Efficient urea synthesis via one-step N–C–N coupling: strong metal–support interaction-driven planar Cu clusters on two-dimensional Mo2C MXene†
Abstract
The electrocatalytic co-reduction of small carbonaceous and nitrogenous molecules recently emerged as a promising strategy to mitigate carbon emissions, facilitate wastewater denitrification, and sustain urea synthesis. However, the development of highly efficient electrocatalysts to accelerate C–N coupling and multiple protonation steps remains challenging. Herein, inspired by the “strong metal–support interaction” (SMSI) concept, we designed novel catalysts, comprised of size-selected Cu clusters anchored on the two-dimensional molybdenum carbide MXene (Cun/Mo2C), for urea production via the co-reduction of nitrate (NO3−) and carbon dioxide (CO2). Our density functional theory (DFT) computations revealed that these Cu clusters are strongly immobilized on the Mo2C substrate by forming planar structures, leading to considerable active sites. Notably, these Cun/Mo2C catalysts demonstrate enhanced activity for urea synthesis through a one-step N–C–N coupling mechanism. This process involves the insertion of CO* into the 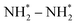 intermediate, facilitated by electrostatic attractions between their nitrogen atoms. In particular, among the catalysts tested, Cu4/Mo2C exhibits superior performance, achieving urea production with a limiting potential of −0.36 V. Furthermore, the competing side reactions, such as CO reduction or release, NH3 production, hydrogen evolution, and surface oxidation, are significantly suppressed, ensuring its high selectivity for urea synthesis. This work not only introduces a novel method for urea electro-synthesis via SMSI between metal clusters and substrate, but also suggests a strategy for synthesizing other organonitrogen compounds.
intermediate, facilitated by electrostatic attractions between their nitrogen atoms. In particular, among the catalysts tested, Cu4/Mo2C exhibits superior performance, achieving urea production with a limiting potential of −0.36 V. Furthermore, the competing side reactions, such as CO reduction or release, NH3 production, hydrogen evolution, and surface oxidation, are significantly suppressed, ensuring its high selectivity for urea synthesis. This work not only introduces a novel method for urea electro-synthesis via SMSI between metal clusters and substrate, but also suggests a strategy for synthesizing other organonitrogen compounds.



 Please wait while we load your content...
Please wait while we load your content...