Enhancing CO2 gasification-reforming of municipal solid waste with Ni/CeO2 and Ni/ZrO2 catalysts†
Abstract
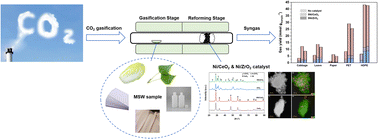
The global energy crisis and environmental sustainability challenges are exacerbated by the rapid increase in population and industrialization, necessitating effective management of municipal solid waste. The CO2 gasification-reforming of municipal solid waste with Ni/CeO2 and Ni/ZrO2 catalysts was conducted in a two-stage fixed-bed reactor. A significant increase in gas production from various waste samples (cabbage, poplar leaves, printed paper, PET, and HDPE) was observed, with the 5% Ni/CeO2 demonstrating higher efficiency than the 5% Ni/ZrO2 catalyst. The structural characterization of the catalysts revealed that Ni was more uniformly dispersed on the CeO2 support compared to ZrO2, resulting in enhanced activity of the 5% Ni/CeO2 catalysts. Further exploration into the optimal nickel loading and the ideal reforming temperature was conducted to maximize the efficiency of the CO2 gasification-reforming. The application of 5% Ni/CeO2 catalysts in the CO2 gasification-reforming of simulated municipal solid waste notably increased CO and total gas yields by 223% and 106%, respectively. This advancement holds promise for new technical approaches in resource utilization and the environmentally friendly processing of municipal solid waste.
- This article is part of the themed collections: 2024 Journal of Materials Chemistry A HOT Papers and Journal of Materials Chemistry A Emerging Investigators 2024


 Please wait while we load your content...
Please wait while we load your content...