 Open Access Article
Open Access ArticleRecent advances in noble metal-free electrocatalysts to achieve efficient alkaline water splitting
Mohammed-Ibrahim
Jamesh
 ag,
Dingqin
Hu
ag,
Jing
Wang
ag,
Dingqin
Hu
ag,
Jing
Wang
 b,
Farah
Naz
ag,
Jianpei
Feng
ag,
Li
Yu
b,
Farah
Naz
ag,
Jianpei
Feng
ag,
Li
Yu
 ag,
Zhao
Cai
ag,
Zhao
Cai
 b,
Juan Carlos
Colmenares
b,
Juan Carlos
Colmenares
 cf,
Duu-Jong
Lee
*d,
Paul K.
Chu
cf,
Duu-Jong
Lee
*d,
Paul K.
Chu
 *e and
Hsien-Yi
Hsu
*e and
Hsien-Yi
Hsu
 *ag
*ag
aSchool of Energy and Environment, Department of Materials Science and Engineering, Centre for Functional Photonics (CFP), City University of Hong Kong, Kowloon Tong, Hong Kong, China. E-mail: sam.hyhsu@cityu.edu.hk
bFaculty of Materials Science and Chemistry, China University of Geosciences (Wuhan), Wuhan 430074, China
cInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, 01224, Warsaw, Poland
dDepartment of Mechanical Engineering, City University of Hong Kong, Kowloon Tong, Hong Kong, China. E-mail: tuclee@cityu.edu.hk
eDepartment of Physics, Department of Materials Science and Engineering, Department of Biomedical Engineering, City University of Hong Kong, Tat Chee Avenue, Kowloon, Hong Kong, China. E-mail: paul.chu@cityu.edu.hk
fEngineering Research Institute (In3), Universidad Cooperativa de Colombia, Medellín 50031, Colombia
gShenzhen Research Institute of City University of Hong Kong, Shenzhen 518057, P. R. China
First published on 9th April 2024
Abstract
Electrochemical water splitting is one of the promising approaches for generating hydrogen. Developing noble metal-free electrocatalysts for the hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) is important to achieve efficient water-splitting. This paper reviews the activity, stability, and durability of recently reported noble metal-free electrocatalysts such as oxides/hydroxides/(oxy)hydroxides/layered double hydroxides, sulfides, selenides, phosphides/phosphates, nitrides, carbon-based electrocatalysts, and alloy/B/V/F/Si based electrocatalysts for the HER and OER in an alkaline environment, including the strategies used to achieve high activity and stability/durability at a current density of ≥1000 mA cm−2. Moreover, this paper discusses the various promising strategies including the fabrication of nanostructured, ultrathin, porous, and nanoporous materials, preparing superaerophobic surfaces, construction of hollow structures, core–shell structures, heterostructures, heterojunctions, or Mott–Schottky heterojunctions, designing facile and/or scalable synthesis routes, creating doping and/or vacancies/defects, fabricating catalysts with high valence state sites, designing medium or high-entropy alloys, and tuning the atomic packing structure, electronic structure, or conductivity to enhance the activity and stability for the HER and/or OER.
1. Introduction
Hydrogen is considered as a promising fuel having low carbon emission and high energy density (142 MJ kg−1). The global hydrogen production market size was estimated at about 120 billion USD in the year 2020, whereas currently, about 95% of the industrial hydrogen production processes such as steam reforming, and coal gasification with extensive greenhouse gas release are not efficient processes, while only about 4% of the H2 production around the world depends on water electrolysis, and the current research studies are being focused on water electrolysis or other strategies to alleviate the utilization of fossil fuels for the production of hydrogen energy.1 Electrochemical water splitting is a promising way for sustainable hydrogen production, especially with renewable electricity such as solar or wind sources.2 Electrochemical water splitting comprises a hydrogen evolution reaction (HER) at the cathode with the involvement of two electrons, and an oxygen evolution reaction (OER) at the anode with the involvement of four electrons in both acid and alkaline environments. Currently, noble electrocatalysts, such as Ir/Ru-based compounds for the OER and Pt for the HER, are considered as promising electrocatalysts for water splitting.3 Nevertheless, their high cost and scarcity limit their uses. Moreover, the currently used OER electrocatalysts for water splitting in acid environments are scarce and expensive,3,4 whereas the cost of the water electrolyzer system can be reduced by using noble metal-free electrocatalysts in the alkaline environment.2b,d,5 To alleviate the gas mixing issues (H2 and O2), proton exchange membrane (PEM) water electrolysis and anion exchange membrane (AEM) water electrolysis are considered as promising water electrolyzers for industrial level application, where the AEM water electrolyzer can operate in an alkaline environment but the PEM water electrolyzer may require acid-stable OER electrocatalysts to operate in an acid environment, which can elevate the cost.6 Therefore, various promising strategies such as improving the gas-evolution behavior, enhancing the conductivity, tuning the electronic structure, increasing the electrochemical surface area, surface reconstruction during the HER/OER, preparing core–shell/hetero/hollow structured materials, preparing amorphous materials, fabricating bimetallic/multi-metallic materials, doping metals/heteroatoms, preparing porous/nanostructured/quantum sized materials, and creating oxygen vacancies/defects are used to achieve high-performance noble metal-free electrocatalysts for the HER and/or OER in an alkaline environment.5,7Developing noble-metal-free electrocatalysts with substantially lower overpotential (η) for HER and OER or significantly lower cell voltage for overall water splitting is highly desirable. Fabrication of transition metal boride electrocatalysts with a 3D hollow structure may alter the electronic structure, provide optimal adsorption energy with intermediates, improve the conductivity, expose abundant active sites, provide high mechanical strength, and facilitate gas evolution, all of which could improve the activity and stability of the HER and OER. For the HER in 1 M KOH, NiMoB hollow foam8 affords an η of −18 mV at −10 mA cm−2, suggesting its outstanding activity. For overall water splitting in 1 M KOH, NiMoB hollow foam//NiMoB hollow foam affords a potential of 1.431 V at 10 mA cm−2, suggesting its good activity. Fabrication of composites containing transition metal selenides and perovskite oxides could enhance the performance of the OER. For the OER in 1 M KOH, LSCO-MoSe2 (ref. 9) affords an η of 39 mV at 10 mA cm−2, suggesting its very high activity, where LSCO is La0.5Sr0.5CoO3−δ perovskite oxide. Moreover, Mn1Ni1Co1-P//Mn1Ni1Co1-P,10 c-NiFe/a-NiFeOOH@NiMo//c-NiFe/a-NiFeOOH@NiMo,11 and Gr-CNTs-Sn4P3//Gr-CNTs-Sn4P3 (ref. 12) exhibit very high activity for overall water splitting in 1 M KOH, while the NiP2-FeP2@Cu nanoarray13 exhibits very high activity for the HER. Thus, several noble metal-free electrocatalysts exhibit substantially much lower η for the HER and OER or significantly much lower cell voltage for overall water splitting.
An electrocatalyst for the HER or OER fabricated at a relatively low cost should operate at low η to deliver a current density for a prolonged period, which is highly desirable for industrial applications. Fabrication of tin-doped nickel sulfide having an ultrathin nanostructure could modify the electronic structure, and facilitate the evolution of gas even at large current densities, and that could boost the performance of the HER and OER. For the OER in 1 M KOH, the Sn-Ni3S2 (ref. 14) affords an η of 267 mV at 100 mA cm−2, and an η of 580 mV at a current density of 1000 mA cm−2, indicating its significantly high activity, while it undergoes negligible decay at a very large current density of 2000 mA cm−2 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its substantially good durability. For the HER in 1 M KOH, it affords an η of −171 mV at −100 mA cm−2, and an η of −570 mV at a current density of −1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a current density of −1000 mA cm−2 after 10
000 cycles of CV, suggesting its substantially good durability. For the HER in 1 M KOH, it affords an η of −171 mV at −100 mA cm−2, and an η of −570 mV at a current density of −1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a current density of −1000 mA cm−2 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its significantly very high durability. For overall water splitting in 1 M KOH, the Sn-Ni3S2//Sn-Ni3S2 affords a potential of 1.46 V at 10 mA cm−2, and ∼2.65 V at a current density of 1000 mA cm−2, suggesting its significantly very high activity. Integrating N-doped carbon with nanostructured transition metal/metal oxide could redistribute the electrons at the heterojunction interface, afford optimal adsorption energy with intermediates, enhance the conductivity, facilitate the evolution of gas, expose abundant active sites, and that could enhance the performance for the HER and OER. For the OER in 1 M KOH, Ni/MoO2@CN15 affords an η of 250 mV at 10 mA cm−2, and an η of 420 mV at a large current density of 1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a large current density of 1000 mA cm−2 for 200 h, suggesting its significantly very high stability. For the HER in 1 M KOH, it affords an η of −33 mV at −10 mA cm−2, and an η of −267 mV at a large current density of −1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a large current density of −1000 mA cm−2 for 200 h, suggesting its significantly very high stability. For overall water splitting in 1 M KOH, Ni/MoO2@CN//Ni/MoO2@CN affords a potential of 1.83 V at 200 mA cm−2, and a potential of 2.02 V at a large current density of 1000 mA cm−2, suggesting its significantly very high activity, while it affords 98.19% retention at 1.7 V for 168 h, suggesting its significantly very high stability. Moreover, C-Ni1−xO/3D printed Ni,16 Fe-Co-CO3-OH,17 FeP/Ni2P,18 Ni2P-Fe2P,19 and Co2N0.67/CoMoO4 (ref. 20) for the HER and OER exhibit high activity and stability in 1 M KOH, while MoS2/Mo2C21 for the HER, and CuS-Ni3S2/CuNi22 for the OER exhibit high activity and stability. Thus, several noble metal-free electrocatalysts exhibit low η at high current densities with high stability for the HER and OER.
000 cycles of CV, suggesting its significantly very high durability. For overall water splitting in 1 M KOH, the Sn-Ni3S2//Sn-Ni3S2 affords a potential of 1.46 V at 10 mA cm−2, and ∼2.65 V at a current density of 1000 mA cm−2, suggesting its significantly very high activity. Integrating N-doped carbon with nanostructured transition metal/metal oxide could redistribute the electrons at the heterojunction interface, afford optimal adsorption energy with intermediates, enhance the conductivity, facilitate the evolution of gas, expose abundant active sites, and that could enhance the performance for the HER and OER. For the OER in 1 M KOH, Ni/MoO2@CN15 affords an η of 250 mV at 10 mA cm−2, and an η of 420 mV at a large current density of 1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a large current density of 1000 mA cm−2 for 200 h, suggesting its significantly very high stability. For the HER in 1 M KOH, it affords an η of −33 mV at −10 mA cm−2, and an η of −267 mV at a large current density of −1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a large current density of −1000 mA cm−2 for 200 h, suggesting its significantly very high stability. For overall water splitting in 1 M KOH, Ni/MoO2@CN//Ni/MoO2@CN affords a potential of 1.83 V at 200 mA cm−2, and a potential of 2.02 V at a large current density of 1000 mA cm−2, suggesting its significantly very high activity, while it affords 98.19% retention at 1.7 V for 168 h, suggesting its significantly very high stability. Moreover, C-Ni1−xO/3D printed Ni,16 Fe-Co-CO3-OH,17 FeP/Ni2P,18 Ni2P-Fe2P,19 and Co2N0.67/CoMoO4 (ref. 20) for the HER and OER exhibit high activity and stability in 1 M KOH, while MoS2/Mo2C21 for the HER, and CuS-Ni3S2/CuNi22 for the OER exhibit high activity and stability. Thus, several noble metal-free electrocatalysts exhibit low η at high current densities with high stability for the HER and OER.
Besides, the fabrication of noble metal-free electrocatalysts by green-chemistry approaches is highly desirable to achieve efficient, green water electrolysis, and that could alleviate or diminish the use or production of hazardous substances.23 Designing energy-efficient synthesis routes (especially at room temperature) for the fabrication of noble metal-free electrocatalysts is one of the promising green-chemistry approaches.23 Chen et al.24 observed that S-FeOOH exhibits enhanced activity and stability for the OER, while it was obtained by immersing cleaned Fe foam for 20 min at room temperature in the solution containing Fe(NO3)3 and Na2S2O3. The redox reactivities of the lattice oxygen and Fe in FeOOH are activated due to S doping. Moreover, Zhao et al.25 observed that NiFe LDH exhibits enhanced activity and stability for the OER, while it was prepared by immersing cleaned, acid-treated Fe foam in NiSO4 solution under ambient conditions. In addition, Liu et al.26 observed that IronCE10s exhibit enhanced activity and stability for the OER, while it was prepared on the cheap iron substrate by applying pulsed potentials for 10 s in NiSO4 electrolyte at 25 ± 1 °C, where the substrate was used as a counter electrode. The utilization of waste as a source for the fabrication of noble metal-free electrocatalysts is one of the promising green chemistry approaches.23 Lu et al.27 observed that WO3@F0.1-C exhibits enhanced activity and stability for the OER, while it was obtained by a plasma-induced assembly method, where perfluorooctanoic acid (a pollutant) is used as an F source. Utilization of renewable raw materials for the fabrication of noble-metal-free electrocatalysts is one of the most promising green-chemistry approaches.23 Huang et al.28 observed that Zn-Fe/Mn@Mn-FeP exhibits enhanced activity and stability for the HER and OER, where the nanostructured phosphide-based electrocatalyst is prepared using relatively low-cost metals such as Fe, Mn, and Zn. Thus, some green chemistry approaches including ambient temperature synthesis, utilization of waste as a source, and using relatively low-cost metals have been used for the fabrication of noble metal-free electrocatalysts for electrochemical water splitting.
Thus, recently, several promising strategies have been used for the fabrication of noble metal-free electrocatalysts to achieve low η at high current densities with high stability for the HER and OER and to achieve substantially much lower η for the HER and OER, while some green chemistry approaches have been applied for the fabrication of noble metal-free electrocatalysts for electrochemical water splitting. However, reviews on the fabrication of various noble metal-free electrocatalysts using several promising strategies along with their catalytic performances for electrochemical water splitting (HER and OER) in alkaline environments have been rarely reported. In this respect, the present paper reviews the activity, stability, and durability of several kinds of recently reported noble metal-free electrocatalysts such as oxides/hydroxides/(oxy)hydroxides/layered double hydroxides, sulfides, selenides, phosphides/phosphates, nitrides, carbon-based electrocatalysts, and alloy/B/V/F/Si based electrocatalysts for the HER and OER in an alkaline environment. In addition, this paper reviews the strategies used to achieve high activity and stability/durability at a current density of ≥1000 mA cm−2 of noble metal-free electrocatalysts for the HER and OER in an alkaline environment. Moreover, this paper reviews some green chemistry approaches used for the fabrication of noble metal-free electrocatalysts for electrochemical water splitting. Finally, this review summarizes several promising strategies used for the fabrication of noble metal-free electrocatalysts to achieve enhanced performance for the HER and OER in alkaline environments.
2. Constructing noble metal-free electrocatalysts for the HER and OER in an alkaline electrolyte
2.1. Constructing oxide/hydroxide/(oxy)hydroxide/layered double hydroxide electrocatalysts
Fabrication of nanostructured transition metal oxide/metal heterojunctions could expose abundant active sites, facilitate the evolution of gas, afford optimal adsorption energy with intermediates, and that could enhance the performance of HER and OER. Guo et al.30 observed that MoO2/Co exhibits enhanced activity for the HER and OER. It was synthesized on Ni foam by hydrothermal treatment followed by annealing under a H2/Ar atmosphere. MoO2/Co exhibits higher activity and lower charge transfer resistance for the HER than the CoMo precursor. It is crystalline containing MoO2, α-Co, and β-Co phases having heterojunction. It contains Mo, Co, and O, which are homogeneously distributed. It possesses an ultrathin porous nanosheet structure. The density functional theory (DFT) calculations reveal that the Gibbs free energy of hydrogen adsorption (ΔGH*) of MoO2/Co (−0.38 eV) is much nearer to the thermoneutral value than pure Co (−1.46 eV), suggesting the optimal hydrogen adsorption/desorption on the MoO2/Co heterojunction, and that could enhance the activity for the HER. The HER in 1 M KOH affords an η of −48 mV at −10 mA cm−2, suggesting its very high activity. Fabricating nanostructured transition metal doped metal oxides with oxygen vacancies could provide optimal adsorption energy with intermediates, and that could boost the performance of the HER and OER. Xue et al.31 observed that Mn6-CoO exhibits enhanced activity and stability for the HER and OER. It was obtained on Ni foam by hydrothermal treatment followed by annealing at 350 °C for 2 h under an Ar atmosphere. Mn6-CoO exhibits higher activity for the HER and OER than CoO, while it exhibits higher electrochemical surface area and lower charge transfer resistance than CoO. Mn6-CoO is composed of Mn-doped CoO. It possesses a hetero-phase (amorphous–crystalline). It contains oxygen vacancies (38.2%). It possesses a rambutan-like morphology, which contains numerous small nanoneedles. The DFT calculations reveal that the oxygen vacancies in Mn-CoO can optimize the adsorption-free energy of H* and HOO* intermediates, suggesting enhanced performance for the HER and OER. The HER in 1 M KOH affords an η of −25.6 mV at −10 mA cm−2, suggesting its significantly very high activity. For overall water splitting in 1 M KOH, Mn6-CoO//Mn6-CoO affords a potential of 1.52 V at 10 mA cm−2, suggesting its very high activity.
Rani et al.32 studied the fabrication of faceted transition bimetallic oxide (CuMn2O4) nanoparticles with oxygen vacancies which can enhance the performance, activity, and stability of the HER. It was prepared by the polyol-mediated annealing method (hydrothermal treatment followed by annealing at 600 °C). The reported activity of faceted CuMn2O4 is higher than that of non-faceted CuMn2O4 for the HER. The prepared sample showed a cubic spinel CuMn2O4 phase, and Mn, Cu, and O are homogeneously distributed. It also has oxygen vacancies. HER active phases can be produced by tuning the calcination parameters such as temperature, gas atmosphere, and time. Wu et al.33 observed that Bi2O3-based catalyst exhibits enhanced activity and stability for the HER. It was prepared on Ni foam by hydrothermal treatment followed by heat treatment at 400 °C for 2 h in air followed by heat treatment at 400 °C in an Ar/H2 atmosphere. It is crystalline containing α-Bi2O3, Bi3Ni, and BiNi alloys, which are homogeneously distributed. It possesses nanosheet morphology. Thus, applying an in situ phase engineering strategy for Bi2O3 on Ni foam could produce HER active sites (BixNi alloys and oxide phases), and that could enhance the performance of the HER.
Dynamically restructuring NixCryO during electrolysis enhanced the porosity for better activity of the OER. Malek et al.34 fabricated NixCryO through hydrothermal treatment followed by calcination. The in situ and electrochemical investigations disclose that the electrochemically active surface area of NixCryO could be increased by the leaching and redistribution of Cr upon electrolysis for a certain time interval, leading to an increase in porosity, and that could enhance the activity for the OER. The OER in 1 M KOH affords an η of 270 mV at 100 mA cm−2, and an η of 320 mV at a high current density of 500 mA cm−2, suggesting its very high activity, while it affords high stability. Fabrication of ultrathin transition bimetallic oxides with abundant oxygen vacancies can afford optimal adsorption energy with intermediates to increase the conductivity, which could also improve the performance of the OER. Yu et al.35 prepared f-Ni0.1Co0.9Ox by the electron beam evaporation method followed by calcination at 160 °C for 30 min using an O2 atmosphere under UV light irradiation. f-Ni0.1Co0.9Ox exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than f-CoOx and f-NiOx. The alloy oxide film (f-Ni0.1Co0.9Ox) is optically transparent with a thickness of about 10 nm (ultrathin). It contains Co, Ni, and O, which are homogeneously distributed. It contains oxygen vacancies. Fabrication of a transition metal oxide/metal oxide hetero interface with abundant high valence ions can provide optimal adsorption energy with intermediates, increase the conductivity, and that could enhance the performance of the OER. Zhang et al.36 observed that Co3−xO4/NiO exhibits enhanced activity and stability for the OER. It was prepared by hydrothermal treatment of the prepared Co3−xO4 with nickel acetate followed by calcination in the air atmosphere. Co3−xO4/NiO exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than Co3−xO4 and Co3O4. It is crystalline containing Co3−xO4 and NiO. It contains a heterointerface. It contains Co, Ni, and O, which are homogeneously distributed. It contains Ni3+, Ni2+, Co3+, and Co2+. It possesses particle morphology (diameter: about 20 nm). Fabrication of a thin layer of a transition metal oxide on ZnO can modulate the electronic structure, and that could enhance the performance of the OER. Nandanapalli et al.37 observed that Co3O4 SS Mesh/ZnO exhibits enhanced activity and stability for the OER (SS Mesh: stainless steel mesh). It was obtained by atomic layer deposition followed by chemical bath deposition followed by annealing followed by electrochemical deposition. Co3O4 SS Mesh/ZnO exhibits higher activity for the OER than SS Mesh/ZnO. It is composed of ZnO nanorods on stainless steel mesh, which are covered by a thin layer of cobalt oxide containing a predominant amount of Co3O4 along with a minor amount of CoO. It is crystalline. It contains Zn, Co, and O, which are homogeneously distributed. It contains Co3+ and Co2+.
Fabrication of nickel–molybdenum oxysulfide with unique morphology as a pre-catalyst is essential, where the electrochemical reduction of the pre-catalyst can produce a highly active robust catalyst containing metallic species for the HER, while the electrochemical oxidation of the pre-catalyst can form a highly active robust catalyst containing high valence species for the OER. Liu et al.38 observed that the HR-NiMoO@Ni exhibits substantially very high activity and stability for the HER, while the OR-NiOOH exhibits significantly very high activity and stability for the OER. The NiMoOS pre-catalyst on Ni foam was synthesized by hydrothermal treatment followed by sulfurization at 350 °C for 2 h. HR-NiMoO@Ni was obtained by applying cathodic CV scans on NiMoOS, whereas OR-NiOOH was obtained by applying anodic CV scans on NiMoOS (CV: cyclic voltammetry). HR-NiMoO@Ni and OR-NiOOH exhibit higher electrochemical surface area and lower charge transfer resistance than NiMoOS. The NiMoOS pre-catalyst is crystalline and is composed of sulfur-doped nickel molybdate (NiMoO4). It contains Mo, Ni, O, and S, which are homogeneously distributed. It possesses nanorod array morphology. HR-NiMoO@Ni is composed of ultrathin cubic Ni metal nanosheets, which are vertically grown on NiMoO nanorods, forming a core–shell structure (core: NiMoO; shell: Ni). It contains abundant defects. OR-NiOOH is composed of quasi-crystalline NiOOH along with a residual amount of S. The atomic ratio of O![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni
Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) S is 3.9
S is 3.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.1, respectively, suggesting the existence of a trace amount of S. It contains Ni3+. It contains abundant defects. It possesses nanorod array morphology. For the OER in 1 M KOH, it affords an η of 213 mV at 10 mA cm−2, and an η of 358 mV at a current density of 1000 mA cm−2, suggesting its significantly very high activity, while it affords reasonable stability at 1000 A m−2 for 100 h, suggesting its significantly very high stability. For the HER in 1 M KOH, HR-NiMoO@Ni affords an η of −96 mV at −10 mA cm−2, and an η of −308 mV at a current density of −1000 mA cm−2, suggesting its substantially very high activity, while it affords reasonable stability at −1000 A m−2 for 100 h, suggesting its significantly very high stability. For overall water splitting in 1 M KOH, OR-NiOOHOER//HR-NiMoO@NiHER affords a potential of 1.51 V at 10 mA cm−2, suggesting its very high activity, while it affords reasonable stability at 1000 A m−2 for 500 h, suggesting its significantly very high stability.
0.1, respectively, suggesting the existence of a trace amount of S. It contains Ni3+. It contains abundant defects. It possesses nanorod array morphology. For the OER in 1 M KOH, it affords an η of 213 mV at 10 mA cm−2, and an η of 358 mV at a current density of 1000 mA cm−2, suggesting its significantly very high activity, while it affords reasonable stability at 1000 A m−2 for 100 h, suggesting its significantly very high stability. For the HER in 1 M KOH, HR-NiMoO@Ni affords an η of −96 mV at −10 mA cm−2, and an η of −308 mV at a current density of −1000 mA cm−2, suggesting its substantially very high activity, while it affords reasonable stability at −1000 A m−2 for 100 h, suggesting its significantly very high stability. For overall water splitting in 1 M KOH, OR-NiOOHOER//HR-NiMoO@NiHER affords a potential of 1.51 V at 10 mA cm−2, suggesting its very high activity, while it affords reasonable stability at 1000 A m−2 for 500 h, suggesting its significantly very high stability.
Designing a facile synthesis route for the fabrication of heterostructured transition metal oxides is essential, which could modify the electronic structure, provide optimal adsorption energy with intermediates, and that could enhance the performance of the HER and OER. Hu et al.1g observed that the MH-TMO (mesoporous and heterostructured transition metal oxides) exhibits enhanced activity and stability for the HER and OER than Fe2O3, and CuO. It was synthesized on Ni foam by one-step hydrothermal treatment. It is crystalline, containing Fe2O3, and CuO. It exhibits a heterostructure. The Fe 2p peak of MH-TMO exhibits a slight negative shift in the high-resolution XPS spectra which confirms the modified electronic structure. It contains oxygen vacancies and mesopores. It exhibits a slightly compressed M–O–M bond, suggesting the electronic interaction between Fe2O3 and CuO. It possesses a sandwich structure, where the Fe2O3 nanosheets are surrounded on either side by CuO polyhedra. The DFT calculations disclose that the Fe site on the MH-TMO heterostructure affords optimal adsorption energy with intermediates. For the OER in 1 M KOH, it affords an η of ∼216 mV at 10 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at 10 mA cm−2 for 100 h, suggesting its very high stability. For the HER in 1 M KOH, it affords an η of ∼−70 mV at −10 mA cm−2, suggesting its very high activity, while it affords reasonable stability at −10 mA cm−2 for 100 h. For overall water splitting in 1 M KOH, MH-TMO//MH-TMO affords a potential of 1.49 V at 10 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at 10 mA cm−2 for 100 h, suggesting its very high stability.
Fabrication of ultrafine transition metal oxide particles/nanostructured transition metal hydroxide heterostructures could modify the electronic structure, afford optimal adsorption energy with intermediates, and that could enhance the performance of the HER. Peng et al.39 observed that MoO3-Co(OH)2@Ag exhibits enhanced activity and stability for the HER. It was prepared on Ag nanowire cloth by a two-step electrodeposition approach. MoO3-Co(OH)2@Ag exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER than MoO3@Ag and Co(OH)2@Ag. It is composed of a Co(OH)2 nanosheet array on Ag nanowires, which is decorated by ultrafine MoO3 particles. It is crystalline. It exhibits a heterostructure. In the high-resolution XPS spectra, the Mo 3d peak of MoO3-Co(OH)2@Ag exhibits a slight negative shift, when compared to that of MoO3@Ag, suggesting a modified electronic structure due to the electronic interaction between MoO3 and Co(OH)2. It contains oxygen vacancies. The DFT calculations reveal that the Gibbs free-energy of the adsorbed H* (ΔGH*) value on MoO3-Co(OH)2 (0.22 eV) is nearer to 0 eV when compared to that of MoO3 (0.71), and Co(OH)2 (1.99), suggesting optimal hydrogen adsorption/desorption on the MoO3-Co(OH)2 heterojunction.
Doping of phosphorus in cobalt molybdate could alter the electronic structure, provide optimal adsorption energy with intermediates, and that could enhance the performance of the HER and OER. Wang et al.40 observed that P-CoMoO4 exhibits enhanced activity and stability for the HER and OER. It was synthesized on Ni foam by hydrothermal treatment followed by phosphorization at 400 °C for 2 h under an Ar atmosphere. P-CoMoO4 exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER and OER than CoMoO4. It is composed of phosphorus-doped monoclinic β-CoMoO4. It has low crystallinity. It contains Co, Mo, O, and P, which are homogeneously distributed. In the high-resolution XPS spectra, the Co 2p peak of P-CoMoO4 exhibits a slight positive shift, when compared to that of CoMoO4, suggesting a modified electronic structure due to the phosphorus doping. It contains Moδ+ (2 < δ < 4), Mo4+, Mo5+, and Mo6+, whereas CoMoO4 without P doping contains only Mo6+. It contains PO43−. It contains oxygen vacancies. It possesses micro-rod array morphology. It undergoes surface reconstruction during the HER and OER. It is reconstructed into Co(OH)2-CoMoO4/P-CoMoO4 during the HER, while it is converted into CoOOH/P-CoMoO4 during the OER. For the HER in 1 M KOH, it affords an η of −44 mV at −10 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at −50 mA cm−2 for 100 h, suggesting its very high stability. For overall water splitting in 1 M KOH, P-CoMoO4//P-CoMoO4 affords a potential of 1.54 V at 10 mA cm−2, suggesting its very high activity.
Fabricating an electrocatalyst on a 3D periodic porous electrode could facilitate the evolution of gas bubbles, reduce bubble coalescence, expose abundant active sites, and that could enhance the activity for the HER and OER even at high current densities. Kou et al.16 reported that the C-Ni1−xO/3D printed Ni exhibits very high activity and stability for the HER and OER in 1 M KOH. It was prepared by 3D printing with solvent evaporation followed by heat treatment followed by coating of the catalyst (Fig. 1): at first, a Ni lattice structure was obtained through a direct ink writing technique, where the paste-like ink was composed of Ni particles, polylactic-co-glycolic acid (PLGA), dichloromethane (DCM), and ethylene glycol butyl ether (EGBE). Then, the printed parts were subjected to drying in air followed by thermal treatment (300 °C for 1 h and then 600 °C for 1 h in an H2 atmosphere) followed by heat treatment (900 °C in an Ar atmosphere for 3 h). Later, nickel oxalate was grown on 3D printed Ni (working electrode) by applying 50 V for 10 minutes in 0.3 M oxalic acid as the electrolyte, where Ni foil was used as a counter electrode. Finally, C-Ni1−xO/3D printed Ni was obtained by heating the nickel oxalate/3D printed Ni at 400 °C for 40 minutes in the Ar atmosphere. C-Ni1−xO/3D printed Ni exhibits higher activity and electrochemical surface area for the HER and OER than C-Ni1−xO/Ni foam. C-Ni1−xO on 3D printed Ni with a periodic porous structure exhibits much higher bubble flow and much less bubble coalescence than C-Ni1−xO on 3D Ni foam with a disordered porous structure. It is composed of 3D printed Ni having periodic pore structures, which is decorated by carbon doped nickel oxide, where the 3D porous structure could facilitate the evolution of gas bubbles, diminish bubble coalescence, and expose abundant active sites, which could enhance the activity for the HER and OER even at large current densities. The C-Ni1−xO/3D printed Ni affords an η of −245 mV at a current density of −1000 mA cm−2 for the HER (Fig. 1h), while it affords an η of 425 mV at a current density of 1000 mA cm−2 for the OER (Fig. 1i) in 1 M KOH, showing significantly high activity.
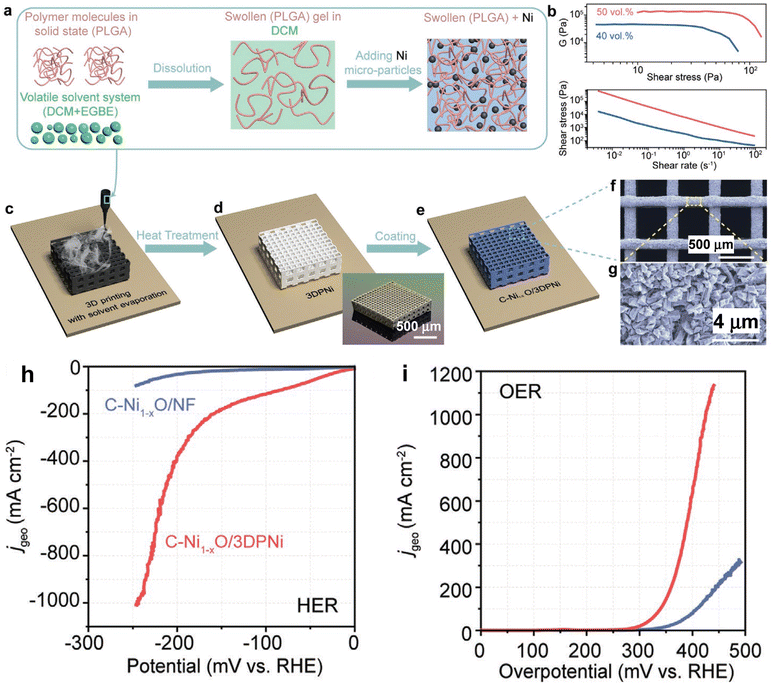 | ||
| Fig. 1 (a) Schematic diagram depicting the ink formulation process; the graph located in the upper part of (b) represents the elastic modulus versus shear stress, and the graph located in the lower part of (b) indicates the shear stress versus shear rate for Ni-based inks with solid loadings of 50 vol% (red color) and 40 vol% (blue color); (c)–(e) schematic pictures depicting the fabrication process of C-Ni1−xO/3D printed Ni, where the inset in (d) shows its corresponding digital photographic image; (f) and (g) SEM images of C-Ni1−xO/3D printed Ni; LSV curves of C-Ni1−xO/3D printed Ni in comparison with C-Ni1−xO/Ni foam for (h) the HER and (i) OER in 1 M KOH (reproduced with permission from ref. 16 Copyright 2020, Wiley-VCH GmbH). | ||
Integrating N-doped carbon with nanostructured transition metal/metal oxide could redistribute intermediates, enhance the conductivity, facilitate the evolution of gas, and expose abundant active sites, and that could enhance the performance of the HER and OER. Qian et al.15 observed that Ni/MoO2@CN exhibits significantly very high activity and stability for the HER and OER. It was prepared on Ni foam by hydrothermal treatment followed by carbonization at 450 °C for 2 h under a H2/Ar atmosphere. Ni/MoO2@CN exhibits higher activity for the HER than MoO2@CN, while it exhibits higher activity for the OER than Ni/MoO2. It is composed of Ni/MoO2, which is encapsulated by N-doped carbon. It exhibits a three-phase heterojunction. It contains Ni, Mo, C, N, and O, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p peak of Ni/MoO2@CN exhibits a slight positive shift, and the Mo 3d peak of Ni/MoO2@CN exhibits a slight negative shift, when compared to that of Ni/MoO2, suggesting a modified electronic structure due to the N doped carbon. It possesses nano-needle morphology. DFT calculations disclose the redistribution of electrons at the triple-phase heterojunction interface, which could provide optimal adsorption energy with intermediates, and could enhance the performance of the HER and OER. For the OER in 1 M KOH, it affords an η of 250 mV at 10 mA cm−2, and an η of 420 mV at a current density of 1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a current density of 1000 mA cm−2 for 200 h, suggesting its significantly very high stability. For the HER in 1 M KOH, it affords an η of −33 mV at −10 mA cm−2, and an η of −267 mV at a current density of −1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a current density of −1000 mA cm−2 for 200 h, suggesting its significantly very high stability. For overall water splitting in 1 M KOH, Ni/MoO2@CN//Ni/MoO2@CN affords a potential of 1.83 V at 200 mA cm−2, and a potential of 2.02 V at a current density of 1000 mA cm−2, suggesting its significantly very high activity, while it affords 98.19% retention at 1.7 V for 168 h, suggesting its significantly very high stability.
The utilization of waste as a source for the green synthesis of electrocatalysts is highly desirable, whereas perfluorooctanoic acid, an aqueous pollutant, can be used as a fluorine source. Lu et al.27 observed that WO3@F0.1-C exhibits enhanced activity and stability for the OER. It was obtained by the plasma-induced assembly method, where perfluorooctanoic acid (a pollutant) is used as an F source. WO3@F0.1-C exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than WO3@C. It is composed of ultra-small WO3 nanoparticles (monoclinic crystals), which are decorated on F-doped graphite sheets. It exhibits an ID/IG ratio of 0.924, suggesting the existence of high defects and low graphitization degree. In the high-resolution XPS spectra, the W 4f peak of WO3@F0.1-C exhibits a slight shift, when compared to that of WO3@C, suggesting a modified electronic structure due to the F doping. The DFT calculations disclose that the energy barrier for OH deprotonation is lowered by the F-doping, and the hydroxyl adsorption is enhanced by WO3.
The fabrication of semi-oxidized transition metal oxide nanoparticles anchored on carbon produces highly active defective metal (oxy)hydroxides during the OER, and that could enhance the performance of the OER. Wang et al.41 observed that Co-CoO/GO (graphene oxide) exhibits enhanced activity and stability for the OER. It was synthesized by sonication for 1 h followed by hydrothermal treatment followed by annealing at 450 °C for 30 min under an Ar atmosphere. Co-CoO/GO exhibits higher activity and lower charge transfer resistance for the OER than Co/GO, and Co3O4/GO. It contains metallic Co, crystalline CoO, and graphene oxide. It contains Co, C, and O, which are homogeneously distributed. It exhibits the formation of defective CoOOH during the OER.
Constructing Mott–Schottky heterojunctions could lead to the spontaneous flow of electrons at the metal–semiconductor heterointerfaces till the work functions reach equilibrium on both sides, and that could modify the electronic structure, provide optimal adsorption energy with intermediates, enhance the conductivity, and that could enhance the performance for the HER and OER. Li et al.42 observed that Ni/CeO2@N-CNFs exhibit enhanced activity and stability for the HER and OER (CNFs: carbon nanofibers). They were synthesized by electrospinning followed by stabilization at 250 °C for 2 h followed by carbonization at 600 °C for 3 h under an N2 atmosphere. The Ni/CeO2@N-CNFs exhibits higher activity and electrochemical surface area for the HER and OER than Ni@N-CNFs and CeO2@N-CNFs. It is composed of crystalline Ni/CeO2 nanoparticles, which are encased into N-doped carbon nanofibers. It exhibits a heterointerface between Ni and CeO2. It contains Ce, Ni, O, and N, which are homogeneously distributed. In the high-resolution XPS spectra, the Ce 3d peak of Ni/CeO2@N-CNFs exhibits a slight negative shift, when compared to that of CeO2@N-CNFs, while the Ni 2p peak of Ni/CeO2@N-CNFs exhibits a slight positive shift, when compared to that of Ni@N-CNFs, suggesting a modified electronic structure possibly due to the partial transfer of electrons from Ni to CeO2 at the heterointerface in Ni/CeO2@N-CNFs. It contains oxygen vacancies. It is porous. For overall water splitting in 1 M KOH, the Ni/CeO2@N-CNFs//Ni/CeO2@N-CNFs affords a potential of 1.56 V at 10 mA cm−2, suggesting its very high activity, while it undergoes negligible decay for 100 h, suggesting its very high stability.
Reduction and oxidation can occur by applying pulsed potentials on the substrate in the electrochemical method; while utilizing this strategy, a scalable, ultrafast, and energy-efficient synthesis route can be achieved for the fabrication of highly active electrocatalysts at ambient temperature, which is highly desirable for green synthesis. Liu et al.26 observed that the IronCE10s exhibit enhanced activity and stability for the OER, while it was prepared on the cheap iron substrate by applying pulsed potentials for 10 s (+2.4 V for 5 s followed by −2.4 V for 5 s) in NiSO4 electrolyte at 25 ± 1 °C, where the substrate was used as a counter electrode. The IronCE10s exhibits higher activity for the OER than IronWE10s, IronCE5s, IronCE60s, and IronCE360s. It contains Ni, Fe, and O, while it may be iron-doped amorphous nickel hydroxide. The morphology of the prepared samples was a nanosheet array.
The effect of the substrate in the electrodeposition process could alter the conductivity and electrochemical surface area of the electrocatalyst, and that could influence the performance of the OER. Yin et al.44 observed that FeNiOH on Ni foil exhibits enhanced activity and stability for the OER. It was obtained on the cleaned Ni foil by the electrodeposition method at 20 °C for 600 s. FeNiOH on Ni foil exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than FeNiOH on Fe foil. It is an amorphous FeNi hydroxide, which is distributed homogeneously. It possesses nanostructured morphology. For the OER in 1 M KOH, it affords an η of 200 mV at 10 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at 90 mA cm−2 after 1000 cycles of CV, suggesting its high durability.
Fabrication of transition bimetallic hydroxides through a facile synthesis route at ambient temperature could enhance the performance of the OER. Zhuo et al.45 demonstrated a rapid, facile, cost-effective, and scalable (area: 2000 cm2) synthesis route at room temperature for Ni(Fe)(OH)2, where it was synthesized by submerging the pre-cleaned Ni foam for 60 s in 0.2 M Fe(NO3)3 at ambient temperature, while it exhibits high activity and stability for the OER. The incorporation of Fe in Ni hydroxide and the in situ formation of Ni(Fe)OOH from Ni(Fe)(OH)2 during electrolysis are attributed to the enhancement in the performance for the OER. For the OER in 1 M KOH, it affords an η of 240 mV at 10 mA cm−2, and an η of 680 mV at a current density of 1000 mA cm−2, suggesting its significantly high activity, while it affords high stability.
Metal–organic frameworks (MOFs) can be converted into hydroxides/(oxy)hydroxides with abundant oxygen vacancies and mesopores using an alkaline hydrolysis–oxidation strategy with the assistance of an electric field, and that could modify the electronic structure, afford optimal adsorption energy with intermediates, enhance the electrochemical surface area, and that could enhance the performance for the OER. Li et al.46 observed that CoNiFe-OH exhibits enhanced activity and stability for the OER. It was synthesized by the following steps. At first, Co/Ni/Fe MOFs were obtained on Ni foam by hydrothermal treatment. Finally, CoNiFe-OH was obtained using an alkaline hydrolysis–oxidation strategy with the assistance of an electric field (at 1.45 V for 30 min), where Co/Ni/Fe MOFs were used as the working electrode, and 1 M KOH was used as the electrolyte. The CoNiFe-OH exhibits higher activity and electrochemical surface area for the OER than CoNiFe-H. It is polycrystalline, containing Co(OH)2, CoOOH, Ni(OH)2, NiOOH, and FeOOH phases. It contains Fe, Co, Ni, and O, which are homogeneously distributed. It contains oxygen vacancies. It possesses nanostructured ultrathin hexagonal plate-like morphology. It is mesoporous. For the OER in 1 M KOH, it affords an η of 207 mV at 10 mA cm−2, suggesting its very high activity.
Doping of cerium into NiFe hydroxide could increase the valence state of Fe, modify the electronic structure, and afford optimal adsorption energy with intermediates, and that could enhance the performance of the OER. Liu et al.47 observed that Ce-NiFe exhibits enhanced activity and stability for the OER. It was prepared on NiFe foam by the electrodeposition method. Ce-NiFe exhibits higher activity and electrochemical surface area for the OER than NiFe, CeNi, and CeFe. It is an amorphous Ce-doped NiFe hydroxide. It contains Ce, Ni, Fe, and O, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p and Fe 2p peaks of Ce-NiFe exhibit a slight positive shift, when compared to that of NiFe, suggesting a modified electronic structure. It contains high valence Fe ions (Fe > 3+). For the OER in 1 M KOH, it affords an η of 195 mV at 10 mA cm−2, and an η of ∼770 mV at a current density of 1000 mA cm−2, suggesting its very high activity, while it affords reasonable stability at a current density of 1000 mA cm—2 for 100 h, suggesting its very high stability.
Fabrication of nanostructured transition bimetallic carbonate hydroxides could modify the electronic structure, afford optimal adsorption energy with intermediates, facilitate the evolution of gas, and expose abundant active sites, and that could boost the performance for the HER and OER. Hui et al.17 observed that Fe-Co-CO3-OH exhibits significantly very high activity and stability for the HER and OER. It was obtained on Ni foam by the hydrothermal treatment. Fe-Co-CO3-OH exhibits higher activity for the HER and OER than Co-CO3-OH and Fe-CO3-OH. It is Fe-doped Co carbonate hydroxide. It is crystalline. It exhibits a homogeneous distribution of Fe and Co. In the high-resolution XPS spectra, the Co 2p peak of Fe-Co-CO3-OH exhibits a slight shift, when compared to Co-CO3-OH, suggesting a modified electronic structure due to the Fe doping. It possesses nanosheet array morphology. For the OER in 1 M KOH, it affords an η of 228 mV at 10 mA cm−2, and an η of 308 mV at a current density of 1000 mA cm−2, suggesting its substantially very high activity. For the HER in 1 M KOH, it affords an η of −77 mV at −10 mA cm−2, and an η of −256 mV at a current density of −1000 mA cm−2, suggesting its substantially very high activity, while it undergoes negligible decay at a current density of −1000 mA cm−2 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its substantially very high durability. Moreover, Tang et al.48 observed that Co-Mn-CO3-OH exhibits enhanced activity and stability for the HER and OER. It was obtained on Ni foam by hydrothermal treatment. Co-Mn-CO3-OH exhibits higher activity and electrochemical surface area for the HER and OER than Co-CO3-OH and MnCO3. It is Mn-doped Co carbonate hydroxide. It has low crystallinity. It contains Mn, Co, C, and O, which are homogeneously distributed. The number of electrons present in the 3d orbital of the Co atom in Co-Mn-CO3-OH is about 7.30, which is higher than that of Co-CO3-OH, suggesting the modified electronic structure possibly due to the partial transfer of electrons from Mn to Co. It possesses nanosheet array morphology. For the OER in 1 M KOH, it affords an η of 462 mV at a current density of 1000 mA cm−2, suggesting its very high activity.
000 cycles of CV, suggesting its substantially very high durability. Moreover, Tang et al.48 observed that Co-Mn-CO3-OH exhibits enhanced activity and stability for the HER and OER. It was obtained on Ni foam by hydrothermal treatment. Co-Mn-CO3-OH exhibits higher activity and electrochemical surface area for the HER and OER than Co-CO3-OH and MnCO3. It is Mn-doped Co carbonate hydroxide. It has low crystallinity. It contains Mn, Co, C, and O, which are homogeneously distributed. The number of electrons present in the 3d orbital of the Co atom in Co-Mn-CO3-OH is about 7.30, which is higher than that of Co-CO3-OH, suggesting the modified electronic structure possibly due to the partial transfer of electrons from Mn to Co. It possesses nanosheet array morphology. For the OER in 1 M KOH, it affords an η of 462 mV at a current density of 1000 mA cm−2, suggesting its very high activity.
Doping sulfur in Fe (oxy)hydroxide could activate the redox reactivities of the lattice oxygen and Fe, and that could enhance the performance of the OER. Therefore, fabricating sulfur-doped Fe (oxy)hydroxide at ambient temperature through a facile, scalable, energy-efficient, and ultrafast synthesis route is highly desirable for green synthesis. Chen et al.24 observed that S-FeOOH exhibits enhanced activity and stability for the OER. It was obtained by immersing the cleaned Fe foam for 20 min at room temperature in the solution containing Fe(NO3)3 and Na2S2O3. S-FeOOH exhibits higher activity and electrochemical surface area for the OER than FeOOH. It is polycrystalline, containing S-doped β-FeOOH. It exhibits a homogeneous distribution of S. In the high-resolution XPS spectra, the Fe 2p peak of S-FeOOH slightly shifts, when compared to that of FeOOH, suggesting a modified electronic structure due to the S doping. It contains oxygen vacancies. The redox reactivities of the lattice oxygen and Fe in FeOOH are activated due to S doping. It possesses nanosheet array morphology. For the OER in 1 M KOH, it affords an η of 244 mV at 10 mA cm−2, suggesting its very high activity, while it exhibits negligible decay at 20 mA cm−2 for 100 h, suggesting its very high stability.
The role of cations in the electrolyte could influence the activity of the OER. Hou et al.50 observed that the activity of the NiFe-(OOH)-based catalyst for the OER in different electrolytes is in the following order: CsOH > KOH > NaOH > LiOH. The catalytic activity trend is nearly associated with the potential for maximum entropy of the system. The catalytic activity has been nearly related to the variable length of Ni–O bonds in the NiOOH active phase structure.
Fabrication of transition bimetallic alloy/(oxy) hydroxide heterostructures could tune the electronic structure, afford optimal adsorption energy with intermediates, expose abundant active sites, improve charge/mass transfer, facilitate gas evolution, and that could improve the performance for the HER and OER. lv et al.11 observed that c-NiFe/a-NiFeOOH@NiMo (c: crystalline; a: amorphous) exhibits enhanced activity and stability for the HER and OER. It was obtained on carbon fiber cloth by galvanostatic electrodeposition at ∼0 °C followed by cyclic voltammetry treatment followed by potentiostatic electrodeposition. c-NiFe/a-NiFeOOH@NiMo exhibits higher activity and lower charge transfer resistance for the HER and OER than c-NiFe/a-NiFeOOH and NiMo. It contains heterojunctions. It exhibits a core–shell structure, containing NiMo particles as the core, and thin c-NiFe/a-NiFeOOH nanosheets as the shell, where the thin nanosheet is composed of crystalline NiFe nanoflowers (cubic Ni0.36Fe0.64 alloy), which are embedded in amorphous NiFeOOH. a-NiFeOOH contains nanosized pores. The ultrafine NiFe alloy nanocrystals possesses abundant edges and high electronic conductivity, and the amorphous NiFeOOH support possesses abundant defects and nanosized pores, while the NiMo alloy can afford firm grasp to the c-NiFe/a-NiFeOOH nanosheets, and that could modify the electronic structure, provide optimal adsorption energy with intermediates, expose abundant active sites, enhance charge/mass transfer, facilitate gas evolution, which could enhance the performance for the HER and OER. For the OER in 1 M KOH, c-NiFe/a-NiFeOOH@NiMo affords 133.2 mV at 20 mA cm−2, suggesting its very high activity. For the HER in 1 M KOH, it affords an η of −91.9 mV at −20 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, c-NiFe/a-NiFeOOH@NiMo//c-NiFe/a-NiFeOOH@NiMo affords a potential of 1.45 V at 10 mA cm−2, suggesting its very high activity, while it affords 95.86% retention at 100 mA cm−2 for 100 h, suggesting its very high stability.
The fabrication of NiFe-based electrocatalysts for the OER through an electrodeposition process using a deep eutectic solvent (DES) with Ni as a source in the form of nitrate could form multi vacancies upon electrolysis, and that could enhance the performance for the OER. Wei et al.6a fabricated NiFe based electrocatalysts for the OER through a one-step electrodeposition process, where Fe was used as a sacrificial anode and ethaline-based DES with nickel nitrate was used as an electrolyte. The utilization of nitrate ions in the electrodeposition process for the preparation of NiFe based catalysts could generate multiple vacancies (such as O, Ni, and Fe vacancies) during water oxidation, leading to optimal adsorption energies with intermediates, and that could boost the performance for the OER. For the OER in 1 M KOH, it affords an η of 256 mV at 10 mA cm−2, suggesting its high activity.
The electrochemical activation of transition bimetallic LDH can reconstruct the surface, which could generate highly active sites, enhance the surface area, and that could improve the performance of the HER and OER. Pehlivan et al.51 observed that the NiFe LDH activated exhibits enhanced activity for the HER and OER. Crystalline NiFe LDH was synthesized on pre-treated Ni foam by hydrothermal treatment. NiFe LDH activated affords an η of 201 mV at 10 mA cm−2 for the OER, and it affords an η of −189 mV at −10 mA cm−2 for the HER in 1 M KOH, suggesting its very high activity.
Transition bimetallic catalysts having a structure almost similar to the NiFe LDH can be fabricated by the spray pyrolysis deposition technique, and that could enhance the activity and stability of the OER. An et al.52 observed that the NiFe-based catalyst exhibits enhanced activity and stability for the OER. It was obtained on a fluorine-doped tin oxide glass substrate by an ultrasonic spray pyrolysis deposition method. The NiFe-based catalyst aged at 0 min exhibits higher activity and lower charge transfer resistance for the OER than the NiFe-based catalyst aged at 2, 4, and 8 min. It is composed of NiFe oxide and layered hydroxyl nitrate. The structure of the NiFe-based catalyst is almost similar to the that of the typical NiFe LDH. It contains Fe, Ni, O, and N with a film thickness of 100 nm and uniform coral-like stripes.
Fabrication of heterostructured metal (oxy)hydroxide/transition bimetallic LDH through a facile one-step synthesis route is highly desirable. Li et al.53 observed that the FeOOH@NiFe LDH exhibits enhanced activity and stability for the OER. It was synthesized by immersing the cleaned Ni foam in FeCl3 solution at 100 °C for 5 s. FeOOH@NiFe LDH exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than FeOOH and NiFe LDH. It is crystalline, containing β-FeOOH and NiFe LDH phases. It exhibits a heterointerface. It contains Fe, Ni, and O, which are uniformly distributed. It possesses nanosheet array morphology. During the OER, it is converted into highly active FeOOH@β-Ni(Fe)OOH phases. For the OER in 1 M KOH, it affords an η of 210 mV at 10 mA cm−2, suggesting its very high activity, while it undergoes negligible decay for 100 h, suggesting its very high stability.
Negatively charged metal nanoparticles can be integrated with positively charged transition bimetallic LDH due to the electrostatic attraction by the physical blending method, which could modify the electronic structure, and that could enhance the performance of the OER. Wu et al.54 observed that the Ag@NiFe LDH exhibits enhanced activity and stability for the OER. It was prepared by the physical blending method, where Ag@NiFe LDH can be precipitated when adding Ag with NiFe LDH due to the electrostatic attraction between positively charged NiFe LDH and negatively charged Ag nanoparticles, while the NiFe LDH was prepared by hydrothermal treatment. Ag@NiFe LDH exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than Au@NiFe LDH and NiFe LDH. It is crystalline. It is composed of metallic Ag nanoparticles, which are anchored on NiFe LDH nanosheets. It exhibits a heterointerface. In the high-resolution XPS spectra, the Ni 2p and Fe 2p peaks of Ag@NiFe LDH exhibit a slight positive shift, when compared to that of NiFe LDH, while the Ag 3d peaks of Ag@NiFe LDH exhibit a slight negative shift, when compared to that of metallic Ag, suggesting a modified electronic structure possibly due to the partial transfer of electrons from NiFe LDH to Ag.
Doping of cerium into transition bimetallic LDH could modify the electronic structure, and that could enhance the performance of the HER and OER. Dhandapani et al.55 observed that the Ce@NiCo LDH exhibits enhanced activity and stability for the HER and OER. It was obtained on cleaned Ni foam by hydrothermal treatment. Ce@NiCo LDH exhibits higher activity and lower charge transfer resistance for the HER and OER than NiCo LDH. It is 25.5% Ce doped NiCo LDH. It contains Ce, Ni, Co, and O, which are homogeneously distributed. In the high-resolution XPS spectra, the Co 2p peak of Ce@NiCo LDH exhibits a slight shift compared to that of NiCo LDH, suggesting a modified electronic structure. It possesses nano petal morphology.
Heterostructured transition bimetallic phosphide/bimetallic LDH can be obtained by the partial phosphidation of bimetallic LDH, and that could modify the electronic structure, afford optimal adsorption energy with intermediates, which could enhance the activity and stability for the HER and OER. Zheng et al.56 observed that the c-CoFeP/a-CoFe LDH exhibits enhanced activity and stability for the OER, whereas the c-CoMnP/a-CoMn LDH (c: crystalline; a: amorphous) exhibits enhanced activity and stability for the HER. The c-CoFeP/a-CoFe LDH or c-CoMnP/a-CoMn LDH was prepared on pretreated Ni foam by hydrothermal treatment followed by partial phosphidation for 2 h at 250 °C under an Ar atmosphere. The c-CoFeP/a-CoFe LDH exhibits higher activity and electrochemical surface area for the OER than c-CoMnP/a-CoMn LDH, c-CoNiP/a-CoNi LDH, c-CoCuP/a-CoCu LDH, and c-CoZnP/a-CoZn LDH. The c-CoMnP/a-CoMn LDH exhibits higher activity and electrochemical surface area for the HER than c-CoFeP/a-CoFe LDH, c-CoNiP/a-CoNi LDH, c-CoCuP/a-CoCu LDH, and c-CoZnP/a-CoZn LDH. The c-CoFeP/a-CoFe LDH is the crystalline CoFeP decorated amorphous CoFe LDH heterostructure. It possesses a nanostructure. The c-CoMnP/a-CoMn LDH is the crystalline CoMnP decorated amorphous CoMn LDH heterostructure. It possesses a nanostructure. For overall water splitting in 1 M KOH, the c-CoFeP/a-CoFe LDHOER//c-CoMnP/a-CoMn LDHHER affords a potential of 1.498 V at 10 mA cm−2, confirming its very high activity.
Integrating LDH with MXene could modify the electronic structure and provide optimal adsorption energy with intermediates, enhancing the activity and stability of the HER and OER. Yu et al.57 observed that the NiFeLa LDH/v-MXene (vertically aligned MXene) exhibits significantly very high activity and stability for the HER and OER. It was synthesized via the following steps: at first, Ti3C2 MXene nanosheets were prepared by selectively etching Al layers in Ti3AlC2. Then, vertically aligned Ti3C2 MXene was obtained on Ni foam by electrophoretic deposition followed by freeze-drying. Finally, NiFeLa LDH/v-MXene was obtained by electrodeposition. The NiFeLa LDH/v-MXene exhibits higher activity for the HER and OER than NiFe-LDH/v-MXene, NiFeLa LDH/MXene, NiFeLa LDH, and v-MXene. It comprises amorphous NiFeLa LDH nanosheets on crystalline Ti3C2 MXene nanosheets, where the MXene is vertically aligned on Ni foam. It contains Ti, C, Ni, Fe, La, and O, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p peak of NiFeLa LDH/v-MXene exhibits a slight positive shift compared to that of NiFe LDH/v-MXene, suggesting a modified electronic structure. It exhibits super hydrophilicity with a contact angle of 0°. For the OER in 1 M KOH, it affords an η of 191 mV at 10 mA cm−2, suggesting its very high activity. In contrast, it undergoes negligible decay at 100 mA cm−2 for 1200 h, suggesting its robust stability. For the HER in 1 M KOH, it affords an η of −38 mV at −10 mA cm−2, suggesting its very high activity, while it affords reasonable stability at −100 mA cm−2 for 400 h, suggesting its very high stability, and it undergoes negligible decay at −700 mA cm−2 after 2000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, the NiFeLa LDH/v-MXene//NiFeLa LDH/v-MXene affords a potential of 1.48 V at 10 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at 100 mA cm−2 for 400 h, suggesting its robust stability.
2.2. Fabricating chalcogenide (sulfide and selenide) electrocatalysts
Fabrication of cobalt-doped nickel sulfide having an ultrathin nanostructure could modify the electronic structure and facilitate the evolution of gas even at high current densities, boosting the performance of the HER and OER. Jian et al.59 observed that Co-Ni3S2 having an ultrathin nanosheet structure exhibits enhanced activity and stability for the HER and OER even at a current density of 1000 mA cm−2, while it was obtained on pretreated Ni foam by a one-step hydrothermal treatment. Co-Ni3S2 exhibits higher activity for the HER and OER than Ni3S2. It is a Co-doped crystalline Ni3S2. It contains Ni, Co, and S, which are homogeneously distributed. It possesses an ultrathin nanosheet structure. For the OER in 1 M KOH, it affords an η of 310 mV at 50 mA cm−2 and an η of 750 mV at a current density of 1000 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at a current density of 1000 mA cm−2 after 1000 cycles of CV, suggesting its high durability. For the HER in 1 M KOH, it affords an η of −220 mV at −50 mA cm−2 and an η of −850 mV at a current density of −1000 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at a current density of −1000 mA cm−2 after 1000 cycles of CV, suggesting its high durability.
Besides, Jian et al.14 observed that Sn-Ni3S2 exhibits substantially very high activity and stability for the HER and OER. It was synthesized on Ni foam by a one-step hydrothermal treatment. Sn-Ni3S2 exhibits higher activity for the HER and OER than Ni3S2, while it exhibits lower charge transfer resistance for the OER than Ni3S2. It is Sn-doped hexagonal Ni3S2. It contains Sn, Ni, and S, which are homogeneously distributed. It possesses an ultrathin nanosheet structure. For the OER in 1 M KOH, it affords an η of 267 mV at 100 mA cm−2 and an η of 580 mV at a current density of 1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a high current density of 2000 mA cm−2 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its substantially very high durability. For the HER in 1 M KOH, it affords an η of −171 mV at −100 mA cm−2 and an η of −570 mV at a current density of −1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a current density of −1000 mA cm−2 after 10
000 cycles of CV, suggesting its substantially very high durability. For the HER in 1 M KOH, it affords an η of −171 mV at −100 mA cm−2 and an η of −570 mV at a current density of −1000 mA cm−2, suggesting its significantly very high activity, while it undergoes negligible decay at a current density of −1000 mA cm−2 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its significantly very high durability. For overall water splitting in 1 M KOH, Sn-Ni3S2//Sn-Ni3S2 affords a potential of 1.46 V at 10 mA cm−2 and ∼2.65 V at a current density of 1000 mA cm−2, suggesting its significantly very high activity.
000 cycles of CV, suggesting its significantly very high durability. For overall water splitting in 1 M KOH, Sn-Ni3S2//Sn-Ni3S2 affords a potential of 1.46 V at 10 mA cm−2 and ∼2.65 V at a current density of 1000 mA cm−2, suggesting its significantly very high activity.
Moreover, Mao et al.60 observed that FeCo-Ni3S4 exhibits enhanced activity and stability for the OER. It was prepared on pretreated Ni foam by hydrothermal treatment followed by hydrothermal sulfurization. FeCo-Ni3S4 exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than Fe-Ni3S4, Co-Ni3S4, and Ni3S4. It is Fe and Co dual-doped cubic Ni3S4. It contains Ni, S, Fe, and Co, which are homogeneously distributed. It exhibits a higher amount of high valence Ni ions (Ni3+) than Ni3S4. In the high-resolution XPS spectra, the Ni 2p peak of FeCo-Ni3S4 exhibits a slight shift when compared to that of Ni3S4, suggesting a modified electronic structure due to the Fe and Co dual doping. It possesses nanosheet array morphology. For the OER in 1 M KOH, it affords an η of 230 mV at 20 mA cm−2, suggesting its very high activity, while it exhibits 22 mV decay at 20 mA cm−2 for 360 h, suggesting its very high stability, and it undergoes negligible decay at 100 mA cm−2 after 3000 cycles of CV, suggesting its very high durability.
Besides, Zhang et al.61 reported that Fe, Ce-NixSy exhibits enhanced activity and stability for the HER and OER. It was synthesized on the pretreated Ni foam by a one-step hydrothermal treatment. Fe, Ce-NixSy exhibits higher activity and electrochemical surface area for the OER than Fe-NixSy, Ce-NixSy, and NixSy. In comparison, it exhibits higher activity for the HER than Fe-NixSy, Ce-NixSy, and NixSy. It is Ce, Fe dual doped NixSy, where NixSy is crystalline, containing trigonal Ni3S2 and orthorhombic Ni9S8. It contains Ni, Fe, Ce, and S, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p peak of Fe, Ce-NixSy exhibits a slight shift compared to that of NixSy, suggesting a modified electronic structure possibly due to the doping of Fe and Ce. It possesses nano forest array morphology.
Moreover, Huang et al.62 observed that Fe-NiS-NiS2 exhibits enhanced activity and stability for the HER and OER. It was prepared by co-precipitation followed by hydrothermal treatment followed by annealing at 350 °C for 2 h under an N2 atmosphere. Fe-NiS-NiS2 exhibits higher activity and electrochemical surface area for the HER and OER than Fe-NiS2 and NiS-NiS2. It is Fe-doped crystalline NiS-NiS2. It exhibits the heterojunctions at a higher amount of high valence Ni ions (Ni3+) when compared to Fe-NiS2. In the high-resolution XPS spectra, the Ni 2p peak of Fe-NiS-NiS2 exhibits a slight positive shift compared to that of Fe-NiS2, suggesting a modified electronic structure. It possesses a microsphere structure, where the microsphere is composed of nanoplates.
In addition, Fereja et al.63 have observed that W-NiS2/MoO2 exhibits enhanced activity for the HER and OER. It was obtained on cleaned carbon cloth by hydrothermal treatment followed by sulfurization at 400 °C for 2 h under an H2/Ar atmosphere. W-NiS2/MoO2 exhibits higher activity and electrochemical surface area for the HER than NiS2/MoO2, W-NiS2, and W-MoO2. It is W doped NiS2/MoO2. It exhibits the heterojunctions between the NiS2 and MoO2 phases. It contains W, Ni, Mo, S, and O, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p peak of W-NiS2/MoO2 exhibits a slight positive shift compared to that of NiS2. The Mo 3d peak of W-NiS2/MoO2 exhibits a slight negative shift when compared to that of MoO2, suggesting a modified electronic structure in W-NiS2/MoO2 possibly due to the partial transfer of electrons from NiS2 to MoO2, which could be due to the higher electronegativity of Mo when compared to that of Ni. It contains mesopores. For the HER in 1 M KOH, it affords an η of −52 mV at −10 mA cm−2, suggesting its very high activity.
Fabrication of nickel sulfide/molybdenum sulfide as Mott–Schottky heterojunctions could lead to the spontaneous flow of electrons at the metal–semiconductor heterointerfaces till the work functions reach equilibrium on both sides, and that could modify the electronic structure, afford optimal adsorption energy with intermediates, improve the conductivity, which could boost the performance for the HER and OER. Gu et al.64 observed that NiS/MoS2 exhibits enhanced activity and stability for the HER and OER. It was obtained on pre-cleaned carbon cloth by a one-step hydrothermal treatment. NiS/MoS2 exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER than NiS and MoS2. It is crystalline, containing hexagonal NiS and hexagonal MoS2, while it exhibits a heterojunction interface between NiS and MoS2. It contains Mo, Ni, and S, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p peak of NiS/MoS2 exhibits a slight negative shift compared to that of NiS. In contrast, Mo 3d peak of NiS/MoS2 exhibits a slight positive shift compared to that of MoS2, suggesting a modified electronic structure possibly due to the partial transfer of electrons from MoS2 to NiS. It exhibits superior hydrophilicity, having a water contact angle of 15.72°. It possesses a flower-like morphology due to a combination of numerous nanosheets. For the HER in 1 M KOH, it affords an η of −87 mV at −10 mA cm−2, suggesting its very high activity. For water splitting in 1 M KOH, NiS/MoS2//NiS/MoS2 affords a potential of 1.51 V at 10 mA cm−2, suggesting its very high activity. At the same time, it exhibits 99.2% retention for 50 h, suggesting its very high stability. Besides, Yang et al.65 observed that Ni3S2-MoS2 exhibits enhanced activity and stability for the HER and OER. It was synthesized on pretreated Ni foam by a one-step hydrothermal treatment. Ni3S2–MoS2 exhibits higher activity and lower charge transfer resistance for the HER than Ni3S2 and MoS2. It is crystalline, containing Ni3S2 and MoS2 phases. It exhibits heterointerfaces between Ni3S2 and MoS2. It contains Mo, Ni, and S, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p peak of Ni3S2-MoS2 and Mo 3d peak of Ni3S2-MoS2 showed a slight shift as compared to Ni3S2 and MoS2, respectively, illustrating the modified electronic structure possibly due to the partial transfer of electrons between Ni3S2 and MoS2. It exhibits 3D porous flower morphology, where the flower is composed of nanosheets.
Metal (oxy)hydroxides for the OER and metallic species for the HER are formed as the active species due to the dynamic structural reconstruction of heterostructured metallic sulfide/bimetallic sulfide pre-catalysts during water electrolysis to boost the performance for the HER and OER. Wu et al.66 observed that Ni3S2/FeNi2S4 showed high activity and stability for the HER and OER. It was synthesized on NiFe foam by the following steps: first, dual-scale porous NiFe foam was prepared by a redox process. After that, NiFeAl LDH was obtained on the pretreated NiFe foam by hydrothermal treatment. Finally, NiFeAl LDH was converted into Ni3S2/FeNi2S4 by hydrothermal treatment. Ni3S2/FeNi2S4 exhibits higher activity and lower charge transfer resistance for the HER and OER than NiFeAl LDH. It is crystalline, containing Ni3S2 and FeNi2S4 phases. It exhibits heterointerfaces between Ni3S2 and FeNi2S4. It contains Fe, Ni, and S, which are homogeneously distributed, while it exhibits a trace amount of Al (3.4%). Ni3S2/FeNi2S4 act as pre-catalysts, exhibiting dynamic structural reconstruction during water electrolysis, where the metallic Ni0 for the HER and γ-NiOOH for the OER are the active species formed during electrolysis. It possesses porous, nanosheet array morphology. For the OER and HER in 1 M KOH, it affords an η of 201 mV at 10 mA cm−2 and an η of −50 mV at −10 mA cm−2, respectively, showing its better activity. For overall water splitting in 1 M KOH, Ni3S2/FeNi2S4//Ni3S2/FeNi2S4 affords a potential of 1.55 V at 10 mA cm−2, suggesting its very high activity.
Besides, Zhang et al.22 observed that CuS-Ni3S2/CuNi exhibits enhanced activity and stability for the OER. It was obtained on pretreated Ni foam by electrodeposition followed by hydrothermal sulfurization. CuS-Ni3S2/CuNi exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than Ni3S2/Ni. It is crystalline, containing CuS and Ni3S2 phases, where CuS–Ni3S2 is in situ grown on a CuNi alloy. It exhibits heterointerfaces between CuS and Ni3S2. It contains Ni, Cu, and S, which are homogeneously distributed. It contains macropores. For the OER in 1 M KOH, it affords an η of 337 mV at 100 mA cm−2 and an η of 510 mV at a current density of 1000 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at a current density of 1000 mA cm−2 after 5000 cycles of CV, suggesting its high durability.
Moreover, Luo et al.67 observed that MoS2-MoO3−x/Ni3S2 exhibits enhanced activity and stability for the HER. It was prepared on Ni foam by a spontaneous chemical reaction at 80 °C followed by electrodeposition. MoS2-MoO3−x/Ni3S2 exhibits higher activity and lower charge transfer resistance for the HER than MoS2, MoO3−x, and Ni3S2. It comprises a Ni3S2 layer on Ni foam, which is covered by the MoS2–MoO3−x layer on the top. It exhibits a heterostructure. It has low crystallinity. It contains MoS2 with abundant bridging S22− sites, MoO3−x, and Ni3S2, which could afford optimal adsorption energy with intermediates. For the HER in 1 M KOH, it affords an η of −76 mV at −10 mA cm−2, suggesting its very high activity.
In addition, Wang et al.68 observed that NixSy@MnOxHy exhibits enhanced activity and stability for the HER and OER. It was obtained on pretreated Ni foam by hydrothermal sulfurization followed by anodic galvanostatic electrodeposition. NixSy@MnOxHy exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER and OER than NixSy and MnOxHy. It comprises a rod-like NixSy, surrounded by MnOxHy, where NixSy comprises Ni3S2 and NiS phases. It exhibits a heterointerface between NixSy and MnOxHy. In the high-resolution XPS spectra, the Ni 2p peak of NixSy@MnOxHy exhibits a slight positive shift compared to that of NixSy, suggesting a modified electronic structure. Moreover, it contains Mn–O, Mn–Mn, and Mn–S bonds, where the existence of the Mn–S bond suggests the presence of electronic interaction at the heterointerface. It possesses a nanostructure. For the OER in 1 M KOH, it affords reasonable stability at 100 mA cm−2 for 150 h, suggesting its very high stability, and it undergoes negligible decay at 500 mA cm−2 after 5000 cycles of CV, suggesting its high durability. For the HER in 1 M KOH, it affords an η of −179 mV at −10 mA cm−2, suggesting its very high activity, while it affords reasonable stability at −100 mA cm−2 for 100 h, suggesting its very high stability. For overall water splitting in 1 M KOH, NixSy@MnOxHy//NixSy@MnOxHy affords a potential of 1.53 V at 10 mA cm−2, suggesting its very high activity. In contrast, it undergoes negligible decay at 100 mA cm−2 for 100 h, suggesting its very high stability.
Moreover, Che et al.69 observed that (Ni-Fe)Sx/NiFe(OH)y exhibits enhanced activity and stability for the HER and OER. It was obtained on pretreated Ni foam by the one-step electrodeposition method at room temperature. (Ni-Fe)Sx/NiFe(OH)y exhibits higher activity for the HER and OER than Fe0.96S/Fe(OH)3. It is an amorphous (Ni-Fe)Sx/NiFe(OH)y composite. It possesses micro/nano-architecture, and exhibits high wettability with a water contact angle of ≤0°, suggesting its super-aerophobic properties, which could facilitate the evolution of gas. For the OER in 1 M KOH, it affords an η of 199 mV at 10 mA cm−2 and an η of ∼510 mV at a current density of 1000 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, (Ni-Fe)Sx/NiFe(OH)y//(Ni-Fe)Sx/NiFe(OH)y affords a potential of 1.46 V at 10 mA cm−2, suggesting its very high activity.
In addition, Lu et al.70 observed that FeCoSx-PBA exhibits enhanced activity and stability for the OER, where FeCoSx-PBA is composed of amorphous FeCoSx, which is embedded in Prussian blue analog hetero-nanoframes. It possesses a hollow cubic-like morphology, where plenty of nanoparticles are attached to the cubic surface. It contains two forms of S atom, where one is S2− in FeCoSx, while the other is S-doped PBA. In the high-resolution XPS spectra, the Fe 2p peak of FeCoSx-PBA exhibits a slight shift when compared to that of mixed PBA, suggesting a modified electronic structure in FeCoSx-PBA. The following steps are involved in the synthesis: first, PBA was obtained by the precipitation method. Then, mixed PBA was prepared by an ultrasound-assisted ion exchange route. Finally, FeCoSx-PBA was obtained by solvothermal treatment. FeCoSx-PBA exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER PBA and mixed PBA.
The incorporation of Ni in iron thiophosphate could tune the electronic structure and increase the electronic conductivity, and that could enhance the activity for the HER and OER. Tang et al.71 observed that Ni-FePS3 exhibits enhanced activity and stability for the HER and OER in 1 M KOH. It was prepared by impregnation–adsorption followed by annealing at 450 °C for 5 minutes in an Ar atmosphere. The Ni-FePS3 exhibits higher activity for the HER and OER than Co-FePS3, Pd-FePS3, and FePS3. The differential electron density of Ni-FePS3 (Structure 1 as shown in Fig. 2a and Structure 2 as shown in Fig. 2b) are provided to understand the causes of the interaction of Ni atom with FePS3, which enhances the catalysis. The interaction of the Ni atom with FePS3 in Ni-FePS3 leads to the flow of electrons of the Fe atom, preferably towards the opposite Ni–S bonding, which causes a large negative charge on S and lesser electron density on Fe, as shown in Fig. 2a. In the same way, the charge distribution at the Ni–S bond changes when the charge of the S atom increases to −0.45 from −0.38, as shown in Fig. 2b. Moreover, the charge of Fe atoms near the Ni atom linked by edge S atom sharing (Fe-S-Ni) increases to 0.74 from 0.7. Structure 2 is considered a more suitable configuration because it exhibits lower formation energy than Structure 1. The outbound H2O adsorption behavior on Ni sites of Ni-FePS3 for the HER was disclosed by the differential electron density (Fig. 2c). Fig. 2d depicts the calculated Gibbs free energy profiles and the proposed reaction mechanism for the HER. Ni-FePS3 exhibits lower water adsorption energy (0.11 eV) and lesser water dissociation energy barrier (1.2 eV), suggesting its enhanced HER catalysis. In contrast, FePS3 exhibits higher water adsorption energy (0.43 eV) and a more enormous water dissociation energy barrier (1.92 eV), suggesting its sluggish HER catalysis. The water adsorption/dissociation configurations on Ni-FePS3 suggest that water molecules could be adsorbed on the Ni sites, where the water dissociation could be supported by the nearby S sites having increased electron density. Fig. 2e depicts the calculated Gibbs free energy profiles and the proposed reaction mechanism for the OER. The Ni atoms with charge enrichment in Ni-FePS3 could serve as active sites to more suitably weaken the rate-limiting O* intermediates compared to the P sites in the FePS3. Ni-FePS3 comprises the monoclinic phase of FePS3, anchored by atomically dispersed Ni atoms. The characteristic peak corresponding to Ni–Ni bonding at about 2.48 Å was not observed for Ni-FePS3 from the Ni K-edge EXAFS spectra, suggesting the existence of Ni predominantly as a single atomic species in Ni-FePS3. The Fe K-edge XANES spectra disclose that the near-edge absorption energy of Ni-FePS3 is higher than that of FePS3 and Fe foil, suggesting that the average electron density around Fe in Ni-FePS3 is less than that of FePS3 and Fe foil. In the high-resolution XPS spectra of S 2p, Ni-FePS3 exhibits a slight negative shift (0.2 eV) when compared to FePS3, suggesting the modified electronic structure after Ni incorporation in FePS3. The projected density of states discloses the formation of some new electronic states for Ni-FePS3 due to the hybridization of Fe 3d, S 2p, and Ni 3d in the bandgap, which could be due to the charge redistribution, suggesting the higher electronic conductivity after Ni incorporation in FePS3. Ni, Fe, P, and S possess nanosheet morphology and are distributed homogeneously. Thus, incorporation of Ni in FePS3 could modify the electronic structure and improve the electronic conductivity, and that could enhance the activity for the HER and OER.
 | ||
| Fig. 2 Differential electron density (top-view and side-view) of (a) Structure 1 and (b) Structure 2 of Ni-FePS3 (iso-surface value: 0.005 eBohr−3), where the red and green color numbers indicate Bader charge numbers of S and Fe, respectively; (c) differential electron density (top-view and side-view) of water adsorbed on the basal plane of Ni-FePS3 (iso-surface value: 0.002 eBohr−3); Gibbs free energy profiles of Ni-FePS3 in comparison with FePS3 for the (d) HER and (e) OER (reproduced with permission from ref. 71 Copyright 2021, Zhengzhou University). | ||
Besides, Yang et al.72 observed that Ni2P-Ni12P5@Ni3S2 exhibits enhanced activity and stability for the HER. It was obtained on cleaned Ni foam by hydrothermal treatment for simultaneous corrosion and sulfidation followed by phosphidation at 370 °C for 2 h under an N2 atmosphere. Ni2P-Ni12P5@Ni3S2 exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER than Ni2P. It comprises a Ni2P-Ni12P5 nanorod array, which is rooted in Ni3S2 film. It exhibits a heterointerface between Ni2P and Ni12P5 phases. It exhibits super hydrophilicity with a contact angle of 0°. For the HER in 1 M KOH, it affords an η of −32 mV at −10 mA cm−2, suggesting its very high activity.
Moreover, Paudel et al.73 observed that the 1T Co-WS2/NiTe2/Ni exhibits enhanced activity and stability for the HER and OER. It was obtained on pretreated Ni foam by a two-step hydrothermal treatment. The 1T Co-WS2/NiTe2/Ni exhibits higher activity and lower charge transfer resistance for the HER than WS2/NiTe2/Ni, WS2/Ni, and 1T NiTe2/Ni. 1T Co-WS2/NiTe2/Ni exhibits higher activity and electrochemical surface area for the OER than WS2/NiTe2/Ni, WS2/Ni, and 1T NiTe2/Ni. It comprises a metallic NiTe2/Ni nanoskeleton as the core and a Co-WS2 nanosheet layer as the shell. It exhibits a heterointerface between the WS2 and NiTe2 phases. For the HER in 1 M KOH, it affords an η of −88 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, the 1T Co-WS2/NiTe2/Ni//1T Co-WS2/NiTe2/Ni affords a potential of 1.521 V at 10 mA cm−2, suggesting its high activity.
Fabrication of electrocatalysts with high valence state sites could act as favorable reductive centers for large-current-density water splitting. Li et al.74 proposed that a high valence state of Co3+ in the Ni9.5Co0.5-S-FeOx hybrid catalyst can act as a favorable center for a stable and efficient HER compared to the hybrid catalyst with low chemical states. At the same time, it was obtained on Cu foam through a two-step electrodeposition method. For the HER in 1 M KOH at 60 °C, it affords an η of −22 mV at −10 mA cm−2 and an η of −175 mV at a current density of −1000 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH at 60 °C, Ni9.5Co0.5-S-FeOx//Ni9.5Co0.5-S-FeOx affords a potential of 1.730 V at a current density of 1000 mA cm−2, suggesting its very high activity, while it exhibits high stability.
Coupling of the suitable transition metal atoms with the nanostructured Chevrel-phase metal sulfides could cause the delocalization of d-electrons, enhance the charge transfer, afford optimal adsorption energies with intermediates, and that could enhance the performance for the HER and OER. Chandrasekaran et al.75 demonstrated that Co/N-NiMo3S4 exhibits high activity and stability for the HER and OER while it was prepared by hydrothermal treatment. Theoretical and experimental investigation discloses that delocalization of the d-electron on Co/N-NiMo3S4 can occur due to the coupling of Co atoms with edge Ni atoms, leading to enhancement in charge transfer, which could enhance the performance for overall water electrolysis. Moreover, an upshift in the d-band center of Co/N-NiMo3S4 can provide optimal adsorption energies for the intermediates for water dissociation and adsorption, enhancing overall water electrolysis. For the HER in 1 M KOH, it affords an η of −78 mV at −10 mA cm−2 and an η of −307 mV at a high current density of −1000 mA cm−2, suggesting its very high activity. For the OER in 1 M KOH, it affords an η of 186 mV at 50 mA cm−2 and an η of 225 mV at 300 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, Co/N-NiMo3S4//Co/N-NiMo3S4 affords a potential of 1.47 V at 10 mA cm−2, suggesting its very high activity while it exhibits high stability.
Fabrication of transition bimetallic doped selenides could modify the electronic structure and provide optimal adsorption energy with intermediates, enhancing the performance for the HER and OER. Ibraheem et al.77 observed that Fe@Co/Se2 exhibits enhanced activity and stability for the HER and OER. It was obtained by hydrothermal treatment. Fe@Co/Se2 exhibits higher activity and lower charge transfer resistance for the HER and OER than Fe-Se2 and Co-Se2. It is crystalline, containing an Fe, Co dual doped selenide. It contains Co, Fe, and Se, which are homogeneously distributed. In the high-resolution XPS spectra, the Fe 2p peak of Fe@Co/Se2 exhibits a slight positive shift when compared to that of Fe-Se2. In contrast, the Co 2p peak of Fe@Co/Se2 exhibits a slight negative shift when compared to that of Co-Se2, suggesting a modified electronic structure, possibly due to the partial transfer of electrons from the Fe to Co, which could be due to the higher electronegativity of Co, when compared to that of Fe. It contains Fe2+/3+/Co2+ species, which are coupled to selenide utilizing an Fe-coordinated Co-bridged bond. It possesses nanorod morphology. For the OER in 1 M KOH, it affords an η of 200 mV at 10 mA cm−2, suggesting its very high activity. For the HER in 1 M KOH, it affords an η of −78 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, Fe@Co/Se2//Fe@Co/Se2 affords a potential of 1.51 V at 10 mA cm−2, suggesting its very high activity.
Fabrication of nanostructured tungsten incorporated CoSe/Co3O4 could modify the electronic structure, afford optimal adsorption energy with intermediates, facilitate the gas evolution, and expose abundant active sites, and that could enhance the activity and stability for the HER and OER. Balaji et al.78 observed that WCoSe/WCo3O4 exhibits very high activity and stability for the HER and OER. It was synthesized on Ni foam by hydrothermal treatment at ∼160 °C for 16 h followed by selenization at ∼180 °C for 4 h followed by calcination at 300 °C for 2 h in an air atmosphere. WCoSe/WCo3O4 exhibits higher activity and electrochemical surface area for the HER and OER than WSe2/WO2 and CoSe/Co3O4. It possesses a nanosheet-like structure having nanopores (Fig. 3a). It is polycrystalline (Fig. 3b). It contains WSe2, WO2, CoSe, and Co3O4 phases, while it exhibits heterointerfaces (Fig. 3c). It exhibits a high crystalline structure (Fig. 3d). It contains W, Co, Se, and O (Fig. 3e). In the XPS spectra, the W 4f peak for WCoSe/WCo3O4 is slightly shifted towards higher binding energy when compared to that of WSe2/WO2, suggesting a modified electronic structure. For the OER in 1 M KOH, it affords an η of 175 mV at 10 mA cm−2, suggesting its very high activity. For the HER in 1 M KOH, it affords an η of −98 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, WCoSe/WCo3O4//WCoSe/WCo3O4 affords a potential of 1.49 V at 10 mA cm−2, suggesting its very high activity. In contrast, it affords reasonable stability at 100 mA cm−2 for 100 h, suggesting its high stability.
 | ||
| Fig. 3 Characterization of the WCoSe/WCo3O4 heterostructure: (a) TEM image; (b) SAED pattern; (c) HRTEM image; (d) FFT pattern; (e) STEM image and its corresponding EDS elemental mapping (reproduced with permission from ref. 78 Copyright 2022, The Royal Society of Chemistry). | ||
Selenization of stainless steel followed by heat treatment followed by electrochemical oxidation could create abundant oxygen vacancies, increase the Ni content, and that could enhance the performance of the OER. Han et al.79 observed that SS-500-AO exhibits enhanced activity and stability for the OER. SS-500-AO is an electrocatalyst on SUS304 stainless steel, where the catalyst was prepared by selenization followed by heat treatment followed by electrochemical oxidation. It contains abundant oxygen vacancies (76.09%). It contains 51.18% of Ni3+, 12.72% of Ni2+, and 36.1% of metallic Ni. For the OER in 1 M KOH, it affords an η of 284.3 mV at 10 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at 10 mA cm−2 for 160 h, suggesting its very high stability.
Fabrication of composites containing transition metal selenides and perovskite oxides could enhance the performance of the HER and OER. Kim et al.9 observed that LSCO-MoSe2 exhibits enhanced activity for the HER and OER, where LSCO is La0.5Sr0.5CoO3−δ perovskite oxide. It was prepared by a sol–gel process followed by calcination followed by ball milling. Fabrication of composites containing transition metal selenides, N-doped graphene quantum dots, and perovskite oxides could modify the electronic structure, afford optimal adsorption energy with intermediates, enhance the conductivity, and that could enhance the performance for the HER and OER. Cao et al.80 observed that LSC-N-GQDs-MoSe2 exhibits enhanced activity and stability for the HER and OER (LSC: La0.5Sr0.5CoO3−δ; N-GQDs: N-doped graphene quantum dots). It is a composite containing a thin layer of N-GQDs (thickness: 2 to 3 nm) at the interface of LSC and MoSe2. In the high-resolution XPS spectra, the La 3d and Co 2p peaks of LSC-N-GQDs-MoSe2 exhibit a slight positive shift when compared to that of LSC, suggesting a modified electronic structure. LSC-N-GQDs-MoSe2 exhibits higher activity and lower charge transfer resistance for the HER and OER than LSC-MoSe2. For overall water splitting in 1 M KOH, LSC-N-GQDs-MoSe2//LSC-N-GQDs-MoSe2 affords a potential of ∼1.57 V at 10 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at 500 mA cm−2 for 24 h, suggesting its very high stability. The following steps were used in its preparation: at first, N-GQDs were obtained by hydrothermal treatment, and the LSC was synthesized by a sol–gel process followed by calcination. Then, LSC-N-GQDs were obtained by sonication of the LSC and N-GQDs. Later, MoSe2 was obtained by ball milling. Finally, LSC-N-GQDs-MoSe2 was prepared by ball milling the LSC-N-GQDs and MoSe2.
Ion irradiation could activate the inert basal plane of MoSe2, and that could enhance the performance of the HER. Huang et al.81 demonstrated that the activity of MoSe2 nanosheet arrays was activated by He+ ion irradiation, which could generate multiple vacancies in the inert basal planes. DFT studies disclose that the increase in electrical conductivity and reduction in energy barriers for water dissociation and subsequent proton adsorption can be due to the existence of single Mo and single Se vacancies on the basal plane of MoSe2, which could enhance HER performance. For the HER in 1 M KOH, it affords an η of −90 mV at −10 mA cm−2, suggesting its very high activity, while it exhibits high stability at a current density of 1000 mA cm−2.
2.3. Fabricating metal phosphide/phosphate electrocatalysts
Fabrication of nickel phosphide nanoarrays with a superaerophobic surface could facilitate the gas evolution, expose abundant active sites, enhance the conductivity, and that could enhance the performance for the HER. Yu et al.82 observed that Ni2P exhibits enhanced activity and stability for the HER. It was prepared by two-step hydrothermal treatment followed by phosphorization at 300 °C for 30 min under Ar atmosphere. The Ni2P exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER than Ni(OH)2. It is hexagonal Ni2P. It contains Ni and P, which are homogeneously distributed. It possesses nanoarray morphology. It exhibits a superaerophobic surface, which could facilitate the evolution of gas. For the HER in 1 M KOH, it affords an η of −37 mV at −10 mA cm−2 and an η of −306 mV at current density of −1000 mA cm−2, suggesting its very high activity, and it undergoes negligible decay at −1200 mA cm−2 after 5000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, the NiFe LDHOER//Ni2PHER affords a potential of ∼2.05 V at a current density of 1000 mA cm−2, suggesting its very high activity.Fabrication of transition bimetallic phosphides could modify the electronic structure, afford optimal adsorption energy with intermediates, and enhance the performance of the HER and OER. Zhao et al.83 observed that NiCoP exhibits enhanced activity and stability for the HER and OER. It was synthesized by ultrasonic agitation followed by phosphorization at 350 °C for 3 h. NiCoP exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER and OER than CoP. It is quasi-monolayered Ni5%CoP. It contains Ni, Co, and P, which are homogeneously distributed. In the high-resolution XPS spectra, the P 2p peaks of NiCoP exhibit a slight negative shift compared to that of CoP, suggesting a modified electronic structure. It possesses a 2D sub-nanostructure. It contains abundant mesopores. For the OER in 1 M KOH, it affords an η of 259 mV at 10 mA cm−2, suggesting its very high activity, and it undergoes negligible decay at 90 mA cm−2 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its high durability. For the HER in 1 M KOH, it affords an η of −84 mV at −10 mA cm−2, suggesting its very high activity, and it undergoes slight decay at −140 mA cm−2 after 10
000 cycles of CV, suggesting its high durability. For the HER in 1 M KOH, it affords an η of −84 mV at −10 mA cm−2, suggesting its very high activity, and it undergoes slight decay at −140 mA cm−2 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, the NiCoP//NiCoP affords a potential of 1.48 V at 10 mA cm−2, suggesting its very high activity. Moreover, Song et al.84 observed that CoNiP exhibits enhanced activity for the HER and OER. It was prepared by ion exchange/etching of ZIF-67 (Co-based zeolitic imidazolate frameworks) followed by phosphidation. CoNiP exhibits higher activity for the HER and OER than that of CoNi LDH. It is crystalline, containing nanostructured CoNiP. It contains Ni, Co, P, and C, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p and Co 2p peaks of CoNiP exhibit a slight shift compared to that of CoNi LDH, suggesting a modified electronic structure.
000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, the NiCoP//NiCoP affords a potential of 1.48 V at 10 mA cm−2, suggesting its very high activity. Moreover, Song et al.84 observed that CoNiP exhibits enhanced activity for the HER and OER. It was prepared by ion exchange/etching of ZIF-67 (Co-based zeolitic imidazolate frameworks) followed by phosphidation. CoNiP exhibits higher activity for the HER and OER than that of CoNi LDH. It is crystalline, containing nanostructured CoNiP. It contains Ni, Co, P, and C, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p and Co 2p peaks of CoNiP exhibit a slight shift compared to that of CoNi LDH, suggesting a modified electronic structure.
Nanostructured Fe-doped CoP can be prepared by the phosphorization of a bimetallic Prussian blue analog, and that could facilitate the evolution of gas, enhance the conductivity, which could enhance the performance for the HER and OER. Cao et al.85 observed that the Fe-CoP exhibits enhanced activity and stability for the HER and OER. It was obtained on pretreated Ni foam by co-precipitation followed by phosphorization at 400 °C for 3 h under an N2 atmosphere. Fe-CoP exhibits higher activity and lower charge transfer resistance for the HER and OER than CoFe2O4. It is Fe-doped CoP. It contains Fe, Co, P, O, and C, which are homogeneously distributed. It possesses a hierarchical nanoporous structure, where the crystalline nanoparticles are embedded in amorphous carbon layers. It possesses a high specific surface area of 177 m2 g−1. It contains mesopores and macropores. For the OER in 1 M KOH, it affords an η of 190 mV at 10 mA cm−2 and an η of 428 mV at a current density of 1000 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at 1000 mA cm−2 for 30 h, suggesting its very high stability. For the HER in 1 M KOH, it affords an η of −78 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, Fe-CoP//Fe-CoP affords a potential of 1.49 V at 10 mA cm−2, suggesting its very high activity.
Fabrication of the nanostructured trimetallic MnNiCo phosphide could modify the electronic structure, provide optimal adsorption energy with intermediates, facilitate the evolution of gas, enhance the conductivity, expose abundant active sites, and that could enhance the performance for the HER and OER. Salem et al.10 observed that Mn1Ni1Co1-P exhibits enhanced activity and stability for the HER and OER. It was obtained on Ni foam by electrodeposition followed by plasma phosphidation at 250 °C for 3 h under a PH3/Ar atmosphere. Mn1Ni1Co1-P exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER and OER than MnNiCo-OH. It is trimetallic Mn1Ni1Co1-P, containing hexagonal NiCoP. It contains Ni, Mn, Co, and P, which are homogeneously distributed. In the high-resolution XPS spectra, the P 2p peak of Mn1Ni1Co1-P exhibits a slight negative shift when compared to that of phosphorus, suggesting a modified electronic structure possibly due to the strong interaction between the metals and the P atom. It possesses an interconnected nanosheet structure. For the OER in 1 M KOH, it affords an η of 289 mV at 10 mA cm−2, suggesting its very high activity, and it undergoes negligible decay at 10 mA cm−2 after 5000 cycles of CV, suggesting its high durability. For the HER in 1 M KOH, it affords an η of −14 mV at −10 mA cm−2, suggesting its substantially very high activity, and it undergoes negligible decay at −10 mA cm−2 after 5000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, Mn1Ni1Co1-P//Mn1Ni1Co1-P affords a potential of 1.48 V at 10 mA cm−2, suggesting its very high activity.
Besides, Wu et al.19 observed that Ni2P-Fe2P exhibits enhanced activity and stability for the HER and OER. The following steps were employed to obtain it. At first, Ni(OH)2 was prepared on cleaned Ni foam by the etching growth method at room temperature. Then, Ni(OH)2 was converted into (Ni,Fe)(OH)2 by an ion-exchange process at room temperature. Finally, (Ni,Fe)(OH)2 was converted into Ni2P-Fe2P by phosphidation at 450 °C for 1.5 h under an Ar atmosphere. Ni2P-Fe2P exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER and OER than Ni(OH)2 and Ni2P-Ni5P4. It is crystalline, containing Ni2P and Fe2P phases. It exhibits a heterostructure. It contains Fe, Ni, and P, which are homogeneously distributed. It possesses microsheet morphology having a thickness of about 7.4 nm. For the OER in 1 M KOH, it affords an η of 218 mV at 10 mA cm−2 and an η of 337 mV at a current density of 1000 mA cm−2, suggesting its very high activity, and it undergoes negligible decay at 1000 mA cm−2 after 3000 cycles of CV, suggesting its high durability. For the HER in 1 M KOH, it affords an η of −128 mV at −10 mA cm−2 and an η of −333 mV at a current density of −1000 mA cm−2, suggesting its very high activity, and it undergoes negligible decay at −1000 mA cm−2 after 3000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, Ni2P-Fe2P//Ni2P-Fe2P affords a potential of 1.561 V at 10 mA cm−2 and ∼1.98 V at a current density of 1000 mA cm−2, suggesting its very high activity.
Moreover, Yu et al.18 observed that FeP/Ni2P exhibits significantly very high activity and stability for the HER and OER. It was prepared on Ni foam by a two-step phosphidation process. FeP/Ni2P exhibits higher activity and electrochemical surface area for the HER than Ni2P*, while it exhibits higher activity and electrochemical surface area for the OER than Ni2P. It exhibits lower charge transfer resistance than Ni2P for the HER and OER. FeP/Ni2P possesses mesopores and/or nanopores (Fig. 4a and b). It possesses nanoparticle morphology. It is crystalline and contains FeP and Ni2P phases (Fig. 4c, d and g). It contains Ni, Fe, and P, which are homogeneously distributed (Fig. 4e). It is a metal phosphide (Fig. 4f). Thus, the nanostructured transition hybrid metal phosphides with pores on 3D Ni foam could modify the electronic structure, provide optimal adsorption energy with intermediates, enhance the conductivity, facilitate the gas evolution, and expose abundant active sites, and that could enhance the activity and stability for the HER and OER. For the OER in 1 M KOH, it affords an η of 154 mV at 10 mA cm−2 and an η of 293 mV at a current density of 1000 mA cm−2, suggesting its outstanding activity, while it undergoes negligible decay at a current density of 1500 mA cm−2 after 5000 cycles of CV, suggesting its outstanding durability. For the HER in 1 M KOH, it affords an η of −14 mV at −10 mA cm−2 and an η of ∼−270 mV at a current density of −1000 mA cm−2, suggesting its outstanding activity, while it undergoes negligible decay at −1000 mA cm−2 after 5000 cycles of CV, suggesting its outstanding durability. For overall water splitting in 1 M KOH, FeP/Ni2P//FeP/Ni2P affords a potential of 1.42 V at 10 mA cm−2 and a potential of ∼1.78 V at a current density of 1000 mA cm−2 (Fig. 4h), suggesting its outstanding activity, while it undergoes negligible decay at 500 mA cm−2 for >40 h (Fig. 4i), suggesting its very high stability.
 | ||
| Fig. 4 Characterization of FeP/Ni2P: (a) and (b) SEM images; (c) SAED pattern; (d) HRTEM image; (e) TEM image and its corresponding EDX elemental mapping; (f) XPS spectrum; (g) XRD pattern; (h) LSV curves of FeP/Ni2P//FeP/Ni2P in comparison with IrO2//Pt for overall water splitting in 1 M KOH; (i) chronopotentiometric curves of FeP/Ni2P at 30, 100, and 500 mA cm−2 in 1 M KOH (reproduced with permission from ref. 18 Copyright 2018, Nature Publishing Group). | ||
In addition, Yu et al.86 observed that Ni2(1−x)Mo2xP exhibits enhanced activity and stability for the HER. It was prepared on pretreated Ni foam by hydrothermal treatment followed by phosphorization at 500 °C for 1 h under an Ar atmosphere. Ni2(1−x)Mo2xP exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER than Ni2P and NiMoO4. The XRD patterns show the prominent peaks for Ni2P and a tiny peak for the NiMoO4 precursor. It contains Mo, Ni, and P, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p peak of Ni2(1−x)Mo2xP exhibits a slight negative shift when compared to that of Ni2P, suggesting the modified electronic structure. It possesses highly porous nanowire array morphology. For the HER in 1 M KOH, it affords an η of −72 mV at −10 mA cm−2 and an η of −294 mV at a current density of −1000 mA cm−2, suggesting its very high activity, while it affords reasonable stability for 160 h, suggesting its very high stability, and it undergoes negligible decay at −700 mA cm−2 after 5000 cycles of CV, suggesting its high durability.
Besides, Yuan et al.87 observed that S-Co2P@Ni2P exhibits enhanced activity and stability for the HER and OER. It was synthesized on cleaned Ti mesh by hydrothermal treatment followed by an ion-exchange process followed by a chemical deposition method followed by phosphidation at 320 °C for 2 h under an Ar atmosphere. S-Co2P@Ni2P exhibits higher activity for the HER and OER than Co2P@Ni2P. It is a S-doped Co2P@Ni2P heterostructure, where the Co2P nanowire is the core, and the Ni2P nanosheets are the shell. It exhibits a heterointerface between the Co2P and Ni2P phases. In the high-resolution XPS spectra, the Ni 2p and Co 2p peaks of S-Co2P@Ni2P exhibit slight shift when compared to that of Co2P@Ni2P, suggesting a modified electronic structure possibly due to the partial transfer of electrons from Co2P to Ni2P after S doping, which could be due to the higher electronegativity of Ni, when compared to that of Co. From the ultraviolet photoelectron spectroscopy, the value of the work function for S-Co2P is 4.03 eV, which is lower than that of 4.78 eV for Ni2P, suggesting the existence of a heterostructure, which could cause interfacial electron redistribution till the work functions reach equilibrium on both sides. For the OER in 1 M KOH, it undergoes negligible decay at 600 mA cm−2 after 1000 cycles of CV, suggesting its high durability. For the HER in 1 M KOH, it affords an η of −43 mV at −10 mA cm−2, suggesting its very high activity, and it undergoes negligible decay at −700 mA cm−2 after 1000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, S-Co2P@Ni2P//S-Co2P@Ni2P affords a potential of 1.52 V at 10 mA cm−2, suggesting its very high activity.
Active sites of nickel phosphide can be tuned by pulse-reverse electrodeposition, which could enhance the performance of the HER and OER. Kim et al.88 observed that the NiP film exhibits enhanced activity and stability for the HER and OER. It was prepared by pulse-reverse electrodeposition, where the dissolution step can modulate the chemical states of P in the NiP film. The enhanced activity for the HER of NiP film can be achieved by enhancing the generation of NixP by inhibiting the oxidation of P to POx− in the pulse-reverse electrodeposition, the dissolution step should be as minimal as possible. On the other hand, the enhanced activity for the OER of the NiP film can be achieved by increasing the dissolution amount in the pulse-reverse electrodeposition.
Phosphorus doping can play a crucial role in the formation of a nanoporous surface, where the nanoporous surface can be formed by the electrochemical etching of a phosphorus-doped alloy, which could expose abundant active sites and that could improve the performance of the HER and OER. Feng et al.89 observed that e-FeCoNiCu-P exhibits enhanced activity and stability for the HER and OER, where e-FeCoNiCu-P is electrochemically etched FeCoNiCu-P. The following steps were involved in its preparation: first, FeCoNiCu foil as a medium entropy alloy was obtained by the arc melting process. Then, FeCoNiCu-P was obtained by phosphidation of the pretreated FeCoNiCu foil. Finally, e-FeCoNiCu-P was prepared by electrochemical etching of FeCoNiCu-P. e-FeCoNiCu-P exhibits higher activity for the HER and OER than FeCoNiCu-P. It possesses a nanoporous surface, which could expose abundant active sites and enhance the performance of the HER and OER.
Besides, Li et al.90 observed that Ni5P4@FeP exhibits enhanced activity and stability for the OER. It was obtained on Ni foam by immersion for about 5 s (corrosion process) followed by phosphorization at 350 °C for 1 h under an N2 atmosphere. Ni5P4@FeP exhibits higher activity and lower charge transfer resistance for the OER than Ni5P4 and FeP. It is crystalline, while it exhibits a heterointerface between Ni5P4 and FeP phases. It possesses nanosheet array morphology. It is reconstructed into NiFe2O4 during the OER at low oxidation potentials, while NiFe2O4 is partially reconstructed into Ni/FeOOH at large oxidation potentials. Thus, Ni5P4@FeP is reconstructed into Ni/FeOOH@NiFe2O4 during the OER, where this genuinely active species has high structural reversibility. For the OER in 1 M KOH, it affords an η of 205 mV at 10 mA cm−2, suggesting its very high activity. In contrast, it undergoes negligible decay at 100 mA cm−2 for 100 h, suggesting its very high stability.
Fabricating nanostructured phosphide-based electrocatalysts using relatively low-cost metals such as Fe, Mn, and Zn is highly desirable for green synthesis, which could modify the electronic structure, provide optimal adsorption energy with intermediates, facilitate the gas evolution, and that could enhance the performance for the HER and OER. Huang et al.28 observed that Zn-Fe/Mn@Mn-FeP exhibits enhanced activity and stability for the HER and OER. It was prepared by the etching of Fe70Mn28Zn2 for 1 h followed by annealing at 400 °C for 1 h in air followed by etching for 1 h followed by phosphorization at 500 °C for 3 h in an Ar atmosphere. Zn-Fe/Mn@Mn-FeP exhibits higher activity and electrochemical surface area for the HER than Fe/Mn@Mn-FeP, Zn-Fe@Zn-FeP, and Fe@FeP. Zn-Fe/Mn@Mn-FeP exhibits higher activity for the OER than Fe/Mn@Mn-FeP, Zn-Fe@Zn-FeP, and Fe@FeP. It is Zn-Fe/Mn@Mn-FeP. It contains orthorhombic FeP, cubic Fe, and Mn, where the Zn phase is not observed, possibly due to its low content. It contains an Mn–P bond. In the high-resolution XPS spectra, the Fe 2p peak of Zn-Fe/Mn@Mn-FeP exhibits a slight positive shift when compared to that of metallic Fe, and the Mn 2p peak of Zn-Fe/Mn@Mn-FeP exhibits a slight positive shift, when compared to that of metallic Mn, suggesting a modified electronic structure. It contains Mn, Zn, Fe, and P, which are homogeneously distributed. It possesses ultrathin nanosheet morphology. For the HER in 1 M KOH, it affords an η of −165 mV at −10 mA cm−2, suggesting its very high activity. S-doped NiFeP can be derived from the phosphorization of MOF, and that could modify the electronic structure, provide optimal adsorption energy with intermediates, downshift the d-band center from the Fermi level, which could enhance the performance for the HER and OER. Li et al.91 observed that S-NiFeP-20 exhibits enhanced activity and stability for the HER, while S-NiFeP-10 exhibits enhanced activity and stability for the OER. S-NiFeP-20 or S-NiFeP-10 was prepared on pretreated carbon cloth by hydrothermal treatment (for MOF preparation) followed by phosphorization at 300 °C for 2 h under an Ar atmosphere. It is S-doped NiFeP. In the high-resolution XPS spectra, the Fe 2p peak of S-NiFeP exhibits a slight positive shift compared to NiFeP, suggesting a modified electronic structure due to S doping. The DFT calculations disclose that the S doping in NiFeP plays a crucial role in the formation of optimal ΔG values for the formation of intermediates on Ni atoms, and that enhances the performance of the OER. S-NiFeP-20 downshifts the d-band center from the Fermi level, and that enhances the performance for the HER. In 1 M KOH, S-NiFeP-20 affords an η of −56 mV at −10 mA cm−2 for the HER, while S-NiFeP-10 affords an η of 201 mV at 10 mA cm−2 for the OER, suggesting their very high activity. For overall water splitting in 1 M KOH, S-NiFeP-10OER//S-NiFeP-20HER affords a potential of 1.5 V at 10 mA cm−2, suggesting its very high activity.
Besides, Kumar et al.13 observed that the NiP2-FeP2@Cu nanoarray exhibits enhanced activity and stability for the HER. The following steps were involved in its preparation: at first, a Cu nanoarray was grown on pretreated Cu foam by chemical oxidation followed by calcination followed by electrochemical reduction. Then, NiFe LDH was grown on the Cu nanoarray by electrodeposition. Finally, the NiP2-FeP2@Cu nanoarray was obtained by the phosphidation of the NiFe LDH@Cu nanoarray at 350 °C for 2 h under an Ar atmosphere. The NiP2-FeP2@Cu nanoarray on Cu foam exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER than NiP2-FeP2 on carbon fiber paper. It is NiP2-FeP2 on a Cu nanoarray. It exhibits heterointerfaces between NiP2 and FeP2 phases. It contains Ni, Fe, P, and Cu, which are homogeneously distributed. The high-resolution XPS spectra of Cu 2p for the NiP2-FeP2@Cu nanoarray obtained before and after Ar etching disclose the existence of Cu0 along with a small peak, where the small peak is attributed to Cuδ+, which could be due to the coupling between NiP2-FeP2 and the Cu nanoarray. For the HER in 1 M KOH, it affords an η of −23.6 mV at −10 mA cm−2 and an η of −357 mV at a current density of −1000 mA cm−2, suggesting its substantially very high activity, while it affords reasonable stability at −1000 mA cm−2 for 50 h, suggesting its very high stability.
Moreover, Zhang et al.92 observed that Ni2P/NC exhibits enhanced activity and stability for the HER. It was synthesized by a coordination reaction followed by calcination followed by phosphorization followed by etching. Ni2P/NC exhibits higher activity for the HER than Ni2P. It is composed of nickel phosphide nanoparticles, which are anchored on N-doped porous carbon nanorods. It contains N, C, Ni, and P, which are homogeneously distributed. In the high-resolution XPS spectra, the Ni 2p peak of Ni2P/NC exhibits a slight positive shift compared to that of Ni2P, suggesting a modified electronic structure, possibly due to the partial transfer of electrons between Ni2P and NC. It possesses nanorod morphology, having abundant mesopores.
In addition, Liu et al.93 observed that NiCoFe-P/C exhibits enhanced activity and stability for the HER and OER. It was synthesized on pretreated Ni foam by hydrothermal treatment followed by carbonization at 450 °C for 2 h in N2 followed by phosphorization at 350 °C for 2 h in N2. NiCoFe-P/C exhibits higher activity and electrochemical surface area for the HER than NiCoFe-ZIF and NiCoFe-C. NiCoFe-P/C exhibits higher activity for the OER than NiCoFe-ZIF and NiCoFe-C. It is composed of 0D NiCoFe-P quantum dots, which are anchored on 2D porous carbon, where NiCoFe-P comprises Ni2P, CoP, and Fe2P phases. It exhibits heterointerfaces. In the high-resolution XPS spectra, the Ni 2p peak of NiCoFe-P/C is slightly less than that of Ni2+, suggesting a modified electronic structure. For the HER in 1 M KOH, it affords an η of −87 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, NiCoFe-P/C//NiCoFe-P/C affords a potential of 1.55 V at 10 mA cm−2, suggesting its very high activity. The surface properties of the materials can be improved by plasma treatment.94 Fabrication of Co-Nb bimetallic phosphide on plasma-modified carbon cloth could modify the electronic structure, provide optimal adsorption energy with intermediates, enhance the conductivity, and that could enhance the performance for the HER. Xiang et al.95 observed that CoP3-Nb2P/PCC exhibits enhanced activity and stability for the HER (PCC: plasma modified carbon cloth). The following steps were involved in its preparation: first, plasma-modified carbon-cloth was obtained by dielectric barrier discharge atmospheric pressure plasma treatment on cleaned carbon cloth. Then, the CoO-NbO/PCC was prepared by hydrothermal treatment. Finally, CoP3-Nb2P/PCC was obtained by phosphorization at 550 °C for 2 h in N2. CoP3-Nb2P/PCC exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER than CoO-NbO/PCC. It comprises the cubic phase of CoP3 and the orthorhombic phase of Nb2P on plasma-modified carbon cloth. It contains Nb, Co, and P, which are homogeneously distributed. It possesses drum-like morphology. DFT calculations and in situ Raman spectra results disclose that the enhanced performance for the HER of CoP3-Nb2P/PCC is attributed to the synergistic effect between Nb and Co phosphide. For the HER in 1 M KOH, it affords an η of −111 mV at −10 mA cm−2 and an η of −375 mV at a current density of −1000 mA cm−2, suggesting its very high activity, and it affords undergoes negligible decay at −500 mA cm−2 after 5000 cycles of CV, suggesting its high durability.
Besides, Bai et al.6e observed that Fe2P/Co@NPC exhibits enhanced activity and stability for the HER and OER. It was synthesized by hydrothermal treatment followed by carbonization at 950 °C for 3 h under an Ar atmosphere. Fe2P/Co@NPC exhibits higher activity and lower charge transfer resistance for the HER and OER than Co@NC and Fe2P@NPC. It is composed of Fe2P and Co nanoparticles, which are embedded in P and N-incorporated porous carbon. It contains Fe, Co, C, N, and P, which are homogeneously distributed. It contains pyridinic N, pyrrolic N, and graphitic N. It contains micropores and mesopores. It exhibits a high specific surface area of 547 m2 g−1.
Moreover, Singh et al.96 observed that NiP2/NbP@CNTs exhibit enhanced activity and stability for the HER and OER (CNTs: carbon nanotubes). It was obtained on Ni foam by electrodeposition followed by chemical vapor deposition followed by hydrothermal treatment followed by phosphorization at 300 °C for 2 h under an Ar atmosphere. NiP2/NbP@CNTs exhibit higher activity and electrochemical surface area for the HER and OER than NbP@CNTs and NiP2@CNTs. It comprises carbon nanotubes as the core and NiP2/NbP nanosheets as the shell, while it also contains a small amount of Nb2Ni9P phase. It exhibits a heterointerface between the NbP and NiP2 phases. In the high-resolution XPS spectra, the Ni 2p peak of NiP2/NbP@CNTs exhibits a slight negative shift when compared to that of NiP2@CNTs, the Nb 3d peak of NiP2/NbP@CNTs exhibits a slight positive shift, when compared to that of pure NbP, suggesting a modified electronic structure, possibly due to the partial transfer of electrons from Ni to Nb, which could be due to the higher electronegativity of Ni, when compared to that of Nb. For overall water splitting in 1 M KOH, NiP2/NbP@CNTs//NiP2/NbP@CNTs affords a potential of 1.51 V at 10 mA cm−2, suggesting its very high activity.
In addition, Chu et al.97 observed that Ni-CoP/Co2P@NC exhibits enhanced activity and stability for the HER, while the activated Ni-CoP/Co2P@NC exhibits enhanced activity and stability for the OER. Ni-CoP/Co2P@NC was prepared by epitaxial growth followed by pyrolysis. The activated Ni-CoP/Co2P@NC was obtained by electrochemical activation of Ni-CoP/Co2P@NC, where the activation forms the thin cobalt oxyhydroxide layer as the active species for the OER on the surface of Ni-CoP/Co2P@NC. Ni-CoP/Co2P@NC exhibits higher activity and lower charge transfer resistance for the HER than CoP/Co2P@NC. In comparison, the activated Ni-CoP/Co2P@NC exhibits higher activity and lower charge transfer resistance for the OER than Ni-CoP/Co2P@NC and CoP/Co2P@NC. Ni-CoP/Co2P@NC exhibits numerous interfaces along with defects between the 2D nanosheets and 3D nanoparticles. It contains Co2P and CoP phases. Graphitized carbon layers cover the cobalt phosphides. In the high-resolution XPS spectra, the Co 2p peak of Ni-CoP/Co2P@NC exhibits a slight negative shift compared to that of CoP/Co2P@NC, suggesting a modified electronic structure. It contains Ni, Co, C, P, O, and N, which are homogeneously distributed. For overall water splitting in 1 M KOH, the activated-Ni-CoP/Co2P@NCOER//Ni-CoP/Co2P@NCHER affords a potential of 1.59 V at 10 mA cm−2, suggesting its very high activity. In contrast, it undergoes negligible decay at 1.6 V for 400 h, suggesting its very high stability.
Besides, Riyajuddin et al.12 observed that Gr-CNTs-Sn4P3 exhibit enhanced activity and stability for overall water splitting (Gr: graphene; CNTs: carbon nanotubes). It was prepared on pretreated Ni foam by thermal chemical vapor deposition followed by immersion in ferric nitrate solution for 12 h followed by thermal chemical vapor deposition followed by electrochemical deposition followed by phosphorization through solvothermal treatment followed by acid treatment. Gr-CNTs-Sn4P3 exhibits higher activity for the OER than Gr-CNTs. It is composed of Sn4P3 with a rhombohedral structure, which is grown on a carbon matrix, where the carbon matrix is composed of graphene and multi-walled carbon nanotubes. It exhibits a heterojunction interface between CNTs and Sn4P3. In the high-resolution XPS spectra, the C 1s peak of Gr-CNTs-Sn4P3 exhibits a slight positive shift when compared to that of Gr-CNTs, suggesting a modified electronic structure, possibly due to the partial transfer of electrons between the carbon matrix and Sn4P3. It possesses a flower-like morphology. It exhibits a superhydrophilic surface with a contact angle of 0°, suggesting its high surface wettability. For the OER in 1 M KOH, it affords an η of 169 mV at 20 mA cm−2, suggesting its very high activity, while it affords ∼96% retention for 105 h, suggesting its very high stability, and it undergoes negligible decay at 200 mA cm−2 after 5000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, Gr-CNTs-Sn4P3//Gr-CNTs-Sn4P3 affords a potential of 1.482 V at 10 mA cm−2, suggesting its very high activity.
Moreover, Vijayakumar et al.98 observed that CoP-NC@NiFeP exhibits enhanced activity and stability for the HER and OER. It was prepared by precipitation followed by carbonization followed by pyro-phosphatization followed by hydrothermal treatment followed by pyro-phosphatization. CoP-NC@NiFeP exhibits higher activity for the HER and OER than CoP-NC and Co3O4-NC. It is composed of CoP-NC as a core and NiFeP as a shell. It contains crystalline CoP and NiFeP phases, while it contains N-doped amorphous carbon. It contains graphitic N, pyridinic N, and pyrrolic N. It contains Co, Ni, Fe, C, N, P, and O, which are homogeneously distributed. It possesses a coral-like morphology.
CoxP/NC can be obtained from melamine-modified ZIF-9, while the surface modification of ZIF-9 with melamine can generate robust Co coordination sites on the surface of the ZIF-9 by coordination of Co with N donor sites in the melamine, and that could enhance the performance of the OER (ZIF-9: cobalt benzimidazole zeolitic imidazolate framework). Liu et al.99 observed that CoxP/NC-melamine exhibits enhanced activity and stability for the OER. The following steps are involved in its preparation. First, ZIF-9 was obtained by the microwave hydrothermal treatment. Then, ZIF-9-melamine was prepared by modifying ZIF-9 with melamine at 100 °C for 1 h. Finally, CoxP/NC-melamine was obtained by phosphidation at 700 °C for 2 h under an Ar atmosphere. The surface engineering of ZIF-9 using melamine constructs robust Co coordination sites on the surface of the ZIF-9 via coordination of Co with N donor sites in the melamine. DFT calculations disclose that the ZIF-9 is surface modified using melamine at coordinatively unsaturated Co sites and at coordinatively saturated Co sites through a thermodynamically favored ligand exchange process between melamine and benzimidazole in ZIF-9. CoxP/NC prepared using melamine exhibits higher activity and electrochemical surface area for the OER than CoxP/NC prepared using hexamethylenetetramine, CoxP/NC prepared using phenanthroline, and CoxP/NC. It is composed of CoxP nanoparticles, which are dispersed on the carbon rods. It contains Co2P and CoP phases. It contains graphitic N, pyridinic N, Co–Nx, and pyrrolic N. It contains Co, P, N, and C, which are homogeneously distributed.
Developing a synthesis route at an industrial scale for efficient electrocatalysts for the HER and OER is highly desirable for industrial applications. Hundred-gram scale production of the Cu3P-Cu2O heterostructure integrated with N, P dual doped porous carbon can be achieved by carbonization of Cu2+-containing ion-exchange resins with KOH, and that could modify the electronic structure and provide abundant active sites, which could enhance the performance for the HER and OER. Zhu et al.100 observed that Cu3P-Cu2O/NPC exhibits enhanced activity and stability for the HER and OER. Hundred-gram scale production of Cu3P-Cu2O/NPC was achieved by carbonization of Cu2+-containing ion-exchange resins with KOH, where the pyrolysis was performed for 1 h at 850 °C under an N2 atmosphere. Cu3P-Cu2O/NPC exhibits higher activity for the HER and OER than Cu2O/NPC and Cu3P/NPC. Cu3P-Cu2O/NPC exhibits lower charge transfer resistance for the OER than Cu2O/NPC and Cu3P/NPC. It is composed of Cu3P-Cu2O nanoparticles, which are embedded in N,P dual-doped porous carbon. It exhibits a heterointerface between hexagonal Cu3P and cubic Cu2O. It contains quaternary N, pyridinic N, and pyrrolic N. For the OER in 1 M KOH, it affords an η of 286 mV at 10 mA cm−2, suggesting its very high activity, and it undergoes negligible decay at 150 mA cm−2 after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its high durability. For the HER in 1 M KOH, it affords an η of ∼−155 mV at −10 mA cm−2, suggesting its very high activity, and it undergoes negligible decay at −45 mA cm−2 after 20
000 cycles of CV, suggesting its high durability. For the HER in 1 M KOH, it affords an η of ∼−155 mV at −10 mA cm−2, suggesting its very high activity, and it undergoes negligible decay at −45 mA cm−2 after 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, Cu3P-Cu2O/NPC//Cu3P-Cu2O/NPC affords a potential of 1.57 V at 10 mA cm−2, suggesting its very high activity, while it affords 98.19% retention at 1.7 V for 168 h, suggesting its very high stability.
000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, Cu3P-Cu2O/NPC//Cu3P-Cu2O/NPC affords a potential of 1.57 V at 10 mA cm−2, suggesting its very high activity, while it affords 98.19% retention at 1.7 V for 168 h, suggesting its very high stability.
Besides, Ding et al.101 observed that Fe2P-CoP/CeO2 exhibits enhanced activity and stability for the HER and OER. It was synthesized by co-precipitation followed by refluxing followed by phosphidation (300 °C for 2 h under an N2 atmosphere). Fe2P-CoP/CeO2 exhibits higher activity and electrochemical surface area for the HER and OER than Fe2P-CoP and CeO2. It contains a higher amount of oxygen vacancies than Fe2P-CoP. It is crystalline and contains Fe2P, CoP, and CeO2 phases. It contains a triphase heterojunction. It contains Co, Fe, P, O, and Ce, which are homogeneously distributed. It possesses a porous nanocube structure having mesopores and micropores. In the high-resolution XPS spectra, the peaks corresponding to the Fe–P bond and Co–P bond of Fe2P-CoP/CeO2 show a slight positive shift when compared to that of Fe2P-CoP, while the peaks of Ce 3d in Fe2P-CoP/CeO2 show a slight negative shift when compared to that of CeO2, suggesting the modified electronic structure and strong electronic interaction at the triphase heterojunction, where the partial electrons could be transferred between Co/Fe and Ce. The ΔGH* value of Fe2P-CoP/CeO2 was calculated to be −0.03 eV (Fig. 5a), suggesting its enhanced intrinsic HER catalytic activity. The water dissociation energy of Fe2P-CoP/CeO2 (0.48 eV) is lower than that of CoP, Fe2P, and Fe2P-CoP (Fig. 5b), suggesting its accelerated HER kinetics. As shown in Fig. 5c, the charge density difference of Fe2P-CoP and Fe2P-CoP/CeO2 discloses the increase in localized charge density at the Fe2P–CoP interface, suggesting the strong interaction of Fe2P with CoP. In contrast, charge density has been redistributed at the three-phase heterojunction interface, possibly due to the introduction of CeO2 with Fe2P-CoP, suggesting the enhanced intrinsic electrocatalytic activity. For the HER in 1 M KOH, it affords an η of −45 mV at −10 mA cm−2, suggesting its outstanding activity. For overall water splitting in 1 M KOH, Fe2P-CoP/CeO2//Fe2P-CoP/CeO2 affords a potential of 1.52 V at 10 mA cm−2, suggesting its very high activity, while it affords reasonable stability at 500 mA cm−2 for 40 h, suggesting its very high stability.
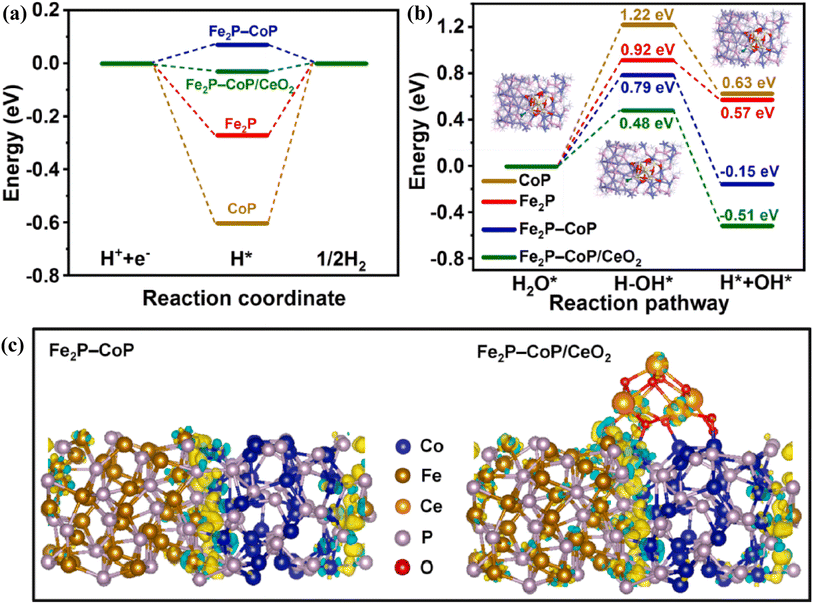 | ||
| Fig. 5 (a) Gibbs free energy profiles for the HER on Fe2P-CoP/CeO2 in comparison with Fe2P, CoP, and Fe2P-CoP; (b) water dissociation capability of Fe2P-CoP/CeO2 in comparison with Fe2P, CoP, and Fe2P-CoP (insets depict the final, transient, and initial state geometries of Fe2P-CoP/CeO2 for water dissociation); (c) charge density difference plot of Fe2P-CoP/CeO2 in comparison with Fe2P-CoP, where the yellow color indicates electron accumulation, and the cyan color represents electron depletion (reproduced with permission from ref. 101 Copyright 2022, Elsevier B.V.). | ||
Moreover, Peng et al.102 observed that Fe2P-WO2.92 exhibits enhanced activity and stability for the OER. It was synthesized on pretreated Ni foam with hydrothermal treatment, followed by phosphating at 350 °C for 2 h under an N2 atmosphere. Fe2P-WO2.92 exhibits higher activity and electrochemical surface area for the OER than Fe2P and WO2.92. It is composed of monoclinic WO2.92 and hexagonal Fe2P phases. It contains oxygen vacancies. It contains W, Fe, P, C, and O, which are homogeneously distributed. It possesses a porous structure. For the OER in 1 M KOH, it affords an η of 215 mV at 10 mA cm−2, suggesting its very high activity, while it affords 93% retention at 100 mA cm−2 for 60 h, suggesting its very high stability.
In addition, Chen et al.103 observed that Co(OH)2/NiPx exhibits enhanced activity and stability for the OER. It was prepared on a cleaned carbon cloth by a two-step electrodeposition approach. Co(OH)2/NiPx exhibits higher activity and electrochemical surface area for the OER than NiPx and Co(OH)2. It is composed of Co(OH)2 and NiPx. In the high-resolution XPS spectra, the Co 2p peak of Co(OH)2/NiPx exhibits a slight shift compared to that of Co(OH)2, suggesting a modified electronic structure. It possesses nanosheet array morphology. It exhibits a highly hydrophilic surface with a contact angle of 18.9°, suggesting its high surface wettability.
Besides, Cheng et al.104 observed that CoP/FeOOH exhibits enhanced activity and stability for the OER. The following steps were involved in its preparation: at first, Co3O4 nanofibers were synthesized by electrospinning, followed by calcination, followed by annealing. Then, CoP was obtained by phosphorization of Co3O4 for 2 h at 400 °C under an Ar atmosphere. Finally, CoP/FeOOH was prepared by stirring the CoP in ethanol with FeCl3 and NH4HCO3 for 6h at room temperature. CoP/FeOOH exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than CoP and FeOOH. It is composed of a thin amorphous FeOOH layer, which is covered on CoP porous nanofiber. It exhibits a heterointerface between CoP and FeOOH. In the high-resolution XPS spectra, the Co 2p peak of CoP/FeOOH exhibits a slight shift compared to that of CoP, suggesting a modified electronic structure.
Fabrication of a nickel phosphate microprism with unique nanochannels through a one-step solvothermal method could expose more active sites, enhancing HER performance. Calcinating the nickel phosphate microprism followed by incorporating Fe could increase the proportion of high valence Ni ions, modify the electronic structure, and enhance the performance of the OER. Zhao et al.105 observed that VSB/NiPO exhibits enhanced activity and stability for the HER, while Fe-VSB/NiPO-500 exhibits enhanced activity and stability for the OER. VSB/NiPO is a nickel phosphate microprism. The microprism is mainly composed of Versailles-Santa Barbara-5, which is a kind of nickel phosphate molecular sieve having the chemical formula of Ni20[(OH)12(H2O)6][(HPO4)8(PO4)4]·12H2O. VSB/NiPO possesses unique nanochannels, which could expose more active sites and that could enhance the performance for the HER. VSB/NiPO exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER than Ni(OH)2 and Ni(PO3)2. VSB/NiPO was prepared on the pretreated Ni foam by a solvothermal method. VSB/NiPO-500 was obtained by the calcination of VSB/NiPO at 500 °C. Fe-VSB/NiPO-500 was prepared by immersing VSB/NiPO-500 in Fe(NO3)3 solution at room temperature for 5 min. In the high-resolution XPS spectra, the proportion of Ni3+ in Fe-VSB/NiPO-500 is 92.86%, which is much higher than that of VSB/NiPO (59.52%), suggesting the modified electronic structure after Fe doping, which could be due to the partial transfer of electrons between Fe and VSB/NiPO-500. Fe-VSB/NiPO-500 with a higher proportion of high valence Ni ions and a modified electronic structure could enhance the performance of the OER. Fe-VSB/NiPO-500 exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than VSB/NiPO-500. For the OER in 1 M KOH, Fe-VSB/NiPO-500 affords an η of ∼227 mV at 50 mA cm−2, suggesting its very high activity, while it affords 98.2% retention at 50 mA cm−2 for 100 h, suggesting its very high stability. For the HER in 1 M KOH, it affords an η of −58 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, Fe-VSB/NiPO-500OER//VSB/NiPOHER affords a potential of 1.487 V at 10 mA cm−2, suggesting its very high activity. In contrast, it affords 96.44% retention at 100 mA cm−2 for 100 h, suggesting its very high stability.
2.4. Constructing nitride electrocatalysts
Cobalt nitride coupled with N-doped carbon with a porous nanostructure could modify the electronic structure, afford optimal adsorption energy with intermediates, increase the conductivity, and that could enhance the performance of the HER and OER. Song et al.106 observed that CoN@NC exhibits enhanced activity and stability for the HER and OER. The following steps were involved in its preparation: at first, the Co precursor was obtained on cleaned Ni foam by a vaporization deposition approach at 70 °C for 1 h. Finally, CoN@NC was prepared by calcinating the Co precursor under an NH3 atmosphere at 300 °C for 1 h. CoN@NC exhibits higher activity and lower charge transfer resistance for the HER and OER than the Co precursor. It is composed of crystalline CoN, which is wrapped by an amorphous N-doped carbon layer. It contains Co-N, pyrrolic-N, pyridinic-N, and graphitic-N. It contains Co, C, and N, which are homogeneously distributed. It contains defects. It possesses porous nanosheet morphology. It exhibits the formation of CoOOH as the active species during the OER, which could be due to the partial oxidation of CoN in CoN@NC. For the HER in 1 M KOH, it affords an η of −61 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, CoN@NC//CoN@NC affords a potential of 1.53 V at 10 mA cm−2, suggesting its very high activity.Fabrication of nanostructured antiperovskite nitrides could modify the electronic structure, enhance the conductivity, expose abundant active sites, and that could enhance the performance of the HER and OER. Zhu et al.107 observed that CoN0.73Co3 exhibits enhanced activity and stability for the HER, and CuNCo3 exhibits enhanced activity and stability for the OER. CoN0.73Co3 or CuNCo3 was prepared by hydrothermal treatment followed by annealing at 420 °C for 2 h under an NH3 atmosphere. CoN0.73Co3 exhibits higher activity and lower charge transfer resistance for the HER than CuNCo3. CuNCo3 exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than CoN0.73Co3. CoN0.73Co3 is antiperovskite CoN0.73Co3 having a cubic crystal structure. It contains Co and N, which are homogeneously distributed. It possesses nanowire-like morphology. CuNCo3 is antiperovskite CuNCo3, having a cubic crystal structure. It contains Co, Cu, and N, which are homogeneously distributed. It possesses nanosheet morphology. For the HER in 1 M KOH, it affords an η of −31 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, CuNCo3OER//CoN0.73Co3HER affords a potential of 1.53 V at 10 mA cm−2, suggesting its very high activity.
Besides, Hu et al.20 observed that Co2N0.67/CoMoO4 exhibits enhanced activity and stability for the HER and OER. It was prepared on cleaned carbon cloth by hydrothermal treatment followed by hydrothermal treatment followed by annealing at 400 °C for 5 h under an NH3 atmosphere. Co2N0.67/CoMoO4 exhibits higher activity and lower charge transfer resistance for the HER and OER than Co2N0.67 and CoMoO4. It exhibits a heterointerface between Co2N0.67 and CoMoO4 phases. It contains Mo, Co, O, and N, which are homogeneously distributed. It contains Co2+, Mo4+, Co–N and pyridinic N. It possesses nanosheet morphology, where the nanosheet is composed of numerous nanoparticles. For the HER in 1 M KOH, it affords an η of −63 mV at −10 mA cm−2 and an η of −315 mV at high current density of −1000 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, Co2N0.67/CoMoO4//Co2N0.67/CoMoO4 affords a potential of 1.71 V at 100 mA cm−2 and 1.98 V at a current density of 1000 mA cm−2, suggesting its very high activity.
Fabricating the Mott–Schottky heterointerface comprising metallic and semiconductor heterojunctions could tune the interfacial charge polarization and provide an optimal band structure, boosting the intrinsic activity for the HER. Pi et al.108 observed that Mo5N6-MoS2-HCNRs (HCNRs: hollow carbon nanoribbons) exhibit outstanding activity and stability for the HER. It was prepared by hydrothermal treatment followed by chemical polymerization followed by hydrothermal treatment (220 °C for 36 h) followed by thermal nitridation (750 °C under an NH3 atmosphere). Mo5N6-MoS2-HCNRs exhibit higher activity and electrochemical surface area for the HER than MoS2-HCNRs and Mo5N6-HCNRs. It is composed of Mo5N6-MoS2 nanosheets, which are grown on hollow carbon nanoribbons. It is polycrystalline, which contains Mo5N6 and 2H-MoS2 phases with heterointerfaces. It contains Mo, N, S, and C, which are homogeneously distributed. In the high-resolution XPS spectra, the Mo 3d peak of Mo4+ (Fig. 6a) and S 2p peak of S2− (Fig. 6b) of Mo5N6-MoS2-HCNRs exhibit slight positive shifts when compared to those of MoS2-HCNRs, suggesting a modified electronic structure, where partial electrons could be transferred from MoS2 to Mo5N6. The Mo K-edge XANES spectra (Fig. 6c) disclose that the valence state of Mo in Mo5N6-MoS2-HCNRs is almost closer to that of Mo foil, while the Mo5N6-MoS2-HCNRs exhibit a lower valence state than MoS2-HCNRs and MoO3. In the FT-EXAFS curves (Fig. 6d), the MoS2-HCNRs exhibit peaks for Mo–Mo, Mo–S, and Mo–O, whereas the Mo5N6-MoS2-HCNRs exhibit peaks for Mo–N (1.5 Å), Mo–Mo, Mo–S, and Mo–O with a slight shift of positions for Mo atoms. In the radial distance K-space of Mo K-edge EXAFS (Fig. 6e), the WT intensity maximum of Mo5N6-MoS2-HCNRs is at 6.0 Å corresponding to the Mo–S bond in pristine MoS2, which is higher than that of the 5.6 Å in 2H-MoS2/HCNRs, suggesting the generation of the N–Mo–S triatomic interface and synergistic electronic coupling. The work functions of 2H-MoS2 and Mo5N6 are 5.83 and 7.62 eV, respectively, where the former corresponds to semiconductor characteristics and the latter to metallic characteristics. On integrating 2H–MoS2 with Mo5N6 (Fig. 6f), a Mott–Schottky heterojunction comprising metallic Mo5N6 and semiconductor 2H-MoS2 can be formed, which can cause the transfer of valence electrons from 2H-MoS2 to Mo5N6 till the system reaches equilibrium, and that could boost the HER kinetics. For the HER in 1 M KOH, it affords an η of −53 mV at −10 mA cm−2 and an η of −315 mV at a current density of −1000 mA cm−2, suggesting its outstanding activity. In contrast, it undergoes negligible decay for 33 h, suggesting its very high stability.
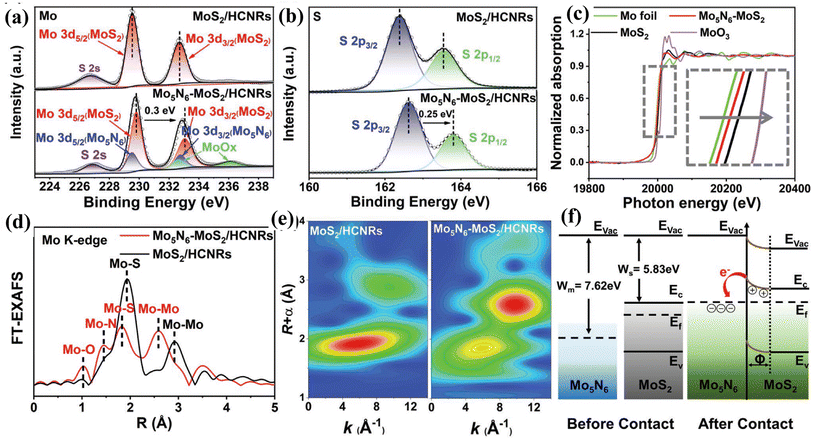 | ||
| Fig. 6 High-resolution XPS spectra of (a) Mo 3d and (b) S 2p obtained from Mo5N6-MoS2-HCNRs in comparison with 2H-MoS2/HCNRs; (c) Mo K-edge XANES spectrum of Mo5N6-MoS2/HCNR in comparison with Mo foil, MoO3, and 2H-MoS2/HCNRs; (d) FT-EXAFS curves and (e) WT-EXAFS of Mo5N6-MoS2-HCNRs in comparison with 2H-MoS2/HCNRs, disclosing the generation of the N–Mo–S triatomic interface and in-plane electronic coupling between MoS2 and Mo5N6; (f) schematic illustration depicts the energy band of the Schottky-type Mo5N6-MoS2 heterojunction, where the electron is transferred from 2H-MoS2 to Mo5N6 due to the metallic Mo5N6/semiconducting MoS2 heterojunction (Evac = vacuum energy, Eg = energy gap, Ec = conduction band, Ef = Fermi level, Ev = valence band, Φ = depletion region, and W = work function) (reproduced with permission from ref. 108 Copyright 2022, Wiley-VCH GmbH). | ||
Besides, Zhai et al.109 fabricated a heterostructured NiMoN/NiFe LDH array through hydrothermal treatment followed by nitridation followed by electrodeposition, where the amorphous NiFe LDH nanosheets on NiMoN nanorods could modify the electronic structure, enhance the conductivity, improve the charge transport, and facilitate the evolution of gas. For the OER in 1 M KOH, it affords an η of 266 mV at a current density of 1000 mA cm−2, suggesting its very high activity, while it affords high stability. The design of a lamella-heterostructured nanoporous self-supported catalyst could expose abundant active sites, facilitate electron transfer, enhance conductivity, and enhance the performance of the OER. Zeng et al.110 demonstrated a self-supported catalytic bimetallic iron–cobalt alloy/oxyhydroxide and cerium oxynitride (FeCo/CeO2−xNx) having a nanoporous lamella-heterostructure as the high-performance catalyst for the OER. The catalyst was prepared by alloying/de-alloying lamella-nanostructured eutectic intermetallic compounds, followed by subsequent thermal nitridation. The 3D nanoporous architecture could expose abundant active sites, while the heterostructure could facilitate mass transport and electron transfer. For the OER in 1 M KOH, it affords an η of 360 mV at a current density of >3900 mA cm−2, suggesting its very high activity, while it shows robust stability at a current density of ∼1900 mA cm−2 for >1000 h.
2.5. Constructing carbon-based electrocatalysts
Fabrication of a Ni–C hybrid nanosheet array with dual nanoislands (one is metallic Ni and the other is metallic Ni coated by C) could modify the electronic structure, enhance the conductivity, expose abundant active sites, and that could enhance the performance for the HER and OER. Zhu et al.111 observed that the Ni–C hybrid nanosheet array exhibits enhanced activity for the HER and OER. The following steps were involved in its synthesis: at first, Ni MOF was prepared on pretreated Ni foam by hydrothermal treatment. Finally, Ni–C was obtained by annealing the Ni MOF at 450 °C for 2 h under an N2 atmosphere. The Ni–C hybrid nanosheet array exhibits higher activity and lower charge transfer resistance for the HER than the Ni@C nanosheet array, Ni nanosheet array, and Ni MOF. It is a Ni–C hybrid nanosheet array, where the nanosheet comprises uniformly dispersed nanoparticles. It exhibits dual nanoislands on nanosheets, where one is a metallic Ni particle, and the other is a metallic Ni core with a graphitic C shell. It contains Ni and C, which are homogeneously distributed. For the HER in 1 M KOH, it affords an η of −37 mV at −10 mA cm−2, suggesting its very high activity, and it undergoes almost negligible decay at −190 mA cm−2 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, Ni–C//Ni–C affords a potential of ∼1.45 V at 50 mA cm−2, suggesting its very high activity.
000 cycles of CV, suggesting its high durability. For overall water splitting in 1 M KOH, Ni–C//Ni–C affords a potential of ∼1.45 V at 50 mA cm−2, suggesting its very high activity.
Constructing a well-controlled core–shell heterostructured electrocatalyst with a superaerophobic surface could provide optimal adsorption energy with intermediates, and facilitate the evolution of gas, and that could enhance the activity and stability of the OER and HER. Hu et al.112 observed that KT-Ni(0)@Ni(II)-TPA exhibits outstanding activity for the OER and it exhibits enhanced activity for the HER, and it was prepared by hydrothermal treatment (Fig. 7a), where a terephthalic acid (TPA)-regulated etching strategy is used on Ni foam for the preparation of a karst topography (KT) featured electrode containing core–shell structured Ni(0)@Ni(II)-TPA. KT-Ni(0)@Ni(II)-TPA exhibits higher activity for the OER than Ni(0)@Ni(II)-TPA and Ni(II)-TPA. It is a karst topography featured electrode containing core–shell structured Ni(0)@Ni(II)-TPA, where the Ni(0) as a core is surrounded by a thin amorphous Ni(II) complex as a shell with the thickness of ∼3.0 nm (Fig. 7f and g). The karst topography (Fig. 7a–c) is composed of arrays of tall towers and low valleys, where the tall tower is ∼120 nm in height (Fig. 7d) and ∼700 nm in length (Fig. 7e). It possesses a superaerophobic surface. It contains Ni, O, and C (Fig. 7h), which are homogeneously distributed. For the OER in 1 M KOH, it affords an η of 197 mV at 10 mA cm−2 and an η of 380 mV at a current density of 1500 mA cm−2, suggesting its outstanding activity, while it affords reasonable stability at an η of 206 mV to 370 mV for >45 h, suggesting its very high stability.
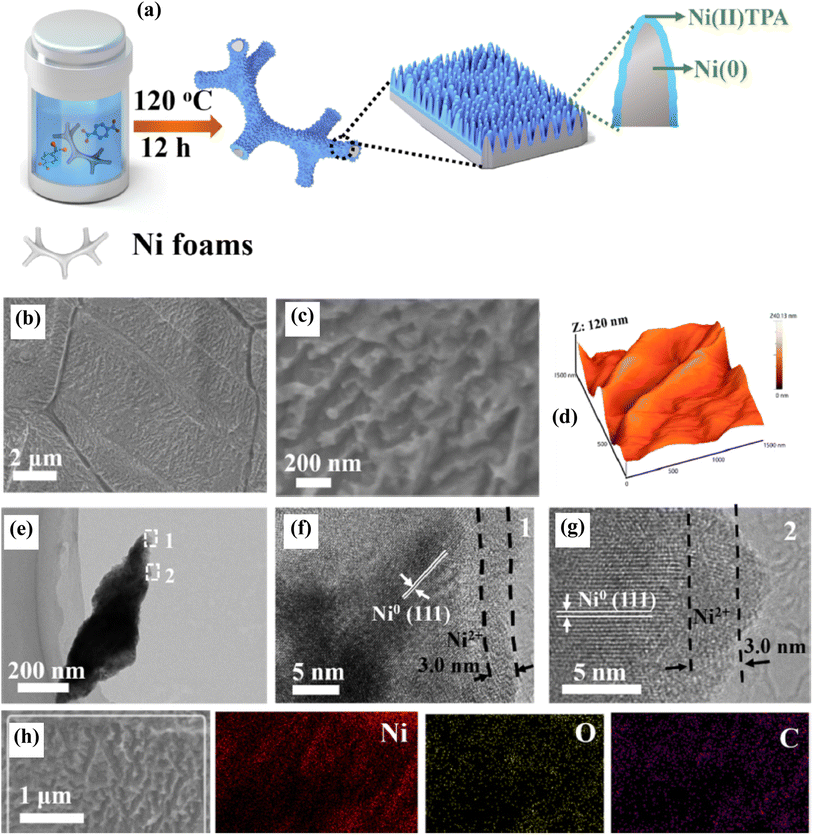 | ||
| Fig. 7 Synthesis and characterization of KT-Ni(0)@Ni(II)-TPA: (a) chematic diagram depicting the preparation process through a hydrothermal reaction using TPA; (b and c) SEM images; (d) 3D surface architecture obtained using AFM; (e) TEM image; (f) and (g) HRTEM images; (h) SEM image and its corresponding elemental mapping (reproduced with permission from ref. 112 Copyright 2021, Elsevier B.V.). | ||
Fabrication of a metallic Co and N dual doped carbon nanosheet array on copper foam could modify the electronic structure, improve the conductivity, expose more active sites, and enhance the performance for the HER. Xin et al.113 observed that CoNC exhibits enhanced activity for the HER. The following steps were involved in its synthesis: at first, ZIF-L was grown on pretreated Cu foam by immersing in an aqueous solution of Co(NO3)2 and 2-methylimidazole for 1 h at room temperature. The recrystallized ZIF-L was obtained by immersing it in a fresh aqueous solution of Co ion and 2-methylimidazole. Finally, CoNC was obtained by pyrolyzing the recrystallized ZIF-L at 500 °C for 2 h under an Ar atmosphere. The CoNC on Cu foam exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER than CoNC on Ni foam. It is a metallic Co and N dual-doped carbon nanosheet array. It contains graphitic N, pyridinic N, pyrrolic N, and Co–N. For the HER in 1 M KOH, it affords an η of −64 mV at −10 mA cm−2, suggesting its very high activity.
ZIF-67 represents a Co-based zeolitic imidazolate framework. The introduction of water in the synthesis of modified-ZIF-67 could alter the morphology and phase of the modified-ZIF-67, generating CoOOH with abundant oxygen vacancies as active species during the OER. In contrast, the heat and acid treatments of modified ZIF-67 could form active species for the HER. Lei et al.114 observed that M-Co-CN exhibits enhanced activity for the HER, while the activated-modified-ZIF-67 exhibits enhanced activity and stability for the OER. The modified-ZIF-67 was obtained by the precipitation method at 0 °C, where a de-ionized water/methanol/isobutanol mixture solution was used. M–Co–CN was synthesized by heat treatment of modified-ZIF-67 at 900 °C for 2 h under an Ar atmosphere followed by acid treatment. The activated-modified-ZIF-67 was obtained by the electrochemical activation process of modified-ZIF-67. The morphology and crystalline phase of the modified ZIF-67 are changed when compared to that of ZIF-67, which could be due to the introduction of water molecules in the synthesis of modified ZIF-67. The modified ZIF-67 possesses nanorod-like morphology, whereas ZIF-67 possesses particle morphology. Moreover, the crystalline phase of modified ZIF-67 is entirely different from that of ZIF-67. In the high-resolution O 1s XPS spectrum of modified-ZIF-67, the peaks at 532.0 eV and 535.2 eV are ascribed to the –OH and absorbed H2O, respectively, while the peak corresponding to adsorbed H2O for modified-ZIF-67 exhibits a slight shift when compared to that of ZIF-67. The modified ZIF-67 contains Co, C, O, and N, which are homogeneously distributed. The formation of CoOOH with abundant oxygen vacancies on modified-ZIF-67 during the OER process is much easier when compared to that of ZIF-67, which could be due to the change in the morphology and crystalline phase of the modified-ZIF-67. M-Co-CN exhibits higher activity for the HER than Co–CN. The activated-modified-ZIF-67 exhibits higher activity and lower charge transfer resistance for the OER than modified-ZIF-67, activated-ZIF-67, and ZIF-67. For the OER in 1 M KOH, the activated-modified-ZIF-67 affords an η of 175 mV at 10 mA cm−2, suggesting its very high activity, while it affords 96.6% retention at 100 mA cm−2 for 100 h, suggesting its very high stability. For the HER in 1 M KOH, M–Co–CN affords an η of −80 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, the activated-modified-ZIF-67OER//M-Co-CNHER affords a potential of 1.51 V at 10 mA cm−2, suggesting its very high activity, while it affords 93.7% retention at 20 mA cm−2 for 100 h, suggesting its very high stability.
Fabrication of Co and N dual-doped carbon nanotubes having an abundance of atomically dispersed low-coordinate Co–N sites could provide optimal adsorption energy with intermediates, enhancing HER performance. Ding et al.115 observed that Co-900-A exhibits enhanced activity for the HER. Co-900-A represents acid-treated Co-900, where Co-900 indicates Co and N co-doped carbon nanotubes with an abundance of atomically dispersed low-coordinate Co–N sites. Co-900-A contains Co, C, and N, which are homogeneously distributed. Co-900 was prepared by the precipitation method followed by pyrolysis at 950 °C for 1 h under an N2 atmosphere, followed by stirring with reflux for 4 h and heat treatment at 900 °C for 1 h under an N2 atmosphere.
Besides, Liu et al.116 observed that Ni-Mo2C-0.67 exhibits enhanced activity and stability for the HER. It was prepared on a Ni plate cathode by electrodeposition in molten LiF-NaF-KF-Na2MoO4-K2CO3 salts at 750 °C. Ni-Mo2C-0.67 is Ni-doped Mo2C. It contains Mo2+, Mo3+, Mo4+, and Mo6+, where Mo2+ and Mo3+ could be ascribed to Mo–C in Mo2C, while Mo4+ and Mo6+ could be attributed to MoO2 and MoO3, respectively, possibly due to the oxidation in air. It possesses a porous 3D flower-like morphology, which is composed of nanosheets. The contact angle of Ni-Mo2C-0.67 is 0°, suggesting its superhydrophilic surface and high wettability, which could enhance the electrolyte interaction and facilitate the gas evolution. The DFT calculations disclose that the ΔGH* value of optimized Ni-Mo2C-0.67 was calculated to be −0.13 eV, suggesting its enhanced intrinsic HER catalytic activity. For the HER in 1 M KOH, it affords an η of −151 mV at −10 mA cm−2, suggesting its very high activity, while it affords reasonable stability for 100 h, suggesting its very high stability.
Moreover, Xu et al.117 observed that Fe/Mo2C-NCOER//Ni/Mo2C-NCHER exhibits enhanced activity and stability for overall water splitting. Ni/Mo2C-NC or Fe/Mo2C-NC was prepared on pretreated Ni foam by the impregnation method followed by carbonization (500 °C for 2 h followed by 750 °C for 2 h) under an Ar atmosphere. Ni/Mo2C-NC exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER than Mo2C-NC, Fe/Mo2C-NC, and Co/Mo2C-NC. In comparison, Fe/Mo2C-NC exhibits higher activity for the OER than Mo2C-NC, Ni/Mo2C-NC, and Co/Mo2C-NC. Ni/Mo2C-NC comprises an Ni/Mo2C heterostructure, which is embedded in N-doped carbon sheets. In the high-resolution XPS spectra, the Mo 3d peak of Ni/Mo2C-NC exhibits a slight negative shift when compared to that of Mo2C-NC, suggesting a modified electronic structure in the Ni/Mo2C-NC possibly due to the partial transfer of electrons from Ni to Mo2C, which could be due to the higher electronegativity of Mo, when compared to that of Ni. For overall water splitting in 1 M KOH, Fe/Mo2C-NCOER//Ni/Mo2C-NCHER affords a potential of 1.66 V at 100 mA cm−2, suggesting its very high activity.
In addition, Yuan et al.118 observed that Co/Mo2C@C exhibits enhanced activity and stability for the HER and OER. It was prepared by stirring, followed by heat treatment for 3 h at 850 °C in an H2/Ar atmosphere. Co/Mo2C@C exhibits higher activity and lower charge transfer resistance for the HER and OER than Mo2C@C. It comprises a cubic Co and hexagonal β-Mo2C heterostructure supported on carbon. The carbon could be graphitic carbon or disordered carbon. In the high-resolution XPS spectra, the Mo 3d peak of Co/Mo2C@C exhibits a slight negative shift when compared to that of Mo2C@C, suggesting a modified electronic structure in Co/Mo2C@C possibly due to the partial transfer of electrons from Co to Mo2C, which could be due to the higher electronegativity of Mo, when compared to that of Co. As a result, the antibonding orbitals of Mo can be filled by the accumulated electrons on Mo2C, which could alter the adsorption of H2O* and H*, enhancing the intrinsic HER catalytic activity. The Mo in Mo2C@C is completely oxidized after the OER. On the other hand, the oxidation of Mo in Co/Mo2C@C is prevented after the OER, where Co is oxidized into CoOOH, suggesting the self-sacrifice effect of Co. The dissolution and oxidation of Mo2C in Co/Mo2C@C could be prevented by Co, where the formation of high valence Co ion and the well-preserved Mo2C could lead to high stability for the OER. It contains micropores. For the HER in 1 M KOH, it affords an η of −98 mV at −10 mA cm−2, suggesting its very high activity.
Besides, Li et al.119 observed that Co-MoC/Mo2C exhibits enhanced activity and stability for the HER. It was synthesized by hydrothermal treatment followed by the impregnation method followed by annealing (400 °C for 2 h followed by 750 °C for 2 h) under an Ar atmosphere. Co-MoC/Mo2C exhibits higher activity (η at −10 mA cm−2), higher electrochemical surface area, and lower charge transfer resistance for the HER than Ni-MoC/Mo2C, Fe-MoC/Mo2C, Cu-MoC/Mo2C, and MoC. It is a Co-MoC/Mo2C heterostructure. It exhibits heterogeneous interfaces between α-MoC and β-Mo2C phases. It contains Co, Mo, C, and N, which are homogeneously distributed. It contains Mo2+, Mo3+, Mo4+, and Mo6+. In the high-resolution XPS spectra, the Co 2p peak of Co-MoC/Mo2C exhibits a slight shift compared to that of Co-Mo2C, suggesting a modified electronic structure. It contains pores (about 5.6 nm). It possesses 2D stripe morphology. For the HER in 1 M KOH, it affords an η of −82 mV at −10 mA cm−2, suggesting its very high activity.
Moreover, Gong et al.120 observed that Mo2C-CoO@NC exhibits enhanced activity and stability for the HER and OER. It was obtained by electrospinning followed by pre-oxidation at 250 °C for 3 h in air and carbonization at 800 °C for 3 h under an N2 atmosphere. It comprises hexagonal β-Mo2C and cubic CoO, which are encapsulated in N-doped carbon nanofiber. It contains pyrrolic-N, pyridinic-N, and graphitic-N. It contains a Mo–N bond, suggesting the existence of interaction between Mo2C and N-doped carbon. It contains Mo, Co, N, C, and O, which are homogeneously distributed. For the OER in 1 M KOH, it affords an η of 222 mV at 10 mA cm−2, suggesting its very high activity.
In addition, Sun et al.121 observed that Co2P/Mo2C@NC exhibits enhanced activity and stability for the HER and OER. It was prepared by heating the SiO2@CoPMo@dopamine complex for 6 h at 800 °C under an N2 atmosphere followed by acid treatment (HCl followed by HF). Co2P/Mo2C@NC exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the HER and OER than Co2P@NC and Mo2C@NC. It comprises a Co2P/Mo2C heterojunction, which is supported on N-doped carbon. It contains a Mo–N bond, suggesting the existence of interaction between Mo2C and N-doped carbon. It contains Mo2+, Mo4+, and Mo6+. It contains Mo, Co, P, C, and N, which are homogeneously distributed. It possesses a porous structure. The Co2P/Mo2C@NC//Co2P/Mo2C@NC electrolyzer using AsGa solar cells exhibits a solar-to-hydrogen conversion efficiency of 18.1%. For the OER in 1 M KOH, it affords an η of 209 mV at 10 mA cm−2, suggesting its very high activity. For the HER in 1 M KOH, it affords an η of −86 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, Co2P/Mo2C@NC//Co2P/Mo2C@NC affords a potential of 1.55 V at 10 mA cm−2, suggesting its very high activity.
Besides, Luo et al.21 observed that MoS2/Mo2C exhibits enhanced activity and stability for the HER. It was prepared on pretreated Ti foil by hydrothermal treatment followed by chemical vapor deposition at 750 °C with CH4. MoS2/Mo2C exhibits higher activity and lower charge transfer resistance for the HER than MoS2. MoS2/Mo2C is composed of MoS2 microspheres, and the microspheres comprised MoS2 nanosheets, where the β-Mo2C nanoparticles are decorated on the edges of MoS2 nanosheets. It contains S, Mo, and C, which are homogeneously distributed. The contact angle of MoS2/Mo2C is ∼0°, suggesting its superhydrophilic surface and high wettability, which could enhance the electrolyte interaction. At the same time, it exhibits a small gas bubble releasing size (<0.2 mm), which could facilitate the gas evolution. For the HER in 1 M KOH, it affords an η of ∼−120 mV at −10 mA cm−2, and an η of −220 mV at a current density of −1000 mA cm−2, suggesting its very high activity, and it undergoes negligible decay at −10 mA cm−2 after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles of CV, suggesting its high durability.
000 cycles of CV, suggesting its high durability.
2.6. Constructing alloy/B/V/F/Si-based electrocatalysts
Fabrication of alloy ribbons by tuning the atomic packing structure could enhance the electron transferring efficiency, reduce the electron binding energy, and enhance the activity for the HER. Liu et al.122 observed that Ni56.5Co35Ti8.5 exhibits enhanced activity for the HER. The following steps were involved in the preparation of the Ni56.5Co35Ti8.5 alloy ribbon: initially, the master alloy was obtained by arc-melting the elements (purity: >99.9 wt%). Finally, the alloy ribbon was obtained by re-melting the master alloy in a quartz tube using induction melting, followed by injecting it onto a roller having a spinning tangent speed of about 40 m s−1. Ni56.5Co35Ti8.5 exhibits higher activity and lower charge transfer resistance for the HER than Ni57.5Co35Ti7.5, Ni50Co37Ti13, and Ni46Co35.5Ti18.5. The enhanced activity of Ni56.5Co35Ti8.5 for the HER is ascribed to low electron binding energy and low atomic packing density.Anodization followed by electrochemical activation of the Fe60(CoNi)30Cr10 medium entropy alloy could generate an optimal metal (oxy)hydroxide layer as active sites on the surface, enhance the conductivity, increase the surface wettability, expose abundant active sites, and that could enhance the activity for the OER. Park et al.123 observed that Fe62.5(CoNi)27.5Cr10 exhibits enhanced activity for the HER, while the activated Fe60(CoNi)30Cr10 exhibits enhanced activity for the OER. The activated Fe60(CoNi)30Cr10 was prepared by the anodization of Fe60(CoNi)30Cr10 followed by CV activation. Fe62.5(CoNi)27.5Cr10 exhibits higher activity for the HER than Fe60(CoNi)30Cr10, Fe57.5(CoNi)32.5Cr10, CoCrNi, and CoCrFeMnNi. Fe60(CoNi)30Cr10 exhibits higher activity for the OER than Fe62.5(CoNi)27.5Cr10, Fe57.5(CoNi)32.5Cr10, CoCrNi, and CoCrFeMnNi, while the activated Fe60(CoNi)30Cr10 exhibits higher activity and lower charge transfer resistance for the OER than pristine Fe60(CoNi)30Cr10 and anodized Fe60(CoNi)30Cr10. Fe60(CoNi)30Cr10 is a medium entropy alloy. The contact angle of activated Fe60(CoNi)30Cr10 is 35°, suggesting its hydrophilic surface and high wettability, which could enhance the electrolyte interaction. The activated Fe60(CoNi)30Cr10 contains an optimal metal (oxy)hydroxide layer as active sites on the surface of Fe60(CoNi)30Cr10. For the OER in 1 M NaOH, the activated Fe60(CoNi)30Cr10 affords an η of 187 mV at 10 mA cm−2, suggesting its very high activity.
Polymetallic MOF can be obtained by a facile precipitation route at room temperature, while the CoNiCuMnAl high-entropy alloy wrapped in ultra-thin carbon can be prepared by the pyrolysis of the polymetallic MOF followed by acid treatment, and that could generate a metal (oxy)hydroxide as active sites during the OER, enhance the conductivity, and that could enhance the performance for the OER. Wang et al.124 observed that CoNiCuMnAl@C exhibits enhanced activity and stability for the OER. It was obtained by room temperature precipitation followed by pyrolysis at 700 °C for 2 h under an Ar2/H2 atmosphere followed by acid treatment. CoNiCuMnAl@C exhibits higher activity for the OER than CoNiCuMnAl MOF, NiMnCuAl@C, CoMnCuAl@C, CoNiCuMn@C, CuMnAl@C, and CoNiMn@C. It possesses a core–shell structure, where the face-centered cubic CoNiCuMnAl high-entropy alloy as the core is wrapped in ultra-thin carbon as the shell. It exhibits the generation of a metal (oxy)hydroxide as an active species during the OER. For the OER in 1 M KOH, it affords an η of 215 mV at 10 mA cm−2, suggesting its very high activity.
The Ni30Co30Cr10Fe10Al18W2 high-entropy alloy possesses a unique eutectic dual-phase microstructure comprising face-centered cubic (FCC) and ordered aluminum-enriched body-centered cubic (BCC) phases. Selective etching of the aluminum-enriched BCC phase of Ni30Co30Cr10Fe10Al18W2 could construct a three-dimensional porous architecture and increase the oxidation state of the alloying elements, which could expose abundant active sites and enhance the performance of the HER and OER. Han et al.125 observed that hea-d48h exhibits enhanced activity and stability for the HER and OER. hea-d48h is a high-entropy alloy (HEA), which is de-alloyed for 48 h, where the Ni30Co30Cr10Fe10Al18W2 high-entropy alloy comprises FCC and ordered aluminum-enriched BCC phases. In comparison, three-dimensional porous architecture is constructed by de-alloying (selective etching of the BCC phase) the high-entropy alloy for 48 h. The following steps were involved in the preparation of the Ni30Co30Cr10Fe10Al18W2 high-entropy alloy: at first, the master alloy ingot was obtained by vacuum arc melting. Finally, the high-entropy alloy was obtained by re-melting the ingot four times followed by drop-casting into a copper mold. The high-entropy alloy Ni30Co30Cr10Fe10Al18W2 de-alloyed for 48 h (hea-d48h) exhibits higher activity for the HER than bare Ni30Co30Cr10Fe10Al18W2, Ni30Co30Cr10Fe10Al18W2 de-alloyed for 12 h, Ni30Co30Cr10Fe10Al18W2 de-alloyed for 24 h, and Ni30Co30Cr10Fe10Al18W2 de-alloyed for 72 h. The high-entropy alloy Ni30Co30Cr10Fe10Al18W2 de-alloyed for 48 h (hea-d48h) exhibits higher activity and electrochemical surface area for the OER than bare Ni30Co30Cr10Fe10Al18W2, Ni30Co30Cr10Fe10Al18W2 de-alloyed for 12 h, Ni30Co30Cr10Fe10Al18W2 de-alloyed for 24 h, and Ni30Co30Cr10Fe10Al18W2 de-alloyed for 72 h. High-resolution XPS spectra disclose that both the bare Ni30Co30Cr10Fe10Al18W2 alloy and Ni30Co30Cr10Fe10Al18W2 de-alloyed for 48 h contain Cr, Fe, Al, Ni, Co, and W, suggesting the existence of all the elements after acid etching. However, the peaks corresponding to Cr, Fe, Al, Ni, Co, and W of Ni30Co30Cr10Fe10Al18W2 de-alloyed for 48 h exhibit a positive shift, when compared to that of bare Ni30Co30Cr10Fe10Al18W2 alloy, suggesting the formation of high valence ions after acid etching. The de-alloying of Ni30Co30Cr10Fe10Al18W2 for 48 h causes a hierarchically porous structure, which could expose abundant active sites. For overall water splitting in 1 M KOH, hea-d48h//hea-d48h affords a potential of 1.615 V at 10 mA cm−2, suggesting its very high activity, while it affords 99.13% retention for 100 h, suggesting its very high stability.
BO33− incorporated NiFe LDH could be fabricated by a facile electrodeposition method, while the incorporation of BO33− in NiFe LDH could tune the electronic structure of the Fe atoms by filling into the oxygen vacancies, increase the oxidation state and coordination number of Fe atoms, enhance the conductivity, and that could enhance the performance for the OER. Liao et al.126 observed that the NiFeB exhibits enhanced activity and stability for the OER. It was prepared on pretreated Ni foam by a one-step electrodeposition process by applying potentials for 10 cycles of CV. NiFeB exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than NiFe LDH. NiFeB is composed of crystalline NiFe LDH, which BO33− decorates. It contains B, Fe, Ni, and O, which are homogeneously distributed. In the high-resolution XPS spectra, the Fe 2p peak of NiFeB exhibits a slight positive shift when compared to that of NiFe LDH, suggesting a modified electronic structure in NiFeB, possibly due to the partial transfer of electrons from Fe to BO33−. It exhibits a lesser amount of oxygen vacancies when compared to NiFe LDH. These results suggest that the incorporation of BO33− in NiFe LDH could tune the electronic structure of the Fe atoms by filling into the oxygen vacancies. Moreover, incorporating BO33− in NiFe LDH increases the coordination numbers of Fe atoms. It possesses ultrathin nanosheet morphology. For the OER in 1 M KOH, it affords an η of 201 mV at 10 mA cm−2, suggesting its very high activity. In contrast, it affords reasonable stability at 500 mA cm−2 for 100 h, suggesting its high stability.
Fabrication of transition metal boride electrocatalysts with a 3D hollow structure could modify the electronic structure, provide optimal adsorption energy with intermediates, improve the conductivity, expose abundant active sites, provide high mechanical strength, and facilitate the gas evolution, which could enhance the activity and stability of the HER and OER. Liu et al.8 observed that the NiMoB hollow foam exhibits outstanding activity and stability for the HER and OER. It was synthesized by the following steps (Fig. 8a). At first, the bare melamine sponge was activated at 35 °C for 10 minutes, where the bare 3D melamine sponge possessed a smooth surface (Fig. 8b and f), which was deposited by nanoparticles after activation (Fig. 8c and g). Then, NiMoB was formed by electroless plating at 40 °C for 4 h (Fig. 8d and h). Finally, NiMoB hollow foam was obtained by calcination at 400 °C for 4 h in an N2 atmosphere (Fig. 8e and i), where the hollow space is observed between the inner wall of NiMoB and carbon fiber (Fig. 8i). The NiMoB hollow foam exhibits higher activity and electrochemical surface area for the HER and OER than NiMoB on the melamine sponge. It contains Ni, Mo, and B (Fig. 8j), which are homogeneously distributed. The atomic ratio of Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mo
Mo![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) B of the NiMoB hollow foam is 1
B of the NiMoB hollow foam is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.03
0.03![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.23. It possesses an amorphous and crystalline state (Fig. 8k and l), where the inner part is mainly composed of a crystalline phase, and the outer layer is an amorphous state (thickness: ≈3 nm). It contains Ni2B, Ni3B, Ni, MoB2, and Mo phases. It exhibits a density of 0.2 g cm−3, which is almost nearer to that of nickel foam. It can withstand a pressure of 2.37 MPa during mechanical compression tests, suggesting its high mechanical stability. It exhibits a sheet resistance of 3.56 Ω sq−1, which is less than that of the nickel foam (4.53 Ω sq−1), suggesting its high electrical conductivity. It exhibits low gas mass transfer resistance. For the OER in 1 M KOH, it affords an η of 230 mV at 10 mA cm−2, and an η of ∼460 mV at a current density of 1000 mA cm−2, suggesting its outstanding activity, while it undergoes negligible decay at 600 mA cm−2 after 500 cycles of CV, suggesting its very high durability. For the HER in 1 M KOH, it affords an η of −18 mV at −10 mA cm−2, and an η of ∼−420 mV at a huge current density of −1000 mA cm−2, suggesting its outstanding activity, while it undergoes negligible decay at −1000 mA cm−2 after 500 cycles of CV, suggesting its very high durability. For overall water splitting in 1 M KOH, the NiMoB hollow foam//NiMoB hollow foam affords a potential of 1.431 V at 10 mA cm−2, suggesting its very high activity, while it affords reasonable stability at 5000 mA cm−2 for 20 h, suggesting its very high stability.
0.23. It possesses an amorphous and crystalline state (Fig. 8k and l), where the inner part is mainly composed of a crystalline phase, and the outer layer is an amorphous state (thickness: ≈3 nm). It contains Ni2B, Ni3B, Ni, MoB2, and Mo phases. It exhibits a density of 0.2 g cm−3, which is almost nearer to that of nickel foam. It can withstand a pressure of 2.37 MPa during mechanical compression tests, suggesting its high mechanical stability. It exhibits a sheet resistance of 3.56 Ω sq−1, which is less than that of the nickel foam (4.53 Ω sq−1), suggesting its high electrical conductivity. It exhibits low gas mass transfer resistance. For the OER in 1 M KOH, it affords an η of 230 mV at 10 mA cm−2, and an η of ∼460 mV at a current density of 1000 mA cm−2, suggesting its outstanding activity, while it undergoes negligible decay at 600 mA cm−2 after 500 cycles of CV, suggesting its very high durability. For the HER in 1 M KOH, it affords an η of −18 mV at −10 mA cm−2, and an η of ∼−420 mV at a huge current density of −1000 mA cm−2, suggesting its outstanding activity, while it undergoes negligible decay at −1000 mA cm−2 after 500 cycles of CV, suggesting its very high durability. For overall water splitting in 1 M KOH, the NiMoB hollow foam//NiMoB hollow foam affords a potential of 1.431 V at 10 mA cm−2, suggesting its very high activity, while it affords reasonable stability at 5000 mA cm−2 for 20 h, suggesting its very high stability.
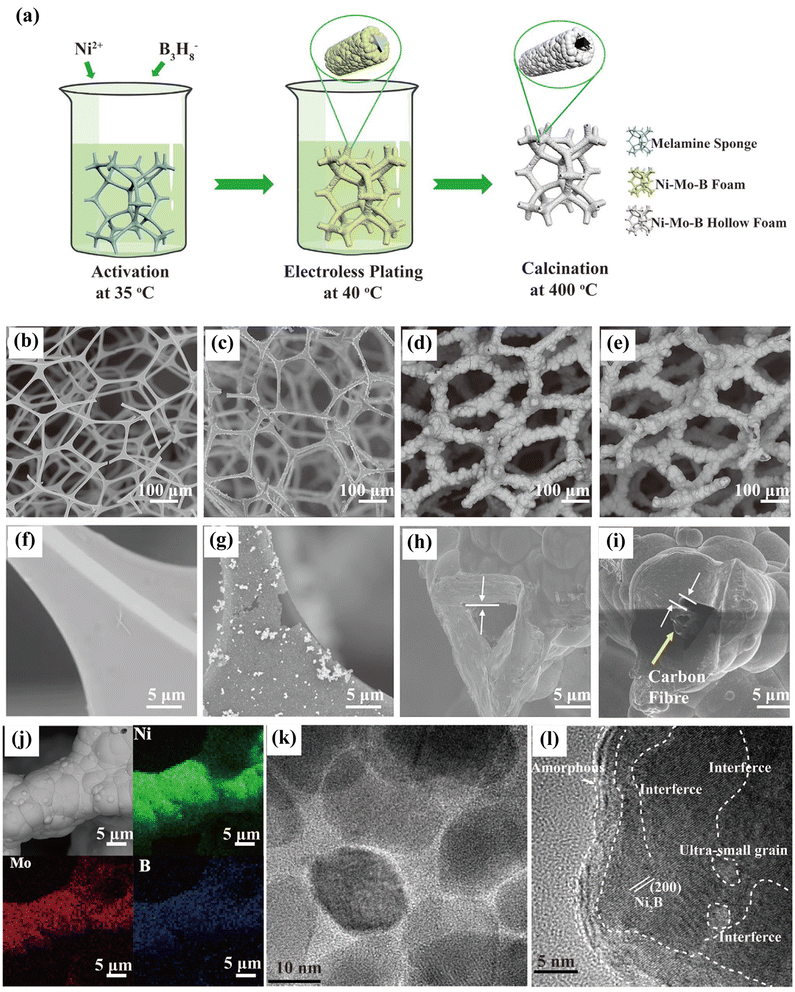 | ||
| Fig. 8 (a) Schematic diagram depicting the fabrication process of NiMoB hollow foam; (b) and (f) SEM images of bare melamine sponge; (c) and (g) SEM images of activated melamine sponge; (d) and (h) SEM images of Ni-Mo-B foam; (e) and (i) SEM images of NiMoB hollow foam; (j) SEM image and the corresponding EDX elemental mapping of NiMoB hollow foam; (k) and (l) TEM images of NiMoB hollow foam (reproduced with permission from ref. 8 Copyright 2021, Wiley-VCH GmbH). | ||
Fabrication of nanostructured NiFeV hydroxide on the V3O7 nanofiber could modify the electronic structure, increase the oxidation state of Fe, enhance the conductivity, and expose more active sites, which could enhance the performance of the OER. Zhang et al.127 observed that the NiFeV nanofibers exhibit enhanced activity and stability for the OER. It was synthesized by a two-step hydrothermal treatment. The NiFeV nanofibers exhibit higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than NiFeV and NiFe. The NiFeV nanofibers are composed of NiFeV hydroxide nanosheets as shells, which are vertically grown on the V3O7 nanofiber as the core. It exhibits a heterointerface between NiFeV hydroxide and V3O7. In the high-resolution XPS spectra, the Fe 2p peak of NiFeV nanofibers exhibits a slight positive shift when compared to that of NiFeV and NiFe, suggesting a modified electronic structure in NiFeV nanofibers with the formation of high valence Fe ions (Fe > 3+), possibly due to the partial transfer of electrons from Fe to V. It contains V3+, V4+, and, V5+. In contrast, the NiFeV nanofibers exhibit a higher amount of low oxidation state vanadium (V3+), when compared to that of NiFeV, suggesting the more partial transfer of electrons to the V in NiFeV nanofiber rather than in NiFeV, possibly due to the interfacial atom-substitution strategy. For the OER in 1 M KOH, it affords an η of 181 mV at 10 mA cm−2, suggesting its very high activity.
Fabrication of NiFe-based vanadates with a moderate heterophase by a facile co-precipitation method could create abundant oxygen vacancies, enhance the conductivity, and generate hydroxide as active species by surface reconstruction during the OER, which could enhance the performance for the OER. Shao et al.128 observed that NiFe-VOx exhibits enhanced activity and stability for the OER. It was synthesized by a facile co-precipitation method. NiFe-VOx exhibits higher activity and lower charge transfer resistance for the OER than NiCo-VOx and CoFe-VOx. NiFe-VOx exhibits a moderate heterophase, comprising both crystalline and amorphous structures. It contains Ni2FeV3O11 and Ni2FeVO6 phases. It contains V, Fe, Ni, and O, which are homogeneously distributed. It contains abundant oxygen vacancies. It possesses nanoparticle morphology, which is further composed of small nanosheets. It exhibits the formation of hydroxide, possibly NiFe hydroxide, as an active species during the OER.
Fabrication of the Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N heterojunction could modify the electronic structure, enhance the conductivity, and that could enhance the performance for the HER and OER, while it could generate a cmetal(oxy)hydroxide layer as active species by surface reconstruction during the OER, and that could enhance the performance for the OER. Lu et al.129 observed that Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N exhibits enhanced activity and stability for the HER and OER. It was synthesized by stirring followed by hydrothermal treatment followed by calcination at 500 °C for 8 h in an NH3 atmosphere. Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N exhibits higher activity and lower charge transfer resistance for the HER and OER than Ni3FeN, Ni3Fe0.75V0.25N, and Ni3Fe/Ni3FeN. It is an Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N heterojunction, while it contains Ni3FeN and Ni3Fe phases, where V is incorporated in both phases. It exhibits a heterointerface. It contains V, Fe, Ni, and N, which are homogeneously distributed. It exhibits surface reconstruction during the OER, where a thin amorphous NiFeOOH layer is formed on the surface of the Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N heterojunction.
Fabrication of hybrid metal hydroxyl fluorides (Co0.21Fe0.28(OH)F) could modify the electronic structure, generate a larger Co2+/Co3+ ratio, provide optimal adsorption energy with intermediates, enhance the conductivity, expose abundant active sites, and that could enhance the performance for the HER and OER. Li et al.130 observed that Co0.21Fe0.28(OH)F exhibits enhanced activity and stability for the HER and OER. It was obtained on pretreated Ni foam by one-step hydrothermal treatment at 120 °C for 3 h. Co0.21Fe0.28(OH)F exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than Co0.14Fe0.78(OH)F and Co0.41Fe0.14(OH)F. In comparison, it exhibits higher activity for the HER than Co0.14Fe0.78(OH)F and Co0.41Fe0.14(OH)F. It is a hybrid metal hydroxyl fluoride (Co0.21Fe0.28(OH)F), containing FeO(OH), Co(OH)F, and Ni(OH)2 phases. It contains Fe, Co, O, F, and Ni, which are homogeneously distributed, where a small amount of Ni comes from the Ni foam substrate. The Co2+/Co3+ ratio is 1.94 for Co0.21Fe0.28(OH)F, which is larger than that of 1.38 for Co0.14Fe0.78(OH)F, and 1.30 for Co0.41Fe0.14(OH)F, suggesting the exposure of more Co2+ ions due to the presence of an optimal proportion of Co and Fe in Co0.21Fe0.28(OH)F. It exhibits the formation of metal hydroxide (metal(oxy)hydroxide or metal hydroxide) as active species by surface reconstruction during the OER. It possesses nanosheet array morphology. For the OER in 1 M KOH, it affords an η of 195 mV at 10 mA cm−2, suggesting its very high activity, while it undergoes negligible decay at 20 mA cm−2 for 120 h, suggesting its very high stability. For the HER in 1 M KOH, it affords an η of −73 mV at −10 mA cm−2, suggesting its very high activity. For overall water splitting in 1 M KOH, Co0.21Fe0.28(OH)F//Co0.21Fe0.28(OH)F affords a potential of 1.53 V at 10 mA cm−2, suggesting its high activity.
Fabrication of carbon-confined iron–nickel alloy/iron fluoride could modify the electronic structure, enhance the conductivity, expose abundant active sites, and enhance the performance of the OER. Li et al.131 observed that C/O-FeNi/FeF2 exhibits enhanced activity and stability for the OER. It was synthesized by hydrothermal treatment (for the formation of the Fe2Ni MIL MOF) followed by carbonization at 800 °C for 1 h in N2 (for the formation of FeNi-800 °C) followed by fluorination at 450 °C for 2 h in N2 (for the formation of C/O-FeNi/FeF2). C/O-FeNi/FeF2 exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than FeNi-800 °C and Fe2Ni MIL MOF. It is composed of oxygen-doped FeNi/FeF2, confined by carbon. In the high-resolution XPS spectra, the Fe 2p peak corresponding to Fe2+ of C/O-FeNi/FeF2 exhibits a slight positive shift when compared to that of FeNi-800 °C, suggesting a modified electronic structure. It could exhibit the formation of metal(oxy)hydroxide by surface reconstruction during the OER.
An electrochemical tuning process comprising lithiation and rapid delithiation of the nanostructured NiSi could form a core–shell structure. In contrast, the subsequent electrochemical activation could increase the oxygen vacancies, enhance the conductivity, provide optimal adsorption energy with intermediates, increase the electrochemical surface area, and that could enhance the performance of the HER and OER. Chang et al.132 observed that SAEHER-ECT-NiSi exhibits enhanced activity and stability for the HER, while SAEOER-ECT-NiSi exhibits enhanced activity and stability for the OER. In SAEHER-ECT-NiSi, the NiSi nanowires prepared by CVD are subjected to ECT followed by SAEHER, where ECT is an electrochemical tuning process comprising lithiation and rapid delithiation of NiSi. At the same time, SAEHER is a self-adaptive evolution that involves conducting multiple linear sweep voltammetry on ECT-NiSi under HER conditions. In SAEOER-ECT-NiSi, the NiSi nanowires prepared by CVD are subjected to ECT followed by SAEOER, where ECT is an electrochemical tuning process comprising lithiation and rapid delithiation of NiSi. At the same time, SAEOER is a self-adaptive evolution that involves conducting multiple linear sweep voltammetry on ECT-NiSi under OER conditions. SAEHER-ECT-NiSi exhibits higher activity and lower charge transfer resistance for the HER than NiSi nanowires. SAEOER-ECT-NiSi exhibits higher activity, higher electrochemical surface area, and lower charge transfer resistance for the OER than NiSi nanowires. The NiSi with a core–shell structure is formed by the ECT process of the NiSi nanowires, where the intact core is surrounded by an amorphous shell dispersed with nanoparticles. The SAEHER-ECT-NiSi contains oxidized Ni, metallic Ni, oxidized Si, and metallic Si, which is almost similar to that of ECT-NiSi. In contrast, the self-adaptive evolution on ECT-NiSi under HER conditions increases the oxygen vacancies. In the high-resolution Ni 2p, Si 2p, and O 1s XPS spectra of SAEOER-ECT-NiSi, a considerable decrease in the metallic Ni and Si peak intensities, an increase in the oxidized Ni and Si peak intensities, and an increase in the peak intensity for oxygen vacancies are observed, when compared to those of ECT-NiSi. At the same time, the formation of the Ni(OH)2 phase is also observed in SAEOER-ECT-NiS. Tables 1 and 2 provide the overpotential to achieve −10 mA cm−2 for the HER of various reported noble metal-free electrocatalysts in alkaline electrolytes. Tables 3 and 4 provide the long-term stability of various reported noble metal-free electrocatalysts for the HER in alkaline electrolytes. Table 5 provides the durability of various reported noble metal-free electrocatalysts for the HER in alkaline electrolytes. Tables 6 and 7 provide the overpotential to achieve 10 mA cm−2 for the OER of various reported noble metal-free electrocatalysts in alkaline electrolytes. Tables 8 and 9 provide the long-term stability of various reported noble metal-free electrocatalysts for the OER in alkaline electrolytes. Table 10 provides the durability of various reported noble-metal-free electrocatalysts for the OER in alkaline electrolytes. Tables 11 and 12 provide the potential to achieve 10 mA cm−2 for overall water splitting of different kinds of reported noble-metal-free bifunctional electrocatalysts in alkaline electrolytes. Tables 13 and 14 provide the long-term stability of various reported noble-metal-free electrocatalysts for overall water splitting in alkaline electrolytes. Table 15 provides the overpotential to achieve −1000 mA cm−2 for the HER of various reported noble-metal-free electrocatalysts in alkaline electrolytes. Table 16 provides the overpotential to achieve 1000 mA cm−2 for the OER of various reported noble-metal-free electrocatalysts in alkaline electrolytes. Table 17 provides the potential to achieve 1000 mA cm−2 for overall water splitting of different kinds of reported noble-metal-free bifunctional electrocatalysts in alkaline electrolytes.
| Section 1 | Section 2 | ||||
|---|---|---|---|---|---|
| HER electrocatalysts | η at −10 mA cm−2 (mV) | Ref. | HER electrocatalysts | η at −10 mA cm−2 (mV) | Ref. |
| a η: overpotential; Ref.: references; PCC: plasma modified carbon cloth; HCNRs: hollow carbon nanoribbons; c-NiFe/a-NiFeOOH@NiMo: crystalline-NiFe/amorphous-NiFeOOH@NiMo; LDH: layered double hydroxide; VSB/NiPO: nickel phosphate microprism, where the microprism is mainly composed of Versailles-Santa Barbara-5, which is a kind of nickel phosphate molecular sieve having the chemical formula of Ni20[(OH)12(H2O)6][(HPO4)8(PO4)4]·12H2O; MH-TMO: mesoporous and heterostructured transition metal oxides; hea-d48h: it is a high-entropy alloy (hea), which is de-alloyed for 48 h, where the Ni30Co30Cr10Fe10Al18W2 high-entropy alloy comprises FCC and ordered aluminum-enriched BCC phases, while three dimensional porous architecture is constructed by de-alloying (selective etching of the BCC phase) the high-entropy alloy for 48 h; N-CNFs: N-doped carbon nanofibers; hnsa: hybrid nanosheet array; M-Co-CN: it was synthesized by the heat treatment of modified-ZIF-67 followed by acid treatment, where ZIF-67 represents a Co-based zeolitic imidazolate framework. b ∼−90 mV at −100 mA cm−2. c −91.9 mV at −20 mA cm−2. d −124 mV at −100 mA cm−2. | |||||
| Mn1Ni1Co1-P | −14 | 10 | Fe@Co/Se2 | −78 | 77 |
| FeP/Ni2P | −14 | 18 | Fe-CoP | −78 | 85 |
| NiMoB hollow foam | −18 | 8 | M-Co-CN | −80 | 114 |
| NiP2-FeP2@Cu nanoarray | −23.6 | 13 | Co-MoC/Mo2C | −82 | 119 |
| Mn6-CoO | −25.6 | 31 | NiCoP | −84 | 83 |
| CoN0.73Co3 | −31 | 107 | Co2P/Mo2C@NC | −86 | 121 |
| Ni2P-Ni12P5@Ni3S2 | −32 | 72 | NiCoFe-P/C | −87 | 93 |
| Ni/MoO2@CN | −33 | 15 | NiS/MoS2 | −87 | 64 |
| Ni-C hnsa | −37 | 111 | 1T Co-WS2/NiTe2/Ni | −88 | 73 |
| Ni2P | −37 | 82 | MoSe2@NiCo2Se4 | −89 | 76 |
| NiFeLa LDH/v-MXene | −38 | 57 | C-Ni1−xO/3D printed Ni | ∼−90b | 16 |
| S-Co2P@Ni2P | −43 | 87 | c-NiFe/a-NiFeOOH@NiMo | −91.9c | 11 |
| P-CoMoO4 | −44 | 40 | HR-NiMoO@Ni | −96 | 38 |
| Fe2P-CoP/CeO2 | −45 | 101 | WCoSe/WCo3O4 | −98 | 78 |
| MoO2/Co | −48 | 30 | Co/Mo2C@C | −98 | 118 |
| Ni3S2/FeNi2S4 | −50 | 66 | Ni/CeO2@N-CNFs | −100 | 42 |
| W-NiS2/MoO2 | −52 | 63 | hea-d48h | −101 | 125 |
| Mo5N6-MoS2-HCNRs | −53 | 108 | Ni3S2-MoS2 | −103 | 65 |
| Zn-Fe/Mn@Mn-FeP | −53 | 28 | NiP | −107 | 88 |
| S-NiFeP-20 | −56 | 91 | CoNiP | −107.56 | 84 |
| VSB/NiPO | −58 | 105 | CoP3-Nb2P/PCC | −111 | 95 |
| CoN@NC | −61 | 106 | Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N | −113 | 129 |
| Co2N0.67/CoMoO4 | −63 | 20 | Mo2C-CoO@NC | −115 | 120 |
| CoNC | −64 | 113 | Faceted CuMn2O4 | −116 | 32 |
| MH-TMO | ∼−70 | 1g | Ni-CoP/Co2P@NC | −117 | 97 |
| Ni2(1−x)Mo2xP | −72 | 86 | MoS2/Mo2C | ∼−120 | 21 |
| Co0.21Fe0.28(OH)F | −73 | 130 | (Ni-Fe)Sx/NiFe(OH)y | −124d | 69 |
| MoS2-MoO3−x/Ni3S2 | −76 | 67 | Fe,Ce-NixSy | −125 | 61 |
| Fe-Co-CO3-OH | −77 | 17 | Bi2O3 based | −127 | 33 |
| HER electrocatalysts | η at −10 mA cm−2 (mV) | Electrolyte | Ref. |
|---|---|---|---|
| a η: overpotential; LDH: layered double hydroxide; Co-900-A: it represents acid treated Co-900, where Co-900 indicates Co and N co-doped carbon nanotubes with an abundance of low-coordinate Co–N sites; e-FeCoNiCu-P: electrochemically etched FeCoNiCu-P; CNTs: carbon nanotubes; c-CoMnP/a-CoMn LDH: crystalline CoMnP decorated amorphous CoMn LDH; KT-Ni(0)@Ni(II)-TPA: a terephthalic acid (TPA)-regulated etching strategy is used for the preparation of a karst topography (KT) featured electrode containing core-shell structured Ni(0)@Ni(II)-TPA; LSC/LSCO: La0.5Sr0.5CoO3−δ; N-GQDs: N-doped graphene quantum dots; SAEHER-ECT-NiSi: NiSi is subjected to ECT followed by SAEHER, where ECT is an electrochemical tuning process comprising lithiation and rapid delithiation of NiSi, while SAEHER is a self-adaptive evolution that involves conducting multiple linear sweep voltammetry on ECT-NiSi under HER conditions. b −131 mV at −100 mA cm−2. c −134 mV at −50 mA cm−2. d −137 mV at −20 mA cm−2. e −156 mV at −100 mA cm−2. f −163 mV at −50 mA cm−2. g –170.3 mV at −100 mA cm−2. h −171 mV at −100 mA cm−2. i −220 mV at −50 mA cm−2. j −425 mV at −500 mA cm−2. | |||
| Ni2P-Fe2P | −128 | 1 M KOH | 19 |
| Ni/Mo2C-NC | −131b | 1 M KOH | 117 |
| Ce@NiCo LDH | −134c | 1 M KOH | 55 |
| NiP2/NbP@CNTs | −137d | 1 M KOH | 96 |
| MoO3-Co(OH)2@Ag | −142 | 1 M KOH | 39 |
| Ni-Mo2C-0.67 | −151 | 1 M KOH | 116 |
| Cu3P-Cu2O/NPC | ∼−155 | 1 M KOH | 100 |
| SAEHER-ECT-NiSi | −156e | 1 M KOH | 132 |
| CoP-NC@NiFeP | ∼−163f | 1 M KOH | 98 |
| e-FeCoNiCu-P | −165 | 1 M KOH | 89 |
| Fe-NiS-NiS2 | −167 | 1 M KOH | 62 |
| c-CoMnP/a-CoMn LDH | −170.3g | 1 M KOH | 56 |
| Sn-Ni3S2 | −171h | 1 M KOH | 14 |
| Co3O4 | −177 | 1 M KOH | 29 |
| NixSy@MnOxHy | −179 | 1 M KOH | 68 |
| Co-Mn-CO3-OH | −180 | 1 M KOH | 48 |
| NiFe LDHactivated | −189 | 1 M KOH | 51 |
| Co-Ni3S2 | −192 | 1 M NaOH | 58 |
| Ni2P/NC | −201 | 1 M KOH | 92 |
| Co-Ni3S2 | −220i | 1 M KOH | 59 |
| KT-Ni(0)@Ni(II)-TPA | −228 | 1 M KOH | 112 |
| Fe2P/Co@NPC | −235 | 1 M KOH | 6e |
| Co-900-A | −246 | 1 M KOH | 115 |
| LSCO-MoSe2 | −260 | 1 M KOH | 9 |
| Ni-FePS3 | −356 | 1 M KOH | 71 |
| Fe62.5(CoNi)27.5Cr10 | −378 | 1 M NaOH | 123 |
| LSC-N-GQDs-MoSe2 | −409 | 1 M KOH | 80 |
| Ni56.5Co35Ti8.5 | −425j | 1 M KOH | 122 |
| HER electrocatalysts | Chr amp | Chr pot | Dur (h) | Remark after the stability test | Ref. |
|---|---|---|---|---|---|
| a η: overpotential; Ref.: references; Dur: duration of the stability test; Chr pot: chronopotentiometry; Chr amp: chronoamperometry; NA: not applicable; CD: current density; PCC: plasma modified carbon cloth; HCNRs: hollow carbon nanoribbons; CNTs: carbon nanotubes; LDH: layered double hydroxide; VSB/NiPO: nickel phosphate microprism, where the microprism is mainly composed of Versailles-Santa Barbara-5, which is a kind of nickel phosphate molecular sieve having the chemical formula of Ni20[(OH)12(H2O)6][(HPO4)8(PO4)4]·12H2O; MH-TMO: mesoporous and heterostructured transition metal oxides; SAEHER-ECT-NiSi: NiSi is subjected to ECT followed by SAEHER, where ECT is an electrochemical tuning process comprising lithiation and rapid delithiation of NiSi, while SAEHER is a self-adaptive evolution that involves conducting multiple linear sweep voltammetry on ECT-NiSi under HER conditions; hnsa: hybrid nanosheet array. | |||||
| NiFeLa LDH/v-MXene | NA | Yes | 400 | Reasonable stability at a CD of −100 mA cm−2 | 57 |
| Ni/MoO2@CN | NA | Yes | 200 | Negligible decay at a CD of −1000 mA cm−2 | 15 |
| Ni2(1−x)Mo2xP | Yes | NA | 160 | Reasonable stability | 86 |
| P-CoMoO4 | NA | Yes | 100 | Negligible decay at a CD of −50 mA cm−2 | 40 |
| MH-TMO | NA | Yes | 100 | Reasonable stability at a CD of −10 mA cm−2 | 1g |
| HR-NiMoO@Ni | NA | Yes | 100 | Reasonable stability at a CD of −1000 A m−2 | 38 |
| Ni-Mo2C-0.67 | Yes | NA | 100 | Reasonable stability | 116 |
| NixSy@MnOxHy | NA | Yes | 100 | Reasonable stability at a CD of −100 mA cm−2 | 68 |
| Cu3P-Cu2O/NPC | Yes | NA | 90 | 96% retention | 100 |
| Zn-Fe/Mn@Mn-FeP | NA | Yes | 80 | Negligible decay at a CD of −10 mA cm−2 | 28 |
| Mn6-CoO | Yes | NA | 60 | 83.6% retention | 31 |
| Co2N0.67/CoMoO4 | NA | Yes | 60 | Reasonable stability at a CD of −100 mA cm−2 | 20 |
| Sn-Ni3S2 | Yes | NA | 60 | Negligible decay at an η of −223 mV | 14 |
| NiP2-FeP2@Cu nanoarray | NA | Yes | 50 | Reasonable stability at a CD of −1000 mA cm−2 | 13 |
| Ni-C hnsa | Yes | NA | 50 | Negligible decay at an η of −59 mV | 111 |
| CoP3-Nb2P/PCC | Yes | NA | 50 | 93.6% retention | 95 |
| Faceted CuMn2O4 | Yes | NA | 50 | Negligible decay | 32 |
| Ni-CoP/Co2P@NC | Yes | NA | 50 | Negligible decay | 97 |
| CoP-NC@NiFeP | NA | Yes | 50 | Reasonable stability at a CD of −50 mA cm−2 | 98 |
| Mn1Ni1Co1-P | Yes | NA | 48 | 92% retention | 10 |
| CoN0.73Co3 | Yes | NA | 48 | 81% retention | 107 |
| Co/Mo2C@C | Yes | NA | 48 | 90.1% retention at an η of −98 mV | 118 |
| (Ni-Fe)Sx/NiFe(OH)y | NA | Yes | 48 | Negligible decay at a CD of −120 mA cm−2 | 69 |
| Ni2P-Fe2P | NA | Yes | 48 | Reasonable stability at a CD of −100/−500 mA cm−2 | 19 |
| WCoSe/WCo3O4 | NA | Yes | >45 | Reasonable stability at a CD of −100 mA cm−2 | 78 |
| Fe,Ce-NixSy | NA | Yes | 40 | Negligible decay at a CD of −10 mA cm−2 | 61 |
| SAEHER-ECT-NiSi | Yes | NA | 35 | Negligible decay at an η of −250 mV | 132 |
| NiCoFe-P/C | Yes | NA | 34 | Negligible decay | 93 |
| Mo5N6-MoS2-HCNRs | Yes | NA | 33 | Negligible decay | 108 |
| NiP2/NbP@CNTs | Yes | NA | 33 | ∼88.6% retention at an η of −247 mV | 96 |
| Fe2P-CoP/CeO2 | Yes | NA | 30 | Negligible decay | 101 |
| VSB/NiPO | NA | Yes | >30 | 94.1% retention at a CD of −100 mA cm−2 | 105 |
| Fe-CoP | NA | Yes | 30 | Negligible decay at a CD of −10 mA cm−2 | 85 |
| Co2P/Mo2C@NC | Yes | NA | 30 | Negligible decay | 121 |
| Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N | Yes | NA | 30 | Reasonable stability | 129 |
| Co-Ni3S2 | Yes | NA | 30 | Negligible decay at an η of −462 mV | 59 |
| HER electrocatalysts | Chr amp | Chr pot | Dur (h) | Remark after the stability test | Ref. |
|---|---|---|---|---|---|
| a η: overpotential; Ref.: references; Dur: duration of the stability test; Chr pot: chronopotentiometry; Chr amp: chronoamperometry; NA: not applicable; CD: current density; LDH: layered double hydroxide; N-CNFs: N-doped carbon nanofibers. | |||||
| MoO3-Co(OH)2@Ag | NA | Yes | 29 | Reasonable stability at a CD of −100 mA cm−2 | 39 |
| Ni/CeO2@N-CNFs | Yes | NA | 27 | Negligible decay at an η of −135 mV | 42 |
| CoN@NC | NA | Yes | 25 | Reasonable stability at a CD of −10 mA cm−2 | 106 |
| NiS/MoS2 | Yes | NA | 25 | Reasonable stability | 64 |
| FeP/Ni2P | NA | Yes | 24 | Negligible decay at a CD of −100 mA cm−2 | 18 |
| Ni2P-Ni12P5@Ni3S2 | Yes | NA | 24 | Negligible decay | 72 |
| Ni2P | Yes | NA | 24 | Reasonable stability at an η of −240 mV to −650 mV | 82 |
| MoO2/Co | Yes | NA | 24 | Negligible decay | 30 |
| S-NiFeP-20 | Yes | NA | 24 | Reasonable stability | 91 |
| 1T Co-WS2/NiTe2/Ni | NA | Yes | 24 | 97% retention at a CD of −50 mA cm−2 | 73 |
| MoSe2@NiCo2Se4 | Yes | NA | 24 | Negligible decay at an η of −135 mV | 76 |
| Ni3S2-MoS2 | NA | Yes | 24 | Reasonable stability at a CD of −10 mA cm−2 | 65 |
| MoS2/Mo2C | Yes | NA | 24 | Negligible decay | 21 |
| Ni/Mo2C-NC | Yes | NA | 24 | Reasonable stability | 117 |
| Ce@NiCo LDH | Yes | NA | 24 | Reasonable stability | 55 |
| S-Co2P@Ni2P | Yes | NA | 20 | Negligible decay | 87 |
| Co0.21Fe0.28(OH)F | NA | Yes | 20 | Negligible decay at a CD of −20 mA cm−2 | 130 |
| Fe@Co/Se2 | NA | Yes | 20 | Negligible decay at a CD of −10 mA cm−2 | 77 |
| Co-MoC/Mo2C | Yes | NA | 20 | Reasonable stability | 119 |
| Bi2O3 based | Yes | NA | 20 | 85.4% retention | 33 |
| Co-Ni3S21 M NaOH | NA | Yes | 18 | Negligible decay | 58 |
| Fe2P/Co@NPC | NA | Yes | >16 | Negligible decay at a CD of −10 mA cm−2 | 6e |
| Co3O4 | NA | Yes | >15 | Negligible decay at a CD of −100 mA cm−2 | 29 |
| NiCoP | Yes | NA | 12 | 87% retention at an η of −84 mV | 83 |
| Fe-NiS-NiS2 | Yes | NA | 12 | Reasonable stability | 62 |
| Ni2P/NC | Yes | NA | 12 | 92% retention | 92 |
| MoS2-MoO3−x/Ni3S2 | NA | Yes | 11 | Reasonable stability at a CD of −100 mA cm−2 | 67 |
| Co-Mn-CO3-OH | NA | Yes | 10 | Negligible decay at a CD of −10 mA cm−2 | 48 |
| HER electrocatalysts | Cycles of CV | Remark after the durability test | Ref. |
|---|---|---|---|
| a PCC: plasma modified carbon cloth; N-CNFs: N-doped carbon nanofibers; hnsa: hybrid nanosheet array; HCNRs: hollow carbon nanoribbons; hea-d48h: it is a high-entropy alloy (hea), which is de-alloyed for 48 h, where the Ni30Co30Cr10Fe10Al18W2 high-entropy alloy comprises FCC and ordered aluminum-enriched BCC phases, while three dimensional porous architecture is constructed by de-alloying (selective etching of the BCC phase) the high-entropy alloy for 48 h. | |||
| Cu3P-Cu2O/NPC | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Negligible decay at −45 mA cm−2 | 100 |
| Sn-Ni3S2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Negligible decay at −1000 mA cm−2 | 14 |
| Ni-C hnsa | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Almost negligible decay at −190 mA cm−2 | 111 |
| MoS2/Mo2C | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Negligible decay at −10 mA cm−2 | 21 |
| NiCoP | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Slight decay at −140 mA cm−2 | 83 |
| Fe-Co-CO3-OH | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Negligible decay at −1000 mA cm−2 | 17 |
| Ni2(1−x)Mo2xP | 5000 | Negligible decay at −700 mA cm−2 | 86 |
| CoP3-Nb2P/PCC | 5000 | Almost negligible decay at −500 mA cm−2 | 95 |
| Mn1Ni1Co1-P | 5000 | Negligible decay at −10 mA cm−2 | 10 |
| FeP/Ni2P | 5000 | Negligible decay at −1000 mA cm−2 | 18 |
| Ni2P | 5000 | Negligible decay at −1200 mA cm−2 | 82 |
| Mn6-CoO | 3000 | 9 mV decay | 31 |
| Co2N0.67/CoMoO4 | 3000 | Slight decay at −200 mA cm−2 | 20 |
| Ni2P-Fe2P | 3000 | Negligible decay at −1000 mA cm−2 | 19 |
| Co2P/Mo2C@NC | 3000 | Negligible decay at −90 mA cm−2 | 121 |
| Ni/CeO2@N-CNFs | 3000 | Negligible decay at −125 mA cm−2 | 42 |
| MoO2/Co | 3000 | Very slight decay at −225 mA cm−2 | 30 |
| NiFeLa LDH/v-MXene | 2000 | Negligible decay at −700 mA cm−2 | 57 |
| Mo5N6-MoS2-HCNRs | 2000 | Almost negligible decay at −100 mA cm−2 | 108 |
| NiS/MoS2 | 2000 | Negligible decay at −175 mA cm−2 | 64 |
| Fe@Co/Se2 | 2000 | Negligible decay at −10 mA cm−2 | 77 |
| Ni3S2/FeNi2S4 | 2000 | Negligible decay at −300 mA cm−2 | 66 |
| Mo2C-CoO@NC | 2000 | Very slight decay at −200 mA cm−2 | 120 |
| P-CoMoO4 | 1000 | Negligible decay | 40 |
| Zn-Fe/Mn@Mn-FeP | 1000 | Negligible decay at −200 mA cm−2 | 28 |
| Co/Mo2C@C | 1000 | Negligible decay at −50 mA cm−2 | 118 |
| Fe,Ce-NixSy | 1000 | Negligible decay at −225 mA cm−2 | 61 |
| Co-Ni3S2 | 1000 | Negligible decay at −1000 mA cm−2 | 59 |
| S-NiFeP-20 | 1000 | Negligible decay | 91 |
| S-Co2P@Ni2P | 1000 | Negligible decay at −700 mA cm−2 | 87 |
| Hea-d48h | 1000 | Negligible decay at −10 mA cm−2 | 125 |
| NiMoB hollow foam | 500 | Negligible decay at −1000 mA cm−2 | 8 |
| Section 1 | Section 2 | ||||
|---|---|---|---|---|---|
| OER electrocatalysts | η at 10 mA cm−2 (mV) | Ref. | OER electrocatalysts | η at 10 mA cm−2 (mV) | Ref. |
| a η: overpotential; Ref.: references; NA: not applicable; c-NiFe/a-NiFeOOH@NiMo: crystalline-NiFe/amorphous-NiFeOOH@NiMo; e-FeCoNiCu-P: electrochemically etched FeCoNiCu-P; CNTs: carbon nanotubes; LDH: layered double hydroxide; VSB/NiPO: nickel phosphate microprism, where the microprism is mainly composed of Versailles-Santa Barbara-5, which is a kind of nickel phosphate molecular sieve having the chemical formula of Ni20[(OH)12(H2O)6][(HPO4)8(PO4)4]·12H2O; KT-Ni(0)@Ni(II)-TPA: a terephthalic acid (TPA)-regulated etching strategy is used for the preparation of a karst topography (KT) featured electrode containing core–shell structured Ni(0)@Ni(II)-TPA; LSC/LSCO: La0.5Sr0.5CoO3−δ; MH-TMO: mesoporous and heterostructured transition metal oxides; hea-d48h: it is a high-entropy alloy (hea), which is de-alloyed for 48 h, where the Ni30Co30Cr10Fe10Al18W2 high-entropy alloy comprises FCC and ordered aluminum-enriched BCC phases, while three dimensional porous architecture is constructed by de-alloying (selective etching of the BCC phase) the high-entropy alloy for 48 h; N-CNFs: N-doped carbon nanofibers; act-modified-ZIF-67: activated modified Co-based zeolitic imidazolate framework; NiFeV nf: NiFeV nanofibers; IronCE10s: the catalyst was prepared on an iron substrate by applying pulsed potentials for 10 seconds, where the substrate was used as a counter electrode; FeCoSx-PBA: FeCoS embedded in a Prussian blue analogue hetero-nanoframes; Gr: graphene. b 133.2 mV at 20 mA cm−2. c 169 mV at 20 mA cm−2. d 184 mV at 20 mA cm−2. e ∼227 mV at 50 mA cm−2. f 227 mV at 50 mA cm−2. g 230 mV at 20 mA cm−2. h 248.2 mV at 20 mA cm−2. i 250 mV at 50 mA cm−2. j 257 mV at 100 mA cm−2. k 260 mV at 20 mA cm−2. l 260 mV at 50 mA cm−2. m 267 mV at 100 mA cm−2. n 270 mV at 50 mA cm−2. o 270 mV at 50 mA cm−2. p 270 mV at 50 mA cm−2. | |||||
| LSCO-MoSe2 | 39 | 9 | Fe,Ce-NixSy | 227f | 61 |
| c-NiFe/a-NiFeOOH@NiMo | 133.2b | 11 | Fe-Co-CO3-OH | 228 | 17 |
| FeP/Ni2P | 154 | 18 | FeCo-Ni3S4 | 230g | 60 |
| Gr-CNTs-Sn4P3 | 169c | 12 | Ni/CeO2@N-CNFs | 230 | 42 |
| WCoSe/WCo3O4 | 175 | 78 | NiMoB hollow foam | 230 | 8 |
| act-modified-ZIF-67 | 175 | 114 | Co(OH)2/NiPx | 236 | 103 |
| NiFeV nf | 181 | 127 | Co3O4 | 236 | 29 |
| Zn-Fe/Mn@Mn-FeP | 184d | 28 | IronCE10s | 239.5 | 26 |
| NiFe LDH | 186 | 25 | Co3−xO4/NiO | 240 | 36 |
| 1 M NaOHFe60(CoNi)30Cractivated10 | 187 | 123 | CoN@NC | 240 | 106 |
| Fe-CoP | 190 | 85 | S-FeOOH | 244 | 24 |
| NiFeLa LDH/v-MXene | 191 | 57 | Ag@NiFe LDH | 246 | 54 |
| Ce-NiFe | 195 | 47 | Fe2P-CoP/CeO2 | 248 | 101 |
| Co0.21Fe0.28(OH)F | 195 | 130 | NiP2/NbP@CNTs | 248.2h | 96 |
| KT-Ni(0)@Ni(II)-TPA | 197 | 112 | CoP/FeOOHElectrolyte NA | 250 | 104 |
| (Ni-Fe)Sx/NiFe(OH)y | 199 | 69 | Ni/MoO2@CN | 250 | 15 |
| FeNiOH | 200 | 44 | e-FeCoNiCu-P | 250 | 89 |
| Fe@Co/Se2 | 200 | 77 | Ce@NiCo LDH | 250i | 55 |
| NiFeB | 201 | 126 | C/O-FeNi/FeF2 | 253 | 131 |
| S-NiFeP-10 | 201 | 91 | Co/Mo2C@C | 254 | 118 |
| NiFe LDHactivated | 201 | 51 | NiFe based | 255 | 52 |
| Ni3S2/FeNi2S4 | 201 | 66 | MoSe2@NiCo2Se4 | 255 | 76 |
| Ni5P4@FeP | 205 | 90 | NiCoFe-P/C | 257j | 93 |
| CoNiFe-OH | 207 | 46 | NiCoP | 259 | 83 |
| Co2P/Mo2C@NC | 209 | 121 | P-CoMoO4 | 260k | 40 |
| FeOOH@NiFe LDH | 210 | 53 | CuNCo3 | 260 | 107 |
| OR-NiOOH | 213 | 38 | Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N | 260l | 129 |
| CoNiCuMnAl@C | 215 | 124 | FeCoSx-PBA | 266 | 70 |
| Fe2P-WO2.92 | 215 | 102 | NiFe-VOx | 267 | 128 |
| MH-TMO | ∼216 | 1g | Sn-Ni3S2 | 267m | 14 |
| Ni2P-Fe2P | 218 | 19 | Hea-d48h | 270n | 125 |
| Mo2C-CoO@NC | 222 | 120 | Fe-NiS-NiS2 | 270o | 62 |
| Fe-VSB/NiPO-500 | ∼227e | 105 | CoP-NC@NiFeP | 270p | 98 |
| OER electrocatalysts | η at 10 mA cm−2 (mV) | Ref. |
|---|---|---|
| a η: overpotential; c-CoFeP/a-CoFe LDH: crystalline CoFeP decorated amorphous CoFe LDH; act-Ni-CoP/Co2P@NC: activated Ni-CoP/Co2P@NC; SS-500-AO: it is an electrocatalyst on SUS304 stainless steel, where the catalyst was prepared by selenization followed by heat treatment followed by electrochemical oxidation; SAEOER-ECT-NiSi: NiSi is subjected to ECT followed by SAEOER, where ECT is an electrochemical tuning process comprising lithiation and rapid delithiation of NiSi, while SAEOER is a self-adaptive evolution that involves conducting multiple linear sweep voltammetry on ECT-NiSi under OER conditions; N-GQDs: N-doped graphene quantum dots; LSC/LSCO: La0.5Sr0.5CoO3−δ; CoxP/NC-melamine: CoxP/NC was prepared through a melamine assisted synthesis route. b 271.7 mV at 100 mA cm−2. c 290 mV at 30 mA cm−2. d 293 mV at 100 mA cm−2. e 294 mV at 30 mA cm−2. f 296 mV at 100 mA cm−2. g 300 mV at ∼701 A g−1. h 301 mV at 50 mA cm−2. i 310 mV at 50 mA cm−2. j 326 mV at 100 mA cm−2. k ∼330 mV at 100 mA cm−2. l 337 mV at 100 mA cm−2. m ∼340 mV at 100 mA cm−2. n ∼350 mV at 20 mA cm−2. o ∼350 mV at 50 mA cm−2. p 372 mV at 50 mA cm−2. q ∼410 mV at 100 mA cm−2. r 500 mV at 100 mA cm−2. | ||
| c-CoFeP/a-CoFe LDH | 271.7b | 56 |
| act-Ni-CoP/Co2P@NC | 272 | 97 |
| MoO2/Co | 280 | 30 |
| f-Ni0.1Co0.9Ox | 282 | 35 |
| Co2N0.67/CoMoO4 | 283 | 20 |
| SS-500-AO | 284.3 | 79 |
| Cu3P-Cu2O/NPC | 286 | 100 |
| Ni-FePS3 | 287 | 71 |
| Mn1Ni1Co1-P | 289 | 10 |
| Co3O4 SS Mesh/ZnO0.1 M KOH | 290 | 37 |
| 1T Co-WS2/NiTe2/Ni | 290c | 73 |
| Fe/Mo2C-NC | 293d | 117 |
| Co-Mn-CO3-OH | 294e | 48 |
| SAEOER-ECT-NiSi | 296f | 132 |
| WO3@F0.1-C | 298 | 27 |
| NiFe-(OOH)-based0.1 M KOH | 300g | 50 |
| Mn6-CoO | 301h | 31 |
| LSC-N-GQDs-MoSe2 | 302 | 80 |
| NiP | 305 | 88 |
| Defect-FeOOHactivated | 307 | 49 |
| Annealed α-Ni(OH)2 | 310 | 43 |
| Co-Ni3S2 | 310i | 59 |
| CoxP/NC-melamine | 312 | 99 |
| NixSy@MnOxHy | 326j | 15 |
| S-Co2P@Ni2P | ∼330k | 87 |
| Fe2P/Co@NPC | 331 | 6e |
| CuS-Ni3S2/CuNi | 337l | 22 |
| C-Ni1−xO/3D printed Ni | ∼340m | 16 |
| CoNiP | ∼350n | 84 |
| Ni3S2-MoS2 | ∼350o | 65 |
| Co-CoO/GO | 372p | 41 |
| NiS/MoS2 | ∼410q | 64 |
| Co-Ni3S21 M NaOH | 500r | 58 |
| OER electrocatalysts | Chr amp | Chr pot | Dur (h) | Remark after the stability test | Ref. |
|---|---|---|---|---|---|
| a η: overpotential; Ref.: references; Dur: duration of the stability test; Chr pot: chronopotentiometry; Chr amp: chronoamperometry; NA: not applicable; CD: current density; CNTs: carbon nanotubes; LDH: layered double hydroxide; VSB/NiPO: nickel phosphate microprism, where the microprism is mainly composed of Versailles-Santa Barbara-5, which is a kind of nickel phosphate molecular sieve having the chemical formula of Ni20[(OH)12(H2O)6][(HPO4)8(PO4)4]·12H2O; KT-Ni(0)@Ni(II)-TPA: a terephthalic acid (TPA)-regulated etching strategy is used for the preparation of a karst topography (KT) featured electrode containing core–shell structured Ni(0)@Ni(II)-TPA; MH-TMO: mesoporous and heterostructured transition metal oxides; hea-d48h: it is a high-entropy alloy (hea), which is de-alloyed for 48 h, where the Ni30Co30Cr10Fe10Al18W2 high-entropy alloy comprises FCC and ordered aluminum-enriched BCC phases, while three dimensional porous architecture is constructed by de-alloying (selective etching of the BCC phase) the high-entropy alloy for 48 h; act-Ni-CoP/Co2P@NC: activated Ni-CoP/Co2P@NC; act-modified-ZIF-67: activated modified Co-based zeolitic imidazolate framework; NiFeV nf: NiFeV nanofibers; SS-500-AO: it is an electrocatalyst on SUS304 stainless steel, where the catalyst was prepared by selenization followed by heat treatment followed by electrochemical oxidation; Gr: graphene. | |||||
| NiFeLa LDH/v-MXene | NA | Yes | 1200 | Negligible decay at a CD of 100 mA cm−2 | 57 |
| FeCo-Ni3S4 | NA | Yes | 360 | 22 mV decay at a CD of 20 mA cm−2 | 60 |
| Ni/MoO2@CN | NA | Yes | 200 | Negligible decay at a CD of 1000 mA cm−2 | 15 |
| NiFe LDH | NA | Yes | 192 | Reasonable stability at a CD of 100 mA cm−2 | 25 |
| SS-500-AO | NA | Yes | 160 | Negligible decay at a CD of 10 mA cm−2 | 79 |
| NixSy@MnOxHy | NA | Yes | 150 | Reasonable stability at a CD of 100 mA cm−2 | 68 |
| Co0.21Fe0.28(OH)F | NA | Yes | 120 | Negligible decay at a CD of 20 mA cm−2 | 130 |
| Gr-CNTs-Sn4P3 | NA | Yes | 105 | ∼96% retention | 12 |
| FeOOH@NiFe LDH | NA | Yes | 100 | Negligible decay | 53 |
| NiFeB | NA | Yes | 100 | Reasonable stability at a CD of 500 mA cm−2 | 126 |
| Ce-NiFe | NA | Yes | 100 | Reasonable stability at a CD of 1000 mA cm−2 | 47 |
| S-FeOOH | NA | Yes | 100 | Negligible decay at a CD of 20 mA cm−2 | 24 |
| Ni5P4@FeP | NA | Yes | 100 | Negligible decay at a CD of 100 mA cm−2 | 90 |
| OR-NiOOH | NA | Yes | 100 | Reasonable stability at a CD of 1000 A m−2 | 38 |
| Fe-VSB/NiPO-500 | NA | Yes | 100 | 98.2% retention at a CD of 50 mA cm−2 | 105 |
| MH-TMO | NA | Yes | 100 | Negligible decay at a CD of 10 mA cm−2 | 1g |
| act-modified-ZIF-67 | NA | Yes | 100 | 96.6% retention at a CD of 100 mA cm−2 | 114 |
| Cu3P-Cu2O/NPC | Yes | NA | 90 | Negligible decay | 100 |
| Zn-Fe/Mn@Mn-FeP | NA | Yes | 80 | Negligible decay at a CD of 50 mA cm−2 | 28 |
| Co3−xO4/NiO | Yes | NA | 60 | Reasonable stability at 1.47 V | 36 |
| CoNiFe-OH | NA | Yes | 60 | Negligible decay at a CD of 10 mA cm−2 | 46 |
| Fe2P-WO2.92 | NA | Yes | 60 | 93% retention at a CD of 100 mA cm−2 | 102 |
| Mn6-CoO | Yes | NA | ∼60 | 85.2% retention | 31 |
| Hea-d48h | Yes | NA | 60 | Negligible decay | 125 |
| Sn-Ni3S2 | Yes | NA | 60 | Negligible decay at an η of 438 mV | 14 |
| Defect-FeOOHactivated | NA | Yes | 55 | Reasonable stability at a CD of 10 mA cm−2 | 49 |
| act-Ni-CoP/Co2P@NC | Yes | NA | 50 | Negligible decay | 97 |
| WCoSe/WCo3O4 | NA | Yes | 50 | Reasonable stability at a CD of 100 mA cm−2 | 78 |
| Ni3S2/FeNi2S4 | Yes | NA | 50 | Negligible decay | 66 |
| (Ni-Fe)Sx/NiFe(OH)y | NA | Yes | 50 | Negligible decay at a CD of 100 mA cm−2 | 69 |
| CuNCo3 | Yes | NA | 48 | 86% retention | 107 |
| Ni-FePS3 | NA | Yes | 48 | Negligible decay at a CD of 10 mA cm−2 | 71 |
| Co/Mo2C@C | Yes | NA | 48 | 92.3% retention at an η of 254 mV | 118 |
| Mn1Ni1Co1-P | Yes | NA | 48 | Reasonable stability | 10 |
| Ni2P-Fe2P | NA | Yes | 48 | Negligible decay at a CD of 100/500 mA cm−2 | 19 |
| Co2N0.67/CoMoO4 | NA | Yes | >45 | Reasonable stability at a CD of 100 mA cm−2 | 20 |
| KT-Ni(0)@Ni(II)-TPA | Yes | NA | >45 | Reasonable stability at an η of 206 mV to 370 mV | 112 |
| NiFeV nf | NA | Yes | >40 | Reasonable stability | 127 |
| NiFe based | NA | Yes | 40 | Reasonable stability at a CD of 5 mA cm−2 | 52 |
| P-CoMoO4 | NA | Yes | 40 | Negligible decay at a CD of 50 mA cm−2 | 40 |
| Fe,Ce-NixSy | NA | Yes | 40 | Reasonable stability at a CD of 10 mA cm−2 | 61 |
| CoP-NC@NiFeP | NA | Yes | 40 | Negligible decay at a CD of 50 mA cm−2 | 98 |
| OER electrocatalysts | Chr amp | Chr pot | Dur (h) | Remark after the stability test | Ref. |
|---|---|---|---|---|---|
| a η: overpotential; Ref.: references; Dur: duration of the stability test; Chr pot: chronopotentiometry; Chr amp: chronoamperometry; NA: not applicable; CD: current density; SAEOER-ECT-NiSi: NiSi is subjected to ECT followed by SAEOER, where ECT is an electrochemical tuning process comprising lithiation and rapid delithiation of NiSi, while SAEOER is a self-adaptive evolution that involves conducting multiple linear sweep voltammetry on ECT-NiSi under OER conditions; CNTs: carbon nanotubes; LDH: layered double hydroxide; FeCoSx-PBA: FeCoS embedded in Prussian blue analogue hetero-nanoframes; N-CNFs: N-doped carbon nanofibers; CoxP/NC-melamine: CoxP/NC was prepared through a melamine assisted synthesis route. | |||||
| SAEOER-ECT-NiSi | Yes | NA | 35 | Negligible decay at an η of 470 mV | 132 |
| NiCoFe-P/C | Yes | NA | 34 | Negligible decay | 93 |
| NiP2/NbP@CNTs | Yes | NA | 33 | ∼88.2% retention at an η of 274.1 mV | 96 |
| Co2P/Mo2C@NC | Yes | NA | 30 | Negligible decay | 121 |
| Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N | Yes | NA | 30 | Reasonable stability | 129 |
| Fe2P-CoP/CeO2 | Yes | NA | 30 | Negligible decay | 101 |
| Co-Ni3S2 | Yes | NA | 30 | Negligible decay at an η of 465 mV | 59 |
| Fe-CoP | NA | Yes | 30 | Negligible decay at a CD of 1000 mA cm−2 | 85 |
| Annealed α-Ni(OH)2 | NA | Yes | 26 | Negligible decay at a CD of 10 mA cm−2 | 43 |
| CoN@NC | NA | Yes | 25 | Reasonable stability at a CD of 10 mA cm−2 | 106 |
| WO3@F0.1-C | NA | Yes | 24 | Negligible decay | 27 |
| f-Ni0.1Co0.9Ox | Yes | NA | 24 | Reasonable stability at a potential of 1.53 V | 35 |
| CoNiCuMnAl@C | NA | Yes | 24 | Negligible decay at a CD of 10 mA cm−2 | 124 |
| 1T Co-WS2/NiTe2/Ni | NA | Yes | 24 | Reasonable stability at a CD of 50 mA cm−2 | 73 |
| S-NiFeP-10 | Yes | NA | 24 | Reasonable stability | 91 |
| Co-Ni3S21 M NaOH | NA | Yes | 24 | Negligible decay at a CD of 50 mA cm−2 | 58 |
| Fe/Mo2C-NC | Yes | NA | 24 | Reasonable stability | 117 |
| Ce@NiCo LDH | Yes | NA | 24 | Reasonable stability at a potential of 1.55 V | 55 |
| FeP/Ni2P | NA | Yes | 24 | Negligible decay at 100 mA cm−2 | 18 |
| FeNiOH | Yes | NA | 20 | Negligible decay at a potential of 1.46 V | 44 |
| CoP/FeOOHElectrolyte NA | NA | Yes | 20 | Reasonable stability at a CD of 10 mA cm−2 | 104 |
| S-Co2P@Ni2P | Yes | NA | 20 | Negligible decay | 87 |
| Fe@Co/Se2 | NA | Yes | 20 | Negligible decay at a CD of 10 mA cm−2 | 77 |
| Ag@NiFe LDH | Yes | NA | >19 | Reasonable stability | 54 |
| Co-Mn-CO3-OH | NA | Yes | 18 | Negligible decay at a CD of 50 mA cm−2 | 48 |
| C/O-FeNi/FeF2 | Yes | NA | 16 | Reasonable stability | 131 |
| Fe2P/Co@NPC | NA | Yes | >16 | Reasonable stability at a CD of 10 mA cm−2 | 6e |
| Co3O4 | NA | Yes | >15 | Negligible decay at a CD of 100 mA cm−2 | 29 |
| CuS-Ni3S2/CuNi | NA | Yes | 15 | Negligible decay at a CD of 100 mA cm−2 | 22 |
| NiFe-VOx | NA | Yes | 12 | 24.2 mV decay at a CD of 10 mA cm−2 | 128 |
| Co-CoO/GO | Yes | NA | 12 | 93% retention at a potential of 1.71 V | 41 |
| NiCoP | Yes | NA | 12 | 99% retention at an η of 259 mV | 83 |
| Fe-NiS-NiS2 | Yes | NA | 12 | Reasonable stability at an η of 270 mV | 62 |
| Co(OH)2/NiPx | NA | Yes | >10 | Reasonable stability at a CD of 10 mA cm−2 | 103 |
| FeCoSx-PBA | Yes | NA | 10 | 98.3% retention | 70 |
| Ni/CeO2@N-CNFs | Yes | NA | >10 | Negligible decay | 42 |
| Co3O4 SS Mesh/ZnO0.1 M KOH | Yes | NA | 7 | Reasonable stability | 37 |
| CoxP/NC-melamine | NA | Yes | 4 | Reasonable stability at a CD of 10 mA cm−2 | 99 |
| OER electrocatalysts | Cycles of CV | Remark after the durability test | Ref. |
|---|---|---|---|
| a NA: not applicable; CNTs: carbon nanotubes; LDH: layered double hydroxide; N-CNFs: N-doped carbon nanofibers; c-NiFe/a-NiFeOOH@NiMo: crystalline-NiFe/amorphous-NiFeOOH@NiMo; hea-d48h: it is a high-entropy alloy (hea), which is de-alloyed for 48 h, where the Ni30Co30Cr10Fe10Al18W2 high-entropy alloy comprises FCC and ordered aluminum-enriched BCC phases, while three dimensional porous architecture is constructed by de-alloying (selective etching of the BCC phase) the high-entropy alloy for 48 h; Gr: graphene. | |||
| Cu3P-Cu2O/NPC | 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Negligible decay at 150 mA cm−2 | 100 |
| NiCoP | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Negligible decay at 90 mA cm−2 | 83 |
| Sn-Ni3S2 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
Negligible decay at 2000 mA cm−2 | 14 |
| NixSy@MnOxHy | 5000 | Negligible decay at 500 mA cm−2 | 68 |
| Gr-CNTs-Sn4P3 | 5000 | Negligible decay at 200 mA cm−2 | 12 |
| Mn1Ni1Co1-P | 5000 | Negligible decay at 10 mA cm−2 | 10 |
| FeP/Ni2P | 5000 | Negligible decay at 1500 mA cm−2 | 18 |
| CuS-Ni3S2/CuNi | 5000 | Negligible decay at 1000 mA cm−2 | 22 |
| CoNiCuMnAl@C | 3000 | Negligible decay at 90 mA cm−2 | 124 |
| FeCo-Ni3S4 | 3000 | Negligible decay at 100 mA cm−2 | 60 |
| Co2P/Mo2C@NC | 3000 | Negligible decay at 90 mA cm−2 | 121 |
| Mn6-CoO | 3000 | 13 mV decay | 31 |
| Co2N0.67/CoMoO4 | 3000 | Negligible decay | 20 |
| Ni/CeO2@N-CNFs | 3000 | Negligible decay at 60 mA cm−2 | 42 |
| Ni2P-Fe2P | 3000 | Negligible decay at 1000 mA cm−2 | 19 |
| (Ni-Fe)Sx/NiFe(OH)y | 3000 | Negligible decay at 100 mA cm−2 | 69 |
| c-NiFe/a-NiFeOOH@NiMo | 2000 | Negligible decay at 10 mA cm−2 | 11 |
| Ni-FePS3 | 2000 | Negligible decay at 100 mA cm−2 | 71 |
| Fe@Co/Se2 | 2000 | Negligible decay at 10 mA cm−2 | 77 |
| Ni3S2/FeNi2S4 | 2000 | Negligible decay at 300 mA cm−2 | 66 |
| Mo2C-CoO@NC | 2000 | Negligible decay at 75 mA cm−2 | 120 |
| FeOOH@NiFe LDH | 1000 | Negligible decay at 300 mA cm−2 | 53 |
| C/O-FeNi/FeF2 | 1000 | Negligible decay at 50 mA cm−2 | 131 |
| f-Ni0.1Co0.9Ox | 1000 | Slight decay at 100 mA cm−2 | 35 |
| FeNiOH | 1000 | Negligible decay at 90 mA cm−2 | 44 |
| Ni5P4@FeP | 1000 | Negligible decay at 350 mA cm−2 | 90 |
| Co3O4 SS Mesh/ZnO0.1 M KOH | 1000 | Negligible decay at 10 mA cm−2 | 37 |
| CoP/FeOOHElectrolyte NA | 1000 | Slight decay at 90 mA cm−2 | 104 |
| S-Co2P@Ni2P | 1000 | Negligible decay at 600 mA cm−2 | 87 |
| S-NiFeP-10 | 1000 | Negligible decay | 91 |
| Hea-d48h | 1000 | Negligible decay at 125 mA cm−2 | 125 |
| Zn-Fe/Mn@Mn-FeP | 1000 | Negligible decay at 150 mA cm−2 | 28 |
| Fe-NiS-NiS2 | 1000 | Negligible decay at 350 mA cm−2 | 62 |
| Fe,Ce-NixSy | 1000 | Negligible decay at 225 mA cm−2 | 61 |
| Co/Mo2C@C | 1000 | Negligible decay at 10 mA cm−2 | 118 |
| Co-Ni3S2 | 1000 | Negligible decay at 1000 mA cm−2 | 59 |
| NiMoB hollow foam | 500 | Negligible decay at 600 mA cm−2 | 8 |
| Bifunctional electrocatalysts for the HER and OER | Overall water splitting (V at 10 mA cm−2) | Electrolyte | Ref. |
|---|---|---|---|
| a nb: non-bifunctional electrocatalyst; c-NiFe/a-NiFeOOH@NiMo: crystalline-NiFe/amorphous-NiFeOOH@NiMo; CNTs: carbon nanotubes; LDH: layered double hydroxide; c-CoMnP/a-CoMn LDH: crystalline CoMnP decorated amorphous CoMn LDH; c-CoFeP/a-CoFe LDH: crystalline CoFeP decorated amorphous CoFe LDH; VSB/NiPO: nickel phosphate microprism, where the microprism is mainly composed of Versailles-Santa Barbara-5, which is a kind of nickel phosphate molecular sieve having the chemical formula of Ni20[(OH)12(H2O)6][(HPO4)8(PO4)4]·12H2O; MH-TMO: mesoporous and heterostructured transition metal oxides; M-Co-CN: it was synthesized by the heat treatment of modified-ZIF-67 followed by acid treatment, where ZIF-67 represents the Co-based zeolitic imidazolate framework; act-modified-ZIF-67: activated modified Co-based zeolitic imidazolate framework; hnsa: hybrid nanosheet array; Gr: graphene. b ∼1.45 V at 50 mA cm−2. | |||
| FeP/Ni2P | 1.42 | 1 M KOH | 18 |
| NiMoB hollow foam | 1.431 | 1 M KOH | 8 |
| c-NiFe/a-NiFeOOH@NiMo | 1.45 | 1 M KOH | 11 |
| Ni-C hnsa | ∼1.45b | 1 M KOH | 111 |
| Sn-Ni3S2 | 1.46 | 1 M KOH | 14 |
| (Ni-Fe)Sx/NiFe(OH)y | 1.46 | 1 M KOH | 69 |
| NiCoP | 1.48 | 1 M KOH | 83 |
| NiFeLa LDH/v-MXene | 1.48 | 1 M KOH | 57 |
| Mn1Ni1Co1-P | 1.48 | 1 M KOH | 10 |
| Gr-CNTs-Sn4P3 | 1.482 | 1 M KOH | 12 |
| Co3O4 | 1.483 | 1 M KOH | 29 |
| nbFe-VSB/NiPO-500OER//VSB/NiPOHER | 1.487 | 1 M KOH | 105 |
| MH-TMO | 1.49 | 1 M KOH | 1g |
| WCoSe/WCo3O4 | 1.49 | 1 M KOH | 78 |
| Fe-CoP | 1.49 | 1 M KOH | 85 |
| nbc-CoFeP/a-CoFe LDHOER//c-CoMnP/a-CoMn LDHHER | 1.498 | 1 M KOH | 56 |
| nbS-NiFeP-10OER//S-NiFeP-20HER | 1.5 | 1 M KOH | 91 |
| NiS/MoS2 | 1.51 | 1 M KOH | 64 |
| NiP2/NbP@CNTs | 1.51 | 1 M KOH | 96 |
| nbOR-NiOOHOER//HR-NiMoO@NiHER | 1.51 | 1 M KOH | 38 |
| Fe@Co/Se2 | 1.51 | 1 M KOH | 77 |
| nbact-modified-ZIF-67OER//M-Co-CNHER | 1.51 | 1 M KOH | 114 |
| Mn6-CoO | 1.52 | 1 M KOH | 31 |
| S-Co2P@Ni2P | 1.52 | 1 M KOH | 87 |
| Fe2P-CoP/CeO2 | 1.52 | 1 M KOH | 101 |
| 1T Co-WS2/NiTe2/Ni | 1.521 | 1 M KOH | 73 |
| NixSy@MnOxHy | 1.53 | 1 M KOH | 68 |
| nbCuNCo3OER//CoN0.73Co3HER | 1.53 | 1 M KOH | 107 |
| CoN@NC | 1.53 | 1 M KOH | 106 |
| Co0.21Fe0.28(OH)F | 1.53 | 1 M KOH | 130 |
| P-CoMoO4 | 1.54 | 1 M KOH | 40 |
| NiCoFe-P/C | 1.55 | 1 M KOH | 93 |
| Co2P/Mo2C@NC | 1.55 | 1 M KOH | 121 |
| Ni3S2/FeNi2S4 | 1.55 | 1 M KOH | 66 |
| Bifunctional electrocatalysts for the HER and OER | Overall water splitting (V at 10 mA cm−2) | Electrolyte | Ref. |
|---|---|---|---|
| a nb: non-bifunctional electrocatalyst; N-CNFs: N-doped carbon nanofibers; LDH: layered double hydroxide; e-FeCoNiCu-P: electrochemically etched FeCoNiCu-P; LSC/LSCO: La0.5Sr0.5CoO3−δ; N-GQDs: N-doped graphene quantum dots; hea-d48h: it is a high-entropy alloy (hea), which is de-alloyed for 48 h, where the Ni30Co30Cr10Fe10Al18W2 high-entropy alloy comprises FCC and ordered aluminum-enriched BCC phases, while three dimensional porous architecture is constructed by de-alloying (selective etching of the BCC phase) the high-entropy alloy for 48 h; SAEHER-ECT-NiSi: NiSi is subjected to ECT followed by SAEHER, where ECT is an electrochemical tuning process comprising lithiation and rapid delithiation of NiSi, while SAEHER is a self-adaptive evolution that involves conducting multiple linear sweep voltammetry on ECT-NiSi under HER conditions; SAEOER-ECT-NiSi: NiSi is subjected to ECT followed by SAEOER, where ECT is an electrochemical tuning process comprising lithiation and rapid delithiation of NiSi, while SAEOER is a self-adaptive evolution that involves conducting multiple linear sweep voltammetry on ECT-NiSi under OER conditions; act-Ni-CoP/Co2P@NC: activated Ni-CoP/Co2P@NC. b 1.63 V at 100 mA cm−2. c 1.66 V at 100 mA cm−2. d 1.71 V at 100 mA cm−2. e 1.738 V at 100 mA cm−2. f ∼1.76 V at 20 mA cm−2. g 1.79 V at 50 mA cm−2. h ∼1.8 V at 250 mA cm−2. i 1.83 V at 200 mA cm−2. j 1.87 V at 20 mA cm−2. | |||
| Ni/CeO2@N-CNFs | 1.56 | 1 M KOH | 42 |
| Fe,Ce-NixSy | 1.56 | 1 M KOH | 61 |
| Mo2C-CoO@NC | 1.56 | 1 M KOH | 120 |
| Ni2P-Fe2P | 1.561 | 1 M KOH | 19 |
| LSC-N-GQDs-MoSe2 | ∼1.57 | 1 M KOH | 80 |
| W-NiS2/MoO2 | 1.57 | 1 M KOH | 63 |
| CoP-NC@NiFeP | 1.57 | 1 M KOH | 98 |
| Cu3P-Cu2O/NPC | 1.57 | 1 M KOH | 100 |
| Co-Ni3S2 | 1.58 | 1 M KOH | 59 |
| Fe-NiS-NiS2 | 1.59 | 1 M KOH | 62 |
| nbact-Ni-CoP/Co2P@NCOER//Ni-CoP/Co2P@NCHER | 1.59 | 1 M KOH | 97 |
| Co/Mo2C@C | 1.59 | 1 M KOH | 118 |
| Hea-d48h | 1.615 | 1 M KOH | 125 |
| nbSAEOER-ECT-NiSiOER//SAEHER-ECT-NiSiHER | 1.63b | 1 M KOH | 132 |
| nbFe/Mo2C-NCOER//Ni/Mo2C-NCHER | 1.66c | 1 M KOH | 117 |
| Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N | 1.66 | 1 M KOH | 129 |
| Ni3S2-MoS2 | 1.67 | 1 M KOH | 65 |
| Ce@NiCo LDH | 1.68 | 1 M KOH | 55 |
| Co-Mn-CO3-OH | 1.68 | 1 M KOH | 48 |
| MoO2/Co | 1.683 | 1 M KOH | 30 |
| Ni-FePS3 | 1.71 | 1 M KOH | 71 |
| Co2N0.67/CoMoO4 | 1.71d | 1 M KOH | 20 |
| Fe2P/Co@NPC | 1.73 | 1 M KOH | 6e |
| e-FeCoNiCu-P | 1.738e | 1 M KOH | 89 |
| CoNiP | ∼1.76f | 1 M KOH | 84 |
| Zn-Fe/Mn@Mn-FeP | 1.79g | 1 M KOH | 28 |
| nbNiFe LDHOER//Ni2PHER | ∼1.8h | 1 M KOH | 82 |
| Ni/MoO2@CN | 1.83i | 1 M KOH | 15 |
| Co-Ni3S2 | 1.87j | 1 M NaOH | 58 |
| Bifunctional electrocatalysts for the HER and OER | Chr amp | Chr pot | Dur (h) | Remark after the stability test | Ref. |
|---|---|---|---|---|---|
| a Ref.: references; Dur: duration of the stability test; Chr pot: chronopotentiometry; Chr amp: chronoamperometry; NA: not applicable; CD: current density; c-NiFe/a-NiFeOOH@NiMo: crystalline-NiFe/amorphous-NiFeOOH@NiMo; CNTs: carbon nanotubes; LDH: layered double hydroxide; c-CoMnP/a-CoMn LDH: crystalline CoMnP decorated amorphous CoMn LDH; c-CoFeP/a-CoFe LDH: crystalline CoFeP decorated amorphous CoFe LDH; VSB/NiPO: nickel phosphate microprism, where the microprism is mainly composed of Versailles-Santa Barbara-5, which is a kind of nickel phosphate molecular sieve having the chemical formula of Ni20[(OH)12(H2O)6][(HPO4)8(PO4)4]·12H2O; MH-TMO: mesoporous and heterostructured transition metal oxides; hea-d48h: it is a high-entropy alloy (hea), which is de-alloyed for 48 h, where the Ni30Co30Cr10Fe10Al18W2 high-entropy alloy comprises FCC and ordered aluminum-enriched BCC phases, while three dimensional porous architecture is constructed by de-alloying (selective etching of the BCC phase) the high-entropy alloy for 48 h; SAEHER-ECT-NiSi: NiSi is subjected to ECT followed by SAEHER, where ECT is an electrochemical tuning process comprising lithiation and rapid delithiation of NiSi, while SAEHER is a self-adaptive evolution that involves conducting multiple linear sweep voltammetry on ECT-NiSi under HER conditions; SAEOER-ECT-NiSi: NiSi is subjected to ECT followed by SAEOER, where ECT is an electrochemical tuning process comprising lithiation and rapid delithiation of NiSi, while SAEOER is a self-adaptive evolution that involves conducting multiple linear sweep voltammetry on ECT-NiSi under OER conditions; N-CNFs: N-doped carbon nanofibers; act-Ni-CoP/Co2P@NC: activated Ni-CoP/Co2P@NC; M-Co-CN: it was synthesized by the heat treatment of modified-ZIF-67 followed by acid treatment, where ZIF-67 represents the Co-based zeolitic imidazolate framework; act-modified-ZIF-67: activated modified Co-based zeolitic imidazolate framework; Gr: graphene; nb: non-bifunctional electrocatalyst. | |||||
| nbOR-NiOOHOER//HR-NiMoO@NiHER | NA | Yes | 500 | Reasonable stability at a CD of 1000 A m−2 | 38 |
| NiFeLa LDH/v-MXene | NA | Yes | 400 | Negligible decay at a CD of 100 mA cm−2 | 57 |
| nbact-Ni-CoP/Co2P@NCOER//Ni-CoP/Co2P@NCHER | Yes | NA | 400 | Negligible decay at a potential of 1.6 V | 97 |
| Ni/MoO2@CN | NA | Yes | 300 | Negligible decay at a CD of 1000 mA cm−2 | 15 |
| Cu3P-Cu2O/NPC | Yes | NA | 168 | 98.19% retention at a potential of 1.7 V | 100 |
| NixSy@MnOxHy | NA | Yes | 100 | Negligible decay at a CD of 100 mA cm−2 | 68 |
| c-NiFe/a-NiFeOOH@NiMo | NA | Yes | 100 | 95.86% retention at a CD of 100 mA cm−2 | 11 |
| nbFe-VSB/NiPO-500OER//VSB/NiPOHER | NA | Yes | 100 | 96.44% retention at a CD of 100 mA cm−2 | 105 |
| MH-TMO | NA | Yes | 100 | Negligible decay at a CD of 10 mA cm−2 | 1g |
| Hea-d48h | Yes | NA | 100 | 99.13% retention | 125 |
| Ni/CeO2@N-CNFs | Yes | NA | 100 | Negligible decay | 42 |
| WCoSe/WCo3O4 | NA | Yes | 100 | Reasonable stability at a CD of 100 mA cm−2 | 78 |
| nbact-modified-ZIF-67OER//M-Co-CNHER | NA | Yes | 100 | 93.7% retention at a CD of 20 mA cm−2 | 114 |
| Fe,Ce-NixSy | NA | Yes | 90 | Reasonable stability at a CD of 20 mA cm−2 | 61 |
| nbc-CoFeP/a-CoFe LDHOER//c-CoMnP/a-CoMn LDHHER | NA | Yes | 85 | 29.1 mV decay at a CD of 100 mA cm−2 | 56 |
| Zn-Fe/Mn@Mn-FeP | NA | Yes | 80 | Reasonable stability at a CD of 50 mA cm−2 | 28 |
| P-CoMoO4 | NA | Yes | 72 | Negligible decay at a CD of 50 mA cm−2 | 40 |
| Sn-Ni3S2 | Yes | NA | ∼70 | Negligible decay at a potential of 2.26 V | 14 |
| Gr-CNTs-Sn4P3 | Yes | NA | 65 | 96% retention at a potential of 1.482 V | 12 |
| Fe2P/Co@NPC | NA | Yes | 60 | Reasonable stability at a CD of 10 mA cm−2 | 6e |
| nbSAEOER-ECT-NiSiOER//SAEHER-ECT-NiSiHER | Yes | NA | 55 | Negligible decay at a potential of 1.75 V | 132 |
| NiS/MoS2 | Yes | NA | 50 | 99.2% retention | 64 |
| NiP2/NbP@CNTs | Yes | NA | 50 | Negligible decay | 96 |
| Co2N0.67/CoMoO4 | NA | Yes | 50 | Reasonable stability at a CD of 500 mA cm−2 | 20 |
| Mn1Ni1Co1-P | Yes | NA | 50 | 96% retention at a potential of 1.48 V | 10 |
| Ni3S2/FeNi2S4 | Yes | NA | 50 | Negligible decay at a potential of 2 V | 66 |
| Fe-CoP | NA | Yes | 50 | Negligible decay at a CD of 10 mA cm−2 | 85 |
| Bifunctional electrocatalysts for the HER and OER | Chr amp | Chr pot | Dur (h) | Remark after the stability test | Ref. |
|---|---|---|---|---|---|
| a nb: non-bifunctional electrocatalyst; Ref.: references; Dur: duration of the stability test; Chr pot: chronopotentiometry; Chr amp: chronoamperometry; NA: not applicable; CD: current density; LDH: layered double hydroxide; e-FeCoNiCu-P: electrochemically etched FeCoNiCu-P; LSC/LSCO: La0.5Sr0.5CoO3−δ; N-GQDs: N-doped graphene quantum dots. | |||||
| 1T Co-WS2/NiTe2/Ni | Yes | NA | 48 | 94% retention | 73 |
| nbCuNCo3OER//CoN0.73Co3HER | Yes | NA | 48 | 90% retention | 107 |
| Co/Mo2C@C | Yes | NA | 48 | 90.2% retention at a potential of 1.59 V | 118 |
| Ni3Fe0.75V0.25/Ni3Fe0.75V0.25N | Yes | NA | 40 | Reasonable stability at a potential of 2 V | 129 |
| CoP-NC@NiFeP | NA | Yes | 40 | Reasonable stability at a CD of 50 mA cm−2 | 98 |
| Fe2P-CoP/CeO2 | NA | Yes | 40 | Reasonable stability at a CD of 500 mA cm−2 | 101 |
| Ni2P-Fe2P | NA | Yes | >40 | Reasonable stability at a CD of 500 mA cm−2 | 19 |
| FeP/Ni2P | NA | Yes | >40 | Negligible decay at a CD of 500 mA cm−2 | 18 |
| Ce@NiCo LDH | Yes | NA | 36 | Reasonable stability at a potential of 1.68 V | 55 |
| Co2P/Mo2C@NC | Yes | NA | 30 | Negligible decay | 121 |
| Fe@Co/Se2 | NA | Yes | 30 | Negligible decay at a CD of 10 mA cm−2 | 77 |
| Co-Ni3S2 | Yes | NA | 30 | Negligible decay at a potential of 1.58 V | 59 |
| Mn6-CoO | NA | Yes | 28 | Reasonable stability at a CD of 10 mA cm−2 | 31 |
| CoN@NC | NA | Yes | 25 | Reasonable stability at a CD of 10 mA cm−2 | 106 |
| Mo2C-CoO@NC | Yes | NA | >25 | Reasonable stability at a potential of 1.9 V | 120 |
| NiCoFe-P/C | Yes | NA | 24 | Reasonable stability | 93 |
| e-FeCoNiCu-P | NA | Yes | 24 | Negligible decay at a CD of 100 mA cm−2 | 89 |
| LSC-N-GQDs-MoSe2 | NA | Yes | 24 | Negligible decay at a CD of 500 mA cm−2 | 80 |
| nbS-NiFeP-10OER//S-NiFeP-20HER | Yes | NA | 24 | Reasonable stability at a potential of 1.5 V | 91 |
| Co-Ni3S21 M NaOH | NA | Yes | 24 | Negligible decay | 58 |
| nbFe/Mo2C-NCOER//Ni/Mo2C-NCHER | Yes | NA | 24 | Reasonable stability at a potential of 1.66 V | 117 |
| Co0.21Fe0.28(OH)F | NA | Yes | 20 | Negligible decay at a CD of 20 mA cm−2 | 130 |
| NiMoB hollow foam | NA | Yes | 20 | Reasonable stability at a CD of 5000 mA cm−2 | 8 |
| nbNiFe LDHOER//Ni2PHER | Yes | NA | >15 | Reasonable stability at a potential of 2.1 V | 82 |
| NiCoP | Yes | NA | 12 | 98% retention | 83 |
| Fe-NiS-NiS2 | Yes | NA | 12 | Reasonable stability at a potential of 1.59 V | 62 |
| Co-Mn-CO3-OH | NA | Yes | >12 | Negligible decay at a CD of 10 mA cm−2 | 48 |
| HER electrocatalysts | η at −1000 mA cm−2 (mV) | Electrolyte | Ref. |
|---|---|---|---|
| a HCNRs: hollow carbon nanoribbons. | |||
| MoS2/Mo2C | −220 | 1 M KOH | 21 |
| C-Ni1−xO/3D printed Ni | −245 | 1 M KOH | 16 |
| Fe-Co-CO3-OH | −256 | 1 M KOH | 17 |
| Ni/MoO2@CN | −267 | 1 M KOH | 15 |
| FeP/Ni2P | ∼−270 | 1 M KOH | 18 |
| Ni2(1−x)Mo2xP | −294 | 1 M KOH | 86 |
| Ni2P | −306 | 1 M KOH | 82 |
| HR-NiMoO@Ni | −308 | 1 M KOH | 38 |
| Mo5N6-MoS2-HCNRs | −315 | 1 M KOH | 108 |
| Co2N0.67/CoMoO4 | −315 | 1 M KOH | 20 |
| Ni2P-Fe2P | −333 | 1 M KOH | 19 |
| NiP2-FeP2@Cu nanoarray | −357 | 1 M KOH | 13 |
| NiMoB hollow foam | ∼−420 | 1 M KOH | 8 |
| Sn-Ni3S2 | −570 | 1 M KOH | 14 |
| Co-Ni3S2 | −850 | 1 M KOH | 59 |
| OER electrocatalysts | η at 1000 mA cm−2 (mV) | Electrolyte | Ref. |
|---|---|---|---|
| a η: overpotential; KT-Ni(0)@Ni(II)-TPA: a terephthalic acid (TPA)-regulated etching strategy is used for the preparation of a karst topography (KT) featured electrode containing core–shell structured Ni(0)@Ni(II)-TPA. b 380 mV at 1500 mA cm−2. | |||
| FeP/Ni2P | 293 | 1 M KOH | 18 |
| Fe-Co-CO3-OH | 308 | 1 M KOH | 17 |
| Ni2P-Fe2P | 337 | 1 M KOH | 19 |
| OR-NiOOH | 358 | 1 M KOH | 38 |
| KT-Ni(0)@Ni(II)-TPA | 380b | 1 M KOH | 112 |
| Ni/MoO2@CN | 420 | 1 M KOH | 15 |
| C-Ni1−xO/3D printed Ni | 425 | 1 M KOH | 16 |
| Fe-CoP | 428 | 1 M KOH | 85 |
| NiMoB hollow foam | ∼460 | 1 M KOH | 8 |
| Co-Mn-CO3-OH | 462 | 1 M KOH | 48 |
| (Ni-Fe)Sx/NiFe(OH)y | ∼510 | 1 M KOH | 69 |
| CuS-Ni3S2/CuNi | 510 | 1 M KOH | 22 |
| Sn-Ni3S2 | 580 | 1 M KOH | 14 |
| Co-Ni3S2 | 750 | 1 M KOH | 59 |
| Ce-NiFe | ∼770 | 1 M KOH | 47 |
| Bifunctional electrocatalysts for the HER and OER | Overall water splitting (V at 1000 mA cm−2) | Electrolyte | Ref. |
|---|---|---|---|
| a nb: non-bifunctional electrocatalyst. | |||
| FeP/Ni2P | ∼1.78 | 1 M KOH | 18 |
| Ni2P-Fe2P | ∼1.98 | 1 M KOH | 19 |
| Co2N0.67/CoMoO4 | 1.98 | 1 M KOH | 20 |
| Ni/MoO2@CN | 2.02 | 1 M KOH | 15 |
| nbNiFe LDHOER//Ni2PHER | ∼2.05 | 1 M KOH | 82 |
| Sn-Ni3S2 | ∼2.65 | 1 M KOH | 14 |
| NiMoB hollow foam | ∼3.8 | 1 M KOH | 8 |
3 Summary and outlook
One of the promising approaches for generating hydrogen is electrochemical water splitting. At the same time, efficient water-splitting can be achieved by developing noble metal-free electrocatalysts for the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER). The activity, stability, and durability of several kinds of recently reported noble metal-free electrocatalysts such as oxides/hydroxides/(oxy)hydroxides/layered double hydroxides, sulfides, selenides, phosphides/phosphates, nitrides, carbon-based electrocatalysts, and alloy/B/V/F/Si based electrocatalysts for the HER and OER in an alkaline environment have been reviewed in this paper. In addition, this paper reviews the strategies used to achieve high activity and stability/durability, including at a high current density of ≥1000 mA cm−2 of noble metal-free electrocatalysts for the HER and OER in an alkaline environment.The following several promising strategies have been used for the noble metal-free electrocatalysts to attain enhanced performance for the HER and/or OER in alkaline environments (Fig. 9):
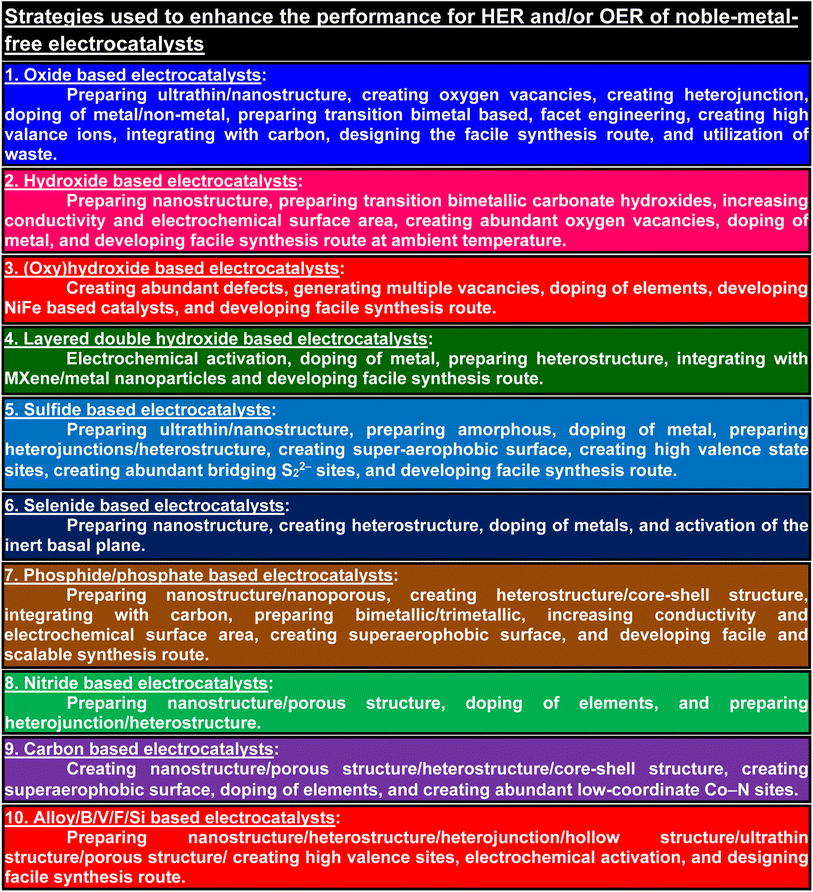 | ||
| Fig. 9 Strategies used to enhance the performance for the HER and/or OER of noble metal-free electrocatalysts. | ||
(a) Fabricating oxide based noble metal-free electrocatalysts enhanced the performance for the HER and OER, while various strategies including preparing ultrathin/nanostructures, creating oxygen vacancies, creating heterojunctions, doping of metals/non-metals, preparing transition bimetallic oxide based catalysts, facet engineering, creating high valence ions, integrating with carbon, designing the facile synthesis route, and utilization of waste enhanced the performance.1g,15-16,27,29-42
(b) Fabricating hydroxide based noble metal-free electrocatalysts enhanced the performance for the HER and OER, while various strategies including preparing nanostructures, preparing transition bimetallic carbonate hydroxides, increasing the conductivity and electrochemical surface area, creating abundant oxygen vacancies, doping of metals, and developing facile synthesis routes at ambient temperature enhanced the performance.17,26,43–48
(c) Fabricating (oxy)hydroxide based noble metal-free electrocatalysts enhanced the performance for the HER and OER, while various strategies including creating abundant defects, generating multiple vacancies, doping of elements, developing NiFe based catalysts, and developing facile synthesis routes enhanced the performance.6a,11,24,49-50
(d) Fabricating layered double hydroxide based noble metal-free electrocatalysts enhanced the performance for the HER and OER, while various strategies including electrochemical activation, doping of metals, preparing heterostructures, integrating with MXene/metal nanoparticles and developing facile synthesis routes enhanced the performance.25,51–57
(e) Fabricating sulfide based noble metal-free electrocatalysts enhanced the performance for the HER and OER, while various strategies including preparing ultrathin/nanostructures, preparing amorphous sulfide based catalysts, doping of metals, preparing heterojunctions/heterostructures, creating super-aerophobic surfaces, creating high valence state sites, creating abundant bridging S22− sites, and developing facile synthesis routes enhanced the performance.14,22,58–75
(f) Fabricating selenide based noble metal-free electrocatalysts enhanced the performance for the HER and OER, while various strategies including preparing nanostructures, creating heterostructures, doping of metals, and activation of the inert basal plane enhanced the performance.9,76–81
(g) Fabricating phosphide/phosphate based noble metal-free electrocatalysts enhanced the performance for the HER and OER, while various strategies including preparing nanostructures/nanoporous, creating heterostructures/core–shell structures, integrating with carbon, preparing bimetallic/trimetallic, increasing the conductivity and electrochemical surface area, creating superaerophobic surfaces, and developing facile and scalable synthesis routes enhanced the performance.6e,10,13,18-19,28,82-93,95-105
(h) Fabricating nitride based noble metal-free electrocatalysts enhanced the performance for the HER and OER, while various strategies including preparing nanostructures/porous structures, doping of elements, and preparing heterojunctions/heterostructures enhanced the performance.20,106–110
(i) Fabricating carbon based noble metal-free electrocatalysts enhanced the performance for the HER and OER, while various strategies including creating nanostructures/porous structures/heterostructures/core–shell structures, creating superaerophobic surfaces, doping of elements, and creating abundant low-coordinate Co–N sites enhanced the performance.21,111–121
(j) Fabricating alloy/B/V/F/Si based noble metal-free electrocatalysts enhanced the performance for the HER and OER, while various strategies including preparing nanostructures/heterostructures/heterojunctions/hollow structures/ultrathin structures/porous structures/creating high valence sites, electrochemical activation, and designing facile synthesis routes enhanced the performance.8,122–132
The stage has been developed for the noble metal-free electrocatalysts for the HER and OER to afford superior performance in practical applications. Hence, the vital factors governing the performance of noble metal-free electrocatalysts should be considered in future research: (1) recently, some green chemistry approaches, including ambient temperature synthesis,24–26 utilization of waste as a source,27 and using relatively low-cost metals,28 have been used for the fabrication of noble metal-free electrocatalysts for electrochemical water splitting. However, efficient green chemistry approaches for fabricating efficient noble metal-free electrocatalysts for the HER and OER are considerably limited. Hence, more progress is needed to construct efficient noble metal-free electrocatalysts for the HER and OER using various green chemistry approaches.
(2) Noble metal-free bifunctional electrocatalysts for overall water splitting exhibit low cell voltage at much higher current densities with high stability, which are substantially minimal. Therefore, additional progress is obviously needed to fulfill the requirement of industrial water electrolysis.
Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors acknowledge financial support from the Research Grants Council of Hong Kong (Grant nos. CityU 21203518 and F-CityU106/18), Innovation and Technology Commission (Grant no. MHP/104/21), Shenzhen Science Technology and Innovation Commission (Grant nos. JCYJ20210324125612035, R-IND12303, and R-IND12304), City University of Hong Kong (Grant no. 7005289, 7005580, 7005720, 9667213, 9667229, 9680331 and 9678291). The author M. I. J. thanks Dr Luo Yang, Dr Huang Chao, and Dr Wang Bin from the City University of Hong Kong. The author M.I.J. thanks Prof. Penghui Li, Shenzhen Institutes of Advanced Technology. DQH is thankful for the financial support from the Hong Kong SAR Government for the Postdoctoral Hub Program of the Innovation and Technology Fund (Project No. 9446002) under the STEM Professorship scheme.References
-
(a) H.-Y. Wang, M.-L. Sun, J.-T. Ren and Z.-Y. Yuan, Adv. Energy Mater., 2023, 13, 2203568 CrossRef CAS
; (b) I. Roger, M. A. Shipman and M. D. Symes, Nat. Rev. Chem, 2017, 1, 0003 CrossRef CAS
; (c) J. Guo, Y. Zheng, Z. Hu, C. Zheng, J. Mao, K. Du, M. Jaroniec, S.-Z. Qiao and T. Ling, Nat. Energy, 2023, 8, 264–272 CrossRef CAS
; (d) W. Tong, M. Forster, F. Dionigi, S. Dresp, R. Sadeghi Erami, P. Strasser, A. J. Cowan and P. Farràs, Nat. Energy, 2020, 5, 367–377 CrossRef CAS
; (e) Y. Tang, C. H. Mak, J. Zhang, G. Jia, K.-C. Cheng, H. Song, M. Yuan, S. Zhao, J.-J. Kai, J. C. Colmenares and H.-Y. Hsu, Adv. Mater., 2023, 35, 2207835 CrossRef CAS PubMed
; (f) Y. Tang, C. H. Mak, R. Liu, Z. Wang, L. Ji, H. Song, C. Tan, F. Barrière and H.-Y. Hsu, Adv. Funct. Mater., 2020, 30, 2006919 CrossRef CAS
; (g) F. Hu, D. Yu, M. Ye, H. Wang, Y. Hao, L. Wang, L. Li, X. Han and S. Peng, Adv. Energy Mater., 2022, 12, 2200067 CrossRef CAS
; (h) L. Yuan, C. Han, M.-Q. Yang and Y.-J. Xu, Int. Rev. Phys. Chem., 2016, 35, 1–36 Search PubMed
.
-
(a) J. A. Turner, Science, 2004, 305, 972–974 CrossRef CAS PubMed
; (b) M. I. Jamesh, J. Power Sources, 2016, 333, 213–236 CrossRef CAS
; (c) M.-I. Jamesh and X. Sun, J. Power Sources, 2018, 400, 31–68 CrossRef CAS
; (d) J. Luo, J.-H. Im, M. T. Mayer, M. Schreier, M. K. Nazeeruddin, N.-G. Park, S. D. Tilley, H. J. Fan and M. Grätzel, Science, 2014, 345, 1593–1596 CrossRef CAS PubMed
.
- C. C. L. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2015, 137, 4347–4357 CrossRef CAS PubMed
.
- L. C. Seitz, C. F. Dickens, K. Nishio, Y. Hikita, J. Montoya, A. Doyle, C. Kirk, A. Vojvodic, H. Y. Hwang, J. K. Norskov and T. F. Jaramillo, Science, 2016, 353, 1011–1014 CrossRef CAS PubMed
.
- M. I. Jamesh and M. Harb, J. Energy Chem., 2021, 56, 299–342 CrossRef CAS
.
-
(a) Z. Wei, M. Guo and Q. Zhang, Appl. Catal., B, 2023, 322, 122101 CrossRef CAS
; (b) J. Sun, Z. Zhang and X. Meng, Appl. Catal., B, 2023, 331, 122703 CrossRef CAS
; (c) X. Kang, F. Yang, Z. Zhang, H. Liu, S. Ge, S. Hu, S. Li, Y. Luo, Q. Yu, Z. Liu, Q. Wang, W. Ren, C. Sun, H.-M. Cheng and B. Liu, Nat. Commun., 2023, 14, 3607 CrossRef CAS PubMed
; (d) C. Huang, Q. Zhou, L. Yu, D. Duan, T. Cao, S. Qiu, Z. Wang, J. Guo, Y. Xie, L. Li and Y. Yu, Adv. Energy Mater., 2023, 13, 2301475 CrossRef CAS
; (e) Y. Bai, Y. Wang, Z. Qiao, Y. Yang, L. Deng, C. Li, X. Chen, S. Wang, Y. Huang, X. Zhang and D. Cao, J. Mater. Chem. A, 2022, 10, 16037–16045 RSC
.
-
(a) M.-I. Jamesh and M. Harb, Mater. Sci. Energy Technol., 2020, 3, 780–807 Search PubMed
; (b) M. I. Jamesh, J. Power Sources, 2020, 448, 227375 CrossRef
; (c) M.-I. Jamesh and X. Sun, J. Energy Chem., 2019, 34, 111–160 CrossRef
; (d) M.-I. Jamesh, Y. Kuang and X. Sun, ChemCatChem, 2019, 11, 1550–1575 CrossRef CAS
; (e) T. T. T. Toan, D. M. Nguyen, A. Q. Dao, V. T. Le and Y. Vasseghian, Mol. Catal., 2023, 538, 113001 CrossRef CAS
; (f) S.-H. Li, M.-Y. Qi, Z.-R. Tang and Y.-J. Xu, Chem. Soc. Rev., 2021, 50, 7539–7586 RSC
; (g) K. Mensah-Darkwa, D. N. Ampong, E. A. Tsiwah, A. Kumar, M. A. Nartey, P. Aggrey, F. O. Agyemang and R. K. Gupta, Mol. Catal., 2023, 547, 113375 CrossRef CAS
; (h) R. Khosa, E. Pervaiz, U. Abdullah, M. Ali, U. Sohail and A. Shakoor, Mol. Catal., 2022, 528, 112514 CrossRef CAS
; (i) Y. Chen, J. Xu, M. Jiang, L. Wang, R. Ma, Y. Chen, Z.-H. Xie, P. Munroe, F. Hu, L. Li and S. Peng, Adv. Energy Mater., 2024, 14, 2303450 CrossRef CAS
; (j) Y. Ye, J. Xu, X. Li, Y. Jian, F. Xie, J. Chen, Y. Jin, X. Yu, M.-H. Lee, N. Wang, S. Sun and H. Meng, Adv. Mater., 2024, 2312618 CrossRef PubMed
; (k) H. Li, G. Yan, H. Zhao, P. C. Howlett, X. Wang and J. Fang, Adv. Mater., 2024, 2311272 CrossRef PubMed
; (l) G. Y. Jang, S. Kim, J. Choi, J. Park, S. An, J. Baek, Y. Li, T.-K. Liu, E. Kim, J. H. Lee, H. Wang, M. Kim, H.-S. Cho, X. Zheng, J. S. Yoo, K. Seo and J. H. Park, Adv. Energy Mater., 2024, 2303924 CrossRef CAS
; (m) H. He, Q. Zhang, Z. Wang, S. Pan, Y. Zhao and X. Zhang, Adv. Energy Mater., 2024, 2303713 CrossRef CAS
; (n) F. M. Yap, J. Y. Loh, S.-F. Ng and W.-J. Ong, Adv. Energy Mater., 2024, 2303614 CrossRef
; (o) H. Sun, B. Yao, Y. Han, L. Yang, Y. Zhao, S. Wang, C. Zhong, J. Chen, C.-P. Li and M. Du, Adv. Energy Mater., 2024, 2303563 CrossRef CAS
; (p) J. Huang, N. Hales, A. H. Clark, N. S. Yüzbasi, C. N. Borca, T. Huthwelker, T. J. Schmidt and E. Fabbri, Adv. Energy Mater., 2024, 2303529 CrossRef CAS
; (q) F. T. Haase, E. Ortega, S. Saddeler, F.-P. Schmidt, D. Cruz, F. Scholten, M. Rüscher, A. Martini, H. S. Jeon, A. Herzog, U. Hejral, E. M. Davis, J. Timoshenko, A. Knop-Gericke, T. Lunkenbein, S. Schulz, A. Bergmann and B. Roldan Cuenya, Energy Environ. Sci., 2024, 17, 2046–2058 RSC
; (r) Y.-N. Zhou, F.-T. Li, B. Dong and Y.-M. Chai, Energy Environ. Sci., 2024, 17, 1468–1481 RSC
; (s) C. Feng, Y. Zhou, M. Chen, L. Zou, X. Li, X. An, Q. Zhao, P. Xiaokaiti, A. Abudula, K. Yan and G. Guan, Appl. Catal. B: Environ. Energy, 2024, 349, 123875 CrossRef CAS
; (t) X. Xiao, Y. Wei, S. Song, B. McElhenny, F. Zhang, X. Jiang, Y. Zhang, S. Chen, M. Wang, Y. Shen and Z. Ren, Appl. Catal. B: Environ. Energy, 2024, 349, 123871 CrossRef CAS
; (u) F. Wang, X. Zhao, Y. Li, L. Liang, K. Sasaki, Q. Hao, W. Yuan, S. Li and H. Liu, Appl. Catal. B: Environ. Energy, 2024, 348, 123830 CrossRef CAS
; (v) J.-T. Ren, L. Chen, H.-Y. Wang, W.-W. Tian, S.-X. Zhai, Y. Feng and Z.-Y. Yuan, Appl. Catal. B: Environ. Energy, 2024, 347, 123817 CrossRef CAS
; (w) Z. Zhao, Z. Li, Z. Zhang and X. Meng, Appl. Catal. B: Environ. Energy, 2024, 347, 123805 CrossRef CAS
; (x) Z. Zang, Y. Ren, C. Fan, Y. Cheng, L. Li, X. Yu, X. Yang, Z. Lu, X. Zhang and H. Liu, Appl. Catal. B: Environ. Energy, 2024, 123912 CrossRef CAS
.
- H. Liu, X. Li, L. Chen, X. Zhu, P. Dong, M. O. L. Chee, M. Ye, Y. Guo and J. Shen, Adv. Funct. Mater., 2022, 32, 2107308 CrossRef CAS
.
- S.-W. Kim, J.-K. Kim, H. J. Kim, C. T. Cao, N. Khen Oh, Y. Yang, H.-C. Song, M. Shim, H. S. Park and J. M. Baik, Nano Energy, 2022, 95, 107023 CrossRef CAS
.
- K. E. Salem, A. A. Saleh, G. E. Khedr, B. S. Shaheen and N. K. Allam, Energy Environ. Mater., 2023, 6, e12324 CrossRef CAS
.
- Q. lv, B. Yao, W. Zhang, L. She, W. Ren, L. Hou, Y. Fautrelle, X. Lu, X. Yu and X. Li, Chem. Eng. J., 2022, 446, 137420 CrossRef CAS
.
- S. Riyajuddin, M. Pahuja, P. K. Sachdeva, K. Azmi, S. Kumar, M. Afshan, F. Ali, J. Sultana, T. Maruyama, C. Bera and K. Ghosh, ACS Nano, 2022, 16, 4861–4875 CrossRef CAS PubMed
.
- A. Kumar, V. Q. Bui, J. Lee, A. R. Jadhav, Y. Hwang, M. G. Kim, Y. Kawazoe and H. Lee, ACS Energy Lett., 2021, 6, 354–363 CrossRef CAS
.
- J. Jian, L. Yuan, H. Qi, X. Sun, L. Zhang, H. Li, H. Yuan and S. Feng, ACS Appl. Mater. Interfaces, 2018, 10, 40568–40576 CrossRef CAS PubMed
.
- G. Qian, J. Chen, T. Yu, J. Liu, L. Luo and S. Yin, Nano-Micro Lett., 2022, 14, 20 CrossRef CAS PubMed
.
- T. Kou, S. Wang, R. Shi, T. Zhang, S. Chiovoloni, J. Q. Lu, W. Chen, M. A. Worsley, B. C. Wood, S. E. Baker, E. B. Duoss, R. Wu, C. Zhu and Y. Li, Adv. Energy Mater., 2020, 10, 2002955 CrossRef CAS
.
- L. Hui, Y. Xue, D. Jia, H. Yu, C. Zhang and Y. Li, Adv. Energy Mater., 2018, 8, 1800175 CrossRef
.
- F. Yu, H. Zhou, Y. Huang, J. Sun, F. Qin, J. Bao, W. A. Goddard, S. Chen and Z. Ren, Nat. Commun., 2018, 9, 2551 CrossRef PubMed
.
- L. Wu, L. Yu, F. Zhang, B. McElhenny, D. Luo, A. Karim, S. Chen and Z. Ren, Adv. Funct. Mater., 2021, 31, 2006484 CrossRef CAS
.
- Y. Hu, Z. Luo, M. Guo, J. Dong, P. Yan, C. Hu, T. T. Isimjan and X. Yang, Chem. Eng. J., 2022, 435, 134795 CrossRef CAS
.
- Y. Luo, L. Tang, U. Khan, Q. Yu, H.-M. Cheng, X. Zou and B. Liu, Nat. Commun., 2019, 10, 269 CrossRef PubMed
.
- N. Zhang, Y. Gao, Y. Mei, J. Liu, W. Song and Y. Yu, Inorg. Chem. Front., 2019, 6, 293–302 RSC
.
- M.-I. Jamesh, A. Akila, D. Sudha, K. Gnana Priya, V. Sivaprakash and A. Revathi, Sustainability, 2022, 14, 16359 CrossRef CAS
.
- X. Chen, Q. Wang, Y. Cheng, H. Xing, J. Li, X. Zhu, L. Ma, Y. Li and D. Liu, Adv. Funct. Mater., 2022, 32, 2112674 CrossRef CAS
.
- W. Zhao, H. Xu, H. Luan, N. Chen, P. Gong, K. Yao, Y. Shen and Y. Shao, Adv. Energy Mater., 2022, 12, 2102372 CrossRef CAS
.
- S. Liu, G. Han, J. Zhang, H. Wang and X. Huang, Chem. Eng. J., 2022, 428, 130688 CrossRef CAS
.
- K. Lu, Z. Wang, Y. Wu, X. Zhai, C. Wang, J. Li, Z. Wang, X. Li, Y. He, T. An, K. Yang, D. Yang, F. Yu and B. Dai, Chem. Eng. J., 2023, 451, 138590 CrossRef CAS
.
- L. Huang, R. Yao, X. Wang, S. Sun, X. Zhu, X. Liu, M. G. Kim, J. Lian, F. Liu, Y. Li, H. Zong, S. Han and X. Ding, Energy Environ. Sci., 2022, 15, 2425–2434 RSC
.
- C. Chen, Z. Zhou, J. Liu, B. Zhu, H. Hu, Y. Yang, G. Chen, M. Gao and J. Zhang, Appl. Catal., B, 2022, 307, 121209 CrossRef CAS
.
- Y. Guo, X. Liu, Y. Zang, Y. Wu, Q. Zhang, Z. Wang, Y. Liu, Z. Zheng, H. Cheng, B. Huang, Y. Dai and P. Wang, J. Mater. Chem. A, 2022, 10, 17297–17306 RSC
.
- H. Xue, A. Meng, T. Yang, Z. Li and C. Chen, J. Energy Chem., 2022, 71, 639–651 CrossRef CAS
.
- B. J. Rani, A. Sivanantham, T. S. Shridharan, T. Runfa and I. S. Cho, J. Mater. Chem. A, 2022, 10, 17710–17720 RSC
.
- Z. Wu, J. Mei, Q. Liu, S. Wang, W. Li, S. Xing, J. Bai, J. Yang, W. Luo, O. Guselnikova, A. P. O'Mullane, Y. Gu, Y. Yamauchi, T. Liao and Z. Sun, J. Mater. Chem. A, 2022, 10, 808–817 RSC
.
- A. Malek, Y. Xue and X. Lu, Angew. Chem., Int. Ed., 2023, 62, e202309854 CrossRef CAS PubMed
.
- X. Yu, C. Hu, P. Ji, Y. Ren, H. Zhao, G. Liu, R. Xu, X. Zhu, Z. Li, Y. Ma and L. Ma, Appl. Catal., B, 2022, 310, 121301 CrossRef CAS
.
- Y.-C. Zhang, C. Han, J. Gao, J. Wu, X.-D. Zhu and J.-J. Zou, Chem. Eng. J., 2022, 446, 137036 CrossRef
.
- K. R. Nandanapalli, D. Mudusu, R. Karuppannan, Y.-B. Hahn and S. Lee, Chem. Eng. J., 2022, 429, 132360 CrossRef CAS
.
- J. Liu, W. Qiao, Z. Zhu, J. Hu and X. Xu, Small, 2022, 18, 2202434 CrossRef CAS PubMed
.
- H. Peng, K. Zhou, Y. Jin, Q. Zhang, J. Liu and H. Wang, Chem. Eng. J., 2022, 429, 132477 CrossRef CAS
.
- J. Wang, J. Hu, C. Liang, L. Chang, Y. Du, X. Han, J. Sun and P. Xu, Chem. Eng. J., 2022, 446, 137094 CrossRef CAS
.
- L. Wang, Y. Pan, D. Wu, X. Liu, L. Cao, W. Zhang, H. Chen, T. Liu, D. Liu, T. Chen, T. Ding, Y. Wang, C. Ding, C. Kang, C. Li, J. He and T. Yao, J. Mater. Chem. A, 2022, 10, 20011–20017 RSC
.
- T. Li, J. Yin, D. Sun, M. Zhang, H. Pang, L. Xu, Y. Zhang, J. Yang, Y. Tang and J. Xue, Small, 2022, 18, 2106592 CrossRef CAS PubMed
.
- S. Tao, Q. Wen, W. Jaegermann and B. Kaiser, ACS Catal., 2022, 12, 1508–1519 CrossRef CAS
.
- Z. Yin, R. He, Y. Zhang, L. Feng, X. Wu, T. Wågberg and G. Hu, J. Energy Chem., 2022, 69, 585–592 CrossRef CAS
.
- Y. Zhuo, D. Liu, L. Qiao, S. Chen, J. Lu, W. F. IP, H. Pan and Z. Wang, Adv. Energy Mater., 2023, 13, 2301921 CrossRef CAS
.
- X. Li, M. Hou, X. Qu, Y. Zhang and M. Li, Small, 2022, 18, 2104863 CrossRef CAS PubMed
.
- J. Liu, Y. Liu, X. Mu, H. Jang, Z. Lei, S. Jiao, P. Yan, M. G. Kim and R. Cao, Adv. Funct. Mater., 2022, 32, 2204086 CrossRef CAS
.
- T. Tang, W.-J. Jiang, S. Niu, N. Liu, H. Luo, Y.-Y. Chen, S.-F. Jin, F. Gao, L.-J. Wan and J.-S. Hu, J. Am. Chem. Soc., 2017, 139, 8320–8328 CrossRef CAS PubMed
.
- Y. Hu, J. Zhou, L. Li, Z. Hu, T. Yuan, C. Jing, R. Liu, S. Xi, H. Jiang, J.-Q. Wang and L. Zhang, J. Mater. Chem. A, 2022, 10, 602–610 RSC
.
- S. Hou, L. Xu, X. Ding, R. M. Kluge, T. K. Sarpey, R. W. Haid, B. Garlyyev, S. Mukherjee, J. Warnan, M. Koch, S. Zhang, W. Li, A. S. Bandarenka and R. A. Fischer, Angew. Chem., Int. Ed., 2022, 61, e202201610 CrossRef CAS PubMed
.
- I. B. Pehlivan, N. A. Saguì, J. Oscarsson, Z. Qiu, W. Zwaygardt, M. Lee, M. Mueller, S. Haas, L. Stolt, M. Edoff and T. Edvinsson, J. Mater. Chem. A, 2022, 10, 12079–12091 RSC
.
- N. An, H. Tian, Y. Zhou, Y. Zou, H. Xiu, Y. Cao, Y. Wang, J. Li, D. Liu and Y. Kuang, J. Energy Chem., 2022, 66, 657–665 CrossRef CAS
.
- Y. Li, Y. Wu, M. Yuan, H. Hao, Z. Lv, L. Xu and B. Wei, Appl. Catal., B, 2022, 318, 121825 CrossRef CAS
.
- L. Wu, J. Zhang, S. Wang, Q. Jiang, R. Feng, S. Ju, W. Zhang and F. Song, Chem. Eng. J., 2022, 442, 136168 CrossRef CAS
.
- H. N. Dhandapani, D. Mahendiran, A. Karmakar, P. Devi, S. Nagappan, R. Madhu, K. Bera, P. Murugan, B. R. Babu and S. Kundu, J. Mater. Chem. A, 2022, 10, 17488–17500 RSC
.
- K. Zheng, J. Ren, X. Li, G. Li, L. Jiao and C. Xu, Chem. Eng. J., 2022, 441, 136031 CrossRef CAS
.
- M. Yu, J. Zheng and M. Guo, J. Energy Chem., 2022, 70, 472–479 CrossRef CAS
.
- Y. Li, Y. Duan, K. Zhang and W. Yu, Chem. Eng. J., 2022, 433, 134472 CrossRef CAS
.
- J. Jian, L. Yuan, H. Li, H. Liu, X. Zhang, X. Sun, H. Yuan and S. Feng, Chem. Res. Chin. Univ., 2019, 35, 179–185 CrossRef CAS
.
- X. Mao, Y. Liu, Z. Chen, Y. Fan and P. Shen, Chem. Eng. J., 2022, 427, 130742 CrossRef CAS
.
- X. Zhang, Y. Qiu, Q. Li, X. Ji and J. Liu, J. Power Sources, 2022, 522, 231004 CrossRef CAS
.
- S. Huang, Q. Zhang, P. Xin, J. Zhang, Q. Chen, J. Fu, Z. Jin, Q. Wang and Z. Hu, Small, 2022, 18, 2106841 CrossRef CAS PubMed
.
- S. L. Fereja, P. Li, Z. Zhang, J. Guo, Z. Fang, Z. Li, S. He and W. Chen, Chem. Eng. J., 2022, 432, 134274 CrossRef
.
- C. Gu, G. Zhou, J. Yang, H. Pang, M. Zhang, Q. Zhao, X. Gu, S. Tian, J. Zhang, L. Xu and Y. Tang, Chem. Eng. J., 2022, 443, 136321 CrossRef CAS
.
- S. Yang, Y. Guo, Y. Zhao, L. Zhang, H. Shen, J. Wang, J. Li, C. Wu, W. Wang, Y. Cao, S. Zhuo, Q. Zhang and H. Zhang, Small, 2022, 18, 2201306 CrossRef CAS PubMed
.
- Y. Wu, Y. Li, M. Yuan, H. Hao, X. San, Z. Lv, L. Xu and B. Wei, Chem. Eng. J., 2022, 427, 131944 CrossRef CAS
.
- M. Luo, S. Liu, W. Zhu, G. Ye, J. Wang and Z. He, Chem. Eng. J., 2022, 428, 131055 CrossRef CAS
.
- P. Wang, Y. Luo, G. Zhang, Z. Chen, H. Ranganathan, S. Sun and Z. Shi, Nano-Micro Lett., 2022, 14, 120 CrossRef CAS PubMed
.
- Q. Che, Q. Li, Y. Tan, X. Chen, X. Xu and Y. Chen, Appl. Catal., B, 2019, 246, 337–348 CrossRef CAS
.
- M. Lu, L. An, J. Yin, J. Jin, R. Yang, B. Huang, Y. Hu, Y.-Q. Zhao and P. Xi, J. Mater. Chem. A, 2022, 10, 19757–19768 RSC
.
- C. Tang, D. He, N. Zhang, X. Song, S. Jia, Z. Ke, J. Liu, J. Wang, C. Jiang, Z. Wang, X. Huang and X. Xiao, Energy Environ. Mater., 2022, 5, 899–905 CrossRef CAS
.
- H. Yang, P. Guo, R. Wang, Z. Chen, H. Xu, H. Pan, D. Sun, F. Fang and R. Wu, Adv. Mater., 2022, 34, 2107548 CrossRef CAS PubMed
.
- D. R. Paudel, U. N. Pan, R. B. Ghising, P. P. Dhakal, V. A. Dinh, H. Wang, N. H. Kim and J. H. Lee, Nano Energy, 2022, 102, 107712 CrossRef CAS
.
- S. Li, Y. Liu, K. Feng, C. Li, J. Xu, C. Lu, H. Lin, Y. Feng, D. Ma and J. Zhong, Angew. Chem., Int. Ed., 2023, 62, e202308670 CrossRef CAS PubMed
.
- S. Chandrasekaran, T. Ma, Z. Hu, Q. Liu, C. Zhan, Y. Li, C. Bowen, H. Lu and Y. Liu, Appl. Catal., B, 2023, 338, 123007 CrossRef CAS
.
- A. Majumdar, P. Dutta, A. Sikdar, H. Lee, D. Ghosh, S. N. Jha, S. Tripathi, Y. Oh and U. N. Maiti, Small, 2022, 18, 2200622 CrossRef CAS PubMed
.
- S. Ibraheem, G. Yasin, A. Kumar, M. A. Mushtaq, S. Ibrahim, R. Iqbal, M. Tabish, S. Ali and A. Saad, Appl. Catal., B, 2022, 304, 120987 CrossRef CAS
.
- R. Balaji, T. T. Nguyen, K. Harish, N. H. Kim and J. H. Lee, J. Mater. Chem. A, 2022, 10, 3782–3792 RSC
.
- M. H. Han, Y.-J. Ko, S. Y. Lee, C. Lim, W. H. Lee, M. W. Pin, J. H. Koh, J. Kim, W. Kim, B. K. Min and H.-S. Oh, J. Power Sources, 2022, 521, 230953 CrossRef CAS
.
- C. T. Cao, S.-W. Kim, H. J. Kim, R. Purbia, S. H. Kim, D. Kim, K. J. Choi, H. Park and J. M. Baik, Nano Energy, 2022, 96, 107117 CrossRef CAS
.
- L. Huang, G. Wei, J. Wang, D. Li, S. Jia, S. Wu, T. Jiang, Y. Guo, Y. Liu and F. Ren, Adv. Energy Mater., 2023, 13, 2300651 CrossRef CAS
.
- X. Yu, Z.-Y. Yu, X.-L. Zhang, Y.-R. Zheng, Y. Duan, Q. Gao, R. Wu, B. Sun, M.-R. Gao, G. Wang and S.-H. Yu, J. Am. Chem. Soc., 2019, 141, 7537–7543 CrossRef CAS PubMed
.
- L. Zhao, M. Wen, Y. Tian, Q. Wu and Y. Fu, J. Energy Chem., 2022, 74, 203–211 CrossRef CAS
.
- Y. Song, W. Xie, Y. Song, H. Li, S. Li, S. Jiang, J. Y. Lee and M. Shao, Appl. Catal., B, 2022, 312, 121400 CrossRef CAS
.
- L.-M. Cao, Y.-W. Hu, S.-F. Tang, A. Iljin, J.-W. Wang, Z.-M. Zhang and T.-B. Lu, Advanced Science, 2018, 5, 1800949 CrossRef PubMed
.
- L. Yu, I. K. Mishra, Y. Xie, H. Zhou, J. Sun, J. Zhou, Y. Ni, D. Luo, F. Yu, Y. Yu, S. Chen and Z. Ren, Nano Energy, 2018, 53, 492–500 CrossRef CAS
.
- W. Yuan, T. Jiang, X. Fang, Y. Fan, S. Qian, Y. Gao, N. Cheng, H. Xue and J. Tian, Chem. Eng. J., 2022, 439, 135743 CrossRef CAS
.
- B. K. Kim, M. J. Kim and J. J. Kim, Appl. Catal., B, 2022, 308, 121226 CrossRef CAS
.
- R. Feng, Z. Ye, Q. Jiang, C. Li, J. Gu and F. Song, J. Power Sources, 2022, 542, 231774 CrossRef CAS
.
- Y. Li, Y. Wu, H. Hao, M. Yuan, Z. Lv, L. Xu and B. Wei, Appl. Catal., B, 2022, 305, 121033 CrossRef CAS
.
- S. Li, L. Wang, H. Su, A. N. Hong, Y. Wang, H. Yang, L. Ge, W. Song, J. Liu, T. Ma, X. Bu and P. Feng, Adv. Funct. Mater., 2022, 32, 2200733 CrossRef CAS
.
- X. Zhang, G. Ma, L. Shui, G. Zhou and X. Wang, J. Energy Chem., 2022, 72, 88–96 CrossRef CAS
.
- R. Liu, X.-R. Shi, Y. Wen, X. Shao, C. Su, J. Hu and S. Xu, J. Energy Chem., 2022, 74, 149–158 CrossRef CAS
.
- M.-I. Jamesh, Lubricants, 2022, 10, 255 CrossRef CAS
.
- H. Xiang, W. Chen, T. Li, J. Huang, G. Chen, T. Gong and K. Ken Ostrikov, Chem. Eng. J., 2022, 446, 137419 CrossRef CAS
.
- S. Singh, D. C. Nguyen, N. H. Kim and J. H. Lee, Chem. Eng. J., 2022, 442, 136120 CrossRef CAS
.
- H. Chu, P. Feng, B. Jin, G. Ye, S. Cui, M. Zheng, G.-X. Zhang and M. Yang, Chem. Eng. J., 2022, 433, 133523 CrossRef CAS
.
- E. Vijayakumar, S. Ramakrishnan, C. Sathiskumar, D. J. Yoo, J. Balamurugan, H. S. Noh, D. Kwon, Y. H. Kim and H. Lee, Chem. Eng. J., 2022, 428, 131115 CrossRef CAS
.
- H. Liu, S. Yang, J. Ma, M. Dou and F. Wang, Nano Energy, 2022, 98, 107315 CrossRef CAS
.
- J. Zhu, E. Jiang, X. Wang, Z. Pan, X. Xu, S. Ma, P. Kang Shen, L. Pan, M. Eguchi, A. K. Nanjundan, J. Shapter and Y. Yamauchi, Chem. Eng. J., 2022, 427, 130946 CrossRef CAS
.
- X. Ding, J. Yu, W. Huang, D. Chen, W. Lin and Z. Xie, Chem. Eng. J., 2023, 451, 138550 CrossRef CAS
.
- Q. Peng, Q. He, Y. Hu, T. T. Isimjan, R. Hou and X. Yang, J. Energy Chem., 2022, 65, 574–582 CrossRef CAS
.
- M. Chen, H. Li, C. Wu, Y. Liang, J. Qi, J. Li, E. Shangguan, W. Zhang and R. Cao, Adv. Funct. Mater., 2022, 32, 2206407 CrossRef CAS
.
- J. Cheng, B. Shen, Y. Song, J. Liu, Q. Ye, M. Mao and Y. Cheng, Chem. Eng. J., 2022, 428, 131130 CrossRef CAS
.
- J. Zhao, Y. Zhang, H. Guo, H. Zhang, J. Ren and R. Song, Small, 2022, 18, 2200832 CrossRef CAS PubMed
.
- J. Song, D. Yu, X. Wu, D. Xie, Y. Sun, P. Vishniakov, F. Hu, L. Li, C. Li, M. Yu. Maximov, K. M. El-Khatib and S. Peng, Chem. Eng. J., 2022, 437, 135281 CrossRef CAS
.
- L. Zhu, C. Li, H. Li, H. Li, Z. Wu, Y. Huang, X. Zhu and Y. Sun, J. Mater. Chem. A, 2022, 10, 15520–15527 RSC
.
- C. Pi, X. Li, X. Zhang, H. Song, Y. Zheng, B. Gao, A. Kızılaslan, P. K. Chu and K. Huo, Small, 2022, 18, 2201137 CrossRef CAS PubMed
.
- P. Zhai, C. Wang, Y. Zhao, Y. Zhang, J. Gao, L. Sun and J. Hou, Nat. Commun., 2023, 14, 1873 CrossRef CAS PubMed
.
- S.-P. Zeng, H. Shi, T.-Y. Dai, Y. Liu, Z. Wen, G.-F. Han, T.-H. Wang, W. Zhang, X.-Y. Lang, W.-T. Zheng and Q. Jiang, Nat. Commun., 2023, 14, 1811 CrossRef CAS PubMed
.
- Y. Zhu, J. Zhang, Q. Qian, Y. Li, Z. Li, Y. Liu, C. Xiao, G. Zhang and Y. Xie, Angew. Chem., Int. Ed., 2022, 61, e202113082 CrossRef CAS PubMed
.
- Q. Hu, Z. Wang, X. Huang, Y. Qin, H. Yang, X. Ren, Q. Zhang, J. Liu, M. Shao and C. He, Appl. Catal., B, 2021, 286, 119920 CrossRef CAS
.
- Y. Xin, F. Wang, L. Chen, Y. Li and K. Shen, Green Chem., 2022, 24, 6544–6555 RSC
.
- Z. Lei, X. Jin, J. Li, Y. Liu, J. Liu, S. Jiao and R. Cao, J. Energy Chem., 2022, 65, 505–513 CrossRef CAS
.
- R. Ding, Y. Chen, X. Li, Z. Rui, K. Hua, Y. Wu, X. Duan, X. Wang, J. Li and J. Liu, Small, 2022, 18, 2105335 CrossRef CAS PubMed
.
- W. Liu, X. Wang, J. Qu, X. Liu, Z. Zhang, Y. Guo, H. Yin and D. Wang, Appl. Catal., B, 2022, 307, 121201 CrossRef CAS
.
- Y. Xu, J. Yang, T. Liao, R. Ge, Y. Liu, J. Zhang, Y. Li, M. Zhu, S. Li and W. Li, Chem. Eng. J., 2022, 431, 134126 CrossRef CAS
.
- S. Yuan, M. Xia, Z. Liu, K. Wang, L. Xiang, G. Huang, J. Zhang and N. Li, Chem. Eng. J., 2022, 430, 132697 CrossRef CAS
.
- J. Li, R. Ge, P. Lan, J. Yang, J. Feng, Y. Li, S. Li, B. Liu and W. Li, J. Mater. Chem. A, 2022, 10, 10493–10502 RSC
.
- T. Gong, J. Zhang, Y. Liu, L. Hou, J. Deng and C. Yuan, Chem. Eng. J., 2023, 451, 139025 CrossRef CAS
.
- P. Sun, Y. Zhou, H. Li, H. Zhang, L. Feng, Q. Cao, S. Liu, T. Wågberg and G. Hu, Appl. Catal., B, 2022, 310, 121354 CrossRef CAS
.
- X. Liu, P. Zou, L. Song, B. Zang, B. Yao, W. Xu, F. Li, J. Schroers, J. Huo and J.-Q. Wang, ACS Catal., 2022, 12, 3789–3796 CrossRef CAS
.
- H. Park, J. W. Bae, T. H. Lee, I. J. Park, C. Kim, M. G. Lee, S. A. Lee, J. W. Yang, M.-J. Choi, S. H. Hong, S. Y. Kim, S. H. Ahn, J. Y. Kim, H. S. Kim and H. W. Jang, Small, 2022, 18, 2105611 CrossRef CAS PubMed
.
- S. Wang, W. Huo, F. Fang, Z. Xie, J. K. Shang and J. Jiang, Chem. Eng. J., 2022, 429, 132410 CrossRef CAS
.
- X. Han, Q. Chen, Q. Chen, Q. Wu, Z. Xu, T. Zheng, W. Li, D. Cui, Z. Duan, J. Zhang, J. Li, H. Li, Z. Wang, J. Wang and Z. Xia, J. Mater. Chem. A, 2022, 10, 11110–11120 RSC
.
- H. Liao, G. Ni, P. Tan, Y. Liu, K. Chen, G. Wang, M. Liu and J. Pan, Appl. Catal., B, 2022, 317, 121713 CrossRef CAS
.
- B. Zhang, Z. Wu, W. Shao, Y. Gao, W. Wang, T. Ma, L. Ma, S. Li, C. Cheng and C. Zhao, Angew. Chem., Int. Ed., 2022, 61, e202115331 CrossRef CAS PubMed
.
- W. Shao, M. Xiao, C. Yang, M. Cheng, S. Cao, C. He, M. Zhou, T. Ma, C. Cheng and S. Li, Small, 2022, 18, 2105763 CrossRef CAS PubMed
.
- M. Lu, S. Kong, S. Yan, P. Zhou, T. Yu and Z. Zou, J. Mater. Chem. A, 2022, 10, 12391–12399 RSC
.
- Y. Li, X. Zhai, C. Fan, X. Chen, Y. Liu, J. Yang, L. Chen, G. Ge and J. Zhang, J. Mater. Chem. A, 2022, 10, 11774–11783 RSC
.
- M. Li, S. Wang, X. Wang, X. Tian, X. Wu, Y. Zhou, G. Hu and L. Feng, Chem. Eng. J., 2022, 442, 136165 CrossRef CAS
.
- W. J. Chang, E. S. Sim, J. Kwon, S. Jang, D. Y. Jeong, T. Song, N. Oh, H. W. Jang, Y.-C. Chung and W. I. Park, Chem. Eng. J., 2022, 434, 134668 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2024 |
