Open Access Article
Open Access ArticleThe global burden of plastics in oral health: prospects for circularity, sustainable materials development and practice
Albert
Ong†
 a,
Jerald Y. Q.
Teo†
a,
Jerald Y. Q.
Teo†
 a,
David C.
Watts
a,
David C.
Watts
 b,
Nikolaos
Silikas
b,
Nikolaos
Silikas
 b,
Jason Y. C.
Lim
b,
Jason Y. C.
Lim
 *ac and
Vinicius
Rosa
*ac and
Vinicius
Rosa
 *de
*de
aInstitute of Materials Research and Engineering (IMRE), Agency for Science, Technology and Research (A*STAR), 2 Fusionopolis Way, Innovis #08-03, Singapore 138634, Republic of Singapore. E-mail: jason_lim@imre.a-star.edu.sg
bSchool of Medical Sciences, University of Manchester, Manchester M13 9PL, UK
cDepartment of Materials Science and Engineering, National University of Singapore (NUS), 9 Engineering Drive 1, 117576, Singapore
dFaculty of Dentistry, National University of Singapore, 11 Lower Kent Ridge Road, 119085, Singapore. E-mail: vini@nus.edu.sg
eORCHIDS: Oral Care Health Innovations and Design Singapore, National University of Singapore, 11 Lower Kent Ridge Road, 119085, Singapore
First published on 16th February 2024
Abstract
Plastics are indispensable and ubiquitous materials in oral healthcare and dental applications, favored for their diversity in structure and properties, low cost, durability, chemical and water resistance, ease of processing, and shaping. However, ancillary plastics are used for short periods or even once due to hygiene concerns and convenience, and insufficient attention has been given to their unsustainable current usage and end-of-life. Thus, the amount of plastic waste generated by consumers and clinicians is staggering and projected to increase unabatedly for the foreseeable future. With recent advances in plastics recycling and sustainable polymers, it is time to consider alternatives to tackle dentistry's growing plastic waste problem. This Perspectives article highlights the sources and scale of dental plastic wastage, followed by a multi-pronged consideration of material and practical interventions for this issue. On the materials front, we discuss emerging approaches and alternative sustainable polymers to address the unsustainable end-of-life of existing petroleum-based dental plastics/polymers and enable material circularity. On the practical front, we discuss strategies for sustainable plastic usage, which must be implemented alongside complementary material approaches. These approaches highlight the abundant unrealized opportunities for developing a circular economy around dental plastics while reducing the environmental footprint of modern dentistry.
Sustainability spotlightThe increasing access to oral healthcare pressures the demand for the use of plastics in dentistry globally, intensifying concerns about their unsustainable use and environmental impact. This Perspective article emphasizes plastics' vital role in maintaining oral health at both consumer and clinical levels and opportunities for sustainable designs, up/recycling strategies, and product development to foster innovations and economic growth, aligning with UN Sustainable Development Goal 3 (Good Health and Well-being). Within Goal 8 (Decent Work and Economic Growth). Simultaneously, Goal 13 (Climate Action) underscores the urgency of transitioning to sustainable polymers and circular economy practices, reducing the environmental footprint of modern dentistry. This work represents a pivotal step towards achieving these sustainability goals, balancing health, economic, and environmental considerations in oral healthcare. |
1. Introduction
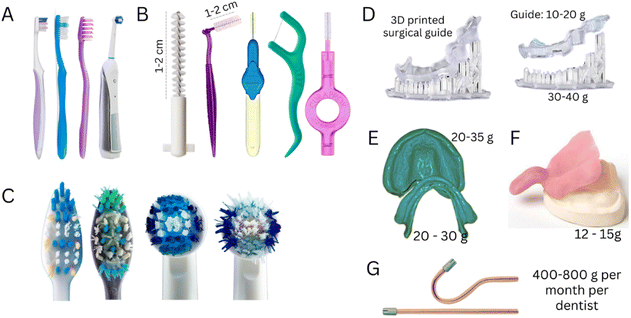
Oral health is an essential and integral component of overall health and well-being, encompassing the condition of the orofacial organs and tissues (enamel, dentine, gum, bone, glands, muscles) that enable both physiological functions (e.g., eating, breathing, and speaking) and influence psychological and social dimensions (e.g., self-confidence, well-being, expression of emotions and feelings, and interpersonal relationships).1,2 Total oral health is achieved by preserving healthy oral tissues, preventing the onset and curing diseases, and preserving re-established healthy states.3 In this regard, oral health demands life-long attention and care by individuals, dentists and healthcare system.1Synthetic polymeric materials, more commonly known as ‘plastics’, are widely used to maintain or re-establish oral health in routine personal oral care and clinical dentistry because of their high versatility and ability to be tailored to specific requirements and properties (Fig. 1). Clinically, dentists rely on a myriad of polymers (e.g., vinyl acrylics, polystyrene, epoxies, polycarbonates, polyethylene, polyvinyl acetate, polysulfides, polysilicon, polyethers, and acrylates) for preventive, restorative, and regenerative therapies such as impression taking procedures, printing models, manufacturing of intraoral devices (crowns, bridges, dentures, clear aligners, splints), restorations, implants, etc. Polymers are also widely used for manufacturing clinical instruments (spatulas, cheek retractors, saliva ejectors), containers (trays, containers), and artificial teeth for training.4,5
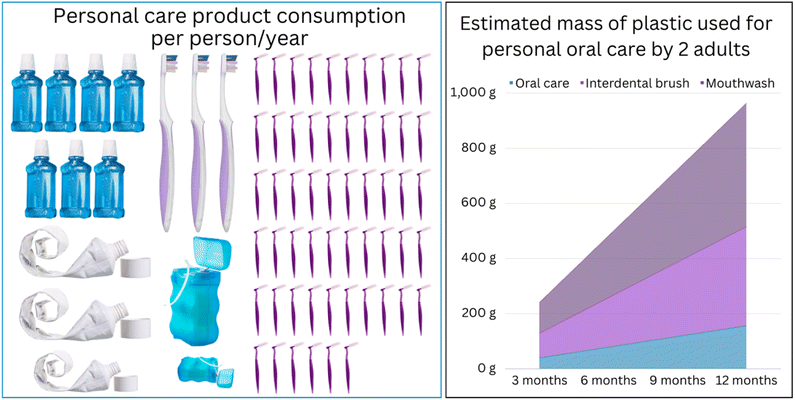
In addition to the polymers used by clinicians, consumers use high volumes of plastics in oral care products due to the increased awareness about the importance of maintaining good oral health. Plastics are polymeric materials in the solid “glassy state” at room or oral temperatures, where they have sufficient stiffness and strength to fulfill various design requirements.6 The size of the global toothbrush market was valued at USD 18.7 billion in 2023 and is projected to reach USD 25.7 billion in 2030. Likewise, the toothpaste market is expected to increase from USD 29 billion in 2021 to USD 40 billion by 2028, with a demand of 19–20 billion toothpaste tubes.7–9 The scale of global plastic consumption for individuals' oral care needs can be estimated using proxy information. For instance, the American Dental Association (ADA) recommends changing the toothbrush every three to four months (or sooner depending on the bristles integrity10). Anecdotal estimates suggest that an individual who brushes their teeth three times a day using a pea-sized amount of toothpaste (0.25 g per dose) would consume 273 g or 2.7 tubes (100 g per tube) of toothpaste a year. This translates to approximately 85 g of plastic waste generated per person annually (15 g per empty tube of toothpaste and 45 g for three toothbrushes) (Fig. 2). If one adds the use of mouthwashes, the mass of plastic waste would increase by 600 g. However, this is only a conservative estimate as it disregards the mass of packaging and the variations in individual usage, frequency of product replacement, and designs. Furthermore, improvements in education, access to essential oral health services, and higher life expectancies will contribute to expanding the oral health market.
Besides the oral care products targeted directly at consumers, dentistry has experienced an increased demand for treatments with higher aesthetic appeals. In this regard, advancements in computer-aided design/computer-aided manufacturing (CAD/CAM) and 3D printing technologies have increased the pressure on polymer usage to match the higher demand for prostheses enabled via digital workflows. Likewise, advancements in orthodontic treatment and teeth whitening have created opportunities for patients to use intraoral trays and aligners with enhanced comfort and predictable outcomes. As a result, the use of flexible and transparent polymers [e.g., polyurethane (PU), polyethylene terephthalate glycol (PETG), and ethylene-vinyl acetate (EVA)] is anticipated to be on the rise.
With these growing trends, the environmental pressure is expected to increase due to the expansion of the dental industry, increased consumption of (disposable) oral health products (Fig. 2), as well as the lack of alternatives made of sustainable polymers (vide infra). While improving sustainability in different aspects of dental practice has started to receive attention in recent years,11–13 examining aspects such as energy usage,14 carbon footprint,15 resource management,16 and environmental impact,17 the massive scale of plastic waste in the dental industry has never been addressed in detail. The following section provides an overview of the diversity of plastic usage for personal and clinical oral care before we consider strategies for achieving greater sustainability for these indispensable and ubiquitous materials for dentistry.
1.1 The state of existing polymer usage in dentistry
The handle can be produced by different polymers such as polyethylene (PE), polypropylene (PP), and polyvinyl chloride (PVC), which are affordable, durable, and lightweight materials that can be easily molded into different shapes with varying degrees of flexibility.18,19 Moreover, these non-porous polymers generally do not absorb water, which makes them a more hygienic choice for toothbrush handles. PE and PP are also used to fabricate tongue cleaners. Although these polymeric materials can be mechanically recycled and reprocessed into new products,19 it often results in the deterioration of material quality and performance.20
The bristles can come in different stiffness as the applications vary from cleaning gingiva and teeth (extra soft and soft) to acrylic dentures (hard).21,22 Hence, different types of polymers are used to produce bristles because of their ability to be molded into different shapes, durability, tear strength, flexibility, resistance to biodegradation, and affordability.18
The nylon family is perhaps the most commonly used polymer to produce bristles. Nylon 6,6 (polyhexamethylene adipamide) has a high degree of crystallinity, which confers high modulus and tensile strength.18 An advantage of using nylon bristles to clean oral tissues is that nylon is hygroscopic and becomes more flexible and less brittle as it absorbs water from the environment, giving it good water bend recovery properties. Nylon 6–12, in particular, is highly durable and has good water absorption properties.23,24 Additionally, it has a higher degree of stiffness than other types of nylon. It absorbs water at a low rate, thereby maintaining the stiffness of the bristles when wet for a longer time.18,25 Regardless of manufacturer and consumer preferences, bristles of different types of nylon are effective in removing debris, bacteria, and biofilms from oral tissues.21,26
Other polymers that can be used to produce bristles are polyethylene (PE) and polypropylene (PP). These are widely available lightweight polymers that can produce bristles at low cost. The PE bristles tend to be softer and less stiff than those manufactured with nylon, which can be more comfortable for consumers with sensitive gums.27 However, a disadvantage of PE bristles is their tendency to wear out faster than nylon ones.19 Finally, PP bristles have higher wear and tear resistance and are less prone to bending than nylon ones,22,27,28 which can be a clinical disadvantage when aiming at extra gentle movements and friction on oral tissues. Table 1 summarises the mechanical properties of polymers typically found in consumer dental products.
| Property | PE | PP | PVC | Nylon-6,6 | Nylon-6,12 | Polyester |
|---|---|---|---|---|---|---|
| Modulus of elasticity (GPa) | 0.4–0.7 (low density), 0.9–1.2 (high density) | 1.6–2.4 | 2.0–3.0 (unplasticized), 0.4–0.7 (plasticized) | 2.8–3.2 | 2.5–3.5 | 1.00–10.6 |
| Water absorption (%, by weight) | 0.1–0.3 | 0.1–0.3 | 0.2–0.5 | 0.8–1.5 | 0.4–1.0 | 0.2–2.0 |
| Coefficient of friction (<0.3 = low, 0.3–0.6 = moderate, 0.6–1.0 = high) | Low | Low/moderate | Low/moderate | Low | Low | Low |
| Melting point (°C, varies according to type and molecular weight) | 110–130 (low density), 130–160 (high density) | 165–170 | 80–140 | 190–238 | 215–220 | 150–290 |
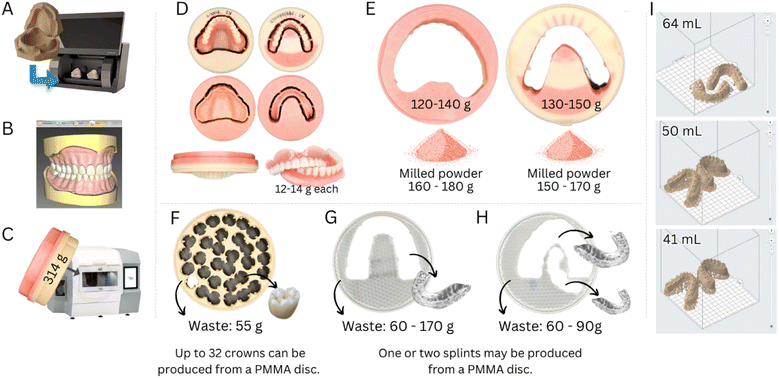
The demand for removable partial prostheses and dentures will inevitably grow with an aging population and higher life expectancy. However, despite the many advancements in developing intraoral scanners, traditional impression-taking procedures with trays loaded with polymeric impression materials [e.g., polyvinyl siloxane (PVS) or polydimethylsiloxane (PDMS)] will maintain their popularity. Our simulations of clinical procedures show that a trivial impression-taking with a stainless steel tray can consume from 150 to 400 g of PDMS.
The fabrication of artificial teeth, denture bases and complete dentures, trays, orthodontic retainers, occlusal splints, and printed or milled casts commonly employs PMMA due to its ease of manipulation, low solubility in saliva and oral fluids, color tailoring, cost-effectiveness and a high degree of conversions (>90%), resulting in resins that present satisfactory mechanical properties and chemical stability.29–31 The material is available as kits containing a powder and liquid, solutions (3D printing), blocks, and discs (CAD/CAM). The latter are highly cross-linked materials with superior mechanical properties.30
Polycarbonate (PC) has high mechanical strength, stiffness, toughness, good chemical resistance, and an acceptable aesthetic appearance.32,33 As a clinical substitute for PMMA, PC offer advantages such as reduced solubility, comparable hardness, enhanced mechanical strength, and fracture toughness.32,34 Therefore, PC has been indicated for fabricating dental guards, prostheses, orthodontic brackets, and temporary crowns.32,33,35
Polyetheretherketone (PEEK) is also used to produce frameworks for removable and fixed dental prostheses by CAD/CAM due to its promising mechanical properties, high inertness and chemical stability in the oral environment, and excellent biocompatibility and aesthetic properties.36,37 Despite the highly optimized workflows, clinics and labs produce a high volume of polymer waste. Manufacturing a complete denture from a PMMA disc via a CAD/CAM can produce up to 300 g of polymer waste. Meanwhile, a highly optimized milling process designed to yield a very high number of individual crowns per PMMA disc (from 25 to 32 crowns per disc) still results in a wastage of 50 to 60 g of polymer (Fig. 3).
Polyurethane (PU) is commonly used to fabricate intraoral appliances (e.g., retainers, night guards, and clear aligners) due to its high biocompatibility, durability, and ability to be molded into complex shapes. In addition, PUs can be used as scaffolds for tissue engineering or as an adhesive to replace bone cement.39 PU is composed of repeating urethane linkages, which consist of a carbamate group linked to two organic groups (R), one on each end [(R1–NH–CO–O–R2)n]. The final properties of PU depend on the final chemical structure of the repeating units.40
Polyethylene terephthalate glycol (PETG) is a non-crystalline co-polyester of polyethylene terephthalate (PET). It is widely used to fabricate clear aligners as it is transparent, resistant to chemical changes, and presents high elongation at break, tensile strength, and modulus of elasticity (approx. 50 MPa and 1 GPa, respectively).41 Notably, PETG allows for high versatility in fabricating clear aligners via thermoforming or 3D printing.42
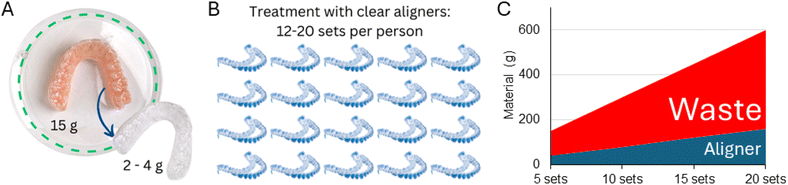
The increasing demand for aesthetic dental treatments, advancements in technology and alternative materials to traditional metal braces have promoted the emergence of the orthodontic clear aligners market, which is expected to grow from USD 4.1 billion in 2022 to approximately USD 32.2 billion in 2030.43 This market growth will add pressure on the environment through the surge in the consumption of polymers for the fabrication of aligners. Aligners are often fabricated using a PU sheet (approximately 15 g) shaped over a model of the patient's teeth using a heat-vacuum forming device. The mandibular and maxillary teeth aligners weigh approximately 1.5–3.0 g and 3.0–4.0 g, respectively (excluding packaging and shipping, Fig. 4). Generally, one aligner or, in some specific designs, two aligners can be obtained per sheet, resulting in 8.0 to 13.5 g of polymer wastage (61 to 90% of the sheet mass when one or two aligners are produced from a single sheet, respectively). Similar wastage is observed in manufacturing trays for teeth whitening. It must be highlighted that these masses of plastic vary according to the size of the mouth, aligner sheet thickness, treatment plan, and design requirements.
1.2 Considerations and opportunities for sustainable polymers in dentistry
It can be seen from Section 1.1 that there is massive plastic usage and waste generated by the consumption of personal oral care products or consumables routinely used in clinical dental practice. These do not include routine personal protective equipment (PPEs) such as gowns, aprons, gloves, and masks, which are single-use and traditionally made from petroleum-derived resources. The magnitude of the consumption can be illustrated with the pre-pandemic data from the Department of Health and Social Care (DHSC, United Kingdom) that distributed monthly 10–15 million aprons units, 40–45 thousand eye protection units, 1–2 million face masks and 145–150 million gloves.44,45 While reusing and reducing the consumption of plastics may be possible to some extent (such as in the case of unnecessary packaging), the need for keeping high-standards of infection control and maintaining hygienic practices to prevent cross-contamination makes the reduction in consumption largely impractical, especially concerning PPE. To compound the challenge further, the diversity of synthetic polymers with different properties suited for various dental applications, as aforementioned, makes providing a one-size-fits-all solution for the current unsustainable plastic usage in dentistry extremely challenging. Nonetheless, there are several possibilities for reducing the environmental impact of polymers, which we will discuss in this Perspectives article.There are limited options for recycling the excess and leftover plastics produced by clinical practice and consumers of oral care products: some of these polymers may, in theory, be recovered and converted into non-medical products, such as turning disposable polypropylene gowns into containers. However, mechanical recycling has limitations: inevitable material degradation during the process eventually relegates these plastics to landfills or incinerators.46 Unfortunately, many plastics used in oral care and dentistry are not recycled due to the difficulty of separating different polymers from composite products (such as the blends used to produce oral care products) or the risks of contamination by body fluids arising from usage. Nonetheless, there have been advancements in the use of high-density polyethylene (HDPE) for producing recyclable toothpaste tubes, and this may translate into packaging innovation with less plastic waste.9,47 There are opportunities to reduce plastic waste through greater product design simplicity and by reconsidering what is essential for personal oral hygiene. Compared to manual brushes with wider heads containing a large number of bristles, leveraging on rotation–oscillation movements to achieve greater cleaning effectiveness can be accomplished even with a reduced number of bristles in the smaller toothbrush head.48,49 In addition, substantial unexploited opportunities exist in repurposing dental-relevant polymers as feedstock for chemical upcycling into industrially relevant small molecule chemicals or, to a lesser extent, functional materials. Chemical upcycling of plastic waste transforms these post-use polymers into new products of higher value, thus placing this low-cost and readily-abundant waste material at the beginning of the value chain instead of at the end.50–53 This approach can reduce our reliance on petroleum sources to produce these essential chemicals, whose industrial demand may prove financially viable to sustain a possible post-use plastics industry. Alternatively, several classes of polymers in current use (e.g., nylons) are also amenable for closed-loop recycling,54 where the polymers are first broken down into their constituent monomers, which can then be repolymerized to form virgin polymers. Therefore, in Section 2, we highlight relevant chemical methods for achieving these transformations that can be applied to the most common classes of polymers used in dentistry. In addition, we briefly discuss the possibilities of harnessing biodegradation to break down dental polymers and exploit the emerging field of synthetic biology for valorizing waste dental plastics into high-value chemicals and polymers.
While chemical upcycling can repurpose and keep existing dental polymers within circular material loops, there is also a simultaneous need to develop the next generation of new sustainable dental polymers. In Section 3, we discuss the practical and material considerations for designing such polymers suited for dentistry. We also highlight several notable classes of polymers that may potentially find useful applications in dentistry. These include biodegradable polymers that can be produced from renewable biomass sources, which reduces reliance on petroleum feedstock, and polymers with inherent circularity built into their chemical structures to be depolymerized with the appropriate physicochemical triggers. However, it should be emphasized that the chemical upcycling of existing polymers and the development of new sustainable polymers are complementary in sustainable dentistry, and one approach should not be pursued at the expense of the other. The development of new polymers can be hampered by the economics and logistics of production, and their differences in properties compared to existing dental polymers can prove to be barriers to widespread adoption in practice. Meanwhile, most of the current polymers of dental relevance are produced from non-renewable petroleum sources that can be depleted in the future. However, some (e.g., polyolefins) can also be produced from biomass sources on substantial scales.55 Finally, we conclude this Perspectives with recommendations for sustainable polymer usage in dentistry that will apply to both existing and emerging classes of dental polymers. We hope the approaches discussed herein may inspire and guide new approaches for a more sustainable future of polymers and plastics in dentistry.
2. New opportunities for dental polymers' end-of-life
The sustainable end-of-life of dental polymers and plastics requires collecting and sorting materials for chemical recycling and upcycling, given the polymer-specific chemistries.Clinically, the collection of single-use plastic PPE is straightforward, as these can be disposed of in designated waste bins and sorted according to their types (gloves, gowns, hair covers, and masks) and decontaminated before any further chemical upcycling (sustainable decontamination of dental waste is discussed in ref. 56). On the other hand, plastic waste from lab procedures not contaminated with oral fluids from usage is of high purity with known composition; thus, it can be segregated by material type. Therefore, in our opinion, the latter is suitable for chemical recycling/upcycling without further sorting or treatment.
Personal hygiene products such as toothbrushes, tongue cleaners, and toothpaste tubes are commonly classified as municipal solid waste and are contaminated with other types of waste. Recovering the polymers from hygiene products for recycling/upcycling can be difficult. To compound the challenge, hygiene products are made of different types of polymers (e.g., handles and bristles of toothbrushes), and separating them from each other can be cumbersome and costly. It may, therefore, be more feasible to produce products made of sustainable alternatives (see Section 3) with simpler single-component designs to allow natural biodegradability and avoid environmental contamination.9,47,57
2.1 Opportunities for chemical recycling and upcycling of dental plastics
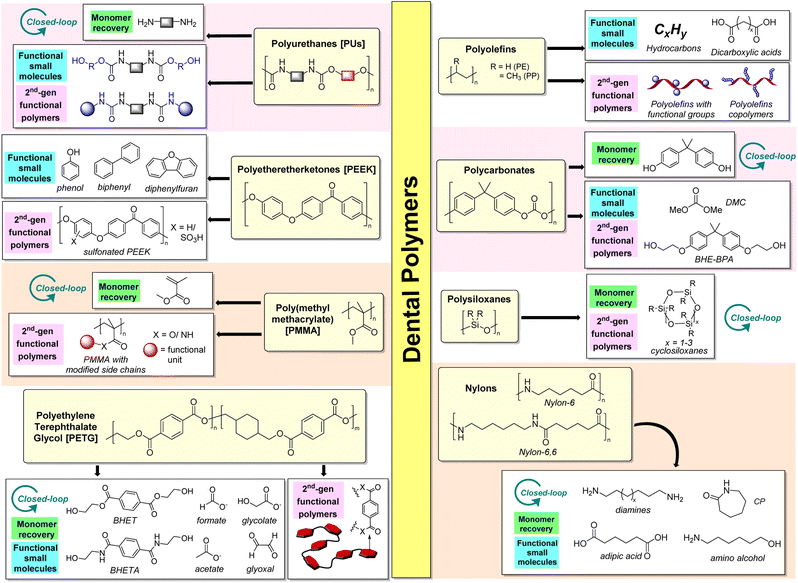
In recent years, there has been a growing customer awareness regarding the environmental impact of long-lasting polymers. This increases the pressure to develop sustainable technologies and establish a circular economy for plastics, including those used in the health industry. The range of new technologies to recycle polymer waste is growing rapidly, and the revenues from new polymer recycling technologies are expected to reach USD 162 billion by 2030 globally.58 Although plastic waste sorting and collection are currently unsolved problems, chemical recycling/upcycling must occur hand-in-hand to create a viable and financially sustainable post-use economy for these sorted plastics. Recent progress in unsupervised machine learning and hyperspectral imaging has made it possible to distinguish and group unidentified plastic samples composed of 12 distinct polymers, including those commonly employed in dentistry (e.g., PEEK, PMMA, and PET).59 Therefore, integrating machine learning with polymer chemistry may enable predictive tools to distinguish different polymer types commonly encountered in clinical and consumer products. Fig. 5 summarises the range of possibilities for dental plastics in use today.Closed-loop PU recycling can be achieved by first subjecting PUs to hydrolysis, which generates polyols, CO2, and the corresponding diamine. The diamine can then be converted to isocyanate starting materials with phosgene and re-polymerized with the polyols obtained to regenerate the original PU.71,72 Hydrolysis processes in superheated water under 250 °C (ref. 74 and 75) have shown great promise in converting PUF wastes within 30 minutes to give a two-phase liquid with a polyol phase and an aqueous phase that contains toluene diamines (72–86% yield). In addition, PU hydrolysis can be performed in a CO2/water mixture at 190 °C for 24 hours, where the carbonic acid formed activates the carbamate groups for the reaction to occur.76 However, due to the high energetic demands of PU hydrolysis, the reaction can be challenging to perform on commercial scales.77
Unlike PUs, the options for upcycling PEEK into industrially-relevant small molecules are limited because it lacks chemically-labile bonds. Therefore, it may be preferable to upcycle PEEK into functional polymeric materials through aromatic sulfonation by reacting the polymer in sulfuric acid83 or chlorosulfonic acid.84
Depending on the degree of sulfonation (DS), the resulting sulfonated polymer can become water-soluble and be used as a polyelectrolyte.84 The sulfonic acid groups can also act as chemical crosslinking sites for reactions with polyols to form polymer electrolyte membranes with high strength, thermal stability, and excellent proton conductivity,85 making them promising candidates for fuel cell applications. Sulfonated PEEK can also be used as polymer electrolytes with the potential for energy storage in lithium-ion batteries.86
The ester side chains of PMMA are amenable to hydrolysis, alcoholysis, and aminolysis to afford carboxylic acids, functionalized esters, and amides, respectively, which can be repurposed for different applications. For instance, the reaction of PMMA with diamines forms polymers terminated with primary amines92 that can be suitable for the immobilization of biomolecules such as DNA for biomedical applications. Hils et al.93 also demonstrated the synthesis of a triple-responsive (pH, temperature, CO2) poly(N,N-diethylaminoethyl methacrylamide) by PMMA amidation with N,N-diethylethylenediamine. With trivalent counterions such as [Fe(CN)6]3−, both an upper and lower critical solution temperature-type phase behavior is achievable at pH 8 and 9. Indeed, the inherent possibilities for functional material production from recovered waste PMMA are vast.
Polyesters like PET and PETG can be transesterified using glycols or alcohols and transamidated by reacting with amines. This enables the aromatic terephthalate component to be recovered for chemical recycling into new PET or other polymers such as polybutylene terephthalate (PBT).94 For example, glycolysis using EG can be performed on PET to recover bis(hydroxyethyl)-terephthalate (BHET), which is performed commercially through IBM's VolCat process95 and can be repolymerized to form virgin PET. Aminolysis of PET using ethanolamine can also yield N,N′-bis(2-hydroxyethyl)terephthalamide (BHETA), which is a useful precursor for the synthesis of new polycarbonates, polyesters, and polyurethanes. Indeed, polyurethanes synthesized from PET-derived BHETA have recently been shown to be useful as polymer electrolytes for energy storage in prototype lithium-ion batteries.96 Glycolysis, alcoholysis, and aminolysis are facilitated by different classes of catalysts, such as Lewis acid metal complexes such as aluminum triisopropoxide97 and dibutyltin oxide,98 deep-eutectic solvents (e.g., 1,3-dimethylurea/zinc acetate)99 and organocatalysts (e.g., 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD)100). More recently, Jehanno et al.101 demonstrated a sustainable PET chemical recycling process using an ionic salt comprising TBD and methanesulfonic acid (MSA). This solventless method completely depolymerized PET in less than 2 hours, producing a BHET yield of 91%. The same BHET monomer recovered was then subjected to repolymerization with the same TBD![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MSA catalyst, affording PET with similar thermal properties. Similarly, these methods could conceivably be applied to the closed-loop chemical recycling of PETG.
MSA catalyst, affording PET with similar thermal properties. Similarly, these methods could conceivably be applied to the closed-loop chemical recycling of PETG.
In recent years, a series of innovative catalytic procedures has demonstrated the feasibility of converting PET into other high-value small molecule chemicals. Through photoreforming, the ethylene glycol component of PET can be transformed into glyoxal, glycolate, acetate, and formate with concomitant H2 evolution, while the terephthalate component can be recovered.102,103 Electrocatalysis can also convert PET to terephthalate, potassium diformate, and H2.104 While not yet demonstrated using PETG, one may envisage that the aliphatic EG and CHDM components can be similarly oxidized to form value-added aliphatic compounds.
In recent years, there has been considerable interest in the post-synthetic modification of polyolefins via C–H functionalization chemistry, which introduces new functional groups on the polymers that can imbue them with new properties for new applications. For instance, Hartwig and coworkers have recently demonstrated that organometallic catalytic oxidation of PE to install carbonyl and hydroxyl groups can create polymers with enhanced adhesion, which can be used for creating new blends and malleable thermosets.109 Selective carbonyl group installation on PEs can also be achieved using aldehydes and oxygen gas.110 These reactive functional groups can also serve as convenient reactive sites for grafting other functional units onto the polymer backbone for new applications. Installing hydroxyl groups onto the side chains of isotactic PP can allow polycaprolactone to be grafted onto these polymers, which can be used to compatibilise immiscible polymer blends.111 Carbonyl and chlorinated groups on post-synthetically-functionalized PE can also be exploited for reaction with short-chain polyamines, forming cationic amphiphilic polymers with antifungal properties.112
Alcoholysis of PCs can allow for the recovery of functional small-molecule organic compounds. For instance, dimethyl carbonate (DMC) can be obtained from the methanolysis of PCs, which can also be facilitated using ionic liquids121,122 and organocatalysts such as 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD).115 These processes also liberate BPA, which can be re-polymerized with DMC to regenerate virgin PCs. In addition, DMC is also useful as a solvent exempted by the U.S. EPA as a volatile organic compound123 and can potentially replace hydrocarbon and halogenated solvents such as parachlorobenzotrifluoride. Additionally, glycolysis of PCs with ethylene glycol can produce bis(hydroxyethyl) ether of BPA (BHE-BPA), which is useful for synthesizing alternative polymers such as PUs.124
Other than thermal cracking, a number of alternative (catalytic) strategies have been developed in recent years. Alcoholysis can be performed with DMC in methanol using potassium fluoride as a catalyst to yield alkoxy(oligo)siloxanes,128 which can be transformed to (poly)siloxanes by hydrolysis. The affinity of silicon towards fluoride can also be exploited by using tetrabutylammonium fluoride to break down PDMS into cyclosiloxanes in a simple process under mild conditions.129 The cyclic product mixture could be repolymerized via acid- or base-catalyzed ring-opening polymerization to form silicones, demonstrating the possibility of closed-loop polymer recycling.
For nylon-6, it is possible to recover the cyclic monomer ε-caprolactam (CP), which can be subjected to ring-opening polymerization for closed-loop chemical recycling. Kamimura et al. have demonstrated that nylon-6, in the presence of 4-dimethylaminopyridine (DMAP) catalyst in ionic liquids, can afford high yields of CP after 6 hours at 300 °C.134 Other than ionic liquids, supercritical alcohols,135 and subcritical water136 can also afford high yields of CP with short reaction times.
Besides these approaches, Milstein and coworkers have reported that hydrogenative depolymerization of various nylons can occur using H2 in the presence of a ruthenium pincer catalyst to afford amino alcohols and oligoamides.137 Valuably, the authors demonstrated that the hydrogenated product mixture of monomers and oligomers can be dehydrogenated to reform a poly(oligo)amide, potentially completing a closed loop for the chemical recycling of amide. While still only in a proof-of-concept stage and not ready for practical adoption, this is the first demonstration that a sustainable, green, and atom-economic hydrogenation-based process can be used for closed-loop polyamide recycling.
2.2 Biodegradation of existing dental plastics
Plastic biodegradation by microorganisms offers an alternative route for the end-of-life treatment of dental plastics. Although this offers an energetically-mild approach to the degradation of polymers, the process is often very slow under ambient conditions. However, it can be sped up under industrial composting conditions. Indeed, advances in synthetic biology, such as genome editing, and our understanding of biological metabolism pathways can allow the engineering of new microorganism strains that can achieve biotransformations traditionally unachievable with natural microbial strains. Depending on the plastics, some of the depolymerized products can be assimilated as feedstock for the production of high-value commodities such as small molecule chemicals (that cannot be easily produced from chemical upcycling approaches) and even bioplastics such as poly(hydroxyalkanoates) (PHAs) (vide infra).138 This section provides a brief snapshot of the possibilities of how biodegradation can impact polymer end-of-life in dentistry.Amongst commonly-used dental polymers, PUs and nylons are the most susceptible to biodegradation by microorganisms. Aspergillus tubingensis139 is a species of fungi that grows on PU films, which results in cracking and erosion of the PU surface, with accompanying chemical degradation observed. Nylon-6 and nylon-6,6 can be broken down by marine bacteria such as Bacillus cereus, which use the polymer as the sole carbon source at 35 °C in a mineral salt medium (pH 7.5), albeit over three months.140 Analysis revealed that the degradation process introduces new functional groups such as terminal amides, aldehydes, and carboxylic acids onto the polymer structure, decreases the polymer's crystallinity, and reduces the average molecular weight of nylon-6 and nylon-6,6 by 42% and 31%, respectively. Other than bacteria, lignolytic fungi such as Phanerochaete chrysosporium can also degrade nylon-6 polymers. As reported by U. Klun et al.141, this species attaches itself to the polymer fibers, using them as a nitrogen source. After three months of exposure, the molar mass of the polymer was reduced by 50%, and physical grooves can be seen by scanning electron microscopy, showing the polymer's physical degradation.
Besides simply breaking down the polymers, biological approaches can be combined with chemical approaches to synthesize useful products from dental-relevant polymers, which is not easily achievable by either method alone. It is possible to synthesize compounds with antioxidant and anti-inflammatory properties, such as protocatechuic acid, from hydrolyzed PET's terephthalic acid (TA).142 Although not yet demonstrated using PETG, it is likely that the TA obtained from chemical or enzymatic hydrolysis of the polymer can be repurposed in a similar manner. In addition, polyethylene (PE) can be subjected to chemical pyrolysis to form a hydrocarbon wax before being chemically oxidized to form a fatty acid mixture that can be used as a substrate for bacterial PHA production.143 The medium chain-length (MCL)-PHAs obtained were largely amorphous, with a molecular weight of approximately 150 kDa and polydispersity index of 1.9, suggesting that these materials could find applications as elastomeric additives in coatings and adhesives. Similarly, MCL-PHAs can also be produced from TA as the sole carbon source.144
Despite the above possibilities, the challenges of biodegradation of dental polymers, viz. slow kinetics, need to be duly addressed before they can become a major player in the end-of-life treatment. This highlights the need for further advances in synthetic biology and the development of a new generation of sustainable polymers produced exclusively from renewable feedstock that are more susceptible to microbial degradation than petroleum sources. These, however, need to be segregated from existing plastic recyclates as they can act as contaminants and reduce the quality of the resulting recycled plastics.
2.3 Perspectives
Chemical recycling and upcycling of existing post-use dental plastics are often hampered by their durability and chemical resistance, as these were originally designed and formulated for utility rather than material circularity. A number of strategies discussed in Section 2.1 require the use of a bulk solvent phase, harsh reaction conditions, or hazardous reagents to achieve their targeted chemical transformation, which is inevitable due to the plastics' unreactivity. Solvents, in particular, pose a significant problem, as they often comprise the largest component of any reaction and are very rarely recycled for reuse. Nonetheless, several recent developments have greatly improved the sustainability of these chemical processes: (1) the use of more environmentally-benign solvents such as supercritical water; (2) avoiding the use of solvents altogether; (3) the usage of more benign reagents such as O2 for oxidations rather than (stoichiometric) oxidants; (4) alternative reactive modalities such as photo- and electrocatalysis rather than relying on traditional thermal chemistries, which has been shown to allow reactions that traditionally require harsh conditions to proceed under much milder, near-ambient conditions.145,146For plastic upcycling to become a practical reality, sustainable and economic factors must be considered. Sustainable methods are critical to avoid creating a more extensive environmental problem through waste generation than the problem it is attempting to solve. Economically, the products need to be of sufficient high value with demand that is commensurate with the scale of plastic waste produced. In this regard, higher-value chemicals (e.g., organic acids), which can be fed directly into existing industrial processes for use as precursors or ingredients, are potentially viable. For (polymeric) materials derived from plastics, their end-of-life needs to be also considered: can these products be suitably upcycled without having to dispose them unsustainably in landfills or incinerators? Notably, these issues impact the field of plastics recycling/upcycling as a whole and are not just related to dental polymers. With the significant global scientific attention to new sustainable chemical recycling/upcycling strategies and biocatalytic processes, rapid advances toward achieving sustainable plastic upcycling can be expected in the coming years.
3. Next-generation sustainable dental polymers: requirements, considerations, and possibilities
Along with the rest of the world, dentistry has a responsibility to find solutions for alternative polymers to address the global plastic waste crisis. The research breakthroughs expected of the plastics industry is to make materials more biodegradable while maintaining strength and durability that rival traditional commodity polymers, viz., the material should not start to degrade during its service and shelf life.147,148 Thus, the challenge in developing sustainable products lies in balancing their ease of degradation with practicality, durability, and biocompatibility. Toothbrush handles made of bamboo, as an alternative to traditional PP, serve as an interesting example to help minimize environmental impact (based on life cycle assessment).149 Nonetheless, research and development in the hitherto underexplored field of sustainable dentistry are needed to afford new scope and opportunities based on green chemistry at all levels. Aside from specific properties that need to be met for a targeted area of dental application (e.g., toothbrush bristles should have high flexibility and effectively reach and clean all areas of the teeth and gums), polymers for oral care consumer products should generally satisfy the following criteria:• Durability: to withstand frequent use over time (e.g., as toothbrush bristles and tongue cleaners).
• Hygiene: materials should ideally be non-porous, resistant to bacteria adherence, and easily cleaned.
• Variety of processing designs: need to be easily molded into various shapes to cater to different oral care needs, durability and rigidity requirements.
In this section, we will consider the essential material properties (Table 2) and consumer and clinical requirements for dental polymers (Section 3.1) and discuss the possibility of replacing existing dental plastics with more sustainable alternatives (Sections 3.2 and 3.3). Table 2 summarises the key material characteristics of existing dental polymers that need to be satisfied for potential sustainable replacement candidates.
| Polymer | Use | Characteristics |
|---|---|---|
| Nylon | Toothbrush bristles and handles | Thermoplastic, stiff, chemically/heat-resistant, durable, good wear properties |
| Low- and high-density polyethylene (LDPE, HDPE) | Toothpaste tubes | Thermoplastic, flexible, durable, lightweight, and chemically resistant. It can be extruded, allowing for the production of tubular structures |
| Thermoplastic elastomers (TPE) | Tongue cleaners | Easily molded, flexible, high stretchability (allowing conformation to tongue surface without permanent shape deformation) |
| Polyetheretherketone (PEEK) | Complete dentures, removable prostheses, implants | Thermoplastic, high stiffness, biocompatibility, excellent geometrical stability, and resistant to many common chemicals used in oral healthcare products |
| Polymethyl methacrylate (PMMA) | Dental prosthesis (bridges and veneers) | High biocompatibility, mechanical strength, and stiffness, easy to polish, and can be colored to match the natural color of the teeth |
| Polyurethane (PU) | Dental prostheses and implants, orthodontic aligners, occlusal splints, and night guards | High biocompatibility and wear resistance. It can be formed into various shapes (multiple levels of flexibility and rigidity), is light-weighted, has ease of fabrication, and has good chemical resistance to common dental disinfectants and cleaning agents, tunable properties |
| Polycarbonate (PC) | Dental guards, sports mouth guards, clear aligners, and other dental prostheses | High biocompatibility, mechanical strength, and stiffness. Good impact strength and chemical resistance to common dental disinfectants and cleaning agents |
| Polydimethylsiloxane (PDMS) | Impression materials, custom dental trays, dental guards, and orthodontic appliances | High tear resistance and elastic recovery. Its excellent flexibility allows PDMS to conform to the shape of oral tissues for accurate impressions. High chemical resistance to common dental disinfectants and cleaning agents |
3.1 Consumer and clinical requirements for dental polymers
The development of new polymeric materials can be targeted to three segments: (i) consumer care (toothbrushes, containers for personal oral care products), (ii) materials for patient use (impression materials, prosthetic and restorative materials, intraoral devices), and (iii) adjunct devices (saliva ejectors, individual trays). Each segment will have specific requirements that will influence the development and adoption of alternative materials to fit their purpose. For instance, nylon has been the material of choice for producing toothbrush bristles because of its hygroscopic capabilities that modulate the bristles' rigidity. However, no specific requirements are expected from the polymers used to manufacture the bristles and the other parts of the toothbrush. Nonetheless, the toothbrush handle and the neck are expected to be light and smooth (to decrease microbial contamination) and allow flexibility to cushion the forces the user applies when brushing to avoid unnecessary stress to oral soft tissues and breakage.On the other hand, alternative polymers that target direct patient applications need to consider specific requirements. For instance, materials used in direct restorations (crowns, bridges) and occlusal splints (bruxism) must present high mechanical properties (e.g., fracture strength) and resistance to degradation by oral fluids, biofilms, pH variation, food, and beverages. Likewise, new polymers for impression need to fulfill the criteria established by organizations such as the American Dental Association (ADA) and International Standardization Organization (ISO), summarised in Table 3.
| Manual toothbrushes – resistance of tufted portion to deflection (ISO 22254:2005) |
|---|
| Since the perception of stiffness differs amongst countries, stiffness may be classified differently |
| Elastomeric impression materials (ISO 4823:2015) | |||||||
|---|---|---|---|---|---|---|---|
| Type: body | Consistency (test disc diam, mm) | Strain-in compression% | Detail reproduction (line width, μm) | Linear dimensional change (max%) | Elastic recovery (min%) | ||
| min | max | min | max | ||||
| 0: putty | — | 35 | 0.8 | 20 | 75 | 1.5 | 96.5 |
| 1: heavy | — | 35 | 50 | ||||
| 2: medium | 31 | 41 | 2.0 | 20 | 20 | ||
| 3: light | 36 | — | 20 | ||||
| Base polymers-part 1: denture base polymers (ISO 20795-1:2013) | |||||
|---|---|---|---|---|---|
| Curing mode (type) | Ultimate flexural strength (min MPa) | Flexural modulus (min MPa) | Residual monomer (max% mass fraction) | Sorption (μg mm−3) | Solubility (μg mm−3) |
| Heat (1), light (4), or microwave (5) | 65 | 2000 | 2.2 | 32 | 1.6 |
| Autopolymerizable (2) | 60 | 1500 | 2.5 | 8.0 | |
| Base polymers-part 2: orthodontic base polymers (ISO 20795-2:2013) | |||||
|---|---|---|---|---|---|
| Types | Ultimate flexural strength (min MPa) | Flexural modulus (min MPa) | Residual monomer (max% mass fraction) | Sorption (μg mm−3) | Solubility (μg mm−3) |
| All | 50 | 1500 | 5 | 32 | 5 |
However, not all materials for direct patient applications require such high compliance standards. For instance, individual trays or surgical guides are primarily prepared with polymers that provide pieces with high rigidity. Different requirements apply to polymers used to fabricate orthodontic aligners and athletic mouthguards. Although both applications require flexibility, aligners must be transparent and be able to withstand the wear and tear of daily use. In addition, clear aligners must present a time-dependent relationship between stress and strain to deform and deliver the forces for tooth movement as planned by the clinician.42 It must be highlighted that developing new polymers capable of delivering steady forces to the teeth over time is needed. The current polymers used for fabricating clear aligners experience an exponential reduction in force over time, resulting in significantly less force after a few hours from installation, which compromises teeth movement.150
In contrast, athletic mouthguards must have high elastic behavior to absorb impact. Still, they may require less wear resistance and are used for a shorter time than aligners. In these cases, long-term wear resistance is not a clinical concern. Therefore, new recyclable/upcyclable polymers that fulfill the “rigidity/flexibility requirement” (and transparency in some cases) at a reasonable cost could become viable clinical alternatives. In the following sections, we will consider some emerging bioplastics (Section 3.2) and new strategies for engineering polymer recyclability (Section 3.3) relevant to dental applications.
3.2 Alternative bioplastics
Bioplastics are polymers wholly or partially synthesized from renewable bio-based feedstock, rather than petroleum. While drop-in bioplastics such as bio-polyethylene can be produced from monomers derived from biomass sources, this class of bioplastics will not be considered herein as they are physically and chemically indistinguishable from their petroleum-derived counterparts. Instead, our discussion will be focused on compostable bioplastics such as polylactic acid (PLA) and poly(hydroxyalkanoates) (PHAs). Although commonly touted as a sustainable replacement for existing petroleum-based plastics, being produced from biological sources does not equate to biodegradability. Indeed, PLA is not biodegradable under ambient conditions but is considered compostable, as it can be broken down under defined and controlled conditions within specific timeframes.151 Nonetheless, should biodegradable and compostable polymers enter mainstream dentistry, their waste needs to be adequately segregated from those of existing petroleum plastics, as they are often treated as impurities and can complicate plastic recycling streams. Cost aside, much remains to be improved for bioplastics before they can claim a significant market presence. Nonetheless, it is important to consider the possibilities of such materials to replace existing plastics in dentistry, which may be especially pertinent for personal dental products (e.g., toothbrushes and tongue cleaners) that cannot be easily separated from municipal waste streams. Amongst bioplastics, PLA and PHAs offer great potential as replacements for some existing petroleum-based polymers (Fig. 6).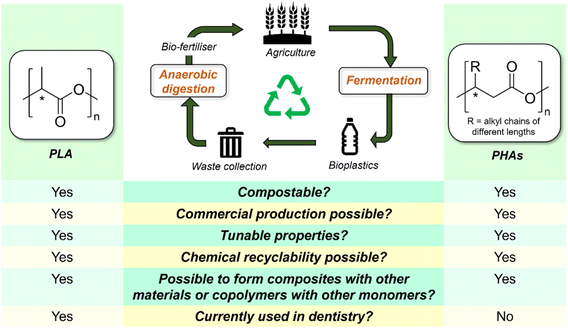 | ||
| Fig. 6 Bioplastics with circular life cycles can potentially replace some existing plastics in dentistry. | ||
At the time of writing, PLA is amongst the most produced bioplastics in the world. Its monomer (lactic acid) can be derived from the fermentation of plant starch from corn, sugarcane, and cassava or from sugars by Lactobacillales bacteria. PLA is highly biocompatible and has been affirmed by the US FDA as a suitable material for medical applications. Indeed, PLA has broad applicability for provisional crowns, complete dentures, and orthodontic devices in dentistry. Although PLA can be produced by polycondensation of lactic acid, the resulting polymeric materials were of ill-defined molecular weight and microstructure.152 Therefore, ring-opening polymerization (ROP) of the cyclic lactide monomer is now more frequently used for PLA synthesis, as it allows for better control of the end product in terms of molecular weight, dispersity, well-defined polymer chain-ends, and desired tacticity.153 Modern advances in catalysis have enabled lactide ROP to be performed under mild conditions using Lewis acidic organometallic complexes154–156 and organocatalysts,157,158 often with good stereocontrol. The properties of PLA can be tuned to reduce brittleness, facilitate processing, and improve their mechanical properties through stereocomplexation,159,160 surface treatment,161 and formulation of composites with various additives,162,163 such that their properties can resemble those of commodity petroleum plastics such as polystyrene and PET. Similarly, appropriate composite formulation and post-synthetic processing techniques can extend the application of PLA-based materials to more diverse dentistry uses.
Poly(hydroxyalkanoates) (PHAs) are a family of biogenic polyesters (Fig. 6) produced by numerous bacteria in nature, such as Cupriavidus necator and Ralstonia eutropha, as an intercellular carbon and energy-storage reservoir.164,165 Natural PHAs feature exclusively (R)-configuration at the chiral center (e.g., isotactic) owing to their fermentative synthesis,165 while synthetic PHAs produced from ROP of cyclic esters and β-lactones are less stereo-regular. The properties of PHAs depend not only on polymer molecular weight but also the identity of their pendant alkyl chains: while short chain length (SCL: C3–C5) PHAs such as poly(3-hydroxybutyrate) [P(3HB)] are highly crystalline, stiff and brittle, with high melting point of ∼175 °C, medium chain length (MCL C6–C14) PHAs are more amorphous, flexible and have lower strength. The diversity of these structures and the possibilities of copolymerizing hydroxyalkanoate monomers with other monomers enable their properties to be customized for different applications. For example, copolymerizing 3-hydroxybutyrate (3-HB) with 25 mol% hydroxyvaleriate (HV) units resulted in the decrease of the melting point of the resulting material (PHBV) to 137 °C,164,166,167 compared with P(3HB). Besides improved thermal processability, its impact strength was enhanced by an order of magnitude, making its overall properties comparable to PP's. Under aerobic conditions, PHB degrades completely to CO2, water, and humus, whereas under an anaerobic environment, CH4 is produced.167 While naturally-occurring PHB is easily degraded by microorganisms due to its exclusive (R)-configuration of the side chains, synthetic PHBs consisting of a mixture of (R)- and (S)-stereoblocks exhibit different degradation rates; (S)-units undergo a slower enzymatic degradation than (R)-units.168,169 This implies that the material's lifetime can be potentially modified by controlling the stereoregularity during polymer synthesis. Chemical degradation of PHAs can yield products such as crotonic acid, which can be used to produce other high-value chemicals such as poly(crotonic acids) and crotonate esters.170 Alternatively, it was shown that MCL-PHAs can be thermally degraded to 2-alkenoic acids, which can be utilized as a feedstock for further PHA biosynthesis, potentially offering a strategy for PHA recycling.171 PHAs are biocompatible, and many of them have been used for various biomedical applications, such as scaffolds for tissue engineering, wound dressings, medical implants, antimicrobial membranes, and drug delivery platforms.172,173 Although not currently used as dental materials, there is significant potential for these bioplastics to be used in this field.
In addition to PLA and PHAs, considerable recent research has been done in developing sugar-derived biomass-based alternatives to conventional oil-based plastics. A bio-PC nanocomposite containing isosorbide (derived from D-glucose) and cellulose nanocrystals that possessed excellent mechanical strength was reported as a potential replacement for BPA-based PCs.174 Alternatively, PET can potentially be replaced with poly(ethylene furanoate) (PEF), which contains the biomass-derived furan-dicarboxylic acid monomer (derived from fructose) as a replacement for the petroleum-derived terephthalic acid component of PET. Compared with PET, PEF production has potentially lower greenhouse gas emissions,175 and this bioplastic has higher gas barrier properties to gases such as water vapor and oxygen.176 Such is the promise of PEF that it is now produced on commercial scales by Avantium. Despite the possibility of these bioplastics replacing oil-based dental polymers in use today, such as BPA-containing PCs and PETG, their end-of-life may not be very different. Although accelerated biodegradation tests have shown that PEF degrades faster than PET at 58 °C,177 PEF breakdown under more realistic natural conditions is yet to be studied. If the purpose of replacing existing dental polymers, especially for personal care products, with bioplastics is to reduce environmental impact through more facile natural biodegradation, these bioplastics may not necessarily be suitable.
Despite the promise of these bioplastics, their costs of production are currently incomparable to those of petroleum-based polymers, which inevitably hinders their more widespread adoption and usage. This applies even to PLA and PHAs. The cost of PHAs, for instance, can be 5–10 times those of existing petroleum-based plastics, associated with complex bioprocessing techniques and challenging downstream product purification.178 In this regard, there is considerable effort in reducing the cost of PHA production, such as using cheaper carbon sources for biosynthesis179 (e.g., industrial and municipal wastes, which include waste polystyrene).180 Beyond cost, other factors also affect the feasibility of bioplastic production and adoption: environmental concerns over agricultural practices, land usage, and competition with food crop production, such as that for PLA feedstock production, can potentially outweigh their benefits over petroleum-based polymers. Such factors can conspire to sound the death knell for bioplastics/biodegradable polymers: despite the promise of Bionolle™, a biodegradable polyester produced by Showa Denko with similar processibility as LDPE and has potential for its monomers (succinic acid and 1,4-butanediol) to be produced from biomass sources,181 its production was terminated in 2016. This was attributed to economic reasons and delays in environmental regulations.182
3.3 Synthetic polymers with inherent recyclability
Other than bioplastics, recent years have also witnessed the development of inherently-recyclable polymers and covalent adaptable networks (CANs)-crosslinked polymers containing reversible covalent bonds that solve the end-of-life issue and provide a direct approach to establishing a circular economy. As shown in Fig. 7, these polymers are designed such that their chemical bonds can be broken on demand upon applying specific physicochemical triggers (e.g., high temperature and specific chemicals). For inherently-recyclable polymers, these properties enable monomer recovery that can then be used in repolymerization. At the same time, CANs allow post-usage reprocessibility and recyclability. Although these polymers show great promise as circular materials, most have not yet made it to commercial production. Nonetheless, we must consider them as potential replacements for existing oil-based polymers where applicable for dentistry, even though the biodegradability of most of these polymers has yet to be tested.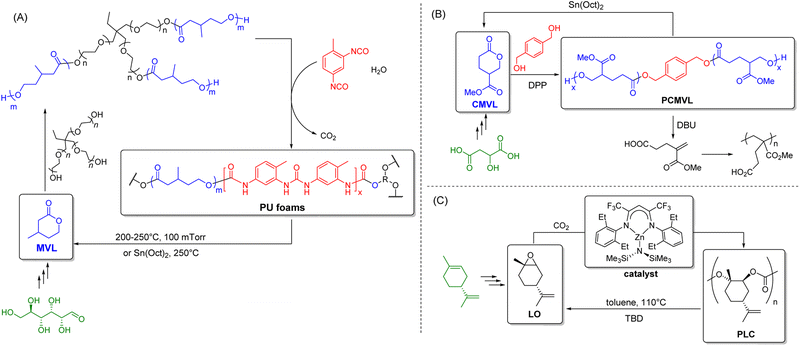 | ||
| Fig. 7 Synthesis of chemically recyclable polymers using renewable sources: (A) flexible PU foams from MVL;183 (B) PCMVL from CMVL;185 and (C) PLC from LO.186,190 | ||
For such polymers, specific chemistries are needed to enable material circularity; it is perhaps more feasible for them to be replacements for clinical settings rather than in personal domestic dental use, where segregating the waste materials can be more feasibly achieved. Regardless, these recyclable polymers should be hydrolytically stable, resistant to common chemical constituents of food and dental products (e.g., Cl−), and preferably produced from sustainable/biomass resources. Herein, we highlight some possibilities that require specific thermal and/or chemical conditions for achieving bond dynamics that are typically not encountered in common dental practice.
The synthesis of chemically recyclable polymers from renewable feedstock has gained immense popularity over the past decade. In particular, substituted polyvalerolactones produced from renewable monomers such as 4-carbomethoxyvalerolactone (CMVL) and β-methyl-δ-valerolactone (MVL) have been documented. Hillmyer et al. reported the synthesis of bio-based and chemically recyclable flexible polyurethane (PU) foams using hydroxy telechelic PMVL to replace petroleum-derived polyols (Fig. 7A).183 These materials not only rival petroleum-derived PUs in performance but also the crosslinked PMVL PUs, which can also be chemically depolymerized to recover the MVL monomer in high purity and yield. An MVL yield of up to 97% and ≥95% purity was achieved when PMVL PU foam was subjected to 200–250 °C and 100 mTorr conditions. Although not essential, the depolymerization could be accelerated by adding Sn(oct)2. To close the recycling loop, the MVL recovered was used to synthesize new PMVL polyols, which were shown to be identical to an analogous sample prepared from virgin monomers.
Malic acid is a promising renewable and abundantly available feedstock that can be produced microbially from biomass as an intermediate in Kreb's cycle.184 Hoye et al. synthesized the CMVL monomer from malic acid via a two-step synthesis. Ring-opening transesterification polymerization (ROTEP) of CMVL then produced the novel substituted polyvalerolactone PCMVL (Fig. 7B).185 This material was found to be semicrystalline with a glass transition temperature of −18 °C, with two melting temperatures at 68 and 86 °C. Furthermore, this polyester can be chemically recycled by two complementary pathways: (1) reverse ROTEP or backbiting depolymerization to CMVL (87% yield), or (2) eliminative process to form a methacrylate-like monomer (88% yield), which can undergo polymerization to give a new polymethacrylate derivative.
Copolymerization of limonene oxide (LO) with CO2 to produce bio-based poly(limonene carbonate) (PLC) has also attracted much attention recently (Fig. 7C).186–188 LO is bio-derived from limonene, a monoterpene commonly found in the peel of citrus fruits.189 The depolymerization of PLC was recently demonstrated by Sablong et al. using 1,5,7-triazabicyclo[4.4.0]dec-5-ene (TBD).190 At 110 °C in toluene, the strong organic base deprotonates the OH-terminated PLC. This leads to a fast degradation back to LO monomer via successive backbiting reactions. This bio-based polymer can be a truly sustainable material due to its quantitative depolymerization to monomers and the use of non-toxic and cheap CO2 as a building block for copolymer synthesis.
The vast majority of the polymers we have considered thus far are thermoplastics, as they are mainly linear polymers that interact with each other primarily through intermolecular forces that can be overcome by heat to form viscoelastic liquids. An alternative class of recyclable polymers is the CANs – an exciting family of renewable plastics with end-of-life recyclability that offer high strength, stability, and chemical resistance compared to traditional thermoset plastics.191 Unlike thermoplastics, thermosets are 3D crosslinked polymers with permanent covalent bonds throughout their structure that cannot easily be broken even when subjected to elevated temperatures, such that the polymer thermally decomposes before melting. In contrast, CANs contain dynamic covalent bonds that can be broken and formed reversibly under suitable conditions, making up the 3D polymer network. A large number of CANs have been developed in recent years, and the interested reader is referred to recent reviews for a more in-depth treatment of these materials.191 CANs may potentially be used as recyclable and reprocessible alternatives to the current commonplace thermosets, such as the PUs and PMMA used in dentistry today. Although most CANs reported are based on petroleum feedstock, an emerging generation of bio-based CANs is gaining popularity, utilizing raw materials derived from sugars, organic acids, oxygenated biopolymers, lignin, natural rubber, and hydrocarbon-rich biomass such as vegetable oils.192
3.4 Perspectives
For the dental industry, as with many other applications, the dominant motivations for adopting new polymers are the practical benefits (e.g., utility, cost, convenience), while sustainability considerations often take a back seat. At the time of writing, considerable challenges need to be surmounted before sustainable dental polymers can find practical deployment. The issue of cost is paramount, as more expensive materials and their (segregated) disposal will ultimately be passed on to patients and consumers. As aforementioned, even the most widely produced bioplastics, PHAs and PLAs, are presently more costly than petroleum-based plastics. The cost problem is expected to be even more acute for polymers with inherent recyclability, as these often require more specialized and expensive monomers for production. Despite this drawback, continued advancement in the production of bioplastics and sustainable alternative polymers will likely drive costs down, offering new possibilities in dental applications.Should some of these sustainable polymers enter the dental polymer toolkit in the future, they should be segregated from existing recyclable plastics after use, as their presence can act as contaminants and reduce the quality of recyclables. However, the diversity in new sustainable polymer classes discussed in Section 3.3 can unnecessarily complicate waste disposal, even at the clinical level by trained practitioners, due to the specific chemistries needed for circularity. This could ultimately result in low adoption or inadvertent backfiring. The problem is further complicated by the diverse material properties required for different dental applications, as a recyclable polymer suited for one application may not be so for another. To overcome this, we suggest that greater emphasis be placed on developing sustainable polymers that possess inherent material diversity and allow ease of property tunability. In this regard, the diverse family of PHAs may be the most promising existing class of polymers, despite being currently overlooked in dentistry, which can all be subjected to composting post-usage. The possibility of composting also makes PHA-based dental products suitable for domestic use. A similar consideration should drive the development of inherently recyclable polymers for dentistry, achieving the greatest diversity in material properties for each type of reversible polymerization chemistry.
4. Beyond the materials: practices for more sustainable dental plastics consumption
Plastic waste in dentistry arising from the consumption of single-use items such as PPEs and disposable clinical tools, packaging materials, consumer care products, leftovers from laboratory procedures, and long-lasting materials for short-lived applications presents a significant environmental concern. Addressing this issue requires advancing technologies for re-using or re-purposing existing polymers, developing less durable materials with desired properties for short-lived clinical applications, and optimizing lab workflows to decrease wastage from manufacturing procedures.It is noteworthy that while dentistry contributes significantly to plastic waste, the broader healthcare industry has a more substantial impact, contributing to approximately 4.4% of global net emissions.193 In Europe, plastic constitutes approximately 36% of healthcare waste.194 Notably, the global medical plastics market was estimated at US$52.9 billion in 2023 and is expected to keep growing with an annual growth rate of 7.4%.195 Hence, breakthroughs to decrease the environmental burden at the manufacturing, clinical, and consumer levels in dentistry also hold the potential for adoption in other medical sectors, all of which face increasing pressure to decrease their waste footprint.
One plausible solution is collecting and segregating single-use clinical materials, such as PPE, saliva ejectors, material packaging, and containers. It is worth highlighting that a new business model for dental practices has emerged in the last two decades, from individual practices (single clinician) to the consolidation of several dental practitioners under the same roof with centralized administration, whether private or public.196 Hence, the collection and segregation of clinical wastage (contaminated or not) can be adopted as part of the clinical operations procedures and guidelines. An impressive example of how this has been feasibly achieved, since the Minamata Convention on Mercury became effective in 2017, dentists from around the world have already managed to segregate the very minute pieces of amalgam that may need to be removed from teeth in a collective effort to reduce mercury emissions and minimize mercury-related health and environmental risks. Hence, dental professionals are not alien to the potential environmental hazards triggered by materials used clinically, and a culture of clinical recycling can be conceivably and feasibly implemented.
Another area that can be readily developed and implemented is the smart design and optimized manufacturing practices for dental appliances and products for consumer oral care, leveraging improved manufacturing precision and more powerful simulation tools. For instance, our simulations show that optimizing the positioning of virtual models before the 3D printing procedures can reduce the amount of polymer consumed for model fabrication by 35% (Fig. 2). Hence, developing software for automated positioning can reduce the amount consumed and decrease the wastage arising from eliminating auxiliaries such as supporting pillars and model bases.
A similar call to action can be made to research and develop more innovative designs for consumer oral care products, clinical tools, and packaging. While the usage of powered toothbrushes will likely continue increasing, manual toothbrushes will remain popular due to their lower unit price and maintenance cost.197 Therefore, manufacturers must continue innovating product designs to develop toothbrushes with less plastic while maintaining cleaning effectiveness. Likewise, manufacturers can innovate to promote a more eco-friendly approach to dental clinical products. For instance, the design and materials used to fabricate the current saliva ejectors are similar to those proposed in the original patent applications in the 1940s. Dentistry embraces technological advancements, evidenced by the high acceptance of novel equipment for care delivery and the adoption of digital planning and 3D-printed solutions. Hence, clinicians worldwide have a fair chance of accepting novel eco-friendly designs that fulfill essential clinical functions satisfactorily.
5. Conclusions
The ideal chemical and physical properties of existing petroleum-based plastics have made them indispensable for developing durable and hygienic materials for consumer products and dental tools and for delivering clinical care and treatments by dental professionals. The high versatility and widespread use of plastics, combined with the humongous volume of products consumed daily by the general population and dentists to maintain and restore oral health, positions dentistry as a significant contributor to the global plastic waste crisis. Thus, there is a need for greater awareness and endless opportunities for innovation on multiple fronts to address the massive plastic wastage issue in oral care. Recent advances in recycling and upcycling technologies can aid in improving material circularity. At the same time, developing sustainable polymers can reduce the accumulation and environmental recalcitrance of long-lasting materials used for short-lived applications. Progress and shifts towards these advancements will not occur instantaneously. Hence, adopting more sustainable practices and improving the designs of established clinical tools and products can serve as a bridge for decreasing the environmental burden in the interim. Globally, there is a growing awareness of the importance of maintaining good oral health and comprehensive dental care. Therefore, research and innovation need to address the rising consumption of plastics in dentistry, simultaneously enabling the improvement of global oral health without the corresponding environmental impact. We hope this Perspectives article spurs much-needed innovations and advances in this direction.Conflicts of interest
There are no conflicts to declare.Acknowledgements
J. Y. C. Lim acknowledges the MTC Programmatic Fund (Project number M22K9b0049) and the A*STAR Central Research Fund for generous financial support of this work. The authors thank Mr Joseph Xu Weijian for discussing laboratory materials and procedures.Notes and references
- WHO World Health Organization, Oral health, https://www.who.int/health-topics/oral-health/#tab=tab_1, accessed 13/01/ 2024 Search PubMed.
- W. D. F. FDI, FDI's definition of oral health, https://www.fdiworlddental.org/fdis-definition-oral-health, accessed 13/01/ 2024 Search PubMed.
- P. T. Alpert, Oral Health: The Oral-Systemic Health, Home Health Care Manag. Pract., 2017, 29, 56–59 CrossRef.
- D. Rokaya, V. Srimaneepong, J. Sapkota, J. Qin, K. Siraleartmukul and V. Siriwongrungson, J. Adv. Res., 2018, 14, 25–34 CrossRef CAS PubMed.
- X. Xu, L. He, B. Zhu, J. Li and J. Li, Polym. Chem., 2017, 8, 807–823 RSC.
- L. Desidery and M. Lanotte, in Plastic Waste for Sustainable Asphalt Roads, 2022, pp. 3–28, DOI:10.1016/b978-0-323-85789-5.00001-0.
- The Insight Partners, Toothpaste Market Size, Share, Growth Report 2028, 2022 Search PubMed.
- Fortune Business Insights, Toothbrush Market Size, Share & COVID-19 Impact Analysis, by Product Type (Manual and Electric), by Bristle Type (Soft, Medium, and Firm), End-User (Adult and Children), and Regional Forecast, 2022-2029, 2022 Search PubMed.
- Colgate-Palmolive, Colgate® Launches its Groundbreaking Recyclable Toothpaste Tube with “Recycle Me!” Packaging in the U.S., https://www.colgatepalmolive.com/en-us/who-we-are/stories/recyclable-toothpaste-tube-recycle-me-packaging-us, accessed 14/04/ 2024 Search PubMed.
- E. S. T. R. Department of Scientific Information, ADA Science & Research Institute, LLC., Toothbrushes, https://www.ada.org/resources/research/science-and-research-institute/oral-health-topics/toothbrushes#:%7E:text=Toothbrushesshouldbereplacedapproximately,asthebristlesbecomeworn, accessed January 08, 2023 Search PubMed.
- N. Martin, M. Sheppard, G. Gorasia, P. Arora, M. Cooper and S. Mulligan, J. Dent., 2021, 112, 103735 CrossRef PubMed.
- B. Duane, J. Fisher, P. Ashley, S. Saget and E. Pasdeki-Clewer, in Sustainable Dentistry: Making a Difference, ed. B. Duane, Springer International Publishing, Cham, 2022, pp. 1–17, DOI:10.1007/978-3-031-07999-3_1.
- F. D. I. World Dental Federation, Int. Dent. J., 2018, 68, 10–11 CrossRef PubMed.
- B. Duane, S. Harford, I. Steinbach, R. Stancliffe, J. Swan, R. Lomax, E. Pasdeki-Clewer and D. Ramasubbu, Br. Dent. J., 2019, 226, 367–373 CrossRef PubMed.
- B. Duane, M. B. Lee, S. White, R. Stancliffe and I. Steinbach, Br. Dent. J., 2017, 223, 589–593 CrossRef CAS PubMed.
- J. Grose, J. Richardson, I. Mills, D. Moles and M. Nasser, Br. Dent. J., 2016, 220, 187–191 CrossRef CAS PubMed.
- D. Byrne, S. Saget, A. Davidson, H. Haneef, T. Abdeldaim, A. Almudahkah, N. Basquille, A. M. Bergin, J. Prida, A. Lyne and B. Duane, Br. Dent. J., 2022, 233, 317–325 CrossRef PubMed.
- E. Baur, T. A. Osswald and N. Rudolph, Plastics Handbook, 2019 Search PubMed.
- M. A. Spalding and A. M. Chatterjee, Handbook of Industrial Polyethylene and Technology, 2017 Search PubMed.
- J. Hopewell, R. Dvorak and E. Kosior, Philos. Trans. R. Soc., B, 2009, 364, 2115–2126 CrossRef CAS PubMed.
- C. d. M. S. Souza, L. O. Sakae, P. M. A. Carneiro, R. A. Esteves and T. Scaramucci, J. Dent., 2021, 105, 1–6 CrossRef PubMed.
- H. R. Rawls, N. J. Mkwayi-Tulloch and M. E. Krull, Dent. Mater., 1990, 6, 111–117 CrossRef CAS PubMed.
- D. Muhammad and M. Asaduzzaman, in Composites and Their Properties, 2012, ch. 14, DOI:10.5772/48246.
- M. Watanabe, M. Karasawa and K. Matsubara, Wear, 1968, 12, 185–191 CrossRef.
- MatWeb, Material Property Data, https://www.matweb.com/index.aspx, accessed January 8, 2023 Search PubMed.
- M. Keller, G. Keller, T. Eller, L. Sigwart, V. Wiesmüller, R. Steiner, V. Offermanns and I. Kapferer-Seebacher, Clin. Oral Investig., 2023, 27, 603–611 CrossRef PubMed.
- B. Hamza, M. Tanner, P. Körner, T. Attin and F. J. Wegehaupt, Int. J. Dent. Hyg., 2021, 19, 355–359 CrossRef PubMed.
- A. Acherkouk, M. Götze, A. Kiesow, A. Ramakrishnan, S. Sarembe, T. Lang and P. Gaengler, BMC Oral Health, 2022, 22, 1–10 CrossRef PubMed.
- M. S. Zafar, Polymers, 2020, 12, 1–35 CrossRef PubMed.
- G. Alp, S. Murat and B. Yilmaz, J. Prosthodontics, 2019, 28, e491–e495 Search PubMed.
- S. Aati, Z. Akram, B. Shrestha, J. Patel, B. Shih, K. Shearston, H. Ngo and A. Fawzy, Dent. Mater., 2022, 38, 57–67 CrossRef CAS PubMed.
- A. Tichy, M. Simkova, J. Schweiger, P. Bradna and J.-F. Güth, Materials, 2021, 14 CrossRef CAS PubMed.
- D. Edelhoff, J. Schweiger, O. Prandtner, J. Trimpl, M. Stimmelmayr and J. F. Guth, Quintessence Int., 2017, 48, 181–191 Search PubMed.
- R. Lin and J. S. C.-H. Yu, Shanghai Kouqiang Yixue, 2019, 28, 467–471 Search PubMed.
- M. Watanabe, T. Hase and Y. Imai, Dent. Mater. J., 2001, 20, 353–358 CrossRef CAS PubMed.
- B. Siewert, M. Plaza-Castro, N. Sereno and M. Jarman-Smith, in PEEK Biomaterials Handbook, 2019, pp. 333–342, DOI:10.1016/b978-0-12-812524-3.00020-x.
- B. Wang, M. Huang, P. Dang, J. Xie, X. Zhang and X. Yan, Polymers, 2022, 14 Search PubMed.
- L. Vovelle and J. M. Martin, in Interface Dynamics, Proceedings of the 14th Leeds-Lyon Symposium on Tribology, 1987, pp. 255–258, DOI:10.1016/s0167-8922(08)71073-3.
- K. Lei, Q. Zhu, X. Wang, H. Xiao and Z. Zheng, ACS Biomater. Sci. Eng., 2019, 5, 5489–5497 CrossRef CAS PubMed.
- F. M. de Souza, P. K. Kahol and R. K. Gupta, in Polyurethane Chemistry: Renewable Polyols and Isocyanates, 2021, pp. 1–24, DOI:10.1021/bk-2021-1380.ch001.
- P. Latko-Durałek, K. Dydek and A. Boczkowska, J. Polym. Environ., 2019, 27, 2600–2606 CrossRef.
- Y. M. Bichu, A. Alwafi, X. Liu, J. Andrews, B. Ludwig, A. Y. Bichu and B. Zou, Bioact. Mater., 2023, 22, 384–403 Search PubMed.
- G. V. Research, Clear Aligners Market Size, Share & Trends Analysis Report by Age (Adults, Teens), by End-Use (Hospitals, Standalone Practices, Group Practices, Others), by Region, and Segment Forecasts, 2023–2030, Report GVR-4-68038-960-9, 2022 Search PubMed.
- D. Westgarth, BDJ In Practice, 2021, 34, 12–16 Search PubMed.
- Department of Health and Social Care, Personal Protective Equipment (PPE) Strategy: stabilise and build resilience, https://www.gov.uk/government/publications/personal-protective-equipment-ppe-strategy-stabilise-and-build-resilience/personal-protective-equipment-ppe-strategy-stabilise-and-build-resilience, accessed 20/01/ 2024 Search PubMed.
- J. Y. C. Lim, T. N. B. Truong, J. Y. Q. Teo, C.-G. Wang and Z. Li, in Circularity of Plastics, ed. Z. Li, J. Y. C. Lim and C.-G. Wang, Elsevier, 2023, pp. 1–34, DOI:10.1016/B978-0-323-91198-6.00010-3.
- Unilever, Unilever to introduce recyclable toothpaste tubes, https://www.unilever.com/news/press-and-media/press-releases/2021/unilever-to-introduce-recyclable-toothpaste-tubes/, accessed 20/01/ 2024 Search PubMed.
- M. Yaacob, H. V. Worthington, S. A. Deacon, C. Deery, A. D. Walmsley, P. G. Robinson and A. M. Glenny, Cochrane Database Syst. Rev., 2014, 2014, CD002281 Search PubMed.
- E. X. Goh and L. P. Lim, Oral Health Prev. Dent., 2017, 15, 23–32 Search PubMed.
- C. Jehanno, J. W. Alty, M. Roosen, S. De Meester, A. P. Dove, E. Y. X. Chen, F. A. Leibfarth and H. Sardon, Nature, 2022, 603, 803–814 CrossRef CAS PubMed.
- X. Zhao, B. Boruah, K. F. Chin, M. Đokić, J. M. Modak and H. S. Soo, Adv. Mater., 2021, 2100843 Search PubMed.
- J. Y. Q. Teo, A. Ong, T. T. Y. Tan, X. Li, X. J. Loh and J. Y. C. Lim, Green Chem., 2022, 24, 6086–6099 RSC.
- A. Ong, J. Y. Q. Teo, Z. Feng, T. T. Y. Tan and J. Y. C. Lim, ACS Sustain. Chem. Eng., 2023, 11, 12514–12522 CrossRef CAS.
- C. Alberti, R. Figueira, M. Hofmann, S. Koschke and S. Enthaler, ChemistrySelect, 2019, 4, 12638–12642 CrossRef CAS.
- V. Siracusa and I. Blanco, Polymers, 2020, 12, 1–17 Search PubMed.
- B. Duane, N. Armstrong, S. Harford, Viviana, A. Pinhas, H. Ahmed and D. Ramasubbu, in Sustainable Dentistry: Making a Difference, ed. B. Duane, Springer International Publishing, Cham, 2022, pp. 117–139, DOI:10.1007/978-3-031-07999-3_7.
- A. Borunda, How your toothbrush became a part of the plastic crisis, https://www.nationalgeographic.com/environment/article/story-of-plastic-toothbrushes, accessed 20 May 2023 Search PubMed.
- IDTechEx, Green Technology and Polymer Recycling: Market Analysis 2020-2030 Technology for a Sustainable Circular Economy in Plastic Waste, UK, 2020 Search PubMed.
- M. L. Henriksen, C. B. Karlsen, P. Klarskov and M. Hinge, Vib. Spectrosc., 2022, 118 CrossRef CAS.
- M. M. A. Nikje and M. Nikrah, J. Macromol. Sci., Part A: Pure Appl.Chem., 2007, 44, 613–617 CrossRef.
- M. Murai, M. Sanou, T. Fujimoto and F. Baba, J. Cell. Plast., 2003, 39, 15–27 CrossRef CAS.
- X. Wang, H. Chen, C. Chen and H. Li, Fibers Polym., 2011, 12, 857 CrossRef CAS.
- J. Borda, G. Pásztor and M. Zsuga, Polym. Degrad. Stab., 2000, 68, 419–422 CrossRef CAS.
- A. Elidrissi, O. Krim, S. Ouslimane, M. Berrabeh and R. Touzani, J. Appl. Polym. Sci., 2007, 105, 1623–1631 CrossRef CAS.
- S. Xue, M. Omoto, T. Hidai and Y. Imai, J. Appl. Polym. Sci., 1995, 56, 127–134 CrossRef.
- S. Chuayjuljit, C. Norakankorn and V. Pimpan, J. Met., Mater. Miner., 2002, 12, 19–22 CAS.
- M. M. Alavi Nikje, M. Nikrah and M. Haghshenas, Polym. Bull., 2007, 59, 91–104 CrossRef CAS.
- P. Zhu, Z. B. Cao, Y. Chen, X. J. Zhang, G. R. Qian, Y. L. Chu and M. Zhou, Environ. Technol., 2014, 35, 2676–2684 CrossRef CAS PubMed.
- D. Simón, A. M. Borreguero, A. de Lucas, C. Gutiérrez and J. F. Rodríguez, in Environment, Energy and Climate Change I: Environmental Chemistry of Pollutants and Wastes, ed. E. Jiménez, B. Cabañas and G. Lefebvre, Springer International Publishing, Cham, 2014, pp. 229–260, DOI:10.1007/698_2014_275.
- C. Molero, A. de Lucas and J. F. Rodríguez, Polym. Degrad. Stab., 2006, 91, 894–901 CrossRef CAS.
- P. Zahedifar, L. Pazdur, C. M. L. Vande Velde and P. Billen, Sustainability, 2021, 13, 3583 CrossRef CAS.
- A. Kemona and M. Piotrowska, Polymers, 2020, 12, 1752 CrossRef CAS PubMed.
- M. B. Martin, Process for Converting the Decomposition Products of Polyurethane and Novel Compositions Thereby Obtained, US Pat., 4110266, 1978 Search PubMed.
- L. R. Mahoney, S. A. Weiner and F. C. Ferris, Environ. Sci. Technol., 1974, 8, 135–139 CrossRef CAS.
- Z. Dai, B. Hatano, J.-i. Kadokawa and H. Tagaya, Polym. Degrad. Stab., 2002, 76, 179–184 CrossRef CAS.
- S. Motokucho, A. Yamaguchi, Y. Nakayama, H. Morikawa and H. Nakatani, J. Polym. Sci., Part A: Polym. Chem., 2017, 55, 2004–2010 CrossRef CAS.
- D. Simón, A. M. Borreguero, A. de Lucas and J. F. Rodríguez, Waste Manage., 2018, 76, 147–171 CrossRef PubMed.
- P. Patel, A. A. Stec, T. R. Hull, M. Naffakh, A. M. Diez-Pascual, G. Ellis, N. Safronava and R. E. Lyon, Polym. Degrad. Stab., 2012, 97, 2492–2502 CrossRef CAS.
- S. M. Kurtz, in PEEK Biomaterials Handbook, 2012, pp. 75–79, DOI:10.1016/b978-1-4377-4463-7.10006-5.
- L. O. Dandy, G. Oliveux, J. Wood, M. J. Jenkins and G. A. Leeke, Polym. Degrad. Stab., 2015, 112, 52–62 CrossRef CAS.
- P. Patel, T. R. Hull, R. W. McCabe, D. Flath, J. Grasmeder and M. Percy, Polym. Degrad. Stab., 2010, 95, 709–718 CrossRef CAS.
- Y. Shibasaki, J.-i. Kadokawa, H. Tagaya, B. Hatano and C. Kato, J. Mater. Cycles Waste Manage., 2004, 6, 1–5 CrossRef.
- P. Xing, G. P. Robertson, M. D. Guiver, S. D. Mikhailenko, K. Wang and S. Kaliaguine, J. Membr. Sci., 2004, 229, 95–106 CrossRef CAS.
- F. Trotta, E. Drioli, G. Moraglio and E. B. Poma, J. Appl. Polym. Sci., 1998, 70, 477–482 CrossRef CAS.
- S. D. Mikhailenko, G. P. Robertson, M. D. Guiver and S. Kaliaguine, J. Membr. Sci., 2006, 285, 306–316 CrossRef CAS.
- M. Guo, M. Zhang, D. He, J. Hu, X. Wang, C. Gong, X. Xie and Z. Xue, Electrochim. Acta, 2017, 255, 396–404 CrossRef CAS.
- J. F. W. Kaminsky, J. Anal. Appl. Pyrolysis, 1991, 19, 311–318 CrossRef.
- E. D. Segui and B. C. Alarcon, US Pat., US2858255A, 1958 Search PubMed.
- A. Sasaki, N. Kikuya, T. Ookubo and M. Hayashida, US Pat., US8304573B2, 2012 Search PubMed.
- K. Koyanagi, T. Masaki, S. Morooka and H. Saito, Japan Pat., JP3410343B2, 1997 Search PubMed.
- M. J. S. J. M. Olazar, A. T. Aguayo, J. M. Arandes and J. Bilbao, Chem. Eng. J., 1993, 51, 53–60 CrossRef CAS.
- F. Fixe, M. Dufva, P. Telleman and C. B. Christensen, Nucleic Acids Res., 2004, 32, e9 CrossRef CAS PubMed.
- C. Hils, E. Fuchs, F. Eger, J. Schobel and H. Schmalz, Chemistry, 2020, 26, 5611–5614 CrossRef CAS PubMed.
- SABIC Introduces LNP™ ELCRIN™ IQ Upcycled Compounds to Extend Useful Life of PET Bottles and Help Reduce Plastic Waste https://www.sabic.com/en/news/20177-sabic-introduces-lnp-elcrin-iq-upcycled-compounds-to-extend-useful-life-of-pet-bottles-and-help-reduce-plastic-waste, accessed 21 May 2023.
- B. Allen, G. Breyta, J. Garcia, G. Jones and J. L. Hedrick, Polyester Digestion: VOLCAT, IBM, 2018 Search PubMed.
- M. Y. Tan, L. Goh, D. Safanama, W. W. Loh, N. Ding, S. W. Chien, S. S. Goh, W. Thitsartarn, J. Y. C. Lim and D. W. H. Fam, J. Mater. Chem. A, 2022, 10, 24468–24474 RSC.
- H. Kurokawa, M.-a. Ohshima, K. Sugiyama and H. Miura, Polym. Degrad. Stab., 2003, 79, 529–533 CrossRef CAS.
- M. E. Tawfik and S. B. Eskander, Polym. Degrad. Stab., 2010, 95, 187–194 CrossRef CAS.
- B. Liu, W. Fu, X. Lu, Q. Zhou and S. Zhang, ACS Sustain. Chem. Eng., 2019, 7, 3292–3300 CrossRef CAS.
- K. Fukushima, O. Coulembier, J. M. Lecuyer, H. A. Almegren, A. M. Alabdulrahman, F. D. Alsewailem, M. A. Mcneil, P. Dubois, R. M. Waymouth, H. W. Horn, J. E. Rice and J. L. Hedrick, J. Polym. Sci., Part A: Polym. Chem., 2011, 49, 1273–1281 CrossRef CAS.
- C. Jehanno, I. Flores, A. P. Dove, A. J. Müller, F. Ruipérez and H. Sardon, Green Chem., 2018, 20, 1205–1212 RSC.
- T. Uekert, H. Kasap and E. Reisner, J. Am. Chem. Soc., 2019, 141, 15201–15210 CrossRef CAS PubMed.
- X. Gong, F. Tong, F. Ma, Y. Zhang, P. Zhou, Z. Wang, Y. Liu, P. Wang, H. Cheng, Y. Dai, Z. Zheng and B. Huang, Appl. Catal., B, 2022, 307, 121143 CrossRef CAS.
- H. Zhou, Y. Ren, Z. Li, M. Xu, Y. Wang, R. Ge, X. Kong, L. Zheng and H. Duan, Nat. Commun., 2021, 12, 4679 CrossRef CAS PubMed.
- A. Tennakoon, X. Wu, A. L. Paterson, S. Patnaik, Y. Pei, A. M. LaPointe, S. C. Ammal, R. A. Hackler, A. Heyden, I. I. Slowing, G. W. Coates, M. Delferro, B. Peters, W. Huang, A. D. Sadow and F. A. Perras, Nat. Catal., 2020, 3, 893–901 CrossRef CAS.
- E. Bäckström, K. Odelius and M. Hakkarainen, ACS Sustainable Chem. Eng., 2019, 7, 11004–11013 CrossRef.
- E. Bäckström, K. Odelius and M. Hakkarainen, Ind. Eng. Chem. Res., 2017, 56, 14814–14821 CrossRef.
- C.-F. Chow, W.-L. Wong, K. Y.-F. Ho, C.-S. Chan and C.-B. Gong, Chem. - Eur. J., 2016, 22, 9513–9518 CrossRef CAS PubMed.
- L. Chen, K. G. Malollari, A. Uliana, D. Sanchez, P. B. Messersmith and J. F. Hartwig, Chem, 2021, 7, 137–145 CAS.
- J. Y. Q. Teo, C. W. S. Yeung, T. T. Y. Tan, W. W. Loh, X. J. Loh and J. Y. C. Lim, Green Chem., 2022, 24, 6287–6294 RSC.
- C. Bae, J. F. Hartwig, N. K. Boaen Harris, R. O. Long, K. S. Anderson and M. A. Hillmyer, J. Am. Chem. Soc., 2005, 127, 767–776 CrossRef CAS PubMed.
- C. W. S. Yeung, M. H. Periayah, J. Y. Q. Teo, E. T. L. Goh, P. L. Chee, W. W. Loh, X. J. Loh, R. Lakshminarayanan and J. Y. C. Lim, Macromolecules, 2023, 56, 815–823 CrossRef CAS.
- E. Diamanti-Kandarakis, J.-P. Bourguignon, L. C. Giudice, R. Hauser, G. S. Prins, A. M. Soto, R. T. Zoeller and A. C. Gore, Endocr. Rev., 2009, 30, 293–342 CrossRef CAS PubMed.
- M. Watanabe, T. Hase and Y. Imai, Dent. Mater. J., 2001, 20, 353–358 CrossRef CAS PubMed.
- T. Do, E. R. Baral and J. G. Kim, Polymer, 2018, 143, 106–114 CrossRef CAS.
- D. S. Achilias, E. V. Antonakou, E. Koutsokosta and A. A. Lappas, J. Appl. Polym. Sci., 2009, 114, 212–221 CrossRef CAS.
- G. Grause, K. Sugawara, T. Mizoguchi and T. Yoshioka, Polym. Degrad. Stab., 2009, 94, 1119–1124 CrossRef CAS.
- X. Song, F. Liu, L. Li, X. Yang, S. Yu and X. Ge, J. Hazard. Mater., 2013, 244–245, 204–208 CrossRef CAS PubMed.
- L. Li, F. Liu, Z. Li, X. Song, S. Yu and S. Liu, Fibers Polym., 2013, 14, 365–368 CrossRef CAS.
- E. Quaranta, Appl. Catal., B, 2017, 206, 233–241 CrossRef CAS.
- F. Liu, Z. Li, S. Yu, X. Cui and X. Ge, J. Hazard. Mater., 2010, 174, 872–875 CrossRef CAS PubMed.
- F. Liu, L. Li, S. Yu, Z. Lv and X. Ge, J. Hazard. Mater., 2011, 189, 249–254 CrossRef CAS PubMed.
- US EPA, Technical Air Pollution Resources, https://www.epa.gov/technical-air-pollution-resources, accessed 20 May 2023 Search PubMed.
- C.-H. Lin, H.-Y. Lin, W.-Z. Liao and S. A. Dai, Green Chem., 2007, 9, 38–43 RSC.
- W. Patnode and D. F. Wilcock, J. Am. Chem. Soc., 1946, 68, 358–363 CrossRef CAS.
- G. Deshpande and M. E. Rezac, Polym. Degrad. Stab., 2002, 76, 17–24 CrossRef CAS.
- G. Saevarsdottir, T. Magnusson and H. Kvande, J. Sustain. Metall., 2021, 7, 848–857 CrossRef.
- M. Okamoto, S. Suzuki and E. Suzuki, Appl. Catal., A, 2004, 261, 239–245 CrossRef CAS.
- B. Rupasinghe and J. C. Furgal, ACS Appl. Polym. Mater., 2021, 3, 1828–1839 CrossRef CAS.
- C. Jehanno, M. M. Pérez-Madrigal, J. Demarteau, H. Sardon and A. P. Dove, Polym. Chem., 2019, 10, 172–186 RSC.
- U. Klun and A. Kržan, Polym. Adv. Technol., 2002, 13, 817–822 CrossRef CAS.
- S. R. Shukla, A. M. Harad and D. Mahato, J. Appl. Polym. Sci., 2006, 100, 186–190 CrossRef CAS.
- U. Češarek, D. Pahovnik and E. Žagar, ACS Sustainable Chem. Eng., 2020, 8, 16274–16282 CrossRef PubMed.
- A. Kamimura and S. Yamamoto, Org. Lett., 2007, 9, 2533–2535 CrossRef CAS PubMed.
- A. Kamimura, Y. Oishi, K. Kaiso, T. Sugimoto and K. Kashiwagi, ChemSusChem, 2008, 1, 82–84 CrossRef CAS PubMed.
- J. Chen, G. Liu, L. Jin, P. Ni, Z. Li, H. He, Y. Xu, J. Zhang and J. Dong, J. Anal. Appl. Pyrolysis, 2010, 87, 50–55 CrossRef CAS.
- A. Kumar, N. von Wolff, M. Rauch, Y.-Q. Zou, G. Shmul, Y. Ben-David, G. Leitus, L. Avram and D. Milstein, J. Am. Chem. Soc., 2020, 142, 14267–14275 CrossRef CAS PubMed.
- N. Wierckx, M. A. Prieto, P. Pomposiello, V. de Lorenzo, K. O'Connor and L. M. Blank, Microb. Biotechnol., 2015, 8, 900–903 CrossRef PubMed.
- S. Khan, S. Nadir, Z. U. Shah, A. A. Shah, S. C. Karunarathna, J. Xu, A. Khan, S. Munir and F. Hasan, Environ. Pollut., 2017, 225, 469–480 CrossRef CAS PubMed.
- M. Sudhakar, C. Priyadarshini, M. Doble, P. Sriyutha Murthy and R. Venkatesan, Int. Biodeterior. Biodegrad., 2007, 60, 144–151 CrossRef CAS.
- U. Klun, J. Friedrich and A. Kržan, Polym. Degrad. Stab., 2003, 79, 817–822 CrossRef.
- M. Sasoh, E. Masai, S. Ishibashi, H. Hara, N. Kamimura, K. Miyauchi and M. Fukuda, Appl. Environ. Microbiol., 2006, 72, 1825–1832 CrossRef CAS PubMed.
- M. W. Guzik, T. Nitkiewicz, M. Wojnarowska, M. Sołtysik, S. T. Kenny, R. P. Babu, M. Best and K. E. O'Connor, Waste Manage., 2021, 135, 60–69 CrossRef CAS PubMed.
- S. T. Kenny, J. N. Runic, W. Kaminsky, T. Woods, R. P. Babu, C. M. Keely, W. Blau and K. E. O'Connor, Environ. Sci. Technol., 2008, 42, 7696–7701 CrossRef CAS PubMed.
- A. Ong, Z. C. Wong, K. L. O. Chin, W. W. Loh, M. H. Chua, S. J. Ang and J. Y. C. Lim, Chem. Sci., 2024, 15, 1061–1067 RSC.
- H. Zhou, Y. Ren, Z. Li, M. Xu, Y. Wang, R. Ge, X. Kong, L. Zheng and H. Duan, Nat. Commun., 2021, 12, 4679 CrossRef CAS PubMed.
- J. Baranwal, B. Barse, A. Fais, G. L. Delogu and A. Kumar, Polymers, 2022, 14, 983 CrossRef CAS PubMed.
- A.-C. Albertsson and M. Hakkarainen, Science, 2017, 358, 872–873 CrossRef CAS PubMed.
- A. Lyne, P. Ashley, S. Saget, M. Porto Costa, B. Underwood and B. Duane, Br. Dent. J., 2020, 229, 303–309 CrossRef PubMed.
- G. Siciliani, F. Mollica, A. Arreghini, V. Mazzanti, E. Martines and L. Lombardo, Angle Orthod., 2016, 87, 11–18 CrossRef PubMed.
- S. Deconinck and B. de Wilde, Benefits and Challenges of Bio- and Oxo-Degradable Plastics: A Comparative Literature Study, PlasticsEurope, Brussels, Belgium, 2013 Search PubMed.
- H. R. Kricheldorf, Chemosphere, 2001, 43, 49–54 CrossRef CAS PubMed.
- S. Dutta, W. C. Hung, B. H. Huang and C. C. Lin, in Synthetic Biodegradable Polymers, Springer, Berlin, Heidelberg, 1st edn, 2012, vol. 245 Search PubMed.
- K. M. Stridsberg, M. Ryner and A.-C. Albertsson, in Degradable Aliphatic Polyesters, Springer Berlin Heidelberg, Berlin, Heidelberg, 2002, pp. 41–65, DOI:10.1007/3-540-45734-8_2.
- M. H. Chisholm, N. W. Eilerts, J. C. Huffman, S. S. Iyer, M. Pacold and K. Phomphrai, J. Am. Chem. Soc., 2000, 122, 11845–11854 CrossRef CAS.
- M. H. Chisholm, J. Gallucci and K. Phomphrai, Chem. Commun., 2003, 48–49, 10.1039/B208679D.
- K. Fukushima and K. Nozaki, Macromolecules, 2020, 53, 5018–5022 CrossRef CAS.
- J. Y. C. Lim, N. Yuntawattana, P. D. Beer and C. K. Williams, Angew. Chem., Int. Ed., 2019, 58, 6007–6011 CrossRef CAS PubMed.
- H. Tsuji, Macromol. Biosci., 2005, 5, 569–597 CrossRef CAS PubMed.
- S. R. Andersson, M. Hakkarainen, S. Inkinen, A. Södergård and A.-C. Albertsson, Biomacromolecules, 2010, 11, 1067–1073 CrossRef CAS PubMed.
- M. Källrot, U. Edlund and A.-C. Albertsson, Biomacromolecules, 2007, 8, 2492–2496 CrossRef PubMed.
- L. Ranakoti, B. Gangil, S. K. Mishra, T. Singh, S. Sharma, R. A. Ilyas and S. El-Khatib, Materials, 2022, 15, 1–29 CrossRef PubMed.
- X. Li, Y. Lin, M. Liu, L. Meng and C. Li, J. Appl. Polym. Sci., 2023, 140, e53477 CrossRef CAS.
- S. Mecking, Angew. Chem., Int. Ed., 2004, 43, 1078–1085 CrossRef CAS PubMed.
- R. Reichardt and B. Rieger, in Synthetic Biodegradable Polymers, Springer, Berlin, Heidelberg, 1st edn, 2012, vol. 245 Search PubMed.
- L. L. Wallen and W. K. Rohwedder, Environ. Sci. Technol., 1974, 8, 576–579 CrossRef CAS.
- W. D. Luzier, Proc. Natl. Acad. Sci. U. S. A., 1992, 89, 839–842 CrossRef CAS PubMed.
- J. E. Kemnitzer, S. P. McCarthy and R. A. Gross, Macromolecules, 1992, 25, 5927–5934 CrossRef CAS.
- C. Jaimes, R. Dobreva-Schué, O. Giani-Beaune, F. Schué, W. Amass and A. Amass, Polym. Int., 1999, 48, 23–32 CrossRef CAS.
- Y. Aoyagi, K. Yamashita and Y. Doi, Polym. Degrad. Stab., 2002, 76, 53–59 CrossRef CAS.
- S. Sato, N. Ishii, Y. Hamada, H. Abe and T. Tsuge, Polym. Degrad. Stab., 2012, 97, 329–336 CrossRef CAS.
- D.-M. Miu, M. C. Eremia and M. Moscovici, Materials, 2022, 15, 1–29 CrossRef PubMed.
- B. S. Thorat Gadgil, N. Killi and G. V. N. Rathna, Med. Chem. Commun., 2017, 8, 1774–1787 RSC.
- S.-A. Park, Y. Eom, H. Jeon, J. M. Koo, E. S. Lee, J. Jegal, S. Y. Hwang, D. X. Oh and J. Park, Green Chem., 2019, 21, 5212–5221 RSC.
- A. J. J. E. Eerhart, A. P. C. Faaij and M. K. Patel, Energy Environ. Sci., 2012, 5, 6407–6422 RSC.
- S. K. Burgess, D. S. Mikkilineni, D. B. Yu, D. J. Kim, C. R. Mubarak, R. M. Kriegel and W. J. Koros, Polymer, 2014, 55, 6870–6882 CrossRef CAS.
- G.-J. Gruter, Technology and Markets Day: Path to the Future (Avantium), https://www.avantium.com/wp-content/uploads/2019/06/20190606-Technology-Day_CTO_Gert-Jan_Gruter_breakout_final_.pdf, accessed 24 May 2023 Search PubMed.
- G.-Q. Chen, X.-Y. Chen, F.-Q. Wu and J.-C. Chen, Adv. Ind. Eng. Polym. Res., 2020, 3, 1–7 Search PubMed.
- D. H. Vu, D. Åkesson, M. J. Taherzadeh and J. A. Ferreira, Bioresour. Technol., 2020, 298, 122393 CrossRef CAS PubMed.
- B. Johnston, I. Radecka, D. Hill, E. Chiellini, V. I. Ilieva, W. Sikorska, M. Musioł, M. Zięba, A. A. Marek, D. Keddie, B. Mendrek, S. Darbar, G. Adamus and M. Kowalczuk, Polymers, 2018, 10, 1–22 CrossRef PubMed.
- Y. Ichikawa and T. Mizukoshi, in Synthetic Biodegradable Polymers, Springer, Berlin, Heidelberg, 1st edn, 2012, vol. 245 Search PubMed.
- SDK to Terminate Production and Sale of Biodegradable Plastic, https://www.sdk.co.jp/english/news/15030/16250.html, accessed 21 May 2023.
- D. K. Schneiderman, M. E. Vanderlaan, A. M. Mannion, T. R. Panthani, D. C. Batiste, J. Z. Wang, F. S. Bates, C. W. Macosko and M. A. Hillmyer, ACS Macro Lett., 2016, 5, 515–518 CrossRef CAS PubMed.
- Z. Chi, Z.-P. Wang, G.-Y. Wang, I. Khan and Z.-M. Chi, Crit. Rev. Biotechnol., 2016, 36, 99–107 CrossRef CAS PubMed.
- G. W. Fahnhorst and T. R. Hoye, ACS Macro Lett., 2018, 7, 143–147 CrossRef CAS PubMed.
- C. M. Byrne, S. D. Allen, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2004, 126, 11404–11405 CrossRef CAS PubMed.
- M. Reiter, S. Vagin, A. Kronast, C. Jandl and B. Rieger, Chem. Sci., 2017, 8, 1876–1882 RSC.
- O. Hauenstein, M. Reiter, S. Agarwal, B. Rieger and A. Greiner, Green Chem., 2016, 18, 760–770 RSC.
- R. Ciriminna, M. Lomeli-Rodriguez, P. Demma Carà, J. A. Lopez-Sanchez and M. Pagliaro, Chem. Commun., 2014, 50, 15288–15296 RSC.
- C. Li, R. J. Sablong, R. A. T. M. van Benthem and C. E. Koning, ACS Macro Lett., 2017, 6, 684–688 CrossRef CAS PubMed.
- J. Zheng, Z. M. Png, S. H. Ng, G. X. Tham, E. Ye, S. S. Goh, X. J. Loh and Z. Li, Mater. Today, 2021, 51, 586–625 CrossRef CAS.
- X.-L. Zhao, P.-X. Tian, Y.-D. Li and J.-B. Zeng, Green Chem., 2022, 24, 4363–4387 RSC.
- J. Karliner, S. Slotterback, R. Boyd, B. Ashby and K. Steele, Health Care's Climate Footprint, Health Care Without Harm, 2019 Search PubMed.
- Centre for Sustainable Healthcare, World Environment Day 2023: Solutions for Healthcare Plastic Pollution, https://sustainablehealthcare.org.uk/blog/world-environment-day-2023-healthcare-plastic-pollution, accessed 20/01/ 2024 Search PubMed.
- Grand View Research, Medical Plastic Market Size, Share & Trends Analysis Report By Product (PE, PP, PC, LCP, PPSU, PES, PEI, PMMA), By Application (Medical Device Packaging, Medical Components, Mobility Aids), by Region, and Segment Forecasts, 2024 - 2030, 2023 Search PubMed.
- J. R. Cole, W. W. Dodge, J. S. Findley, S. K. Young, B. D. Horn, K. L. Kalkwarf, M. M. Martin Jr and R. L. Winder, J Dent Educ, 2015, 79, 465–471 CrossRef PubMed.
- T. Tawichai, Electric Toothbrushes Thrive in Developed Markets, https://www.euromonitor.com/article/electric-toothbrushes-thrive-in-developed-markets, accessed 08 Jun, 2023 Search PubMed.
Footnote |
| † These authors contributed equally to the work. |
| This journal is © The Royal Society of Chemistry 2024 |