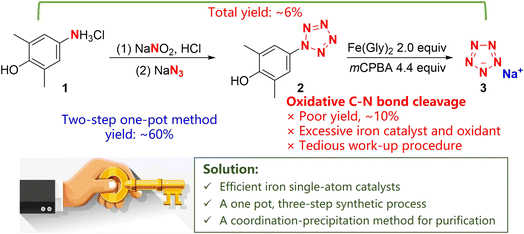
In 2017, some of us first synthesized ambient stable pentazolate (cyclo-N5−) salts, which have attracted great interest in the field of high-energy-density materials (HEDMs) owing to their potential high energy and unique energy release mode.1 In the last five years, sodium pentazolate [NaN5(H2O)]·2H2O (NaN5) has been used as a raw material for the synthesis of various cyclo-N5− complexes, including pentazolate metallic and non-metallic salts, co-crystals and 3D metal-inorganic frameworks, via metathesis reactions (Fig. 1).2 Furthermore, cyclo-N5−, as a rare and preferred ligand for building topology architectures, provides new possibilities in coordination chemistry.2i Therefore, the use of NaN5 as a basic raw material for all N5− based energetic materials is a fundamental of pentazolate chemistry.At present, NaN53 is synthesized via the C–N bond cleavage of arylpentazole 2, which is produced from arylamine hydrochloride (2,6-dimethyl-4-aminophenol hydrochloride, 1) through a diazotization and cyclization reaction (Scheme 1). The yield of arylpentazole 2 is about 60%, while the yield of the C–N bond cleavage step is poor (∼10%); thus, the total yield is only ∼6% with respect to arylamine hydrochloride 1. In the C–N bond cleavage step, a tedious work-up procedure and excessive iron catalyst and oxidant usage are the norm, thereby making this approach unsuitable for large-scale synthesis.1a Hence, it is appealing and desirable to improve the synthetic process of NaN5 for the achievement of its efficient scaled-up synthesis.
Hang's group revealed the mechanism of the oxidative cleavage of the C–N bond of 2: Fe(Gly)2 reacts with mCPBA to form a Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate, which can realize the oxidative C–N bond cleavage of 2 to form NaN5. DFT calculations indicate that the Fe(IV)
O intermediate, which can realize the oxidative C–N bond cleavage of 2 to form NaN5. DFT calculations indicate that the Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate plays a crucial role in the reaction, since it greatly reduces the reaction activation energy.3 It is well known that some iron-based enzymes with a FeN4 structure (such as cytochrome P450 or hemin) can activate oxygen to form Fe(IV)
O intermediate plays a crucial role in the reaction, since it greatly reduces the reaction activation energy.3 It is well known that some iron-based enzymes with a FeN4 structure (such as cytochrome P450 or hemin) can activate oxygen to form Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species for the oxidation of organic substrates under mild conditions.4 According to the bionic theory, a series of N-doped carbon-supported iron single-atom catalysts with enzyme-like FeNx sites have been developed for selective oxidation reactions.5 Hence, an N-doped carbon-supported iron single-atom catalyst with FeNx sites could exhibit good performance in the C–N bond cleavage of 2 for the effective preparation of NaN5.
O species for the oxidation of organic substrates under mild conditions.4 According to the bionic theory, a series of N-doped carbon-supported iron single-atom catalysts with enzyme-like FeNx sites have been developed for selective oxidation reactions.5 Hence, an N-doped carbon-supported iron single-atom catalyst with FeNx sites could exhibit good performance in the C–N bond cleavage of 2 for the effective preparation of NaN5.
In addition, the unstable compound 2 must be purified using acetone washing at −60 °C in the original preparation process, which greatly reduces the yield of 2 owing to the decomposition and dissolution of 2 in acetone. The separation and purification of NaN5 requires column chromatography, which is costly and inefficient, and makes the process difficult to scale up. According to these results, we propose three strategies for the upgrading of this synthetic process: (1) an iron single-atom catalyst with highly active FeNx sites could be applied instead of Fe(Gly)2 for the selective oxidative cleavage of the C–N bond to improve the activity of Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. (2) The synthesis of 3 could be directly completed via a one-pot, three-step method, which would avoid the decomposition of 2 during the work-up process. (3) Pentazolate ions could be converted into cobalt pentazolate precipitates (CoN5) via a coordination-precipitation strategy, which can be separated by simple filtration and transformed into other pentazolate salts through metathesis reactions.6
O. (2) The synthesis of 3 could be directly completed via a one-pot, three-step method, which would avoid the decomposition of 2 during the work-up process. (3) Pentazolate ions could be converted into cobalt pentazolate precipitates (CoN5) via a coordination-precipitation strategy, which can be separated by simple filtration and transformed into other pentazolate salts through metathesis reactions.6
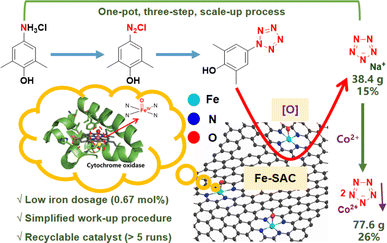
Along this line, we have developed a pyrolysis-milling approach for the synthesis of N-doped carbon-supported iron single-atom catalysts (Fe1@NC-x), which display much better performance than Fe(Gly)2 and hemin owing to their high activity and the stability of enzyme-like FeN4 single-atom sites. These materials can also achieve a one-pot, three-step process for the scaled-up synthesis of NaN5 (38.4 g) and CoN5 (77.6 g), providing several advantages, including a much lower dosage (0.67 mol%) of the recyclable iron catalyst (>5 runs), a simplified work-up procedure and higher pentazolate yields (Fig. 2). To the best of our knowledge, this is the first example of single-atom catalysis for scaled-up pentazolate synthesis.
Results and discussion
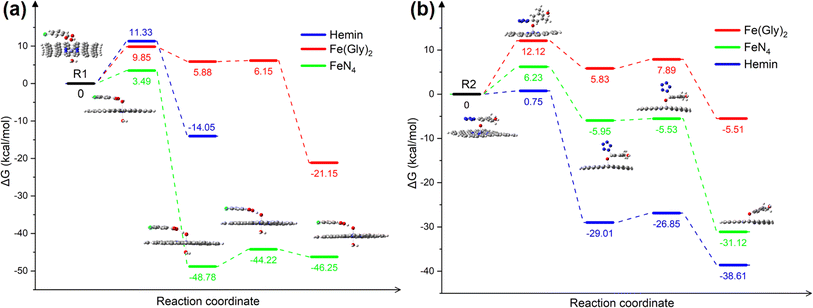
An iron single-atom catalyst supported on N-doped carbon material was prepared using a pyrolysis-milling strategy (Fig. 3a). A mixture of melamine and carbon black was calcined at x °C (x = 700, 800 and 900) under a nitrogen atmosphere to prepare a nitrogen-doped carbon material (NC-x). Then, Fe1@NC-x was prepared by ball-grinding NC-x with FeSO4·7H2O. As shown in Fig. 3b–d, no iron nanoparticles are observed for any Fe1@NC-x catalysts. There are only two broad peaks at 25° and 43°, which can be attributed to the (002) and (100) crystal planes of graphitic carbon, respectively (Fig. S1†).7 These results suggest that no highly crystalline Fe-containing species exist in these Fe1@NC-x catalysts.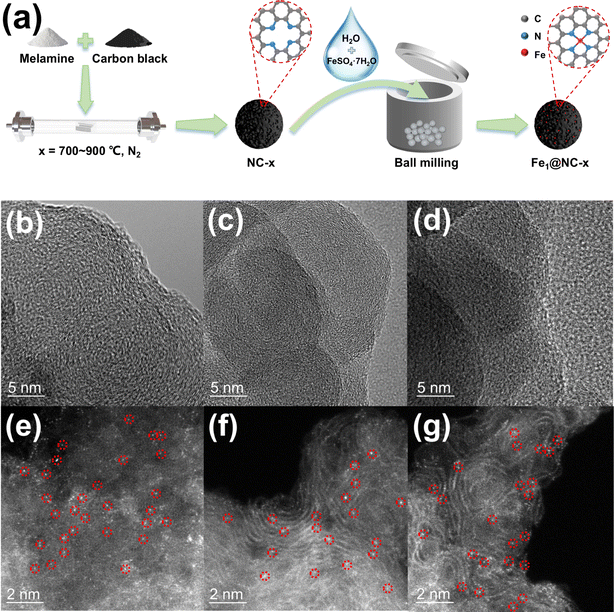 | ||
| Fig. 3 (a) Synthesis route of Fe1@NC-x; HRTEM images of Fe1@NC-700 (b), Fe1@NC-800 (c), Fe1@NC-900 (d); AC-HAADF-STEM images of Fe1@NC-700 (e), Fe1@NC-800 (f), and Fe1@NC-900 (g). | ||
According to Raman spectra (Fig. S2†), the D (defect) and G (graphite) bands of Fe1@NC-x were observed at about 1336 cm−1 and 1582 cm−1, respectively. The ID/IG value of Fe1@NC-700 is higher than that of the other two catalysts, indicating that Fe1@NC-700 has the most defects.8 The Fe contents of Fe1@NC-700, Fe1@NC-800 and Fe1@NC-900 are 0.82 wt%, 0.73 wt% and 0.65 wt%, respectively (Table S1†). The BET surface area and aperture of the Fe1@NC-x catalysts are shown in Fig. S3† and Table S2.† Fe1@NC-700 has the highest BET surface area (68.41 m2 g−1) and largest average aperture (38.56 nm) among these catalysts, which is consistent with Raman results.9
The surface chemical states of these catalysts were measured using X-ray photoelectron spectroscopy (XPS). The N 1s spectrum of Fe1@NC-x could be deconvoluted into four peaks centered at binding energies of 398.4, 399.4, 400.5 and 403.2 eV, which were assigned to pyridinic N, Fe–N, pyrrole N and N-oxide, respectively (Fig. S4†).10 The N content of the three catalysts was calculated by semi-quantitative analysis of XPS results (Table S3†). Among the three catalysts, Fe1@NC-700 has the highest N content, which further explains the reason for its high defect degree and corresponds to Raman and BET characterization data.11 The defects on the carbon material are of great significance for capturing N species, which can anchor more iron species to generate FeNx sites. Moreover, defects enlarge the surface area of the catalysts, which is beneficial to expose more active sites and thus increase interaction between the catalyst and reactive species. Furthermore, Fe1@NC-700 has the highest Fe–N content, which has a positive correlation with the number of active Fe sites, further elucidating the reason for its high catalytic activity.12
Aberration-corrected high annular angle dark-field scanning TEM (AC-HAADF-STEM) and X-ray absorption fine structure (XAFS) spectroscopy were also employed to study the Fe configurations of Fe@NC-x catalysts at the atomic level. As shown in Fig. 3e–g, isolated bright spots (marked by red circles) show good dispersion in these catalysts, proving that iron species exist in these catalysts as single atomic iron sites.
The energy absorption edge of Fe1@NC-700 lies between the energy absorption boundaries of Fe foil and Fe2O3, indicating that the Fe valence state is between 0 and +3 (Fig. 4a).13 Fourier-transformed k3-weighted extended X-ray absorption fine structure (FT-EXAFS) spectra are shown in Fig. 4b. Fe1@NC-700 exhibits only a main peak at 1.6 Å, which is attributed to the Fe–N bond, further confirming that the Fe species of Fe1@NC-700 mainly exist in the form of single Fe atom sites.14 According to the results of fitting using the program FEFF6, the coordination number of the single Fe atom is about 3.9 (Table S4†), and the fitting curve of the FeN4 sites is basically consistent with this catalyst curve (Fig. 4c); thus, iron single-atom sites should mainly be FeN4 coordination structures.15
 | ||
| Fig. 4 (a and b) XANES and FT EXAFS spectra at the Fe K-edge of Fe1@NC-700, Fe foil, FePc and Fe2O3. (c) Corresponding fitting of the EXAFS spectrum of Fe1@NC-700 in R space. | ||
With these iron catalysts in hand, their catalytic activities were studied in a one-pot three-step process for the synthesis of NaN5 (Table 1), whose structure was confirmed using MS, IR and ion chromatography (Fig. S5–S7 and S11a†). Fe(Gly)2 could not achieve this three-step, one-pot process (entry 1), since its Fe ions would dissociate from the ligand under acidic conditions. Hemin, with its stable FeN4 sites, could achieve the selective oxidation and cleavage of C–N bonds to synthesize NaN5 under acidic conditions, with a yield of 10%. When FeCl2 was used as the catalyst, no desired product was obtained, indicating that the Fe ions without N coordination have no catalytic activity for the reaction (entry 3). The yields of Fe1@NC-x are much higher than those of Fe(Gly)2, hemin and FeCl2 (entries 1–6), since their Fe sites are anchored into NC to form stable FeN4 single-atom sites, which exhibit excellent catalytic oxidation performance.16 In addition to the FeN4 sites, the porous structure of Fe1@NC-700 promotes the reaction to a certain extent, because it is beneficial for the interaction between reactants (or intermediates) and catalysts.
| Entry | Catalyst | Catalyst (g) | mCPBAb (g) | Yieldc (%) |
|---|---|---|---|---|
| a Reaction conditions: step 1: 87 mmol 2,6-dimethyl-4-aminophenol hydrochloride (15.1 g), 96 mmol HCl, 90 mmol NaNO2, THF, −5 °C, 0.5 h; step 2: 90 mmol NaN3, MeOH, −40 °C, 2 h; step 3: catalyst, mCPBA, MeCN, −15 °C, 12 h. b m-Chloroperbenzoic acid. c Yields were calculated based on arylamine hydrochloride 1. | ||||
| 1 | Fe(Gly)2 | 42 | 33 | 0 |
| 2 | Hemin | 56 | 33 | 10 |
| 3 | FeCl2 | 4 | 33 | 0 |
| 4 | Fe1@NC-800 | 4 | 33 | 16 |
| 5 | Fe1@NC-900 | 4 | 33 | 14 |
| 6 | Fe1@NC-700 | 4 | 33 | 18 |
| 7 | Fe1@NC-700 | 2 | 33 | 13 |
| 8 | Fe1@NC-700 | 3 | 33 | 16 |
| 9 | Fe1@NC-700 | 5 | 33 | 18 |
| 10 | Fe1@NC-700 | 4 | 16.5 | 10 |
| 11 | Fe1@NC-700 | 4 | 24.8 | 16 |
| 12 | Fe1@NC-700 | 4 | 41.3 | 18 |
| 13 | Fe1@NC-700-2 | 4 | 33 | 17 |
| 14 | Co1@NC-700 | 4 | 33 | 1 |
| 15 | NC-700 | 4 | 33 | 0 |
| 16 | — | 4 | 33 | 0 |
Among the three Fe1@NC-x catalysts, Fe1@NC-700 exhibits the best performance because it has the most iron single-atom sites (entries 4–6). The dosages of Fe1@NC-700 and mCPBA were also optimized, and the combination of 4 g Fe1@NC-700 and 33 g mCPBA in the 87 mmol scale reaction was the best option (entries 6–12). Fe1@NC-700-2 and Co@NC-700 were synthesized via the same preparation methods using FeCl3 and CoSO4·7H2O as metal precursors. Fe1@NC-700-2 displays similar catalytic activity to Fe1@NC-700 (entries 6 vs. 13), while only trace NaN5 can be obtained in the case of Co1@NC-700 (entry 14), since it cannot form a Co(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate. Blank experiments verify that the iron sites are the catalytically active center of this reaction (entries 15 vs. 16).
O intermediate. Blank experiments verify that the iron sites are the catalytically active center of this reaction (entries 15 vs. 16).
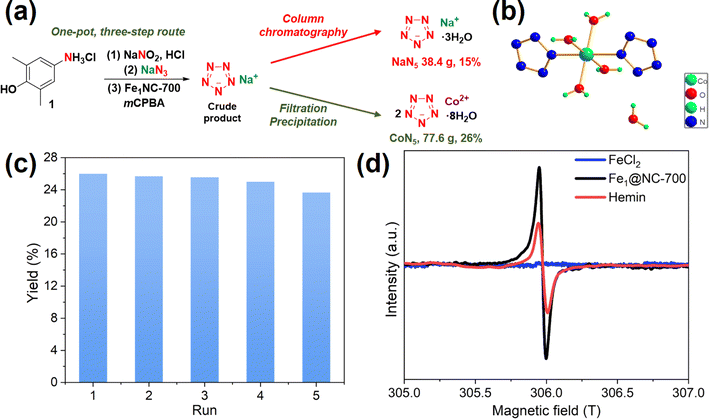
To further highlight the potential of this catalyst, we increased the reaction scale 20 times (1.74 mol, 302 g of 1). Fe1@NC-700 maintained good catalytic activity, and the yield of NaN5 reached 15% (Fig. 5a). Nevertheless, the work-up procedure for the separation and purification of NaN5 is tedious, including multiple room-temperature vacuum rotary evaporations for water removal and column chromatography, which results in an ultra-long post-treatment time, the use of excessive organic solvents, high energy consumption, and loss and decomposition of NaN5 (Table 2, entry 2).
| Entry | Route | Purification | Cat. Fe (g) | Solventb (L) | PTc (h) | Yieldd (%) | Puritye (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: step 1: 1.74 mol 2,6-dimethyl-4-aminophenol hydrochloride (302 g), 1.92 mol HCl, 1.80 mol NaNO2, THF, −5 °C, 1 h; step 2: 1.80 mol NaN3, MeOH, −40 °C, 2 h; step 3: catalyst, 660 g mCPBA, MeCN, −15 °C, 12 h. b Post-processing solvent usage. c Post-processing time. d Yields were calculated based on arylamine hydrochloride 1. e Product purity was determined using ion chromatography. | |||||||
| 1 | Two-pot | Column chromatography | Fe(Gly)2 (840) | 33.0 | 26 | 7 (NaN5) | 70 |
| 2 | One-pot | Column chromatography | Fe1@NC-700 (80) | 31.0 | 20 | 15 (NaN5) | 90 |
| 3 | One-pot | Precipitation and filtration | Fe1@NC-700 (80) | 6.5 | 7 | 26 (CoN5) | 98 |
To eliminate these issues, a coordination-precipitation strategy has been developed. Organic acid salts in the reaction solution are first converted into organic acids by adjusting the pH and further removed by extraction. Then, pentazolate anions (N5−) cooperate with Co2+ salts to yield CoN5 precipitate ([Co(H2O)4(N5)2]·4H2O), which can be separated by simple filtration (Fig. 5b, S8–S10, S11b; Tables S5 and S6†). Furthermore, CoN5 can replace NaN5 as the basic raw material for other pentazolate salts.6 A 26% yield of CoN5 (77.6 g) was afforded through the coordination-precipitation strategy.
To verify the stability and recyclability of Fe1@NC-700, the scaled-up synthesis of CoN5 was chosen as the model reaction. The substrate (arylamine hydrochloride) was completely converted into pentazolate and other by-products. Fe1@NC-700 should be able to adsorb some by-products generated during the reaction, such as phenols, mCPBA and m-chlorobenzoic acid. After the reaction was completed, the weight of the filtered recovered catalyst increased by 9% after drying, which confirmed our hypothesis. Therefore, ethanol washing is necessary to remove adsorbates during the catalyst recovery process. However, pentazolate salts have good water solubility, so they should not be adsorbed on the catalyst. As shown in Fig. 5c, Fe1@NC-700 could still maintain high catalytic activity after five runs. A comparison of different synthetic routes for the scaled-up pentazolate synthesis is provided in Table 2. Compared to the other two routes, the one-pot CoN5 synthetic route has a higher yield and purity and shorter post-processing time and uses less Fe catalyst and post-processing solvent.
To confirm the Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate in this process, a low-temperature (77 K) electron paramagnetic resonance (EPR) experiment was carried out. Rhombic signals were observed after the reaction of hemin and Fe1@NC-700 with excess mCPBA, thereby demonstrating the existence of a Fe(IV)
O intermediate in this process, a low-temperature (77 K) electron paramagnetic resonance (EPR) experiment was carried out. Rhombic signals were observed after the reaction of hemin and Fe1@NC-700 with excess mCPBA, thereby demonstrating the existence of a Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate.17 The EPR singlet intensity of Fe1@NC-700 is higher than that of hemin, suggesting that Fe1@NC-700 is more conducive to the generation of Fe(IV)
O intermediate.17 The EPR singlet intensity of Fe1@NC-700 is higher than that of hemin, suggesting that Fe1@NC-700 is more conducive to the generation of Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O than hemin. No singlet was detected in the case of FeCl2, which means that it failed to react with mCPBA to afford Fe(IV)
O than hemin. No singlet was detected in the case of FeCl2, which means that it failed to react with mCPBA to afford Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. This can explain why FeCl2 has no catalytic performance in this reaction.
O. This can explain why FeCl2 has no catalytic performance in this reaction.
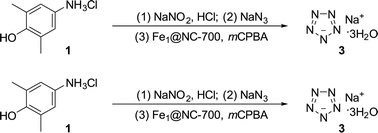
The Gibbs free energy profiles for the reaction of iron catalysts (hemin, Fe(Gly)2 and FeN4) with mCPBA are also illustrated in Fig. 6a and S12–S14.† The energy barrier for the reaction of FeN4 (the catalytically active site of Fe1@NC-700) with mCPBA to generate Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is the lowest (3.49 kcal mol−1). This further proves that the formation of Fe(IV)
O is the lowest (3.49 kcal mol−1). This further proves that the formation of Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O over Fe1@NC-700 is easier than for other catalysts (hemin and Fe(Gly)2). Fig. 6b shows the free energy diagram for the selective oxidative cleavage of the C–N bond. According to the Gibbs free energy profiles of these two reactions, the energy barriers of iron-catalyzed reactions for NaN5 synthesis follow the order Fe1@NC-700 (6.23 kcal mol−1) < Fe(Gly)2 (9.85 kcal mol−1) < hemin (11.33 kcal mol−1).
O over Fe1@NC-700 is easier than for other catalysts (hemin and Fe(Gly)2). Fig. 6b shows the free energy diagram for the selective oxidative cleavage of the C–N bond. According to the Gibbs free energy profiles of these two reactions, the energy barriers of iron-catalyzed reactions for NaN5 synthesis follow the order Fe1@NC-700 (6.23 kcal mol−1) < Fe(Gly)2 (9.85 kcal mol−1) < hemin (11.33 kcal mol−1).
According to above presented results, the superior catalytic performance of Fe1@NC-700 can be attributed to following aspects. (1) It has stable FeN4 enzyme-like catalytic active sites, which promote the formation of a Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate and maintain catalytic activity even under weakly acidic conditions and after multiple cycles. (2) It has a higher surface area with more defects and the FeN4 sites than Fe1@NC-800 and Fe1@NC-900, all of which can boost its catalytic activity.
O intermediate and maintain catalytic activity even under weakly acidic conditions and after multiple cycles. (2) It has a higher surface area with more defects and the FeN4 sites than Fe1@NC-800 and Fe1@NC-900, all of which can boost its catalytic activity.
Conclusions
In summary, an iron single-atom catalyst (Fe1@NC-700) with the FeN4 sites has been fabricated using a pyrolysis-milling strategy and can achieve scaled-up pentazolate synthesis (38.4 g, 15% yield for NaN5; 77.6 g, 26% yield for CoN5) via a three-step, one-pot process with a 0.67 mol% iron dosage. The superior catalytic performance of Fe1@NC-700 can be attributed to two key factors. (1) It possesses stable FeN4 enzyme-like catalytic active sites as evidenced by EPR and DFT calculation results, which promote the formation of an Fe(IV)![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O intermediate and maintain catalytic activity even under weakly acidic conditions and after five cycles. (2) It has a higher surface area and more defects and the FeN4 sites than Fe1@NC-800 and Fe1@NC-900 based on BET, Raman, ICP and XPS results, all of which can boost its catalytic activity. This innovative work integrates single-atom catalysis with the synthesis of energetic materials, offering a novel approach for the scalable production of pentazolates and broadening the application scope of single-atom catalysis.
O intermediate and maintain catalytic activity even under weakly acidic conditions and after five cycles. (2) It has a higher surface area and more defects and the FeN4 sites than Fe1@NC-800 and Fe1@NC-900 based on BET, Raman, ICP and XPS results, all of which can boost its catalytic activity. This innovative work integrates single-atom catalysis with the synthesis of energetic materials, offering a novel approach for the scalable production of pentazolates and broadening the application scope of single-atom catalysis.
Data availability
Data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (22275090 and 22105102) and Young Elite Scientist Sponsorship Program by CAST (YESS20210074).References
-
(a) Y. Xu, Q. Wang, C. Shen, Q. Lin, P. Wang and M. Lu, A series of energetic metal pentazolate hydrates, Nature, 2017, 549, 78–81 CrossRef CAS PubMed
; (b) C. Zhang, C. Sun, B. Hu, C. Yu and M. Lu, Synthesis and characterization of the pentazolate anion cyclo-N5− in (N5)6(H3O)3(NH4)4Cl, Science, 2017, 355, 374–376 CrossRef CAS PubMed
; (c) P. Wang, Q. Lin, Y. Xu and M. Lu, Pentazole anion cyclo-N5−: a rising star in nitrogen chemistry and energetic materials, Sci. China: Chem., 2018, 61, 1355–1358 CrossRef CAS
; (d) C. Zhang, C. Sun, B. Hu and M. Lu, Investigation on the stability of multisubstituted arylpentazoles and the influence on the generation of pentazolate anion, J. Energ. Mater., 2016, 34, 103–111 CrossRef CAS
.
-
(a) Y. Xu, L. Tian, D. Li, P. Wang and M. Lu, A series of energetic cyclo-pentazolate salts: rapid synthesis, characterization, and promising performance, J. Mater. Chem. A, 2019, 7, 12468–12479 RSC
; (b) Y. Xu, P. Wang, Q. Lin and M. Lu, A carbon-free inorganic-metal complex consisting of an all-nitrogen pentazole anion, a Zn(II) cation and H2O, Dalton Trans., 2017, 46, 14088–14093 RSC
; (c) Y. Xu, Q. Lin, P. Wang and M. Lu, Syntheses, Crystal Structures and Properties of a Series of 3D Metal–Inorganic Frameworks Containing Pentazolate Anion, Chem.–Asian J., 2018, 13, 1669–1673 CrossRef CAS PubMed
; (d) Y. Yuan, Y. Xu, Q. Xie, D. Li, Q. Lin, P. Wang and M. Lu, Pentazolate coordination polymers self-assembled by in situ generated [Pb4(OH)4]4+ cubic cations trapping cyclo-N5−, Dalton Trans., 2022, 51, 5801–5809 RSC
; (e) Y. Xu, L. Ding, F. Yang, D. Li, P. Wang, Q. Lin and M. Lu, LiN5: A novel pentazolate salt with high nitrogen content, Chem. Eng. J., 2022, 429, 132399 CrossRef CAS
; (f) L. Tian, Y. Xu, Q. Lin, P. Wang and M. Lu, Syntheses of Energetic cyclo-Pentazolate Salts, Chem.–Asian J., 2019, 14, 2877–2882 CrossRef CAS PubMed
; (g) Y. Xu, Z. Xu, X. Zhang, T. Hou and M. Lu, [Na4(N5)4(H2O)2]·H2O·2MeOH: a honeycomb-like sodium pentazolate framework with helical chains, CrystEngComm, 2022, 24, 4853–4856 RSC
; (h) P. Wang, Y. Xu, Q. Lin and M. Lu, Recent advances in the syntheses and properties of polynitrogen pentazolate anion cyclo-N5− and its derivatives, Chem. Soc. Rev., 2018, 47, 7522–7538 RSC
; (i) Y. Cao, Y. Liu and W. Zhang, Pentazolate Anion: A Rare and Preferred Five-Membered Ligand for Constructing Pentasil-Zeolite Topology Architectures, Angew. Chem., Int. Ed., 2024, 63, e202317355 CrossRef CAS PubMed
.
- F. Shang, R. Liu, J. Liu, P. Zhou, C. Zhang, S. Yin and K. Han, Unraveling the Mechanism of cyclo-N5− Production through Selective C–N Bond Cleavage of Arylpentazole with Ferrous Bisglycinate and m-Chloroperbenzonic Acid: A Theoretical Perspective, J. Phys. Chem. Lett., 2020, 11, 1030–1037 CrossRef CAS PubMed
.
-
(a) J. Qin, B. Han, X. Liu, W. Dai, Y. Wang, H. Luo, X. Lu, J. Nie, C. Xian and Z. Zhang, An enzyme-mimic single Fe-N3 atom catalyst for the oxidative synthesis of nitriles via C–C bond cleavage strategy, Sci. Adv., 2022, 8, eadd1267 CrossRef CAS PubMed
; (b) L. Jiao, H. Yan, Y. Wu, W. Gu, C. Zhu, D. Du and Y. Lin, When Nanozymes Meet Single-Atom Catalysis, Angew. Chem., Int. Ed., 2020, 59, 2565–2576 CrossRef CAS PubMed
; (c) F. Zhu, G.-P. Lu, F. Wang, E. Ren, Y. Yu and Y. Lin, Iron catalyzed organic reactions in water: A “nature-like” synthesis, Curr. Opin. Green Sustainable Chem., 2023, 40, 100754 CrossRef CAS
; (d) R.-Y. Lu, S.-J. Jiang, Y.-G. Xu, Q.-H. Lin, M. Lu and P.-C. Wang, Thermal hazard assessment of one-step synthesis pentazolate anion by calorimetry data, J. Therm. Anal. Calorim., 2023, 148, 12175–12183 CrossRef CAS
; (e) X. N. Wang, T. Liu, M. X. Chen, Q. Liang, J. Jiang, L. Chen, K. L. Fan, J. H. Zhang and L. Z. Gao, An Erythrocyte-Templated Iron Single-Atom Nanozyme for Wound Healing, Adv. Sci., 2023, 11, 2307844 CrossRef PubMed
; (f) X. Chen, W. Y. Lu, T. F. Xu, N. Li, D. D. Qin, Z. X. Zhu, G. Q. Wang and W. X. Chen, A bio-inspired strategy to enhance the photocatalytic performance of g-C3N4 under solar irradiation by axial coordination with hemin, Appl. Catal., B, 2017, 201, 518–526 CrossRef CAS
.
-
(a) F. Wang, F. Zhu, E. Ren, G. Zhu, G.-P. Lu and Y. Lin, Recent Advances in Carbon-Based Iron Catalysts for Organic Synthesis, Nanomaterials, 2022, 12, 3462 CrossRef CAS PubMed
; (b) Y. Lin, F. Wang, J. Yu, X. Zhang and G.-P. Lu, Iron single-atom anchored N-doped carbon as a ‘laccase-like’ nanozyme for the degradation and detection of phenolic pollutants and adrenaline, J. Hazard. Mater., 2022, 425, 127763 CrossRef CAS PubMed
; (c) K. Sun, H. Shan, H. Neumann, G.-P. Lu and M. Beller, Efficient iron single-atom catalysts for selective ammoxidation of alcohols to nitriles, Nat. Commun., 2022, 13, 1–9 Search PubMed
; (d) X. Cui, H. Li, Y. Wang, Y. Hu, L. Hua, D. Deng and X. Bao, Room-temperature methane conversion by graphene-confined single iron atoms, Chem, 2018, 4, 1902–1910 CrossRef CAS
; (e) D. Deng, X. Chen, L. Yu, X. Wu, Q. Liu, Y. Liu, X. Pan and X. Bao, A single iron site confined in a graphene matrix for the catalytic oxidation of benzene at room temperature, Sci. Adv., 2015, 1, e1500462 CrossRef PubMed
; (f) W. Liu, L. Zhang, X. Liu, X. Liu, X. Yang, S. Miao, W. Wang, A. Wang and T. Zhang, Discriminating catalytically active FeNx species of atomically dispersed Fe–N–C catalyst for selective oxidation of the C–H bond, J. Am. Chem. Soc., 2017, 139, 10790–10798 CrossRef CAS PubMed
.
-
(a) X. Wang, Z. Dong, R. Yang, S. Zhou and Z. Ye, Syntheses and Characterization of two cyclo-pentazolate salts, Z. Anorg. Allg. Chem., 2021, 647, 681–685 CrossRef CAS
; (b) L. Chen, C. Yang, H. Hu, L. Shi, C. Zhang, C. Sun, C. Gao, Y. Du and B. Hu, Synthesis and characterization of cyclo-pentazolate salts of iron (III) and aluminum (III), CrystEngComm, 2022, 24, 8152–8159 RSC
.
- W. Ni, Z. Liu, Y. Zhang, C. Ma, H. Deng, S. Zhang and S. Wang, Electroreduction of Carbon Dioxide Driven by the Intrinsic Defects in the Carbon Plane of a Single FeN4 Site, Adv. Mater., 2021, 33, e2003238 CrossRef PubMed
.
-
(a) J. Ribeiro-Soares, M. Oliveros, C. Garin, M. David, L. Martins, C. Almeida, E. Martins-Ferreira, K. Takai, T. Enoki and R. Magalhães-Paniago, Structural analysis of polycrystalline graphene systems by Raman spectroscopy, Carbon, 2015, 95, 646–652 CrossRef CAS
; (b) J. Zhang, H. Zhou, J. W. Zhu, P. Hu, C. Hang, J. L. Yang, T. Peng, S. C. Mu and Y. H. Huang, Facile Synthesis of Defect-Rich and S/N Co-Doped Graphene-Like Carbon Nanosheets as an Efficient Electrocatalyst for Primary and All-Solid-State Zn–Air Batteries, ACS Appl. Mater. Interfaces, 2017, 9, 24545–24554 CrossRef CAS PubMed
; (c) T. S. Zhou, Y. Zhou, R. G. Ma, Z. Z. Zhou, G. H. Liu, Q. Liu, Y. F. Zhu and J. C. Wang, Nitrogen-doped hollow mesoporous carbon spheres as a highly active and stable metal-free electrocatalyst for oxygen reduction, Carbon, 2017, 114, 177–186 CrossRef CAS
.
- J. W. Zhu and S. C. Mu, Defect Engineering in Carbon-Based Electrocatalysts: Insight into Intrinsic Carbon Defects, Adv. Funct. Mater., 2020, 30, 2001097 CrossRef CAS
.
-
(a) L. Jiao, G. Wan, R. Zhang, H. Zhou, S. Yu and H. Jiang, From metal–organic frameworks to single-atom Fe implanted N-doped porous carbons: efficient oxygen reduction in both alkaline and acidic media, Angew. Chem., Int. Ed., 2018, 130, 8661–8665 CrossRef
; (b) A. Zitolo, V. Goellner, V. Armel, M. T. Sougrati, T. Mineva, L. Stievano, E. Fonda and F. Jaouen, Identification of catalytic sites for oxygen reduction in iron-and nitrogen-doped graphene materials, Nat. Mater., 2015, 14, 937–942 CrossRef CAS PubMed
; (c) W. Liu, L. Zhang, W. Yan, X. Liu, X. Yang, S. Miao, W. Wang, A. Wang and T. Zhang, Single-atom dispersed Co–N–C catalyst: structure identification and performance for hydrogenative coupling of nitroarenes, Chem. Sci., 2016, 7, 5758–5764 RSC
; (d) L. Zhang, A. Wang, W. Wang, Y. Huang, X. Liu, S. Miao, J. Liu and T. Zhang, Co–N–C catalyst for C-C coupling reactions: on the catalytic performance and active sites, ACS Catal., 2015, 5, 6563–6572 CrossRef CAS
.
-
(a) J. W. Zhu, H. Zhou, C. T. Zhang, J. Zhang and S. C. Mu, Dual active nitrogen doped hierarchical porous hollow carbon nanospheres as an oxygen reduction electrocatalyst for zinc–air batteries, Nanoscale, 2017, 9, 13257 RSC
; (b) P. P. Su, W. J. Huang, Q. G. Du and J. Yang, Fe atoms anchored on defective nitrogen doped hollow carbon spheres as efficient electrocatalysts for oxygen reduction reaction, Nano Res., 2021, 14, 1069–1077 CrossRef CAS
; (c) O. Y. Podyacheva, S. V. Cherepanova, A. I. Romanenko, A. N. Suboch, A. V. Puzynin and Z. R. Ismagilov, Nitrogen doped carbon nanotubes and nanofibers: Composition, structure, electrical conductivity and capacity properties, Carbon, 2017, 122, 475–483 CrossRef CAS
; (d) E. V. Suslova, K. I. Maslakov, S. V. Savilov, A. S. Ivanov, L. Lu and V. V. Lunin, Study of nitrogen-doped carbon nanomaterials by bomb calorimetry, Carbon, 2016, 102, 506–512 CrossRef CAS
; (e) E. V. Suslova, S. V. Savilov, J. Ni, V. V. Lunin and S. M. Aldoshin, The enthalpies of formation of carbon nanomaterials as a key factor for understanding their structural features, Phys. Chem. Chem. Phys., 2017, 19, 2269–2275 RSC
.
-
(a) G.-P. Lu, K. Sun, Y. Lin, Q. Du, J. Zhang, K. Wang and P. Wang, Single-atomic-site iron on N-doped carbon for chemoselective reduction of nitroarenes, Nano Res., 2022, 15, 603–611 CrossRef CAS
; (b) G.-P. Lu, H. Shan, Y. Lin, K. Zhang, B. Zhou, Q. Zhong and P. Wang, A Fe single atom on N, S-doped carbon catalyst for performing N-alkylation of aromatic amines under solvent-free conditions, J. Mater. Chem. A, 2021, 9, 25128–25135 RSC
.
- W.-C. Cheong, W. Yang, J. Zhang, Y. Li, D. Zhao, S. Liu, C. Chen and Y. Li, Isolated iron single-atomic site-catalyzed chemoselective transfer hydrogenation of nitroarenes to arylamines, ACS Appl. Mater. Interfaces, 2019, 11, 33819–33824 CrossRef CAS PubMed
.
- S. Tian, M. Hu, Q. Xu, W. Gong, W. Chen, J. Yang, D. Wang and Y. Li, Single-atom Fe with Fe1N3 structure showing superior performances for both hydrogenation and transfer hydrogenation of nitrobenzene, Sci. China Mater., 2020, 64, 642–650 CrossRef
.
-
(a) Y. Li, Y. Zhou, X. Ma and H. Jiang, A metal–organic framework-templated synthesis of γ-Fe2O3 nanoparticles encapsulated in porous carbon for efficient and chemoselective hydrogenation of nitro compounds, Chem. Commun., 2016, 52, 4199–4202 RSC
; (b) Y. Liu, W. Zhang, Y. Zheng, K. Wu, P. Dong, R. He, N. Lu and J. Mao, Single-atom Fe–N4 site for the hydrogenation of nitrobenzene: theoretical and experimental studies, Dalton Trans., 2021, 50, 7995–8001 RSC
.
-
(a) T.-Y. Su, G.-P. Lu, K.-K. Sun, M. Zhang and C. Cai, ZIF-derived metal/N-doped porous carbon nanocomposites: efficient catalysts for organic transformations, Catal. Sci. Technol., 2022, 12, 2106–2121 RSC
; (b) X. Zhang, G.-P. Lu, K. Wang, Y. Lin, P. Wang and W. Yi, Lignin-derived Zn single atom/N-codoped porous carbon for α-alkylation of aromatic ketones with alcohols via borrowing hydrogen strategy, Nano Res., 2022, 15, 1874–1881 CrossRef CAS
; (c) X. Zhang, Q. Zhang, J. Reng, Y. Lin, Y. Tang, G. Liu, P. Wang and G.-P. N. Lu, S Co-Coordinated Zinc Single-Atom Catalysts for N-Alkylation of Aromatic Amines with Alcohols: The Role of S-Doping in the Reaction, Nanomaterials, 2023, 13, 445 CrossRef CAS PubMed
.
-
(a) X. Wei, S. Song, W. Song, Y. Wen, W. Xu and C. Zhu, Tuning iron spin states in single-atom nanozymes enables efficient peroxidase mimicking, Chem. Sci., 2022, 13, 13574–13581 RSC
; (b) K. Hou, J. Börgel, H. Z. H. Jiang, H. Kwon, R. C. Rohde, J. W. Taylor and J. R. Long, Reactive high-spin iron(IV)-oxo sites through dioxygen activation in a metal–organic framework, Science, 2023, 382, 547–553 CrossRef CAS PubMed
; (c) G. E. Cutsail, R. Banerjee, D. B. Rice, O. M. Stepanic, J. D. Lipscomb and S. DeBeer, Determination of the iron (IV) local spin states of the Q intermediate of soluble methane monooxygenase by Kβ X-ray emission spectroscopy, J. Biol. Inorg. Chem., 2022, 27, 573–582 CrossRef PubMed
; (d) A. R. Mcdonald and Lawrence Que Jr, High-valent nonheme iron-oxo complexes: Synthesis, structure, and spectroscopy, Coord. Chem. Rev., 2013, 257, 414–428 CrossRef CAS
; (e) M. Puri and Lawrence Que Jr, Toward the Synthesis of More Reactive S = 2 Non-Heme Oxoiron (IV) Complexes, Acc. Chem. Res., 2015, 48, 2443–2452 CrossRef CAS PubMed
; (f) F. Zhu, Y. Yu, H. Qiu, G.-P. Lu, Z. Chen, J. Hu and Y. Lin, S-Doping Regulated Iron Spin States in Fe–N–C Single-Atom Material for Enhanced Peroxidase-Mimicking Activity at Neutral pH, Small, 2024, 2311848 CrossRef PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta03312d |
| This journal is © The Royal Society of Chemistry 2024 |