Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Extraction of cellulose from restaurant food waste†
Matthew T.
Garnett
 ,
Harrish Kumar
Senthil Kumar
,
Bryan S.
Beckingham
and
Symone L. M.
Alexander
,
Harrish Kumar
Senthil Kumar
,
Bryan S.
Beckingham
and
Symone L. M.
Alexander
 *
*
Department of Chemical Engineering, Auburn University, 212 Ross Hall, Auburn, AL 36849, USA. E-mail: sla0044@auburn.edu
First published on 25th November 2023
Abstract
Dietary fiber provides organisms with key nutrients and allows for transport of small molecules and metabolic products. Due to being biocompatible, sustainable, and positively influencing microbial communities, dietary fiber is utilized in the design of many materials in applications such as biomedical or agricultural. In this work, the feasibility of using randomly collected, mixed food waste from a local restaurant as a feedstock for extracting native cellulose is explored. The extraction procedure adapts previously utilized acid/base extraction procedures for the extraction of cellulose from single source fruit and vegetables and is tailored in both sequencing and concentration to account for the complexity of the feedstock. Despite being collected at random over a period of a year, extraction of cellulose from restaurant waste led to products with reproducible yield and chemical properties. FTIR spectroscopy and XRD revealed that the extracted cellulose has a chemical structure similar to commercially available cellulose products, but that the extracted cellulose was less crystalline, due to the presence of lower molecular weight species. Thermal analysis confirmed that the extracted cellulose contained lower molecular weight species and residual lignin, indicating a trade-off between yield and purity when using a complex feedstock such as mixed food waste in current extraction methodologies. Besides obtaining cellulose, other biopolymers, specifically pectin, hemicellulose, and lignin, can be recovered as viable products. This research demonstrates the feasibility of diverting real-world food waste streams from local restaurants to provide a sustainable, environmentally friendly feedstock for the extraction of biopolymers and to decrease the production of greenhouse gases in landfills.
Sustainability spotlightIn recent years, there has been a shift to utilize biopolymers, such as cellulose, in the production of many different materials. These biopolymers are in abundance and found naturally in plants. The goal of our work is to design a sustainable, environmentally friendly platform to extract biopolymers from real-world, mixed food waste that can be utilized in many different applications. Using restaurant food waste as a feedstock to our process decreases waste being placed into landfills and ultimately helps drive down the emission of greenhouse gases. Our work emphasizes the importance of the following sustainability goals of the UN: climate action, industry, innovation, and infrastructure. |
Introduction
Every year, one-third of all the food produced worldwide is discarded as waste. If the food waste is taken to a landfill, it can take between a few weeks to several years for full decomposition, leading to long-term production of harmful greenhouse gases like methane.1,2 Approximately 72.5 billion kilograms is generated in the United States alone, with 9.9 to 15 billion kilograms being generated from restaurants.3,4 Therefore, significant efforts have been made over the past few decades to find alternative ways to utilize food waste. Specifically, 45% of food waste is made up of fruits and vegetables, which can be turned into viable feedstocks for useful biopolymers.5,6Fruits and vegetables are composed of dietary fiber and natural polymers such as cellulose, lignin, pectin, and hemicellulose.7 Each of these biopolymers play an important role in the growth of plants. Cellulose is the most abundant polymer in the world; it gives structural stability to the cell wall and is non-digestible by humans due to the absence of cellulase enzymes that can degrade the material.8–10 Lignin, like cellulose, helps enhance the cell wall's rigid structure, and allows for transport of key minerals into the plant via diffusion.11 Another biopolymer, pectin, supports cell attachment in the cell wall, facilitates plant growth and development, and helps define the porosity of the cell wall due to its crosslinking behavior.12,13 Additionally, hemicellulose is important in structural support of plants, plant growth, and cell expansion.14,15 The favorable characteristics of fibrous biopolymers make them valuable feedstocks to produce biodegradable and biocompatible biomaterials if they can be extracted from food waste.16
The extraction of cellulose has been the major focus of efforts to turn plant and food waste into viable feedstocks. On a large scale, cellulose is primarily extracted from wood and cotton.17 Typically, cellulose is extracted from wood chips using the Kraft pulping process, which isolates cellulose from lignin and hemicellulose using sodium hydroxide and sodium sulfide.18–20 Another common way to extract cellulose from wood chips is the sulfite pulping process, which uses sulfurous acid and bisulfite ions to help rid the system of lignin.21 Both of these processes produce a wood pulp that is mainly composed of cellulose fibers. The Kraft pulping process produces higher strength pulp from a variety of wood sources compared to the sulfite process, but the sulfite process produces a brighter pulp with bleaching.22 These industrial scale processes also have the potential to extract cellulose from food waste, which is a more sustainable and environmentally friendly feedstock than wood or cotton.6
With the increasing need to make fruit and vegetable food waste valuable and viable, other extraction processes have been developed to extract cellulose from the discarded peel or pulp of produce. For example, single source feedstocks such as apples, kale, carrots, tomatoes, and cucumbers have been used to extract cellulose via a series of sequential heated washing steps with water, acid, base, and a bleaching agent to obtain pure cellulose.6,23 Water soluble compounds are removed during the hot water wash, pectin is removed during the acid treatment, hemicellulose is removed during the basic step, and lignin is removed during the bleaching step.6,24 Using different singular source feedstocks of fruits or vegetables yields different amounts of cellulose during the extraction. For example, cucumbers yielded a higher cellulose content at 16.13 g per 100 g of dry pomace compared to tomatoes at 8.60 g per 100 g of dry pomace.6 However, utilizing single source feedstocks (i.e., from only apples or only carrots) does not adequately represent the composition of the waste streams from local restaurants and homes. Therefore, there is a critical need to investigate extraction of cellulose and other valuable biopolymers from uncontrolled mixed, waste streams.
In this work, we investigate the feasibility of using existing processes for cellulose extraction to obtain cellulose from mixed food waste streams. We collected mixed fruit and vegetable waste at random from a local juice bar to provide a more realistic approach to repurposing food waste that is being directed to landfills. We altered the extraction techniques to reproducibly obtain cellulose from the acid/base extraction procedure, with the potential to also obtain valuable biopolymers such as pectin, hemicellulose, and lignin for the production of biomaterials. Finally, we compare the extracted cellulose to commercially available fibrous and microcrystalline cellulose from cotton linter pulp and discuss future outlooks for using real world waste streams to recover valuable biopolymers and lower the impact of food waste on the environment.
Materials and methods
Materials
An assortment of fruit and vegetable waste was obtained from I Love Juice Bar® located in Auburn, Alabama. Hydrochloric acid (ACS reagent, 37%) and sodium hydroxide (ACS reagent, >97%) pellets were purchased from Millipore Sigma. Hydrogen peroxide (J.T. Baker, ACS Reagent 30%) was purchased from VWR. 1 M cupriethylediamine solution was purchased from Sycamore Life Sciences.Collection and processing of food waste from the I Love Juice Bar®
Over the period of a year, our lab collected approximately 22.7 kg of food waste from I Love Juice Bar® to utilize as a feedstock to the acid/base extraction procedure. ESI Table S1† lists all fresh fruits and vegetables served on their menu. Our group contacted the restaurant to request waste at random, to simulate randomly generated waste feedstocks with variable composition. The waste consisted of rinds, pomace, peels, skin, and occasionally a whole fruit or vegetable. The mixed food waste was brought back to the lab and processed as depicted in ESI Fig. S1.† Any barcode/product identification stickers were removed from the peels of the waste prior to dehydrating. Then, the assorted food waste was placed in a Presto Dehydro Food Dehydrator for approximately fourteen hours. The dried food waste was then placed in a Goldenwall Grinder at 28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for approximately four minutes to allow the food waste to pulverize into a powder. The generated powder was recovered from the grinder, placed in a bottle, and stored in the chemical storage cabinet at room temperature.
000 rpm for approximately four minutes to allow the food waste to pulverize into a powder. The generated powder was recovered from the grinder, placed in a bottle, and stored in the chemical storage cabinet at room temperature.
Extraction of cellulose from food waste powder
Approximately 300 g of food waste was boiled in 3 L of boiling water for 20 minutes. At the conclusion of the time interval, the solution is filtered using a grade 100 cheese cloth bag to remove water soluble compounds. Immediately, the filtered solids are deposited in another 3 L of boiling water, and the boiling water step is repeated once. Then, the filtered solids were placed in a solution of 1 M HCl at 85 °C for 30 minutes under constant stirring at 250 rpm using an IKA RW 14 Overhead Stirrer before being filtered using grade 100 cheese cloth. This process is repeated once more with the molarity of the HCl solution reduced to 0.5 M. Next, the filtered solids are placed in a solution of 1 M NaOH at 85 °C for 30 minutes under constant stirring at 250 rpm before being filtered using grade 100 cheese cloth. Then, the filtered solids were washed with deionized water until the solids had a neutral pH to prevent an accelerating oxidation reaction caused by the addition of hydrogen peroxide.24 Finally, the neutralized filtered solids are added to 3% hydrogen peroxide solution under constant stirring at 250 rpm and a temperature of 70 °C for 1 hour; after the 1 hour, the residual solution is filtered. The bleaching step is repeated once more, and then the filtered solids are freeze-dried using a LabConco FreeZone Freeze-Dryer.Fourier transform infrared (FTIR) spectroscopy
FTIR spectroscopy was used to evaluate the overall purity of the sample using a Thermo Scientific Nicolet 6700 Spectrometer. The extracted cellulose sample was compared to the stock native cellulose (medium, cotton linter) and microcrystalline cellulose samples purchased from Sigma Aldrich. Both samples were prepared as powder and were visualized over the range 4000–350 cm−1.Determination of molecular weight and degree of polymerization
The established TAPPI T230-99 protocol at 25 °C with Ubbelohde viscometer was used to determine molecular weight and the degree of polymerization of the cellulose solution.25–27 A 0.5 M cupriethylenediamine solution with 0.250 g of cellulose was prepared and was allowed to stir at 25 °C for 10 minutes prior to the viscosity measurement. This step ensures that the cellulose has fully dissolved in the 0.5 M cupriethylenediamine (CED) solution. Once the cellulose had fully dissolved in solution, 10 mL was placed in the Ubbelohde viscometer and the time for the solution to move from one designated point to the other was recorded. The viscosity can be determined from the time using eqn (1)25,28| ηsolution = cρt | (1) |
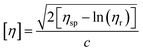 | (2) |
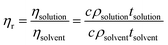 | (3) |
| ηsp = ηr − 1 | (4) |
| [η] = 3.85 × 10−2 (MW)0.76 | (5) |
| [η] = 1.75 (DP)0.69 | (6) |
Scanning electron microscopy (SEM)
The macrostructure of the extracted cellulose was examined using a ThermoFisher Scientific Phenom ProX Desktop Scanning Electron Microscope at 430X and 5700X with a voltage of 5 kV. Prior to imaging, the sample was sputter coated with a gold layer using a Q150T ES Plus Electron Microscopy Sciences Sputter Coater.X-ray diffraction (XRD)
X-ray diffraction (XRD) was performed on the control fibrous and microcrystalline cellulose and extracted cellulose using a Proto manufacturing AXRD powder diffraction system with Cu Kα radiation (λ = 1.5418 Å). Samples were scanned at a rate of 2.4° min−1 (Δ2θ = 0.0139°, dwell time = 5 s) from 15° to 50° (2θ) at 30 mA and 40 kV. The data obtained from the AXRD was processed using OriginPro 2022 (OriginLab). The crystallinity index (CrI) of the samples analyzed was determined using Segal's method as shown in eqn (7)30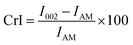 | (7) |
The mean size of the ordered (crystalline) (τ) domain was also determined using Scherrer's equation (eqn (8))31
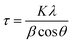 | (8) |
Differential Scanning Calorimetry (DSC)
The glass transition temperature was examined using a TA Instruments Q20 Differential Scanning Calorimeter over the range of 20–180 °C using a ramp of 5 °C min−1 in an inert, nitrogen atmosphere. The samples were prepared by weighing out 5–10 mg of sample and placed in an aluminium sample pan.Thermogravimetric analysis (TGA)
The degradation of the extracted cellulose sample was examined over a temperature range of 30–600 °C using a TA Instruments Q500 Thermogravimetric Analyzer at a heating ramp of 10 °C min−1 from 30–600 °C using an inert, nitrogen atmosphere. The samples were prepared by weighing out 5–10 mg of sample and placed in a platinum sample pan.Results and discussion
The feedstock for the extraction experiment was 300 grams of a variety of different fruits and vegetable pulp, skin, and rinds from I Love Juice Bar® (see ESI Table S1†). I Love Juice Bar®, located in Auburn, AL, is a franchised restaurant (1 of 24 located across the United States) that serves fresh and homemade smoothies, juices, bowls, energy shots, and soups. Over the period of a year, our lab collected approximately 22.7 kg of food waste from I Love Juice Bar®, to utilize as a feedstock to our acid/base extraction procedure. Our group would contact the restaurant to request waste at random to simulate randomly generated waste feedstocks with variable composition. The restaurant employees placed the waste discarded from preparation of fruit and vegetable-based menu items in a large trash bag that was separate from any other waste (i.e., customer trash, grain, or meat waste). All of the fruits and vegetables in the waste were raw and were the product of juicing, chopping, peeling, etc. At the end of the business day, our group retrieved the garbage bag of waste and placed it in cold storage to prevent the fruits and vegetables from decaying prior to use. From the fruits and vegetable listed in Table S1,† the waste consisted of rinds, pomace, peels, skin, and occasionally a whole fruit or vegetable. Every batch of waste collected contained different compositions of fruits and vegetables, which simulated real-world waste streams. As intended, we do not know the exact composition of the waste as it depended on what customers ordered that day and the rotating menu of the restaurant.To make a homogeneous waste stream, the waste was dried and powderized. Initially, we followed the common extraction procedure which uses a sequence of boiling in hot water, an acid wash, a base wash, and a bleaching step.6 Specifically, the extraction procedure had the following parameters: 10 minutes boiling water step, 1 M HCl step for 1 hour, 1 M NaOH step for 1 hour, and a 2% sodium hypochlorite (NaClO) bleaching step for 30 minutes. However, this procedure was designed for a single food source and was ineffective in extracting cellulose from the variety of food waste obtained from I Love Juice Bar®. To develop an extraction procedure for cellulose from a mixed food waste stream, process variables such as sequence, concentration, and bleaching agent were varied. After each variation, the extracted cellulose was compared to commercially available cotton linter pulp using Fourier-transform Infrared Spectroscopy (FTIR) spectroscopy. FTIR spectroscopy allows for specific molecules to be identified via functional groups that are determined through the spectra peaks corresponding to the molecular stretching and bending vibrational bands and their intensities.
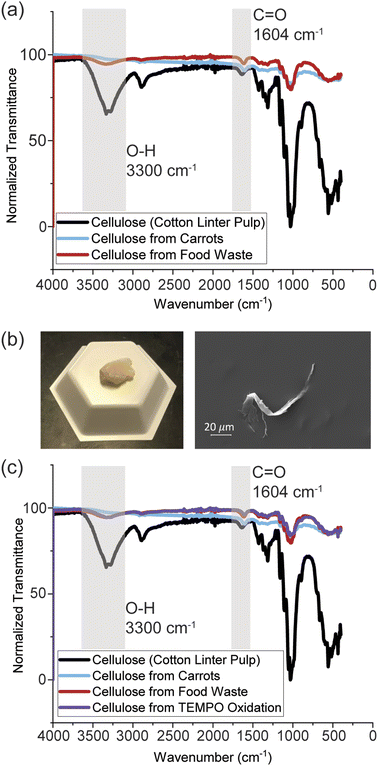
Fig. 1a displays the FTIR spectra for the extracted cellulose from mixed food waste compared to the commercial cellulose from cotton linter pulp and cellulose from a single source (carrots). Two major bands of interest occur in the identification of cellulose around 3300 and 1604 cm−1 for both samples, which represent the stretching of –OH and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, respectively.32,33 Also present is the distinct cellulose absorption region between 1250 and 950 cm−1.34 As shown in Fig. 1a, there is a decrease in transmittance of the hydroxyl group at 3300 cm−1 for the extracted cellulose from mixed food waste and carrots compared to the commercial cellulose. As reported in previous literature, cellulose fibers degrade via oxidation when NaClO is used at concentrations of 2% and temperatures of 70 °C or higher.35,36 Therefore, TEMPO oxidation was performed on the commercial cellulose (Fig. 1c) to determine whether the extracted cellulose was oxidizing due to the bleaching step. The intensity of the hydroxyl and carbonyl groups for the commercial cellulose that underwent TEMPO oxidation match the extracted cellulose, indicating that the extracted cellulose is being oxidized during bleaching step. Additionally, as shown in Fig. 1b, the sample was yellow compared to the bleached white color expected from cellulose products. The yellow coloring present in the sample is due the residual lignin not being fully extracted during the bleaching step and related to oxidization.37 These results indicate that bleaching with NaClO is not an effective bleaching agent for extracting cellulose from mixed food waste due to failure to remove lignin without oxidizing the desired cellulose. Additionally, NaClO is toxic to the environment and counteracts the purpose of a sustainable way to extract cellulose from food waste.
O, respectively.32,33 Also present is the distinct cellulose absorption region between 1250 and 950 cm−1.34 As shown in Fig. 1a, there is a decrease in transmittance of the hydroxyl group at 3300 cm−1 for the extracted cellulose from mixed food waste and carrots compared to the commercial cellulose. As reported in previous literature, cellulose fibers degrade via oxidation when NaClO is used at concentrations of 2% and temperatures of 70 °C or higher.35,36 Therefore, TEMPO oxidation was performed on the commercial cellulose (Fig. 1c) to determine whether the extracted cellulose was oxidizing due to the bleaching step. The intensity of the hydroxyl and carbonyl groups for the commercial cellulose that underwent TEMPO oxidation match the extracted cellulose, indicating that the extracted cellulose is being oxidized during bleaching step. Additionally, as shown in Fig. 1b, the sample was yellow compared to the bleached white color expected from cellulose products. The yellow coloring present in the sample is due the residual lignin not being fully extracted during the bleaching step and related to oxidization.37 These results indicate that bleaching with NaClO is not an effective bleaching agent for extracting cellulose from mixed food waste due to failure to remove lignin without oxidizing the desired cellulose. Additionally, NaClO is toxic to the environment and counteracts the purpose of a sustainable way to extract cellulose from food waste.
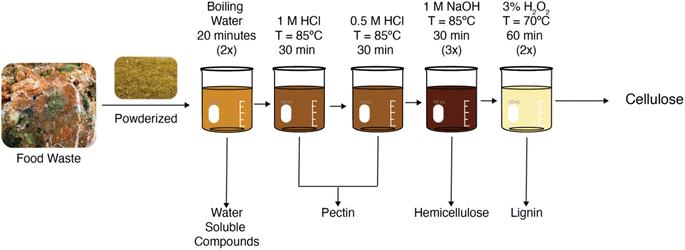
To prevent yellowing from the carbonyl groups and decrease the environmental toxicity of the extraction process, the procedure was modified by changing the bleaching agent and bleaching time and altering the sequence and time of the boiling water, acid, and base steps. The updated extraction process is depicted in Fig. 2.
In the modified procedure, the powderized food waste underwent two repetitions of hot water boiling and stirring for 20 minutes. This maximized the removal of water-soluble compounds in the first step compared to prior procedures. Next, the pulp underwent two sequential acid washing steps at 85 °C. In the first acid step, the pulp was heated and stirred in 1 M HCl for 30 minutes followed by vacuum filtration. The pulp then underwent another acid washing step in 0.5 M HCl for 30 minutes. Replacing the acid solution halfway through the procedure enabled fresh HCl to interact with the food waste, allowed for higher removal of pectin from the sample, and facilitated filtration. Next, the pulp underwent three repetitions of heating and stirring in 1 M NaOH steps for thirty minutes. This modification to the previous procedure allowed the remaining pulp to interact with NaOH longer and allowed for fresh 1 M NaOH to be introduced every thirty minutes to remove as much hemicellulose as possible.
The most significant change to the procedure was the bleaching step. As mentioned previously, the NaClO solution oxidized the cellulose and did not remove enough lignin to prevent yellowing. Also, NaClO is more hazardous to the environment compared to other bleaching agents. For these reasons, the bleaching agent was changed to hydrogen peroxide and the bleaching time was increased. Specifically, the pulp underwent two repetitions of bleaching with 3% hydrogen peroxide for an hour. Hence, the pulp spent an hour and a half longer in the bleaching step and the solution was changed at the halfway point. This allowed for higher lignin removal and was indicated by a colorless appearance of the final cellulose product. With this procedure, the final product was determined to be approximately 12.59 ± 2.79 g on average, and the cellulose yield was 4.19 ± 0.93%, calculated using the eqn (9) (ESI Table S2†). This data is based on seven extraction runs with seven random batches of mixed food waste collected over the period of a year from the I Love Juice Bar®.
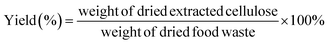 | (9) |
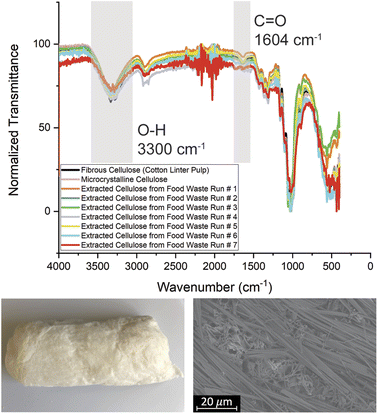
In previous literature, the cellulose yield ranged from 8–16%.6 However, these experiments controlled the fruit and vegetable feedstock such that the feedstocks were known to have higher initial cellulosic content, resulting in higher cellulose yields. In our experiment, a randomized, mixed food waste stream was utilized that more accurately reflects the randomized food waste received from a restaurant. After determining the yield, the extracted cellulose from the new procedure was analyzed using FTIR spectroscopy and compared to cotton linter pulp from Sigma Aldrich (Fig. 3).
The extracted cellulose and commercial cellulose have similar bands and intensities in the characteristic hydroxyl (3300 cm−1) and carbonyl (1604 cm−1) regions, indicating that the chemical structure of the extracted cellulose is comparable to native cellulose from cotton linter pulp. The transmittance of the cellulose samples varied only slightly between extractions that occurred throughout the year from randomly collected batches of food waste, which can be attributed to the differing levels of water content in the samples that may have absorbed water after drying.38 To confirm the cause of the slight variations, FTIR spectroscopy was performed on both a freeze-dried cellulose sample and wet cellulose sample that was air-dried (ESI Fig. S2†). The main difference between the two samples was the stretching vibrations at 3300 cm−1 due to the presence of –OH groups from water molecules.
The molecular weight (MW) and degree of polymerization (DP) were calculated after determining the intrinsic viscosity for both an extracted and a fibrous control cellulose sample using the data collected from the Ubbelohde viscometer (ESI Table S3†). The extracted cellulose's MW and DP were determined to be 1323 Da and 11, respectively, while the control cellulose's MW and DP were determined to be 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 422 Da and 105, respectively. Based on these results, there is an order of magnitude difference between both the MW and DP for the extracted and control cellulose samples. The lower molecular weight of the extracted cellulose is expected due to the source materials – fruits and vegetables (extracted) vs. cotton (control) – and due to differences in processing conditions.6 The extracted cellulose sample underwent processing conditions such as pulping, chopping, blending, pulverizing, boiling, and chemical treatment, all of which can influence the final MW and DP.
422 Da and 105, respectively. Based on these results, there is an order of magnitude difference between both the MW and DP for the extracted and control cellulose samples. The lower molecular weight of the extracted cellulose is expected due to the source materials – fruits and vegetables (extracted) vs. cotton (control) – and due to differences in processing conditions.6 The extracted cellulose sample underwent processing conditions such as pulping, chopping, blending, pulverizing, boiling, and chemical treatment, all of which can influence the final MW and DP.
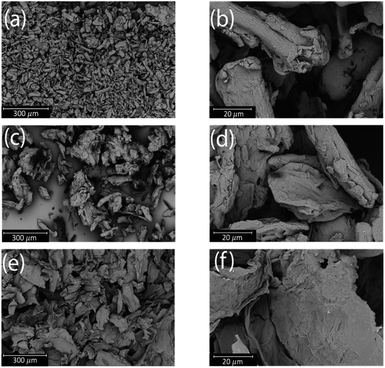
The morphology of the extracted cellulose was investigated using SEM to determine if the extracted cellulose was cellulose nanofibers, cellulose nanocrystals, or microcrystalline cellulose (Fig. 4). Cellulose nanocrystals (CNC) have a crystalline structure that is often rod or whisker shaped with good mechanical properties. However, retrieval of CNC requires substantial chemical treatment to obtain high purity.39,40 Cellulose nanofibers (CNF) consist of fibers with a higher surface area, increased strength, and higher elastic modulus without the need of extensive chemical pretreatment.39 Microcrystalline cellulose (MCC) is often found as microfibril bundles with a crystalline structure.40 Both CNC and MCC have crystalline structures, but they vary in the acid treatment used to obtain the cellulose. CNC usually is produced with sulfuric acid (H2SO4), while MCC is produced with hydrochloric acid (HCl).41 As shown in Fig. 4, the microstructure of the extracted cellulose is similar to both cotton linter pulp (fibrous) and microcrystalline cellulose. Thus, additional methods such as XRD were needed to characterize the structure of the extracted cellulose.
To evaluate the solid-state structure with respect to the relative proportion of crystalline and amorphous regions, XRD was performed on the three cellulose samples: cotton linter pulp (fibrous) control, microcrystalline cellulose, and the cellulose extracted from mixed food waste (Fig. 5). From these spectra, the crystallinity index was determined by eqn (7). The crystallinity index of the extracted cellulose samples compared to the control cellulose is based on the relative intensity of the crystalline and amorphous diffraction. A peak was present at 22.5 (2θ) for all samples which corresponds to the presence of type I cellulose. The absence of the doublet peak at 22.5 (2θ) suggests absence of type II cellulose. These 002 plane diffraction peaks were compared with the intensity the amorphous diffraction at 18 (2θ). Both the microcrystalline and fibrous cellulose control samples show a crystallinity index of 61%, whereas the extracted cellulose shows a much lower crystallinity index of 16% (ESI Table S4†). Eqn (8) (Scherrer equation) was used to determine the mean size of the ordered crystalline domain where distinct differences were observed. The microcrystalline control's mean size of ordered domain was 45.0 Å, fibrous control had 75.2 Å, and the extracted cellulose had a mean size of 64.4 Å. To further explore the difference in crystallinity for the extracted cellulose compared to both MCC and the fibrous control, thermal analysis was performed.
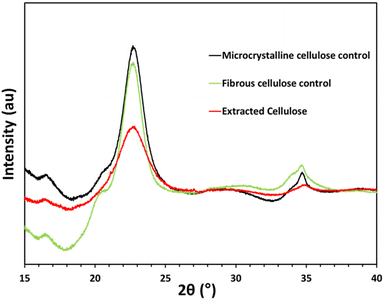 | ||
| Fig. 5 XRD pattern of (black) microcrystalline cellulose control, (red) extracted cellulose and (green) fibrous cellulose control. XRD patterns have been offset for comparison. | ||
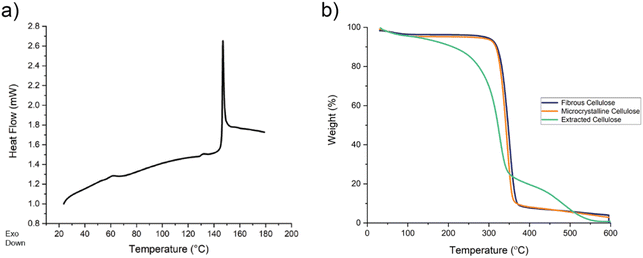
Differential scanning calorimetry (DSC) was performed on the extracted cellulose to determine the glass transition temperature (Tg). As shown in Fig. 6a, two glass transition temperatures are observed around 58.5 °C and 131 °C. According to the literature, microcrystalline cellulose has three glass transition temperatures: 132.5 °C, 159 °C, and 184 °C.42 Hemicellulose has a glass transition temperature at 40 °C, while lignin can display glass transition temperatures between 50–100 °C.43 Therefore, the sample is not pure cellulose and has fractions of other components such as lignin that could be reducing the crystallinity of the extracted cellulose by disrupting the crystal packing structure of the cellulose molecules.
Thermogravimetric analysis (TGA) was performed on the microcrystalline and fibrous controls and on the extracted cellulose product to further investigate the thermal properties of the extracted cellulose (Fig. 6b). Extracted cellulose shows a 6.21% weight loss between 30 °C and 150 °C, while the controls show little to no weight loss. The weight loss during this initial phase indicates that the extracted cellulose likely has light volatile components that are not present in the controls.44 At 320 °C, rapid decomposition of extracted cellulose is observed, indicated by a 70.4% weight loss, while the controls show rapid decomposition at 340 °C. Additionally, extracted cellulose shows an additional step decrease in weight loss at 350 °C, indicating residual lignin may be present in the sample.45 Therefore, TGA indicates that the decreased crystallinity of the extracted cellulose observed in XRD could be due to lower molecular weight species that disrupt the packing structure of cellulose leading to more amorphous regions. To obtain cellulose products with crystallinities similar to commercially available MCC and fibrous cotton linter pulp, the extraction procedure could be adjusted to reduce residual low molecular weight species. However, there is a balance between achieving a given crystallinity and product yield with the current extraction process.
The current methodology and results indicate that a higher yield of cellulose can be achieved for a single source feedstock (between 8.60–16.13%) compared to a mixed source feedstock that ranged in yield between 2.85–5.32% using an acid/base extraction process.6 Much of the cellulose loss can be attributed to the numerous exchanges and filtration steps required for this extraction methodology. It also is important to note that in previous extraction procedures fresh, singular food sources that had high cellulosic content were targeted, which does not adequately represent a waste feedstock. Therefore, our procedure more adequately represents this waste stream for real world application. When the previously developed single source feedstock acid/base extraction procedure was performed on a mixed source feedstock, the desired cellulose product was oxidized due to the bleaching agent and process temperature. When we modified the sequence, concentration, temperature, and bleaching agent of the extraction procedure, the chemical structure of the cellulose from mixed food waste via FTIR matched commercial products as well as the cellulose extracted from a single source feedstock.32,33 Changing the bleaching agent from NaClO to 3% hydrogen peroxide prevented the oxidation of cellulose, but also served as a more environmentally friendly alternative, and the lower temperature of operation provides energy and cost savings. Additionally, the acid and base waste streams effectively neutralize each other and result in salt water that can be poured down the drain. The extracted cellulose from a single source displayed a crystallinity index value between 48.97–68.73% like commercially available alternatives.6 However, the extracted cellulose from a mixed source feedstock has a lower crystallinity index value of 16%, which can be attributed to the presence of lower molecular weight species. Additionally, the extracted cellulose has not undergone any additional processing techniques to enhance crystallinity, so it is likely the crystallinity can be improved.46,47 Due to the complexity of a mixed waste feedstock, use of the acid/base extraction procedure is a balance between yield and purity and may not be the best approach for a mixed waste stream. However, further modifications can be made to the acid/base extraction procedure to help increase both the crystallinity and the yield. Nonetheless, this procedure is the first of its kind utilizing real-world, mixed food waste with acid/base extraction, and is a promising pathway to obtain cellulose and other value-added biopolymers for commercial use. Future work will explore emerging extraction techniques such as supermass colloiders, which use mechanical separation techniques, reduce the need for concentrated chemicals, and facilitate extraction from complex feedstocks without sacrificing yield and molecular weight to obtain purity – creating a more energy efficient process.48,49
Conclusions
In this work, we explored the feasibility of using randomly collected, mixed food waste from a local restaurant as a feedstock for extracting native cellulose. The complexity of the mixed food waste feedstock required significant alteration to the extraction protocol used for single, fresh food feedstocks and required a change in the bleaching agent to remove lignin and prevent oxidation. Upon optimization of the extraction process, cellulose with a chemical structure similar to that of commercially available cellulose products was obtained. However, the extracted cellulose had lower molecular weight and crystallinity than the commercially available cellulose, likely due to the source of cellulose (fruits and vegetables vs. cotton), the processing method, and due to the presence of lower molecular weight species as evidenced by TGA and DSC.The consistency and reproducibility of the extracted cellulose from mixed food waste that was obtained at random from a local restaurant is promising for diverting waste from landfills. Mixed food waste streams provide a more realistic approach to repurposing food waste and, though cellulose was the focus of this work, recovery of the waste filtrate streams throughout the process enables collection of additional valuable biopolymers, such as pectin, hemicellulose, and lignin. Recovery of these biopolymers and improvement of quality of the extracted cellulose from the waste filtrate streams will be the focus of future work.
Author contributions
Conceptualization, S. L. M. A.; methodology, M. T. G. and S. L. M. A.; software, M. T. G.; validation, M. T. G., H. K. S. K., B. S. B, and S. L. M. A.; formal analysis, M. T. G. and H. K. S. K.; investigation, M. T. G., H. K. S. K., and S. L. M. A.; writing – original draft, M. T. G.; writing – review and editing, M. T. G., H. K. S. K., B. S. B, and S. L. M. A.; supervision, S. L. M. A.; funding acquisition, S. L. M. A.Conflicts of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.Acknowledgements
M. T. G. and S. L. M. A. would like to acknowledge Samuel Ginn College of Engineering and the Department of Chemical Engineering at Auburn University for funding and support of this project. We would like to thank the former I Love Juice Bar® in Auburn, Alabama for supplying mixed food waste for this project. We would like to thank the Beckingham Polymer Research Group for allowing us to use their DSC and Pravin Parasakthi Aravindhan for his assistance with the DSC. This work was supported by the facilities in the Auburn University Center for Polymers and Advanced Composites (CPAC). We would like to thank Dr Ramsis Farag and Tripp Hinkle for their assistance with FTIR spectroscopy, TGA, and using the Ubbelohde viscometer.References
- I. Priyambada and I. Wardana, Sustinere: Journal of Environment and Sustainability, 2018, 2(3), 156–167 Search PubMed.
- S. To, C. Coughenour and J. Pharr, Int. J. Environ. Res. Public Health, 2019, 16, 10 Search PubMed.
- R. Nicastro and P. Carillo, Int. J. Environ. Res. Public Health, 2021, 13, 10 Search PubMed.
- D. Gunders and J. Bloom, Wasted: How America Is Losing Up To 40 Percent Of Its Food From Farm To Fork To Landfill, Natural Resources Defense Council, 2017, vol. 18, pp. 1–58 Search PubMed.
- K. Roka, Encyclopaedia of the UN Sustainable Development Goals, 2020, pp. 216–227 Search PubMed.
- M. Szymanska-Chargot, M. Chylinska, K. Gdula, A. Koziol and A. Zdunek, Polymers, 2017, 9, 10 CrossRef PubMed.
- G. Dhillon, S. Kaur and S. Brar, Renew. Sust. Energ. Rev., 2013, 27, 789–805 CrossRef.
- J. McNamara, J. Morgan and J. Zimmer, Annu. Rev. Biochem., 2015, 84, 895–921 CrossRef CAS PubMed.
- T. Li, C. Chen, A. Brozena, J. Zhu, L. Xu, C. Driemeier, O. Rojas, A. Isogai, L. Wågberg and L. Hu, Nature, 2021, 590(7844), 47–56 CrossRef CAS PubMed.
- J. Ganster and H. Fink, Bio-Based Plastics: Materials and Applications, 2014, pp. 35–62 Search PubMed.
- J. Cummings, Gut, 1984, 25(25), 805–810 CrossRef CAS PubMed.
- Q. Liu, L. Luo and L. Zheng, Int. J. Mol. Sci., 2018, 19, 2 Search PubMed.
- C. Xiao and C. Anderson, Front. Plant Sci., 2013, 4(3), 1–7 Search PubMed.
- T. Vanitha and M. Khan, Role of Pectin in Food Processing and Food Packaging, Pectins - Extraction, Purification, Characterization and Applications, IntechOpen, 2020, vol. 1, pp. 1–178 Search PubMed.
- H. Scheller and P. Ulvskov, Annu. Rev. Plant Biol., 2010, 61, 263–289 CrossRef CAS PubMed.
- S. Ranganathan, S. Dutta, J. Moses and C. Anandharamakrishan, Heliyon, 2020, 6, 9 CrossRef PubMed.
- R. Naomi, R. Idrus and M. Fauzi, Int. J. Environ. Res. Public Health, 2020, 17, 6803 CrossRef CAS PubMed.
- E. Malachowska, M. Dubowik, A. Lipkiewicz, K. Przybysz and P. Przybysz, Sustainability, 2020, 12(17), 1–12 CrossRef.
- C. Chen, C. Duan, J. Li, Y. Liu, X. Ma, L. Zheng, J. Stavik and Y. Ni, BioResources, 2016, 11(2), 5553–5564 Search PubMed.
- X. Wang, H. Li, Y. Cao and Q. Tang, Bioresour. Technol., 2011, 102(17), 7959–7965 CrossRef CAS PubMed.
- P. Fatchi and Y. Ni, ACS Symp. Ser., 2011, 1067, 409–441 CrossRef.
- G. Smook, Handbook for Pulp and Paper Technologists, 3rd edn, Angus Wilde Publications, 2002 Search PubMed.
- T. Wang and Y. Zhao, Carbohydr. Polym., 2021, 253, 8 Search PubMed.
- P. Hart, C. Houtman and K. Hirth, Tappi J., 2013, 12(7), 59–65 CAS.
- Tappi. Viscosity of Pulp (Capillary Viscometer Method) (T230-99), Pulp Properties Committee of the Process and Product Quality Division, 1999 Search PubMed.
- J. Zhou, L. Zhang and J. Cai, J. Polym. Sci. B Polym. Phys., 2004, 42, 2 CrossRef.
- I. Fareez, N. Ibrahim, W. Yaacob, N. Razali, A. Jasni and F. Aziz, Cellulose, 2018, 25, 4407–4421 CrossRef CAS.
- M. Masuelli, J. Polym. Biopolym. Phys. Chem., 2018, 6(1), 13–25 CrossRef CAS.
- T. Gillespie and M. Hulme, J. Appl. Polym. Sci., 1969, 13, 2031–2032 CrossRef CAS.
- L. Segal, J. Creely, A. Martin and C. Conrad, Text. Res. J., 1959, 29(10), 786–794 CrossRef CAS.
- P. Scherrer, Nachrichten von Der Gesellschaft Der Wissenschaften Zu Göttingen, Mathematisch-Physikalische Klasse, 1918, vol. 2, pp. 98–100 Search PubMed.
- H. Zhang, C. Yang, W. Zhou, Q. Luan, W. Li, Q. Deng, X. Dong, H. Tang and F. Huang, ACS Sustain. Chem. Eng., 2018, 6(11), 13924–13931 CrossRef CAS.
- N. Jia, S. Li, M. Ma, J. Zhu and R. Sun, BioResources, 2011, 6(2), 1186–1195 CAS.
- S. Doncea, R. Ion, R. Fierascui, E. Bacalum, A. Bunaciu and H. Aboul-Enein, Instrum. Sci. Technol., 2010, 38(1), 96–106 CrossRef CAS.
- G. M. Nabar and J. A. Rathod, J. Soc. Ind. Research, 1949, 8B, 154–156 CAS.
- C. Aurelia, A. Murdiati and A. Ningrum, Pak. J. Nutr., 2018, 18(2), 193–200 CrossRef.
- K. Ahn, S. Zaccaron, N. Zwirchmayr, H. Hettegger, A. Hofinger, M. Bacher, U. Henniges, T. Hosoya, A. Potthast and T. Rosenau, Cellulose, 2019, 26, 429–444 CrossRef CAS.
- S. Cichosz, A. Masek and K. Dems-Rudnicka, Sci. Rep., 2022, 12 Search PubMed.
- A. Barkane, E. Kampe, O. Platnieks and S. Gaidukovs, Nanomaterials, 2021, 11, 7 CrossRef PubMed.
- F. dos Santos, G. Iulianelli and M. Tavares, Polym. Test., 2017, 61, 280–288 CrossRef CAS.
- M. Holtzapple, Encyclopedia of Food Sciences and Nutrition, 2003, pp. 998–1007 Search PubMed.
- S. Gharaibeh, W. Obeidat and N. Al-Zoubi, e-Polymers, 2022, 22(1), 536–543 CrossRef CAS.
- L. Kong, Z. Zhao, Z. He and S. Yi, Results Phys., 2017, 7(1), 914–919 CrossRef.
- M. Kok and E. Ozgur, Energy Sources, 2017, 39(2), 134–139 CrossRef CAS.
- P. Balasubramanian, S. Ramalingam, M. Javid and J. Rao, J. Am. Leather Chem. Assoc., 2018, 113, 311–317 CAS.
- N. Park, S. Choi, J. E. Oh and D. Y. Hwang, Carbohydr. Polym., 2019, 223, 116114 CrossRef PubMed.
- H. Kargarzadeh, I. Ahmad, I. Abdullah, A. Dufresne, S. Y. Zainudin and R. M. Sheltami, Cellulose, 2012, 19, 855–866 CrossRef CAS.
- J. Parlucha and R. Razal, J. Trop. For. Sci., 2022, 34(2), 159–169 Search PubMed.
- Y. Nan, D. Gomez-Maldonado, M. Iglesias, D. Whitehead and M. Peresin, Cellulose, 2023, 30, 3639–3651 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3su00192j |
| This journal is © The Royal Society of Chemistry 2024 |