Open Access Article
Open Access ArticleComputational discovery of stable Na-ion sulfide solid electrolytes with high conductivity at room temperature†
Seong-Hoon
Jang
 *ab,
Randy
Jalem
*ab,
Randy
Jalem
 b and
Yoshitaka
Tateyama
b and
Yoshitaka
Tateyama
 bc
bc
aInstitute for Materials Research, Tohoku University, 2-1-1 Katahira, Aoba-ku, Sendai, 980-8577, Japan. E-mail: jang.seonghoon.b4@tohoku.ac.jp
bResearch Center for Energy and Environmental Materials (GREEN), National Institute for Materials Science (NIMS), 1-1 Namiki, Tsukuba, Ibaraki 305-0044, Japan
cLaboratory for Chemistry and Life Science, Tokyo Institute of Technology, 4259 Nagatsuta, Midori-ku, Yokohama, 226-8501, Japan
First published on 5th August 2024
Abstract
The search for inorganic solid electrolytes suitable for the realization of solid-state batteries with structural stability and high ion conductivity at room temperature remains a significant challenge. In this study, we employed a multi-stage density functional theory molecular dynamics (DFT-MD) sampling workflow, focusing on Na-ion sulfides  with trivalent (M) and pentavalent (M′) metal ions and an expanded selection of parent structures (Ω). This led to the identification of two promising sampling spaces (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4). The predictions were validated through multi-temperature DFT-MD calculations, wherein σNa,300K ≳ 10−3 S cm−1 are attained within a thermodynamic phase stability range of 9 < Ehull < 25 meV per atom (Ehull is convex hull decomposition energy): Na4Ga0.5P0.5S4, Na3.75Ga0.375P0.625S4, Na4.25Ga0.625P0.375S4, Na3.75Si0.75Ta0.25S4, Na3.625Si0.625Ta0.375S4, and Na3.5Si0.5Ta0.5S4. These compounds are highly suggested for experimental synthesis and investigation. Moreover, our brute-force and highly generalized sampling technique is expected to be applicable in uncovering other solid electrolyte classes, thus potentially contributing to the advancement of solid-state battery technology.
with trivalent (M) and pentavalent (M′) metal ions and an expanded selection of parent structures (Ω). This led to the identification of two promising sampling spaces (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4). The predictions were validated through multi-temperature DFT-MD calculations, wherein σNa,300K ≳ 10−3 S cm−1 are attained within a thermodynamic phase stability range of 9 < Ehull < 25 meV per atom (Ehull is convex hull decomposition energy): Na4Ga0.5P0.5S4, Na3.75Ga0.375P0.625S4, Na4.25Ga0.625P0.375S4, Na3.75Si0.75Ta0.25S4, Na3.625Si0.625Ta0.375S4, and Na3.5Si0.5Ta0.5S4. These compounds are highly suggested for experimental synthesis and investigation. Moreover, our brute-force and highly generalized sampling technique is expected to be applicable in uncovering other solid electrolyte classes, thus potentially contributing to the advancement of solid-state battery technology.
The quest to identify inorganic solid electrolytes (SEs) suited for solid-state batteries, characterized by structural stability, including experimental synthesizability and high ion conductivity at room temperature, remains a longstanding challenge. Among the various classes of solid electrolytes under investigation, Na-ion sulfides have recently gained significant attention. This increased interest can be attributed to the abundant presence of Na in the earth's crust and excellent mechanical performance while interfacing with active electrodes, such as formability, processability, and low elastic moduli.1–4 Moreover, the discovery of the high room-temperature Na-ion conductivity σNa,300K = 3.2 × 10−2 S cm−1 in Na2.88Sb0.88W0.12S4 underscores the potential for further advancements in the material search in this class,5 with other notable series.3,6–10
While numerous high-throughput sampling techniques have been rigorously developed for the material search of SEs,11–14 we built our own multi-stage density functional theory molecular dynamics (DFT-MD) sampling workflow in a bid to efficiently find stable Na-ion sulfides with high σNa,300K as demonstrated in our previous study.15 Encompassing various  ions [M and M′ denote two distinct metal ions characterized by varying valence states, denoted as ν(M) and ν(M′), respectively, and n, m, and m′ are the contents of Na-, M-, and M′-ions, respectively] while maintaining parent structures Ω (given by NanΩMmS4; nΩ is the content of Na ions in the structure), our analysis yielded that a significant proportion of several promising candidates with σNa,300K > 10−3 S cm−1 features ν(M) = 3 and ν(M′) = 5, M = Si with ν(M) = 4, and M = Ta with ν(M′) = 5.
ions [M and M′ denote two distinct metal ions characterized by varying valence states, denoted as ν(M) and ν(M′), respectively, and n, m, and m′ are the contents of Na-, M-, and M′-ions, respectively] while maintaining parent structures Ω (given by NanΩMmS4; nΩ is the content of Na ions in the structure), our analysis yielded that a significant proportion of several promising candidates with σNa,300K > 10−3 S cm−1 features ν(M) = 3 and ν(M′) = 5, M = Si with ν(M) = 4, and M = Ta with ν(M′) = 5.
In this study, leveraging the methodology and knowledge from our previous study,15 we aim to expand the scope of candidate parent structures Ω considering the promising combinations of (M,M′), freeing Ω from a direct association with M (Ω = NanΩMΩS4; MΩ is the host metal ion for Ω, not necessarily being M). This new approach will broaden the exploration scope for material space, thereby enhancing the efficiency of identifying uncharted but synthesizable materials possessing superior target properties such as σNa,300K. Our objective was to investigate whether (M,M′) may exhibit increased stability in different polymorphs. Initially, we thoroughly explored the material space (M,M′,Ω) while maintaining a fixed value of m = m′ = 0.5 for 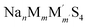 : 112 cases of (M,M′,Ω) in total. This selection, driven by the maximization of mixing entropy, resulted in the attainment of the highest value of σNa,300K as reported in the preceding study.15 Within this framework, we identified two stable sampling spaces (Si,Ta,Na4SiS4) and (Ga,P,Na4SiS4), both showing high promise for σNa,300K. Subsequently, we extended our exploration by varying the values of m and m′ within (Si,Ta,Na4SiS4) and (Ga,P,Na4SiS4). This expanded approach resulted in the discovery of crystal structures with not only promising σNa,300K but also significantly decreased convex hull decomposition energy per atom Ehull, thereby greatly improving our ability to predict stable crystal frameworks Ω capable of accommodating M and M′.
: 112 cases of (M,M′,Ω) in total. This selection, driven by the maximization of mixing entropy, resulted in the attainment of the highest value of σNa,300K as reported in the preceding study.15 Within this framework, we identified two stable sampling spaces (Si,Ta,Na4SiS4) and (Ga,P,Na4SiS4), both showing high promise for σNa,300K. Subsequently, we extended our exploration by varying the values of m and m′ within (Si,Ta,Na4SiS4) and (Ga,P,Na4SiS4). This expanded approach resulted in the discovery of crystal structures with not only promising σNa,300K but also significantly decreased convex hull decomposition energy per atom Ehull, thereby greatly improving our ability to predict stable crystal frameworks Ω capable of accommodating M and M′.
Sampling protocol
We established a sampling protocol, illustrated in Fig. 1, and briefly outlined here, with further details provided in Discussion S1.† The sampling space (M,M′,Ω) encompasses combinations of trivalent M (Al, Ga, and In), pentavalent metal ions M′ (P, V, Nb, Sb, and Ta), as well as (M,M′) = (Si,Ta), and seven different Ω, namely Na5AlS4,10,16 with which Na5GaS4 is isostructural,17 Na5InS4,18 Na4.5Al0.5Si0.5S4,10 Na4SiS4,6,10,19,20 Na4SnS4,7,9,21 Na3VS4,8,22,23 and Na3SbS4.5,24–26 Initially, we generated a substantial number of random site arrangements for supercells to represent (M,M′,Ω), resulting in a total dataset size of ndata = 5, 290, 074, 920. From these arrangements, we selected fewer than six with the lowest Ewald coulombic energies EEwald for each (M,M′,Ω): ndata = 469.27–29 In the subsequent step of DFT geometry optimizations, we fully relaxed the site positions and lattice parameters for the selected arrangements. The cell structure with the lowest DFT energy EDFT, or equivalently, the lowest Ehull, was identified to determine the most suitable Ω for (M,M′): ndata = 16. Then, we conducted a DFT-MD sampling, named the single-temperature “long-time” diagnosis, for the selected (M,M′,Ω), estimating
supercells to represent (M,M′,Ω), resulting in a total dataset size of ndata = 5, 290, 074, 920. From these arrangements, we selected fewer than six with the lowest Ewald coulombic energies EEwald for each (M,M′,Ω): ndata = 469.27–29 In the subsequent step of DFT geometry optimizations, we fully relaxed the site positions and lattice parameters for the selected arrangements. The cell structure with the lowest DFT energy EDFT, or equivalently, the lowest Ehull, was identified to determine the most suitable Ω for (M,M′): ndata = 16. Then, we conducted a DFT-MD sampling, named the single-temperature “long-time” diagnosis, for the selected (M,M′,Ω), estimating  (represented as σNa,300K values in this step). This was achieved by performing DFT-MD calculations with a time step of τ = 1 fs over a simulation time of τ = 250 ps at a constant temperature of T = 300 K. Two criteria were applied for selecting promising samples: Ehull < 25 meV per atom, which is comparable to the case of Li10GeP2S12 (Ehull = 19 meV per atom),30 and
(represented as σNa,300K values in this step). This was achieved by performing DFT-MD calculations with a time step of τ = 1 fs over a simulation time of τ = 250 ps at a constant temperature of T = 300 K. Two criteria were applied for selecting promising samples: Ehull < 25 meV per atom, which is comparable to the case of Li10GeP2S12 (Ehull = 19 meV per atom),30 and 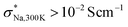 . Based on these criteria, we selected two sampling spaces, namely (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4), for further analysis in the multi-temperature diagnosis.
. Based on these criteria, we selected two sampling spaces, namely (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4), for further analysis in the multi-temperature diagnosis.
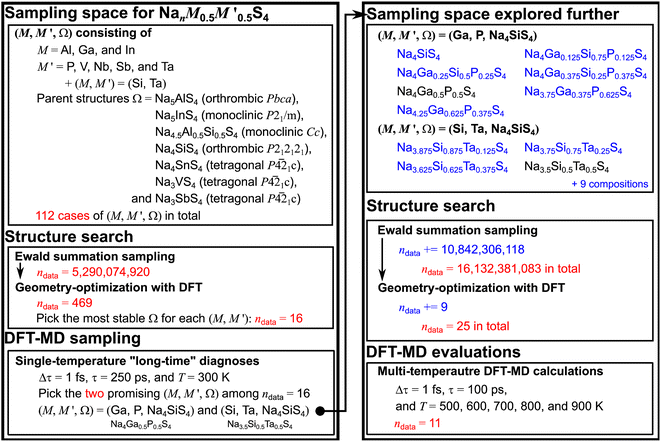 | ||
Fig. 1 Sampling protocol to identify stable Na-ion sulfide solid electrolytes with high conductivity at room temperature. The protocol comprises two parts: sampling for  and further sampling within two material space (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4) (For denotations, please refer to the main text. Details are provide in Discussion S1.†). and further sampling within two material space (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4) (For denotations, please refer to the main text. Details are provide in Discussion S1.†). | ||
Next, we employed 11 compositions with varying m (and correspondingly, m′) for (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4) by adding 9 compositions (Na4SiS4, Na4Ga0.125Si0.75P0.125S4, Na4Ga0.25Si0.5P0.25S4, Na4Ga0.375Si0.125P0.375S4, Na3.75Ga0.375P0.625S4, Na4.25Ga0.625P0.375S4, Na3.875Si0.875Ta0.125S4, Na3.75Si0.75Ta0.25S4, and Na3.625Si0.625Ta0.375S4) to the existing 2 compositions (Na4Ga0.5P0.5S4 and Na3.5Si0.5Ta0.5S4). The same procedure for the structure search was executed again. We generated a substantial number of random site arrangements for these supercells by adding a dataset size of ndata = 10, 842, 306, 118 for the 9 compositions. From these arrangements, we selected the lowest EEwald case for each composition by adding ndata = 9.27–29 In the subsequent step of DFT geometry optimizations, we fully relaxed the site positions and lattice parameters for the selected arrangements. Then, we estimated σNa,300K and Na-ion activation energies Ea by performing DFT-MD simulations with τ = 1 fs and τ = 100 ps at different temperatures T = 500, 600, 700, 800, and 900 K.
Promising material spaces
The results of the geometry optimizations for and Na3.5Si0.5Ta0.5S4 are presented in Table 1. Among the various combinations of M and M′, it was observed that M = Al or Ga, Ω = Na4SiS4 tends to be the most stable, except for M′ = Sb, in which Ω = Na4SnS4 appeared to be more stable. Notably, with M = In, stability was exclusively observed in Ω = Na4SnS4. In terms of the calculated Ehull values, the majority of the optimized cell structures exhibited Ehull < 25 meV per atom, with a few exceptions, such as Na4In0.5P0.5S4 (Ehull = 30.8 meV per atom), Na4In0.5V0.5S4 (Ehull = 35.9 meV per atom), and Na4In0.5Ta0.5S4 (Ehull = 26.0 meV per atom). Additional cases of Ω are provided in Table S1.† Furthermore, the results from the single-temperature “long-time” diagnosis indicated that Na4Ga0.5P0.5S4 and Na3.5Si0.5Ta0.5S4 meet Ehull < 25 meV per atom and
and Na3.5Si0.5Ta0.5S4 are presented in Table 1. Among the various combinations of M and M′, it was observed that M = Al or Ga, Ω = Na4SiS4 tends to be the most stable, except for M′ = Sb, in which Ω = Na4SnS4 appeared to be more stable. Notably, with M = In, stability was exclusively observed in Ω = Na4SnS4. In terms of the calculated Ehull values, the majority of the optimized cell structures exhibited Ehull < 25 meV per atom, with a few exceptions, such as Na4In0.5P0.5S4 (Ehull = 30.8 meV per atom), Na4In0.5V0.5S4 (Ehull = 35.9 meV per atom), and Na4In0.5Ta0.5S4 (Ehull = 26.0 meV per atom). Additional cases of Ω are provided in Table S1.† Furthermore, the results from the single-temperature “long-time” diagnosis indicated that Na4Ga0.5P0.5S4 and Na3.5Si0.5Ta0.5S4 meet Ehull < 25 meV per atom and  .
.
 and Na3.5Si0.5Ta0.5S4 whose structures were relaxed by using DFT with parent structures Ω that stabilized most (giving the lowest Ehull for each composition). The two compositions satisfying Ehull < 25 meV per atom and
and Na3.5Si0.5Ta0.5S4 whose structures were relaxed by using DFT with parent structures Ω that stabilized most (giving the lowest Ehull for each composition). The two compositions satisfying Ehull < 25 meV per atom and  are boldened: Na4Ga0.5Ta0.5S4 and Na3.5Si0.5Ta0.5S4. Lattice constants α, β, and γ were close to 90°. In the first row, the compositions per unit cell are presented in parentheses. In the last row, the room-temperature Na-ion conductivities
are boldened: Na4Ga0.5Ta0.5S4 and Na3.5Si0.5Ta0.5S4. Lattice constants α, β, and γ were close to 90°. In the first row, the compositions per unit cell are presented in parentheses. In the last row, the room-temperature Na-ion conductivities  are presented, which were estimated by performing the single-temperature “long-time” diagnosis with τ = 1 fs, τ = 250 ps, and T = 300 K, and “—” denotes the absence of observed Na-ion migrations)
are presented, which were estimated by performing the single-temperature “long-time” diagnosis with τ = 1 fs, τ = 250 ps, and T = 300 K, and “—” denotes the absence of observed Na-ion migrations)
| Composition | Ω | a (Å) | b (Å) | c (Å) | V (Å3) | E hull (meV per atom) | |
|---|---|---|---|---|---|---|---|
| Na4Al0.5P0.5S4 (Na96Al12P12S96) | Na4SiS4 | 41.87 | 8.917 | 13.86 | 5174 | 14.6 | 4.10 × 10−3 |
| Na4Al0.5V0.5S4 (Na96Al12V12S96) | Na4SiS4 | 41.90 | 8.780 | 14.08 | 5181 | 16.8 | 3.25 × 10−3 |
| Na4Al0.5Nb0.5S4 (Na96Al12Nb12S96) | Na4SiS4 | 42.20 | 8.951 | 14.15 | 5346 | 17.2 | — |
| Na4Al0.5Sb0.5S4 (Na96Al12Sb12S96) | Na4SnS4 | 15.74 | 15.74 | 13.86 | 3434 | 13.9 | 1.52 × 10−3 |
| Na4Al0.5Ta0.5S4 (Na96Al12Ta12S96) | Na4SiS4 | 42.23 | 8.954 | 14.14 | 5346 | 19.4 | — |
| Na 4 Ga 0.5 P 0.5 S 4 (Na 96 Ga 12 P 12 S 96 ) | Na 4 SiS 4 | 41.76 | 8.977 | 13.87 | 5201 | 15.8 | 1.03 × 10 −2 |
| Na4Ga0.5V0.5S4 (Na96Ga12V12S96) | Na4SiS4 | 42.02 | 8.807 | 14.05 | 5200 | 17.3 | 3.04 × 10−3 |
| Na4Ga0.5Nb0.5S4 (Na96Ga12Nb12S96) | Na4SiS4 | 42.24 | 8.989 | 14.13 | 5366 | 17.7 | 1.10 × 10−3 |
| Na4Ga0.5Sb0.5S4 (Na96Ga12Sb12S96) | Na4SnS4 | 15.76 | 15.76 | 13.90 | 3449 | 15.5 | 3.73 × 10−4 |
| Na4Ga0.5Ta0.5S4 (Na96Ga12Ta12S96) | Na4SiS4 | 42.30 | 8.997 | 14.11 | 5369 | 19.7 | 2.35 × 10−4 |
| Na4In0.5P0.5S4(Na96In12P12S96) | Na4SnS4 | 15.93 | 15.93 | 13.65 | 3464 | 30.8 | 1.66 × 10−3 |
| Na4In0.5V0.5S4 (Na96In12V12S96) | Na4SnS4 | 15.83 | 15.83 | 13.82 | 3464 | 35.9 | 5.16 × 10−5 |
| Na4In0.5Nb0.5S4 (Na96In12Nb12S96) | Na4SnS4 | 15.84 | 15.84 | 13.97 | 3503 | 23.5 | 8.41 × 10−3 |
| Na4In0.5Sb0.5S4 (Na96In12Sb12S96) | Na4SnS4 | 15.92 | 15.92 | 13.96 | 3536 | 17.4 | — |
| Na4In0.5Ta0.5S4 (Na96In12Ta12S96) | Na4SnS4 | 15.85 | 15.85 | 13.97 | 3507 | 26.0 | 2.44 × 10−5 |
| Na 3.5 Si 0.5 Ta 0.5 S 4 (Na 84 Si 12 Ta 12 S 96 ) | Na 4 SiS 4 | 41.65 | 8.937 | 14.08 | 5241 | 24.1 | 3.34 × 10 −2 |
As illustrated in our previous study, 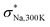 is limited by the short timescale for site-to-site jumps at low T, however, it serves as a computationally efficient metric for identifying promising candidates with high σNa,300K.15 Besides, in our previous study, given the fixed v(M) and v(M′), we identified two key descriptors for achieving high σNa,300K: the average widest Na–3S solid angle max(ΩNaSx) for NaSx polyhedra and the average Na–S bond length dNa–S.15 These descriptors with high values would facilitate the release of self-diffusing Na-ions from the cages of NaSx. Notably, Na4Ga0.5P0.5S4 and Na3.5Si0.5Ta0.5S4 exhibit high values for not only
is limited by the short timescale for site-to-site jumps at low T, however, it serves as a computationally efficient metric for identifying promising candidates with high σNa,300K.15 Besides, in our previous study, given the fixed v(M) and v(M′), we identified two key descriptors for achieving high σNa,300K: the average widest Na–3S solid angle max(ΩNaSx) for NaSx polyhedra and the average Na–S bond length dNa–S.15 These descriptors with high values would facilitate the release of self-diffusing Na-ions from the cages of NaSx. Notably, Na4Ga0.5P0.5S4 and Na3.5Si0.5Ta0.5S4 exhibit high values for not only  but also max(ΩNaSx) and dNa–S (see Fig. 2), indicating their potential for excellent σNa,300K. Based on these observations, our focus shifted towards investigating (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4) with varying m.
but also max(ΩNaSx) and dNa–S (see Fig. 2), indicating their potential for excellent σNa,300K. Based on these observations, our focus shifted towards investigating (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4) with varying m.
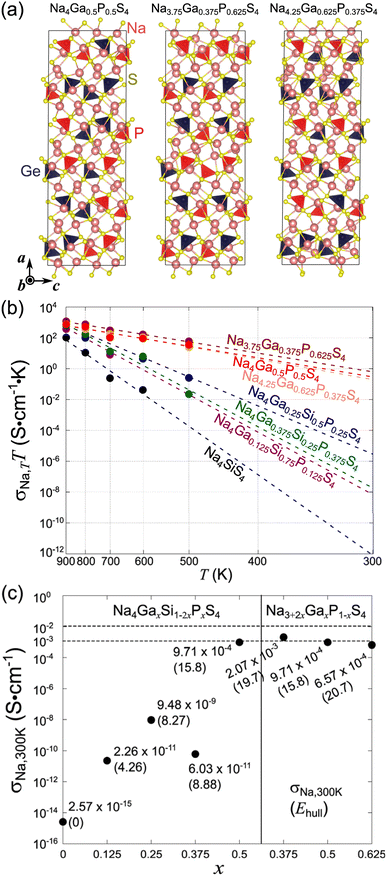
The outcomes of the geometric optimizations conducted for the 11 compositions across (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4) are presented in Table 2 as well. It is noteworthy that a decrease in the Si content (that is, the deviation from Ω) results in an increase in the value of Ehull. For all the investigated compositions, Ehull remains below 25 meV per atom, signifying their structural (meta)stability and the feasibility of their synthesis. We represent several examples of the visualized crystal structures in Fig. 3a and 4a. In addition, their bandgap energies Eg, a metric for electron-insulating properties, exhibited high values: around 3 eV for most cases.
| Composition | a (Å) | b (Å) | c (Å) | V (Å3) | E hull (meV per atom) | E g (eV) |
|---|---|---|---|---|---|---|
| Na4SiS4 (Na96Si24S96) | 41.61 | 8.791 | 13.88 | 5077 | 0 | 4.03 |
| Na4Ga0.125Si0.75P0.125S4 (Na96Ga3Si18P3S96) | 41.71 | 8.820 | 13.88 | 5106 | 4.26 | 2.98 |
| Na4Ga0.25Si0.5P0.25S4 (Na96Ga6Si12P6S96) | 41.86 | 8.843 | 13.87 | 5136 | 8.27 | 3.12 |
| Na4Ga0.375Si0.25P0.375S4 (Na96Ga9Si6P9S96) | 41.60 | 8.912 | 13.88 | 5145 | 8.88 | 3.12 |
| Na4Ga0.5P0.5S4 (Na96Ga12P12S96) | 41.76 | 8.977 | 13.87 | 5201 | 15.8 | 2.44 |
| Na3.75Ga0.375P0.625S4 (Na90Ga9P15S96) | 40.81 | 8.953 | 13.93 | 5091 | 19.7 | 3.00 |
| Na4.25Ga0.625P0.375S4 (Na102Ga15P9S96) | 42.21 | 8.996 | 13.98 | 5308 | 20.7 | 2.92 |
| Na3.875Si0.875Ta0.125S4 (Na93Si21Ta3S96) | 41.66 | 8.782 | 13.95 | 5102 | 5.20 | 3.03 |
| Na3.75Si0.75Ta0.25S4 (Na90Si18Ta6S96) | 41.75 | 8.782 | 14.00 | 5134 | 9.26 | 3.02 |
| Na3.625Si0.625Ta0.375S4 (Na87Si15Ta9S96) | 41.77 | 8.767 | 14.10 | 5134 | 14.7 | 2.70 |
| Na3.5Si0.5Ta0.5S4 (Na84Al12Ta12S96) | 41.65 | 8.937 | 14.08 | 5241 | 24.1 | 2.85 |
In Table S2,† we provide the values for σNa,T and DNa,T obtained through multi-temperature DFT-MD calculations for (M,M′,Ω) = (Ga,P,Na4SiS4). Furthermore, in Fig. S1a–g,† we present mean squared displacement (MSD) curves, most of which exhibit linear responses against the sampled time intervals τMSD. As indicated in their insets, the trajectories at T = 500 K showed limited interconnectivity until the Si-ion content becomes zero. The interconnected trajectories, indicative of site-to-site jumps, are noticeable in Na4Ga0.5P0.5S4, Na3.75Ga0.375P0.625S4, and Na4.25Ga0.625P0.375S4. This observed trend is also reflected in the Arrhenius plot, where we estimated the interpolated Ea and the extrapolated σNa,300K (see Fig. 3b). For these three samples, Ea was suppressed to less than 350 meV, while σNa,300K either exceeded or remained around 10−3 S cm−1. We note that a possible explanation of an order of magnitude discrepancy between  and σNa,300K ≈ 10−3 S cm−1 for Na4Ga0.5P0.5S4 is the insufficient timescale for site-to-site jumps considered in
and σNa,300K ≈ 10−3 S cm−1 for Na4Ga0.5P0.5S4 is the insufficient timescale for site-to-site jumps considered in 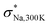 . It is noteworthy that a decrease in the Na-ion content, as seen in Na3.75Ga0.375P0.625S4, resulted in a suppression of Ea and an enhancement of σNa,300K, likely due to the creation of the additional free space for Na-ion self-diffusions. As illustrated in Fig. 3c, achieving σNa,300K ≳ 10−3 S cm−1 for (M,M′,Ω) = (Ga,P,Na4SiS4) would be realized at the expense of decreased phase stability (15 < Ehull < 21 meV per atom, which are relative to zero decomposition energy of the pristine structure Ω): Na4Ga0.5P0.5S4, Na3.75Ga0.375P0.625S4, and Na4.25Ga0.625P0.375S4.
. It is noteworthy that a decrease in the Na-ion content, as seen in Na3.75Ga0.375P0.625S4, resulted in a suppression of Ea and an enhancement of σNa,300K, likely due to the creation of the additional free space for Na-ion self-diffusions. As illustrated in Fig. 3c, achieving σNa,300K ≳ 10−3 S cm−1 for (M,M′,Ω) = (Ga,P,Na4SiS4) would be realized at the expense of decreased phase stability (15 < Ehull < 21 meV per atom, which are relative to zero decomposition energy of the pristine structure Ω): Na4Ga0.5P0.5S4, Na3.75Ga0.375P0.625S4, and Na4.25Ga0.625P0.375S4.
In Table S2,† we also provide the values for σNa,T and DNa,T obtained through multi-temperature DFT-MD calculations for (M,M′,Ω) = (Si,Ta,Na4SiS4). Additionally, in Fig. S2a–d,† we present linear MSD curves against τMSD. As indicated in their insets, even at T = 500 K, trajectories exhibited interconnectivity, even for the relatively low doping levels of the Ta-ion. Notably, the interconnected features were predominantly observed around Ta-ions, wherein Na vacancies exist. This trend is reflected in the Arrhenius plot (see Fig. 4b). For instance, in the case of Na3.875Si0.875Ta0.125S4, Ea = 413 meV and σNa,300K = 4.93 × 10−5 S cm−1 were estimated. Furthermore, with an increase in the Ta-ion doping level (or a decrease in the Na-ion content), Ea was further suppressed, accompanied by an enhancement of σNa,300K. In the case of Na3.5Si0.5Ta0.5S4, Ea = 215 meV and σNa,300K = 1.35 × 10−2 S cm−1 were estimated. As illustrated in Fig. 4c, achieving σNa,300K ≳ 10−3 S cm−1 for (M,M′,Ω) = (Si,Ta,Na4SiS4) would be realized at the expense of decreased phase stability (9 < Ehull < 25 meV per atom): Na3.75Si0.75Ta0.25S4, Na3.625Si0.625Ta0.375S4, and Na3.5Si0.5Ta0.5S4. The high values of σNa,300K ≳ 10−3 S cm−1 for Na4Ga0.5P0.5S4 and Na3.5Si0.5Ta0.5S4 partly justify the use of m = m′ = 0.5 in the initial step of the sampling protocol. We also discuss the electrochemical stability windows for the 11 compositions in the Discussion S2.†
Descriptors for convex hull decomposition energy
We established a multivariate linear regression model to discern descriptors influencing Ehull, leveraging a dataset of given in the structure search step, encompassing various Ω = NanΩMΩS4; the data are presented in Table S1† (ndata = 110). The proposed model comprises three descriptors:
given in the structure search step, encompassing various Ω = NanΩMΩS4; the data are presented in Table S1† (ndata = 110). The proposed model comprises three descriptors: | (1) |
The model exhibits a substantial R2-value (R2 = 0.711) and an F-value of 87.0 with a significantly low p-value (p < 0.001), while all t-tests for the constant term and the three coefficients revealed p < 0.001. The variance inflation factors (VIF) were sufficiently small, indicating an absence of multicollinearity issues: VIF = 1.02, 1.06, and, 1.08 for the first, second, and third coefficients, respectively. Here, r(M) denotes the Shannon ionic radius for a metal ion M.31,32 The data plot for this model is presented in Fig. 5.
 | ||
| Fig. 5 Data plot for the multivariate regression model against Ehull calculated by using DFT (presented in Table S1†) with a substantial R2-value (R2 = 0.711). For the model, eqn (1) was adopted with ndata = 110. | ||
The first term n − nΩ suggests that Ehull would increase if the Na-ion sites are added (rather than omitted) during structural modifications from the pristine Ω. The second term  suggests that Ehull would increase when the average valence for M and M′ deviates from that of MΩ. The third term
suggests that Ehull would increase when the average valence for M and M′ deviates from that of MΩ. The third term 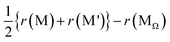 suggests that Ehull would increase with a larger average ionic radius for M and M′ compared to that of MΩ. In light of these findings, two sampling spaces, namely (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4), with high σNa,300K, are deemed appealing for experimental realizations. For Na4Ga0.5P0.5S4 with Ehull = 15.8 meV per atom, it is given that n − nΩ = 0,
suggests that Ehull would increase with a larger average ionic radius for M and M′ compared to that of MΩ. In light of these findings, two sampling spaces, namely (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4), with high σNa,300K, are deemed appealing for experimental realizations. For Na4Ga0.5P0.5S4 with Ehull = 15.8 meV per atom, it is given that n − nΩ = 0, 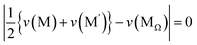 , and
, and  . Similarly, for Na3.5Si0.5Ta0.5S4Ehull = 24.1 meV per atom, it is given that n − nΩ = −0.5,
. Similarly, for Na3.5Si0.5Ta0.5S4Ehull = 24.1 meV per atom, it is given that n − nΩ = −0.5,  , and
, and  . The numerical analyses elucidate that a combination of Ga, P, and Ta would constitute an appropriate blend for the parent structure Na4SiS4.
. The numerical analyses elucidate that a combination of Ga, P, and Ta would constitute an appropriate blend for the parent structure Na4SiS4.
Summary and outlook
In this study, we employed the multi-stage sampling protocol11 to identify promising Na-ion sulfides characterized by high σNa,300K within the constraints of limited choices for M and M′, but with an expanded selection of parent structures Ω to effectively pinpoint unexplored yet synthesizable materials with superior conductivity σNa,300K. Our approach began with the identification of Ω that stabilizes each combination of (M,M′) most. Subsequently, circumventing the tedious tasks of the exhaustive access for the sampling spaces, we efficiently predicted that (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4), characterized by wide max(ΩNaSx) and long dNa–S, have the potential to achieve high σNa,300K through the single-temperature “long-time” diagnosis.
characterized by high σNa,300K within the constraints of limited choices for M and M′, but with an expanded selection of parent structures Ω to effectively pinpoint unexplored yet synthesizable materials with superior conductivity σNa,300K. Our approach began with the identification of Ω that stabilizes each combination of (M,M′) most. Subsequently, circumventing the tedious tasks of the exhaustive access for the sampling spaces, we efficiently predicted that (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4), characterized by wide max(ΩNaSx) and long dNa–S, have the potential to achieve high σNa,300K through the single-temperature “long-time” diagnosis.
These predictions were subsequently validated through multi-temperature DFT-MD calculations. Notably, σNa,300K ≳ 10−3 S cm−1 were attainable within a range of 9 < Ehull < 25 meV per atom: Na4Ga0.5P0.5S4, Na3.75Ga0.375P0.625S4, Na4.25Ga0.625P0.375S4, Na3.75Si0.75Ta0.25S4, Na3.625Si0.625Ta0.375S4, and Na3.5Si0.5Ta0.5S4. Based on our observations, we expect that the co-doping of Ga, P, and Ta into the parent structure Na4SiS4, leading to the formation of compositions Na4+g−p−tGagSi1−g−p−tPpTatS4, would present an intriguing avenue for further investigation in future studies. The limitation of this study should be noted also; although σNa,300K were optimized at m = m′ = 0.5, as observed in Na4Ga0.5P0.5S4 and Na3.5Si0.5Ta0.5S4, other choices of m (m′) in the initial step of the sampling protocol are worth exploring. Future studies should assess these alternatives.
We believe that these two identified sampling spaces, characterized by both thermodynamic stability and fast Na-ion conductivity, warrant further experimental investigations. Additionally, this brute-force sampling technique has the potential to explore other classes of solid electrolytes, which could be pivotal in the ongoing advancement of solid-state battery technology.
Data availability
Data for this article, including crystal structures and molecular dynamics results for (M,M′,Ω) = (Ga,P,Na4SiS4) and (Si,Ta,Na4SiS4) are available at github at https://github.com/JerryGarcia1995/NasulfidesExt. The data supporting this article have been included as part of the ESI† as well.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported in part by MEXT as “Program for Promoting Research on the Supercomputer Fugaku” grant number JPMXP1020230325, Data Creation and Utilization Type Material Research and Development Project grant number JPMXP1122712807 and Materials Processing Science project (“Materealize”) grant number JPMXP0219207397, and by JSPS KAKENHI grant numbers JP21K14729 and JP24H02203, as well as JST through ALCA-SPRING grant number JPMJAL1301, COI-NEXT grant number JPMJPF2016, and GteX Program Japan grant number JPMJGX23S4. The calculations were performed on the supercomputers at NIMS (Numerical Materials Simulator) and the supercomputer Fugaku at the RIKEN through the HPCI System Research Project (project IDs: hp230154 and hp230205). Visualization for crystal structures was made with the VESTA software.33 Plots were generated using gnuplot 5.4.34References
- N. Yabuuchi, K. Kubota, M. Dahbi and S. Komaba, Research Development on Sodium-Ion Batteries, Chem. Rev., 2014, 114(23), 11636–11682, DOI:10.1021/cr500192f.
- H. Pan, Y.-S. Hu and L. Chen, Room-Temperature Stationary Sodium-Ion Batteries for Large-Scale Electric Energy Storage, Energy Environ. Sci., 2013, 6(8), 2338–2360, 10.1039/c3ee40847g.
- A. Hayashi, A. Sakuda and M. Tatsumisago, Development of Sulfide Solid Electrolytes and Interface Formation Processes for Bulk-Type All-Solid-State Li and Na Batteries, Front. Energy Res., 2016, 4, 25, DOI:10.3389/fenrg.2016.00025.
- F. Li, Z. Wei, A. Manthiram, Y. Feng, J. Ma and L. Mai, Sodium-Based Batteries: From Critical Materials to Battery Systems, J. Mater. Chem. A, 2019, 7(16), 9406–9431, 10.1039/c8ta11999f.
- A. Hayashi, N. Masuzawa, S. Yubuchi, F. Tsuji, C. Hotehama, A. Sakuda and M. Tatsumisago, A Sodium-Ion Sulfide Solid Electrolyte with Unprecedented Conductivity at Room Temperature, Nat. Commun., 2019, 10(1), 5266, DOI:10.1038/s41467-019-13178-2.
- N. Tanibata, K. Noi, A. Hayashi and M. Tatsumisago, Preparation and Characterization of Highly Sodium Ion Conducting Na3PS4–Na4SiS4 Solid Electrolytes, RSC Adv., 2014, 4(33), 17120–17123, 10.1039/c4ra00996g.
- J. W. Heo, A. Banerjee, K. H. Park, Y. S. Jung and S. Hong, New Na-Ion Solid Electrolytes Na4-xSn1-xSbxS4 (0.02 ≤ x ≤ 0.33) for All-Solid-State Na-Ion Batteries, Adv. Energy Mater., 2018, 8(11), 1702716, DOI:10.1002/aenm.201702716.
- Y. He, F. Lu and X. Kuang, Enhanced Sodium Ion Conductivity in Na3VS4 by P-Doping, RSC Adv., 2019, 9(67), 39180–39186, 10.1039/c9ra08900d.
- S. Xiong, Z. Liu, L. Yang, Y. Ma, W. Xu, J. Bai and H. Chen, Anion and Cation Co-Doping of Na4SnS4 as Sodium Superionic Conductors, Mater. Today Phys., 2020, 15, 100281–100288, DOI:10.1016/j.mtphys.2020.100281.
- S. Harm, A. Hatz, C. Schneider, C. A. Hoefer, C. Hoch and B. V. Lotsch, Finding the Right Blend: Interplay between Structure and Sodium Ion Conductivity in the System Na5AlS4-Na4SiS4, Front. Chem., 2020, 8, 90, DOI:10.3389/fchem.2020.00090.
- Z. Zhu, I.-H. Chu and S. P. Ong, Li3Y(PS4)2 and Li5PS4Cl2: New Lithium Superionic Conductors Predicted from Silver Thiophosphates using Efficiently Tiered Ab Initio Molecular Dynamics Simulations, Chem. Mater., 2017, 29(6), 2474–2484, DOI:10.1021/acs.chemmater.6b04049.
- A. D. Sendek, Q. Yang, E. D. Cubuk, K.-A. N. Duerloo, Y. Cui and E. J. Reed, Holistic Computational Structure Screening of More Than 12000 Candidates for Solid Lithium-Ion Conductor Materials, Energy Environ. Sci., 2017, 10, 306–320, 10.1039/C6EE02697D.
- S. Muy, J. Voss, R. Schlem, R. Koerver, S. J. Sedlmaier, F. Maglia, P. Lamp, W. G. Zeier and S.-H. Yao, High-Throughput Screening of Solid-State Li-Ion Conductors Using Lattice-Dynamics Descriptors, iScience, 2019, 16, 270–282, DOI:10.1016/j.isci.2019.05.036.
- R. Jalem, Y. Tateyama, K. Takada and S.-H. Jang, Multiobjective Solid Electrolyte Design of Tetragonal and Cubic Inverse-Perovskites for All-Solid-State Lithium-Ion Batteries by High-Throughput Density Functional Theory Calculations and AI-Driven Methods, J. Phys. Chem. C, 2023, 127(35), 17307–17323, DOI:10.1021/acs.jpcc.3c02801.
- S.-H. Jang, Y. Tateyama and R. Jalem, High-Throughput Data-Driven Prediction of Stable High-Performance Na-Ion Sulfide Solid Electrolytes, Adv. Funct. Mater., 2022, 32(48), 2206036, DOI:10.1002/adfm.202206036.
- A. Brown and B. Tani, Powder X-Ray Diffraction Identification of Some New Phases in the Na2S-Al2S3 System, Mater. Res. Bull., 1987, 22(8), 1029–1037, DOI:10.1016/0025-5408(87)90231-5.
- S. Balijapelly, P. Sandineni, A. Adhikary, N. Gerasimchuk, A. V. Chernatynskiy and A. Choudhury, Ternary Alkali Ion Thiogallates, A5GaS4 (A = Li and Na), with Isolated Tetrahedral Building Units and Their Ionic Conductivities, Dalton Trans., 2021, 50(21), 7372–7379, 10.1039/d1dt00766a.
- B. Eisenmann and A. Hofmann, Crystal Structure of Pentasodium Tetrathioindate(III), Na5InS4, Z. Kristallogr. – Cryst. Mater., 1991, 197(1–2), 169–170, DOI:10.1524/zkri.1991.197.1-2.169.
- N. Tanibata, K. Noi, A. Hayashi, N. Kitamura, Y. Idemoto and M. Tatsumisago, X-Ray Crystal Structure Analysis of Sodium-Ion Conductivity in 94Na3PS4·6Na4SiS4 Glass-Ceramic Electrolytes, ChemElectroChem, 2014, 1(7), 1130–1132, DOI:10.1002/celc.201402016.
- N. Tanibata, A. Hayashi and M. Tatsumisago, Improvement of Rate Performance for All-Solid-State Na15Sn4/Amorphous TiS3 Cells Using 94Na3PS4·6Na4SiS4 Glass-Ceramic Electrolytes, J. Electrochem. Soc., 2015, 162(6), A793–A795, DOI:10.1149/2.0011506jes.
- J.-C. Jumas, E. Philippot, F. Vermot-Gaud-Daniel, M. Ribes and M. Maurin, Etude de la tétracoordination de l’etain dans deux orthothiostannates: Na4SnS4 et Ba2SnS4 (α), J. Solid State Chem., 1975, 14(4), 319–327, DOI:10.1016/0022-4596(75)90050-x.
- M. V. Peskov and V. A. Blatov, Comparative Crystal-Chemical Analysis of d-Metal Sulfides, Selenides, and Tellurides and Binary Compounds, Russ. J. Inorg. Chem., 2006, 51(4), 590–598, DOI:10.1134/s0036023606040140.
- K. O. Klepp and G. Gabl, New Complex Sulfides of the VA-Metals: Preparation and Crystal Structure of Na3VS4 (With a Note on the Crystal Structure of the Low Temperature Modification of Na3PO4), Eur. J. Solid State Inorg. Chem., 1997, 34(10), 1143–1154 CAS.
- H. Graf and H. Schäfer, Zur Strukturchemie der Alkalisalze der Tetrathiosäuren der Elemente der 5. Hauptgruppe, Z. Anorg. Allg. Chem., 1976, 425(1), 67–80, DOI:10.1002/zaac.19764250109.
- H. Wang, Y. Chen, Z. D. Hood, G. Sahu, S. P. Amaresh, J. K. Keum, K. An and C. Liang, An Air-Stable Na3SbS4 Superionic Conductor Prepared by a Rapid and Economic Synthetic Procedure, Angew. Chem., 2016, 55(30), 8551–8555, DOI:10.1002/anie.201601546.
- A. Banerjee, K. H. Park, J. W. Heo, Y. J. Nam, C. K. Moon, S. M. Oh, S.-T. Hong and Y. S. Jung, Na3SbS4: A Solution Processable Sodium Superionic Conductor for All-Solid-State Sodium-Ion Batteries, Angew. Chem., Int. Ed., 2016, 55(33), 9634–9638, DOI:10.1002/anie.201604158.
- P. P. Ewald, Die Berechnung Optischer und Elektrostatischer Gitterpotentiale, Ann. Phys., 1921, 369(3), 253–287, DOI:10.1002/andp.19213690304.
- A. Y. Toukmaji and J. A. Board, Ewald Summation Techniques in Perspective: A Survey, Comput. Phys. Commun., 1996, 95(2–3), 73–92, DOI:10.1016/0010-4655(96)00016-1.
- S. Jang, R. Jalem and Y. Tateyama, EwaldSolidSolution: A High-Throughput Application to Quickly Sample Stable Site Arrangements for Ionic Solid Solutions, J. Phys. Chem. A, 2023, 127(27), 5734–5744, DOI:10.1021/acs.jpca.3c00076.
- K. Persson, Materials Data on Li10Ge(PS6)2 (SG:1) by Materials Project. MaterialsProject, 2016, DOI:10.17188/1284960, accessed 2024-03-21.
- R. D. Shannon, Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides, Acta Crystallogr., 1976, A32, 751–767, DOI:10.1107/S0567739476001551.
- A. A. B. Baloch, S. M. Alqahtani, F. Mumtaz, A. H. Muqaibel, S. N. Rashkeev and F. H. Alharbi, Extending Shannon's Ionic Radii Database Using Machine Learning, Phys. Rev. Mater., 2021, 5(4), 043804, DOI:10.1103/PhysRevMaterials.5.043804.
- K. Momma and F. Izumi, VESTA 3 for Three-Dimensional Visualization of Crystal, Volumetric and Morphology Data, J. Appl. Crystallogr., 2011, 44(6), 1272–1276, DOI:10.1107/S0021889811038970.
- T. Williams and C. Kelley, gnuplot 5.4: an Interactive Plotting Programhttp://www.gnuplot.info/docs_5.4/Gnuplot_5_4.pdf, accessed 2023-09-29.
Footnote |
| † Electronic supplementary information (ESI) available: Details of sampling protocol, comprehensive dataset for lattice constants, unit cell volumes, and convex hull decomposition energies for (M,M′,Ω) at m = m′ = 0.5, and the results of the multi-temperature diagnosis. See DOI: https://doi.org/10.1039/d4ta02522a |
| This journal is © The Royal Society of Chemistry 2024 |