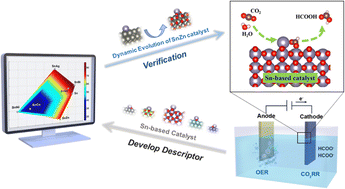
The electrochemical carbon dioxide reduction reaction (CO2RR) using renewable energy sources is a promising solution for mitigating CO2 emissions. In particular, CO2RR to formate represents a commercially profitable target. However, a comprehensive understanding of the catalytic mechanisms of Sn-based catalysts under reaction conditions, including the real-time structural evolution of catalysts and the role of all key reaction intermediates in influencing the CO2RR selectivity, is still lacking. The current study reports a framework to study the selectivity preference of Sn-based bimetallic catalysts using a combination of electrochemical measurements, in situ characterization, and density functional theory (DFT) calculations. The addition of a second metal (Co, Ni, Ag, Zn, Ga, Bi) was found to play a vital role in affecting the CO2RR performance. In situ X-ray absorption near edge structure (XANES) measurements revealed a dynamic evolution in the Sn valence state induced by different secondary metals. A multidimensional descriptor involving all the key reaction intermediates was developed to assess formate selectivity using a 2-dimensional volcano plot. This research offers an effective framework for understanding CO2RR catalytic selectivity by considering both the real-time structural evolution of catalysts and all the key intermediates involved.