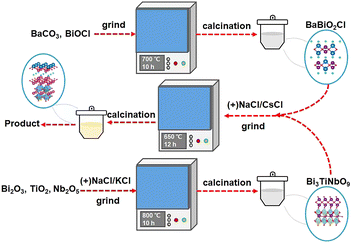
New insights into the synthesis of Sillén–Aurivillius oxyhalides: molten salts induce interlayer halogen competing reaction†
Yunxiang
Zhang
ab,
Chenliang
Zhou
a,
Shishi
Xu
a,
Hazem
Abdelsalam
 ac,
Zhichao
Mu
a,
Wei
Chen
a,
Zhili
Chen
a,
Xiangyu
Cheng
a,
Diab
Khalafallah
ad and
Qinfang
Zhang
ac,
Zhichao
Mu
a,
Wei
Chen
a,
Zhili
Chen
a,
Xiangyu
Cheng
a,
Diab
Khalafallah
ad and
Qinfang
Zhang
 *ab
*ab
aSchool of Materials Science and Engineering, Yancheng Institute of Technology, Yancheng 224051, China. E-mail: qfangzhang@gmail.com
bKey Laboratory for Ecological-Environment Materials of Jiangsu Province, Yancheng Institute of Technology, Yancheng, 224051, PR China
cTheoretical Physics Department, National Research Centre, El-Buhouth Str., Dokki, 12622, Giza, Egypt
dMechanical Design and Materials Department, Faculty of Energy Engineering, Aswan University, P. O. Box 81521, Aswan, Egypt
First published on 21st November 2023
Abstract
Numerous studies disclosed that molten salts provide a special liquid reaction environment, accelerate the diffusion rate of active elements, and promote the formation of high-purity materials in the molten-salt synthesis method. Herein, the interlayer halogen-competing reactions between molten salts and halogen layers during the formation of BaBi4TiNbO11X (X = Cl, Br) oxyhalides were discovered for the first time. The liquid environment provided by molten salts can facilitate the full migration of halogen atoms between molten salts and halogen layers and form an even distribution of halogen atoms, which is ascribed to the weak van der Waals forces of the halogen layers between [BaBiO2]+ and [Bi2O2]2+ blocks. By employing this mechanism, BaBi4TiNbO11(Cl1−xBrx) with different x contents could be easily obtained. DFT calculations showed that the mixed halogen oxyhalides, such as BaBi4TiNbO11(Cl0.5Br0.5), introduce new chemical bonds between the O and Bi atoms, which increase their contribution to lowest occupied/unoccupied orbitals, thus regulating the energy band and accelerating the separation and transfer of photogenerated charge carriers. Consequently, the optimized BaBi4TiNbO11(Cl0.5Br0.5) sample showed a superior degradation rate of RhB compared to the traditional P25 photocatalyst, and its O2 evolution performance was also enhanced compared with other blank samples.
1. Introduction
With the acceleration of modern industrialization and the increase in global energy consumption, the energy crisis and environmental pollution have become increasingly prominent, seriously endangering the survival and development of human beings.1–3 Thus, the photocatalytic technology, an emerging technology, that utilizes solar energy to drive chemical reactions, such as water splitting, hydrogen evolution, oxygen evolution, CO2 reduction, degradation of organic pollutants, and reduction of heavy metal ions, has attracted attention from researchers around the world due to its advantages of mild reaction conditions and clean and abundant energy sources.4–6 In the past few decades, the most widely studied catalyst is titanium dioxide (TiO2), which has the advantages of strong oxidation capacity, low toxicity, low cost, and easy synthesis.7,8 However, the band gap (Eg) of traditional anatase TiO2 is wide, i.e., about 3.2 eV, and only responds to UV light, severely limiting the generation of photogenerated charge carriers. In this case, one promising method to extend its light absorption is narrowing the Eg values of semiconductors.9,10 In general, this often causes a mismatch between the band energy position and the redox potential of the target substances, which will weaken the reaction driving force of semiconductors. Therefore, it is of great research value to explore new visible light catalysts.Nowadays, the majority of photocatalysts that satisfy the requirements of wide spectral absorption range and matching redox potential are nitrides/nitrogen oxides and sulfides/sulfur oxides.11,12 The non-oxide elements of these semiconductor materials will react with the holes generated under light irradiation near the valence band maximum (VBM), resulting in self-decomposition, deactivation and reduction in their photocatalytic stability. Over the past few decades, a range of semiconductor materials has been widely explored for solar energy applications with the aim of developing highly efficient and stable photocatalysts.13–15 Among them, bismuth (Bi)-based Sillén–Aurivillius oxyhalides, such as Bi4MO8X (M = Nb, Ta; X = Cl, Br, and I), have been confirmed as promising photocatalysts owing to their unique electronic structure and excellent catalytic performance.16–20 On the one hand, the VBM in Sillén–Aurivillius oxyhalides is mainly composed of O 2p orbitals, not Cl 3p, Br 4p, and I 5p orbitals, making them robust against self-decomposition during the photocatalytic process. On the other hand, the stronger hybridization between Bi 6s and O 2p orbitals can promote the VBM to shift to a more negative level, which lowers the Eg value and broadens the spectral absorption of Sillén–Aurivillius oxyhalides. Furthermore, the non-centrosymmetric (NSC) structure of Sillén–Aurivillius oxyhalides originating from the distortion of the BO6 octahedron in the perovskite blocks can form internal electric fields (IEF), facilitate the separation of photogenerated charge carriers and lessen the recombination of electron–hole pairs.21,22 Thus, based on the above-mentioned advantages, Sillén–Aurivillius oxyhalides have attracted extensive attention.
At present, the mainstream route for growing Sillén–Aurivillius oxyhalides is solid-state reaction (SSR), which is significantly influenced by the level of halogen precursor and calcination temperature.16,22 When the stoichiometric precursor materials are calcined at high temperature, highly crystalline Sillén–Aurivillius oxyhalides can be formed. However, high temperature also induces the loss of halogen [X] elements, creating deep-level defects, which serve as recombination centers, hindering the transport of photogenerated charge carriers and reducing the photocatalytic performance.23,24 Based on this, the molten-salt synthesis (MSS) method has been widely used for the fabrication of Sillén–Aurivillius oxyhalides given that it can offer a special liquid reaction environment that expedites the diffusion rate of reactive elements and motivates the formation of high-purity and uniform reaction products.25,26 Because Sillén–Aurivillius oxyhalides contain halogen elements (F, Cl, Br, and I), most Bi-based materials are grown with the same molten salt as halogen. For example, Zhizhong Chen et al. used NaCl/CsCl as the molten salt to synthesize Bi4NbO8Cl.27 This method can avoid the influence of the halogen elements of the molten salt based on the synthetic target substance. In addition, other researchers adopted Cl-based flux (NaCl/CsCl, NaCl/KCl) to fabricate Bi4NbO8Br from a mixture of Ta2O5, BiOBr, and Bi2O3 at high calcination temperature.28,29 However, a higher temperature during the calcination process causes the full diffusion of the elements, which will inevitably affect synthetic materials.25 Therefore, it is necessary to study the effect of the chosen molten salt material on the target product.
In this work, a series of novel Sillén–Aurivillius oxyhalides, BaBi4TiNbO11X (X = Cl, Br), based on different molten salt materials was investigated to confirm the competing reaction of the interlayer halogen. Initially, we determined the existence of halogen competing reactions in the synthesis of BaBi4TiNbO11X by X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), and X-ray photoelectron spectroscopy (XPS). In combination with the investigation of material properties for Sillén–Aurivillius oxyhalides, a possible mechanism of interlayer halogen competing reaction between molten salts and the halogen layers was proposed. Furthermore, the experimental characterization and theoretical calculations confirmed that the hybrid halogen in Sillén–Aurivillius oxyhalides can optimize their energy band structure to facilitate the separation and transfer of the photogenerated charge carriers, promoting their photocatalytic activities in O2 evolution and the degradation of organic pollutants. This study aimed to offer new insights into the interlayer halogen competing reaction between molten salts and the halogen layers.
2. Experimental methods
2.1. Materials
All the chemical reagents employed in this work were of analytical grade and directly use without further purification, and all purchased from Shanghai Aladdin Reagent Co., LTD.2.2. Preparation of photocatalysts
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 to prepare the BaBiO2X precursor at 700 °C for 10 h by the traditional SSR method.
1 to prepare the BaBiO2X precursor at 700 °C for 10 h by the traditional SSR method.
2.3. Characterization
The surface morphologies of the BaBi4TiNbO11X samples were determined via scanning electron microscopy (SEM). X-ray diffraction (XRD) with a Cu Kα source was employed to characterize the crystal structure of the as-prepared samples. The microscopic characteristics of the BaBi4TiNbO11X samples were analyzed via transmission electron microscopy (TEM). The information on surface chemical elements (Ba, Bi, Ti, Nb, O, Cl, Br, and C elements) was collected via X-ray photoelectron spectroscopy (XPS) with an Al Kα source. The light absorption properties of the BaBi4TiNbO11X samples were measured by UV-vis diffuse reflectance spectroscopy (DRS). The structural composition of the BaBi4TiNbO11X oxyhalides was determined by Raman spectroscopy using a 514 nm laser. Fourier transform infrared (FTIR) spectroscopy was used to confirm the characteristic functional groups present in the BaBi4TiNbO11X oxyhalides. The separation efficiency of photogenerated electrons and holes was analyzed by photoluminescence spectroscopy (PL, Hitachi F-4600), transient photocurrent responses, and electrochemical impedance spectroscopy (EIS) (CHI-660D Electrochemical Workstation, Chenhua Instruments Co., Ltd, China). In addition, the electrochemical workstation was also employed for the electrochemical and photoelectrochemical measurements of the BaBi4TiNbO11X oxyhalides, as described in the previous studies.9,22,312.4. Photocatalytic activity measurements
The photocatalytic performance of the BaBi4TiNbO11X samples was evaluated by oxygen (O2) evolution and RhB degradation under visible light. The light source in the experiment was a 300 W Xe lamp with a 420 nm cutoff filter (Beijing China Education Au-light Co., Ltd). In the O2 evolution experiment, about 100 mg BaBi4TiNbO11X powder was mixed with an AgNO3 solution (250 mL, 5 mmol L−1) and thoroughly stirred at room temperature. Before visible light irradiation, the reactor for O2 evolution was vacuumized five times and purified with Ar (99.999%) to remove the residual O2. Then, the Xe lamp was turned on. The rate of O2 evolution was measured from the O2 concentration by liquid chromatography once every hour.For the process of RhB degradation under visible light, 100 mg BaBi4TiNbO11X powder was suspended in an RhB aqueous solution (100 mL, 5 mg L−1). Firstly, the as-obtained mixed solution was kept in a dark room with magnetic stirring for 0.5 h. Subsequently, the reactor containing the mixed solution was moved under an Xe lamp for the photocatalytic degradation experiments. Furthermore, the reaction solution of about 4 mL was withdrawn from the reactor once every 15 min to determine the degradation efficiencies of RhB. In the trapping experiment, 0.5 mmol of EDTA-2Na, tert-butyl alcohol (t-BuOH and TBA), and benzoquinone (BQ) was employed to capture the h+, ˙OH, and ˙O2−, respectively, and this process was similar to that of the RhB degradation process.
2.5. Computational methodology
All DFT calculations were performed using the Vienna ab initio simulation package (VASP).32,33 The generalized gradient approximation (GGA) was employed to describe the electron exchange and correlation in the Perdew–Burke–Ernzerhof (PBE) functional.34 The PBE functional was used only for structure optimization but for the band structure calculation we used the Heyd–Scuseria–Ernzerhof (HSE06) functional due to its accurate description of the electronic structure.35,36 The plane-wave kinetic energy cutoff was set to 520 eV. The considered conversion criteria for the energies and the forces on the individual atoms were 10−5 eV and 10−3 eV Å−1, respectively. In the partial density of states (PDOS) calculations, Gaussian smearing was used. A gamma-centered K-point mesh of 8 × 8 × 2 was considered for the optimization and PDOS calculations. For better visualization of the structure properties, we measured scanning tunneling microscopy (STM) images with a tip distance of 1.5 Å.3. Results and discussion
3.1. Material properties of BaBi4TiNbO11X (X = Cl, Br) nanoplates
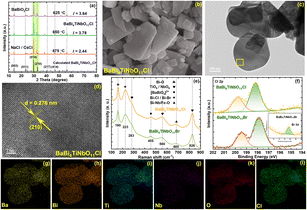
XRD was employed to characterize the phase purity and crystal structure of the as-prepared samples. The XRD patterns of BaBi4TiNbO11Cl synthesized from the raw materials of BaBiO2Cl and Bi3TiNbO9 based on NaCl/CsCl molten salts are presented in Fig. 2a. Clearly, the main diffraction peaks of all the BaBi4TiNbO11Cl samples located at the 2θ values of (003), (011), (014), (210), (217), and (414) are consistent with the DFT results reported in our previous work.22 The results suggest that BaBi4TiNbO11Cl oxyhalides can be synthesized over a wide temperature range. To determine the optimal synthesis temperature, the value of peak intensity ratio (I(014)/(210)) between the primary peak (014) and the secondary peak (210) was calculated for the different samples. The values of I(014)/(210) for the BaBi4TiNbO11Cl samples fabricated at 625 °C, 650 °C, and 675 °C are 3.64, 3.78, and 2.44, respectively. The stronger peak intensity ratio of the samples indicates that the BaBi4TiNbO11Cl oxyhalide fabricated at 650 °C grows along the (014) crystal faces easily. Simultaneously, the full width at half maximum (FWHM) of the (014) peak for the BaBi4TiNbO11Cl samples fabricated at 625 °C, 650 °C, and 675 °C is 0.2128, 0.2021, and 0.3443, respectively, and thus the crystallinity of the sample prepared at 650 °C is the best. Based on the above-mentioned discussion, it can be concluded that the optimal synthesis temperature for the BaBi4TiNbO11Cl oxyhalides is 650 °C. To reveal the morphological characteristics of the as-prepared samples, SEM was applied to the BaBi4TiNbO11Cl oxyhalide obtained at 750 °C. It can be observed that the sample displayed irregular rectangular nanoplates, as shown in Fig. 2b. This phenomenon is ascribed to the melted NaCl/CsCl molten salts, which provide fast transfer channels for the reaction elements and motivates the formation of high-purity and uniform reaction products. Furthermore, TEM was used to analyze the microstructure characteristics of the oxyhalide material. The TEM image in Fig. 2c shows that BaBi4TiNbO11Cl possessed the typical layered stack structure. Then, the area inside the yellow box was magnified. The corresponding high-resolution TEM image of this sample in Fig. 2d shows that it possesses uniform and clear lattice fringes of 0.276 nm, consistent with the d-spacing value of the (210) crystal plane. In addition, according to the TEM-EDS elemental mappings in Fig. 2g–l, Ba, Bi, Ti, Nb, O, and Cl, respectively, are uniformly distributed throughout the nanoplate, confirming that these elements are present on the photocatalysts and the successful preparation of BaBi4TiNbO11Cl.Afterwards, the raw material BaBiO2Cl was replaced with BaBiO2Br to grow BaBi4TiNbO11Br oxyhalides via the same process. The crystal lattice structure and diffraction patterns are shown in Fig. S1.† The diffraction peaks of the samples are consistent with the DFT results for BaBi4TiNbO11Br, suggesting that no impurities were formed in BaBi4TiNbO11Br at 650 °C. Combined with the SEM and TEM results in Fig. S2 and S3,† respectively, it was proven that BaBi4TiNbO11Br was successfully synthesized. In addition, the vibrational signature of BaBi4TiNbO11Br and BaBi4TiNbO11Cl was described in the range of 100 to 900 cm−1 by Raman spectroscopy, as shown in Fig. 2e. The Raman shifts at 160 and 455 cm−1 can be related to the Bi–O bond, while that at 223, 455, and 685 cm−1 are ascribed to the Bi–X (X = Cl, Br) bond, [Bi2O2]2+ layers, and Bi–Ti/Nb–O bonds, respectively.37–39 Meanwhile, the Raman shifts at 560 and 826 cm−1 are assigned to the Nb–O bonds in the NbO6 octahedra, further confirming the successful synthesis of the BaBi4TiNbO11X (X = Cl, Br) oxyhalides.
However, the Cl element was found in the TEM-EDS elemental mappings of BaBi4TiNbO11Br, as shown in Fig. S4.† Simultaneously, the component of the Cl element, as presented in Table S1 (the components of oxyhalide materials from TEM-EDS in Fig. S4),† is about 6.87%, which is about 15.27 times higher than that of the Br element. This indicates that both Cl and Br elements are present in the BaBi4TiNbO11X oxyhalides prepared with BaBiO2Br and Bi3TiNbO9 as the precursor materials and NaCl/CsCl as the molten salt. This phenomenon is consistent with the results from the XPS pattern shown in Fig. 2f. Based on previous conclusions, the molten salt only acts as a solvent to provide a liquid phase environment and facilitate the full diffusion of the elements.10,28,29 However, this contradicts the actual results obtained in this work. The reason for this phenomenon is that a large number of Cl elements from the NaCl/CsCl molten salt substituted the halogen elements (Br) in the precursor of BaBiO2Br to participate in the formation of the BaBi4TiNbO11X oxyhalides.
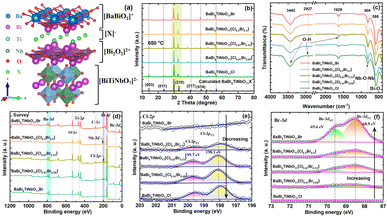
The crystal structure of BaBi4TiNbO11X is presented in Fig. 3a. It can be found that BaBi4TiNbO11X (X = Cl, Br) is a Sillén–Aurivillius perovskite oxyhalide material, which consists of a unit cell comprised of slabs of fluorite-type [Bi2O2]2+ layer inter-grown with [BiTiNbO7]2−, [BaBiO2]+, and [X]− layers along the P21cn space group. Simultaneously, along the c-axis direction, the different layers between [BaBiO2]+ and [Bi2O2]2+ are connected by X ions via van der Waals forces.40 As is known, van der Waals forces, which are formed from the distribution of electric charges, are one of the weakest forces between molecules.41 Therefore, during the preparation of the Sillén–Aurivillius perovskite oxyhalide by high-temperature calcination in previous works, the halogens, such as Cl, Br, and I, are easily lost, forming defects.16,22,42 Thus, to further confirm the influence of the molten salts on the Sillén–Aurivillius perovskite oxyhalide, different molar ratios of precursor materials were used to fabricate BaBi4TiNbO11X, and further details on the precursors and naming are shown in Table S1.† In the case of BaBi4TiNbO11Cl, the raw materials of BaBiO2Cl and Bi3TiNbO9 were used to fabricate BaBi4TiNbO11X with molten salts of NaCl/CsCl. For the other samples, the raw materials were BaBiO2Br and Bi3TiNbO9 and the content of molten salts of NaCl/CsCl was also decreased from 20 mmol to 0.5 mmol. Furthermore, the BaBi4TiNbO11Br sample was synthesized from the raw materials of BaBiO2Br and Bi3TiNbO9 based on the molten salts of NaBr/CsBr.
Then, the crystal structures and phase purity of the as-prepared samples were characterized by XRD, as shown in Fig. 3b and S5.† The main peaks located at the 2θ values of (014), (210), and (414) for all the samples are consistent with the XRD pattern calculated using DFT, implying that the synthetic BaBi4TiNbO11X possessed high crystallinity and purity. In addition, the FTIR spectra of the as-synthesized BaBi4TiNbO11X samples showed primary absorption bands in the range of 400–4000 cm−1, as depicted in Fig. 3c. The peaks observed at about 3442 cm−1, 2927 cm−1, and 1629 cm−1 can be attributed to the O–H stretching vibration.43 The Bi–O stretching vibrations were identified based on the peak at 586 cm−1.44 The other peak located at 808 cm−1 can be attributed to the Nb–O–Nb stretching vibration.45 The similar peaks in the FTIR spectra of the BaBi4TiNbO11X samples fabricated using different molar ratios of precursor materials suggest that the functional groups in the Sillén–Aurivillius perovskite oxyhalide did not change.
XPS analysis was performed to investigate the surface chemical state of the elements and confirm the effect of the molten salts on the formation of the Sillén–Aurivillius perovskite oxyhalides. The survey scan of all the samples, as shown in Fig. 3d, show that the BaBi4TiNbO11X perovskite oxyhalide is composed of Ba, Bi, Ti, Nb, O, and Cl (or Br) elements, and the high-resolution XPS spectra of the evolution behaviors of C 1s, Ba 3d, Bi 4f, Ti 2p, Nb 3d, O 1s, and Cl 2p (Br 3d) are also presented in Fig. 3e, f and S6–S11.† The binding energy (BE) of the BaBi4TiNbO11X samples was calibrated by the C 1s peaks at 284.8 eV, as shown in Fig. S6.† The peaks of Ba 3d5/2, Ba 3d3/2, Bi 4f7/2, Bi 4f5/2, Ti 2p3/2, Nb 3d5/2, Nb 3d3/2, O 1s, Cl 2p3/2, Cl 2p1/2, Br 3d5/2, and Br 3d3/2 were located at approximately 779.5 eV, 794.8 eV, 159.0 eV, 164.3 eV, 458.0 eV, 206.6 eV, 209.3 eV, 529.7 eV, 198.1 eV, 199.7 eV, 68.5 eV, and 69.6 eV, respectively, which further confirm the existence of Ba2+, Bi3+, Ti4+, Nb5+, O2−, Cl−, and Br− in BaBi4TiNbO11X. We also found that with an increase in the content of Br in the precursor materials, the intensity of the Cl 2p peak gradually decreased, as shown in Fig. 3e, while the Br 3d peak increased correspondingly, as shown in Fig. 3f, and these results suggest that the content of Br in the BaBi4TiNbO11X perovskite oxyhalide material halogen oxide product is related to the proportion of [Cl]/[Br] in the precursor materials. Simultaneously, the x value in the BaBi4TiNbO11(Cl1−xBrx) samples was calculated from the XPS peak table based on the content of elements. The x value of the BaBi4TiNbO11X samples was calculated to be 0, 9.8, 35.6, 50.8, and 100, which is close to that of the Br content in the precursor materials (0, 9, 33.3, 50, and 100), respectively. This calculated result suggests that the ratio of halogens in the precursor materials (molten salts and solutes) determined the x value of the final product. Namely, the stoichiometric ratios of the samples with different halogen molar ratios of precursor materials ([Br]/([Br] + [Cl])) (0%, 9%, 33.3%, 50%, and 100%) was BaBi4TiNbO11Cl, BaBi4TiNbO11(Cl0.91Br0.09), BaBi4TiNbO11(Cl0.67Br0.33), BaBi4TiNbO11(Cl0.5Br0.5), and BaBi4TiNbO11Br, respectively.
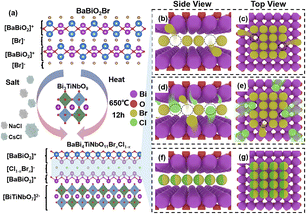
3.2. Halogen-competing reaction in BaBi4TiNbO11(Cl1−xBrx) Sillén–Aurivillius perovskite oxyhalides
In general, molten salts, such as NaCl/CsCl and NaBr/CsBr, can provide a special liquid reaction environment that expedites the diffusion rate of reactive elements and induces the formation of high-purity and uniform reaction products. During this process, the molten salt only plays an auxiliary role in the synthesis of the materials and does not participate in the reaction. According to our previous work,22 a suitable calcination temperature can facilitate the evolution and formation of layered BaBi4TiNbO11X perovskite oxyhalide via the MSS method in a muffle furnace. Herein, a novel method was proposed using the BaBiO2X structure as the template to form the BaBi4TiNbO11(Cl1−xBrx) perovskite oxyhalide by intercalating the [BiTiNbO7] octahedron between the [BaBiO2]+ layer and the halogen [X]− atomic layer through the MSS method, as depicted in Fig. 4a. Due to the proportion of [Cl]/[Br] in the precursor materials affecting the x value, the growth mechanisms for BaBi4TiNbO11(Cl1−xBrx) were proposed to explain the halogen-competing reactions in the perovskite halogens, as shown in Fig. 4b–g.Herein, the BaBiO2Br and Bi3TiNbO9 solutes and NaCl/CsCl solvents (molten salts) were used as representatives to clarify the halogen diffusion path during the formation of BaBi4TiNbO11(Cl1−xBrx). If the NaCl/CsCl molten salts only provide the liquid reaction environment and do not participate in the chemical reaction, the final production of perovskite oxyhalide is BaBi4TiNbO11Br, as shown in Fig. 4b and c. However, the halogen [X] layer in the crystal structure of the Sillén–Aurivillius perovskite oxyhalide connects the [BaBiO2]+ and [Bi2O2]2+ layers by van der Waals force along the c-axis direction. van der Waals forces are one of the weakest forces between molecules, which induce the formation of unstable [X] atoms in the halogen layer and cause [X] vacancies (VX), and thus some Br atoms spill out of the Sillén–Aurivillius perovskite oxyhalide into NaCl/CsCl molten salts (process I). In addition, during the high-temperature calcination process, a large number of molten salt atoms, such as Na, K, and Cl, surrounded the synthetic BaBi4TiNbO11Br products. Given that the Cl and Br atoms are located in the same main group and have similar chemical properties, the highly active Cl atoms will diffuse from the molten salt and occupy the Br atomic sites (VX) in the halogen oxides (process II), as described in Fig. 4d and e. Simultaneously, the high calcination temperature of the samples also promotes the migration of atoms currently located in the halogen lattice site into the molten salts (process III). Furthermore, the halogen atoms that migrate to the molten salts, such as Br, may also transition to VX again (process IV). Processes I–V occur continuously between the molten salts and perovskite oxyhalide during the high-temperature calcination process, resulting in the same proportion of [Cl]/[Br] in the molten salts as that in the halogen [X] atomic layer, as presented in Fig. 4f and g. Briefly, the weak van der Waals forces between the [BaBiO2]+ and [Bi2O2]2+ layers of BaBi4TiNbO11(Cl1−xBrx) induce the full interaction of halogens to diffuse inside and outside the perovskite oxyhalide lattice, which form an even distribution of halogen atoms between the molten salts and the halogen layers.
3.3. Optical and electrochemical properties of Sillén–Aurivillius perovskite oxyhalide
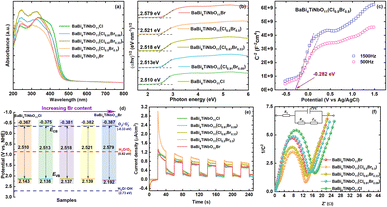
Generally, the band gap (Eg) of photocatalysts plays a significant role in the efficiency of their catalytic performance, especially for light absorption and the recombination of photogenerated carriers.22 Thus, the UV-vis spectra of the BaBi4TiNbO11X samples were recorded to analyze their optical properties. As demonstrated in Fig. 5a, the absorption edges of all the BaBi4TiNbO11X samples are approximately 460 nm, indicating that the BaBi4TiNbO11X oxyhalide is a type of visible light photocatalyst and halogen substitution (from Cl to Br) has little effect on its light absorption. To better compare the Eg values of the samples with different halogen contents, the conventional Kubelka–Munk function was applied to the BaBi4TiNbO11X oxyhalides, as follows:9,30,46| (αℏν)1/m = A(ℏν − Eg) | (1) |
Afterwards, the energy band structure of the as-prepared BaBi4TiNbO11(Cl1−xBrx) material was determined based on the Mott–Schottky (M–S) curves between the liquid and semiconductor material. As presented in Fig. 5c and S12,† all the sample curves show positive slopes of capacitance, and this the BaBi4TiNbO11X oxyhalides are n-type semiconductors.47 According to the M–S diagram, the flat band (Efb) potential of the BaBi4TiNbO11Cl, BaBi4TiNbO11(Cl0.91Br0.09), BaBi4TiNbO11(Cl0.67Br0.33), BaBi4TiNbO11(Cl0.5Br0.5), and BaBi4TiNbO11Br samples were calculated to be −0.267, −0.275, −0.281, −0.282, and −0.287 eV vs. Ag/AgCl, respectively. According to previous works,22,48 the conduction band (CB) of a semiconductor material is about 0.1 eV more negative than that of its Efb position. Based on this, the CBM of the BaBi4TiNbO11Cl, BaBi4TiNbO11(Cl0.91Br0.09), BaBi4TiNbO11(Cl0.67Br0.33), BaBi4TiNbO11(Cl0.5Br0.5), and BaBi4TiNbO11Br samples was −0.367, −0.375, −0.381, −0.382, and −0.387 eV, respectively. The valence band (VB) potential can be calculated using the following equation:
| EVB = ECB + Eg | (2) |
Simultaneously, transient photocurrent and electrochemical impedance spectroscopy (EIS) measurements were performed to disclose the separation and transport performance of photo-generated charges (electrons and holes). The diffused electrons from photoexcited carrier separation can generate a photocurrent signal, and thus transient photocurrent measurement can be used to demonstrate the separation efficiency of charge carriers. The photocurrent responses of all the samples, as shown in Fig. 5e, indicate that the BaBi4TiNbO11(Cl0.5Br0.5) sample has the highest photocurrent intensity and the fastest charge separation efficiency in response to visible light, which can be related to the substituting of Br for Cl in the BaBi4TiNbO11(Cl1−xBrx) oxyhalides, and more details about this result will be discussed through DFT calculations later. Then, the transfer behavior of electron–hole pairs was examined by EIS, as shown in Fig. 5f. Among the samples, BaBi4TiNbO11(Cl0.5Br0.5) also displayed the smallest Nyquist semicircle diameter, which reveals the lowest charge transfer resistance of the BaBi4TiNbO11(Cl1−xBrx) oxyhalides. The photoelectrochemical results of transient photocurrent and EIS confirm that the energy band structure of BNB is conducive to facilitate the separation and transfer performance of the photogenerated charge carriers, which is expected to promote the photocatalytic reactions for BaBi4TiNbO11(Cl0.5Br0.5).
3.4. Photocatalytic activities of BaBi4TiNbO11(Cl1−xBrx) oxyhalides
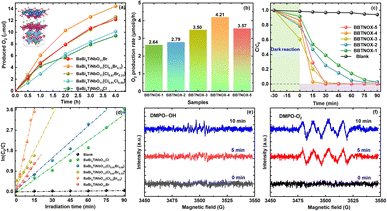
The photocatalytic performance of the as-prepared BaBi4TiNbO11(Cl1−xBrx) photocatalysts was assessed using the time course of O2 evolution in aqueous 5 mM AgNO3 solution under visible light irradiation. As indicated in Fig. 6a, the BaBi4TiNbO11(Cl0.5Br0.5) sample exhibited the best photocatalytic oxygen evolution efficiency among samples, indicating that halogen substitution in Sillén–Aurivillius oxyhalide enhances their photo-absorption, resulting in a more efficient photocatalytic performance. For a clearer comparison of the differences in performance, the corresponding photocatalytic rates of O2 production were estimated, as shown in Fig. 6b. BaBi4TiNbO11Cl and BaBi4TiNbO11Br showed an O2 production rate of 2.64 and 3.57 μmol g−1 h−1, respectively. Comparatively, the BaBi4TiNbO11(Cl0.5Br0.5) sample displayed the highest photocatalytic activity among the oxyhalide photocatalysts, and its rate is 4.21 μmol g−1 h−1. This enhanced photocatalytic performance can be ascribed to the excellent separation and transfer of photogenerated charges, as exhibited in Fig. 5.Furthermore, the degradation of 5 mg per L rhodamine B (RhB) under visible light irradiation was also employed to evaluate the photocatalytic activities of the Sillén–Aurivillius oxyhalide. Fig. 6c shows the photocatalytic efficiencies for the degradation of RhB by the BaBi4TiNbO11(Cl1−xBrx) samples, where the kinetic analysis of RhB photo-degradation was followed using the equation η = (C0 − C)/C0 × 100% (C0 and C are the initial and reacted concentration of RhB solution at different sampling times). It can be seen that the catalyst-free samples exhibited no obvious variation over time, which means that the RhB organic pollutant is relatively stable under solar irradiation. Although all the samples could degrade the RhB pollutant within 90 min, respectively, a significant enhancement in photocatalytic efficiency for the BaBi4TiNbO11(Cl0.5Br0.5) sample was observed. Then, the reaction kinetic constants of the different samples in Fig. 6d were determined according to the following equation:
| Ln(C0/C) = kt | (3) |
The degradation mechanisms of organic pollutants were investigated by radical scavenging experiments. Each scavenger was added before the photodegradation experiment. Among the many scavengers, TBA, BQ, and EDTA-2Na were incorporated as a quencher of hydroxyl radicals, singlet oxygens, and holes, respectively, and the process is similar to that for degrading RhB solution. The results of the trapping experiment are provided in Fig. S14.† After the addition of the EDTA-2Na quencher, the degradation efficiency for the organic pollutant decreased sharply, and it was only degraded by 16.9% in 120 min. This observation in the trapping experiment suggests that h+ as the active species plays a major role in the RhB degradation process. Thus, the degradation of organic pollutants for Sillén–Aurivillius oxyhalide materials is driven by photocatalytic oxidation rather than dye sensitization in this work. Furthermore, the degradation efficiency of the RhB solution also decreased by half after the addition of BQ, which reveals that the ˙O2− radical was generated and played a role in the process. Meanwhile, it can be found that the photocatalytic degradation efficiency of RhB has no significant change when TBA was supplied as the scavenger. Hence, the ˙OH radicals hardly affected the degradation process of RhB solution. The reason for this phenomenon is that the redox potential of H2O/˙OH (2.73 eV) is more positive than the EVB positive of BaBi4TiNbO11(Cl0.5Br0.5) (2.139 eV) in the reaction solution, and thus the photogenerated h+ cannot drive the production of ˙OH radicals, as revealed in Fig. 5d. Furthermore, electron spin resonance (ESR) was further employed to evaluate the active species (˙OH and ˙O2−) during the photocatalytic process. By adding the trapping agent of DMPO, the radicals of ˙OH and ˙O2− were captured. As depicted in Fig. 6c and d, the signals of the ˙OH and ˙O2− radicals were not detected in the dark (0 min). When light was applied to the surface of the photocatalyst, the ˙OH radicals had no significant change and the ˙O2− radicals were clearly observed. This phenomenon proves that the ˙O2− radicals played an important role in the photocatalytic process, which is in agreement with the results of the trapping experiment.
3.5. Photocatalytic activities in BaBi4TiNbO11(Cl1−xBrx) oxyhalides
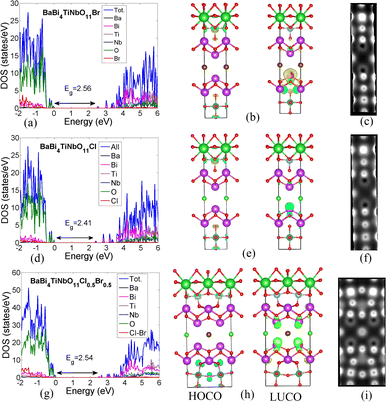
Herein, we studied the electronic properties of three Sillén–Aurivillius oxyhalides, namely, BaBi4TiNbO11X (X = Br, Cl, and Cl0.5Br0.5). The partial density of states (PDOS), the highest occupied crystal orbital (HOCO), and the lowest unoccupied crystal orbital (LUCO) of BaBi4TiNbO11Br are shown in Fig. 7a–c, while that for BaBi4TiNbO11Cl is given in Fig. 7d–f and BaBi4TiNbO11(Cl0.5Br0.5) in Fig. 7g–i, respectively. To account for both the Cl and Br in the Sillén–Aurivillius oxyhalides, we constructed a superlattice from 38 atoms with one Cl and one Br atom, as shown in Fig. 7h. The peaks of the PDOS in Fig. 7a show that the high energy-occupied bands are mainly contributed by the O atoms, with very low contribution from the Br and Ti atoms. Alternatively, the unoccupied bands (hole states) are mainly contributed by the Bi atoms, followed by a lower contribution by O and Br atoms. The same was also observed from the PDOS of BaBi4TiNbO11Cl, as shown in Fig. 7d. The distributions of HOCO on the O and Ti atoms (see HOCO in Fig. 7b and e) and the LUCO on the Bi and O atoms (see LUCO Fig. 7b and e) confirm the results obtained from PDOS.Regarding the band gap, it was noted that the band gap decreases from Eg = 2.56 eV in BaBi4TiNbO11Br to Eg = 2.41 eV in BaBi4TiNbO11Cl. The lower electronegativity of Br compared to that of Cl leads to a higher electronegativity difference (between O and Br atoms) in BaBi4TiNbO11Br, which increases its ionicity, decreases the orbital overlap, and eventually increases the band gap. Thus, by decreasing the electronegativity of the halogen, the band gap decreases. We tested this assumption by calculating the energy gaps of BaBi4TiNbO11F and BaBi4TiNbO11I, which were found to be 2.21 and 2.93 eV, respectively.
The presence of two halogens with equal ratios as in BaBi4TiNbO11(Cl0.5Br0.5) resulted in noticeable changes in the structure and electronic properties of the samples. It was observed from the supercell, as shown in Fig. 7h, that the O atoms around the transition metals (Ti and Nb) form new chemical bonds with the Bi atoms, which did not exist in the structures with a single halogen. These new bonds enhanced the contribution of the O-atoms (see the lower part of the supercell in Fig. 7h) to the HOCO, and consequently boosted the interactivity of these atoms. Another difference observed in the LUCO is that it is distributed on the Bi atoms on both sides of the halogens (see LUCO in Fig. 7h). Therefore, incorporating two halogens increases the contribution of the different O and Bi atoms in HOCO and LUCO, respectively, and then increases the interactivity of the whole structure. According to the density of states, the band gap of BaBi4TiNbO11(Cl0.5Br0.5) equals 2.54 eV, which is higher than that of BaBi4TiNbO11Cl but lower than that of BaBi4TiNbO11Br, in accordance with the experimental results.
Imaging surfaces using STM is an important characterization tool given that it provides important information about the material structure. The STM images of the three Sillén–Aurivillius compounds are shown in Fig. 7c, f and i. It can be seen that the O-atoms produce bright spots in the images, which indicate that their electronic states dominate the occupied levels. Also, around the Bi- and Nb-atoms, empty black spots inside bright ones are observed, which correspond to the Bi- and Nb-atoms between the O-atoms, respectively. The Br, Cl, and Cl0.5Br0.5 halogens could also be identified by STM through their less bright but recognizable spots, as seen in the plots. The brightest spots observed in all the cases (see the bright spot surrounded by lower bright four spots in the upper part of Fig. 7c) represent the electronic density from both the Ti- and O-atoms.
3.6. Proposed mechanism for the degradation of BaBi4TiNbO11X oxyhalides
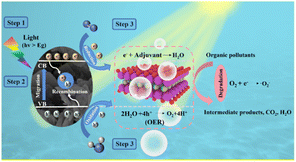
According to the above-mentioned experimental and calculated results, the potential mechanism for the enhancement in the photo-catalytic performance of the BaBi4TiNbO11X oxyhalides is shown in Fig. 8. Under visible light irradiation, photons with energy larger than the Eg of the semiconductor can produce e−–h+ pairs in the semiconductor material (eqn (4)). Afterward, the e− is excited to the CB, and the h+ is transported to the VB of the semiconductor material. The hybridization of the halogens in oxyhalides can increase the contribution to the low-energy orbitals and facilitate the separation and transfer performance of the photogenerated charge carriers, promoting the photocatalytic reaction. Given that the EVB of the semiconductor material (about 2.14 eV) is more positive than that of the H2O/O2 redox potential (0.82 eV), and the photo-generated h+ can promote the reaction of H2O to form O2 (eqn (5)), the excess e− is quenched by the adjuvant material. This is the mechanism for the photocatalytic process of O2 evolution by BaBi4TiNbO11X.Furthermore, given that the ECB of BaBi4TiNbO11X at about −0.38 eV is more negative than the redox potential of O2/˙O2− (−0.33 eV vs. NHE), the excited e− will combine with O2 to form ˙O2− (eqn (7)) during the degradation process of RhB pollutant. Simultaneously, the redox potential of H2O/˙OH (2.73 eV) is more positive than the EVB of the semiconductor material, as shown in Fig. 5d, indicating that H2O can be converted to ˙OH, which was confirmed by the radical scavenging experiments. Finally, the e− in the CB, the generated ˙O2−, and the h+ in the VB will participate in the redox reaction to degrade the organic pollutant molecules (eqn (8)). This is the mechanism for the photocatalytic degradation of organic pollutants by the BaBi4TiNbO11X oxyhalides. Based on the above-mentioned analyses, the photocatalytic mechanism of the BaBi4TiNbO11X oxyhalides can be described as follows:
| BaBi4TiNbO11X + hν → e− + h+ | (4) |
| 2H2O + 4h+ → O2 + 4H+ | (5) |
| e− + adjuvant → H2O | (6) |
| O2 + e− → ˙O2− | (7) |
| ˙O2− + h+ + e− + organic pollutant → intermediate product + CO2 + H2O | (8) |
4. Conclusions
In this contribution, novel Sillén–Aurivillius BaBi4TiNbO11X (X = Cl, Br) oxyhalides with a tunable halogen ratio were prepared via the molten-salt synthesis method. Both Cl and Br elements were detected in BaBi4TiNbO11Br fabricated with BaBiO2Br and Bi3TiNbO9 as precursor materials and NaCl/CsCl as the molten salt demonstrated that the molten salt not only provides a liquid reaction environment but also participates in the chemical reaction. This is ascribed to the weak van der Waals forces between the [BaBiO2]+ and [Bi2O2]2+ layers of BaBi4TiNbO11(Cl1−xBrx), which induced the full interaction of halogens to diffuse inside and outside the perovskite oxyhalide lattice, forming an even distribution of halogen atoms between the molten salts and the halogen layers in oxyhalides. By employing this mechanism, BaBi4TiNbO11(Cl1−xBrx) with different x contents could be easily obtained. Simultaneously, DFT calculations were performed to investigate the structural and electronic properties of the three Sillén–Aurivillius oxyhalides, namely, BaBi4TiNbO11X (X = Br, Cl, and Cl0.5Br0.5). The HSE calculations showed that the structures are semiconductors with band gaps equal to 5.41, 2.54, and 2.56 eV, respectively, which is agreement with the experimental Eg of the samples. The partial density of states and the visualization of the highest occupied crystal orbitals show that the low energy occupied orbitals are mainly from O, and then from the Ti atoms. Alternatively, the low energy unoccupied ones are contributed mainly by Bi followed by O atoms. Employing two chalcogens as in BaBi4TiNbO11Cl0.5Br0.5 led to the formation of additional Bi–O bonds and slight change in structure. These additional bonds enhanced the contributions from the different O/Bi atoms to the highest occupied/lowest unoccupied orbitals, which boosted the interactivity of the whole structure. The simulated scanning tunneling microscopy images were used for the characterization and identification of the prepared samples. Thus, the BaBi4TiNbO11(Cl0.5Br0.5) sample exhibited an excellent RhB degradation performance compared with the commercial photocatalyst P25. Meanwhile, it also showed a significant photocatalytic O2 evolution performance owning to the reinforced separation and transfer performance of the photogenerated charge carriers. By the rational design of the energy band structure via the interlayer halogen-competing reaction induced by the precursor molten salts, this work developed an exceptional Sillén–Aurivillius photocatalyst family under visible light irradiation.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (No. 12274361), the Natural Science Foundation of Jiangsu Province (BK20211361), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_3145) and the school-level research projects of Yancheng Institute of Technology (xjr2019028, xjr2019059).Notes and references
- H. Mai, D. Chen, Y. Tachibana, H. Suzuki, R. Abe and R. A. Caruso, Chem. Soc. Rev., 2021, 50, 13692–13729 RSC.
- Y. Zhang, L. Shi, Z. Wang, H. Dai, Z. Hu, S. Zhou, H. Chen, X. Feng, J. Zhu, Y. Sun, W. Liu and Q. Zhang, Sol. Energy, 2021, 227, 334–342 CrossRef CAS.
- Y. Zhang, Y. Zhang, X. Chen, S. Wang, Q. Gao, M. Wu, Z. Wang, J. Ao, Y. Sun, W. Liu and Q. Zhang, Mater. Sci. Semicond. Process., 2022, 140, 111569 Search PubMed.
- C. Sun, J. Yang, M. Xu, Y. Cui, W. Ren, J. Zhang, H. Zhao and B. Liang, Chem. Eng. J., 2022, 427, 131564 CrossRef CAS.
- C. Liu, Y. Zhang, J. Wu, H. Dai, C. Ma, Q. Zhang and Z. Zou, J. Mater. Sci. Technol., 2022, 114, 81–89 CrossRef CAS.
- X. Lu, L. Quan, H. Hou, J. Qian, Z. Liu and Q. Zhang, J. Alloys Compd., 2022, 925, 166552 CrossRef CAS.
- H. Zhang, J. Cai, Y. Wang, M. Wu, M. Meng, Y. Tian, X. Li, J. Zhang, L. Zheng, Z. Jiang and J. Gong, Appl. Catal., B, 2018, 220, 126–136 CrossRef CAS.
- S. Lee and S. Park, J. Ind. Eng. Chem., 2013, 19, 1761–1769 CrossRef CAS.
- J. Chen, Y. Gu, S. Xu, Y. Zhang, Z. Zhang, L. Shi, Z. Mu, C. Zhou, J. Zhang and Q. Zhang, Catalysts, 2023, 13, 751 CrossRef CAS.
- X. Tao, Y. Zhao, L. Mu, S. Wang, R. Li and C. Li, Adv. Energy Mater., 2018, 8, 1701392 CrossRef.
- J. Akter, M. A. Hanif, M. A. Islam, K. P. Sapkota, I. Lee and J. R. Hahn, J. Environ. Chem. Eng., 2021, 9, 106831 CrossRef CAS.
- Y. Chen, W. Zhong, F. Chen, P. Wang, J. Fan and H. Yu, J. Mater. Sci. Technol., 2022, 121, 19–27 CrossRef CAS.
- X. Sun, L. Shi, H. Huang, X. Song and T. Ma, Chem. Commun., 2020, 56, 11000–11013 RSC.
- Z. Zhu, F. Chen, N. Tian, Y. Zhang and H. Huang, Curr. Opin. Green Sustainable Chem., 2022, 37, 100669 CrossRef CAS.
- A. Kumar, A. Kumar and V. Krishnan, ACS Catal., 2020, 10, 10253–10315 CrossRef CAS.
- K. Ogawa, A. Nakada, H. Suzuki, O. Tomita, M. Higashi, A. Saeki, H. Kageyama and R. Abe, ACS Appl. Mater. Interfaces, 2019, 11, 5642–5650 CrossRef CAS PubMed.
- A. Sharma, S. K. Kanth, S. Xu, N. Han, L. Zhu, L. Fan, C. Liu and Q. Zhang, J. Alloys Compd., 2022, 895, 162576 CrossRef CAS.
- J. Sun, N. Han, Y. Gu, X. Lu, L. Si and Q. Zhang, Catalysts, 2020, 10, 1425 CrossRef CAS.
- C. Hu, F. Chen and H. Huang, Angew. Chem., Int. Ed., 2023, e202312895 CAS.
- Z. Zhu, H. Huang, L. Liu, F. Chen, N. Tian, Y. Zhang and H. Yu, Angew. Chem., Int. Ed., 2022, 61, e202203519 CrossRef CAS PubMed.
- M. Banoo, R. S. Roy, M. Bhakar, J. Kaur, A. Jaiswal, G. Sheet and U. K. Gautam, Nano Lett., 2022, 22, 8867–8874 CrossRef CAS PubMed.
- S. Xu, Y. Zhang, Y. Wang, J. Chen, C. Zhou, Z. Mu, Z. Zhang, J. Zhang, J. Wang and Q. Zhang, J. Alloys Compd., 2023, 952, 169932 CrossRef CAS.
- Y. Gu, F. Yu, J. Chen and Q. Zhang, Catalysts, 2022, 12, 221 CrossRef CAS.
- L. Shi, X. Wu, C. Xu, Q. Bai, Z. Nie, C. Ji, Y. Zhang, Z. Yin, S. Zhang, X. Qu and F. Du, J. Mater. Chem. C, 2021, 9, 2784–2792 RSC.
- A. Majumdar, U. Ghosh and A. Pal, J. Alloys Compd., 2022, 900, 163400 CrossRef CAS.
- Y. Xu, Y. You, H. Huang, Y. Guo and Y. Zhang, J. Hazard. Mater., 2020, 381, 121159 CrossRef CAS PubMed.
- Z. Chen, R. Xu, S. Ma, Y. Ma, Y. Hu, L. Zhang, Y. Guo, Z. Huang, B. Wang, Y. Y. Sun, J. Jiang, R. Hawks, R. Jia, Y. Xiang, G. C. Wang, E. A. Wertz, J. Tian, D. Gall, X. Chen, V. Wang, L. Gao, H. Zhu and J. Shi, Adv. Funct. Mater., 2022, 32, 2206343 CrossRef CAS.
- R. Xu, Y. Wang, D. Cui, K. Du, Z. Shi, H. Zhang, Z. Xu, W. Hao and Y. Du, Sol. RRL, 2022, 6, 2200365 CrossRef CAS.
- C. Hu, H. Huang, F. Chen, Y. Zhang, H. Yu and T. Ma, Adv. Funct. Mater., 2019, 30, 1908168 CrossRef.
- Y. Zhang, S. Xu, Z. Mu, K. Liu, J. Chen, C. Zhou, Y. Yao, X. Chen, L. Shi, Z. Wang, Y. Sun, W. Liu and Q. Zhang, Vacuum, 2022, 206, 111569 CrossRef CAS.
- N. Han, S. Xu and Q. Zhang, J. Mater. Sci., 2022, 57, 2870–2882 CrossRef CAS.
- G. Kresse and J. Furthmüller, Phys. Rev. B: Condens. Matter Mater. Phys., 1996, 54, 11169–11186 CrossRef CAS PubMed.
- G. Kresse and J. Furthmüller, Comput. Mater. Sci., 1996, 6, 15–50 CrossRef CAS.
- J. P. Perdew, K. Burke and M. Ernzerhof, Phys. Rev. Lett., 1996, 77, 3865–3868 CrossRef CAS PubMed.
- J. Heyd, G. E. Scuseria and M. Ernzerhof, J. Chem. Phys., 2003, 118, 8207–8215 CrossRef CAS.
- F. Fuchs, J. Furthmüller, F. Bechstedt, M. Shishkin and G. Kresse, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 115109 CrossRef.
- S. S. Kim, J. W. Kim and C. M. Raghavan, New Phys.: Sae Mulli, 2015, 65, 542–549 Search PubMed.
- A. Prasetyo, B. Mihailova, V. Suendo and A. A. Nugroho, J. Appl. Phys., 2015, 117, 064102 CrossRef.
- Z. Wei, J. Liu, W. Fang, Z. Qin, Z. Jiang and W. Shangguan, Catal. Sci. Technol., 2018, 8, 3774–3784 RSC.
- X. Wu, C. Zhang, Z. Nie, Y. Zhang, Q. Bai, Z. Yin, S. Zhang, X. Qu, F. Du and L. Shi, Sep. Purif. Technol., 2022, 292, 120969 CrossRef CAS.
- Q. Li, V. Rudolph, B. Weigl and A. Earl, Int. J. Pharm., 2004, 280, 77–93 CrossRef CAS PubMed.
- N. Han and Q. Zhang, Appl. Surf. Sci., 2021, 562, 150215 CrossRef CAS.
- X. Qu, M. Liu, H. Zhai, X. Zhao, L. Shi and F. Du, J. Alloys Compd., 2019, 810, 151919 CrossRef CAS.
- N. Thanabodeekij, E. Gulari and S. Wongkasemjit, Powder Technol., 2005, 160, 203–208 CrossRef CAS.
- X. Qu, M. Liu, Z. Gao, H. Zhai, W. Ren, L. Shi and F. Du, Appl. Surf. Sci., 2020, 506, 144688 CrossRef CAS.
- Y. Zhang, Z. Hu, S. Lin, S. Cheng, Z. He, C. Wang, Z. Zhou, Y. Sun and W. Liu, ACS Appl. Energy Mater., 2020, 3, 9963–9971 CrossRef CAS.
- Z. Liu, H. Wang, J. Duan and B. Hou, Ceram. Int., 2022, 48, 24777–24787 CrossRef CAS.
- S. Li, B. Xue, J. Chen, Y. Liu, J. Zhang, H. Wang and J. Liu, Sep. Purif. Technol., 2021, 254, 117579 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3ta05438a |
| This journal is © The Royal Society of Chemistry 2024 |