Open Access Article
Open Access ArticleTransparent and colour-neutral luminescent solar concentrators using bright Eu3+ supramolecular cages towards photovoltaic windows†
Irene
Motta
 a,
Gregorio
Bottaro
a,
Gregorio
Bottaro
 *bc,
Maria
Rando
*bc,
Maria
Rando
 a,
Marzio
Rancan
a,
Marzio
Rancan
 *bc,
Roberta
Seraglia
d and
Lidia
Armelao
*bc,
Roberta
Seraglia
d and
Lidia
Armelao
 ace
ace
aDepartment of Chemical Sciences (DiSC), University of Padova, via F. Marzolo 1, 35131, Padova, Italy
bInstitute of Condensed Matter Chemistry and Technologies for Energy (ICMATE), National Research Council (CNR), c/o Department of Chemical Sciences (DiSC), University of Padova, via F. Marzolo 1, 35131, Padova, Italy
cNational Interuniversity Consortium of Materials Science and Technology (INSTM), Florence, Italy
dInstitute of Condensed Matter Chemistry and Technologies for Energy (ICMATE), National Research Council (CNR), Corso Stati Uniti, 4, 35127, Padova PD, Italy
eDepartment of Chemical Sciences and Materials Technologies (DSCTM), National Research Council (CNR), Piazzale A. Moro 7, 00185, Roma, Italy
First published on 3rd June 2024
Abstract
The integration of photovoltaic (PV) devices into the built environment has become a key factor for the development of energy efficient buildings. Luminescent solar concentrators (LSCs) are well suited for this application, as they could be installed over architectural elements inaccessible to conventional PVs. In the present work, we report the synthesis of super-bright [Eu2L4]2− cages bearing bis-β-diketonate ligands and their subsequent embedding in polymethylmethacrylate (PMMA) by casting of 5 × 5 × 0.27 cm3 planar LSCs. With a measured average visible transmittance (AVT) of 92%, the proposed LSCs possess excellent transparency and aesthetic quality fit for glass destined for neutral colour applications. Moreover, the obtained materials absorb from 60% to 90% of solar UV radiation, exposure to which is harmful for human health and responsible for fading of building interiors. The performance of devices attained by edge-coupling LSC slabs with monocrystalline silicon solar cells matches that of classic Eu3+-based concentrators. Nonetheless, the employment of super-bright [Eu2L4]2− luminophores allows the reduction of the Eu3+ ion content of our devices down to 0.01% wt. This denotes a most efficient use of a valuable material like europium in the developed LSC-PVs, as Eu3+ weight contents reported in relevant literature were one to two orders of magnitude higher.
Introduction
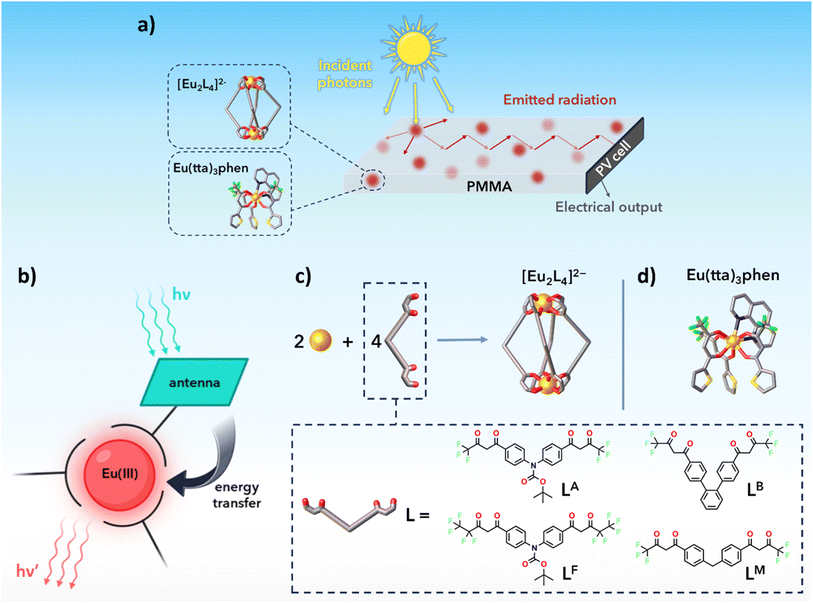
Developing energy-efficient infrastructures towards decarbonisation of buildings1,2 requires innovative renewable energy technologies. Among these, building-integrated photovoltaics aim to directly integrate PV systems into constructions as a replacement for conventional building components.3 Luminescent solar concentrators (LSCs) are light-management devices that have largely been proposed for the realization of transparent or semi-transparent BIPVs.4–8 They consist of slabs of glassy or polymeric material doped or coated with luminescent species (luminophores), whose emission can be stimulated by sunlight incident on the slate's surface. The emitted radiation is then waveguided towards the edges of the device, where thin strips of conventional PV material collect it and convert it into electrical power (Fig. 1a). The versatility in materials, shapes and coloration makes LSCs suitable for installation over a wide range of architectures, where constrains in terms of functionality and appearance limit or prevent the introduction of classic PV modules. This becomes especially true in the case of windows, for which tunability in colour and transparency is highly requested. The latest efforts in designing LSCs suitable for photovoltaic windows have been directed towards highly transparent and colourless materials, to ensure seamless integration in most buildings destined for housing and working.3,9–12 Provided the use of highly transparent matrices, among which PMMA is the most popular option in LSC studies, the choice of luminophore plays a determinant role in the appearance and optical quality of the LSC. While a broad absorption spectrum is generally a desirable feature in LSC emitters, absorption of visible light reaches a trade-off with the coloration of the device. Therefore, UV or NIR selective absorbers are necessary to produce low- or non-tinted materials. Lanthanide antenna complexes (Fig. 1b) are suitable candidates to obtain transparent and colourless LSCs, as the ligand absorption range covers, in most cases, the UV spectral region.13 This class of luminophores presents other advantages, such as intense sensitized emission and very large pseudo-Stokes shifts (>200 nm). Zero overlap between the luminophore absorption and emission is highly desirable for LSC scale-up, since re-absorption losses are one of the main drawbacks encountered with larger modules, thus hindering their development beyond the lab-scale.6,14Complexes of rare earth ions with β-diketonates have become a widespread choice due to the versatility in molecular design conferred by this class of ligands, and the relative ease in their synthesis and functionalization.15,16 The literature on lanthanide-based LSCs is mainly centred on β-diketonates,17–19 and more specifically Eu3+ and Tb3+ since they display the highest photoluminescence quantum yields (PLQY) among the rare earth series.20
Herein, we report the synthesis and characterization of highly transparent LSCs based on a series of supramolecular, binuclear lanthanide bis-β-diketonates with the general formula [Ln2L4]2− (L = LA, LB, LM, LF) and structure depicted in Fig. 1c. Eu(tta)3phen (tta = thenoyltrifluoroacetone and phen = 1,10-phenanthroline, Fig. 1d) is also included in the study, due to its documented use in the literature regarding lanthanide-based LSCs.17,18 PMMA has been adopted as the host medium, due to its high transmittance in the UV and in the visible region. Emitters have been dissolved in a methyl methacrylate (MMA)/PMMA syrup that has been then poured into a casting mould and successively polymerized to prepare the LSCs. Spectroscopic characterisation of the luminophores has been conducted before and after embedment in the polymeric matrix. Average visible transmission (AVT), colour rendering index (CRI) and CIELAB coordinates have been derived from the transmittance spectra of the fabricated LSCs. The prepared LSCs were used to study the behaviour of the devices obtained by edge-coupling the luminescent planar waveguides with monocrystalline Si solar cells. Device performances have been evaluated according to the latest published protocols21,22 and terminology23 used in LSC research. A perspective on the literature regarding Eu3+-based concentrators is also offered, with the aim to assess the stand of the present study over the past-decade results.
Experimental
Experimental details for luminophore synthetic procedures, single crystal X-ray diffraction and mass spectrometry are given in the ESI.†LSC fabrication
The LSCs have been prepared through bulk radical polymerization of methyl methacrylate (MMA) solutions containing the desired amount of luminophore, 20 wt% of commercial polymethylmethacrylate powder (PMMA, AMW = 350![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) and 0.1 wt% of azobisisobutyronitrile (AIBN). The polymerization has been conducted in a water bath set at 60 °C for 18 hours, where the casting solution had previously been transferred into a casting mold composed of two glass plates clamped together, separated by a PVC plastic gasket. After extraction of the polymerised tile from the mold, the gasket has been cut off and the edges have been polished to yield the finished LSC.
000 g mol−1) and 0.1 wt% of azobisisobutyronitrile (AIBN). The polymerization has been conducted in a water bath set at 60 °C for 18 hours, where the casting solution had previously been transferred into a casting mold composed of two glass plates clamped together, separated by a PVC plastic gasket. After extraction of the polymerised tile from the mold, the gasket has been cut off and the edges have been polished to yield the finished LSC.
Photophysical characterization
UV/vis absorption spectra were recorded on a CARY5000 double-beam spectrophotometer in the 300–800 nm range, with a spectral bandwidth of 2 nm. Transmittance (T(λ)) and reflectance (R(λ)) spectra were recorded on the same instrument, equipped with a poly(tetrafluoroethylene)-coated integration sphere, and with the same parameters. Absorptance (A(λ)) spectra have been calculated as A(λ) = 1 − T(λ) − R(λ).Photoluminescence spectra were collected on a Horiba JobinYvon Fluorolog-3 spectrofluorimeter equipped with double-grating monochromators on the excitation side and iHR320 spectrograph on the emission side. A R928P Hamamatsu photomultiplier or Horiba Sincerity CCD detectors were employed. A 450 W Xe arc lamp was used as the excitation source. PLQY measurements were performed on the same instrument equipped with a poly(tetrafluoroethylene)-coated integration sphere, using a 90° excitation-collection geometry.
Electrical characterization
Current–voltage (I–V) curves of the LSC-PVs were collected under simulated solar irradiation (100 mW cm−2, AM1.5G) with a Keithley 2450 Graphical Source Measure Unit (SMU). Monocrystalline Si cells coupled to the LSCs were purchased from IXYS Corporation (series IXOLAR™ SolarBIT, model KXOB22-12X1F). External quantum efficiency (EQE) measurements were performed on a custom-assembled setup, composed of a UV/vis transmitting optical fibre (Ocean Insight QP1000-2-UV/Vis, 300–1100 nm) coupled to the Horiba JobinYvon Fluorolog-3 spectrofluorimeter, and an appropriate sample and PV cell holder. Incident light was focused on the LSC surface (spot diameter 1 mm) through a lens doublet. The focusing optics have been mounted on a XYZ linear stage allowing alignment of the light spot and scanning over the whole sample surface (25 mm2). Incident power readings were acquired through a Gentec-EO Pronto Si power meter, while current readings were collected with a Keithley 2450 Graphical SourceMeter SMU. In both J–V and EQE measurements, LSCs and Si cells were optically coupled with optical grade silicone grease.Results and discussion
Luminophore synthesis
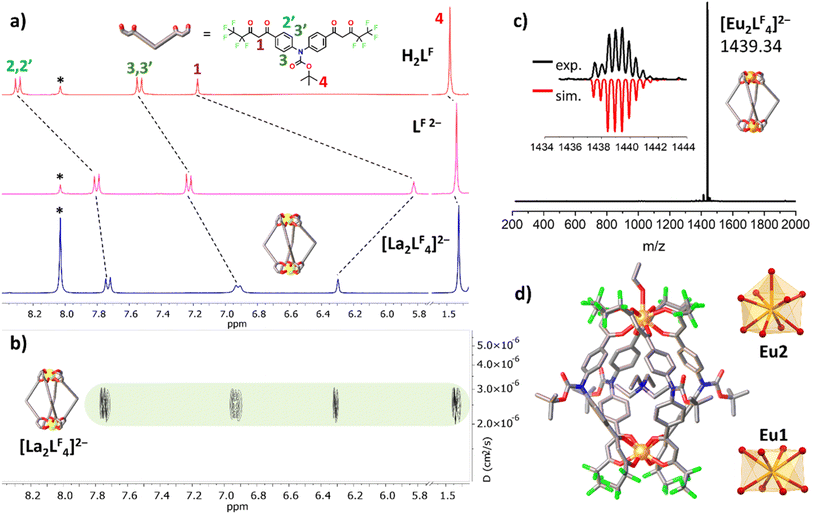
Ligands LB, LM and LA (Fig. 1c) were prepared as previously reported.24,25 The ligand LF (Fig. 1c) is a modified version of LA bearing an additional –CF2– unit in the fluorinated terminal chains, to increase the cage solubility. This new ligand was prepared by a two step procedure (Cu(I) catalysed N–C coupling reaction followed by Claisen condensation) and fully characterized by NMR (Fig. 2a), single crystal XRD (Fig. S1 and Table S1†) and electrospray mass spectrometry (ESI-MS, Fig. S3†). Deprotonation of the ligand with tetraethylammonium hydroxide (TEAOH) and subsequent addition of a Eu3+ inorganic salt leads to the self-assembly of quadruple-stranded cages. We already reported elsewhere24,25 the detailed synthesis and characterization (NMR, ESI-MS and single crystal XRD) for the cages based on LB, LM and LA. The synthesis and characterization of the new {[Eu2LF4](NEt4)2} cage is here discussed. The formation of the cage has been followed and assessed through 1H-NMR spectroscopy. The spectra of the ligand (H2LF, LF![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2−) and [La2LF4]2− analogue are displayed in Fig. 2a. Upon deprotonation, all ligand signals experience an upfield shift. The most pronounced effect is seen in proton H1, located at the α position of the β-diketonate moiety. Following La3+ ion coordination, the cage exhibits a single set of signals, consistent with the ligand's C2 symmetry and the mean C4 symmetry of the quadruple-stranded [La2LF4]2− architecture. To provide dimensional information, diffusion-ordered NMR spectroscopy (DOSY) was conducted, as shown in Fig. 2b. The calculated hydrodynamic diameter for [La2LF4]2− is 22.2 Å. The Eu3+ cage gives a very clean ESI-MS spectrum with only one signal due to the [Eu2LF4]2− double-negative charged species (Fig. 2c). The crystal structure of the newly synthetized {[Eu2LF4](NEt4)2} has been determined by single-crystal X-ray diffraction (Fig. 2d and S2†). The H⋯H distances of the tert-butyl groups of opposite ligands are in the range 21.3–22.8 Å. This value agrees with the hydrodynamic diameter calculated from the DOSY experiment (22.2 Å) for the La3+ cage. As previously observed for the [Eu2LM4]2−, [Eu2LB4]2− (ref. 24) and [Eu2LA4]2− cages,25 one of the two tetraethylammonium cations is hosted by the quadruple stranded cage. In such quadruple-stranded Ln3+ cages, the ligand scaffold and guest molecule can influence the formation of helicates or mesocates.26 The cage [Eu2LF4]2− is a helicate with both the right-handed (P, ΔΔ) and left-handed (M, ΛΛ) isomers in a 1
2−) and [La2LF4]2− analogue are displayed in Fig. 2a. Upon deprotonation, all ligand signals experience an upfield shift. The most pronounced effect is seen in proton H1, located at the α position of the β-diketonate moiety. Following La3+ ion coordination, the cage exhibits a single set of signals, consistent with the ligand's C2 symmetry and the mean C4 symmetry of the quadruple-stranded [La2LF4]2− architecture. To provide dimensional information, diffusion-ordered NMR spectroscopy (DOSY) was conducted, as shown in Fig. 2b. The calculated hydrodynamic diameter for [La2LF4]2− is 22.2 Å. The Eu3+ cage gives a very clean ESI-MS spectrum with only one signal due to the [Eu2LF4]2− double-negative charged species (Fig. 2c). The crystal structure of the newly synthetized {[Eu2LF4](NEt4)2} has been determined by single-crystal X-ray diffraction (Fig. 2d and S2†). The H⋯H distances of the tert-butyl groups of opposite ligands are in the range 21.3–22.8 Å. This value agrees with the hydrodynamic diameter calculated from the DOSY experiment (22.2 Å) for the La3+ cage. As previously observed for the [Eu2LM4]2−, [Eu2LB4]2− (ref. 24) and [Eu2LA4]2− cages,25 one of the two tetraethylammonium cations is hosted by the quadruple stranded cage. In such quadruple-stranded Ln3+ cages, the ligand scaffold and guest molecule can influence the formation of helicates or mesocates.26 The cage [Eu2LF4]2− is a helicate with both the right-handed (P, ΔΔ) and left-handed (M, ΛΛ) isomers in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in the unit cell. Finally, the crystal structure evidences that the two Eu centres have different coordination numbers (Fig. 2d). Both the ions are tetrakis-chelated by four β-diketonate groups, but Eu2 also has the ninth site occupied by an ethanol molecule. We previously25 calculated that solvent molecules such as water and ethanol are loosely bonded to the Ln3+ ion and hence can be present or absent according to the experimental conditions. Tables S2 and S3† report the result of the stereochemical analysis by means of continuous shape measures27 showing that the eight-coordinated centre has a square antiprismatic coordination geometry while the nine-coordinated ion has a capped square antiprismatic coordination geometry (Fig. 2d).
1 ratio in the unit cell. Finally, the crystal structure evidences that the two Eu centres have different coordination numbers (Fig. 2d). Both the ions are tetrakis-chelated by four β-diketonate groups, but Eu2 also has the ninth site occupied by an ethanol molecule. We previously25 calculated that solvent molecules such as water and ethanol are loosely bonded to the Ln3+ ion and hence can be present or absent according to the experimental conditions. Tables S2 and S3† report the result of the stereochemical analysis by means of continuous shape measures27 showing that the eight-coordinated centre has a square antiprismatic coordination geometry while the nine-coordinated ion has a capped square antiprismatic coordination geometry (Fig. 2d).
The synthesis of Eu(tta)3phen comprised a first deprotonation of tta using NaOH and consequent addition of the Eu3+ salt to the mixture of phen and deprotonated tta. The desired product was obtained by employing a metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tta
tta![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) NaOH
NaOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) phen molar ratio of 1
phen molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3
3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. ESI-MS (Fig. S4†) confirmed the nature of the synthesised product.
1. ESI-MS (Fig. S4†) confirmed the nature of the synthesised product.
Absorption and emission in MMA solutions
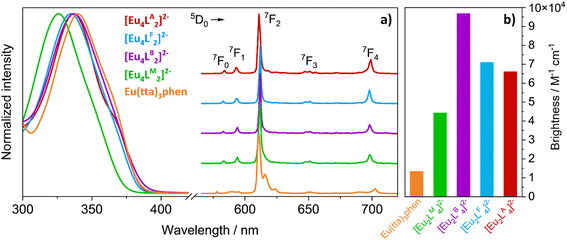
The photophysics of the luminophores has been first investigated in MMA, since it is the reaction solvent used in the preparation of the LSCs.UV/vis absorption and photoluminescence (PL) spectra (Fig. 3a) have confirmed effective sensitization through the antenna effect: absorption of light is carried out entirely by the β-diketonate ligands in the 300–380 nm region, resulting in a ligand-centred excitation, while the sole PL profile observed is that of the Eu3+ ion. The Eu3+ PL spectrum is composed of five sharp peaks, corresponding to the transitions originating from the 5D0 excited state towards the 7FJ (J = 0, 1, 2, 3, and 4) ground state. The 5D0 → 7F2 hypersensitive transition at 612 nm dominates the emission spectrum of Eu3+ β-diketone complexes and is responsible for the red luminescence.15,28 Overlap between absorption and emission is null, with a pseudo-Stokes shift of ≈200 nm.
When selecting the most promising luminescent complexes/molecules for the development of LSCs, we must consider both the values of the photoluminescence quantum yield (PLQY) and the absorption coefficient (ε). Often, seeking the best emitters, there is a tendency to consider only the PLQY. However, this is a partial evaluation since it only takes into account the fraction of emitted photons but does not provide information on the number of absorbed photons (indicated by ε). The parameter that should be maximized is the number of photons emitted per unit area and per unit time. For molecules and complexes in solution, this parameter is well represented by the brightness (B = ε × PLQY),29,30i.e., the product of the absorption coefficient and the emission quantum yield. The luminophores here discussed have similar absorption and emission spectra and hence it is sufficient to compare the brightness values relative to the maximum absorption (Fig. 3b and Table 1). In MMA solutions, [Eu2LB4]2− is the brightest complex of the family with B = 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 M−1 cm−1, followed by [Eu2LA4]2− and [Eu2LF4]2−, having a B of around 70
880 M−1 cm−1, followed by [Eu2LA4]2− and [Eu2LF4]2−, having a B of around 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1, and then [Eu2LM4]2− with B close to 45
000 M−1 cm−1, and then [Eu2LM4]2− with B close to 45![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1. The difference in brightness between Eu(tta)3phen (13
000 M−1 cm−1. The difference in brightness between Eu(tta)3phen (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 M−1 cm−1) and the binuclear lanthanide bis-β-diketonates [Eu2L4]2− (L = LA, LB, LM) is noteworthy, reaching about one order of magnitude. Considering the brightness values in MMA solutions, the most promising candidate for the development of LSCs seems to be [Eu2LB4]2−. Prior to material synthesis and characterization, we investigated the compatibility of the complexes with the PVC gasket used as lateral walls of the LSC casting mould employed for MMA polymerization. Gasket strips (approximately 1 × 3 × 0.3 cm3) were immersed in MMA solutions of the investigated luminophores, at the same concentrations used for LSC castings. After 20 h (approximately the polymerization time) the PVC pieces were removed from the solutions, dried at room temperature and observed under a UV lamp at 365 nm to detect eventual traces of the cages. The PVC strip immersed in the [Eu2LB4]2− solution displayed a markedly brighter luminescence with respect to the others, suggesting a strong affinity between [Eu2LB4]2− and PVC and eventually possible issues during material synthesis. Moreover, we observed that solubility of Eu(tta)3phen in MMA is about two orders of magnitude larger than those of [Eu2LA4]2−, [Eu2LB4]2− and [Eu2LM4]2− (Table 1). To address this aspect, we synthesized ligand LF by slightly modifying LA in order to obtain higher cage solubility in MMA, achieving in the end a twenty-fold increase in it. Once again, the compatibility of the corresponding bright europium complex, [Eu2LF4]2−, with the PVC gasket was investigated, detecting no type of interaction.
500 M−1 cm−1) and the binuclear lanthanide bis-β-diketonates [Eu2L4]2− (L = LA, LB, LM) is noteworthy, reaching about one order of magnitude. Considering the brightness values in MMA solutions, the most promising candidate for the development of LSCs seems to be [Eu2LB4]2−. Prior to material synthesis and characterization, we investigated the compatibility of the complexes with the PVC gasket used as lateral walls of the LSC casting mould employed for MMA polymerization. Gasket strips (approximately 1 × 3 × 0.3 cm3) were immersed in MMA solutions of the investigated luminophores, at the same concentrations used for LSC castings. After 20 h (approximately the polymerization time) the PVC pieces were removed from the solutions, dried at room temperature and observed under a UV lamp at 365 nm to detect eventual traces of the cages. The PVC strip immersed in the [Eu2LB4]2− solution displayed a markedly brighter luminescence with respect to the others, suggesting a strong affinity between [Eu2LB4]2− and PVC and eventually possible issues during material synthesis. Moreover, we observed that solubility of Eu(tta)3phen in MMA is about two orders of magnitude larger than those of [Eu2LA4]2−, [Eu2LB4]2− and [Eu2LM4]2− (Table 1). To address this aspect, we synthesized ligand LF by slightly modifying LA in order to obtain higher cage solubility in MMA, achieving in the end a twenty-fold increase in it. Once again, the compatibility of the corresponding bright europium complex, [Eu2LF4]2−, with the PVC gasket was investigated, detecting no type of interaction.
| ε max × 105/M−1 cm−1 | PLQY | B/M−1 cm−1 | Solubility/mM | |
|---|---|---|---|---|
| a PLQY and εmax were not measured for these samples in the polymeric matrices because [Eu2LB4]2− and [Eu2LM4]2− give rise to issues during material synthesis and were not employed to develop LSC devices (for details see LSC preparation and characterization section). | ||||
| [Eu2LA4]2− | 1.61 (1.65) | 0.41 (0.39) | 66![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 010 010 |
0.0184 |
| [Eu2LF4]2− | 1.42 (1.43) | 0.50 (0.53) | 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
0.321 |
| [Eu2LB4]2− | 1.73 (—a) | 0.56 (—a) | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 880 880 |
0.0195 |
| [Eu2LM4]2− | 1.01 (—a) | 0.48 (—a) | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 480 480 |
0.0216 |
| Eu(tta)3phen | 0.50 (0.53) | 0.27 (0.25) | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
5.02 |
LSC preparation and characterization
All luminophores have been incorporated into PMMA through a water bath casting procedure with the use of an appropriate casting mould (see the Experimental section) to prepare planar square LSCs with an active area of 5 × 5 cm2 and thickness of 0.27 cm. A geometric gain (G) of 4.63 has been calculated through eqn (1), where Ain is the incident solar irradiance area, also referred to as the LSC active area, and Aout is the light collection area, usually the area of the LSC edges.23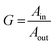 | (1) |
An active area of 25 cm2 has been chosen because it has been proposed as a reasonable standard size for easily comparing results between independent LSC studies on a laboratory scale.22,31 The amount of available luminophores for the castings depended on their solubility in MMA, which was found to be largely different in the investigated luminophores. Maximum solubilities achieved for Eu(tta)3phen and [Eu2LF4]2− in MMA were 0.5 wt% and 0.1 wt%, respectively, while those of the other cages [Eu2LA4]2−, [Eu2LB4]2− and [Eu2LM4]2− were two orders of magnitude lower. Five samples have hence been prepared using each system at 0.005 wt%, and an additional LSC has been prepared for both Eu(tta)3phen and [Eu2LF4]2− at their solubility limit. An undoped PMMA tile has also been fabricated. Labelling of the prepared LSCs is reported in Table S4.†
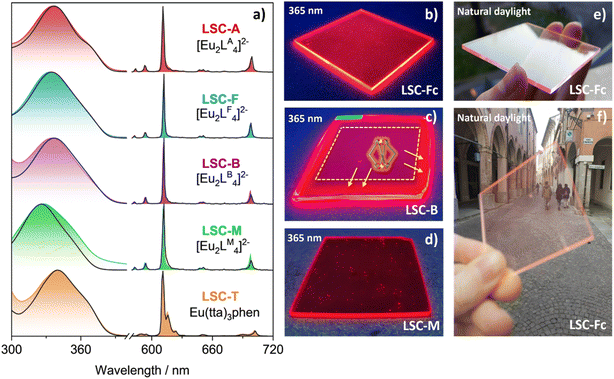
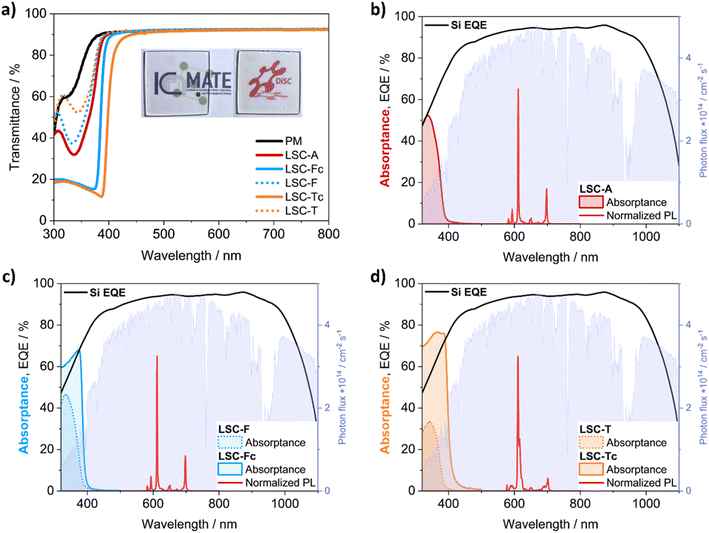
Absorption spectra of the luminophores in PMMA (Fig. 4a) match the absorbance profiles collected for the MMA solutions. Upon UV excitation at λ = 365 nm, all LSCs displayed bright red luminescence and concentration of the emitted light on the tiles edges (Fig. 4b–d). The same light guiding properties could already be appreciated by the naked eye for all doped LSCs under daylight (Fig. 4e and f).
Since the Eu3+ emission spectrum is sensitive to the ion's coordination site28 and displays different shapes accordingly, such a feature has been adopted as a qualitative tool to detect possible alterations in the luminophores' structure caused by embedment into the PMMA matrix. The PL profiles recorded for LSCs and solutions well agree with one another for all the investigated Eu3+ complexes, meaning that neither the PMMA matrix nor material processing affects the structural integrity of such luminophores. Accordingly, absorption coefficients and PLQY are fully consistent between solutions and PMMA tiles (Table 1). The resultant LSC-A, LSC-F and LSC-T were homogeneous and defect-free. Instead, LSC-B showed luminophore segregation towards its edges, as observed under 365 nm excitation (Fig. 4c). Such spatial distribution was ascribed to a preferential absorption of the luminophore into the PVC gasket used to seal the casting mould, thus determining loss of control over the loading of [Eu2LB4]2− into PMMA. This behaviour was already presumed by the reported preliminary PVC swelling tests. When illuminated with UV light, LSC-M (Fig. 4d) exhibits several bright spots dispersed randomly throughout the polymeric matrix. These bright spots are due to the precipitation of [Eu2LM4]2−, likely occurring during polymerization, within the matrix. Such behaviours make these complexes not suitable for device development. We emphasize that material processing may affect single luminophores differently, despite small differences in the molecular structure of the ligands as in the present case. The solubility increase obtained by introducing a –CF2– moiety in the terminal chain of the LA ligand (Fig. 1c) is noteworthy. Hence, the suitability of an emitter for the LSC application should be evaluated based on its characterization after embedment into the matrix of choice.
Considering the application field of the studied materials, their aesthetic appearance is of paramount importance, especially if they are intended as replacements for residential or shop windows. The parameters used to quantitatively assess this aspect are (i) the Average Visible Transmittance (AVT), which measures the amount of visible light transmitted by the LSC, (ii) the Colour Rendering Index (CRI), which quantifies how accurately the colours of objects are reproduced when illuminated with a particular light source, and (iii) (a*, b*) CIELAB coordinates, which define the colour of the material in a perceptually uniform space (a* gives the colour variation along the green-red axis and b* along the yellow-blue one). For the determination of these parameters, AM1.5G and AM1.5G × T(λ) (T(λ) being the transmittance spectrum of the material) are used as source spectra.32
Fig. 5a shows the UV/vis transmittance spectra of the [Eu2LA4]2−, [Eu2LF4]2− and Eu(tta)3phen doped LSCs, as well as that of undoped PMMA. The fabricated LSCs show very high transparency, as they transmit all visible light with AVT ≈ 92%. They also determine minimal visual impact on the transmitted light, as calculated CRI values are above 98 for all samples (Table S5†).
A recent survey on a wide inventory of commercially available and mass-market glass products has provided reference key levels for the aesthetic quality of transparent window products in the glazing industry.10 A CRI ≥ 85, and CIELAB coordinates of −7 < a* < 0 and −3 < b* < 7 have been found as acceptable standards for products destined for neutral colour applications. Besides presenting (a*, b*) coordinates widely within the specified intervals, our samples possess excellent colour and imaging fidelity, where the attribute “excellent” refers to CRI values falling within the 95–100 interval.10 Furthermore, the whole series of doped LSCs shows a CRI and CIELAB coordinates comparable to those of plain PMMA, indicating that the presence of the luminophores does not modify the visible appearance and the light transmitting properties of the pristine material.
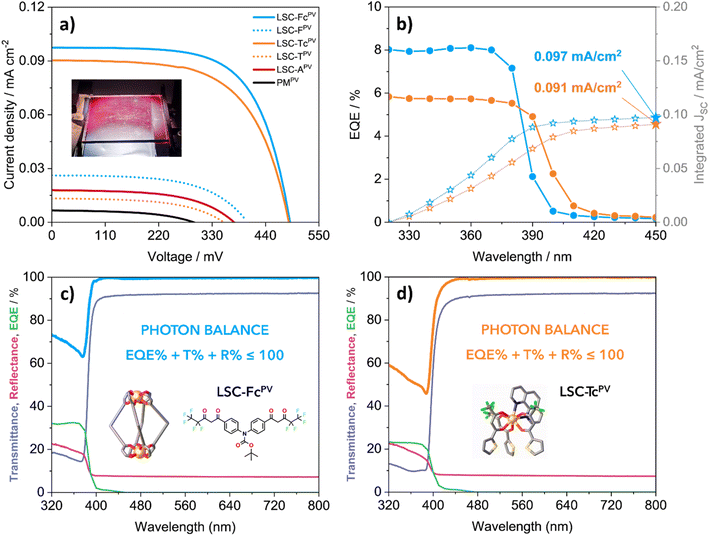
The optimal aesthetic quality of the LSCs containing [Eu2LF4]2− and Eu(tta)3phen remains unchanged even at higher luminophore loadings. LSC-Fc and LSC-Tc both retain high transparency and absence of heavy coloration while a higher luminophore content of 0.1 wt% and 0.5 wt%, respectively, allows them to harvest more light on a wider portion of the UV region and is responsible for a larger total absorptance (eqn (2) and (3)) over the less concentrated samples (Table S5†). Absorption onsets, in fact, shift from 380 nm up to 395 nm and 410 nm, respectively. To better evidence the light absorption capability of the materials it is convenient to refer to the total absorptance ηs,abs, defined as follows:23,33
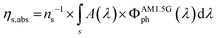 | (2) |
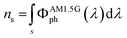 | (3) |
LSC-Fc and LSC-Tc have ηs,abs values of 1.11% and 1.81% (eqn (2) and (3)), respectively, enabling them to absorb up to 90% of the available UV photons. Notably, UV photons in the 280–400 nm range constitute only about 2% of the AM1.5G spectrum when expressed in terms of photon flux.
Given the large gap between absorbing and emitting regions displayed by the investigated LSCs (Fig. 4 and 5), the possibility of self-absorption losses, arising from the sensible luminophore loading increase in LSC-Fc and LSC-Tc, can be safely excluded. Consequently, these two samples are expected to produce significantly higher electrical outputs when coupled to Si cells in the LSC-PV configuration with respect to their less concentrated analogues. Additionally, UV-blocking capabilities are highly desirable for residential and shop windows to prevent fading of furniture, artwork, and flooring. This is typically achieved by applying passive window treatments that function solely by absorbing UV light. Luminescent solar concentrators based on europium antenna complexes offer an innovative approach, transforming passive elements into active ones by converting blocked UV radiation into visible light while also generating electricity.
Device characterization
As previously stated, all functional characterization studies have been conducted in compliance with the guidelines published in recent literature regarding luminescent solar concentrators.21,31 The same terminology reviewed by Warner et al.23 has been here adopted to address the various LSC figures of merit and their related quantities (Table S6;† for labelling of LSC slabs and relative devices see Table S4†). To derive the recommended LSC-PV reporting parameters from experimental data, we developed a Matlab Live Script that can be freely downloaded (see Data availability section).The current density–voltage (J–V) characteristics of the LSC-PVs were investigated by illuminating the LSC active area and optically coupling one of its edges to two monocrystalline, single module Si cells with an active area of 2.2 × 0.7 cm2 each. An apposite cell holder was designed to lodge the 0.27 cm thick LSC edge for the coupling, while covering the PV cell excess area to avoid any direct illumination from the light source. The uncoupled edges were masked with black tape and a matte black background was used.
The J–V curves are shown in Fig. 6a, while the short-circuit current density (JSC), the open-circuit voltage (VOC), the fill factor (FF), the maximum power (PMAX) and the Power Conversion Efficiency (PCE, eqn (4)) are listed in Table 2.w
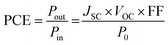 | (4) |
| J SC/mA cm−2 | V OC/mV | FF | P MAX/mW | PCE/% 1 edge (4 edges)b | η int /% | ||
|---|---|---|---|---|---|---|---|
| Experimental | Calculated from EQEa | ||||||
| a Eqn (6). b Calculated by multiplying PCE by 4. c Defined in eqn (7) and (8). | |||||||
| LSC-FcPV | 0.097 | 0.097 | 490 | 0.65 | 0.780 | 0.031 (0.124) | 55 |
| LSC-TcPV | 0.090 | 0.091 | 488 | 0.62 | 0.680 | 0.027 (0.108) | 39 |
| PMPV | 0.007 | — | 292 | 0.50 | 0.024 | 0.001 (0.004) | — |
The highest JSC, VOC and Pmax values were observed for LSC-FcPV and LSC-TcPV indicating that higher luminophore loadings positively affect the electrical outputs. In particular, for LSC-FcPV we observed a maximum output power of 0.780 mW. PCE values for LSC-FcPV and LSC-TcPV stand one order of magnitude higher than those of LSC-APV, LSC-FPV and LSC-TPV.
We focus the following discussion on LSC-FcPV and LSC-TcPV, i.e., on the best performing devices among the series. The data relative to the devices based on lower concentrated LSC tiles can be found in Table S7.†
The two doped samples produce comparable electrical outputs, with slightly better performance exhibited by LSC-FcPV. About 4% of the calculated PCEs arises from simple waveguiding of the incident white light by the PMMA matrix, as deduced from the study of the undoped LSC. Since J–V measurements were performed following the suggested guidelines, only one LSC edge has been coupled with solar cells. A multiplicative correction factor of 4 can be applied to estimate the energy that would be collected if all four edges were PV-coupled.21 The recalculated PCE values would then equal to 0.124% and 0.108% for LSC-FcPV and LSC-TcPV, respectively. It is noteworthy that the former produces higher electrical outputs, even if by a small margin, than the latter although containing one fifth of its wt% concentration of luminophores.
Position dependent External Quantum Efficiency (EQE) spectra (Fig. 6b) have also been collected within the luminophores absorption range by means of a custom-built measurement station. The EQE of a photovoltaic device (eqn (5)) evaluates its capability to convert incident photons into charge carriers, and is proportional to the ratio between the produced electrical current (I(λ)) and the incident electrical power (P(λ)) at each given wavelength, also known as the spectral response (SR(λ)).
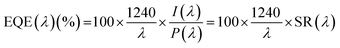 | (5) |
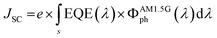 | (6) |
When both EQE and J–V curves are available, a consistency check for LSC-PV devices can be performed by comparing the experimental JSC value with that calculated from the EQE spectrum by means of eqn (6). This comparison is important because it reduces the possibility of photocurrent overestimation due, for example, to a non-perfect masking of the PV cell from direct illumination. Since such artifacts may be difficult to detect, this consistency check should be used to validate the measured data. Integrated JSC values for LSC-FcPV and LSC-TcPV are reported in Fig. 6b and in Table 2. We found a very good agreement between experimental and integrated JSC values, thus validating the experimental data and consequently the whole measurement setup. Furthermore, the good agreement between the measured and calculated JSC values confirms the absence of scattering centres or particulates inside the waveguides, already assessed qualitatively by the naked eye and quantitatively through AVT determination.
The EQE spectra present similar shapes to the absorptance spectra and have the same absorption onsets (Fig. S6†). Any examined LSC-PV device should satisfy the photon balance A(λ) + T(λ) + R(λ) = 1 at all wavelengths, with A(λ), T(λ) and R(λ) being the absorptance, transmittance and reflectance spectrum, respectively. Since the EQE(λ) quantifies how many of the incident photons on the LSC-PV generate a charge carrier pair, its value can at best match that of A(λ), at each wavelength. Given the relation EQE(λ) ≤ A(λ), independent measurements of EQE, transmittance and reflectance should always satisfy the condition EQE(λ) + T(λ) + R(λ) ≤ 1.21,32 This provides a useful consistency check for the photon balance of the device, which is fulfilled for both LSC-FcPV and LSC-TcPV (Fig. 6c and d).
After correction for the four sides, maximum EQE values become ≈32% at 360 nm and ≈23% at 320 nm for LSC-FcPV and LSC-TcPV, respectively. LSC-FcPV displays higher EQE than LSC-TcPV, which yields the larger integrated JSC between the two LSC-PVs, and consequently the higher electrical output (Fig. 6a). Conversely, the latter has a lower EQE but a wider absorption range which allows it to capture an additional portion of the AM1.5G spectrum, wherein the photon population rapidly increases. This produces a J–V curve and a PCE value close to those of LSC-FcPV, despite the considerable gap between EQEs. Widening of absorption bands due to increased luminophore loadings is then a favourable factor for extracting more electrical power, provided that the LSC aesthetic quality is preserved. Further functionalization of [Eu2L4]2− cages oriented towards increased solubility in MMA could facilitate full utilization of the UV region by the proposed LSC-PVs.
Internal photon efficiency (ηint), defined in eqn (7) where nout is the number of photons emitted at the edges and nabs is the number of photons absorbed from the active area, was derived from collected electrical parameters according to a verified method (eqn (8)).23,33
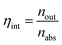 | (7) |
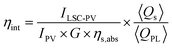 | (8) |
Calculated internal photon efficiencies are 55% and 39% for LSC-FcPV and LSC-TcPV, respectively. Since ηint accounts for the optical losses of the sole LSC (i.e., excluding the coupling to the photovoltaic component), scattering, PLQY and re-absorption are the three main factors contributing to this efficiency. Scattering and re-absorption losses have been found to be absent from our LSC-PVs, so the better ηint of LSC-FcPV is ascribed to the higher PLQY of [Eu2LF4]2− with respect to Eu(tta)3phen.
Furthermore, we performed some preliminary outdoor stability tests on LSC-Fc and LSC-Tc by exposing them, south-facing, to atmospheric ageing in Padova (Italy) from September to November 2023. During this period, the average temperature during the day went from 20–25 °C (Sep, Oct) to 15 °C (Nov). The average (2006–2022) Global Horizontal Irradiation (GHI) in Padova for September, October and November are 4.343, 2.737, and 1.542 kW h m−2, respectively.34 At sample retrieval, a drop in the measured ISC of 20% and 30% was observed for LSC-Fc and LSC-Tc, respectively.
We finally compared the performance of our devices with that of other published Eu3+-based concentrators (Table 3). Being recommended as the primary parameter to describe a LSC-PV, we chose the PCE as a metric for comparison.23 To include precedent studies to the publication of the reporting guidelines,21,23,31 where absent, PCE values have been calculated by us using experimental data provided in the cited references. To accurately compare PCE data, the device structure needs careful consideration: (i) the number of PV-coupled edges should be clearly specified, (ii) an efficient shielding from direct illumination on the edge-coupled photovoltaic cells and a matte black backdrop are required, and (iii) for measurements relative to one edge, the other three should be blackened. This configuration minimizes the contribution of stray light (light not generated by the LSC luminescence) to the photocurrent measurement.
| Luminophore | Matrix | G | Device structure | PCE/% | Eu3+/wt% | |
|---|---|---|---|---|---|---|
| a Value not reported in the publication, calculated based on provided experimental data. | ||||||
| This work | [Eu2LF4](NEt4)2 | PMMA | 4.63 | 5.0 × 5.0 × 0.27 cm3 bulk tile | 0.031 | 0.01 |
| This work | Eu(tta)3phen | PMMA | 4.63 | 5.0 × 5.0 × 0.27 cm3 bulk tile | 0.027 | 0.08 |
| Dev-A (ref. 17) | Eu(tta)3phen | Poly vinylbutyral (PVB) | 6.50 | Film (thickness not specified) deposited onto 7.8 × 7.8 × 0.3 cm3 glass | 0.044 | 0.24 |
| Eu(tta)3Dpbt | 0.050 | 0.20 | ||||
| Dev-B (ref. 19) | [B(TMSP)Im] | PMMA | 7.85 | 5.1 μm thick film deposited onto 7.5 × 2.0 × 0.1 cm3 glass | 0.002 | 2.28 |
| [Eu(tta)4] | ||||||
| Dev-C (ref. 35) | Eu(2mCND)3 | Siloxane-polyether (di-ureasil) | 2.89 | 320 μm thick film deposited onto 5.0 × 5.0 × 0.4 cm3 acrylic | 0.138a | 1.05 |
Concerning the literature examples reported in Table 3, all the data are relative to one edge measurements, but device structures are quite different. While Dev-A has blackened edges and the backdrop is not specified, in Dev-B both edges and background are reflective. The edges of Dev-C are not taped and a white paper foil is used as reflective backdrop, allowing a 92% enhancement of the output power, as declared by the authors of the study.36 To date, finding literature data on devices having analogous structures is quite demanding. The comparison between the results of our study and the literature shows that LSC-FcPV and LSC-TcPV produce electrical outputs of the same order of magnitude of most published Eu3+-based LSC-PVs. This is a significant result, considering that the europium weight content of our devices (0.01 wt% for LSC-FcPV and 0.08 wt% for LSC-TcPV) is inferior to that found in the explored literature down to a one hundred factor.
The presented literature survey compares our LSC-PVs to other analogous Eu3+-based systems with aesthetic properties (high transparency and lack of colour) comparable to ours. Interestingly, Jin et al.37 recently reported LSCs with very similar aesthetic quality, based on a different type of emitter such as Ag,Mn:ZnInS2/ZnS quantum dots (QDs). Moreover, this study followed the suggested reporting guidelines as well, allowing for a direct comparison of device metrics. The QD-based LSCs possess an AVT of 90% and CRI of 95.8, and has a PCE (4 edges) of 0.09%. Such values closely resemble our results, indicating that the devices herein developed rank competitively among the best UV-selective LSC technologies. Owing to the significantly higher intensity of solar radiation in the visible region compared to the ultraviolet region, LSCs based on UV absorbers inherently exhibit lower power conversion efficiencies than LSCs prepared employing visible absorbing luminophores.6,23,38–42 However, this gain in PCE inevitably comes at the expense of a decreased AVT and CRI, and therefore their applicability is strongly dependent on the context of use.
Conclusions
In conclusion, a series of highly transparent LSCs has been developed by incorporating a family of binuclear Eu3+ quadruple-stranded cages with bis-β-diketonate ligands, as well as a reference mononuclear Eu3+ β-diketonate, into PMMA. All the examined complexes retain their structural and photophysical characteristics throughout the study. However, it was possible to obtain viable LSCs only using Eu(tta)3phen and the [Eu2L4]2− cages bearing the LA ligand and its functionalized version LF. A minimal functionalization of the ligand determined a twenty-fold increase in the cage's solubility, which was a key factor in reaching higher luminophore loadings and consequently better light harvesting properties of the LSC.The obtained LSCs appear highly transparent and colourless, with excellent visible transmission and colour rendering properties, comparable to those of widely industrialized glazing products. The increased [Eu2LF4]2− loading makes LSC-FcPV the best performing sample in terms of PCE and EQE, with the latter exceeding 30% in the UV region. The increase in concentration does not affect transparency or colour neutrality of the material. The proposed materials are therefore promising for a seamless integration into buildings as photovoltaic windows, blocking UV light and producing energy while preserving functionality and aesthetics. Finally, the PCEs displayed by LSC-FcPV and LSC-TcPV are in line with those found in the literature regarding Eu3+-based concentrators, but in our case the same performances are obtained with the use of a sensibly lower quantity of the lanthanide ion, down to two orders of magnitude less in terms of Eu3+ weight concentration. Particularly for LSC-FcPV, these results open the way for deployment of LSCs based on europium as highly transparent BIPVs while minimizing its usage.
Data availability
The Matlab Live Script for the calculation of recommended LSC-PV reporting parameters and a sample dataset to test the functionality of the script can be downloaded from the following link: https://it.mathworks.com/matlabcentral/fileexchange/166426-luminescent-solar-concentrators-pv-characterization.Author contributions
Irene Motta; investigation, formal analysis, writing – original draft, writing – review & editing, software. Gregorio Bottaro; conceptualization, investigation, formal analysis, writing – original draft, writing – review & editing, software. Maria Rando; investigation, formal analysis, writing – review & editing. Marzio Rancan; conceptualization, investigation, formal analysis, writing – review & editing. Roberta Seraglia; investigation, formal analysis, writing – review & editing. Lidia Armelao; conceptualization, funding acquisition, supervision, writing – review & editing.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This study is a result of the research project nuovi Concetti, mAteriali e tecnologie per l'iNtegrazione del fotoVoltAico negli edifici in uno scenario di generazione diffuSa' [CANVAS], funded by the Italian Ministry of the Environment and the Energy Security, through the Research Fund for the Italian Electrical System (type-A call, published on G. U. R. I. no. 192 on 18-08-2022). G. B. and M. R. thank the National Research Council PROGETTI@CNR P@CNR_01_TerMoSmart, FOE2020 ‘Capitale naturale e risorse per il futuro dell’Italia’, FOE2022 FuturRaw. L. A. thanks the University of Padova P-DiSC#01-BIRD2021 for financial support. Dr Tobias Stürzer, Bruker AXS (Karlsruhe, Germany), is acknowledged for {[Eu2LF4](NEt4)2} single crystal X-ray data collection.References
- EC REPRESENTATIONS - European Green Deal: Commission proposes to boost renovation and decarbonisation of buildings, https://ec.europa.eu/newsroom/representations/items/730847/default, accessed December 30, 2023.
- European Parliament, Council of the European Union, Directorate-General for Energy: Directive 2010/31/EU of the European Parliament and of the Council of 19 May 2010 on the Energy Performance of Buildings, Off. J. Eur.Union, 2010 Search PubMed , http://data.europa.eu/eli/dir/2010/31/oj, accessed June 7, 2024.
- C. J. Traverse, R. Pandey, M. C. Barr and R. R. Lunt, Nat. Energy, 2017, 2, 849–860 CrossRef.
- M. G. Debije and P. P. C. Verbunt, Adv. Energy Mater., 2012, 2, 12–35 CrossRef CAS.
- F. Meinardi, F. Bruni and S. Brovelli, Nat. Rev. Mater., 2017, 2, 17072 CrossRef CAS.
- B. S. Richards and I. A. Howard, Energy Environ. Sci., 2023, 16, 3214–3239 RSC.
- R. A. S. Ferreira, S. F. H. Correia, A. Monguzzi, X. Liu and F. Meinardi, Mater. Today, 2020, 33, 105–121 CrossRef.
- J. Roncali, Adv. Energy Mater., 2020, 10, 2001907 CrossRef CAS.
- F. Meinardi, S. Ehrenberg, L. Dhamo, F. Carulli, M. Mauri, F. Bruni, R. Simonutti, U. Kortshagen and S. Brovelli, Nat. Photonics, 2017, 11, 177–185 CrossRef CAS.
- C. Yang, M. C. Barr and R. R. Lunt, Phys. Rev. Appl., 2022, 17, 034054 CrossRef CAS.
- V. Fiorini, N. Monti, G. Vigarani, G. Santi, F. Fasulo, M. Massi, L. Giorgini, A. B. Muñoz-García, M. Pavone, A. Pucci and S. Stagni, Dyes Pigm., 2021, 193, 109532 CrossRef CAS.
- K. Lee, H.-D. Um, D. Choi, J. Park, N. Kim, H. Kim and K. Seo, Cell Rep. Phys. Sci., 2020, 1, 100143 CrossRef CAS.
- S. F. H. Correia, V. De Zea Bermudez, S. J. L. Ribeiro, P. S. André, R. A. S. Ferreira and L. D. Carlos, J. Mater. Chem. A, 2014, 2, 5580–5596 RSC.
- L. R. Wilson, B. C. Rowan, N. Robertson, O. Moudam, A. C. Jones and B. S. Richards, Appl. Opt., 2010, 49, 1651–1661 CrossRef PubMed.
- K. Binnemans, in Handbook on the Physics and Chemistry of Rare Earths, Elsevier, 2005, vol. 35, pp. 107–272 Search PubMed.
- K. Wang, in Rare Earth Coordination Chemistry, John Wiley & Sons, Ltd, 2010, pp. 41–89 Search PubMed.
- X. Wang, T. Wang, X. Tian, L. Wang, W. Wu, Y. Luo and Q. Zhang, Sol. Energy, 2011, 85, 2179–2184 CrossRef CAS.
- M. Tonezzer, G. Maggioni, A. Campagnaro, S. Carturan, A. Quaranta, M. della Pirriera and D. Gutierrez Tauste, Prog. Photovoltaics, 2015, 23, 1037–1044 CAS.
- A. R. Frias, M. A. Cardoso, A. R. N. Bastos, S. F. H. Correia, P. S. André, L. D. Carlos, V. de Zea Bermudez and R. A. S. Ferreira, Energies, 2019, 12, 451 CrossRef CAS.
- L. Armelao, S. Quici, F. Barigelletti, G. Accorsi, G. Bottaro, M. Cavazzini and E. Tondello, Coord. Chem. Rev., 2010, 254, 487–505 CrossRef CAS.
- C. Yang, D. Liu and R. R. Lunt, Joule, 2019, 3, 2871–2876 CrossRef.
- C. Yang, H. A. Atwater, M. A. Baldo, D. Baran, C. J. Barile, M. C. Barr, M. Bates, M. G. Bawendi, M. R. Bergren, B. Borhan, C. J. Brabec, S. Brovelli, V. Bulović, P. Ceroni, M. G. Debije, J.-M. Delgado-Sanchez, W.-J. Dong, P. M. Duxbury, R. C. Evans, S. R. Forrest, D. R. Gamelin, N. C. Giebink, X. Gong, G. Griffini, F. Guo, C. K. Herrera, A. W. Y. Ho-Baillie, R. J. Holmes, S.-K. Hong, T. Kirchartz, B. G. Levine, H. Li, Y. Li, D. Liu, M. A. Loi, C. K. Luscombe, N. S. Makarov, F. Mateen, R. Mazzaro, H. McDaniel, M. D. McGehee, F. Meinardi, A. Menéndez-Velázquez, J. Min, D. B. Mitzi, M. Moemeni, J. H. Moon, A. Nattestad, M. K. Nazeeruddin, A. F. Nogueira, U. W. Paetzold, D. L. Patrick, A. Pucci, B. P. Rand, E. Reichmanis, B. S. Richards, J. Roncali, F. Rosei, T. W. Schmidt, F. So, C.-C. Tu, A. Vahdani, W. G. J. H. M. van Sark, R. Verduzco, A. Vomiero, W. W. H. Wong, K. Wu, H.-L. Yip, X. Zhang, H. Zhao and R. R. Lunt, Joule, 2022, 6, 8–15 CrossRef.
- T. Warner, K. P. Ghiggino and G. Rosengarten, Sol. Energy, 2022, 246, 119–140 CrossRef.
- M. Rancan, J. Tessarolo, A. Carlotto, S. Carlotto, M. Rando, L. Barchi, E. Bolognesi, R. Seraglia, G. Bottaro, M. Casarin, G. H. Clever and L. Armelao, Cell Rep. Phys. Sci., 2022, 3, 100692 CrossRef CAS.
- M. Rancan, M. Rando, L. Bosi, A. Carlotto, R. Seraglia, J. Tessarolo, S. Carlotto, G. H. Clever and L. Armelao, Inorg. Chem. Front., 2022, 9, 4495–4505 RSC.
- S. Carlotto, L. Armelao and M. Rancan, Int. J. Mol. Sci., 2022, 23, 10619 CrossRef CAS PubMed.
- M. Pinsky and D. Avnir, Inorg. Chem., 1998, 37, 5575–5582 CrossRef CAS PubMed.
- K. Binnemans, Coord. Chem. Rev., 2015, 295, 1–45 CrossRef CAS.
- K.-L. Wong, J.-C. G. Bünzli and P. A. Tanner, J. Lumin., 2020, 224, 117256 CrossRef CAS.
- International Union of Pure and Applied Chemistry (IUPAC), https://goldbook.iupac.org/terms/view/BT07338, accessed June 7, 2024.
- M. G. Debije, R. C. Evans and G. Griffini, Energy Environ. Sci., 2021, 14, 293–301 RSC.
- C. Yang, D. Liu, M. Bates, M. C. Barr and R. R. Lunt, Joule, 2019, 3, 1803–1809 CrossRef.
- H. Li, K. Wu, J. Lim, H.-J. Song and V. I. Klimov, Nat. Energy, 2016, 1, 16157 CrossRef CAS.
- Italian Solar Radiation Atlas, Italian National Agency for New Technologies, Energy and Sustainable Economic Development, ENEA, https://www.solaritaly.enea.it/indexEn.php, accessed May 23, 2024.
- Y. Wang, Y. Liu, G. Xie, J. Chen, P. Li, Y. Zhang and H. Li, ACS Appl. Mater. Interfaces, 2022, 14, 5951–5958 CrossRef CAS PubMed.
- Y. Liu, N. Li, R. Sun, W. Zheng, T. Liu, H. Li, Y. Chen, G. Liu, H. Zhao, H. Liu and Y. Zhang, Nano Energy, 2021, 85, 105960 CrossRef CAS.
- L. Jin, E. Hamzehpoor, G. S. Selopal, J. Liu, P. Kumar, D. Benetti, X. Tong, D. F. Perepichka, Z. M. Wang and F. Rosei, Small Methods, 2024, 2301695 CrossRef PubMed.
- M. Bartolini, C. Micheletti, A. Picchi, C. Coppola, A. Sinicropi, M. Di Donato, P. Foggi, A. Mordini, G. Reginato, A. Pucci, L. Zani and M. Calamante, ACS Appl. Energy Mater., 2023, 6, 4862–4880 CrossRef CAS PubMed.
- F. Meinardi, F. Bruni, C. Castellan, M. Meucci, A. M. Umair, M. La Rosa, J. Catani and S. Brovelli, Adv. Energy Mater., 2024, 14, 2304006 CrossRef CAS.
- J. Lin, L. Wang, Q. Jing and H. Zhao, Chem. Eng. J., 2024, 481, 148441 CrossRef CAS.
- X. Liu, D. Benetti, J. Liu, L. Jin and F. Rosei, Nano Energy, 2023, 111, 108438 CrossRef CAS.
- C. Yang, M. C. Barr and R. R. Lunt, Phys. Rev. Appl., 2022, 17, 034054 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2349361 and 2349362. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4ta03244f |
| This journal is © The Royal Society of Chemistry 2024 |