Open Access Article
Open Access ArticleAddressing ambient stability challenges in pure FASnI3 perovskite solar cells through organic additive engineering†
Sergio
Galve-Lahoz
ab,
Jesús
Sánchez-Diaz
a,
Carlos
Echeverría-Arrondo
ac,
Jorge
Simancas
 a,
Jhonatan
Rodriguez-Pereira
a,
Jhonatan
Rodriguez-Pereira
 de,
Silver-Hamill
Turren-Cruz
de,
Silver-Hamill
Turren-Cruz
 af,
Juan P.
Martinez-Pastor
af,
Juan P.
Martinez-Pastor
 f,
Iván
Mora-Seró
f,
Iván
Mora-Seró
 *a and
Juan Luis
Delgado
*a and
Juan Luis
Delgado
 *bg
*bg
aInstitute of Advanced Materials (INAM), University Jaume I, Av. Vicent Sos Baynat, s/n, 12071, Castellón de la Plana, Spain. E-mail: sero@uji.es
bPolymat, University of the Basque Country UPV/EHU, 20018 Donostia-San Sebastian, Spain. E-mail: juanluis.delgado@polymat.eu
cInstitute of Chemical Research of Catalonia (ICIQ), Avda. Països Catalans 16, 43007 Tarragona, Spain
dCenter of Materials and Nanotechnologies, Faculty of Chemical Technology, University of Pardubice, nám. Cs. legií 565, Pardubice, 53002, Czech Republic
eCentral European Institute of Technology, Brno University of Technology, Purkynova 123, Brno, 61200, Czech Republic
fInstituto de Ciencia de los Materiales (ICMUV), Universitat de Valencia, 46980 Paterna, Spain
gIkerbasque, Basque Foundation for Science, Bilbao 48013, Spain
First published on 11th July 2024
Abstract
Tin halide perovskite solar cells (Sn-PSCs) have emerged as promising alternatives to their lead-based counterparts. However, their potential for commercialization is threatened by their inherent stability issues and lower efficiencies that have not yet matched those of established technologies. Therefore, in this work we strategically designed and synthesized two novel organic compounds, referred to as OM4 and OM6, which have been successfully incorporated as additives into pure FASnI3 PSCs enhancing the crystal properties and simultaneously acting as protective and passivating agents. Through a systematic exploration of chemical interactions, OM4 and OM6 demonstrated a remarkable ability to improve film morphology and optical properties. Consequently, both additives significantly contributed to the enhancement of the overall device performances and exhibited impressive stabilities in the ambient atmosphere, which is attributed to their hydrophobic nature. Notably, the unencapsulated devices incorporating OM4 and OM6 retained over 80% and 90% of their initial power conversion efficiency (PCE) after 250 hours in the ambient atmosphere at RH = 30%, respectively; in contrast, the devices without any of the organic additives suffered complete degradation after 72 hours under the same conditions. As a result, this work opens new possibilities in the rational design and development of organic additives capable of mitigating the instability and low-performance issues commonly associated with Sn-PSCs.
Introduction
Lead-based perovskite solar cells (Pb-PSCs) have demonstrated great potential to surpass well-established silicon-based solar cells and enter into the photovoltaic market, showing a huge increase in power conversion efficiency (PCE) from the first reported 3.8% in 2009 (ref. 1) to the recently certified >26%.2,3 However, despite their outstanding properties,4–7 the presence of toxic lead (Pb2+)8,9 in these devices hinders their universal commercialization.10,11 To address this issue, researchers have been exploring alternative divalent cations, including germanium (Ge2+),12,13 copper (Cu2+)14 and tin (Sn2+).15–17 Among them, tin has recently emerged as one of the most promising candidates to replace lead in perovskite solar cells due to their chemical similarities.18,19Owing to their close location in the periodic table within the same group, both tin and lead share analogous electronic configurations and atomic sizes (1.49 Å for Pb2+ and 1.35 Å for Sn2+),20 resulting in comparable behaviors as metal cations within perovskite solar cells. In fact, tin-based halide perovskite solar cells (Sn-PSCs) have also demonstrated exceptional properties, including high carrier mobilities,21 high absorption coefficients in the visible region,22 long exciton lifetimes,23 and suitable optimal band gaps within the range of 1.2–1.4 eV.24,25 Furthermore, Sn-based perovskites exhibit a higher level of environmental compatibility than Pb-based perovskites.26 This is primarily attributed to the tendency of the byproducts generated during the degradation of Sn-based perovskites to undergo hydrolysis in water forming SnI4, SnO2, and A2SnI4 (A = methylammonium or formamidinium),27–29 which exhibit lower solubility in water compared to the byproducts derived from Pb-based perovskites, and make Sn-PSCs more suitable candidates for future commercial applications.30
Nevertheless, Sn2+ presents higher Lewis acidity in comparison to its Pb2+ counterpart.31 This disparity in acidity results in accelerated reactions between the perovskite precursors, typically SnI2 and FAI, leading to rapid and uncontrolled crystallization processes.32,33 Consequently, non-homogeneous films with pinhole-rich structures and a high density of defects are usually obtained. It has been widely reported that structural defects may promote the adsorption of water and oxygen molecules onto the perovskite layer, thereby accelerating the degradation process and affecting the stability and operational performance of the final solar cell devices.34,35 Moreover, structural defects can act as non-radiative recombination centers, leading to losses in open-circuit voltage (VOC), which significantly limit the overall performance of the solar cell devices. In this sense, finding new compounds capable of effectively interacting with Sn-PSC precursors to improve the crystallization process and simultaneously passivate the defects formed through the perovskite matrix is essential to obtain high-quality Sn-PSCs.
Another drawback associated with Sn-PSCs is their inherent instability upon exposure to the ambient atmosphere, resulting in fast degradation and significant drops in the device performance. This instability mainly arises from the tendency of Sn2+ to oxidize, in the presence of water or oxygen molecules, to the more stable Sn4+ state.36–38 Subsequently, the proliferation of Sn2+ vacancies within the perovskite lattice gives rise to a detrimental p-type self-doping phenomenon, which can lead to an alteration in the charge transport properties and overall electrical characteristics of the Sn-PSCs, resulting in considerable VOC losses.39,40 To overcome this issue, several approaches have been explored, including effective surface passivation,41–44 the introduction of different reducing agents,45–47 or the formation of stable 2D/3D perovskites, among other methods.48–50 Another interesting methodology widely explored in Pb-PSCs but barely applied to Sn-PSCs to efficiently enhance their stability in air involves the incorporation of hydrophobic compounds, which successfully confer a protective effect on the devices against deleterious external factors.51–54
With these facts in mind, in this work we strategically designed two novel organic additives, namely OM4 and OM6, each possessing two external acrylate moieties attached to the central structures to allow partial polymerization processes. Acrylates are a type of vinyl monomer that can undergo polymerization at moderate temperatures through a process known as thermal polymerization.55–58 This method can take advantage of the intrinsic annealing step necessary for the correct perovskite crystallization process, without the need for additional steps such as UV-light treatments that may damage the perovskite film.59–61 Additionally, OM4 and OM6 were designed with distinct cores to explore the role of different functional groups as additives in Sn-PSCs. As such, OM4 features a pyridine functional group (Lewis base-type), already reported in Sn-PSCs,33,62 whereas OM6 includes a less explored 1,2,4,5-tetrafluorobenzene unit (Lewis acid-type).
After the introduction of the additives into the Sn perovskite films, a significant enhancement in the PCE of the Sn-PSCs was observed. The initial PCE of 7.78% for the pristine FASnI3 (FA, formamidinium) increased to 9.18% and 9.62% upon the addition of OM4 and OM6, respectively. More interestingly, the treated devices displayed exceptional stability after more than 250 h of storage under ambient conditions (at a relative humidity of 30% and a temperature of 25 °C), retaining up to 80% and 90% of their initial PCE for OM4 and OM6 treated devices, respectively. In contrast, pristine FASnI3 devices completely degraded after only 72 h of storage under the same conditions.
Results and discussion
Both OM4 and OM6 were synthesized following the synthetic routes described in Schemes S1 and S2, respectively, in the ESI,† and their chemical structure is illustrated in Fig. 1a and b. More information about the synthetic and characterization details can be found in the ESI (see Fig. S1–S17†).With the aim of understanding the role of the additives in the perovskite film morphology, we conducted scanning electron microscopy (SEM) measurements. Fig. 1c–e show the top-view SEM images of the FASnI3 perovskite films with and without OM4 and OM6. Note that additives were introduced in the perovskite layer during the antisolvent stage63 solved in chlorobenzene (see the ESI† for further details). For clarity, the Sn-based perovskite films treated with OM4 and OM6 will be referred to as Sn-OM4 and Sn-OM6, respectively. In the case of pristine FASnI3, the film exhibited a non-uniform, pinhole-rich morphology due to the rapid and uncontrolled crystallization process typically associated with Sn-based perovskites.33,64 However, upon the incorporation of the different additives, higher quality films were obtained, and the presence of pinholes was significantly reduced in both scenarios. Specifically, Sn-OM4 showed a more compact film with a considerably lower number of pinholes, although some morphological defects were still present. On the other hand, Sn-OM6 exhibited an extremely compact layer with no pinholes and a slight increase in the grain size. To provide a broader perspective on the positive impact of these additives on the perovskite crystallization process, and to demonstrate that the enhancement of the film morphology is not a local coincidence, lower magnification SEM images were obtained and presented in Fig. S18.† However, from the SEM cross-sectional images, we can observe that the incorporation of the additives does not affect the thickness of the perovskite films, as all samples exhibited a similar thickness of around 200 nm (see Fig. S18†). To confirm the presence of the additives within the perovskite film, we carried out Fourier Transform Infrared Spectroscopy (FTIR) on the perovskite films with and without additives, as well as on the individual OM4 and OM6 molecules (see Fig. S19†). The characteristic carbonyl group peak around 1730 cm−1, along with the additional peaks in the fingerprint region, confirms the presence of the additives in the Sn-OM4 and Sn-OM6 films. Additionally, the double peak observed around 1600 cm−1 in the OM4 and OM6 spectra corresponds to the ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2 double bonds present in the acrylate groups. Upon perovskite crystallization and thermal annealing, these peaks disappeared, indicating the polymerization of the materials. However, due to the limited sensitivity of the technique, we cannot entirely rule out the coexistence of single molecules and the polymerized materials in certain regions of the final perovskite film.
CH2 double bonds present in the acrylate groups. Upon perovskite crystallization and thermal annealing, these peaks disappeared, indicating the polymerization of the materials. However, due to the limited sensitivity of the technique, we cannot entirely rule out the coexistence of single molecules and the polymerized materials in certain regions of the final perovskite film.
As previously noted, the rapid reaction between tin(II) iodide (SnI2) and formamidinium iodide (FAI) often results in uncontrolled crystallization processes, yielding low-quality films characterized by random orientations and numerous defects such as pinholes and cracks.65 Intermediate adducts formed between additives and SnI2 or FAI precursors have been found to slow down reaction rates, thereby retarding the crystallization rate and leading to higher quality films.33,38 For a better understanding of the role of OM4 and OM6 in the perovskite crystallization process, we conducted nuclear magnetic resonance (NMR) experiments. All NMR experiments were conducted in deuterated dimethyl sulfoxide-d6 (DMSO-d6) and referenced accordingly. To analyze the interactions with the perovskite precursors, we performed a combination of proton and fluorine nuclear magnetic resonance spectroscopies (1H-NMR and 19F-NMR) for different mixtures. Fig. 1f shows the 1H NMR spectra of FAI, FAI + SnI2, FAI + OM4, and FAI + OM6. In the FAI spectra, the proton peaks located around 7.8 and 8.8 ppm correspond to C–H and N–H protons, respectively. After the addition of SnI2 to the FAI solution, the signal at 8.8 ppm splits into two distinct signals at 8.9 and 8.6 ppm, indicating a change in the charge distribution around N–H protons within the formamidinium group, possibly due to the formation of a hydrogen bond between the amidinium moiety and the SnI2 species. Furthermore, a spiking in the signals at 7.8 and 8.6 is observed, which can be attributed to the presence of HI, likely formed as a consequence of the partial reaction of the SnI2 species with traces of water present in the deuterated solution, as previously reported.66 Interestingly, upon addition of OM4 and OM6 to the FAI solution, the same splitting was observed, suggesting the formation of an adduct between FAI and OM4 and OM6via hydrogen bonding.67 Full range 1H-NMR spectra showing all combinations are presented in Fig. S20 and S21.† To further understand the different behaviors of the pyridine and 1,2,4,5-tetrafluorobenzene units of OM4 and OM6, we also conducted 1H-NMR for OM4 and 19F-NMR for OM6, both with and without SnI2, as depicted in Fig. S22 and S23.† In the case of OM4, two distinct proton signals were observed within the pyridine ring, located at chemical shifts of 8.77 and 8.19 ppm. Upon addition of SnI2 to the deuterated solution, a noticeable downfield shift was observed in both signals, with a more remarkable shift observed in the signal corresponding to the proton adjacent to the nitrogen (N) atom. This shielding effect observed in the pyridine ring's protons suggests an electronic sharing between the lone pair of electrons present on the N atom and the vacant p orbital of the SnI2 species, resulting in the formation of a Lewis acid–base adduct. Furthermore, peaks became narrower and more defined in the presence of SnI2, indicative of greater molecular ordering. On the other hand, the role of the 1,2,4,5-tetrafluorobenzene unit present in OM6 was studied via19F-NMR due to the absence of hydrogen atoms in the ring. Accordingly, the 19F-NMR spectra of OM6 with and without SnI2 were obtained, and a clear upfield shift in the fluorine (F) signal was observed when SnI2 was added, indicating a reduction in the electron density around the fluorinated ring. This spectral alteration also confirms an interaction wherein electronic density is shared between the electron-rich F atoms present in the 1,2,4,5-tetrafluorobenzene unit and the electronically deficient SnI2 species. This phenomenon has been described before using fluorine-rich additives in lead-based PSCs.68 These results point to the presence of interactions between the perovskite precursors and both OM4 and OM6, which in turn modulate the crystallization kinetics, ultimately improving the film quality in the perovskite active layer, as previously observed in the SEM images.
In order to explore the chemical bonding properties of the different FASnI3 perovskite films, with and without additives, X-ray photoelectron spectroscopy (XPS) analysis was employed (see Fig. 1g). As reported elsewhere, the characteristic XPS Sn2+ 3d5/2 peak that corresponds to the FASnI3 perovskite was observed at 487.39 eV.69 Interestingly, with the incorporation of OM4 and OM6, this peak exhibited a small shift towards 487.31 eV and 487.25 eV, respectively. The shift of a specific element in its binding energy peak position typically reflects alterations in its local chemical environment, indicating a variation in the electron density surrounding the nucleus.15,70,71 The contrast between the reference films and the OM4 or OM6 treated films suggests an elevated level of coordination with the 3d orbitals of Sn2+ when OM4 or OM6 is introduced; however, it should be noted that the incorporation of OM6 leads to superior coordination. This observation clarifies the improved crystallization properties observed in the presence of OM6 compared to OM4 and FASnI3 without any additive.
Additionally, to evaluate the role of the organic additives in the inhibition of the oxidation of Sn2+ into Sn4+, the Sn 3d5/2 and 3d3/2 XPS spectra of the FASnI3, Sn-OM4 and Sn-OM6 films were recorded, depicted in Fig. S24.† All the peak binding energies of Sn2+ and Sn4+ are summarized in Table S1†. The Sn4+ content was quantified, revealing a higher value for the pristine FASnI3 film (5,9%) compared to the Sn-OM4 (4.3%) and Sn-OM6 (3.7%) films (see Fig. S25†). These results are in perfect agreement with the expected role of the additives as crystal regulators.
To obtain more information on the impact of the additives on the crystallographic properties of the perovskite films, we performed X-ray diffraction (XRD) analysis. Fig. 2a shows the XRD patterns of the FASnI3 films with and without OM4 and OM6. The addition of additives did not alter the crystal structure of the FASnI3 perovskite film, and in all cases, the XRD patterns exhibited the typical peaks corresponding to the orthorhombic lattice. Concretely, six different diffraction peaks appear at angle values of 14.1°, 24.5°, 28.2°, 31.7°, 37.5°, and 50.5°, which can be ascribed to the (100), (120), (200), (211), (222), and (300) planes, respectively.72 In both Sn-OM4 and Sn-OM6 films, a preferred crystal orientation in the (100) plane was observed as can be inferred from the increase in the relative intensity of the peaks corresponding to this plane.73 Moreover, a clear reduction in the Full Width at Half-Maximum (FWHM) of the (100) and (120) diffraction peaks was noted, indicating a larger crystalline size particularly evident when OM6 was present (see Fig. S26†). Furthermore, it is evident that the addition of OM4 and OM6 does not induce any significant shift in the different diffraction peaks, suggesting that neither of the additives were incorporated into the perovskite crystalline lattice. Instead, they might be located at grain boundaries and crystal surfaces acting as passivating agents.
To investigate the effect of the additives on the optical properties and gain insights into their role in the perovskite film, we conducted ultraviolet-visible absorption (UV-vis), steady-state photoluminescence (PL), and time-resolved PL spectroscopy (TRPL) measurements. Fig. 2b shows the UV-vis absorption spectra. The films treated with OM4 and OM6 showed slightly superior light absorption properties compared to pristine FASnI3 films. This behavior is in good agreement with the morphological results and is attributed to the formation of larger grain domains and the reduced presence of pinholes or cracks in the perovskite film.74 Furthermore, we performed PL and TRPL measurements to study the charge dynamics in the presence of OM4 and OM6 (see Fig. 2c and d respectively). The PL spectrum is shown in Fig. 2c. No significant shift in the bandgap was detected; however, the treated perovskite films exhibited a notorious increase in PL intensity in comparison with the control film, indicating a reduction in the effective non-radiative trap-assisted recombination. Given that the absorption coefficient in Sn-based perovskites can be in the order of 4 × 104 cm−1, and the thickness of the film is around 200 nm, carriers are uniformly photogenerated throughout the whole film thickness, hence trap states located at the grain boundaries can be the dominant non-radiative channels, over those located at the perovskite surface. The TRPL spectra (see Fig. 2d) were fitted with a biexponential decay function using the following equation:
 | (1) |
This systematic characterization highlights the beneficial role of OM4 and OM6 additives in the properties of FASnI3 films. On one hand, they modulate the crystallization kinetics and improve the film quality. On the other hand, XPS characterization reveals a reduction in Sn4+ content, and optoelectronic characterization demonstrates a reduced non-radiative recombination. Since XRD spectra do not show a change in the lattice parameters, we propose that additives may be located at perovskite grain boundaries, suppressing the detrimental non-radiative recombination typical of these defect-rich domains. This behavior suggests a superior performance of Sn-PSCs with light harvesting Sn-perovskite films containing OM4 and OM6 additives.
To validate this potentiality, we evaluated the role of the additives in Sn-PSC performance. Solar cell devices were fabricated adopting an inverted p–i–n configuration that consisted of ITO/PEDOT:PSS/FASnI3/C60/BCP/Ag, as shown in Fig. 3a. As a first step, the concentration for both additives was optimized to 0.40 mM (see Fig. S28 and 29†). The current density–voltage (J–V) curves for the best-performing PSCs in each system are depicted in Fig. 3b and Table S3.† Their corresponding photovoltaic parameters, including VOC, short-circuit current-density (JSC), fill factor (FF) and PCE are summarized in Fig. 3c–f and Table S4.† It is important to note that all the devices underwent a light soaking treatment at 1 sun irradiance in a N2 atmosphere. This post-treatment procedure is incorporated as part of a routine purification process to mitigate the amount of Sn4+ states within the perovskite active layer, as previously demonstrated by our group.45 The control devices showed a maximum efficiency of 7.71% with a JSC of 19.91 mA cm−2, a VOC of 0.581 V, and an FF of 66.59%. The OM4 treated champion device displayed an improved efficiency of 9.25% with a similar JSC of 19.97 mA cm−2, a significantly improved VOC of 0.689 V and an FF of 67.14%. The OM6 treated champion device showed the maximum efficiency of 9.66%, with a similar JSC of 20.04 mA cm−2, a greatly increased VOC of 0.706 V and a slightly increased FF of 68.23%. It is worth mentioning that the photocurrent obtained from the integration of the external quantum efficiency (EQE) is in good agreement with the one obtained from the J–V measurements (see Fig. S30†). The enhancement of the device performance is mainly attributed to the higher VOC presented by the Sn-PSCs treated with the additives. Concretely, the average VOC (see Table S4†) increased from 0.57 V for the pristine FASnI3 perovskite to 0.67 V and 0.71 V for the devices treated with OM4 and OM6, respectively, and consequently resulted in a significant average PCE enhancement of 25% and 37%, respectively. This improvement in VOC, and ultimately in PCE, can be attributed to a reduced carrier recombination within Sn-OM4 and Sn-OM6 perovskite layers. This effect, at the same time, may be ascribed to the formation of more compact and pinhole-free perovskite layers, along with the passivation of the remaining defects present in the matrix,77 as already discussed after the characterization of perovskite layers. Moreover, control devices showed higher hysteresis behavior, which is suppressed upon the additive treatments, as shown in Fig. S31.† This can be linked to a lower trap density, which retards the ion migration across the perovskite film.78 Notably, it is also important to highlight the increase in reproducibility with the use of the additives, especially OM6, as Fig. S32† shows. This better reproducibility can be attributed to the enhanced pinhole-free morphology of Sn-OM6 films (see Fig. 1e).
Moreover, to better understand the role of defects and additives in the improved performance of our solar cells, we investigated FASnI3 surfaces with adsorbed OM4 and OM6 molecules by density functional theory (DFT) simulations. The atomic structures of the input surfaces and output densities of states (DOS) are given in Fig. S33 and S34,† respectively. The DOS (Fig. S34†) include the contribution from N atoms in OM4 molecules and from F atoms in OM6 molecules. Interestingly, the projected DOS reflect a contribution from N atomic orbitals to the conduction band minima which is missing, indeed, for F atoms. The strong localization of photocarriers at N sites can be considered as a plausible reason behind the lower average improvement of solar cell performance reached with the OM4 additive.
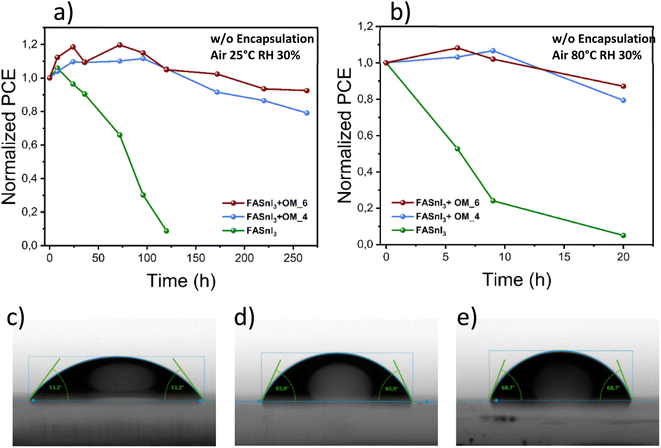
Finally, even more significant than the effect of the additives in the PCE for Sn-PSCs are the implications in the device long-term stability. Sn-PSCs have demonstrated a very good stability under nitrogen conditions,45 but a poor stability under ambient conditions. Consequently, in order to evaluate the stability of our devices under high stress conditions, we carried out shelf-stability tests in an oxygen atmosphere under normal conditions and under thermal stress. All the devices were tested without any encapsulation. Shelf-stability tests were carried out at RH = 30% and 25 °C. After 24 hours, control devices only retained 80% of their initial power conversion efficiency and completely degraded after just 72 hours. On the other hand, OM4 treated devices maintained 80% of their initial PCE for 220 h. More impressively, OM6 treated devices retained over 90% of their initial PCE for more than 250 h, which is among the highest air stabilities reported for pure 3D-phase FASnI3 perovskites (see Fig. 4a and Table S5†). Additionally, we also kept track of the stability of the unencapsulated devices at 80 °C in air and a complete degradation of the control devices was observed after less than 10 h, while the devices containing OM4 or OM6 retained more than 85% of their initial PCE after more than 20 h (see Fig. 4b). This outstanding stability, here reported, is attributed to the high hydrophobicity of the additives, and their intrinsic and widely reported polymerization ability,55–58 which act as protective barriers impeding water molecules from permeating and degrading the perovskite active layer. Also, the presence of hydrophobic aromatic rings within their structure, and additional fluorine atoms in the case of OM6, make both of them exceptional barriers to physically but not electronically isolate the perovskite layer. As shown in Fig. 4c–e, the enhanced hydrophobicity of the Sn-based perovskite films when OM4 or OM6 is present was demonstrated by performing water contact angle measurements. It can be observed that the contact angle of water drops over the pristine FASnI3 film increased from 53.2° to 65.0° and 68.7° when OM4 or OM6 was added, respectively. As expected, the hydrophobicity of the Sn-OM6 film was higher compared to that of Sn-OM4 due to the presence of the well-known highly hydrophobic 1,2,4,5-tetrafluorobenzene unit.79,80
Conclusions
In summary, we developed and synthesized two novel organic additives containing different functional groups – a pyridine unit (OM4) and a 1,2,4,5-tetrafluorobenzene unit (OM6)-, which were incorporated into Sn-based perovskite solar cells as additives. Although both additives showed positive effects in the final performance of the devices, our findings indicated that the fluorinated ring located in OM6 exhibits stronger coordination with the tin perovskite precursors compared to the more widely used pyridine unit (OM4), resulting in enhanced structural, optical and morphological properties. Consequently, a significant improvement in the performance of the solar cell devices was observed; in particular, the average PCE was increased 25% and 37%, with respect to the average PCE of the FASnI3 control cell, when OM4 or OM6 was incorporated as an additive, respectively.Moreover, due to the highly hydrophobic nature of the organic additives, and likely their polymerization capabilities, a notorious enhancement in the stability of the devices was observed. Notably, even under ambient conditions without any encapsulation, the devices containing OM4 and OM6 preserved up to 80% and 90% of their initial PCE, respectively, for over 250 hours, whereas the pristine FASnI3 devices suffered a complete degradation after 72 hours under the same conditions. Hence, this study contributes to the understanding of the role of different functional groups present in organic additives commonly utilized in tin perovskite solar cells, thereby paving the way for future rational molecular design of additives in this field. The additive inclusion strategy is shown to be a successful approach to bypass the limitations of PCE and long-term stability of Sn-PSCs.
Data availability
Data for this article are available at zenodo repository at https://doi.org/10.5281/zenodo.12733691.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially funded by the Generalitat Valenciana via European Union-NextGeneration EU project Print-P (MFA/2022/020). S. H. T. C. gratefully acknowledges funding from the Ministry of Science and Innovation of Spain under Ayudas Ramón y Cajal (RYC2022-035578-I) for financial assistance during this work. J. R. P. acknowledges the Ministry of Education, Youth and Sports of the Czech Republic, for the financial support of XPS measurements using the CEMNAT infrastructure (project LM 2023037). J. L. D. acknowledges Ikerbasque, Basque Foundation for Science, for an “Ikerbasque Research Associate” contract, the Polymat Foundation, and the Spanish Government (PID2021-129084OB-I00 and RED2022-134344-T grants).References
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells, J. Am. Chem. Soc., 2009, 131(17), 6050–6051 CrossRef CAS PubMed.
- J. Park, J. Kim, H.-S. Yun, M. J. Paik, E. Noh, H. J. Mun, M. G. Kim, T. J. Shin and S. I. Seok, Controlled growth of perovskite layers with volatile alkylammonium chlorides, Nature, 2023, 616(7958), 724–730 CAS.
- https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.pdf .
- H. S. Jung and N.-G. Park, Perovskite Solar Cells: From Materials to Devices, Small, 2015, 11(1), 10–25 CrossRef CAS PubMed.
- J. Y. Kim, J.-W. Lee, H. S. Jung, H. Shin and N.-G. Park, High-Efficiency Perovskite Solar Cells, Chem. Rev., 2020, 120(15), 7867–7918 CrossRef CAS PubMed.
- J. Jeong, M. Kim, J. Seo, H. Lu, P. Ahlawat, A. Mishra, Y. Yang, M. A. Hope, F. T. Eickemeyer, M. Kim, Y. J. Yoon, I. W. Choi, B. P. Darwich, S. J. Choi, Y. Jo, J. H. Lee, B. Walker, S. M. Zakeeruddin, L. Emsley, U. Rothlisberger, A. Hagfeldt, D. S. Kim, M. Grätzel and J. Y. Kim, Pseudo-halide anion engineering for α-FAPbI3 perovskite solar cells, Nature, 2021, 592(7854), 381–385 CAS.
- S. Collavini, S. F. Völker and J. L. Delgado, Understanding the Outstanding Power Conversion Efficiency of Perovskite-Based Solar, Cells, 2015, 54(34), 9757–9759 CAS.
- A. Demayo, M. C. Taylor, K. W. Taylor, P. V. Hodson and P. B. Hammond, Toxic effects of lead and lead compounds on human health, aquatic life, wildlife plants, and livestock, CRC Crit. Rev. Environ. Control, 1982, 12(4), 257–305 CrossRef CAS.
- G. Li, Y.-T. Liu, F. Yang, M. Li, Z. Zhang, J. Pascual, Z.-K. Wang, S.-Z. Wei, X.-Y. Zhao, H.-R. Liu, J.-B. Zhao, C.-T. Lin, J.-M. Li, Z. Li, A. Abate and I. Cantone, Biotoxicity of Halide Perovskites in Mice, Adv. Mater., 2024, 36(2), 2306860 CrossRef CAS PubMed.
- N. Moody, S. Sesena, D. W. deQuilettes, B. D. Dou, R. Swartwout, J. T. Buchman, A. Johnson, U. Eze, R. Brenes, M. Johnston, C. L. Haynes, V. Bulović and M. G. Bawendi, Assessing the Regulatory Requirements of Lead-Based Perovskite Photovoltaics, Joule, 2020, 4(5), 970–974 CrossRef.
- G. Schileo and G. Grancini, Lead or no lead? Availability, toxicity, sustainability and environmental impact of lead-free perovskite solar cells, J. Mater. Chem. C, 2021, 9(1), 67–76 RSC.
- I. Kopacic, B. Friesenbichler, S. F. Hoefler, B. Kunert, H. Plank, T. Rath and G. Trimmel, Enhanced Performance of Germanium Halide Perovskite Solar Cells through Compositional Engineering, ACS Appl. Energy Mater., 2018, 1(2), 343–347 CrossRef CAS.
- T. Krishnamoorthy, H. Ding, C. Yan, W. L. Leong, T. Baikie, Z. Zhang, M. Sherburne, S. Li, M. Asta, N. Mathews and S. G. Mhaisalkar, Lead-free germanium iodide perovskite materials for photovoltaic applications, J. Mater. Chem. A, 2015, 3(47), 23829–23832 RSC.
- W. Hu, X. He, Z. Fang, W. Lian, Y. Shang, X. Li, W. Zhou, M. Zhang, T. Chen, Y. Lu, L. Zhang, L. Ding and S. Yang, Bulk heterojunction gifts bismuth-based lead-free perovskite solar cells with record efficiency, Nano Energy, 2020, 68, 104362 CrossRef CAS.
- J. Chen, J. Luo, E. Hou, P. Song, Y. Li, C. Sun, W. Feng, S. Cheng, H. Zhang, L. Xie, C. Tian and Z. Wei, Efficient tin-based perovskite solar cells with trans-isomeric fulleropyrrolidine additives, Nat. Photonics, 2024, 18, 464–470 CrossRef CAS.
- S. Shao, J. Liu, G. Portale, H.-H. Fang, G. R. Blake, G. H. ten Brink, L. J. A. Koster and M. A. Loi, Highly Reproducible Sn-Based Hybrid Perovskite Solar Cells with 9% Efficiency, Adv. Energy Mater., 2018, 8(4), 1702019 CrossRef.
- Z. Chen, J. J. Wang, Y. Ren, C. Yu and K. Shum, Schottky solar cells based on CsSnI3 thin-films, Appl. Phys. Lett., 2012, 101(9), 093901 CrossRef.
- J. Seo, T. Song, S. Rasool, S. Park and J. Y. Kim, An Overview of Lead, Tin, and Mixed Tin–Lead-Based ABI3 Perovskite Solar Cells, Adv. Energy Sustainability Res., 2023, 4(5), 2200160 CrossRef CAS.
- A. Filippetti, S. Kahmann, C. Caddeo, A. Mattoni, M. Saba, A. Bosin and M. A. Loi, Fundamentals of tin iodide perovskites: a promising route to highly efficient, lead-free solar cells, J. Mater. Chem. A, 2021, 9(19), 11812–11826 RSC.
- R. Shannon, Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides, Acta Crystallogr., Sect. A, 1976, 32(5), 751–767 CrossRef.
- L. M. Herz, Charge-Carrier Mobilities in Metal Halide Perovskites: Fundamental Mechanisms and Limits, ACS Energy Lett., 2017, 2(7), 1539–1548 CrossRef CAS.
- N. K. Noel, S. D. Stranks, A. Abate, C. Wehrenfennig, S. Guarnera, A.-A. Haghighirad, A. Sadhanala, G. E. Eperon, S. K. Pathak, M. B. Johnston, A. Petrozza, L. M. Herz and H. J. Snaith, Lead-free organic–inorganic tin halide perovskites for photovoltaic applications, Energy Environ. Sci., 2014, 7(9), 3061–3068 RSC.
- H.-H. Fang, S. Adjokatse, S. Shao, J. Even and M. A. Loi, Long-lived hot-carrier light emission and large blue shift in formamidinium tin triiodide perovskites, Nat. Commun., 2018, 9(1), 243 CrossRef PubMed.
- W. Shockley and H. J. Queisser, Detailed Balance Limit of Efficiency of p-n Junction Solar Cells, J. Appl. Phys., 1961, 32(3), 510–519 CrossRef CAS.
- S. Rühle, Tabulated values of the Shockley–Queisser limit for single junction solar cells, Sol. Energy, 2016, 130, 139–147 CrossRef.
- C. Ponti, G. Nasti, D. Di Girolamo, I. Cantone, F. A. Alharthi and A. Abate, Environmental lead exposure from halide perovskites in solar cells, Trends Ecol. Evol., 2022, 37(4), 281–283 CrossRef CAS PubMed.
- L. Lanzetta, T. Webb, N. Zibouche, X. Liang, D. Ding, G. Min, R. J. E. Westbrook, B. Gaggio, T. J. Macdonald, M. S. Islam and S. A. Haque, Degradation mechanism of hybrid tin-based perovskite solar cells and the critical role of tin (IV) iodide, Nat. Commun., 2021, 12(1), 2853 CrossRef CAS PubMed.
- T. Leijtens, R. Prasanna, A. Gold-Parker, M. F. Toney and M. D. McGehee, Mechanism of Tin Oxidation and Stabilization by Lead Substitution in Tin Halide Perovskites, ACS Energy Lett., 2017, 2(9), 2159–2165 CrossRef CAS.
- D. J. Kubicki, D. Prochowicz, E. Salager, A. Rakhmatullin, C. P. Grey, L. Emsley and S. D. Stranks, Local Structure and Dynamics in Methylammonium, Formamidinium, and Cesium Tin(II) Mixed-Halide Perovskites from 119Sn Solid-State NMR, J. Am. Chem. Soc., 2020, 142(17), 7813–7826 CrossRef CAS PubMed.
- J. Li, H.-L. Cao, W.-B. Jiao, Q. Wang, M. Wei, I. Cantone, J. Lü and A. Abate, Biological impact of lead from halide perovskites reveals the risk of introducing a safe threshold, Nat. Commun., 2020, 11(1), 310 CrossRef CAS PubMed.
- X. Liu, T. Wu, X. Luo, H. Wang, M. Furue, T. Bessho, Y. Zhang, J. Nakazaki, H. Segawa and L. Han, Lead-Free Perovskite Solar Cells with Over 10% Efficiency and Size 1 cm2 Enabled by Solvent–Crystallization Regulation in a Two-Step Deposition Method, ACS Energy Lett., 2022, 7(1), 425–431 CrossRef CAS.
- F. Hao, C. C. Stoumpos, P. Guo, N. Zhou, T. J. Marks, R. P. H. Chang and M. G. Kanatzidis, Solvent-Mediated Crystallization of CH3NH3SnI3 Films for Heterojunction Depleted Perovskite Solar Cells, J. Am. Chem. Soc., 2015, 137(35), 11445–11452 CrossRef CAS PubMed.
- G. Nasti, M. H. Aldamasy, M. A. Flatken, P. Musto, P. Matczak, A. Dallmann, A. Hoell, A. Musiienko, H. Hempel, E. Aktas, D. Di Girolamo, J. Pascual, G. Li, M. Li, L. V. Mercaldo, P. D. Veneri and A. Abate, Pyridine Controlled Tin Perovskite Crystallization, ACS Energy Lett., 2022, 7(10), 3197–3203 CrossRef CAS PubMed.
- J. Hidalgo, W. Kaiser, Y. An, R. Li, Z. Oh, A.-F. Castro-Méndez, D. K. LaFollette, S. Kim, B. Lai, J. Breternitz, S. Schorr, C. A. R. Perini, E. Mosconi, F. De Angelis and J.-P. Correa-Baena, Synergistic Role of Water and Oxygen Leads to Degradation in Formamidinium-Based Halide Perovskites, J. Am. Chem. Soc., 2023, 145(45), 24549–24557 CAS.
- G. Xie, L. Xu, L. Sun, Y. Xiong, P. Wu and B. Hu, Insight into the reaction mechanism of water, oxygen and nitrogen molecules on a tin iodine perovskite surface, J. Mater. Chem. A, 2019, 7(10), 5779–5793 RSC.
- Z. Zhang, Y. Huang, J. Jin, Y. Jiang, Y. Xu, J. Zhu and D. Zhao, Mechanistic Understanding of Oxidation of Tin-based Perovskite Solar Cells and Mitigation Strategies, Angew. Chem., Int. Ed., 2023, 62(45), e202308093 CrossRef CAS PubMed.
- L. Lanzetta, N. Aristidou and S. A. Haque, Stability of Lead and Tin Halide Perovskites: The Link between Defects and Degradation, J. Phys. Chem. Lett., 2020, 11(2), 574–585 CrossRef CAS PubMed.
- J. Pascual, G. Nasti, M. H. Aldamasy, J. A. Smith, M. Flatken, N. Phung, D. Di Girolamo, S.-H. Turren-Cruz, M. Li, A. Dallmann, R. Avolio and A. Abate, Origin of Sn(ii) oxidation in tin halide perovskites, Adv. Mater., 2020, 1(5), 1066–1070 RSC.
- W. Ke, C. C. Stoumpos and M. G. Kanatzidis, “Unleaded” Perovskites: Status Quo and Future Prospects of Tin-Based Perovskite Solar Cells, Adv. Mater., 2019, 31(47), 1803230 CrossRef CAS PubMed.
- C. C. Stoumpos, C. D. Malliakas and M. G. Kanatzidis, Semiconducting Tin and Lead Iodide Perovskites with Organic Cations: Phase Transitions, High Mobilities, and Near-Infrared Photoluminescent Properties, Inorg. Chem., 2013, 52(15), 9019–9038 CrossRef CAS PubMed.
- E. Jokar, C.-H. Chien, A. Fathi, M. Rameez, Y.-H. Chang and E. W.-G. Diau, Slow surface passivation and crystal relaxation with additives to improve device performance and durability for tin-based perovskite solar cells, Energy Environ. Sci., 2018, 11(9), 2353–2362 RSC.
- S. Hu, P. Zhao, K. Nakano, R. D. J. Oliver, J. Pascual, J. A. Smith, T. Yamada, M. A. Truong, R. Murdey, N. Shioya, T. Hasegawa, M. Ehara, M. B. Johnston, K. Tajima, Y. Kanemitsu, H. J. Snaith and A. Wakamiya, Synergistic Surface Modification of Tin–Lead Perovskite Solar Cells, Adv. Mater., 2023, 35(9), 2208320 CrossRef CAS PubMed.
- H. Yan, B. Wang, X. Yan, Q. Guan, H. Chen, Z. Shu, D. Wen and Y. Cai, Efficient passivation of surface defects by lewis base in lead-free tin-based perovskite solar cells, Mater. Today Energy, 2022, 27, 101038 CrossRef CAS.
- Y. Shi, Z. Zhu, D. Miao, Y. Ding and Q. Mi, Interfacial Dipoles Boost Open-Circuit Voltage of Tin Halide Perovskite Solar Cells, ACS Energy Lett., 2024, 1895–1897 CrossRef CAS.
- J. Sanchez-Diaz, R. S. Sánchez, S. Masi, M. Kreĉmarová, A. O. Alvarez, E. M. Barea, J. Rodriguez-Romero, V. S. Chirvony, J. F. Sánchez-Royo, J. P. Martinez-Pastor and I. Mora-Seró, Tin perovskite solar cells with >1,300 h of operational stability in N2 through a synergistic chemical engineering approach, Joule, 2022, 6(4), 861–883 CrossRef CAS PubMed.
- T. Wang, Q. Tai, X. Guo, J. Cao, C.-K. Liu, N. Wang, D. Shen, Y. Zhu, C.-S. Lee and F. Yan, Highly Air-Stable Tin-Based Perovskite Solar Cells through Grain-Surface Protection by Gallic Acid, ACS Energy Lett., 2020, 5(6), 1741–1749 CrossRef CAS.
- F. Hu, C.-H. Chen, Y.-H. Lou, T.-Y. Teng, Y.-R. Shi, Y. Xia, K.-L. Wang, J. Chen, Z.-K. Wang and L.-S. Liao, A vertical antioxidant strategy for high performance wide band gap tin perovskite photovoltaics, J. Mater. Chem. A, 2023, 11(9), 4579–4586 RSC.
- F. Wang, X. Jiang, H. Chen, Y. Shang, H. Liu, J. Wei, W. Zhou, H. He, W. Liu and Z. J. J. Ning, 2D-quasi-2D-3D hierarchy structure for tin perovskite solar cells with enhanced efficiency and stability, Joule, 2018, 2(12), 2732–2743 CrossRef CAS.
- M. Li, W.-W. Zuo, Y.-G. Yang, M. H. Aldamasy, Q. Wang, S. H. T. Cruz, S.-L. Feng, M. Saliba, Z.-K. Wang and A. Abate, Tin Halide Perovskite Films Made of Highly Oriented 2D Crystals Enable More Efficient and Stable Lead-free Perovskite Solar Cells, ACS Energy Lett., 2020, 5(6), 1923–1929 CrossRef CAS.
- B.-B. Yu, Z. Chen, Y. Zhu, Y. Wang, B. Han, G. Chen, X. Zhang, Z. Du and Z. He, Heterogeneous 2D/3D Tin-Halides Perovskite Solar Cells with Certified Conversion Efficiency Breaking 14%, Adv. Mater., 2021, 33(36), 2102055 CrossRef CAS PubMed.
- S. Collavini, M. Saliba, W. R. Tress, P. J. Holzhey, S. F. Völker, K. Domanski, S. H. Turren-Cruz, A. Ummadisingu, S. M. Zakeeruddin, A. Hagfeldt, M. Grätzel and J. L. Delgado, Poly(ethylene glycol)–[60]Fullerene-Based Materials for Perovskite Solar Cells with Improved Moisture Resistance and Reduced Hysteresis, ChemSusChem, 2018, 11(6), 1032–1039 CrossRef CAS PubMed.
- T.-H. Han, J.-W. Lee, C. Choi, S. Tan, C. Lee, Y. Zhao, Z. Dai, N. De Marco, S.-J. Lee, S.-H. Bae, Y. Yuan, H. M. Lee, Y. Huang and Y. Yang, Perovskite-polymer composite cross-linker approach for highly-stable and efficient perovskite solar cells, Nat. Commun., 2019, 10(1), 520 CrossRef CAS PubMed.
- S. Collavini, A. Cabrera-Espinoza and J. L. Delgado, Organic Polymers as Additives in Perovskite Solar Cells, Macromolecules, 2021, 54(12), 5451–5463 CrossRef CAS.
- H. Uceta, A. Cabrera-Espinoza, M. Barrejón, J. G. Sánchez, E. Gutierrez-Fernandez, I. Kosta, J. Martín, S. Collavini, E. Martínez-Ferrero, F. Langa and J. L. Delgado, p-Type Functionalized Carbon Nanohorns and Nanotubes in Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2023, 15(38), 45212–45228 CrossRef CAS PubMed.
- K. Miyazaki and T. Horibe, Polymerization of multifunctional methacrylates and acrylates, J. Biomed. Mater. Res., 1988, 22(11), 1011–1022 CrossRef CAS PubMed.
- Z. Zhu, D. Zhao, C.-C. Chueh, X. Shi, Z. Li and A. K. Y. Jen, Highly Efficient and Stable Perovskite Solar Cells Enabled by All-Crosslinked Charge-Transporting Layers, Joule, 2018, 2(1), 168–183 CrossRef CAS.
- Z. Liu, F. Liu, C. Duan, L. Yuan, H. Zhu, J. Li, Q. Wen, G. I. N. Waterhouse, X. Yang and K. Yan, Polymerization stabilized black-phase FAPbI3 perovskite solar cells retain 100% of initial efficiency over 100 days, Chem. Eng. J., 2021, 419, 129482 CrossRef CAS.
- Y. Xu, G. Liu, J. Hu, G. Wang, M. Chen, Y. Chen, M. Li, H. Zhang and Y. Chen, In Situ Polymer Network in Perovskite Solar Cells Enabled Superior Moisture and Thermal Resistance, J. Phys. Chem. Lett., 2022, 13(16), 3754–3762 CrossRef CAS PubMed.
- A. Farooq, I. M. Hossain, S. Moghadamzadeh, J. A. Schwenzer, T. Abzieher, B. S. Richards, E. Klampaftis and U. W. Paetzold, Spectral Dependence of Degradation under Ultraviolet Light in Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2018, 10(26), 21985–21990 CrossRef CAS PubMed.
- S.-W. Lee, S. Kim, S. Bae, K. Cho, T. Chung, L. E. Mundt, S. Lee, S. Park, H. Park, M. C. Schubert, S. W. Glunz, Y. Ko, Y. Jun, Y. Kang, H.-S. Lee and D. Kim, UV Degradation and Recovery of Perovskite Solar Cells, Sci. Rep., 2016, 6(1), 38150 CrossRef CAS PubMed.
- J. Wei, Q. Wang, J. Huo, F. Gao, Z. Gan, Q. Zhao and H. Li, Mechanisms and Suppression of Photoinduced Degradation in Perovskite Solar Cells, Adv. Energy Mater., 2021, 11(3), 2002326 CrossRef CAS.
- B. Li, X. Wu, H. Zhang, S. Zhang, Z. Li, D. Gao, C. Zhang, M. Chen, S. Xiao, A. K. Y. Jen, S. Yang and Z. Zhu, Efficient and Stable Tin Perovskite Solar Cells by Pyridine-Functionalized Fullerene with Reduced Interfacial Energy Loss, Adv. Funct. Mater., 2022, 32(39), 2205870 CrossRef CAS.
- T. T. Ngo, I. Suarez, G. Antonicelli, D. Cortizo-Lacalle, J. P. Martinez-Pastor, A. Mateo-Alonso and I. Mora-Sero, Enhancement of the Performance of Perovskite Solar Cells, LEDs, and Optical Amplifiers by Anti-Solvent Additive Deposition, Adv. Mater., 2016, 1604056 Search PubMed.
- H. Dong, C. Ran, W. Gao, N. Sun, X. Liu, Y. Xia, Y. Chen and W. Huang, Crystallization Dynamics of Sn-Based Perovskite Thin Films: Toward Efficient and Stable Photovoltaic Devices, Adv. Energy Mater., 2022, 12(1), 2102213 CrossRef CAS.
- X. Meng, Y. Li, Y. Qu, H. Chen, N. Jiang, M. Li, D. J. Xue, J. S. Hu, H. Huang and S. Yang, Crystallization Kinetics Modulation of FASnI(3) Films with Pre-nucleation Clusters for Efficient Lead-Free Perovskite Solar Cells, Angew Chem. Int. Ed. Engl., 2021, 60(7), 3693–3698 CrossRef CAS PubMed.
- W. T. M. Van Gompel, R. Herckens, G. Reekmans, B. Ruttens, J. D'Haen, P. Adriaensens, L. Lutsen and D. Vanderzande, Degradation of the Formamidinium Cation and the Quantification of the Formamidinium–Methylammonium Ratio in Lead Iodide Hybrid Perovskites by Nuclear Magnetic Resonance Spectroscopy, J. Phys. Chem. C, 2018, 122(8), 4117–4124 CrossRef CAS.
- Y. Su, J. Yang, H. Rao, Y. Zhong, W. Sheng, L. Tan and Y. Chen, Environmentally friendly anti-solvent engineering for high-efficiency tin-based perovskite solar cells, Energy Environ. Sci., 2023, 16(5), 2177–2186 RSC.
- J. Pascual, S. Collavini, S. F. Völker, N. Phung, E. Palacios-Lidon, L. Irusta, H.-J. Grande, A. Abate, R. Tena-Zaera and J. L. Delgado, Unravelling fullerene–perovskite interactions introduces advanced blend films for performance-improved solar cells, Sustain. Energy Fuels, 2019, 3(10), 2779–2787 RSC.
- K. Nishimura, M. A. Kamarudin, D. Hirotani, K. Hamada, Q. Shen, S. Iikubo, T. Minemoto, K. Yoshino and S. Hayase, Lead-free tin-halide perovskite solar cells with 13% efficiency, Nano Energy, 2020, 74, 104858 CrossRef CAS.
- T. C. Taucher, I. Hehn, O. T. Hofmann, M. Zharnikov and E. Zojer, Understanding Chemical versus Electrostatic Shifts in X-ray Photoelectron Spectra of Organic Self-Assembled Monolayers, J. Phys. Chem. C, 2016, 120(6), 3428–3437 CrossRef CAS PubMed.
- L. Lanzetta, L. Gregori, L. H. Hernandez, A. Sharma, S. Kern, A. M. Kotowska, A.-H. Emwas, L. Gutiérrez-Arzaluz, D. J. Scurr, M. Piggott, D. Meggiolaro, M. A. Haque, F. De Angelis and D. Baran, Dissociative Host-Dopant Bonding Facilitates Molecular Doping in Halide Perovskites, ACS Energy Lett., 2023, 8(7), 2858–2867 CrossRef CAS.
- F. Hao, C. C. Stoumpos, D. H. Cao, R. P. H. Chang and M. G. Kanatzidis, Lead-free solid-state organic–inorganic halide perovskite solar cells, Nat. Photonics, 2014, 8(6), 489–494 CrossRef CAS.
- G. Zheng, C. Zhu, J. Ma, X. Zhang, G. Tang, R. Li, Y. Chen, L. Li, J. Hu, J. Hong, Q. Chen, X. Gao and H. Zhou, Manipulation of facet orientation in hybrid perovskite polycrystalline films by cation cascade, Nat. Commun., 2018, 9(1), 2793 CrossRef PubMed.
- S. J. Kim, J. Byun, T. Jeon, H. M. Jin, H. R. Hong and S. O. Kim, Perovskite Light-Emitting Diodes via Laser Crystallization: Systematic Investigation on Grain Size Effects for Device Performance, ACS Appl. Mater. Interfaces, 2018, 10(3), 2490–2495 CrossRef CAS PubMed.
- Z. Zhang, L. Wang, A. Kumar Baranwal, S. Razey Sahamir, G. Kapil, Y. Sanehira, M. Akmal Kamarudin, K. Nishimura, C. Ding, D. Liu, Y. Li, H. Li, M. Chen, Q. Shen, T. S. Ripolles, J. Bisquert and S. Hayase, Enhanced efficiency and stability in Sn-based perovskite solar cells by trimethylsilyl halide surface passivation, J. Energy Chem., 2022, 71, 604–611 CrossRef CAS.
- E. V. Péan, S. Dimitrov, C. S. De Castro and M. L. Davies, Interpreting time-resolved photoluminescence of perovskite materials, Phys. Chem. Chem. Phys., 2020, 22(48), 28345–28358 RSC.
- K. T. Cho, S. Paek, G. Grancini, C. Roldán-Carmona, P. Gao, Y. Lee and M. K. Nazeeruddin, Highly efficient perovskite solar cells with a compositionally engineered perovskite/hole transporting material interface, Energy Environ. Sci., 2017, 10(2), 621–627 RSC.
- H. J. Snaith, A. Abate, J. M. Ball, G. E. Eperon, T. Leijtens, N. K. Noel, S. D. Stranks, J. T.-W. Wang, K. Wojciechowski and W. Zhang, Anomalous Hysteresis in Perovskite Solar Cells, J. Phys. Chem. Lett., 2014, 5(9), 1511–1515 CrossRef CAS PubMed.
- A. Kumar, J. Mahato, M. Dixit and G. N. Patwari, Progressive Hydrophobicity of Fluorobenzenes, J. Phys. Chem. B, 2019, 123(47), 10083–10088 CrossRef CAS PubMed.
- S. Valero, T. Soria, N. Marinova and J. L. Delgado, Efficient and stable perovskite solar cells based on perfluorinated polymers, Polym. Chem., 2019, 10(42), 5726–5736 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta03291h |
| This journal is © The Royal Society of Chemistry 2024 |