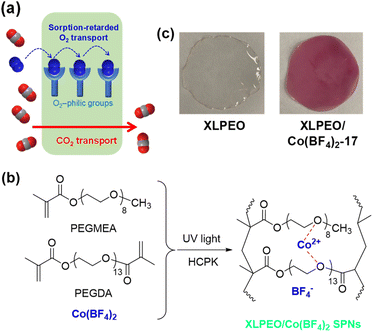
Open Access Article
Open Access ArticleRetarded O2 transport in Co2+-coordinated supramolecular polymer networks for membrane CO2/O2 separations†
Taliehsadat
Alebrahim
,
Narjes
Esmaeili
,
Gengyi
Zhang
and
Haiqing
Lin
 *
*
Department of Chemical and Biological Engineering, University at Buffalo, The State University at New York, Buffalo, New York 14260, USA. E-mail: haiqingl@buffalo.edu
First published on 3rd June 2024
Abstract
Dissociated Co2+ ions in liquids and polymers have been demonstrated to reversibly react with O2 and increase O2 permeability. However, we find a series of Co2+-coordinated supramolecular polymer networks (SPNs) with enormous O2 sorption but retarded diffusion, leading to superior CO2/O2 separation properties. Specifically, Co(BF4)2 is dissolved by cross-linked poly(ethylene oxide) (XLPEO), as validated by Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), and differential scanning calorimetry (DSC). The dissociated Co2+ ions increase O2/CO2 solubility selectivity but decrease its diffusivity selectivity. For example, adding 6.4 mass% Co(BF4)2 in XLPEO increases O2 solubility by 35 times and O2/CO2 solubility selectivity from 0.12 to 5.0, but it decreases O2/CO2 diffusivity selectivity from 0.40 to 0.0058, leading to a CO2/O2 permeability selectivity of 35, above Robeson's upper bound and superior to that of state-of-the-art polymers. This study unravels an exciting platform of metal ion-coordinated supramolecular networks for various molecular separations by harnessing strong affinity but retarded diffusion despite their stability challenge.
Introduction
CO2/O2 separations are of great interest for healthcare and industrial applications. For instance, artificial lungs for CO2 and O2 exchange are critical for blood oxygenation;1,2 atmospheric CO2 and O2 compositions can be manipulated for storing and transporting vegetables and fruit to improve their shelf lifetime;3 CO2 capture from fossil fuel-derived flue gas has been proposed as a critical way to mitigate CO2 emissions to the atmosphere, and the flue gas contains 4–16% O2 that needs to be separated from CO2.4,5 Membrane technology offers a cost- and energy-efficient approach for gas separations. However, CO2 has a kinetic diameter of 3.3 Å, very close to that of O2 (3.46 Å), and therefore, most polymers do not have sufficiently strong size-sieving ability to obtain high diffusivity selectivity and thus permeability selectivity. For example, Matrimid is widely investigated for membrane separations due to its strong size-sieving ability, and it showed a CO2/CH4 selectivity of 35 but a CO2/O2 selectivity of only 3.8 at 35 °C.6Gas separation properties can be often manipulated through interactions between polymers and targeted gases. For example, polymers containing poly(ethylene oxide) (PEO) demonstrate good CO2/O2 separation properties because of their affinity towards CO2, leading to excellent CO2/O2 solubility selectivity.7–9 PEO exhibited a CO2/O2 selectivity of 18, though its CO2/CH4 selectivity was only 20,8 much lower than that of Matrimid.
Co2+ forms complexes with O2, leading to high O2 sorption, and thus, Co2+-based O2-binding carriers have been reported, such as cobalt porphyrins,10 cobalt(II) complexes,11 cobalt(II)-based metal-containing ionic liquid (MCIL),12,13 and Co-based metal–organic frameworks (MOFs).14 These carriers were incorporated into liquid membranes to improve O2/gas separation performance,12,15,16 as shown in Table 1. For example, introducing Co (3-MeOsaltmen) in dimethylacetamide (DMAc) increased O2/N2 selectivity from 1.9 to 25 and achieved O2 permeability as high as 270 barrer (1 barrer = 10−10 cm3(STP) cm cm−2 s−1 cmHg−1).11 However, liquid membranes face instability challenges because of solvent evaporation, pressure-induced liquid loss, and carrier activity loss.
| Facilitated transport membranes | Temp. (°C) | Pressure (bar) | O2 permeability (barrer) | O2/N2 selectivity | ||
|---|---|---|---|---|---|---|
| Polymers/liquids | Cobalt carriers | Carrier content (wt%) | ||||
| a GPU, 1 GPU = 10−6 cm3(STP) cm−2 s−1 cmHg−1. | ||||||
| DMAc11 | Co (3-MeOsaltmen) | 15 | −10 | 0.19 | 270 | 25 |
| Pebax 1657 (ref. 16) | CoPc | 1 | 25 | 2 | 224 | 8.5 |
| Polycarbonate17 | CoSalen | 3 | 5 | 3 | 0.33 | 15 |
| PDMS18 | CoSalen | 20 | 30 | 0.05 | 46a | 7.7 |
| PIM-1 (ref. 19) | mim2Co (NCS)4 | 2.0 | 30 | 2 | 113 | 5.3 |
To overcome the instability of liquid membranes, Co2+-based carriers were dissolved in solid polymers by ion coordination to form supramolecular polymer networks (SPNs) with good mechanical properties.20–22 The dissociated Co2+ can reversibly bind O2, improving O2/N2 separation properties (Table 1). For instance, adding 1 wt% cobalt(II) phthalocyanine (CoPc) in Pebax 1657 (a microphase-separated block copolymer containing PEO and nylon) increased O2/N2 selectivity from 2.9 to 8.5.16 Similar approaches have been adopted to develop Ag+-coordinated SPNs for olefin/paraffin separations. As Ag+ forms complexes with olefins, AgBF4 was dissolved in Pebax to dramatically increase ethylene/ethane selectivity from 1 to 30.23,24
In striking contrast, we report retarded O2 transport in the SPNs prepared from Co(BF4)2 dissociated in crosslinked PEO (XLPEO) synthesized from poly(ethylene glycol) methyl ether acrylate (PEGMEA) and poly(ethylene glycol) diacrylate (PEGDA) (Fig. 1). The Co ions complex with ether oxygens, enabling complete dissociation of the Co salts, as evidenced by transparent and uniform films obtained (Fig. 1c), leading to extremely high O2 sorption. However, instead of improving O2 permeability, increasing the Co(BF4)2 content in XLPEO dramatically decreases O2 permeability and increases CO2/O2 selectivity. The effect of Co ions on the structures and CO2/O2 transport properties of the SPNs is thoroughly investigated to derive structure and property relationships. This study unveils a new mechanism for harnessing strong binding capabilities with a penetrant to lower its diffusivity and permeability and enable its separation from other penetrants.
Experimental
Materials
PEGDA (n = 13; Mn = 700 g mol−1), PEGMEA (n = 9; Mn = 480 g mol−1), 1-hydroxycyclohexyl phenyl ketone (HCPK), and Co(ClO4)2·6H2O were received from Sigma Aldrich Corporation (St. Louis, MO). Co(BF4)2·6H2O was purchased from Thermo Fisher Scientific (Waltham, MA). Gas cylinders of O2, N2, and CO2 (99.99%) were obtained from Airgas Inc. (Buffalo, NY).Preparation of Co2+-coordinated SPNs
To prepare SPNs, PEGDA, PEGMEA, HCPK (0.1 mass% relative to PEGDA and PEGMEA), and Co salt were dissolved in water. The mass ratio of PEGDA to PEGMEA was set at 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 to obtain XLPEO with high gas permeability and good mechanical properties.25 The prepolymer solution was sandwiched between two quartz plates and photopolymerized using UV light with a wavelength of 254 nm at 3.0 mW cm−2 for 5 min.26 The obtained solid films were vacuumed at ≈23 °C for three days or more to remove water and then kept under vacuum before use. The samples are named XLPEO/salt-x, where x represents the mass% of the salt in the dried films.
4 to obtain XLPEO with high gas permeability and good mechanical properties.25 The prepolymer solution was sandwiched between two quartz plates and photopolymerized using UV light with a wavelength of 254 nm at 3.0 mW cm−2 for 5 min.26 The obtained solid films were vacuumed at ≈23 °C for three days or more to remove water and then kept under vacuum before use. The samples are named XLPEO/salt-x, where x represents the mass% of the salt in the dried films.
Characterization of SPNs
Film thickness was measured using a digital micrometer (Mitutoyo Corporation, Kanagawa, JP). An attenuated total reflection-Fourier transform infrared (ATR-FTIR) spectrometer (Vertex 70, Billerica, MA) was utilized at a resolution of 4 cm−1 to investigate the conversion of PEGDA and PEGMEA and the salt state in XLPEO. The SPN structure was examined using an Ultima IV X-ray diffractometer (XRD, Rigaku Corporation, Tokyo, JP) with CuKa radiation (a wavelength of 1.54 Å) at ≈23 °C. Thermal transitions were obtained by Differential Scanning Calorimetry (DSC, Q2000, TA Instruments, New Castle, DE). Two heating cycles were carried out from −90 to 100 °C at 10 °C min−1 in a N2 atmosphere. Glass transition temperature (Tg) was determined as the inflection point of a step change in the second heating cycle. SPN density, ρSP (g cm−3), was determined using an analytical balance equipped with a density kit.26,27Pure-gas permeability of the SPN films was determined using a constant-volume and variable-pressure apparatus at different feed pressures (4.4, 7.9, and 11.3 bar) and 35 °C.28 The measurement followed an order of N2, CO2, and O2. N2 was also re-tested for some samples after O2 to ensure that the samples remained intact after O2 exposure. Gas permeability has an uncertainty of less than 10%, estimated using an error propagation analysis.29 Pure-gas sorption in the SPNs (∼2 g) was determined using a dual-volume and dual-transducer apparatus based on the pressure-decay method at 35 °C and three pressures (∼4.5, ∼7.9, and ∼11.4 bar).30
Results and discussion
Physical and chemical properties of Co2+-coordinated SPNs
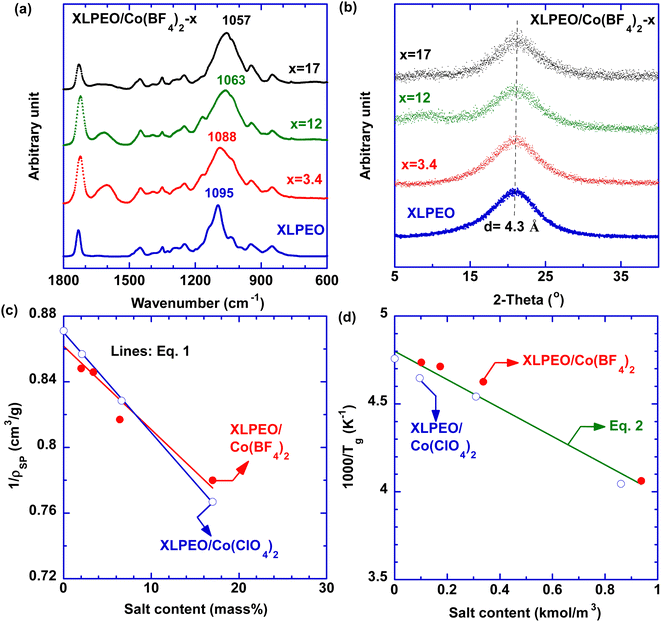
Fig. 2a exhibits FTIR spectra of XLPEO/Co(BF4)2 SPNs. The peak at 1096 cm−1 representing C–O vibration in XLPEO redshifts with increasing Co(BF4)2 content, indicating the weakened C–O bonds caused by the interactions with Co2+.26,31 For example, adding 17 mass% Co(BF4)2 lowers the C–O stretching frequency from 1097 to 1057 cm−1. By contrast, increasing the Co(BF4)2 content has minimal impact on the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching at 1732 cm−1, indicating the absence of strong interactions with Co2+. Similar behaviors have been observed for XLPEO/Co(Cl4)2 (Fig. S1a†). Additionally, the SPNs exhibit a new peak at 623 cm−1 characteristic of free ClO4− anions, confirming the dissociation of Co(ClO4)2 by XLPEO.31
O stretching at 1732 cm−1, indicating the absence of strong interactions with Co2+. Similar behaviors have been observed for XLPEO/Co(Cl4)2 (Fig. S1a†). Additionally, the SPNs exhibit a new peak at 623 cm−1 characteristic of free ClO4− anions, confirming the dissociation of Co(ClO4)2 by XLPEO.31
 | ||
| Fig. 2 Physical properties of SPNs. (a) FTIR spectra and (b) XRD patterns of XLPEO/Co(BF4)2. (c) Correlation between ρSP and the salt content using eqn (1). (d) Correlation between Tg and the salt content using eqn (2). | ||
Fig. 2b and S1b† show that both series of SPNs are amorphous at ≈23 °C, validating complete dissociation of the Co salts by XLPEO. Adding salts has a minimal effect on the d-spacing (4.2 Å). Fig. 2c shows that increasing salt content increases ρSP (Tables 2 and S2†), which can be described using an additive model:31
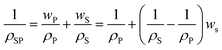 | (1) |
| x (mass%) | r | ρ SP (g cm−3) | T g,SP (°C) | P A (barrer) | Selectivity | ||||
|---|---|---|---|---|---|---|---|---|---|
| N2 | O2 | CO2 | CO2/N2 | CO2/O2 | O2/N2 | ||||
| 0 | — | 1.149 | −63 | 10 | 26 | 510 | 51 | 20 | 2.6 |
| 2 | 259 | 1.179 | −63 | 2.9 | 2.2 | 109 | 38 | 50 | 0.76 |
| 3.4 | 150 | 1.182 | −62 | 1.2 | 0.82 | 33 | 28 | 40 | 0.68 |
| 6.4 | 77 | 1.224 | −57 | 0.49 | 0.40 | 15 | 31 | 38 | 0.82 |
| 17 | 26 | 1.282 | −27 | 0.24 | 0.14 | 8.4 | 35 | 60 | 0.58 |
Fig. S1c and d† present the DSC thermograms of the SPNs. All samples do not show degradation at the temperatures ranging from −90 to 100 °C, indicating their potential applications for typical O2 purification (∼23 °C). XLPEO shows a crystallization peak at −36 °C and a melting peak at −7 °C, which disappear after adding Co(BF4)2 because of the complexation between ethylene oxides and Co2+. The absence of melting peaks at around 0 °C also suggests the absence of water in these films.32 Additionally, increasing salt loading increases Tg,SP (Tables 2 and S2†), which can be described using an empirical equation:26,33
| 1/Tg,SP = 1/Tg,P − acS | (2) |
Gas sorption properties of SPNs
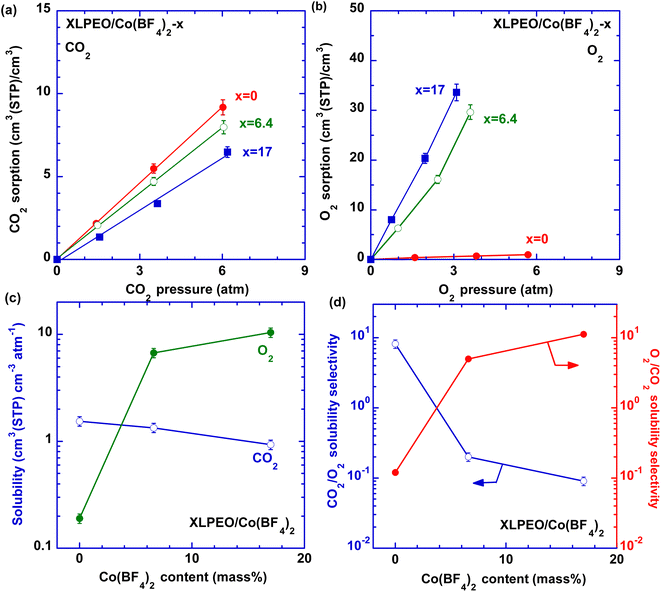
To elucidate the effect of the salt content on gas transport properties, CO2 and O2 sorption isotherms for XLPEO/Co(BF4)2 were obtained at 35 °C and are displayed in Fig. 3a and b. CO2 and O2 solubility can be calculated (Fig. 3c and Table S3†). Increasing Co(BF4)2 loading decreases CO2 solubility and significantly increases O2 solubility. For instance, adding 6.4 mass% Co(BF4)2 in XLPEO increases O2 solubility from 0.19 to 6.7 cm3(STP) cm−3 atm−1 because of the Co2+–O2 complexation and decreases CO2 solubility from 1.5 to 1.3 cm3(STP) cm−3 atm−1 due to the competitive interactions with XLPEO from the Co2+ ions. More importantly, increasing the Co(BF4)2 content decreases CO2/O2 solubility selectivity and significantly increases O2/CO2 solubility selectivity (Fig. 3d and e). In contrast, adding Co(ClO4)2 has minimal impact on O2 solubility (Fig. S2a†). Although the underlying mechanism for missing O2 affinity in XLPEO/Co(ClO4)2 is unclear, it is widely reported that anions and ligands can significantly influence the complexation between Co2+ and O2. For example, Co2+-based carriers with different anions exhibited a wide range of O2 sorption values between 0.06 and 0.90 mol O2 per mol carrier at 16 cmHg.11 Similarly, when various silver salts were dissolved in PEO for C3H6 sorption, the SPNs showed a molar ratio of C3H6 to Ag+ of 0.49 for AgBF4, 0.13 for AgCF3SO3, but only 0.03 for AgNO3;34 anions of bis(trifluoromethylsulfonyl)imide (Tf2N) stabilized Ag+ against H2, but not NO32− anions.35Gas permeation properties of the SPNs
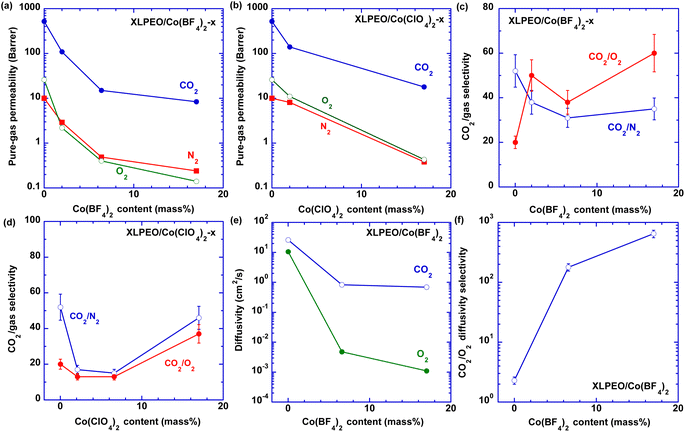
Fig. 4a and b present the effect of the Co(BF4)2 and Co(ClO4)2 content in XLPEO on pure-gas permeability at 35 °C (a typical temperature used for membrane applications), respectively. Increasing the salt loading decreases gas permeability because of the increased Tg,SP. For instance, adding 17 mass% Co(BF4)2 decreases CO2 permeability by 98.4% from 520 to 8.4 barrer and O2 permeability by 99.5% from 26 to 0.14 barrer. Similar behaviors have been observed for XLPEO with the addition of LiClO4, Ni(BF4)2, and Cu(BF4)2 were correlated with the increased Tg,SP.26 Increasing the salt content also decreases CO2/N2 selectivity because of the interactions between Co2+ and XLPEO, which reduce the favorable interactions between CO2 and XLPEO. Importantly, XLPEO/Co(BF4)2 SPNs exhibit unexpected CO2/O2 selectivity. For instance, adding only 3.4 mass% Co(BF4)2 increases CO2/O2 selectivity from 20 to 40, while adding Co(ClO4)2 consistently decreases CO2/O2 selectivity (Table S1†). Interestingly, XLPEO/Co(BF4)2 SPNs even show O2/N2 selectivity less than 1 (Fig. S2b†), which is very unusual for polymeric films.CO2 and O2 diffusion coefficients (DA, cm2 s−1) can be calculated by using DA = PA/SA, and the results are shown in Fig. 4e, f, S2c and Table S3.† Increasing the Co salt content decreases CO2 diffusivity because of the decreased chain flexibility, as indicated by the increased Tg,SP. Importantly, adding 17 mass% Co(BF4)2 increases CO2/O2 diffusivity selectivity from 2.3 to 650, validating the retarded O2 diffusion in the XLPEO/Co(BF4)2 SPNs. In contrast, adding 17 mass% Co(ClO4)2 increases CO2/O2 diffusivity selectivity only to 5.7 (Fig. S2c†).
CO2/O2 separation properties
Fig. 5 exhibits superior CO2/O2 separation properties of the XLPEO/Co(BF4)2 SPNs in Robeson's upper bound plot.36,37 All XLPEO/Co(BF4)2 SPNs (x = 2, 3.4, and 17) exhibit CO2/O2 separation properties above the upper bound.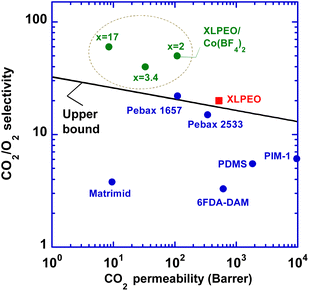 | ||
| Fig. 5 Superior CO2/O2 separation properties in XLPEO/Co(BF4)2 (x = 2, 3.4, and 17) compared with Robeson's upper bound,36,37 and representative polymers, including Matrimid,6 Pebax 1657 and 2533,9 6FDA-DAM,38 PDMS,1 and PIM-1.39 | ||
Our preliminary data show that the SPNs may lose separation properties over time, as shown in Table S4.† After storage in a vacuum for ∼4 months, a freestanding film (XLPEO/Co(BF4)2-5) was tested with CO2 at 50 psig and O2 at 100 psig alternatively for 9 days. CO2 permeability decreased from 75 to 12 barrer, and CO2/O2 selectivity decreased from 100 to 48, presumably because of the instability of Co2+ ions. Though CO2/O2 selectivity is still above the upper bound, the practical applications of these SPNs will need more investigation.
Conclusion
We began this work with a hypothesis that Co2+-based carriers can be incorporated into XLPEO to increase O2 sorption and permeability because of the affinity of O2 towards Co2+. Co(BF4)2 at loadings as high as 17 mass% can be dissociated by XLPEO to form SPNs, as indicated by DSC and XRD results. As expected, increasing Co(BF4)2 content increases Tg,SP and density and decreases gas permeability. Importantly, it dramatically increases O2 solubility and O2/CO2 solubility selectivity. Surprisingly, the XLPEO/Co(BF4)2 SPNs exhibit extremely low O2 permeability due to the retarded O2 diffusion and thus unexpectedly high CO2/O2 permeability selectivity, surpassing Robeson's upper bound and current leading materials for CO2/O2 separation. This study unveils a new series of SPNs with the potential for CO2/O2 separations, and the discovered retarded diffusion may be harnessed to design high-performance materials for various gas and vapor separations.Interestingly, adding Co(ClO4)2 into XLPEO does not improve O2 sorption and decreases CO2/O2 permeability selectivity. We expect that the anion type influences the dissociation of the Co salts and the ability of Co2+ ions to interact with polymers and O2. Additionally, Co2+ is subjected to oxidation and converted to Co3+ without affinity towards O2. Therefore, experimental investigation and computational simulations will be needed to unravel the underlying mechanisms and determine the carrier stability. Future work should also focus on preparing thin-film composite membranes and evaluating them with real gas streams to determine long-term stability.
Author contributions
T. A.: conceptualization, investigation, data curation, writing – original draft preparation; N. E.: data curation and discussion, writing-reviewing and editing; G. Z.: data curation and discussion, writing-reviewing and editing; H. L.: conceptualization, writing-reviewing and editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work received financial support from the U.S. Department of Energy Small Business Technology Transfer Program (DE-SC0020730), the National Energy Technology Laboratory (DE-FE0031736), and the New York State Foundation for Science, Technology and Innovation (NYSTAR).References
- J. Wang, F. Kang, Y. Chen, X. Zhang, X. Jiang, G. He and X. Ruan, Sep. Purif. Technol., 2024, 337, 126461 CrossRef CAS.
- Z. Zhao, W. Gao, Y. Chang, Y. Yang, H. Shen, T. Li and S. Zhao, Adv. Healthcare Mater., 2023, 690, 122183 Search PubMed.
- B. S. Kirkland, R. Clarke and D. R. Paul, J. Membr. Sci., 2008, 324, 119–127 CrossRef CAS.
- T. C. Merkel, X. Wei, Z. He, L. S. White, J. G. Wijmans and R. W. Baker, Ind. Eng. Chem. Res., 2013, 52, 1150–1159 CrossRef CAS.
- J. Wu, F. Hillman, C.-Z. Liang, Y. Jia and S. Zhang, J. Mater. Chem. A, 2023, 11, 17452–17478 RSC.
- T.-S. Chung, S. S. Chan, R. Wang, Z. Lu and C. He, J. Membr. Sci., 2003, 211, 91–99 CrossRef CAS.
- B. Zhu, X. Jiang, S. He, X. Yang, J. Long, Y. Zhang and L. Shao, J. Mater. Chem. A, 2020, 8, 24233–24252 RSC.
- H. Lin and B. D. Freeman, J. Membr. Sci., 2004, 239, 105–117 CrossRef CAS.
- V. I. Bondar, B. D. Freeman and I. Pinnau, J. Polym. Sci., Part B: Polym. Phys., 2000, 38, 2051–2062 CrossRef CAS.
- X. Chen, H. Nishide, K. Oyaizu and E. Tsuchida, J. Phys. Chem. B, 1997, 101, 5725–5729 CrossRef CAS.
- B. M. Johnson, R. W. Baker, S. L. Matson, K. L. Smith, I. C. Roman, M. E. Tuttle and H. K. Lonsdale, J. Membr. Sci., 1987, 31, 31–67 CrossRef CAS.
- Y. Kohno, M. G. Cowan, A. Okafuji, H. Ohno, D. L. Gin and R. D. Noble, Ind. Eng. Chem. Res., 2015, 54, 12214–12216 CrossRef CAS.
- H. Zhao, T. Song, X. Ding, R. Cai, X. Tan and Y. Zhang, J. Membr. Sci., 2023, 679, 121713 CrossRef CAS.
- D. E. Jaramillo, A. Jaffe, B. E. Snyder, A. Smith, E. Taw, R. C. Rohde, M. N. Dods, W. DeSnoo, K. R. Meihaus and T. D. Harris, Chem. Sci., 2022, 13, 10216–10237 RSC.
- H. Nishide, H. Kawakami, S. Toda, E. Tsuchida and Y. Kamiya, Macromolecules, 1991, 24, 5851–5855 CrossRef CAS.
- H. Nagar, P. Vadthya, N. S. Prasad and S. Sridhar, RSC Adv., 2015, 5, 76190–76201 RSC.
- S. H. Chen and J. Y. Lai, J. Appl. Polym. Sci., 1996, 59, 1129–1135 CrossRef CAS.
- W. Choi, P. G. Ingole, H. Li, S. Y. Park, J. H. Kim, H.-K. Lee and I.-H. Baek, Microchem. J., 2017, 132, 36–42 CrossRef CAS.
- J. Han, L. Bai, S. Luo, B. Yang, Y. Bai, S. Zeng and X. Zhang, Sep. Purif. Technol., 2020, 248, 117041 CrossRef CAS.
- E. Khare, N. Holten-Andersen and M. J. Buehler, Nat. Rev. Mater., 2021, 6, 421–436 CrossRef CAS.
- L. Hu, V. T. Bui, S. Fan, W. Guo, S. Pal, Y. Ding and H. Lin, J. Mater. Chem. A, 2022, 10, 10872–10879 RSC.
- Y. Fu, L. Chen, F. Xu, X. Li, Y. Li and J. Sun, J. Mater. Chem. A, 2022, 10, 4695–4702 RSC.
- A. Morisato, Z. J. He, I. Pinnau and T. C. Merkel, Desalination, 2002, 145, 347–351 CrossRef CAS.
- T. C. Merkel, R. Blanc, I. Ciobanu, B. Firat, A. Suwarlim and J. Zeid, J. Membr. Sci., 2013, 447, 177–189 CrossRef CAS.
- H. Lin, E. Van Wagner, J. S. Swinnea, B. D. Freeman, S. J. Pas, A. J. Hill, S. Kalakkunnath and D. S. Kalika, J. Membr. Sci., 2006, 276, 145–161 CrossRef CAS.
- T. Alebrahim, A. Chakraborty, L. Hu, S. Patil, S. Cheng, D. Acharya, C. M. Doherty, A. J. Hill, T. R. Cook and H. Lin, J. Membr. Sci., 2022, 644, 120063 CrossRef CAS.
- T. Alebrahim, L. Huang, H. K. Welgama, N. Esmaeili, E. Deng, S. Cheng, D. Acharya, C. M. Doherty, A. J. Hill, C. Rumsey, M. Trebbin, T. R. Cook and H. Lin, ACS Appl. Mater. Interfaces, 2024, 16, 11116–111124 CrossRef CAS PubMed.
- K. M. Rodriguez, W. Wu, T. Alebrahim, Y. Cao, B. D. Freeman, D. Harrigan, M. Jhalaria, A. Kratochvil, S. Kumar, W. Lee, Y. Lee, H. Lin, J. M. Richardson, Q. Song, B. Sundell, R. Thur, I. Vankelecom, A. Wang, C. Wiscount, J. Xingming and Z. P. Smith, J. Membr. Sci., 2022, 659, 120746 CrossRef.
- P. R. Bevington and D. K. Robinson, Data reduction and error analysis for the physical sciences, McGraw-Hill, Inc., New York, 2nd edn, 1992 Search PubMed.
- T. Tran, Y. Fu, D. Jiang and H. Lin, Macromolecules, 2022, 55, 9860–9867 CrossRef CAS.
- N. Paranjape, P. C. Mandadapu, G. Wu and H. Lin, Polymer, 2017, 111, 1–8 CrossRef CAS.
- T. Tran, C. Lin, S. Chaurasia and H. Lin, J. Membr. Sci., 2019, 574, 299–308 CrossRef CAS.
- C. J. Hawker, F. K. Chu, P. J. Pomery and D. J. T. Hill, Macromolecules, 1996, 29, 3831–3838 CrossRef CAS.
- S. Sunderrajan, B. D. Freeman, C. K. Hall and I. Pinnau, J. Membr. Sci., 2001, 182, 1–12 CrossRef CAS.
- S. Park, O. Morales-Collazo, B. Freeman and J. F. Brennecke, Angew. Chem., Int. Ed., 2022, 61, e202202895 CrossRef CAS PubMed.
- B. D. Freeman, Macromolecules, 1999, 32, 375–380 CrossRef CAS.
- H. Lin and M. Yavari, J. Membr. Sci., 2015, 475, 101–109 CrossRef CAS.
- L. Shao, T.-S. Chung, S. H. Goh and K. P. Pramoda, J. Membr. Sci., 2005, 267, 78–89 CrossRef CAS.
- C. G. Bezzu, A. Fuoco, E. Esposito, M. Monteleone, M. Longo, J. C. Jansen, G. S. Nichol and N. B. McKeown, Adv. Funct. Mater., 2021, 31, 2104474 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4ta02376e |
| This journal is © The Royal Society of Chemistry 2024 |