Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Maximizing polypropylene recovery from waste carpet feedstock: a solvent-driven pathway towards circular economy†
Heider
Salazar
,
Ioan-Alexandru
Baragau‡
,
Zhen
Lu
,
Luis A.
Román-Ramírez
 and
Suela
Kellici
and
Suela
Kellici
 *
*
School of Engineering, London South Bank University, 103 Borough Road, London, SE1 0AA, UK. E-mail: kellicis@lsbu.ac.uk
First published on 2nd April 2024
Abstract
Here we propose a novel approach for the efficient recovery of polypropylene from waste carpet feedstock utilising a solvent based method operating at 160 °C. The findings contribute to advancing sustainable recycling practices for waste carpet materials and offer valuable insight into the recovery of PP which can also be utilised for other complex waste streams.
Sustainability spotlightThis article presents a novel approach for recycling polypropylene (PP) specifically derived from used carpets, with the potential for adaptation to other waste streams. The research conducted in this study holds significant promise for promoting sustainability and advancing circular economy principles. The article focuses on the development of a solvent-driven process for the efficient recovery of PP from waste carpets. The use of a solvent operating at mild temperatures minimizes the risks associated with traditional solvents like toluene, while optimizing the efficiency of PP recovery. This innovative approach not only addresses environmental concerns but potentially can provide economic benefits by reducing the need for virgin PP production. One of the key highlights of this research is the achievement of a high recovery rate of recycled PP that exhibits qualities comparable to virgin polypropylene. This finding holds immense value as it not only contributes to the circular economy by diverting waste from landfills but also provides a sustainable alternative to the production of new plastic materials. |
2.24 Billion tonnes of solid waste are generated annually, of which around 20% is comprised of various forms of plastics where polypropylene (PP) is the largest contributor.1 Plastic pollution is expected to increase by 70% globally by 2050. While the importance of recycling plastic waste is widely recognized, the current recycling rates remain dismally low. Globally, only 9% of plastic production is recycled, and the figure drops to 1% for PP.2 Among the plastic waste generated, synthetic carpets made from PP fibres represent a significant contributor (ca. 5%). PP carpets offer advantages such as water and stain resistance, as well as affordability compared to traditional wool or cotton carpets.3 However, their low resilience and lower resistance to heat and friction make their lifespan of only a few years.3 Waste carpets, including the off-cuts generated during manufacturing, often end up in landfills, where they can contribute to environmental pollution or serve as a feedstock for energy generation through waste incineration.4 Unfortunately, the facilities and technologies required to effectively recycle waste carpets and reclaim high-quality PP materials are scarce. Existing recycling installations tend to rely on energy-intensive processes such as supercritical conditions or pyrolysis, with limited utilization of sustainable technologies.5 Efforts to recycle PP from waste materials have explored the use of organic solvents, including aromatic and toxic solvents (e.g., toluene and other alkylbenzenes), albeit at a laboratory scale.6–8 However, working with such solvents at temperatures sometimes closer to their boiling points presents hazards, and the selectivity of solvents towards PP remains a challenge. To address these issues, this study presents a novel approach for the recycling of PP specifically derived from waste carpets. The focus is on utilizing a proprietary protic ionic liquid (IL) (Fig. S1†) operating at a moderate temperature, minimizing the risks associated with more hazardous solvents and optimizing the efficiency of PP recovery. The investigation involves studying the dissolution kinetics of PP in the novel solvent and comparing it with existing dissolution models following a dissolution–precipitation technique9,10 (see the ESI†). The dissolution behaviour of virgin PP and PP-rich carpet waste fibres (Fig. S2†) in the ionic liquid was investigated by comparing reclaimed PP (Fig. S3†) from carpet waste with virgin PP and is illustrated in Fig. 1. In both cases, the dissolution process commenced immediately, with a gradual increase in the concentration of dissolved PP over time. After approximately 60 min, a plateau was reached, indicating the completion of the dissolution process. It was observed that the dissolved PP experienced slight changes beyond this point, resulting in a dissolution rate ranging between 60% and 90% of the experimental concentration for each specimen. Previous studies11,12 have highlighted the influence of various mechanisms on the dissolution kinetics of polymers. These results may be affected by the typical dissolution behaviour of polymers, where swelling, solvent diffusion, chain disentanglement and dissolution11,12 occur where the polymer is in contact with the solvent (Fig. 2).
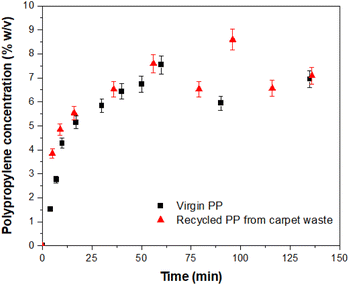 | ||
| Fig. 1 Dissolution behaviour of virgin PP and PP-rich carpet waste in IL. Error bars represent 5% of the given value. | ||
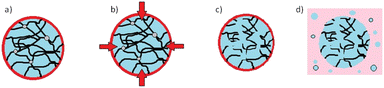 | ||
| Fig. 2 Typical dissolution mechanisms of polymers in solvents describing the different stages; (a) swelling; (b) solvent diffusion; (c) chain disentanglement; and (d) dissolution. | ||
Processes such as the adsorption of species or reactants onto the surface of sorbents can also affect the dynamics by reducing the available surface area.13,14
In the context of this study, it is expected that different physical changes around the particle and transport mechanisms involved would influence the observed behaviour. However, details on the thermodynamic or transport mechanisms of the polymer dissolution were not studied in this paper. A gravimetric protocol was followed, with a manual sampling of aliquots of PP in the solvent at 160 °C as described in the experimental section, and the fitness of such a dissolution process into some existing dissolution models was assessed.
The experimental data were initially modelled assuming pseudo first-order kinetics. The concentration (% w/v) of the recovered PP (either virgin PP or carpet waste PP) at any given time is obtained from eqn (1), for an isothermal constant volume reactor:
| CP = Cf0(1 − e−kt) | (1) |
Table 1 contains the initial concentrations for virgin PP and carpet waste PP, the values of k obtained by fitting of the experimental data to eqn (1) and corresponding coefficient of determination (R2). The R2 show that the dissolution of PP in the solvent can be modelled using the pseudo-first-order rate kinetics. The value of R2 for the carpet waste was higher than that of virgin PP. The results demonstrated that the solvent effectively dissolved PP under the experimental conditions in this study. However, with carpet waste, the solvent is in contact with PP fibres of less than 2 μm in size, compared to a particle size of ∼3 mm in the case of virgin PP. With a smaller polymer particle size, the mechanisms described in Fig. 2 occur more efficiently. Also, the experimental temperature is within the melting temperature found for the material, as detailed later in this study; hence this factor evidently contributes to such behaviour.
| Material | Concentration (% w/v) | C f0 (% w/v) | k | R 2 |
|---|---|---|---|---|
| Virgin PP | 10 | 7.173 | 0.082 | 0.884 |
| Carpet waste | 12 | 7.033 | 0.129 | 0.911 |
Moreover, we compared these results with the dissolution mechanism proposed by Korsmeyer–Peppas, representing a Fickian (or non-Fickian) diffusion modelled by eqn (2).14,16–18
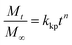 | (2) |
The experimental results for the dissolution of PP in the solvent at 160 °C were compared with the approximate solution given by the Korsmeyer–Peppas model. The R2 values calculated showed that the dissolution also fits the Korsmeyer–Peppas model (Fig. 3 and Table 2).
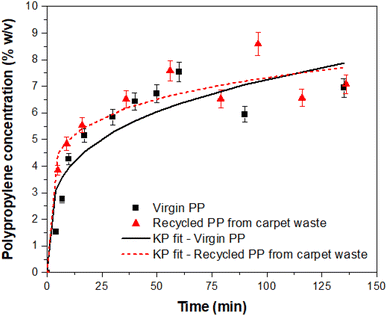 | ||
| Fig. 3 Experimental results of the dissolution of PP in the solvent and comparison with the Korsmeyer–Peppas (KP) model. | ||
| Material | k | n | R 2 |
|---|---|---|---|
| Virgin PP | 2.135 | 0.266 | 0.926 |
| Carpet waste | 3.415 | 0.166 | 0.955 |
The Korsmeyer–Peppas diffusion parameter (n) indicates if the solvent transport or diffusion is Fickian (n ≤ 0.5) or non-Fickian (n > 0.5).19,20 The values for the present study revealed that the dissolution mechanism is Fickian (n < 0.5), and the correlation coefficient R2 indicated a high consistency with the model. These results, together with those obtained with the pseudo-first-order model, suggest that the dissolution of PP in the IL follows a regular diffusion, but also, that there is a time dependency of the dissolution of PP in the solvent at 160 °C. This can be explained by the variable diameter of the PP fibres and their distinct morphology compared to pellets of virgin PP. This is consistent with the values of n; the virgin PP with a more defined spherical shape of the pellets showed a higher value of the diffusion parameter. This coefficient is also affected by the solvent thermodynamic characteristics, with the temperature of dissolution being within the melting point of PP. The higher value of R2 for the dissolution of PP from carpet waste fibres is in correspondence with the faster dissolution compared to virgin PP, as demonstrated by the models analysed in this study.
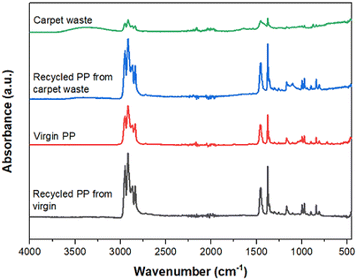
The recovered PP was compared with virgin and carpet waste using FTIR, as shown in the spectra (Fig. 4). It is evident that there are no differences in terms of characteristic bands between them, demonstrating that the chemical structure of the polymer was not affected during the recycling process. It also confirmed that the two different forms of the specimen employed for the analysis, i.e., filaments from the carpet waste and a powder from the virgin and recycled PP, respectively, have not affected the results.
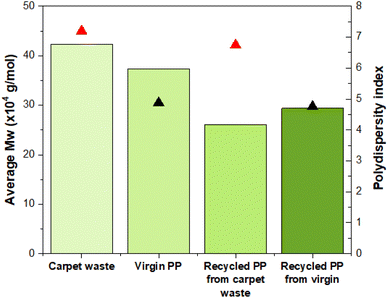
A high-temperature GPC equipped with differential refractive index (DRI), viscometry (VS) and dual angle light scatter (LS 90 + 15) detectors was used to analyse the molecular weight and molecular weight distribution of the PP from the samples. There is an evident difference between the average molecular weight (Mw) values of carpet waste and virgin PP, being larger than their corresponding recycled specimens after the dissolution in the hot solvent (Fig. 5). Interestingly the polydispersity index (PDI) did not vary considerably after the recycling process, maintaining a similar broadness in the molecular weight distribution.
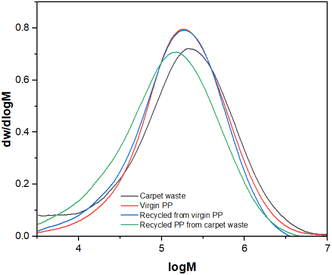
Fig. 6 displays a unimodal and broad (PDI ≥ 2.0) molecular weight distribution21 in all cases, which is consistent with the explained behaviour. No changes were observed in the virgin PP and its recycled version, and the slight displacement observed in the PP recycled from carpet waste can be attributed to the fact that the carpet waste (raw material) did not fully dissolve during the HT-GPC analysis, so the presence of insoluble materials (such as fillers or pigments), with a very high Mw or crosslinked would affect this. Despite this raw material being thermo-mechanically pre-treated (sink-float & drying), the additives used during manufacturing can still be present in the PP fibres.
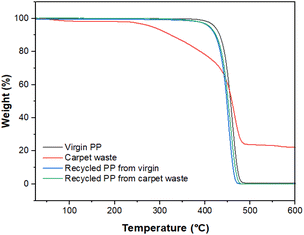
Dynamic TGA was performed using a Mettler Toledo TGA/DSC 3+, heating the sample from 25 °C to 600 °C at a heating rate of 10 °C min−1 under a nitrogen atmosphere (Fig. 7).
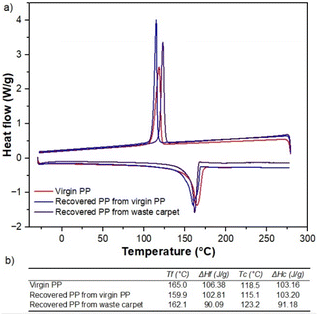
Fig. 8 shows thermograms for samples of the virgin, carpet waste and recycled PP. The sample of carpet waste underwent weight loss before reaching 100 °C, where more volatiles leave the solid matrix, and clearly showed a different behaviour than virgin and recycled PP. The presence of other polymers or additives used during carpet manufacturing and humidity can influence this observation. The weight loss of the recovered polymer was closer than that of the virgin PP (99.05%), indicating that the thermal properties of the reclaimed PP remained unaltered after the recycling process utilised in this study.
DSC tests were performed to assess the melting temperature and enthalpy of the PP specimens. The results presented in Fig. 8a and b confirmed the findings from the TGA analysis. The melting temperatures of the PP samples fall within the value reported for isotactic virgin PP, 160–165 °C (Table S1† and Fig. 8b), and the enthalpies of fusion were within the usual range values found in the literature22,23 for polypropylene (60–260 J g−1).24,25
Conclusions
A new, single-step, eco-friendly polypropylene recovery process from carpet waste using a reusable protic ionic liquid as a selective dissolution environment was reported. High yields of polypropylene with similar qualities and properties to virgin PP were achieved. The process was successfully modelled by two different approaches. The process discussed is a promising candidate as an alternative in the polymer recycling sector.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the European Union (EU) for financial support through the EU Horizon 2020 research and innovation programme under grant agreement no. 820787, project ISOPREP (Ionic Solvent-based Recycling of Polypropylene Products). The authors also gratefully acknowledge WASC (Warwick Analytical Science Centre) at the University of Warwick for their support & assistance in this work. Rotajet (U.K.) is thanked for providing the carpet fibres and Sabanci University (Turkey) for the IL.Notes and references
- Solid Waste Management, https://www.worldbank.org/en/topic/urbandevelopment/brief/solid-waste-management, accessed, Dec. 19, 2022.
- A. Alsabri, F. Tahir and S. G. Al-Ghamdi, Mater. Today: Proc., 2022, 56, 2245 CAS.
- E. K. Çeven, G. Karakan Günaydin and D. KUT, Uludağ Univ. J. Fac. Eng., 2021, 885 CrossRef.
- F. Hussin, M. K. Aroua and M. A. Kassim, Energies, 2021, 14, 24 CrossRef.
- H. Li, H. A. Aguirre-Villegas and R. D. Allen, et al , Green Chem., 2022, 24(23), 8899–9002 RSC.
- J. G. Poulakis and C. D. Papaspyrides, Resour. Conserv. Recycl., 1997, 20(1), 31–41 CrossRef.
- T. W. Walker, N. Frelka and Z. Shen, et al , Sci. Adv., 2020, 6(47), 1 Search PubMed.
- A. Hadi, Energy, Educ., Sci. Technol., 2013, 30, 989 CAS.
- J. Yu, A. del C. Munguía-López, V. S. Cecon, K. L. Sánchez-Rivera, K. Nelson, J. Wu, S. Kolapkar, V. M. Zavala, G. W. Curtzwiler, K. L. Vorst, E. Bar-Ziv and G. W. Huber, Green Chem., 2023, 25, 4723–4734 RSC.
- J. Cavalcante, R. Hardian and G. Szekely, Sustain. Mater. Technol., 2022, 32, e00448 CAS.
- B. A. Miller-Chou and J. L. Koenig, Prog. Polym. Sci., 2003, 28(8), 1223–1270 CrossRef CAS.
- M. Mikula, M. Čeppan, J. Blecha and L. Lapčík, et al , Polym. Test., 1988, 8(5), 339–351 CrossRef CAS.
- M. S. P. Deang, R. I. C. Alindayu and K. V. H. Escasa, et al , Key Eng. Mater., 2020, 841, 59–63 Search PubMed.
- T. P. Kravtchenko, J. Renoir, A. Parker and G. Brigand, Food Hydrocoll., 1999, 13(3), 219–225 CrossRef CAS.
- A. D
![[a with combining cedilla]](https://www.rsc.org/images/entities/char_0061_0327.gif) browski, Adv. Colloid Interface Sci., 2001, 93(1–3), 135–224 CrossRef PubMed.
browski, Adv. Colloid Interface Sci., 2001, 93(1–3), 135–224 CrossRef PubMed. - S. Yadav, A. Asthana and A. K. Singh, et al. , J. Hazard. Mater., 2021, 409, 124840 CrossRef CAS PubMed.
- O. Levenspiel, Chemical Reaction Engineering. John Wiley and Sons, 1999 Search PubMed.
- N. A. Peppas, J. C. Wu and D. Ernst, Macromolecules, 1994, 27(20), 5626–5638 CrossRef CAS.
- L. Ahmed, R. Atif, T. S. Eldeen and I. Yahya, et al , Int. J. Res. Appl. Sci. Eng. Technol., 2019, 8(5), 52–56 Search PubMed.
- A. Celebioglu and T. Uyar, Food Funct., 2020, 11(9), 7626–7637 RSC.
- E. S. Bacaita, B. C. Ciobanu and M. Popa, et al , Phys. Chem. Chem. Phys., 2014, 16(47), 25896–25905 RSC.
- G. Rehage, O. Ernst and J. Fuhrmann, Discuss. Faraday Soc., 1970, 49, 208 RSC.
- C. Auch, M. Harms and K. Mäder, Int. J. Pharm., 2019, 556(2018), 372–382 CrossRef CAS PubMed.
- F. J. Lanyi, N. Wenzke and J. Kaschta, et al , Adv. Eng. Mater., 2020, 22(9), 1900796 CrossRef CAS.
- J. A. Currie, E. M. Petruska and R. W. Tung, Anal. Calorim., 1974, 569–577 CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental methods and characterisation details. See DOI: https://doi.org/10.1039/d3su00270e |
| ‡ National Institute of Materials Physics, 405A Atomistilor Str., Magurele, Ilfov, 077125, Romania. |
| This journal is © The Royal Society of Chemistry 2024 |