Open Access Article
Open Access ArticleSynthesis of high molecular weight poly(ricinoleic acid) via direct solution polycondensation in hydrophobic ionic liquids†
Lulu
Wang‡
,
Hua
Yuan‡
,
Xiaoli
Ma
,
Zijin
Li
,
Haowei
Sun
,
Xiaoyan
Zhang
,
Xiaoyu
Huang
 ,
Qiaohong
Peng
* and
Yeqiang
Tan
,
Qiaohong
Peng
* and
Yeqiang
Tan
 *
*
State Key Laboratory of Bio-Fibers and Eco-Textiles, School of Materials Science and Engineering, Qingdao University, Qingdao, Shangdong 266071, China. E-mail: pengqh@qdu.edu.cn; tanyeqiang@qdu.edu.cn
First published on 10th July 2024
Abstract
Ricinoleic acid, a natural hydroxy fatty acid, is a good candidate for preparing biodegradable polymer elastomers. Herein, high molecular weight poly(ricinoleic acid) (PRA) with a weight average molecular weight up to 122 kDa was successfully synthesized by solution polycondensation of methyl ricinoleate (MR) in hydrophobic ionic liquids, 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide. The influence of monomer concentration, polymerization temperature and time, catalyst and properties of the ionic liquids on the polycondensation was comprehensively studied. Compared with the melt polycondensation, the solution polycondensation of MR in the ionic liquids can achieve much higher molecular weights. The PRA polymerized in ionic liquids shows a 100% cis structure like the natural monomer.
Sustainability spotlightPlastic pollution has emerged as a global crisis, from the manufacturing process to the disposal and incineration of plastic products. Bio-based polymers, made from renewable sources, are being designed to have a minimal carbon footprint, high recyclability, and the ability to biodegrade or compost fully. Herein, we propose a novel synthesis strategy to obtain a bio-based polymer poly(ricinoleic acid) (PRA), which is derived from the inedible castor oil. Using hydrophobic ionic liquids (ILs) as a “green solvent” can largely increase the molecular weight of PRA and thus the performance of the polymer is remarkably improved. The ILs can be easily recovered since phase separation occurs at the late stage of the polycondensation and PRA with high molecular weight is not dissolved in the polar ILs. Thus, the application of ILs does not significantly increase the production cost of PRA. On the contrary, the direct polycondensation process is based on the more available monomer, avoiding the application of high-cost lactone monomers and the ring-opening polymerization process that is sensitive to air and moisture. The synthesis method is expected to address the problems of high production cost as well as poor performance of bio-based polyesters by melt-polycondensation. Consequently, sustainable production for bio-based polymers aligns with the goals of the United Nation's sustainability development goals (SDGs): industry, innovation, and infrastructure (SDG 9), responsible consumption and production (SDG 12), and climate action (SDG 13). |
1. Introduction
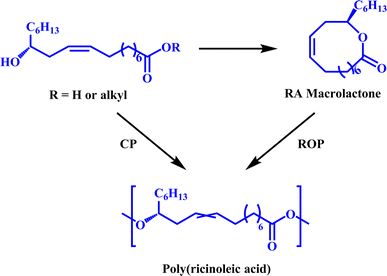
Polyester is one of the most important polymers. Polyesters synthesized from different monomers have different structures and different mechanical and chemical properties, thus polyesters as plastics, fibers, rubbers, adhesives, coatings, etc. show extensive applications.1 However, polyester is generally derived from non-renewable fossil resources such as petroleum and coal. With the growing concern for environmental protection, the development of bio-based polymers has become extremely important.2–4 Poly(ricinoleic acid) (PRA) is derived from castor oil, an inedible vegetable oil, and is transformed through a series of physical and chemical processes.5–8PRA synthesis methods include condensation polymerization (CP)9 and ring-open polymerization (ROP)10,11 (Fig. 1). Although it is easy to obtain PRA with high molecular weight with the latter, ricinoleic acid (RA) needs to be converted first into a large ring monomer, which is difficult to obtain due to the unfavorable intramolecular esterification of the functional groups with long distance. Additionally, the control of the stereochemical structure of PRA via ring-opening metathesis polymerization is not ideal.10,12 Direct polycondensation is a simple and low-cost method for preparing PRA. However, due to the influence of the monomer impurity and harsh polymerization conditions (e.g., high temperature and high vacuum), it is difficult to obtain high molecular weight PRA by direct polycondensation.9 As far as we know, the weight average molecular weight (Mw) of PRA polymerized by thermal polycondensation at 180 °C is only 5600 g mol−1. Enzyme-catalyzed PRA synthesis is really mild and the polymer with Mw higher than 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1 has been polymerized by using an immobilized lipase at 80 °C for 7 days.13 Nevertheless, lipase is expensive and used in large quantities, which hinders its industrialization. The synthesis of low-cost as well as high-molecular weight PRA is the key to realize large-scale and wide-ranging applications. The synthesis of PRA by direct polycondensation catalyzed by trace Lewis acid may be the most efficient method.
000 g mol−1 has been polymerized by using an immobilized lipase at 80 °C for 7 days.13 Nevertheless, lipase is expensive and used in large quantities, which hinders its industrialization. The synthesis of low-cost as well as high-molecular weight PRA is the key to realize large-scale and wide-ranging applications. The synthesis of PRA by direct polycondensation catalyzed by trace Lewis acid may be the most efficient method.
Ionic liquids (ILs), as a new type of environmentally friendly solvent, have their own unique functions in chemical processes compared to conventional organic solvents. In the previous work, we found that hydrophobic bis(trifluoromethanesulfonyl)imide (Tf2N)-anionic ILs as inert solvents for the polycondensation of lactic acid could accelerate the polymerization process, which is ascribed to the activation effect of ions on the functional group of the monomer and the dilution effect of ILs on the viscous polymer melt. Weight average molecular weight as high as 1.32 × 105 g mol−1 was achieved by tuning the monomer concentration, temperature, and the structure of ILs.14 When sulfonic acid-functionalized ILs as solvents were employed in the copolymerization of lactic acid and ε-caprolactone, random copolyesters having high molecular weight were synthesized under moderate conditions and within short polymerization time.15 The acidic ILs act, simultaneously, as a catalyst.15 The catalysis of acidic ILs is dominantly influenced by the type of IL anions. Fradet et al. investigated the polycondensation of aliphatic hydroxy acids as well as diacids and diols in sulfonic acid-functionalized ILs, and non-ignorable etherification of diols was observed in the Tf2N-anionic ILs.16–18 Aliphatic polyethers from diols were successfully synthesized accordingly.19 Polyimides, polyamides, phenol-formaldehyde resins, poly(ethylene terephthalate) (PET), polypeptides, polysulfones (PS), poly(aryl ether ketone)s, poly(phenylene sulfide sulfone), poly-p-phenylene terephthalamide (PPTA), polyetheretherketone (PEEK), etc. have also been successfully synthesized in ILs by polycondensation.20–31 The solubility and functions of ILs play an important role in the polymerization processes.32,33
In this work, high molecular weight PRA was synthesized via solution polycondensation of methyl ricinoleate (MR) in different 1-alkyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([AMIM][Tf2N]) ILs. The effects of monomer concentration, polymerization time, type and dosage of catalyst, and properties of the ILs on the polymerizations were investigated comprehensively. The obtained polymers were characterized by gel permeation chromatography, nuclear magnetic spectroscopy, thermogravimetry, and differential scanning calorimetry. The PRA polymerized in the ILs has high stereoregularity.
2. Experimental part
2.1 Materials
1-methylimidazole (99%), 1-bromohexane (98%), 1-bromodecane (98%), 1-bromotetradecane (98%), and 1-bromooctadecane (98%) were purchased from Aladdin. These reagents were distilled under reduced pressure before use. Lithium bis(trifluoromethanesulfonyl)imide (99%) and titanium(IV) isopropoxide (TIP, 99%) were purchased from Aladdin, tetrabutyl titanate (TBT, 99%) and stannous chloride (SnCl2, 98%) were purchased from Macklin, and toluene (97%), ethyl acetate (97%), dichloromethane (97%), n-hexane (97%), and acetone (97%) were purchased from HuShi. These reagents were used as received. Methyl ricinoleate (MR) (>80%) was purchased from TCI and purified by silica gel chromatography using acetone/hexane (v/v 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9) as an eluent.9
9) as an eluent.9
2.2. Characterization
NMR spectra were recorded on a Bruker AVANCE III HD 400 MHZ spectrometer using deuterated chloroform (CDCl3) and deuterated tetrahydrofuran as solvents and tetramethylsilane (TMS) as an internal standard. Thermogravimetric analysis (TGA) was performed in a nitrogen atmosphere on a TG 209 F3 thermogravimetric analyzer, with a heating rate of 10 °C min−1. Gel permeation chromatography (GPC) was used for the molecular weight test. A Waters E2695 chromatograph equipped with a Waters 410 refractive index detector and a set of Ultrastyragel columns was used. Tetrahydrofuran was used as the mobile phase and the flow rate is 1.0 mL min−1. The system was calibrated using polystyrene standards. Measurement of glass transition temperatures was performed using a DSC 250 differential scanning calorimeter at heating and cooling rates of 10 °C min−1.2.4 Synthesis of ionic liquids
The 1-alkyl-3-methyl-imidazolium bis(trifluorosulfonyl)imide ILs, including 1-hexyl-3-methyl-imidazolium bis(trifluorosulfonyl)imide ([HMIM][NTf2]), 1-decyl-3-methyl-imidazolium bis(trifluorosulfonyl)imide ([DMIM][NTf2]), 1-tetradecyl-3-methyl-imidazolium bis(trifluorosulfonyl)imide ([TdMIM][NTf2]), and 1-octadecyl-3-methyl-imidazolium bis(trifluorosulfonyl)imide ([OdMIM][NTf2]) were synthesized as previously reported (Fig. 2).34 Colorless and transparent ILs were obtained. The structure and purity of the ILs were characterized by 1H NMR.2.5 Synthesis of poly(ricinoleic acid)
PRA was synthesized by solution polycondensation of MR in ILs. Typically, methyl ricinoleate (1.0010 g, 3 mmol), 1-octadecyl-3-methylimidazolium bis(trifluorosulfonyl)imide (1.9701 g, 3 mmol) and catalytic tetrabutyl titanate (0.0101 g, 2.94 × 10−2 mmol) were added into a round-bottom flask and mixed under mechanical stirring. The atmosphere of the flask was replaced with N2 gas to avoid oxidation of the monomer during heating. Then, the flask was placed into an oil bath and heated to 180 °C gradually while stirring. After 10 min, the polycondensation pressure gradually decreased to ca. 100 Pa. The polycondensation of MR was carried out under these conditions for 72 h. After cooling to room temperature, the polymer product was washed with alcohol 3 times and dried in a vacuum oven at room temperature for 48 h. Ultimately, elastic PRA was obtained.3. Results and discussion
Since the NTf2-anionic ILs are hydrophobic and non-coordinating and possess excellent thermal and chemical stability, the monomer MR can be well dissolved in the ILs and thus solution polycondensation of MR in the ILs was successfully performed. NTf2-anionic ILs as inert solvents have shown great improvements in the polycondensation processes.14,32 The polycondensation process of MR is actually composed of countless intermolecular transesterifications between the hydroxyl and ester bond of MR. Common transesterification catalysts such as tetrabutyl titanate (TBT), titanium(IV) isopropoxide (TIP) and SnCl2 were investigated for the solution polycondensation of MR.9,35,36 As shown in Table 1, when titanates are utilized as the catalyst, PRA with much higher molecular weight (Mw up to 75.6 kDa) is obtained at a typical polyesterification temperature (180 °C). In contrast, PRA with Mw lower than 5 kDa is polymerized when SnCl2 is used as the catalyst possibly due to the poor miscibility of SnCl2 in the really hydrophobic mixture of the IL and the monomer. Apparently, non-polar titanates, which have excellent solubility in the mixture, are a more effective catalyst for the polycondensation of MR as compared to SnCl2 and there is no great difference whether applying TBT or TIP as the catalyst, suggesting that Ti acts as the catalytically active center and the substituents have little influence on the polycondensation.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL = 1
IL = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mole, 1 wt% of catalyst relative to the monomer, 72 h)
1 in mole, 1 wt% of catalyst relative to the monomer, 72 h)
| Entry | Temperature (°C) | Catalyst | M n (Da) | M w (Da) | PDI | Yield(%) |
|---|---|---|---|---|---|---|
| 1 | 180 | TBT | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.9 | 85 |
| 2 | 200 | TBT | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
42![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.8 | 83 |
| 3 | 220 | TBT | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.5 | 78 |
| 4 | 180 | TIP | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
73![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.9 | 83 |
| 5 | 180 | SnCl2 | 3200 | 4800 | 1.5 | 25 |
In order to investigate the interactions between the IL, the monomer and the catalyst, 1H NMR spectroscopy of pure MR, MR + IL (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mole), MR + TBT (1
1 in mole), MR + TBT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mole), and MR + IL + TBT (1
1 in mole), and MR + IL + TBT (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mole) was performed (Fig. S1†). The chemical shifts associated with the functional groups of MR, including methine connected to the hydroxyl, and methyl and methylene connected to the ester bond, are located at 3.61, 3.67 and 2.30 ppm, respectively. When MR was completely dissolved in equimolar IL, the chemical shifts of the groups do not change, suggesting weak interaction between the IL and the monomer (Fig. S1a and b†). When an equimolar catalyst was added into the mixture of MR and the IL, the peaks around 3.6 ppm become complicated since the hydroxyl and carbonyl groups may complex with the transition metal Ti(IV) to form the intermediate transition states (Fig. S1d†). The mixture of MR and TBT, without adding the IL, is similar to that mixing with the IL (Fig. S1c†). The titanate shows remarkable catalytic activity for the polycondensation of MR in the IL, since the molecular weight of PRA polymerized in the IL is up to twice that from bulk polymerization. Evidently, the IL plays an important role in this process. Due to the moderate polarity of the IL resembling alcohol,37 the titanate can interact with the anions and cations of the IL through solvation, allowing for excellent miscibility between the titanate and the IL. The anions NTf2 of the IL have weak coordination ability, potentially forming complexes with the catalytically active center Ti.37 Meanwhile, the imidazolium cations of the IL are electrophilic and may interact with the electron-rich oxygen atom in the titanate, enhancing the polarization of the Ti–O bond, thereby exposing the active center more prominently and increasing the catalytic activity of the catalyst.
1 in mole) was performed (Fig. S1†). The chemical shifts associated with the functional groups of MR, including methine connected to the hydroxyl, and methyl and methylene connected to the ester bond, are located at 3.61, 3.67 and 2.30 ppm, respectively. When MR was completely dissolved in equimolar IL, the chemical shifts of the groups do not change, suggesting weak interaction between the IL and the monomer (Fig. S1a and b†). When an equimolar catalyst was added into the mixture of MR and the IL, the peaks around 3.6 ppm become complicated since the hydroxyl and carbonyl groups may complex with the transition metal Ti(IV) to form the intermediate transition states (Fig. S1d†). The mixture of MR and TBT, without adding the IL, is similar to that mixing with the IL (Fig. S1c†). The titanate shows remarkable catalytic activity for the polycondensation of MR in the IL, since the molecular weight of PRA polymerized in the IL is up to twice that from bulk polymerization. Evidently, the IL plays an important role in this process. Due to the moderate polarity of the IL resembling alcohol,37 the titanate can interact with the anions and cations of the IL through solvation, allowing for excellent miscibility between the titanate and the IL. The anions NTf2 of the IL have weak coordination ability, potentially forming complexes with the catalytically active center Ti.37 Meanwhile, the imidazolium cations of the IL are electrophilic and may interact with the electron-rich oxygen atom in the titanate, enhancing the polarization of the Ti–O bond, thereby exposing the active center more prominently and increasing the catalytic activity of the catalyst.
As shown in Table 1, the polymer yields are not very high, near 80%, probably due to the monomer evaporation at the high polymerization temperature and vacuum, polymer residue in the reactors, and the dissolution of the oligomer in the washing solvent.
Fig. 3 shows the influence of catalyst dosage on the solution polycondensation of MR in [OdMIM][NTf2]. Under the same conditions, the highest molecular weight is achieved with a catalyst dosage of 1 wt% relative to the monomer. Since too much catalyst will increase side reactions and make reactions uncontrollable, Mw decreases from 76 kDa to 53 kDa when the catalyst dosage increases to 2 wt%. PRA with Mw less than 10 kDa is obtained when the catalyst dosage is only 0.1 wt%, indicating extremely low catalytic efficiency to use too small amount of catalyst. As a result, the suitable catalyst dosage is 1 wt% as a compromise between the catalytic efficiency and the side reactions.
 | ||
Fig. 3 Solution polycondensation of MR in [OdMIM][NTf2] with different catalyst dosages (conditions: TBT used as the catalyst, MR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [OdMIM][NTf2] = 1 [OdMIM][NTf2] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mole, 180 °C, 72 h). 1 in mole, 180 °C, 72 h). | ||
The polycondensation process of MR was monitored by interval sampling. As shown in Fig. 4, the molecular weight of PRA increases with time during the polymerization. In the early stage, the polycondensation of MR is fast within 48 h, although the polymer is precipitated from the IL after polycondensation about 20 h. Polymer separation will increase the concentration of the terminal groups and makes the polycondensation maintain high enough polymerization rate even when the viscosity of the polymerization system and the extent of the polycondensation increase. However, the polymerization rate after 48 h decreases remarkably due to the fast increase of the polymer molecular weight and the viscosity thereof. Increasing the polycondensation extent significantly reduces the concentration of reactive functional groups. Additionally, high viscosity blocks the access between the functional groups and makes the removal of methanol difficult and thus the reverse reaction to degrade PRA from alcoholysis becomes non-negligible in the polycondensation process. Consequently, the polycondensation is extremely difficult to proceed in the late stage (after ca. 72 h) when the molecular weight of the polyester is high enough.
 | ||
Fig. 4 Change of PRA molecular weights during polymerization (conditions: MR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) [OdMIM][NTf2] = 1 [OdMIM][NTf2] = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mole, 1 wt% of TIP as the catalyst, 180 °C). 1 in mole, 1 wt% of TIP as the catalyst, 180 °C). | ||
We attempted to decrease the viscosity in the late stage by raising the polymerization temperature or increasing the concentration of IL. However, as shown in Table 1, the molecular weight of PRA polymerized at 200 °C and 220 °C is lower than that at 180 °C, which can be attributed to the increasing complex side reactions, such as the elimination or oxidation reactions of the hydroxy groups, the decarboxylation reaction of the ester groups and the oxidation or addition reactions of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds. An obvious proof is that the color of the polymer products changes from light yellow to light brown as the polymerization temperature increases (Fig. S2†). And surprisingly, increasing the concentration of the IL fails to increase the molecular weight (Fig. 5). At the same time, we found that phase separation between the viscous PRA and the transparent IL occurs after about 20 h of polycondensation (Fig. S3†). The viscous PRA polyester with medium molecular weight cannot be dissolved in the IL probably due to the relatively high polarity of the IL. However, the molecular weight achieved from MR
C double bonds. An obvious proof is that the color of the polymer products changes from light yellow to light brown as the polymerization temperature increases (Fig. S2†). And surprisingly, increasing the concentration of the IL fails to increase the molecular weight (Fig. 5). At the same time, we found that phase separation between the viscous PRA and the transparent IL occurs after about 20 h of polycondensation (Fig. S3†). The viscous PRA polyester with medium molecular weight cannot be dissolved in the IL probably due to the relatively high polarity of the IL. However, the molecular weight achieved from MR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL = 1
IL = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mole is a little higher than that without applying IL. When increasing the amount of IL, the functional groups are greatly diluted, leading to an exponential decrease of the polymerization rate and the formation of RA lactones.11 The RA lactones can be evaporated at the polymerization temperature and high vacuum. Thus, the Mw of PRA polymerized in 90 mol% of IL is even lower than 10 kDa. In contrast, when the concentration of IL is about 20 mol%, the Mw of PRA reached 122 kDa, which is about twice that of the melt polycondensation. NMR determination of ethanol-washed PRA proved that a little amount of IL is miscible with PRA even after macroscopic phase separation happened (Fig. S5†). The IL dissolved in the polymer bulk may play a key role in viscosity reduction and facilitates the progress of the polycondensation in the late stage.
1 in mole is a little higher than that without applying IL. When increasing the amount of IL, the functional groups are greatly diluted, leading to an exponential decrease of the polymerization rate and the formation of RA lactones.11 The RA lactones can be evaporated at the polymerization temperature and high vacuum. Thus, the Mw of PRA polymerized in 90 mol% of IL is even lower than 10 kDa. In contrast, when the concentration of IL is about 20 mol%, the Mw of PRA reached 122 kDa, which is about twice that of the melt polycondensation. NMR determination of ethanol-washed PRA proved that a little amount of IL is miscible with PRA even after macroscopic phase separation happened (Fig. S5†). The IL dissolved in the polymer bulk may play a key role in viscosity reduction and facilitates the progress of the polycondensation in the late stage.
 | ||
| Fig. 5 Polycondensation of MR in different amounts of [OdMIM][NTf2] (conditions: TBT as the catalyst, 180 °C, 72 h). | ||
The polycondensation of MR in different ILs was investigated to demonstrate the structural influence of the ILs. As shown in Fig. 6 and Table 2, the molecular weight of PRA increases with the length of the substituted alkyl chain of the imidazoliums. The highest Mw is achieved in [OdMIM][NTf2], which is about 1.4 times that in [HMIM][NTf2]. The repeating unit of PRA also has a long alkyl chain and its carbon number is 18, which is equal to that of the octadecyl substituent of the imidazolium. According to the rule of “like dissolves like”, both PRA and [OdMIM][NTf2] have high hydrophobicity and the long alkyl chains get entangled with each other to enhance their hydrophobic interactions. Compared to the melt polycondensation, much higher molecular weights can be achieved via the solution polycondensation of MR in hydrophobic ILs (Fig. 6). Consequently, the presence of hydrophobic ILs can reduce the viscosity of PRA at the late stage and the better the miscibility between PRA and the ILs is, the higher the molecular weight is achieved. Besides, the imidazolium cations may induce polarization of the end carbonyl groups of the polymer chain to accelerate the polycondensation.14,38
 | ||
Fig. 6 Solution polycondensation of MR in different ILs (conditions: MR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL = 4 IL = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mole, TBT as the catalyst, 180 °C, 72 h). 1 in mole, TBT as the catalyst, 180 °C, 72 h). | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL = 4
IL = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mole, TBT as the catalyst, 180 °C, 72 h)
1 in mole, TBT as the catalyst, 180 °C, 72 h)
| Entry | Ionic liquid | M n (Da) | M w (Da) | PDI | Yield (%) |
|---|---|---|---|---|---|
| 1 | None | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
61![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.4 | 90 |
| 2 | [HMIM][NTf2] | 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 500 |
1.8 | 87 |
| 3 | [DMIM][NTf2] | 55![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
1.8 | 85 |
| 4 | [TdMIM][NTf2] | 53![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
2.0 | 87 |
| 5 | [OdMIM][NTf2] | 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
123![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
1.9 | 88 |
The structure and thermal properties of PRA were determined. Chemical shifts at ca. 5.46 ppm and 5.34 ppm in the 1H NMR spectrum of PRA show that the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds derived from the monomer are not broken during the solution polycondensation of MR in the ILs (Fig. S5†). Moreover, the 13C NMR spectrum of PRA reveals the cis structure of the C
C double bonds derived from the monomer are not broken during the solution polycondensation of MR in the ILs (Fig. S5†). Moreover, the 13C NMR spectrum of PRA reveals the cis structure of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bonds according to the chemical shifts at 132 and 125 ppm and there are no signals of the trans structure (Fig. 7). Since the cis structure is really important to increase the flexibility and cold-resistance of polymers, the PRA with a 100% cis structure is expected to be an excellent elastomer. Differential scanning calorimetry (DCS) reveals that the PRA has a very low glass transition temperature (Tg) of −72.8 °C (Fig. S6†), which is lower than that (−67–−60 °C) from ring-opening metathesis polymerization.10,12 Thermal gravimetric analysis shows that the decomposition temperature of the PRA with 5% weight loss is 319 °C, suggesting good thermal stability of the PRA (Fig. S7†).
C double bonds according to the chemical shifts at 132 and 125 ppm and there are no signals of the trans structure (Fig. 7). Since the cis structure is really important to increase the flexibility and cold-resistance of polymers, the PRA with a 100% cis structure is expected to be an excellent elastomer. Differential scanning calorimetry (DCS) reveals that the PRA has a very low glass transition temperature (Tg) of −72.8 °C (Fig. S6†), which is lower than that (−67–−60 °C) from ring-opening metathesis polymerization.10,12 Thermal gravimetric analysis shows that the decomposition temperature of the PRA with 5% weight loss is 319 °C, suggesting good thermal stability of the PRA (Fig. S7†).
 | ||
Fig. 7
13C NMR spectrum of PRA polymerized in [OdMIM][NTf2] with 1 wt% of TIP (Conditions: MR![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL = 1 IL = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 180 °C, 72 h). 1, 180 °C, 72 h). | ||
The ILs can be readily recovered by decanting the liquid from the reactor owing to the phase separation between the PRA and the ILs at the late stage of the polycondensation. The recovery of the IL is only about 80% and the left IL is miscible with and embedded in the polymer bulk. Taking [OdMIM][NTf2] for instance, the 1H NMR spectrum of the recovered IL shows the intact structure, where the peaks are in good agreement with those of the freshly prepared one (Fig. S3†). In addition, the PRA and the IL maintain their respective characteristic peaks even after polymerization for 72 h at 180 °C (Fig. S4†), which further demonstrates the inert feature of the ILs as a polymerization solvent. Thermal gravimetric analysis of the polymerization mixture exhibits two pyrolysis stages and the pyrolysis temperatures agree well with their individual pyrolysis temperatures (Fig. S6†), indicating good stability of the ILs even at the decomposition temperature (319 °C) of PRA.
The recovered [OdMIM][NTf2] without any further treatment was employed as the polycondensation solvent of MR to evaluate the reusability of the ILs. Under the same polycondensation conditions, the molecular weight of PRA polymerized in the recovered IL after recycling 4 times reduces little as compared to that achieved in the fresh IL (Table 3). Since the recovered ILs are colorless and transparent in appearance and the NMR characterization indicates intact chemical structures, the NTf2-anionic ILs have excellent reusability.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IL = 1
IL = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 in mole, 1 wt% of TBT as the catalyst, 180 °C, 72 h)
1 in mole, 1 wt% of TBT as the catalyst, 180 °C, 72 h)
| Entry | IL | M n (Da) | M w (Da) | PDI | Yield (%) | IL recovery (%) |
|---|---|---|---|---|---|---|
| 1 | Fresh [OdMIM][NTf2] | 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
1.90 | 85 | 89.5 |
| 2 | Recovered [OdMIM][NTf2] | 37![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 700 |
1.74 | 81 | 82 |
| 3 | 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
68![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
2.01 | 86 | 83 | |
| 4 | 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
1.94 | 82 | 83 | |
| 5 | 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 600 |
72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
1.79 | 84 | 79 |
4. Conclusion
PRA with high molecular weights was successfully synthesized by the solution polycondensation of MR in hydrophobic ILs, namely 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide. Under optimal experimental conditions, the Mw of PRA can reach 122 kDa, which is about twice that from the melt polycondensation. The viscosity of the polymer can be greatly reduced to accelerate the polycondensation when the polycondensation is carried out in the IL solvent. Since the IL substituted with a longer alkyl chain has higher hydrophobicity and better miscibility with PRA, the highest molecular weight of PRA is achieved in [OdMIM][NTf2] with the longest alkyl chain. The easily recovered ILs by phase separation show excellent purity and have good reusability when applied as the polycondensation solvent of MR. The polymerized PRA in the ILs has a glass transition temperature as low as −72.8 °C and a decomposition temperature of 319 °C, which is a bio-based degradable elastomer with potential applications. Given the significant improvements the ILs offer in the polycondensation system, future research should focus on understanding the interaction mechanism between the ions of ILs and the monomer and polymer molecules.Data availability
The data underlying this article are available in the article and its online ESI.†Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
The authors thank the National Natural Science Foundation of China (Grant no. 51903130, U23A20587, 52072193, U22A20131 and 52361165657), the Natural Science Foundation of Shandong Province (Grant no. ZR2017PB012, ZR2021J016 and ZR2023YQ040), the Program of Source Innovation of Applied Basic Research in Qingdao City (Grant no. 171124jch), and the Shandong Provincial College Students' innovation and entrepreneurship training program (S202311065122) for financial support.References
- G. Totaro, L. Cruciani, M. Vannini, G. Mazzola, D. Di Gioia, A. Celli and L. Sisti, Synthesis of castor oil-derived polyesters with antimicrobial activity, Eur. Polym. J., 2014, 56, 174–184 CrossRef CAS.
- M. Safari, N. Kasmi, C. Pisani, V. Berthe, A. J. Muller and Y. Habibi, Effect of the structural features of biobased linear polyester plasticizers on the crystallization of polylactides, Int. J. Biol. Macromol., 2022, 214, 128–139 CrossRef CAS PubMed.
- C. Aguilar-Castro, M. D. Gomez, M. G. Nava, J. M. R. García and L. E. L. Uribe, Biobased polyester obtained from bifunctional monomers through metathesis of fatty acids as precursor to synthesis of polyurethanes, J. Appl. Polym. Sci., 2019, 136(8), 47095 CrossRef.
- H. Meheust, J.-F. Le Meins, E. Grau and H. Cramail, Bio-Based Polyricinoleate and Polyhydroxystearate: Properties and Evaluation as Viscosity Modifiers for Lubricants, ACS Appl. Polym. Mater., 2021, 3(2), 811–818 CrossRef CAS.
- Q. Zhang, M. Song, Y. Xu, W. Wang, Z. Wang and L. Zhang, Bio-based polyesters: Recent progress and future prospects, Prog. Polym. Sci., 2021, 120, 101430 CrossRef CAS.
- Y. Xia and R. C. Larock, Vegetable oil-based polymeric materials: synthesis, properties, and applications, Green Chem., 2010, 12(11), 1893–1909 RSC.
- K. R. Kunduru, A. Basu, M. Haim Zada and A. J. Domb, Castor Oil-Based Biodegradable Polyesters, Biomacromolecules, 2015, 16(9), 2572–2587 CrossRef CAS PubMed.
- F. O. Nitbani, P. J. P. Tjitda, H. E. Wogo and A. I. R. Detha, Preparation of Ricinoleic Acid from Castor Oil:A Review, J. Oleo Sci., 2022, 71(6), 781–793 CrossRef CAS.
- R. Slivniak and A. J. Domb, Lactic acid and ricinoleic acid based copolyesters, Macromolecules, 2005, 38(13), 5545–5553 CrossRef CAS.
- J. E. Gautrot and X. X. Zhu, High molecular weight bile acid and ricinoleic acid-based copolyesters via entropy-driven ring-opening metathesis polymerisation, Chem. Commun., 2008, 14, 1674–1676 RSC.
- R. Slivniak and A. J. Domb, Macrolactones and polyesters from ricinoleic acid, Biomacromolecules, 2005, 6(3), 1679–1688 CrossRef CAS PubMed.
- R. Ogawa and M. A. Hillmyer, High molar mass poly(ricinoleic acid) via entropy-driven ring-opening metathesis polymerization, Polym. Chem., 2021, 12, 2253–2257 RSC.
- H. Ebata, K. Toshima and S. Matsumura, Lipase-Catalyzed Synthesis and Curing of High-Molecular-Weight Polyricinoleate, Macromol. Biosci., 2007, 7(6), 757 CrossRef.
- Q. Peng, L. Wei, X. Zhang, Y. Wu, K. Mahmood, Z. Liu and L. Zhang, Direct Polycondensation of L-lactic Acid in Hydrophobic Bis(trifluoromethanesulfonyl)imide-Anionic Ionic Liquids: A Kinetic Study, Eur. Polym. J., 2021, 110692 CrossRef CAS.
- Q. Peng, K. Mahmood, Y. Wu, L. Wang, Y. Liang, J. Shen and Z. Liu, A facile route to realize the copolymerization of L-lactic acid and ε-caprolactone: sulfonic acid-functionalized Brønsted acidic ionic liquids as both solvents and catalysts, Green Chem., 2014, 16(4), 2234–2241 RSC.
- S. Zhang, V. Lemaire, A. Feret, H. Lefebvre, M. Tessier and A. Fradet, Synthesis of linear and hyperbranched polyesters in Bronsted acid ionic liquids, Polym. Chem., 2013, 4(5), 1538–1545 RSC.
- S. Zhang, H. Lefebvre, M. Tessier and A. Fradet, Influence of Bronsted acid ionic liquid structure on hydroxyacid polyesterification, Green Chem., 2011, 13(10), 2786–2793 RSC.
- E.-M. Dukuzeyezu, H. Lefebvre, M. Tessier and A. Fradet, Synthesis of high molar mass poly(12-hydroxydodecanoic acid) in bronsted acid ionic liquids, Polymer, 2010, 51(6), 1218–1221 CrossRef CAS.
- S. Zhang, A. Feret, H. Lefebvre, M. Tessier and A. Fradet, Poly(oxyalkylene) synthesis in Bronsted Acid Ionic Liquids, Chem. Commun., 2011, 47(39), 11092–11094 RSC.
- E. I. Lozinskaya, A. S. Shaplov and Y. S. Vygodskii, Direct polycondensation in ionic liquids, Eur. Polym. J., 2004, 40(9), 2065–2075 CrossRef CAS.
- Y. S. Vygodskii, E. I. Lozinskaya, A. S. Shaplov, K. A. Lyssenko, M. Y. Antipin and Y. G. Urman, Implementation of ionic liquids as activating media for polycondensation processes, Polymer, 2004, 45(15), 5031–5045 CrossRef.
- T. Ogoshi, T. Onodera, T. A. Yamagishi and Y. Nakamoto, Green Polymerization of Phenol in Ionic Liquids, Macromolecules, 2008, 41(22), 8533–8536 CrossRef CAS.
- J. Dou and Z. Liu, Crystallization behavior of poly(ethylene terephthalate)/pyrrolidinium ionic liquid, Polym. Int., 2013, 62(12), 1698–1710 CrossRef CAS.
- J. Dou and Z. Liu, A simple and efficient synthetic method for poly(ethylene terephthalate): phenylalkyl pyrrolidinium ionic liquid as polycondensation medium, Green Chem., 2012, 14(8), 2305–2313 RSC.
- S. D. Zhang, L. D. Goncalves, H. Lefebvre, M. Tessier, B. Rousseau and A. Fradet, Direct Poly(beta-alanine) Synthesis via Polycondensation in Ionic Liquids, ACS Macro Lett., 2012, 1(8), 1079–1082 CrossRef CAS PubMed.
- J. Wang and Z. Liu, An efficient synthetic strategy for high performance polysulfone: ionic liquid/zwitterion as reaction medium, Green Chem., 2012, 14(11), 3204–3210 RSC.
- J. Wang, Y. Wu and Z. Liu, A facile and highly efficient protocol for synthesis of poly(ether sulfone)s in ionic liquid/zwitterion, Chin. J. Polym. Sci., 2016, 34(8), 981–990 CrossRef CAS.
- J. Wang and Z. Liu, Ionic liquids as green reaction media for synthesis of poly(aryl ether ketone)s, Chin. Sci. Bull., 2013, 58(11), 1262–1266 CrossRef CAS.
- D. He, Y. Wu, Z. Liu and T. Zhao, The synthesis of poly(phenylene sulfide sulfone) in ionic liquids at atmospheric pressure, RSC Adv., 2017, 7(63), 39604–39610 RSC.
- S. Dewilde, T. Vander Hoogerstraete, W. Dehaen and K. Binnemans, Synthesis of Poly-p-phenylene Terephthalamide (PPTA) in Ionic Liquids, ACS Sustainable Chem. Eng., 2018, 6(1), 1362–1369 CrossRef CAS.
- H. Q. N. Gunaratne, C. R. Langrick, A. V. Puga, K. R. Seddon and K. Whiston, Production of polyetheretherketone in ionic liquid media, Green Chem., 2013, 15(5), 1166–1172 RSC.
- C. Fu and Z. Liu, Syntheses of high molecular weight aliphatic polyesters in 1-alkyl-3-methylimidazolium ionic liquids, Polymer, 2008, 49(2), 461–466 CrossRef CAS.
- S. Dali, H. Lefebvre, R. El Gharbi and A. Fradet, Synthesis of poly(glycolic acid) in ionic liquids, J. Polym. Sci., Part A: Polym. Chem., 2006, 44(9), 3025–3035 CrossRef CAS.
- J. G. Huddleston, A. E. Visser, W. M. Reichert, H. D. Willauer, G. A. Broker and R. D. Rogers, Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation, Green Chem., 2001, 3(4), 156–164 RSC.
- G. X. Chen, H. S. Kim, E. S. Kim and J. S. Yoon, Synthesis of high-molecular-weight poly(L-lactic acid) through the direct condensation polymerization of L-lactic acid in bulk state, Eur. Polym. J., 2006, 42(2), 468–472 CrossRef CAS.
- S. I. Moon, C. W. Lee, I. Taniguchi, M. Miyamoto and Y. Kimura, Melt/solid polycondensation of L-lactic acid: an alternative route to poly(L-lactic acid) with high molecular weight, Polymer, 2001, 42(11), 5059–5062 CrossRef CAS.
- J. P. Hallett and T. Welton, Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. 2, Chem. Rev., 2011, 111(5), 3508–3576 CrossRef CAS PubMed.
- W. Wang, L. Wu, Y. Huang and B.-G. Li, Melt polycondensation of L-lactic acid catalyzed by 1,3-dialkylimidazolium ionic liquids, Polym. Int., 2008, 57(6), 872–878 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4su00185k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |