Open Access Article
Open Access ArticleA multi-biocatalytic system for effective fumarate synthesis from pyruvate and gaseous CO2†
Mika
Takeuchi
a and
Yutaka
Amao
 *ab
*ab
aGraduate School of Science, Osaka Metropolitan University, 3-3-138 Sugimoto, Sumiyoshi-ku, Osaka 558-8585, Japan
bResearch Centre of Artificial Photosynthesis (ReCAP), Osaka Metropolitan University, 3-3-138 Sugimoto, Sumiyoshi-ku, Osaka 558-8585, Japan. E-mail: amao@omu.ac.jp
First published on 22nd July 2024
Abstract
Fumarate, an unsaturated dicarboxylic acid, is an important material for producing unsaturated polyester resins and biodegradable plastics. Fumarate synthesis from petroleum-derived benzene and butane as starting materials is expected to be replaced by synthesis methods from renewable raw materials. In this work, fumarate synthesis from gaseous CO2 and pyruvate in an aqueous medium using a multi-biocatalytic system consisting of pyruvate carboxylase (PC), malate dehydrogenase (MDH) and fumarase (FUM) in the presence of ATP and NADH is accomplished. The conversion yield of fumarate from pyruvate using this system was estimated to be approximately 16% after 5 h of incubation.
Sustainability spotlightThis research aims to prepare fumarate, the precursor of unsaturated, alkyd and biodegradable polymers, from gaseous CO2 and biomass-derived molecules using multiple biocatalysts in aqueous media under mild conditions compared with the conventional industrial method for the synthesis of precursors of various polymers. Therefore, this research contributes to gaseous CO2 fixation and to the production of alternative plastic precursors for a sustainable society. This system allows CO2 to be fixed in organic molecules and converted into high value-added materials, thus providing long-term storage of gaseous CO2 in molecules. The study is in line with the goals 7, “Affordable and Clean Energy”, and 12, “Responsible Consumption and Production”, of the UN's Sustainable Development Goals. |
Unsaturated polyesters are synthesised by the dehydration condensation of fumaric acid with ethylene glycol.1,2 Also, fumaric acid-modified alkyd resins, derived from polyols and organic acids including dicarboxylic acids or carboxylic acid anhydrides and triglyceride oils, are receiving significant attention in the field of engineering plastics.3 The copolymerisation of succinic acid and 1,4-butanediol, obtained by the hydrogenation and reduction of fumaric acid respectively, yields the biodegradable plastic polybutylene succinate (PBS).4 Thus, fumarate is an important unsaturated dicarboxylic acid to produce unsaturated polyester resins and biodegradable plastics. Fumarate production from CO2 (gaseous CO2 or bicarbonate) and pyruvate using a malate dehydrogenase decarboxylating type enzyme, commonly known as malic enzyme (ME; EC 1.1.1.38), and fumarase (FUM; EC 4.2.1.2) as catalysts in the presence of NADH viaL-malate as an intermediate has been reported as shown in Fig. 1.5,6 The yield of fumarate from pyruvate after 5 h of incubation was estimated to be ca. 11% with this system. ME, which is used in this system to produce the intermediate L-malate, catalyses the binding of CO2 to pyruvate to produce oxaloacetate and the reduction of oxaloacetate to L-malate in the presence of NADH. ME is a useful enzyme for producing L-malate from pyruvate and CO2, but it has the disadvantage that it also catalyses the reduction of pyruvate to L-lactate, which limits the reaction conditions.7 In addition, the efficiency of FUM-catalysed fumarate production requires high concentrations of the intermediate L-malate to be produced in a short incubation time. In other words, improved efficiency of L-malate production is a key prerequisite for a biocatalytic fumarate production system viaL-malate from pyruvate and CO2.
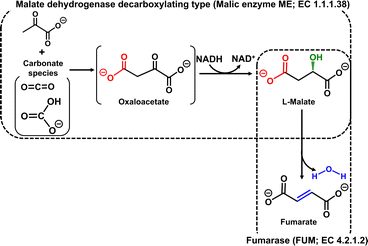 | ||
| Fig. 1 Biocatalytic fumarate production from CO2 (gaseous CO2 or bicarbonate) and pyruvate with ME and FUM in the presence of NADH. | ||
To solve these problems, the present study devised a ‘more haste, less speed’ strategy, in which two enzymes are responsible for each of the two catalytic functions of MDH. One is pyruvate carboxylase (PC; EC 6.4.1.1) for oxaloacetate production by the carboxylation of pyruvate from CO2 in the presence of adenosine triphosphate (ATP).8 The other is malate dehydrogenase (MDH; EC 1.1.1.37) for the reduction of oxaloacetate to produce L-malate in the presence of NADH.9 By using these enzymes and FUM, a fumarate production system can be constructed from pyruvate and CO2 in the presence of ATP and NADH, as shown in Fig. 2.
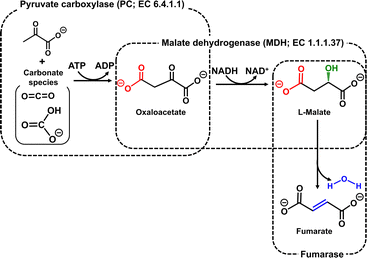 | ||
| Fig. 2 Biocatalytic fumarate production from CO2 (gaseous CO2 or bicarbonate) and pyruvate with PC, MDH and FUM in the presence of ATP and NADH. | ||
In this work, multi-biocatalytic (PC, MDH and FUM) fumarate production from gaseous CO2 and biobased pyruvate as the starting materials in the presence of ATP and NADH was investigated.
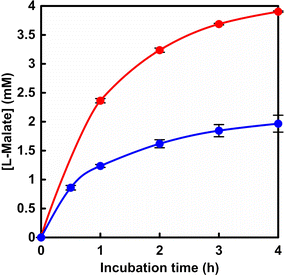
First, L-malate production from gaseous CO2 and pyruvate with PC and MDH in the presence of ATP and NADH was attempted. PC from bovine liver (EC 6.4.1.1) was obtained from Merck KGaA. The molecular weight of a similar PC obtained from chicken liver or pig liver is reported to be 660 kDa.10,11 The MDH recombinant from bacteria (EC 1.1.1.37) was obtained from Oriental Yeast Co., Ltd. The molecular weight of a similar MDH is reported to be 70 kDa.12,13 The sample solution consisted of sodium pyruvate (5.0 mM), manganese chloride (5.0 mM), ATP (5.0 mM), NADH (5.0 mM), acetyl-CoA (1.0 mM), PC (1.0 U) and MDH (10 U) in 5.0 mL of 500 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) buffer (pH 7.2). As shown in Fig. 2, oxaloacetate is produced as an intermediate. Oxaloacetate is unstable and accumulates at high concentrations, decomposing into pyruvate and CO2. Therefore, the activity of MDH was prepared at 10 times the amount of PC to avoid the accumulation of oxaloacetate. Here, acetyl-CoA functions as an allosteric activator for PC and is an essential factor for pyruvate carboxylation.14 In addition, a magnesium ion is required for coordination to ATP and to act as a cofactor in the phosphorylation of bicarbonate in the PC.15 CO2 gas was introduced into the gas phase of the reactor.16 The reaction used an isobaric system as shown in Fig. S1.†16 The total pressure in the reaction system was maintained at 1.01325 × 105 Pa. The reaction was carried out in a thermostatic chamber set at a temperature of 30.5 °C. The amount of L-malate produced was detected by ion chromatography (Metrohm, Eco IC; electrical conductivity detector) with an ion exclusion column (Metrosep Organic Acids 250/7.8 Metrohm; column size: 7.8 × 250 mm; composed of a 9 μm polystyrene-divinylbenzene copolymer with sulfonic acid groups). Details of L-malate quantification by ion chromatography are described in the ESI.† The L-malate concentration was determined from the calibration curve based on the chromatogram of a standard sample (Fig. S2) using eqn (S1).†Fig. 3 shows the time dependence of L-malate production with the system of sodium pyruvate, manganese chloride, ATP, NADH, acetyl-CoA, PC and MDH in HEPES buffer (pH 7.2) filled with gas phase CO2. Fig. S3† shows a chart of an ion chromatogram sampled from the reaction solution of sodium pyruvate, manganese chloride, ATP, NADH, acetyl-CoA, PC and MDH in HEPES buffer filled with gas phase CO2. The time dependence of L-malate production with the system of sodium pyruvate (5.0 mM), magnesium chloride (5.0 mM), NADH (5.0 mM) and ME (0.7 U; EC 1.1.1.38 code: MDH-73-01 obtained from Sulfolobus tokodaii) in HEPES buffer (pH 7.0) filled with gas phase CO2 is also shown in Fig. 3 (blue). The magnesium ion plays a stabilising role by coordinating to pyruvate or the intermediate oxaloacetate, respectively, in the ME.
The reason for comparing the activity of ME (0.7 U) with that of MDH (10 U) is that it was reported that under conditions where the activity of ME was increased by about 10-fold, only L-lactate was produced (no L-malate production was observed).17 As shown in Fig. 3, the concentration of L-malate production increased with incubation time in both reaction systems. By using the dual-biocatalytic (PC and MDH) system, the yield of L-malate from pyruvate was estimated to be ca. 80% after 4 h of incubation. On the other hand, the yield of L-malate from pyruvate was estimated to be ca. 40% after 4 h of incubation using ME. From these results, a drastic yield improvement in L-malate production from CO2 and pyruvate was achieved by using the dual-biocatalytic (PC and MDH) system. Let us discuss each biocatalytic system on the basis of enzymatic kinetic analysis for the production of L-malate using pyruvate as a substrate. In the system with dual-biocatalysis (PC and MDH), enzymatic kinetic analysis was applied to L-malate production from pyruvate and bicarbonate using the two enzymes. The sample solution consisted of sodium pyruvate, manganese chloride (5.0 mM), ATP (2.0 mM), NADH (2.0 mM), acetyl-CoA (1.0 mM), sodium bicarbonate (50 mM), PC (1.0 U) and MDH (10 U) in 5.0 mL of 500 mM HEPES buffer (pH 7.2). The concentration of sodium pyruvate was varied from 0 to 5.0 mM. The amount of L-malate produced was detected by ion chromatography. The initial rate (v0) was calculated from the concentration of L-malate production after 30 min of incubation. ME catalyzed L-malate production from pyruvate and bicarbonate under the following conditions. The sample solution consisted of sodium pyruvate, manganese chloride (5.0 mM), NADH (2.0 mM), sodium bicarbonate (50 mM) and ME (0.7 U) in 5.0 mL of 500 mM HEPES buffer (pH 7.2). The concentration of sodium pyruvate was varied from 0 to 5.0 mM.
Fig. 4 shows the relationship between the pyruvate concentration and initial rate for L-malate production (v0) in the system with dual-biocatalysis (PC and MDH) and with ME.
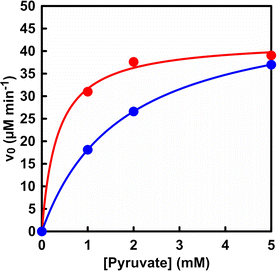 | ||
| Fig. 4 Relationship between the sodium pyruvate concentration and the initial rate for L-malate production (v0). Red circle: dual-biocatalytic (PC and MDH) system. Blue circle: ME. | ||
As shown in Fig. 4, the initial rate tended to increase with increasing pyruvate concentration, followed by a constant rate in both systems. Also, the plot in Fig. 4 obeyed the Michaelis–Menten relationship in both systems. Table 1 shows the kinetic parameter, the Michaelis constant (Km) and the maximum rate (Vmax) of pyruvate concentration for L-malate production in the system with dual-biocatalysis (PC and MDH) and with ME.
| System | K m (mM) | V max (μM min−1) |
|---|---|---|
| a The Km and Vmax values were determined using curve-fitting of the Michaelis–Menten equation (v0 = Vmax [pyruvate])/(Km + [pyruvate]). | ||
| Dual-biocatalytic (PC and MDH) system | 0.37 | 40.0 |
| ME | 1.76 | 50.0 |
As shown in Table 1, there was no noticeable difference in the maximum rate Vmax between the two systems. On the other hand, the Km value of the system with dual-biocatalysis (PC and MDH) was found to be about five times smaller than that of the system with ME. This suggests that the substrate affinity of pyruvate is higher in the system with dual-biocatalysis (PC and MDH) than in the system with ME. In addition, L-malate production is suppressed and L-lactate is produced under low pyruvate concentration conditions in the system with ME, as shown in Fig. S4.† A signal based on L-lactate production was detected on the chart of the ion chromatograph. Thus, effective L-malate production from pyruvate and bicarbonate was accomplished by using the system with dual-biocatalysis (PC and MDH).
Finally, fumarate production from gaseous CO2 and pyruvate with PC, MDH and FUM in the presence of ATP and NADH was attempted. FUM from porcine heart (molecular weight: 200 kDa)18,19 was purchased from Merck Co., Ltd. The sample solution consisted of sodium pyruvate (5.0 mM), manganese chloride (5.0 mM), ATP (5.0 mM), NADH (5.0 mM), acetyl-CoA (1.0 mM), PC (1.0 U), MDH (10 U) and FUM (0.5 U) in 5.0 mL of 500 mM HEPES buffer (pH 7.2). CO2 gas was introduced into the gas phase of the reactor. The reaction used an isobaric system as shown in Fig. S1.† The total pressure in the reaction system was maintained at 1.01325 × 105 Pa. The reaction was carried out in a thermostatic chamber set at a temperature of 30.5 °C. The amount of L-malate and fumarate produced was detected by ion chromatography. Details of fumarate quantification by ion chromatography are described in the ESI.† The fumarate concentration was determined from the calibration curve based on the chromatogram of a standard sample (Fig. S5) using eqn (S2).†
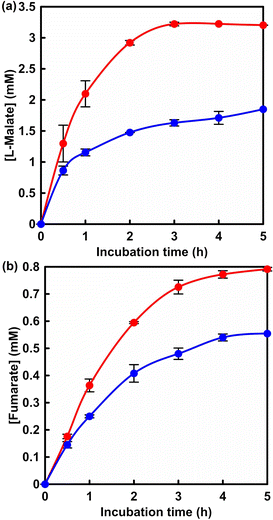
Fig. 5 shows the time dependence of L-malate (a) and fumarate (b) with the system of sodium pyruvate, manganese chloride, ATP, NADH, acetyl-CoA, PC, MDH and FUM in HEPES buffer (pH 7.2) filled with gas phase CO2. Fig. S6† shows a chart of an ion chromatogram sampled from the reaction solution of sodium pyruvate, manganese chloride, ATP, NADH, acetyl-CoA, PC, MDH and FUM in HEPES buffer filled with gas phase CO2 The time dependence of L-malate (a) and fumarate (b) with the system of sodium pyruvate (5.0 mM), magnesium chloride (5.0 mM), NADH (5.0 mM), ME (0.7 U) and FUM (0.5 U) in HEPES buffer (pH 7.0) filled with gas phase CO2 is also shown in Fig. 5 (blue). L-Malate and fumarate concentrations increased with incubation time in both systems. After 5 h of incubation, 3.2 mM of L-malate was produced in the system with multi-biocatalysis (PC, MDH and FUM). On the other hand, 1.7 mM of L-malate was produced in the system with ME and FUM. By using the system with multi-biocatalysis (PC, MDH and FUM), more L-malate production was observed than in the system with ME and FUM. For fumarate production, 0.80 and 0.55 mM of fumarate were produced in the system with multi-biocatalysis (PC, MDH and FUM) and with ME and FUM after 5 h of incubation, respectively. In a catalytic system consisting of ME and FUM, the yield of fumarate from pyruvate was only 11% after 5 h of incubation. After 5 h of incubation, on the other hand, the yield of fumarate from pyruvate increased to 16% in the system with multi-biocatalysis (PC, MDH and FUM). Here, the Km and Vmax of fumarate production based on the FUM-catalysed dehydration of L-malate were calculated to be 0.65 mM and 0.82 μM s−1, respectively.17 In general, three times the Km of the substrate is required for the reaction to proceed consistently at the maximum rate. For efficient fumarate production, thus, an L-malate concentration of more than 2.0 mM is required. In other words, in a multi-biocatalytic system, improving the yield of L-malate production in a short reaction time leads to a subsequent improvement in the efficiency of fumarate production. Therefore, it is expected that increasing the pyruvate concentration of the starting precursor will improve the fumarate production concentration in a multi-biocatalytic system.
In conclusion, the improvement of yield for fumarate synthesis from gaseous CO2 and pyruvate in an aqueous medium using a multi-biocatalytic system consisting of PC, MDH and FUM in the presence of ATP and NADH is accomplished. Especially, the dual-biocatalytic system consisting of PC and MDH drastically improved the yield of L-malate production from pyruvate to about 80% in the presence of ATP and NADH. Moreover, the yield of fumarate production from pyruvate also was improved by using the multi-biocatalytic (PC, MDH and FUM) system. We have reported fumarate production from pyruvate and CO2 gas by adding ME and FUM as catalysts to a visible-light driven NADH regeneration system consisting of triethanolamine, water-soluble zinc porphyrin and a pentamethylcyclopentadienyl coordinated rhodium(III) 2,2′-bipyridyl complex.16,17 As L-malate production is the rate-limiting step in this system, the application of a multi-enzyme (PC, MDH and FUM) is expected to improve the yield of fumarate production with visible-light driven NADH regeneration.17 Furthermore, we plan to incorporate an ATP-regenerating system with polyphosphate kinase (EC 2.7.4.1).20
Data availability
The authors confirm that the data supporting the findings of this manuscript are available within the article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the Grant-in-Aid for Specially Promoted Research (23H05404), Scientific Research (B) (22H01872) and (22H01871), and by the Institute for Fermentation, Osaka (IFO) (G-2023-3-050).Notes and references
- S. Jo, H. Shin, A. K. Shung, J. P. Fisher and A. G. Mikos, Macromolecules, 2011, 34, 2839 CrossRef.
- J. Zhu, Biomaterials, 2010, 31, 4639 CrossRef CAS PubMed.
- C. A. Roa Engel, A. J. J. Straathof, T. W. Zijlmans, W. M. van Gulik and L. A. M. van der Wielen, Appl. Microbiol. Biotechnol., 2008, 78, 379 CrossRef CAS PubMed.
- N. Jacquel, F. Freyermouth, F. Fenouillot, A. Rousseau, J. P. Pascault, P. Fuertes and R. Saint-Loup, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 5301 CrossRef CAS.
- M. Takeuchi and Y. Amao, React. Chem. Eng., 2022, 7, 1931 RSC.
- M. Takeuchi and Y. Amao, RSC. Sustain., 2023, 1, 90 RSC.
- Y. Morimoto, K. Honda, X. Ye, K. Okano and H. Ohtake, J. Biosci. Bioeng., 2014, 117, 147 CrossRef CAS PubMed.
- S. Jitrapakdee, M. St Maurice, I. Rayment, W. W. Cleland, J. C. Wallace and P. V. Attwood, Biochem. J., 2008, 413, 369 CrossRef CAS PubMed.
- Z. D. Wang, B. J. Wang, Y. D. Ge, W. Pan, J. Wang, L. Xu, A. M. Liu and G. P. Zhu, Mol. Biol. Rep., 2011, 38, 1629 CrossRef CAS PubMed.
- G. B. Warren and K. F. Tipton, Biochem. J., 1974, 139, 297 CrossRef CAS PubMed.
- M. C. Scrutton and M. F. Utter, J. Biol. Chem., 1967, 242, 1723 CrossRef CAS PubMed.
- T. K. Sundaram, I. P. Wright and A. E. Wilkinson, Biochemistry, 1980, 19, 2017 CrossRef CAS PubMed.
- Y. D. Ge, Y. T. Guo, L. L. Jiang, H. H. Wang, S. L. Hou and F. Z. Su, Protein J., 2023, 42, 14 CrossRef CAS PubMed.
- K. J. Pedersen, Acta Chem. Scand., 1952, 6, 285 CrossRef CAS.
- A. Adina-Zada, T. N. Zeczycki and P. V. Attwood, Arch. Biochem. Biophys., 2012, 519, 118 CrossRef CAS PubMed.
- M. Takeuchi and Y. Amao, RSC. Sustain., 2023, 1, 1874 RSC.
- M. Takeuchi and Y. Amao, J. Jpn. Petrol. Inst., 2024, 67 DOI:10.1627/jpi.67.167.
- S. Beeckmans and E. Van Driessche, J. Biol. Chem., 1998, 273, 31661 CrossRef CAS PubMed.
- M. Mescam, K. Vinnakota and D. Beard, J. Biol. Chem., 2011, 286, 21100 CrossRef CAS PubMed.
- M. R. Brown and A. Kornberg, Trends Biochem. Sci., 2008, 33, 284 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00486d |
| This journal is © The Royal Society of Chemistry 2024 |