 Open Access Article
Open Access ArticleUnexpected performance of iron(III)chloride in the polymerization of renewable 2,3-butanediol and the depolymerization of poly(ethylene terephthalate)†
Anja
Kirchberg
a,
Sandra
Wegelin
 a,
Leonie
Grutke
a and
Michael A. R.
Meier
a,
Leonie
Grutke
a and
Michael A. R.
Meier
 *ab
*ab
aLaboratory of Applied Chemistry, Institute of Organic Chemistry (IOC), Karlsruhe Institute of Technology (KIT), Straße am Forum 7, 76131 Karlsruhe, Germany. E-mail: m.a.r.meier@kit.edu
bLaboratory of Applied Chemistry, Institute of Biological and Chemical System-Functional Molecular Systems (IBCS-FMS), Karlsruhe Institute of Technology (KIT), Hermann-von-Helmholtz-Platz 1, 76344 Eggenstein-Leopoldshafen, Germany
First published on 22nd December 2023
Abstract
In this study, 2,3-butanediol (BDO), which can be obtained by fermentation, was used in a polycondensation reaction with renewable 2,5-furandicarboxylic acid (FDCA) to obtain a fully renewable polyester. Catalyst screening based on a deconvolution method was performed. 16 different, randomly chosen Lewis acids were screened with the aim to identify the most active catalyst. With iron(III)chloride, the most active catalyst also offering sustainability benefits, further investigations of a polycondensation in melt are presented. Further renewable dicarboxylic acids were (co)polymerized with BDO and FDCA to yield polyesters and copolyesters and to investigate whether FeCl3 was also a suitable catalyst for other polycondensations. Full characterization of all polymers is provided, including 1H NMR and IR spectroscopy as well as differential scanning calorimetry (DSC), size-exclusion chromatography (SEC), and thermogravimetric analysis (TGA). Furthermore, depolymerization reactions of poly(ethylene terephthalate) (PET) materials are performed using the identified catalyst, iron(III)chloride, further demonstrating its versatility.
Sustainability spotlightBiobased and recyclable polymers show potential in reducing both the carbon footprint and the end of life environmental impact of polymeric materials. If additional principles of Green Chemistry can be addressed, as herein shown the use more benign catalysts, overall sustainability can be further improved. In this context, iron(III)chloride was identified as an abundant, less toxic and cheap catalyst alternative to polyester synthesis as well as polyester recycling by depolymerization to monomer. The catalyst performed comparably well and was used to produce a set of renewable polyesters based on 2,3-butanediol (BDO), a seldomly studied diol for polycondensation, with improved properties such as higher molecular weights and better thermal stability. Iron(III)chloride could also be applied to the depolymerization of commercial PET materials, demonstrating its use in a circular economy concept. |
Introduction
The society of the 21st century must deal with inherent challenges, like global warming and depleting fossil resources.1 Virtually all (∼98.5% in 2021) of the 390 million tons of commercial plastics produced annually are derived from fossil fuels, of which less than 9% are currently recycled.2,3 Global plastic pollution is rising tremendously, accompanied by accumulation in landfills as well as natural environments like the oceans. Thus, the world must rapidly change from a fossil-based economy to a plant-based economy by implementing sustainable routes to biobased polymers using renewable feedstock.4Sustainable chemistry was defined as chemistry in which resources, including energy, should be used “at a rate at which they can be replaced naturally, and the generation of wastes cannot be faster than the rate of their remediation.”5 It has been widely recognized that the manufacture of polymers poses hazards, which can be reduced by applying the 12 principles of Green Chemistry. These principles were designed as guideline for scientists to tune their strategies on a molecular level in order to achieve the goal of improved sustainability.6–8 Renewable feedstocks are a promising alternative to petroleum, catalysis can be used to decrease the use of toxic substances as well as the energy consumption and continuous process monitoring can be used to eliminate the generation of waste.9–11
As an alternative to our petroleum-based economy, the biorefinery concept was developed to use renewable resources to produce required commodity chemicals.12–14 Important for this study, short-chain diols and diacids, such as 2,3-butanediol (BDO), 1,4-butanediol, 1,3-propanediol (PG), succinic acid (SA), and adipic acid (AA) can be obtained by fermentation of lignocellulosic biomass.15 BDO represents an interesting diol, which is used in the manufacture of perfumes, moistening agents, and the cosmetic industry, but is not yet widely studied for polymer synthesis.16 The two methyl groups in the BDO structure make this renewable diol interesting for the development of novel polymers, for instance to restrain the crystallization of biobased polyesters.17,18
Polyesters, achievable from various different monomer structures, are manufactured for numerous applications such as beverage bottles, molded plastics parts, and synthetic fibres and films.19,20 The typical route to polyesters is a step-growth polymerization, starting with the reaction of diols with diacids or dimethyl esters to oligomers and secondly, a polycondensation in melt at high temperatures (>200 °C) as well as low pressure (<1 mbar) to obtain the desired high molecular weight polymer.21,22 Poly(ethylene terephthalate) (PET) is the most widely used polyester, mainly known in form of plastic bottles.23,24 Much effort has been taken to produce biobased PET by using bio-EG and bio-terephthalic acid (TPA) as monomers.25,26 The aromatic diacid 2,5-furandicarboxylic acid (FDCA), derived from lignocellulose, shows structural similarities to TPA.27 FDCA was identified by the U.S. Department of Energy as one of the twelve most promising sugar-based building blocks to be used in the development of biobased polymers.28 Biobased poly(ethylene furanoate) (PEF) generally shows similar properties to PET, but PEF has a higher glass transition temperature (Tg) of 86 °C (PET: Tg = 74 °C) and a lower melting temperature (Tm) of 235 °C (PET: Tm = 265 °C).29,30 The novel polymer can be used for the manufacture of biobased bottles, while reducing greenhouse gas emissions.31,32 Despite the already adopted PEF, a large number of further research on FDCA, “the sleeping giant”, in polyester syntheses was reported in literature.33,34 For instance, Zhou et al. reported the synthesis of furan-based polyesters using PG, 1,4-butanediol, 1,6-hexanediol, and 1,8-octanediol in direct polycondensation reactions, revealing a tunable Tg of 21 °C up to 89 °C and Tm of 148 °C up to 210 °C.35 Bikiaris et al. investigated polyesters based on FDCA and longer chain diols (C8 to C10 and C12), leading to ductile polyesters with even lower Tg (<0 °C) and inferior mechanical properties.36 With 1,20-eicosanediol and FDCA, a mainly aliphatic polyester was introduced by Sousa et al. showing a Tm of 107 °C and Tg around 7 °C, with biodegradable properties.37
Furthermore, much effort was taken to tune the properties of PEF through copolymerization with a third renewable dicarboxylic acid. The castor oil derived sebacic acid (SBA) was studied for tuning polyester properties in terms of flexibility, hydrophobicity, durability, and low Tm, which was proven in the introduction of SBA into PEF.38,39 Moreover, the U.S Department of Energy also listed succinic acid (SA) as a future platform chemical derived from renewable resources.28 In a polycondensation reaction with 1,4-butanediol, SA was successfully used for the development of a novel biobased polymer named poly(butylene succinate) (PBS).40,41 The crystalline polyester shows a Tm of 100 °C and a Tg of −32 °C. This increased the interest of the packaging industry to use PBS as biobased and biodegradable alternative to non-biodegradable polyethylene (PE).42,43 Copolymerization with adipic acid (AA) was screened for tuning polyester properties.41,44 AA represents one of the largest commodity chemicals used as pivotal building block for a range of processes in the chemical, pharmaceutical, and food industry, primarily utilized in the polymerization to Nylon 6,6.45–47 Poly(butylene succinate-co-butylene adipate) was investigated by Djonlagic et al. including its enzymatic degradation behavior, showing that the degree of polymer crystallinity had an effect on the degradation rate.48 Furthermore, Sobkowicz et al. investigated copolyesters of PBS, identifying promising candidates for coatings with regards to potential biodegradable paper packaging applications.49
In addition to the research on renewable feedstock for the development of biobased polymers, also catalysis plays an important role in the field of Green Chemistry.6,50 Step-growth polycondensation reactions to polyesters are usually performed under harsh conditions.19 By optimizing the catalytic activity in a process through choosing more efficient, cheap, and environmentally friendlier catalysts, thus by replacing finite elements or toxic catalysts, the sustainability of a process can increase.51 To identify new catalysts faster, Moran et al. developed a catalyst screening method termed deconvolution method.52,53
Here, the deconvolution method was used for the screening of several different Lewis acids in a melt polycondensation reaction of renewable BDO and FDCA, yielding a high molecular weight polyester. FeCl3 was identified as sustainable and cheap catalyst and further studied in copolymerization reactions to tune the properties of the polyester. All polymers were fully characterized. Furthermore, depolymerization reactions of polymeric PET materials, such as beverage bottles, was successfully performed with the identified catalyst FeCl3.
Experimental section
Chemicals
Unless otherwise noted, all listed solvents and reagents were used as received without further purification.Adipic acid (99%, Acros Organics), aluminium(III) chloride (98.5%, Acros Organics), bismuth(III) trifluoromethanesulfonate (99%, Alfa Aesar), boron trifluoride diethyl etherate (technical grade, Sigma-Aldrich), 2,3-butanediol (98%, Sigma-Aldrich), 2,3-butanediol (98%, mixture of racemic and meso forms, Thermo Fisher), cerium(IV)sulfate anhydrous (99.0%, ChemPur), CDCl3 (99.8%, stabilized with silver foils, Eurisotop®), copper(I) chloride (97%, Sigma-Aldrich), dimethyl formamide (VWR Chemicals), DMSO-d6 (99.80%, Eurisotop®), ethanol (HPLC grade, VWR Chemicals), ethyl acetate (HPLC grade), ethylene glycol (≥99.5%, Riedel-de Haën®), furan-2,5-dicarboxylic acid (98%, BLD Pharmatech), 1,1,1,3,3,3-hexafluoro-2-propanol (fluorochem), indium(III) chloride (99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 999%, Sigma-Aldrich), indium(III) trifluoromethanesulfonate (Sigma-Aldrich), iron(III) chloride anhydrous (>97.0%, Fluka), lithium bromide (98%, Fluka), magnesium chloride hexahydrate (99%, Sigma-Aldrich), methanol (VWR Chemicals), 1,2-octanediol (99.91%, BLD Pharmatech Ltd.), phosphomolybdic acid hydrate (Sigma-Aldrich), potassium ferrocyanide trihydrate (98.5%, ThermoFisher), potassium trifluoroacetate (98%, Sigma-Aldrich), propylene glycol (>99.0%, TCI Chemicals), scandium(III) trifluoromethanesulfonate (99.995%, Sigma-Aldrich), sebacic acid (for synthesis, Sigma-Aldrich), silver trifluoromethanesulfonate (≥99%, Sigma-Aldrich), sodium bisulfite (Sigma-Aldrich), succinic acid (99%, Acros Chemicals), therephthalic acid (>99%, Thermo Fisher) THF with 250 ppm butylated hydroxytoluene (≥99.9%, Sigma-Aldrich), tin(II) chloride (98%, Acros Organics), tin(II) 2-ethylhexanoate (95%, Alfa Aesar), titanium(IV) isopropoxide (98%, Acros Chemicals), titanium(IV) n-butoxide (>99%, Alfa Aesar), ytterbium(III) trifluoromethanesulfonate (99,9%, Acros Organics), zinc bromide (>98%, Sigma-Aldrich), zinc(II) trifluoromethanesulfonate (98%, Sigma-Aldrich).
999%, Sigma-Aldrich), indium(III) trifluoromethanesulfonate (Sigma-Aldrich), iron(III) chloride anhydrous (>97.0%, Fluka), lithium bromide (98%, Fluka), magnesium chloride hexahydrate (99%, Sigma-Aldrich), methanol (VWR Chemicals), 1,2-octanediol (99.91%, BLD Pharmatech Ltd.), phosphomolybdic acid hydrate (Sigma-Aldrich), potassium ferrocyanide trihydrate (98.5%, ThermoFisher), potassium trifluoroacetate (98%, Sigma-Aldrich), propylene glycol (>99.0%, TCI Chemicals), scandium(III) trifluoromethanesulfonate (99.995%, Sigma-Aldrich), sebacic acid (for synthesis, Sigma-Aldrich), silver trifluoromethanesulfonate (≥99%, Sigma-Aldrich), sodium bisulfite (Sigma-Aldrich), succinic acid (99%, Acros Chemicals), therephthalic acid (>99%, Thermo Fisher) THF with 250 ppm butylated hydroxytoluene (≥99.9%, Sigma-Aldrich), tin(II) chloride (98%, Acros Organics), tin(II) 2-ethylhexanoate (95%, Alfa Aesar), titanium(IV) isopropoxide (98%, Acros Chemicals), titanium(IV) n-butoxide (>99%, Alfa Aesar), ytterbium(III) trifluoromethanesulfonate (99,9%, Acros Organics), zinc bromide (>98%, Sigma-Aldrich), zinc(II) trifluoromethanesulfonate (98%, Sigma-Aldrich).
Methods
Carousel reactor with temperature range of 0 to 310 °C and vortexing speed of 0 to 1500 rpm purchased from RADLEYS.Differential scanning calorimetry (DSC) of polyesters was performed with the following method and instrument using ∼7 mg of the respective sample: Mettler Toledo DSC stare system. The DSC experiments were carried out under nitrogen atmosphere using 100 μL aluminium crucibles. First, heating from 25 °C to 150 °C (rate 20 °C min−1), then cooling from 150 °C to −10 °C (−20 °C min−1) and subsequently heating from −10 °C to 150 °C (20 °C min−1) per cycle, using 50 mL min−1 N2.
Inductively coupled plasma optical emission spectroscopy (ICP-OES) was used for the measurement of the iron content in the antisolvent used for precipitation of polyesters. Agilent, ICP-OES SVDV 5100 G6010A with CCD-Detector, Autosampler SPS3, G8480 A and cooling system G8481A. For PET materials, first an acid digestion was performed using 104 mg sample in 1 mL water, 1 mL hydrogen peroxide and 6 mL ammonium oxide. Microwave of Ethos.lab MWS Mikrowellensystem-Vertriebs-GmbH. MW-Digestion KöWa: DIN EN 16174, Antimon, Titan: DIN EN ISO 11885.
Infrared (IR) spectroscopy was performed using a Bruker Alpha-p instrument with ATR technology. All spectra were recorded in a frequency range of ṽ = 400–4000 cm−1 with 24 scans per measurement. IR (type of measurement) ṽ/cm−1 = wave number (signal intensity, molecular oscillator assignment).
Nuclear magnetic resonance (NMR) spectroscopy was performed on a Bruker Avance 400 NMR instrument at 400 MHz with 16 scans for 1H NMR and at 101 MHz with 1024 scans for 13C NMR. The chemical shifts are reported in parts per million (ppm) and referenced to the solvent signal of partly deuterated DMSO-d6 at 2.50 ppm (1H NMR) or 39.5 ppm (13C NMR) and of CDCl3 at 7.26 ppm (1H NMR) or 77.2 ppm (13C NMR), respectively. The spin multiplicity and corresponding signal patterns were abbreviated as follows: s = singlet, d = doublet, t = triplet, m = multiplet. Coupling constants (J) were noted in Hertz (Hz). If isomers of a substance were observed, all species which could be assigned were clearly labelled.
Size exclusion chromatography (SEC) of polyesters was performed in 1,1,1,3,3,3-hexafluoro-2-propanol (HFIP) containing 0.10 wt% potassium trifluoroacetate on a Tosoh EcoSEC HLC-8320 SEC system, with a solvent flow of 0.40 mL min−1 at 35 °C and a sample concentration of 1 mg mL−1 injecting 50 μL. The analysis was performed on a two-column system: PSS PFG Micro column (8.00 × 55.0 mm, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å) and PSS PFG Linear S column (8.00 × 300 mm, 50
000 Å) and PSS PFG Linear S column (8.00 × 300 mm, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Å). The system was calibrated with linear poly(methyl methacrylate) standards (Polymer Standard Service, Mp: 800 Da-1600 kDa).
000 Å). The system was calibrated with linear poly(methyl methacrylate) standards (Polymer Standard Service, Mp: 800 Da-1600 kDa).
Solubility tests of polyesters were performed by using 5 mL glass vials with ∼2 mg of sample, adding the respective solvent. Mixing by hand was carried out at room temperature. Solubility was checked visually.
Thermogravimetric analysis (TGA) of all samples was performed on a TA instrument TGA 5500 under nitrogen atmosphere using platinum TGA sample pans. A heating rate of 10 K min−1 in a temperature range from 25 °C to 600 °C was applied.
Results and discussion
Catalyst screening
Lewis acids are typically used in polycondensation reactions to make polyesters, as they ease the nucleophilic attack of alcohol groups.54 For polycondensation reactions, titanium(IV) butoxide (TBO) and titanium(IV) isopropoxide (TTIP) were previously identified as catalysts leading to high Mn polyesters.18,21 The so-called deconvolution method was investigated by Moran et al. to screen catalysts in an efficient and fast way.52 Here, a two-step polycondensation reaction was screened with 16 typical, randomly chosen Lewis acids. The screening conditions were set with the reaction of BDO and FDCA to oligomers at 180 °C, followed by a polycondensation in melt at 200 °C under reduced pressure (see Scheme 1). In each batch, 1.00 mol% of each catalyst was used, the layout of the experiment is shown in Fig. 1 and general considerations regarding this screening method are provided by the developers of the deconvolution method.52 For this screening, an excess of BDO (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 BDO to FDCA) was applied at the start of the reaction to guarantee a mixing of the reactants, a typical approach similar polycondensations. After applying vacuum at a later stage of the polymerization, the excess BDO was continuously removed from the system, leading to the later described high molecule weight polyesters.
1 BDO to FDCA) was applied at the start of the reaction to guarantee a mixing of the reactants, a typical approach similar polycondensations. After applying vacuum at a later stage of the polymerization, the excess BDO was continuously removed from the system, leading to the later described high molecule weight polyesters.
16 Lewis acids were split randomly into batches Ba1 to Ba4. The respective precipitated polyesters 3 from these batches were then analyzed by SEC to screen for the highest Mn polymer, i.e., for the best performing catalyst or catalyst-mixture. Ba1 and Ba3 showed Mn values of ∼9 kDa, whereas polymers obtained from Ba2 and Ba4 were black solids, which were not soluble for any workup or characterization. As the results of Ba2 and Ba4 indicated side reactions, only Ba1 and Ba3 were further deconvoluted into smaller batches, i.e., Ba5 to Ba8, containing two catalysts each (see Fig. 1). Using AlCl3 and MgCl2·6H2O (Ba5) led to the desired polyester with improved Mn of 12 kDa. For Ba6, with InCl3 and CuCl, as well as for Ba8, containing SnCl2 and ZnBr2, a polyester with Mn of 10 kDa was observed. The combination of TTIP and FeCl3 (Ba7) led to a polyester with Mn of 16 kDa.
As Ba7 showed the highest molecular weight, FeCl3 and TTIP were investigated individually as last step of the deconvolution method, revealing FeCl3 as highly potent catalyst. FeCl3 is known to catalyze various reactions, for instance, oxidations, reductions, or cyclizations, with the advantage of a high efficiency, stability, and low cost.55 Here, the comparably non-toxic catalyst FeCl3 was identified to successfully catalyze a polycondensation reaction of BDO and FDCA to the desired polyester with Mn of 18 kDa. The well-known polycondensation catalyst TTIP led to Mn of 15 kDa under these screening conditions, slightly lower than FeCl3. Furthermore, in the study of Thiyagarajan et al., FDCA was polymerized with BDO using TTIP as catalyst, resulting in a Mn of 8 kDa.56 Thus, FeCl3 was identified as a cheaper and more active alternative to TTIP.
All polyesters based on FDCA and BDO catalyzed by FeCl3 exhibited a brownish discoloration, but this was also the case for, i.e., TTIP. This discoloration is reported in the literature for polyesters based on FDCA. A reason for the brown color might be related to side reactions, such as decarboxylation, leftovers of catalyst, or sugar-based impurities, originating from FDCA.18 Therefore, we investigated if the discoloration can be decreased using different reaction conditions. Temperature and time of the esterification reaction between FDCA and BDO were thus changed from 180 °C for 23 h, to 160 °C for 17 h. The reaction time of the subsequent polycondensation reaction in melt was shortened from 23 h at 200 °C (<1 mbar), to 7 h at 215 °C (<1 mbar). Furthermore, the catalyst loading was increased from 1.00 mol% to 1.25 mol% FeCl3. The reduced reaction time paired with increased catalyst loading did not influence the Mn or the color of the desired polyester (see Table 1). Thus, prepurified FDCA (1) and FDCA dimethyl ester (2) were investigated to observe if the discoloration is a result of impurities of FDCA. However, SEC measurements showed a similar Mn of the obtained polymers and similar discoloration was observed in all cases.
| Catalyst | Monomer A | Monomer B | M n in kDa | Ð |
|---|---|---|---|---|
a Condition a: 30.0 mmol of monomer A, 10.0 mmol of monomer B, 1.25 mol% catalyst, 160 °C for 17 h, followed by 215 °C and low pressure (<1 mbar) for 7 h. Precipitation in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ethanol to water.
b Condition b: 30.0 mmol of monomer A, 10.0 mmol of monomer B, 1.00 mol% catalyst, 140 to 180 °C within 4 h, followed by 190 °C for 19 h, followed by 200 °C and low pressure (<1 mbar) for 23 h. Precipitation in methanol. 1 (v/v) ethanol to water.
b Condition b: 30.0 mmol of monomer A, 10.0 mmol of monomer B, 1.00 mol% catalyst, 140 to 180 °C within 4 h, followed by 190 °C for 19 h, followed by 200 °C and low pressure (<1 mbar) for 23 h. Precipitation in methanol.
|
||||
| FeCl3 | BDO | FDCA | 18a | 1.55 |
| TTIP | BDO | FDCA | 14a | 1.95 |
| FeCl3 | BDO | 1 | 19a | 1.85 |
| FeCl3 | BDO | 2 | 17a | 1.53 |
| FeCl3 | BDO | FDCA | 17b | 1.95 |
Thus, different antisolvents were tested for purification. For PBF based on a reaction catalyzed by TTIP, ice-cold methanol seemed to be a suitable anti-solvent, as reported in literature.56 For 3, a mixture of ice-cold water and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) yielded a less brownish polyester solid, compared to using methanol (see Fig. 2). Furthermore, a threefold dissolution and precipitation decreased the discoloration of 3 even further, as depicted in Fig. 3. The multiple precipitation steps did not change the Mn of the polymer. To check if the catalyst FeCl3 was washed out by precipitation in water and ethanol, the antisolvent mixture was analyzed by Prussian blue staining after each precipitation step, depicted in Fig. 2.57
1, v/v) yielded a less brownish polyester solid, compared to using methanol (see Fig. 2). Furthermore, a threefold dissolution and precipitation decreased the discoloration of 3 even further, as depicted in Fig. 3. The multiple precipitation steps did not change the Mn of the polymer. To check if the catalyst FeCl3 was washed out by precipitation in water and ethanol, the antisolvent mixture was analyzed by Prussian blue staining after each precipitation step, depicted in Fig. 2.57
Prussian blue staining showed that FeCl3 was washed out in the first precipitation step, using ethanol and water as antisolvent mixture. To analyze the amount of washed out FeCl3, and to get an idea if the discoloration occurred because of catalyst leftovers in the polyester solid, inductively coupled plasma-optical emission spectroscopy (ICP-OES) was used. In the first precipitation step, 6 to 10 wt% of the used amount of FeCl3 was washed out. The second precipitation step only removed <0.2 wt% and the third precipitation step less than 0.05 wt% (see Fig. 2). ICP-OES measurements thus revealed that most of the used catalyst remained in the polyester solid, yet its color improved significantly, leading to the conclusion that the discoloration was likely caused by other impurities, maybe due to side-reaction of sugar-residues of FDCA, which were removed by precipitation.
The precipitated polyester 3 showed no melting temperature (Tm) or crystallization temperature (Tc) in differential scanning calorimetry (DSC), according to literature.56 However, 3 showed a glass transition temperature (Tg) of 106 °C and a degradation temperature (Td,5%) of 303 °C. Compared to literature, in the report of Thiyagarajan et al., PBF, based on FDCA and BDO, was isolated with a lower Tg of 90 °C and lower Td,5% of 279 °C, compared to the here presented study. Thiyagarajan et al. used reaction temperatures of 260 °C for 4 hours during polymerization in melt and TTIP as catalyst.56 The literature described lower molecular weights might be the reason for the differences observed in thermal properties, further pointing to the benefits of FeCl3 as catalyst. Polyester 3 was soluble in acetone, dichloromethane, HFIP, THF, cyclohexane, and ethyl acetate, whereas being insoluble in water, methanol, and ethanol.
Polyester synthesis
The improved reaction conditions of the polycondensation reaction, as described above, were used in all following reactions. Always, 30.0 mmol of BDO were mixed with 10.0 mmol of a dicarboxylic acid, using 1.25 mol% FeCl3, heating to 160 °C for 17 h, subsequently followed by 215 °C at <1 mbar for 7 h. In addition to FDCA, also SA, SBA, and AA are interesting renewable dicarboxylic acids for the research on novel biobased polymers. SA gained great attention when introducing the dicarboxylic acid to polymerizations with 1,4-butanediol, yielding PBS, which is already implemented in industry.42 While copolymerizing PBS with AA, the obtained polymer showed an increased tensile strength.41 SBA is known for its good flexibility when polymerizing to polyesters.39 However, it is literature known that the Tg decreases with increasing SBA content.38To investigate if the identified catalyst, FeCl3, is also suitable for other polycondensation reactions, further polyesters were thus synthesized using each of the mentioned dicarboxylic acids in polycondensation reactions with BDO, following the same reaction procedure as for 3 (see Scheme 2). The additionally obtained polyesters 6–8 were precipitated in an ice-cold antisolvent mixture of water and ethanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) and dried under vacuum (<1 mbar) before SEC and TGA analysis (see Table 2).
1, v/v) and dried under vacuum (<1 mbar) before SEC and TGA analysis (see Table 2).
| Polymer | M n (kDa) | Ð | DP | T d,5% (°C) | Appearance |
|---|---|---|---|---|---|
a Conditions: 30.0 mmol of BDO, 10.0 mmol of the dicarboxylic acid, 1.25 mol% FeCl3, 160 °C for 17 h, followed by 215 °C and low pressure (<1 mbar) for 7 h. Precipitation in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ethanol to water. 1 (v/v) ethanol to water.
|
|||||
| 3 | 18 | 1.55 | 74.3 | 303 | Brittle solid |
| 6 | 5 | 1.47 | 26.6 | 280 | Viscous liquid |
| 7 | 8 | 1.44 | 36.9 | 326 | Viscous liquid |
| 8 | 10 | 1.47 | 36.7 | 358 | Viscous liquid |
Comparing the properties of the four polyesters, 3 showed the highest Mn with 18 kDa, whereas 6 showed the lowest Mn with 5 kDa, compared to 7 with 8 kDa and 8 with 10 kDa. The degradation temperature of the polyesters increased from 3 with 303 °C (Td,5%) to 358 °C (Td,5%) for 8. Thus, an increasing carbon chain length in the polymer backbone led to an increased thermal stability. The main difference between the obtained polyesters was that 3 was precipitated in form of a polyester solid, whereas 6–8 were isolated as highly viscous liquids.
Copolymerization reactions
As 3 was isolated as solid, but was brittle, we used FDCA and BDO as monomers for copolymerization reactions with a third monomer. We exemplarily chose AA as third monomer and copolymerized it in varying contents. 30 mmol of BDO were thus polymerized with 10 mmol of a dicarboxylic acid mixture, containing FDCA and AA in varying contents, resulting in the following copolyesters 9–15 (see Fig. 3). The copolyesters 9–13 were isolated as slightly brownish solids. A further increase of the AA content to 40% and 60% AA (copolyesters 21 and 22) resulted highly viscous liquids.All copolyesters were characterized using DSC, TGA, and SEC. The Mn and Ð as well as the weight loss of 5% and 15% are listed for each copolyester in Table 3. DSC measurements were performed for all copolyesters, which were obtained as solids, showing no Tm or Tc, which was to be expected according to literature, showing that an increasing content of BDO led to a decreased crystallinity of the polymer.56 A clear trend was observed for Tg (see Fig. 4).
| Copolyester | FDCA content (%) | AA content (%) | M n (kDa) | Ð | DP |
|---|---|---|---|---|---|
a Conditions: 30.0 mmol of BDO, 10.0 mmol of a mixture of FCDA and AA, 1.25 mol% FeCl3, 160 °C for 17 h, followed by 215 °C and low pressure (<1 mbar) for 7 h. Precipitation in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v) ethanol to water. 1 (v/v) ethanol to water.
|
|||||
| 9 | 94 | 6 | 15 | 1.62 | 33.9 |
| 10 | 88 | 12 | 21 | 1.80 | 47.5 |
| 11 | 82 | 18 | 16 | 1.84 | 36.2 |
| 12 | 76 | 24 | 14 | 1.68 | 31.6 |
| 13 | 70 | 30 | 10 | 1.73 | 22.6 |
| 14 | 60 | 40 | 12 | 1.67 | 27.1 |
| 15 | 40 | 60 | 6 | 1.48 | 13.6 |
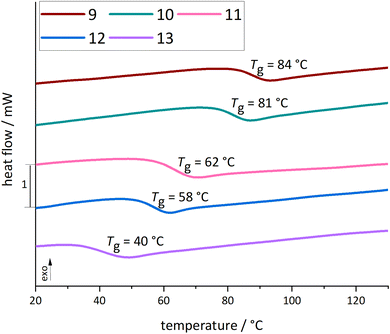 | ||
| Fig. 4 Heating curves of copolyesters 9–13 measured in DSC from −10 to 150 °C in the second scan, showing the glass transition temperature. | ||
For copolyesters 9–13, the Tg varied between 40 °C and 84 °C. The Tg decreased continuously with increasing content of AA. After copolymerization with AA, the obtained copolyesters were, compared to 3, not soluble in cyclohexane anymore, and the introduction of AA reduced the Tg and Td.
Depolymerization of PET using FeCl3
As FeCl3 showed good performance for the synthesis of various polyesters and copolyesters, we were interested if it would also be suitable for the chemical recycling of PET. Typically, a depolymerization of PET is performed via glycolysis, a transesterification with ethylene glycol (EG) at high temperature (180–250 °C).58,59 Thus, PET is transferred into its monomer, bis(hydroxy ethyl)terephthalate (BHET). The efficiency of depolymerization can be compared by dividing the actual amount of isolated BHET compared to the expected amount of BHET upon complete depolymerization.58 Pingale, Palekar, and Shukla identified zinc chloride giving the highest BHET yield with ∼74% after depolymerization of PET bottles with molecular weights varying from 18 up to 20 kDa.60 Here, we performed depolymerization reactions as depicted in Scheme 3. | ||
| Scheme 3 Depolymerization reaction of PET materials using ethylene glycol, catalyzed by FeCl3 to obtain BHET. | ||
The depolymerization reaction conditions of PET were screened by using different temperatures, different amounts of ethylene glycol as well as different catalyst loadings to find the optimal conditions for a high BHET yield. These screenings were performed by using synthesized PET, following the procedure of polyester 3, showing that FeCl3 can also be used for PET synthesis.
Table 4 gives an overview of two sets of screened depolymerization conditions. For both screenings, the reaction temperature was set to 190 °C and the yield of BHET was measured (by weighing the precipitated crystals, see Fig. 5) after a short reaction time of only 3 hours. Different catalyst loadings (1 mol% to 12 mol%) were investigated, using 15 equiv. of EG. Subsequently, the catalyst loading was set to 2 mol% and the equivalents of EG were varied while screening for the highest BHET yield.
| Screening 1 using 15 equiv. EG | Screening 2 using 2 mol% FeCl3 | ||
|---|---|---|---|
| FeCl3 (mol%) | Yield of BHET (%) | EG/equiv. | Yield of BHET (%) |
| a Conditions: 3 hours reaction time at 190 °C. | |||
| 1 | 22 | 15 | 31 |
| 2 | 30 | 23 | 22 |
| 5 | 21 | 31 | 19 |
| 12 | 12 | — | — |
Using 2 mol% FeCl3 in the depolymerization reaction with 1 equiv. PET and 15 equiv. EG led to highest yield of 31% BHET. Fig. 5 shows the 1H NMR spectrum as well as a picture of the obtained BHET solid. Decreasing the reaction temperature from 190 °C to 100 °C also decreased the yield of BHET from 31% to 14%. Thus, the reaction temperature influences the depolymerization of PET as expected, but temperature was not further optimized.
Antimony or titanium catalysts, such as TTIP or titanium(IV)butoxide (TBO), are typically used in the industrial synthesis of PET. Therefore, antimony(III)oxide as well as TBO were tested in the depolymerization of PET for comparison. Using 2 mol% of antimony(III)oxide in the reaction of Scheme 3, a yield of ∼26% BHET was obtained after 3 hours. Using 2 mol% of titanium(IV)butoxide led to a similar yield of ∼26% BHET. Thus, FeCl3 resulted in slightly increased yields of BHET, further confirming its good activity.
Furthermore, commercially available PET materials were tested in the depolymerization reaction with FeCl3. Using this catalyst, a more sustainable and cheap alternative for PET recycling would be provided, as FeCl3 is abundant and the catalyst can furthermore be considered comparably non-toxic. Fig. 6 depicts the investigated materials. Before the depolymerization reaction was performed, these materials were cut into flakes (Fig. 6, bottom).
500 mg of these flakes were used per depolymerization reaction, mixed with 15 equiv. EG and 2 mol% FeCl3, then heated to 190 °C for 3 hours. All materials were successfully depolymerized, resulting in a similar yield of BHET ranging from 65% to 68%. The increased yields of BHET compared to the yields of synthesized PET (with 31% BHET yield, see Table 3) might be caused due to catalyst leftovers from the synthesis in the used materials, as proven by ICP-OES measurements (251 mg kg−1 of antimony were found in the flakes of a coca cola bottle).
Compared to results obtained by of Pingale, Palekar, and Shukla60 a similar mixture of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 14 equiv. PET to EG was used, whereas we used a ratio of 1
14 equiv. PET to EG was used, whereas we used a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15. The reaction time was decreased from 8 hours to 3 hours in the here presented depolymerization, both leading to yields >65% BHET. Thus, in the overall process, FeCl3 gives a sufficient, cheap, and environmentally friendly and cheap catalyst to depolymerize PET materials successfully.
15. The reaction time was decreased from 8 hours to 3 hours in the here presented depolymerization, both leading to yields >65% BHET. Thus, in the overall process, FeCl3 gives a sufficient, cheap, and environmentally friendly and cheap catalyst to depolymerize PET materials successfully.
Conclusion
The deconvolution method was successfully used for the two-step polycondensation reaction of FDCA and BDO, revealing FeCl3 as an active, environmentally friendlier, and cheaper catalyst compared to commonly used TTIP. After polymerization, a solid polyester was obtained with Mn of 18 kDa and a high Tg of 106 °C. Copolymerization reactions were performed to tune the properties of this polyester. The introduction of AA led to a decrease in Tg. Interestingly and importantly, the identified comparably non-toxic and cheap catalyst FeCl3 was further investigated in depolymerization reactions of PET. Here, materials such as PET bottles, bathing shorts and fleece were successfully depolymerized. Thus, FeCl3 was identified as cheap and more sustainable catalyst for polycondensation reactions to polyesters and as well as for depolymerizations, closing the cycle of a circular economy approach.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Our colleagues Masood Esfahani from the working group of Prof. Wilhelm at ITCP-Karlsruhe Institute of Technology and Birgit Huber from the working group of Prof. Théato at SML-Karlsruhe Institute of Technology are gratefully acknowledged for access to and help with analytical measurements. AK and MARM would like to thank the Bundesministerium für Bildung und Forschung (BMBF, project BROWSE, FKZ 031B1053A) for financial support.References
- G. Gwehenberger and M. Narodoslawsky, Process Saf. Environ. Prot., 2008, 86, 321 CrossRef CAS.
- Plastics Europe, Plastics the Facts 2022: An Analysis of European Plastics Production, Demand and Waste Data, Plastics Europe, Brussels, Belgium, 2022 Search PubMed.
- T. Zink, R. Geyer and R. Startz, Law Sci. Adv., 2018, 22, 314 Search PubMed.
- C. Okkerse and H. van Bekkum, Green Chem., 1999, 1, 107 RSC.
- I. T. Horváth, Chem. Rev., 2018, 118, 369 CrossRef PubMed.
- P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301 RSC.
- P. T. Anastas and C. A. Farris, U.S. Environmental Pretection Agency, 1994.
- M. A. Dubé and S. Salehpour, Macromol. React. Eng., 2014, 8, 7 CrossRef.
- P. T. Anastas and M. M. Kirchhoff, Acc. Chem. Res., 2002, 35, 686 CrossRef CAS PubMed.
- P. Anastas and J. C. Warner, Green Chemistry: Theory and Practcice, Oxford University Press, 1998, vol. 39, p. 10 Search PubMed.
- A. Kirchberg, M. Khabazian Esfahani, M.-C. Röpert, M. Wilhelm and M. A. R. Meier, Macromol. Chem. Phys., 2022, 223, 2200010 CrossRef CAS.
- R. Mülhaupt, Macromol. Chem. Phys., 2013, 214, 159 CrossRef.
- S. Octave and D. Thomas, Biochimie, 2009, 91, 659 CrossRef CAS.
- B. Kamm and M. Kamm, Appl. Microbiol. Biotechnol., 2004, 64, 137 CrossRef CAS PubMed.
- R. A. Sheldon, Green Chem., 2014, 16, 950 RSC.
- P. Nachtergaele, S. de Meester and J. Dewulf, J. Chem. Technol. Biotechnol., 2019, 94, 1808 CrossRef CAS.
- M. J. Syu, Appl. Microbiol., 2001, 55, 10 CAS.
- E. Gubbels, L. Jasinska-Walc and C. E. Koning, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 890 CrossRef CAS.
- K. Pang, R. Kotek and A. Tonelli, Prog. Polym. Sci., 2006, 31, 1009 CrossRef CAS.
- U. Edlund and A.-C. Albertsson, Adv. Drug Delivery Rev., 2003, 55, 585 CrossRef CAS PubMed.
- G. Z. Papageorgiou, D. G. Papageorgiou, Z. Terzopoulou and D. N. Bikiaris, Eur. Polym. J., 2016, 83, 202 CrossRef CAS.
- G. Z. Papageorgiou, V. Tsanaktsis and D. N. Bikiaris, Phys. Chem. Chem. Phys., 2014, 16, 7946 RSC.
- J. R. Whinfield and J. T. Dickson, UK Pat., UK 578079, 1946 Search PubMed.
- A. S. Chegolya, V. V. Shevchenko and G. D. Mikhailov, J. Polym. Sci., Polym. Chem. Ed., 1979, 17, 889 CrossRef CAS.
- M. Rabnawaz, I. Wyman, R. Auras and S. Cheng, Green Chem., 2017, 19, 4737 RSC.
- The Coca Cola Company, The Coca-Cola Company Announces Partnerships to Develop Commercial Solutions for Plastic Bottles Made Entirely from Plants, can be found under, https://www.thecoca-colacompany.com, 2011 Search PubMed.
- I. Delidovich, P. J. C. Hausoul, L. Deng, R. Pfützenreuter, M. Rose and R. Palkovits, Chem. Rev., 2016, 116, 1540 CrossRef CAS PubMed.
- T. Werpy and G. Petersen, U.S. Department of Energy, 2004, vol. 1.
- K.-R. Hwang, W. Jeon, S. Y. Lee, M.-S. Kim and Y.-K. Park, Chem. Eng. J., 2020, 390, 124636 CrossRef CAS.
- Avantium, FDCA and PlantMEG Together Make a 100% Plant-Based Plastic PEF, can be found under, https://www.avantium.com/lead-products/, opened, 2023 Search PubMed.
- A. F. Sousa, C. Vilela, A. C. Fonseca, M. Matos, C. S. R. Freire, G.-J. M. Gruter, J. F. J. Coelho and A. J. D. Silvestre, Polym. Chem., 2015, 6, 5961 RSC.
- A. J. J. E. Eerhart, A. P. C. Faaij and M. K. Patel, Energy Environ. Sci., 2012, 5, 6407 RSC.
- X. Fei, J. Wang, X. Zhang, Z. Jia, Y. Jiang and X. Liu, Polymers, 2022, 14, 625 CrossRef CAS PubMed.
- X. Fei, J. Wang, J. Zhu, X. Wang and X. Liu, ACS Sustain. Chem. Eng., 2020, 8, 8471 CrossRef CAS.
- M. Jiang, Q. Liu, Q. Zhang, C. Ye and G. Zhou, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 1026 CrossRef CAS.
- V. Tsanaktsis, G. Z. Papageorgiou and D. N. Bikiaris, J. Polym. Sci., Part A: Polym. Chem., 2015, 53, 2617 CrossRef CAS.
- M. J. Soares, P.-K. Dannecker, C. Vilela, J. Bastos, M. A. Meier and A. F. Sousa, Eur. Polym. J., 2017, 90, 301 CrossRef CAS.
- G. Wang, M. Jiang, Q. Zhang, R. Wang, X. Tong, S. Xue and G. Zhou, Polym. Degrad. Stab., 2017, 143, 1 CrossRef CAS.
- W.-Y. Jeon, M.-J. Jang, G.-Y. Park, H.-J. Lee, S.-H. Seo, H.-S. Lee, C. Han, H. Kwon, H.-C. Lee and J.-H. Lee, et al. , Green Chem., 2019, 21, 6491 RSC.
- L. Aliotta, M. Seggiani, A. Lazzeri, V. Gigante and P. Cinelli, Polymers, 2022, 14, 844 CrossRef CAS.
- J. Xu and B.-H. Guo, Biotechnol. J., 2010, 5, 1149 CrossRef CAS.
- Succinity GmbH, Biobased Polybutylene Succiniate (PBS) – An Attractive Polymer for Biopolymer Compounds, 2016 Search PubMed.
- BioPBS Marketing Group, Mitsubishi Chemical Group, 2017.
- H. Hu, R. Zhang, J. Wang, W. Bin Ying and J. Zhu, Eur. Polym. J., 2018, 102, 101 CrossRef CAS.
- T. Debuissy, E. Pollet and L. Avérous, Polymer, 2016, 99, 204 CrossRef CAS.
- W. Deng, Q. Zhang and Y. Wang, J. Energy Chem., 2015, 24, 595 CrossRef.
- U. Witt, R.-J. Müller and W.-D. Deckwer, J. Environ. Polym. Degrad., 1995, 3, 215 CrossRef CAS.
- M. S. Nikolic and J. Djonlagic, Polym. Degrad. Stab., 2001, 74, 263 CrossRef CAS.
- B. Tan, S. Bi, K. Emery and M. J. Sobkowicz, Eur. Polym. J., 2017, 86, 162 CrossRef CAS.
- S. Y. Tang, R. A. Bourne, R. L. Smith and M. Poliakoff, Green Chem., 2008, 10, 268 RSC.
- X. Zhang, M. Fevre, G. O. Jones and R. M. Waymouth, Chem. Rev., 2018, 118, 839 CrossRef CAS PubMed.
- E. Wolf, E. Richmond and J. Moran, Chem. Sci., 2015, 6, 2501 RSC.
- P. S. Löser, P. Rauthe, M. A. R. Meier and A. Llevot, Philos. Trans. R. Soc., 2020, 378, 20190267 CrossRef PubMed.
- G. N. Lewis, J. Franklin Inst., 1938, 226, 293 CrossRef.
- D. Diaz, P. Miranda, J. Padron and V. Martin, Curr. Org. Chem., 2006, 10, 457 CrossRef.
- S. Thiyagarajan, W. Vogelzang, R. J. I. Knoop, A. E. Frissen, J. van Haveren and D. S. van Es, Green Chem., 2014, 16, 1957 RSC.
- S. K. Suvarna, C. Layton and J. D. Bancroft, Theory and Practice of Histological Techniques, Elsevier, 2018 Search PubMed.
- S. Lalhmangaihzuala, Z. Laldinpuii, C. Lalmuanpuia and K. Vanlaldinpuia, Polymers, 2020, 13, 37 CrossRef PubMed.
- K. Ragaert, L. Delva and K. van Geem, Waste Manage., 2017, 69, 24 CrossRef CAS PubMed.
- N. D. Pingale, V. S. Palekar and S. R. Shukla, J. Appl. Polym. Sci., 2010, 115, 249 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00388d |
| This journal is © The Royal Society of Chemistry 2024 |







