 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Shear thickening in suspensions of particles with dynamic brush layers†
Hojin
Kim
 *ab,
Michael
van der Naald
*ab,
Michael
van der Naald
 ac,
Finn A.
Braaten
ac,
Thomas A.
Witten
ac,
Finn A.
Braaten
ac,
Thomas A.
Witten
 ac,
Stuart J.
Rowan
ac,
Stuart J.
Rowan
 bd and
Heinrich M.
Jaeger
bd and
Heinrich M.
Jaeger
 ac
ac
aJames Franck Institute, The University of Chicago, Chicago, Illinois 60637, USA. E-mail: hojinkim718@gmail.com
bPritzker School of Molecular Engineering, The University of Chicago, Chicago, Illinois 60637, USA
cDepartment of Physics, The University of Chicago, Chicago, Illinois 60637, USA
dDepartment of Chemistry, The University of Chicago, Chicago, Illinois 60637, USA
First published on 25th July 2024
Abstract
Control of frictional interactions among liquid-suspended particles has led to tunable, strikingly non-Newtonian rheology via the formation of strong flow constraints as particles come into close proximity under shear. Typically, these frictional interactions have been in the form of physical contact, controllable via particle shape and surface roughness. We investigate a different route, where molecular bridging between nearby particle surfaces generates a controllable constraint to relative particle movement. This is achieved with surface-functionalized colloidal particles capable of forming dynamic covalent bonds with telechelic polymers that comprise the suspending fluid. At low shear stress this results in particles coated with a uniform polymer brush layer. Beyond an onset stress σ* the telechelic polymers become capable of bridging and generate shear thickening. Over the size range investigated, we find that the dynamic brush layer leads to dependence of σ* on particle diameter that closely follows a power law with exponent −1.76. In the shear thickening regime, we observe an enhanced dilation in measurements of the first normal stress difference N1 and reduction in the extrapolated volume fraction required for jamming, both consistent with an effective particle friction that increases with decreasing particle diameter. These results are discussed in light of predictions for suspensions of hard spheres and of polymer-grafted particles.
1 Introduction
Shear thickening fluids are materials with a viscosity that rises with increasing shear stress or shear rate.1 Dense suspensions of solid particles suspended in a Newtonian liquid have emerged as the prototypical fluid exhibiting strong shear thickening and have been studied extensively over the last two decades. Understanding the flow of suspensions is critically important in both industrial applications and natural processes. Frictional interaction between particles has been implicated as the dominant contribution to dramatic increases in viscosity during shear thickening, whereby applied stress leads to a growing population of particles interacting frictionally and resisting the applied shear.2–5Advances in the understanding of shear thickening in dense suspensions have been facilitated primarily by studying spherical particles as a model system. This is the most straightforward for theoretical calculations and computational modeling as well as being relatively simple to synthesize and measure experimentally. Spherical particle suspensions also have the virtue of making it relatively easy to isolate how changes to particle level characteristics such as particle roughness, particle size, and particle surface chemistry impact shear thickening. For example, by tuning particle roughness,6–9 varying contact friction,10 or incorporating enhanced lubrication hydrodynamics in simulations11,12 it has been shown how the shear thickening response can be controlled. Another way to alter the shear thickening response is to tune the particle size, which controls the stress σ* required to make frictional contact and thus to induce shear thickening. For hard sphere suspensions this stress is found to scale as  , where the effective applied force σ*d2 has to exceed the effective stabilizing force F0 at contact.13,14 Whenever local frictional contacts exist between contacting particles, i.e., whenever σ > σ*, relative movement is hindered and this acts as a stress-activated constraint for the associated degree of freedom.13,15
, where the effective applied force σ*d2 has to exceed the effective stabilizing force F0 at contact.13,14 Whenever local frictional contacts exist between contacting particles, i.e., whenever σ > σ*, relative movement is hindered and this acts as a stress-activated constraint for the associated degree of freedom.13,15
Typically, such stress-induced constraints have their origin in physical contact friction. However, friction can also be created by chemical bridging between suitably functionalized surfaces.16 For example, hydrogen bonding between the particles can enhance the effective friction to the point of inducing shear jamming.17 However, hydrogen bonds are weak and have a very short lifetime. Comparatively new and less studied are suspensions in which the chemistry of particles and suspending polymeric liquid has been designed to enable bridging via dynamic covalent bonds that can be significantly stronger and have longer lifetimes than non-covalent interactions. These polymers have groups at both ends that bond to the particle surfaces.18,19 The effective friction induced by such dynamic covalent bridging has been termed “chemical friction” because the constraint is generated by solvent molecules that chemically attach to contacting particle surfaces in a reversible manner. The shear-induced bridging bond results in constraints resistant to relative particle motions. Such a chemical bond can then be viewed as friction (i.e., as an effective, chemical friction), similar to the physical friction of hard spheres. These dynamic bonds establish quickly and release more slowly, resulting in a longer lifetime. In addition to strong shear thickening such suspensions also can exhibit anti-thixotopic responses owing to the slow release.18,19 The focus of the present work is, however, only on the suspension behavior with increasing applied stress and investigates how dynamic bridging affects the onset of shear thickening, enables a cross-over from lubrication- to friction-dominated rheological behavior, and changes the propensity for jamming. Our experiments show how bridging interactions provide a means of systematically tuning the effective friction in sheared suspensions and, in particular, make it possible to change this effective friction simply by changing particle size.
2 Experiments
2.1 Materials preparation
Five sets of silica spheres with hydroxyl surface groups were purchased from fiber optic center (New Bedford, MA) with diameters in the range 300 nm < d < 2000 nm. These particles were surface-functionalized with thiol groups as reported previously (see ESI†).18 The size of thiol-functionalized particles was measured as d = 340 ± 4 (SP3), 620 ± 10 (SP6), 870 ± 10 (SP9), 1210 ± 40 (SP12), and 1930 ± 90 nm (SP19) using dynamic light scattering.The suspending medium for these spheres was a telechelic polymeric liquid comprised of p-nitrobenzalcyanoacetamide (N-BCAm)-endcapped poly(propylene glycol) (number-averaged molecular weight, Mn ≈ 5300 g mol−1).18 A dynamic brush layer is formed on the surface of the particles via the catalyst free, room temperature dynamic thia-Michael reaction between the thiol and the BCAm group. In contrast to a covalently attached brush layer, the dynamic brush continually forms and releases due to the kinetics of the thia-Michael bond at room temperature (Fig. 1a). The equilibrium constant Keq of this thia-Michael reaction was measured as Keq ≈ 8000 M−1.18 From a thermodynamic perspective, we note that, for all suspensions reported here, the stoichiometric amount of the BCAm Michael-acceptor group is at least 5-fold larger than the surface thiol (1–2 thiol-per-nm2, see the nuclear magnetic resonance spectroscopy study in the ESI†). In this concentration limit and Keq, the telechelic polymers form dynamic bonds with the surface thiol, resulting in the brush layer.
2.2 Rheology measurement
Suspensions with different volume fractions ϕ were prepared by measuring masses of particles and solvents and converting to volumes using their densities. The rheology over all accessible shear stresses, typically σ = 10−1 Pa to σ = 103 Pa, was measured using a rheometer (Anton Paar, MCR 302 or MCR 702) in cone and plate geometry (25 mm diameter, 2° angle). Owing to the high yield stress, the measurements were performed in rate-controlled mode at low stress prior to the shear thickening regime and otherwise using stress control. For suspensions with the smallest particles, d = 340 nm, the rheology was measured in rate-controlled mode throughout, given the particularly high yield stress. The onset stress for shear thickening was determined from the minimum viscosity.3 Results
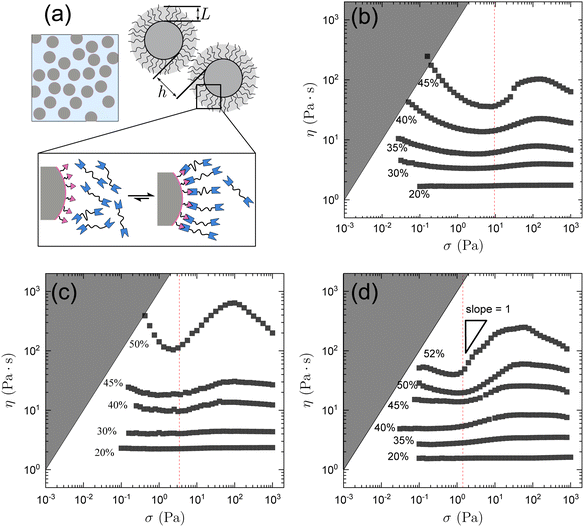
Fig. 1b shows the rheology of SP6 at different volume fractions ϕ defined here using the volume of the thiol-functionalized particles without the attached brush layer of telechelic solvent molecules. For ϕ = 0.30, the suspension exhibits a mild thinning and thickens slightly at larger stresses. The magnitude of shear thickening increases with ϕ, as seen in the slope d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) η/d
η/d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) σ. At ϕ = 0.45 the slope reaches 1, which corresponds to discontinuous shear thickening when viscosity is plotted as a function of shear rate. For all ϕ, the suspension starts to shear thicken at σ* ≈ 10 Pa (red dashed line). Also observed is an increase in the yield stress with increasing ϕ. This is in line with the increased ability to form inter-particle bonds already at rest as the packing fraction becomes high.
σ. At ϕ = 0.45 the slope reaches 1, which corresponds to discontinuous shear thickening when viscosity is plotted as a function of shear rate. For all ϕ, the suspension starts to shear thicken at σ* ≈ 10 Pa (red dashed line). Also observed is an increase in the yield stress with increasing ϕ. This is in line with the increased ability to form inter-particle bonds already at rest as the packing fraction becomes high.
A second shear-thinning regime appears at high stress. While similar behavior has been found in suspensions of anisotropic,20 irregular,21 or soft22 particles, this second thinning in the dynamic suspensions studied here is most likely attributable to the breaking of the bridging interactions at high shear stress. Probing larger particles, SP12 (Fig. 1c) and SP19 (Fig. 1d), analogous behavior is observed. However, the shear thickening behavior for given ϕ becomes weaker with increasing d. Similarly, the low stress yielding behavior is weaker for larger d, even at larger ϕ.
Physically, the onset stress σ* signals the stress level at which the particles have been sheared into sufficiently close distance, h*, to start forming frictional contact. In Fig. 2 we plot the onset stress for shear thickening, σ*, as a function of particle diameter d. Over the size range measured, for our particles (blue symbols) this onset closely follows a power law σ* ∝ dα with best fit exponent α = −1.76. Also shown in that figure are results from prior work13,14,23–28 with particles where the brush layer was attached with permanent covalent bonds (black symbols), and for which a best fit exponent α ≈ −1.9 has been reported.13 We believe that one of the reasons for our data to exhibit very little scatter is that the dynamic brush layers is formed by the exact same N-BCAm-endcapped polymers for all particle sizes.
 | ||
| Fig. 2 Onset stress for shear thickening σ* in suspensions with dynamic covalent bonds (blue diamonds). Also shown are data from prior studies13,14,23–28 using suspension with particles having permanently attached covalent brush layers (black symbols). Solid lines are least-squares fits. | ||
As σ increases, particles are necessarily pushed closer together, thus promoting bridging by the telechelic macromonomers via dynamic bonds at σ > σ*. This generates constraints to relative particle movement, similar to the constraints arising from a network of frictional contacts. In the following, we further explore the dynamic-bond-induced shear thickening state through the first normal stress difference N1 ≡ σxx − σzz. Here σxx and σzz refer to the stress tensor components in the shear flow direction (x) and its gradient direction (z). Prior work has suggested that the sign of N1 distinguishes states where particles interact via lubrication (N1 < 0) or form system-spanning networks of frictional contacts that lead to dilation of the overall sample volume (N1 > 0).3,29,30 In Fig. 3a, we plot N1 − N1,0 for the largest particles, 1930 nm, at different volume fractions 0.3 ≤ ϕ ≤ 0.52, where the initial value N1,0 at the lowest applied shear stress has been subtracted. For ϕ ≤ 0.5, we find N1 < 0 even beyond the stress σ* ≈ 1.3 Pa that indicates the onset of shear thickening. We interpret this as indicating that system-spanning networks of frictional contacts can only form for σ > σ*. This changes once ϕ ≥ 0.52 where now the sign of N1 reverses from negative to positive as σ ≥ σ*.
The effect on N1 of changing particle size is shown in Fig. 3b for fixed ϕ = 0.45. Strikingly, the sign of N1 in the shear thickening regime reverses from negative to positive for particles with d < 1210 nm (see ESI† for other particle packing fractions).
The size-dependent magnitude of the effective friction seen in the behavior of N1 is also observed in the approach to jamming as the particle volume fraction ϕ is increased. This can be seen in plots of the high-stress viscosity ηt, measured at the upper end of the shear thickened state (i.e., the maximum viscosity in the shear thickening regime), as a function of particle volume fraction ϕ, which diverges at a friction-dependent jamming volume fraction ϕμJ.31 The ϕμJ divergence shifts to a lower value with increased interparticle friction and can therefore be taken as another indicator of the effect of dynamic-bond-induced constraints. In Fig. 4 we plot data for the three particle systems measured and fit them to a power-law relation, η = A(1 − ϕ/ϕJ)−n (ref. 31–33) to extract ϕμJ. The fits, with n = 1.7–1.8 (see ESI†), give ϕμJ = 0.54, 0.51, and 0.48 for SP19, SP12 and SP6, respectively.
4 Discussion
For particles coated with a polymer brush layer we can expect that shearing them into contact produces a balance between the applied stress and the counteracting stress from the stiffness of the brush. Balancing the force σ*d2 with the restoring force derived from gradient of the interparticle potential for brush-layer stabilized particles14 gives | (1) |
Different interparticle potentials will produce different scalings σ* ∝ dα of the onset stress as a function of particle diameter d. For example, stabilization by steric interactions with distance-independent forces  leads to σ* ∝ d−2. The data shown Fig. 2 by the black symbols therefore has been interpreted as the result of behavior close to the hard sphere limit. Another much-studied limit is that for compressed semidilute polymer brush layers14,34 with polymer brush thickness L and surface-to-surface distance h (see Fig. 1a). In this case the stabilizing potential gradient between polymer brush layers was found consistent with
leads to σ* ∝ d−2. The data shown Fig. 2 by the black symbols therefore has been interpreted as the result of behavior close to the hard sphere limit. Another much-studied limit is that for compressed semidilute polymer brush layers14,34 with polymer brush thickness L and surface-to-surface distance h (see Fig. 1a). In this case the stabilizing potential gradient between polymer brush layers was found consistent with  . This gradient gives a scaling σ* ∝ d−1.75.14 Intriguingly, this closely matches our data for dynamic brushes. Notably, however, the power law exponent −0.25 in this scaling argument requires semidilute, good solvent conditions for the brush, which are difficult to justify in our system, where we have a very different situation: the particles are dispersed in a melt of N-BCAm endcapped polymers and these polymers also form the dynamic brush layer. In addition, we see that the shear thickening in these suspensions is stress-dependent. The rates of bond association/dissociation do not impact the onset of shear thickening, particularly at this high Keq where the majority of dynamic bonds is in the bonded state, as shown in a previous study19 with a library of benzalcyanoacetamide Michael-acceptors with different Keq.
. This gradient gives a scaling σ* ∝ d−1.75.14 Intriguingly, this closely matches our data for dynamic brushes. Notably, however, the power law exponent −0.25 in this scaling argument requires semidilute, good solvent conditions for the brush, which are difficult to justify in our system, where we have a very different situation: the particles are dispersed in a melt of N-BCAm endcapped polymers and these polymers also form the dynamic brush layer. In addition, we see that the shear thickening in these suspensions is stress-dependent. The rates of bond association/dissociation do not impact the onset of shear thickening, particularly at this high Keq where the majority of dynamic bonds is in the bonded state, as shown in a previous study19 with a library of benzalcyanoacetamide Michael-acceptors with different Keq.
The size dependence of the sign reversal in N1 (Fig. 3b) suggests that the strength of the effective friction produced by the bridging linkers increases with decreasing d. To rationalize this, we consider how particle–particle interactions due to polymer linkages depend on the total energy density associated with bridging. This energy density (ρB) depends on the energy per bridging bond εB, the interparticle contact area A ∼ Ld for d ≫ L, and the number of particles per unit volume, which is proportional to d−3, and it scales as ρB ∼ εBLd/d3 = εBLd−2. As a result, although telechelic polymers on larger particles with less curved surfaces have a stronger attractive pair potential, particularly in the regime of d ≫ Rg,35 the overall bridging energy density scales inversely with particle size, ρB ∼ d−2. Hence, per unit volume smaller particles are more strongly interacting by bridging and behave more frictional, i.e., they experience stronger constraints to relative motion under shear. This then decreases the volume fraction required for the formation of system-spanning frictional networks and thus for the observation of behavior with N1 > 0. Conversely, at fixed ϕ, suspensions of larger particles behave less frictional, thus exhibit less pronounced shear thickening and may not show a transition to positive N1. We also see from Fig. 3 that in suspensions where N1 < 0 initially, there can be a sign reversal at larger shear stresses. We take this as an indication that telechelic linkers make it possible to establish extended network structures even under large applied shear. However, the decrease in viscosity seen in Fig. 1 at these large stress levels suggests that such networks structures deform more easily under shear than hard spheres would.
The fits to the data in Fig. 4 make it possible to estimate the associated effective friction coefficient μ, using the simulation data by Singh et al.36 Taking ϕ∞J ≈ 0.47 as obtained for infinite sliding friction μs = ∞ at fixed rolling friction μr = 0.3,15 the resulting values are μ = 3.5, 0.9, and 0.5 for SP6, SP12, and SP19, respectively. The finding that the extracted effective friction coefficient can be larger than unity again highlights that we are dealing with friction due to chemical bridging, as opposed to physical contact friction for which μ ≤ 1 can typically be expected. We note that it is possible to attribute some of the decrease in ϕμJ with particle diameter d also to the brush layer effectively increasing the volume fraction of particles in the suspension. Accounting for the brush layer would then shift ϕ to ϕeff = ϕ(1 + 2L/d)3. However, this shift is too small to fully explain the observed particle size dependence in ϕμJ (see ESI†).
5 Summary and conclusions
We investigated shear thickening for a class of dense suspensions with particles designed to interact through chemical bridging interactions. In these systems silica particles are surface-functionalized with thiol groups and suspended in a N-BCAm-endcapped poly(propylene glycol). This combination of particle surface and solvent chemistry allows for a dynamic brush layer to be established at the particle surface. At low shear this brush layer stabilizes the particles. As larger stress is applied to the suspension, particles come into sufficiently close proximity that dynamic bridging interactions are formed. These bridges introduce an effective interparticle friction and promote shear thickening. We find that the onset stress of shear thickening for suspensions with dynamic brush layers scales with particle size as d−1.76. It is intriguing that this lines up closely with the theoretical model for covalently-attached brush stabilized particles, which predicts d−1.75 but applies to a rather different regime. The degree to which this model can be generalized is currently unknown. Our results show that bridging interactions can give rise to dilatancy and form system-spanning networks, just as in shear thickening suspensions where the particles interact sterically through direct contact friction. However, owing to the increase in the overall area of contact for fixed brush thickness, and in contrast to steric interactions, the effective bridging-induced friction increases as particle size decreases. Consequently, also the onset of jamming is shifted to smaller ϕμJ for smaller particles.Our findings demonstrate that dynamic bridging interactions provide a direct, highly tunable means for controlling the effective particle friction. For fixed particle and solvent chemistry, this friction can be changed straightforwardly by changing the particle size and it can become as large as or even exceed physical friction produced by particle surface roughness. While we reported on one specific dynamic covalent chemistry, the results introduce a versatile way to design the non-Newtonian rheology of shear thickening fluids, and we expect that this approach can be extended further by tuning the molecular structure of the suspending polymer medium, including the molecular weight (length scale of bridging interaction), persistence length (rolling friction), and thermodynamic reaction equilibrium (effective friction lifetime). Furthermore, the shear-induced bridging discussed here can be seen as a form of adhesive interaction as it shows a long relaxation timescale to break once formed under shear. The distinct stress- and time-dependent conformations of dynamic bonds therefore open a new window to explore particle–polymer composites with adhesive interactions that are dynamic.
Author contributions
HK, SJR, and HMJ conceived the research project; HK and FAB performed the experiments; HK, MvdN, TAW, SJR, and HMJ analyzed the data; all authors participated in the discussion and writing the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Norman Wagner for fruitful discussion. This work was primarily supported by the University of Chicago Materials Research Science and Engineering Center, which is funded by National Science Foundation under award number DMR-2011854. This work was partially supported by National Science Foundation under award DMR-2104694. Parts of this work were carried out at the Soft Matter Characterization Facility and at the MRSEC Characterization Facility at the University of Chicago (award number DMR-2011854).Notes and references
- J. Mewis and N. J. Wagner, Colloidal suspension rheology, Cambridge University Press, 2012 Search PubMed.
- R. Seto, R. Mari, J. F. Morris and M. M. Denn, Phys. Rev. Lett., 2013, 111, 218301 CrossRef PubMed.
- J. R. Royer, D. L. Blair and S. D. Hudson, Phys. Rev. Lett., 2016, 116, 188301 CrossRef PubMed.
- N. Y. Lin, B. M. Guy, M. Hermes, C. Ness, J. Sun, W. C. Poon and I. Cohen, Phys. Rev. Lett., 2015, 115, 228304 CrossRef PubMed.
- C.-P. Hsu, J. Mandal, S. N. Ramakrishna, N. D. Spencer and L. Isa, Nat. Commun., 2021, 12, 1477 CrossRef CAS PubMed.
- D. Lootens, H. Van Damme, Y. Hémar and P. Hébraud, Phys. Rev. Lett., 2005, 95, 268302 CrossRef PubMed.
- L. C. Hsiao, S. Jamali, E. Glynos, P. F. Green, R. G. Larson and M. J. Solomon, Phys. Rev. Lett., 2017, 119, 158001 CrossRef PubMed.
- P. Bourrianne, V. Niggel, G. Polly, T. Divoux and G. H. McKinley, Phys. Rev. Res., 2022, 4, 033062 CrossRef CAS.
- C.-P. Hsu, S. N. Ramakrishna, M. Zanini, N. D. Spencer and L. Isa, Proc. Natl. Acad. Sci. U. S. A., 2018, 115, 5117–5122 CrossRef CAS PubMed.
- Y.-F. Lee, Y. Luo, T. Bai, C. Velez, S. C. Brown and N. J. Wagner, Phys. Fluids, 2021, 33, 033316 CrossRef CAS.
- S. Jamali and J. F. Brady, Phys. Rev. Lett., 2019, 123, 138002 CrossRef CAS PubMed.
- M. Wang, S. Jamali and J. F. Brady, J. Rheol., 2020, 64, 379–394 CrossRef CAS.
- B. M. Guy, M. Hermes and W. C. Poon, Phys. Rev. Lett., 2015, 115, 088304 CrossRef CAS PubMed.
- L.-N. Krishnamurthy, N. J. Wagner and J. Mewis, J. Rheol., 2005, 49, 1347–1360 CrossRef CAS.
- A. Singh, G. L. Jackson, M. van der Naald, J. J. de Pablo and H. M. Jaeger, Phys. Rev. Fluids, 2022, 7, 054302 CrossRef.
- U. Raviv, R. Tadmor and J. Klein, J. Phys. Chem. B, 2001, 105, 8125–8134 CrossRef CAS.
- N. M. James, E. Han, R. A. L. de la Cruz, J. Jureller and H. M. Jaeger, Nat. Mater., 2018, 17, 965–970 CrossRef CAS PubMed.
- G. L. Jackson, J. M. Dennis, N. D. Dolinski, M. van der Naald, H. Kim, C. Eom, S. J. Rowan and H. M. Jaeger, Macromolecules, 2022, 55, 6453–6461 CrossRef CAS PubMed.
- H. Kim, M. van der Naald, N. D. Dolinski, S. J. Rowan and H. M. Jaeger, Soft Matter, 2023, 19, 6797–6804 RSC.
- E. Brown, N. A. Forman, C. S. Orellana, H. Zhang, B. W. Maynor, D. E. Betts, J. M. DeSimone and H. M. Jaeger, Nat. Mater., 2010, 9, 220–224 CrossRef CAS PubMed.
- S. R. Raghavan and S. A. Khan, J. Colloid Interface Sci., 1997, 185, 57–67 CrossRef CAS PubMed.
- S. Jamali, A. Boromand, N. Wagner and J. Maia, J. Rheol., 2015, 59, 1377–1395 CrossRef CAS.
- J. Mewis and J. Vermant, Prog. Org. Coat., 2000, 40, 111–117 CrossRef CAS.
- W. J. Frith, P. Haene, R. Buscall and J. Mewis, J. Rheol., 1996, 40, 531–548 CrossRef CAS.
- T. Strivens, J. Colloid Interface Sci., 1976, 57, 476–487 CrossRef CAS.
- M. Smith, R. Besseling, M. Cates and V. Bertola, Nat. Commun., 2010, 1, 114 CrossRef CAS PubMed.
- L. Isa, PhD thesis, University of Edinburgh, 2008 Search PubMed.
- P. d'Haene, J. Mewis and G. Fuller, J. Colloid Interface Sci., 1993, 156, 350–358 CrossRef.
- D. R. Foss and J. F. Brady, J. Fluid Mech., 2000, 407, 167–200 CrossRef CAS.
- N. Park, V. Rathee, D. L. Blair and J. C. Conrad, Phys. Rev. Lett., 2019, 122, 228003 CrossRef CAS PubMed.
- R. Mari, R. Seto, J. F. Morris and M. M. Denn, J. Rheol., 2014, 58, 1693–1724 CrossRef CAS.
- B. Andreotti, J.-L. Barrat and C. Heussinger, Phys. Rev. Lett., 2012, 109, 105901 CrossRef PubMed.
- E. Lerner, G. Düring and M. Wyart, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 4798–4803 CrossRef CAS PubMed.
- G. H. Fredrickson and P. Pincus, Langmuir, 1991, 7, 786–795 CrossRef CAS.
- X.-X. Meng and W. B. Russel, J. Rheol., 2006, 50, 169–187 CrossRef CAS.
- A. Singh, R. Mari, M. M. Denn and J. F. Morris, J. Rheol., 2018, 62, 457–468 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sm00624k |
| This journal is © The Royal Society of Chemistry 2024 |

![[small gamma, Greek, dot above]](https://www.rsc.org/images/entities/i_char_e0a2.gif)

