Open Access Article
Open Access ArticleUnderstanding the glassy dynamics from melting temperatures in binary glass-forming liquids
Yunhuan
Nie
ab,
Lijin
Wang
*c,
Pengfei
Guan
*a and
Ning
Xu
 *b
*b
aBeijing Computational Science Research Center, Beijing 100193, People's Republic of China. E-mail: pguan@csrc.ac.cn
bHefei National Laboratory for Physical Sciences at the Microscale, CAS Key Laboratory of Microscale Magnetic Resonance and Department of Physics, University of Science and Technology of China, Hefei 230026, People's Republic of China. E-mail: ningxu@ustc.edu.cn
cSchool of Physics and Optoelectronic Engineering, Anhui University, Hefei 230601, People's Republic of China. E-mail: lijin.wang@ahu.edu.cn
First published on 16th January 2024
Abstract
It is natural to expect that small particles in binary mixtures move faster than large ones. However, in binary glass-forming liquids with soft-core particle interactions, we observe the counterintuitive dynamic reversal between large and small particles along with the increase of pressure by performing molecular dynamics simulations. The structural relaxation (dynamic heterogeneity) of small particles is faster (weaker) than large ones at low pressures, but becomes slower (stronger) above a crossover pressure. In contrast, this dynamic reversal never happens in glass-forming liquids with hard-core interactions. We find that the difference of the effective melting temperatures felt by large and small particles can be used to understand the dynamic reversal. In binary mixtures, we derive effective melting temperatures of large and small particles simply from the conversion of units and find that particles with a higher effective melting temperature usually undergo a slower and more heterogeneous relaxation. The presence (absence) of the dynamic reversal in soft-core (hard-core) systems is simply due to the non-monotonic (monotonic) behavior of the melting temperature as a function of pressure. Interestingly, by manipulating the relative softness between large and small particles, we obtain a special case of soft-core systems, in which large particles always have higher effective melting temperatures than small ones. As a result, the dynamic reversal is totally eliminated. Our work provides another piece of evidence of the underlying connections between the properties of non-equilibrium glass-formers and equilibrium crystal-formers.
1 Introduction
The utilization of glassy materials in human civilization has spanned several millennia, yet a comprehensive understanding of the nature of the glass transition and glasses still poses great challenges in physics and materials science.1–8 On approaching the glass transition, supercooled glass-forming liquids exhibit exotic but universal dynamic properties that distinguish them apparently from simple liquids. These universal properties include the super-Arrhenius behavior in the temperature evolution of the structural relaxation, the dynamic heterogeneity,8–21 and the breakdown of the Stokes–Einstein relation,22–32 which have undergone extensive investigations over the past decades and are still ongoing subjects of study.Actually, there have been numerous common dynamic features reported to be shared by a wide swath of glass-formers; however, when going to a quantitative level, one could find that some dynamic properties could exhibit great diversity over glass-formers under different conditions.33–43 For instance, some dynamic properties of glass-formers with different interaction potentials could exhibit vastly different pressure evolutions: it was reported that the glass transition temperature and fragility both increase monotonically when pressure increases in hard-core potential systems,33,34 whereas they exhibit a non-monotonic pressure dependence in soft-core potential systems.35–42,44
What is more complicated is that the dynamics of different particle species in the same glass-forming liquid could differ remarkably, in addition to the temporal–spatial difference in dynamics, i.e., the dynamic heterogeneity. One is that, in binary glass-forming liquids composed of small and large particles, smaller particles usually exhibit faster relaxation rates, which is commonly observed and hence naturally accepted. However, to the best of our knowledge, the reasons behind the faster relaxation of smaller particles remain largely unknown to date. As a result, we cannot yet exclude the possibility that larger particles may have a faster relaxation under certain conditions.
In this work, we systematically compare the dynamics of large and small particles in binary glass-forming liquids. Two widely studied particle interactions, soft-core harmonic and hard-core repulsive Lennard–Jones (RLJ) potentials, are studied. We find that small particles exhibit faster structural relaxations at all pressures in RLJ systems. In harmonic systems, it is the fact, only at low pressures, above a crossover pressure large particles relax unexpectedly faster instead. Interestingly, by constructing effective melting temperatures for large and small particles, respectively, we find that particles with a higher effective melting temperature can always relax more slowly. The correlation between the relaxation and the effective melting temperature of particle species rationalizes the distinct pressure evolution of particle dynamics between harmonic and RLJ systems. Using this correlation, we successfully eliminate the dynamic reversal in harmonic systems by optimizing the relative softness between large and small particles and hence making the effective melting temperature of large particles higher at all pressures.
2 Simulation details
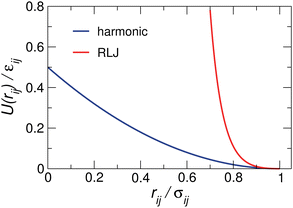
We perform molecular dynamics simulations at constant particle number N, pressure p, and temperature T. A binary mixture of NA A (large) and NB B (small) particles with the same mass m is placed in a three-dimensional cubic cell with side-length L. Periodic boundary conditions are applied in all directions. We use two widely studied particle interactions, namely, harmonic (soft-core)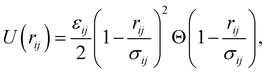 | (1) |
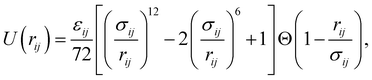 | (2) |
To fight against crystallization, we use a diameter ratio of γ = σA/σB = 1.4 and a concentration ratio quantified by the composition concentration of B particles cB = NB/(NA + NB) = 0.5. We define εAA = (1 + Δ)εAB and εBB = (1 − Δ)εAB with Δ ∈ [−1,1] in order to adjust the relative softness of particle species.45,46 We set the units of mass, length, and energy to be m, σB, and εAB, respectively. The time and temperature are thus in units of σBm1/2εAB−1/2 and εABkB−1 with kB being the Boltzmann constant.
In molecular dynamics simulations, we solve numerically equations of motion of this form:47
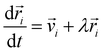 | (3) |
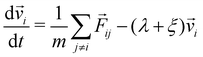 | (4) |
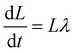 | (5) |
![[v with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0076_20d1.gif) i and
i and ![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) i are the velocity and position of particle i, respectively,
i are the velocity and position of particle i, respectively, ![[F with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0046_20d1.gif) ij = −∇Uij is the force of particle i exerted by particle j, and λ and ξ are the Lagrange multipliers to maintain constant p and T.47
ij = −∇Uij is the force of particle i exerted by particle j, and λ and ξ are the Lagrange multipliers to maintain constant p and T.47
To quantify the structural relaxation, we calculate the self-part of the intermediate scattering function:48
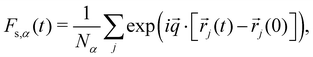 | (6) |
![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) j(t) is the position of particle j at time t, and
j(t) is the position of particle j at time t, and ![[q with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0071_20d1.gif) is chosen in the x-direction with q = |
is chosen in the x-direction with q = |![[q with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0071_20d1.gif) | satisfying the periodic boundary conditions and being approximately the value at the first peak of the static structure factor. The structural relaxation time τα is determined as the time when Fs,α(t) decays to e−1.
| satisfying the periodic boundary conditions and being approximately the value at the first peak of the static structure factor. The structural relaxation time τα is determined as the time when Fs,α(t) decays to e−1.
To characterize the dynamic heterogeneity, we calculate the four-point dynamic susceptibility:49,50
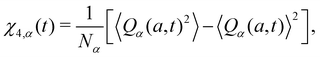 | (7) |
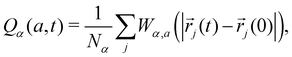 where a = 0.3 and Wα,a(r) = 1 if r ≤ a and 0 otherwise. For glass-forming liquids, χ4,α(t) usually exhibits a non-monotonic time dependence with a maximum χ4,max,α. Here, we use χ4,max,α to quantify dynamic heterogeneity at various temperatures or pressures.
where a = 0.3 and Wα,a(r) = 1 if r ≤ a and 0 otherwise. For glass-forming liquids, χ4,α(t) usually exhibits a non-monotonic time dependence with a maximum χ4,max,α. Here, we use χ4,max,α to quantify dynamic heterogeneity at various temperatures or pressures.
The local bond-orientational order of particle i is defined as 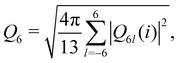 where
where 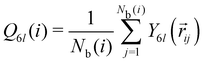 with Nb(i) being the number of nearest neighbors of particle i determined by the Voronoi tessellation and Y6l(
with Nb(i) being the number of nearest neighbors of particle i determined by the Voronoi tessellation and Y6l(![[r with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_0072_20d1.gif) ij) the spherical harmonics. We employ 〈Q6〉 averaged over particles and configurations to quantify the overall structural order.
ij) the spherical harmonics. We employ 〈Q6〉 averaged over particles and configurations to quantify the overall structural order.
The data were collected after the systems had been equilibrated for several to 100 times of τα (depending on the temperature and pressure). We will primarily present the results of systems with N = 1000 particles; however, we have verified partially that there are no apparent finite-size effects on our findings.
3 Results
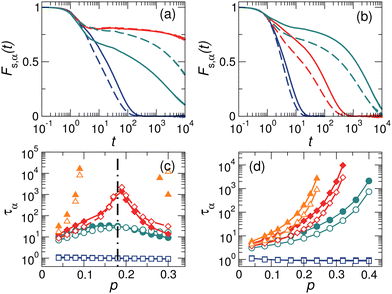
In binary mixtures of large and small particles, the mobility of small particles is intuitively larger than that of larger ones since small particles usually possess larger free volumes. In this section, we will show that this is always true in hard-core RLJ binary glass-forming liquids. In sharp contrast, it is violated in soft-core harmonic systems at high pressures.Fig. 2(a) and (b) compares the intermediate scattering functions of A and B particles, Fs,A(t) and Fs,B(t), for both harmonic and RLJ systems. We vary the pressure at a fixed temperature above the glass transition. Here we first show results for Δ = 0, so that εAA = εBB = εAB. This setup of the characteristic energy scales of the interaction has been widely used in previous modeling of glass-formers.40,51–54 As can be seen from Fig. 2(a) and (b), Fs,B(t) (dashed lines) decays faster than Fs,A(t) (solid lines) at low pressures for both harmonic and RLJ systems, so small particles undergo a faster structural relaxation than large ones, as expected. Fig. 2(b) indicates that Fs,A(t) > Fs,B(t) holds at all pressures for RLJ systems. However, Fig. 2(a) shows that, for harmonic systems, Fs,A(t) < Fs,B(t) when the pressure is above pd ≈ 0.18, so large particles relax counterintuitively faster than small ones. The crossover pressure pd signals the dynamic reversal between large and small particles, at which Fs,A(t) ≈ Fs,B(t), as illustrated by the comparison of the red curves in Fig. 2(a).
In order to verify that the results in Fig. 2(a) and (b) are not limited to some specifically-chosen temperatures, in Fig. 2(c) and (d), we compare τA(p) and τB(p) at different temperatures. At temperatures far beyond the glass transition, the difference between τA(p) and τB(p) is tiny. With the decrease of temperature, an observable gap between τA(p) and τB(p) emerges and grows. For RLJ systems, Fig. 2(d) shows that τA(p) > τB(p) at all temperatures. However, for harmonic systems, there is always a dynamic reversal across a crossover pressure pd, which is roughly insensitive to temperature, as shown in Fig. 2(c).
Fig. 2 raises a couple of questions: (1) what makes harmonic and RLJ systems exhibit so different pressure evolutions, and (2) what determines the crossover pressure pd for harmonic systems? Recent studies suggest that the equilibrium melting temperature plays an unexpected but crucial role in understanding the properties of glasses, such as the glass-forming ability45 and the effective temperature of aging glasses.55 It is interesting to know if the melting temperature can also shed light on the distinct particle dynamics observed here. Next we will show that it actually does and in a robust way.
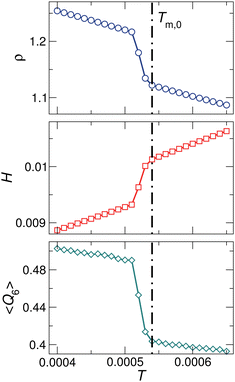
For monodisperse systems, the equilibrium melting temperature is well-defined and can be determined operationally as the temperature at which there is a discontinuous change in the density ρ(T), the enthalpy per particle H(T), or the average bond-orientational order 〈Q6〉 under a constant pressure, as illustrated in Fig. 3. The three parameters are consistent in the determination of the equilibrium melting temperature.56
For binary glass-forming liquids, it seems strange to define melting temperatures and hard to believe such temperature matter. The basic idea is that in binary mixtures large and small particles are under the same pressure p in the common units of εABσB−3; however, in their own units (εAAσA−3 and εBBσB−3, respectively), A and B particles ‘feel’ different pressure values, pA and pB, which can be simply derived from the conversion of units:
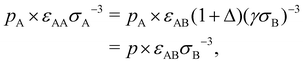 | (8) |
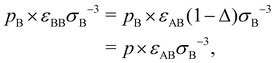 | (9) |
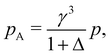 | (10) |
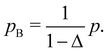 | (11) |
| Tm,A(p) × εABkB−1 = Tm,0(pA) × εAAkB−1, | (12) |
| Tm,B(p) × εABkB−1 = Tm,0(pB) × εBBkB−1. | (13) |
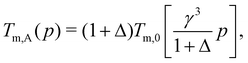 | (14) |
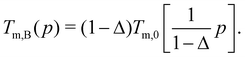 | (15) |
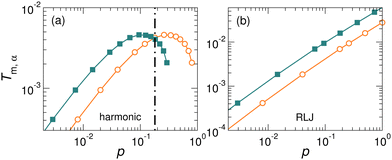
For the case with Δ = 0 shown in Fig. 2, Tm,A(p) = Tm,0(γ3p) and Tm,B(p) = Tm,0(p). Therefore, Tm,A(p) can be simply obtained from Tm,0(p) by multiplying p by γ−3. Fig. 4(a) and (b) compares Tm,A(p) and Tm,B(p) for harmonic and RLJ systems, respectively, with Δ = 0. The remarkable difference between harmonic and RLJ systems is that Tm,0(p) is monotonic for RLJ, but non-monotonic for harmonic, due to their soft- and hard-core natures, respectively.45 Actually, the non-monotonic evolution of the melting temperature with pressure in soft-core potential systems has been studied intensively.42,56–63 Because of this difference, the relative standing between Tm,A(p) and Tm,B(p) shows rather different pressure evolutions between the two systems. For RLJ systems, Tm,A is always larger than Tm,B at all pressures, simply because Tm,0(p) is monotonic. In contrast, because of the non-monotonicity, the Tm,A(p) and Tm,B(p) of harmonic systems intersect at a crossover pressure pm. When p < pm, Tm,A > Tm,B, while Tm,A < Tm,B otherwise in the pressure regime studied here.
It is interesting that the effective melting temperature difference strongly correlates with the relaxation time difference between the two particle species. For RLJ systems, Tm,A(p) > Tm,B(p) at all pressures, and we always have τA(p) > τB(p). For harmonic systems, Tm,A(p) > Tm,B(p) and τA(p) > τB(p) at low pressures, while Tm,A(p) < Tm,B(p) and τA(p) < τB(p) at high pressures. As shown in Fig. 4(a), Tm,A = Tm,B at pm ≈ 0.18, in excellent agreement with pd ≈ 0.18 at which τA = τB. All these suggest that particle species with a higher effective melting temperature undergo a slower structural relaxation.
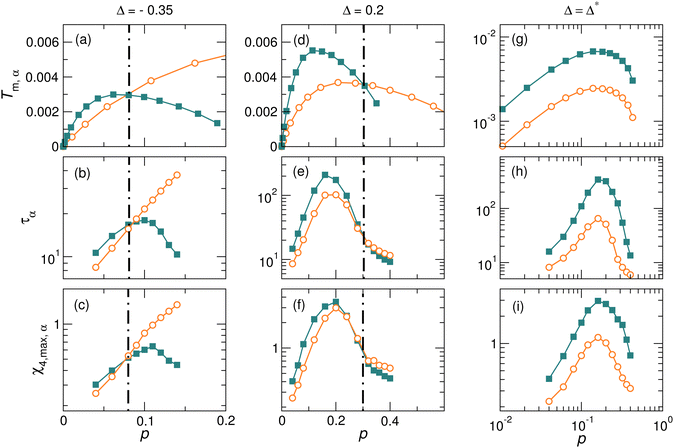
Fig. 2 and 4 show the results for Δ = 0. As can be seen from eqn (14) and (15), Tm,A(p) and Tm,B(p) vary with Δ, so does the crossover pressure pm. In order to see whether there is always a dynamic reversal in harmonic systems and whether pd always agrees with pm, we present results for various Δ values in Fig. 5. For two arbitrarily chosen values of Δ, − 0.35 and 0.2, Fig. 5(a), (b), (d) and (e) indicates that Tm,A(p) and Tm,B(p) intersect at a Δ-dependent pm; meanwhile, the dynamic reversal always occurs at pd(Δ) ≈ pm(Δ). The robust agreement between pd(Δ) and pm(Δ) convincingly supports our argument about the correlation between effective melting temperature and particle dynamics.
The non-monotonicity causes the intersection between Tm,A(p) and Tm,B(p). However, it is not guaranteed that Tm,A(p) and Tm,B(p) have to intersect. There is a special value of Δ, denoted as Δ*, which can be easily derived from the condition of pA = pB, i.e., by equating eqn (10) and (11):
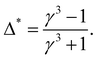 | (16) |
| Tm,A(p) = γ3Tm,B(p). | (17) |
We have compared the dynamics of large and small particles by calculating their structural relaxation times and revealed a connection between the difference in the dynamics and the difference in the effective melting temperatures. In addition, the dynamics usually exhibits spatio-temporal fluctuations in glass-forming liquids, which is referred to as dynamic heterogeneity. It has been well documented40,49 that a slower dynamics usually corresponds to a more heterogeneous dynamics. Since there could be a reversal in the structural relaxation times between large and small particles under certain circumstances, it is interesting to see whether there could be a reversal in the dynamic heterogeneity as well. Furthermore, if the reversal in dynamic heterogeneity exists, does it start at Pm?
We use the maximum of the four-point dynamic susceptibility χ4,max,A and χ4,max,B to quantify the dynamic heterogeneity of A and B particles, respectively. The comparison of χ4,max,A and χ4,max,B is shown in Fig. 5(c) with Δ = −0.35, Fig. 5(f) with Δ = 0.2, and Fig. 5(i) with Δ = Δ*. Interestingly, the evolution of the difference between χ4,max,A(p) and χ4,max,B(p) with pressure follows nearly the same trend as that of the difference between Tm,A(p) and Tm,B(p), in particular χ4,max,A(p) = χ4,max,B(p) at pm where Tm,A(p) = Tm,B(p). Therefore, one could conclude that there is also a good correlation between the effective melting temperature and the dynamic heterogeneity.
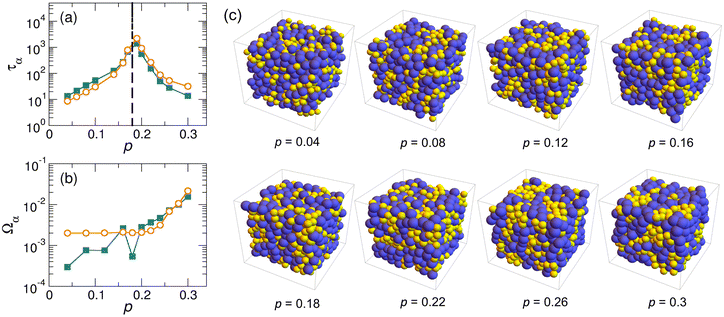
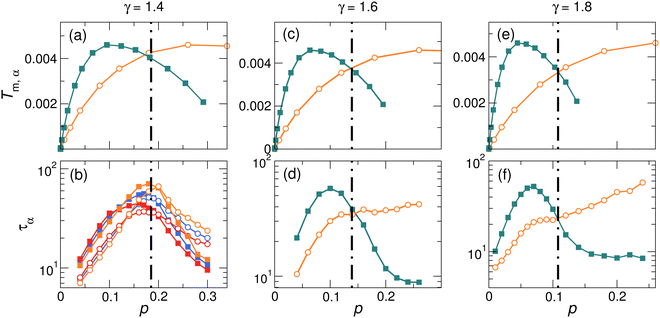
It is worth noting that we have excluded that the dynamic reversal observed in harmonic systems is a direct consequence of the particle demixing; see Fig. 6 and related discussions in the Appendix. Moreover, in Fig. 7 in the Appendix, we also find that our major conclusions do not depend on the diameter ratio or the concentration ratio of A and B particles within an appropriate range. However, in extreme cases where the diameter ratio or the concentration ratio is large and hence the good glass-forming ability64 could not be maintained anymore, we find that our conclusions could not work. Therefore, we believe more work is needed to study systems in these extreme cases.
It is also worthwhile to note that this work demonstrates a strong correlation between the glassy dynamics and the effective melting temperatures. In our previous work,45 the glass-forming ability has been linked to the gap in the effective melting temperatures. To our knowledge, the glassy dynamics and the glass-forming ability are two facets of the glass transition and hence usually studied separately. However, the glass-forming ability could relate closely to the glassy dynamics if combining our results in this work and those in ref. 45, which warrants further studies to investigate.
4 Conclusions
In summary, we compare the dynamics of large and small particles in binary glass-forming liquids with two distinct particle interactions. For soft-core harmonic systems, we observe the unexpected and counterintuitive dynamic reversal at high pressures: small particles relax faster and exhibit weaker dynamic heterogeneity than large ones at low pressures, while large particles relax faster and exhibit weaker dynamic heterogeneity at high pressures. In contrast, the dynamic reversal is absent in hard-core RLJ systems, in which small particles normally undergo a faster relaxation and less heterogeneous dynamics. We find that the difference in effective melting temperatures between large and small particles can rationalize the anomalous dynamic reversal in harmonic systems and the distinct dynamics between the two systems. Our results convincingly suggest that particle species with a higher effective melting temperature will undergo a slower structural relaxation in binary glass-forming liquids. Therefore, the non-monotonic pressure dependence of the melting temperature is the necessary condition for the dynamic reversal to occur. These findings provide us the freedom to manipulate the occurrence of the dynamic reversal and even eliminate it in soft-core systems with a non-monotonic pressure dependence of the melting temperature.It is surprising that the equilibrium melting temperature of monodisperse crystal-formers can take effect to control the dynamics of binary glass-formers. The correlation between the effective melting temperature and the particle dynamics revealed by this work is another piece of evidence of the underlying connections between non-equilibrium systems and their equilibrium counterparts.45,55 Here we are concerned about binary mixtures. Even for polydisperse harmonic systems, if we divide all particles into larger and smaller ones, separated by the average diameter, we expect to see the dynamic reversal as well and interpret it in terms of melting temperatures.
Author contributions
N. X. designed the project. Y. N. performed the simulations. Y. N., L. W., P. G., and N. X. analyzed the data and wrote the paper.Conflicts of interest
There are no conflicts to declare.Appendix
In order to check whether the reported dynamic reversal observed in harmonic systems is due to the phase separation, we quantify the degree of mixing for A particles and B particles using45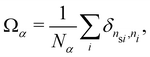 | (18) |
Fig. 6 compares the structure relaxation times of A and B, τA and τB, in (a) and the mixing degree of A and B particles, ΩA and ΩB, in (b) for harmonic systems for Δ = 0. In addition, we also show some snapshots at pressures below, around, and above the pressure at the dynamic reversal. We find it is hard to conclude there is a conclusively direct connection between the dynamic difference and the difference in the mixing degree. However, the demixing (or partial phase separation) seems to become stronger with the increase of pressure at very high pressures,55 and thus future work should check how this affects the particle dynamics.
In the main text, we have focused on γ = 1.4 and cB = 0.5. Fig. 7 shows our further results for cB = 0.4 and cB = 0.6 at fixed γ = 1.4, and for γ = 1.6 and γ = 1.8 at fixed cB = 0.5. For each combination of (γ, cB), the agreement between particle dynamics and effective melting temperature could be reproduced. However, we find that the agreement will disappear when γ or cB becomes large, which deserves further study.
Acknowledgements
We are grateful to L. Berthier for bringing us attention to the dynamic reversal in harmonic systems. Y. N. and P. G. acknowledge the support from the National Natural Science Foundation of China (Grant no. T2325004, 522161160330 and U2230402). N. X. acknowledges the support from the National Natural Science Foundation of China (Grant no. 12334009 and 12074355). Y. N. acknowledges the support from the China Postdoctoral Science Foundation (Grant no. 2021M690328). L. W. acknowledges the support from the National Natural Science Foundation of China (Grant no. 12374202 and 12004001), Anhui Projects (Grant no. 2022AH020009, S020218016, and Z010118169), and Hefei City (Grant no. Z020132009). We also acknowledge Beijing Computational Science Research Center, the Supercomputing Center of the University of Science and Technology of China, Hefei Advanced Computing Center, Beijing Super Cloud Computing Center, and the High-Performance Computing Platform of Anhui University for providing computing resources.References
- C. A. Angell, Science, 1995, 267, 1924 CrossRef CAS PubMed.
- V. Lubchenko and P. G. Wolynes, Annu. Rev. Phys. Chem., 2007, 58, 235 CrossRef CAS PubMed.
- P. G. Debenedetti and F. H. Stillinger, Nature, 2001, 410, 259 CrossRef CAS PubMed.
- J. C. Dyre, Rev. Mod. Phys., 2006, 78, 953 CrossRef CAS.
- L. Berthier and G. Biroli, Rev. Mod. Phys., 2011, 83, 587 CrossRef CAS.
- S. Karmakar, C. Dasgupta and S. Sastry, Annu. Rev. Condens. Matter Phys., 2014, 5, 255 CrossRef CAS.
- D. Chandler and J. P. Garrahan, Annu. Rev. Phys. Chem., 2010, 61, 191 CrossRef CAS PubMed.
- M. D. Ediger, Annu. Rev. Phys. Chem., 2000, 51, 99 CrossRef CAS PubMed.
- E. Flenner, H. Staley and G. Szamel, Phys. Rev. Lett., 2014, 112, 097801 CrossRef PubMed.
- M. Ozawa and G. Biroli, Phys. Rev. Lett., 2023, 130, 138201 CrossRef CAS PubMed.
- M. Adhikari, S. Karmakar and S. Sastry, J. Phys. Chem. B, 2021, 125, 10232 CrossRef CAS PubMed.
- G. Biroli, K. Miyazaki and D. R. Reichman, arXiv, 2022, preprint, arXiv:2209.02825, DOI:10.48550/arXiv.2209.02825.
- A. Tahaei, G. Biroli, M. Ozawa, M. Popović and M. Wyart, Phys. Rev. X, 2023, 11, 031034 Search PubMed.
- R. N. Chacko, F. P. Landes, G. Biroli, O. Dauchot, A. J. Liu and D. R. Reichman, Phys. Rev. Lett., 2021, 127, 048002 CrossRef CAS PubMed.
- Y. Li, Y. Yao and M. P. Ciamarra, Phys. Rev. Lett., 2022, 128, 258001 CrossRef CAS PubMed.
- H. Sillescu, J. Non-Cryst. Solids, 1999, 243, 81 CrossRef CAS.
- L. Berthier, G. Biroli, H.-P. Bouchaud, L. Cipelletti and W. van Saarloos, Dynamical Heterogeneities in Glasses, Colloids, and Granular Media, OUP Oxford, 2011, vol. 150 Search PubMed.
- W. Kob, C. Donati, S. J. Plimpton, P. H. Poole and S. C. Glotzer, Phys. Rev. Lett., 1997, 79, 2827 CrossRef CAS.
- D. N. Perera and P. Harrowell, Phys. Rev. Lett., 1998, 81, 120 CrossRef CAS.
- R. Yamamoto and A. Onuki, Phys. Rev. Lett., 1998, 81, 4915 CrossRef CAS.
- C. Donati, S. Franz, S. C. Glotzer and G. Parisi, J. Non-Cryst. Solids, 2002, 307, 215 CrossRef.
- E. Rössler, Phys. Rev. Lett., 1990, 65, 1595 CrossRef PubMed.
- F. H. Stillinger and J. A. Hodgdon, Phys. Rev. E: Stat. Phys., Plasmas, Fluids, Relat. Interdiscip. Top., 1994, 50, 2064 CrossRef CAS PubMed.
- M. T. Cicerone and M. D. Ediger, J. Chem. Phys., 1996, 104, 7210 CrossRef CAS.
- L. Berthier, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2004, 69, 020201 CrossRef PubMed.
- Y. Jung, J. P. Garrahan and D. Chandler, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2004, 69, 061205 CrossRef PubMed.
- S. K. Kumar, G. Szamel and J. F. Douglas, J. Chem. Phys., 2006, 124, 214501 CrossRef PubMed.
- S. R. Becker, P. H. Poole and F. W. Starr, Phys. Rev. Lett., 2006, 97, 055901 CrossRef PubMed.
- S.-H. Chong and W. Kob, Phys. Rev. Lett., 2009, 102, 025702 CrossRef PubMed.
- S. Sengupta, S. Karmakar, C. Dasgupta and S. Sastry, J. Chem. Phys., 2013, 138, 12A548 CrossRef PubMed.
- P. Charbonneau, Y. Jin, G. Parisi and F. Zamponi, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 15025 CrossRef CAS PubMed.
- A. D. Parmar, S. Sengupta and S. Sastry, Phys. Rev. Lett., 2017, 119, 056001 CrossRef PubMed.
- S. Sastry, Nature, 2001, 409, 164 CrossRef CAS PubMed.
- L. Wang and N. Xu, Phys. Rev. Lett., 2014, 112, 055701 CrossRef PubMed.
- G. Foffi, F. Sciortino, P. Tartaglia, E. Zaccarelli, F. L. Verso, L. Reatto and C. N. Likos, Phys. Rev. Lett., 2003, 90, 238301 CrossRef CAS PubMed.
- E. Zaccarelli, C. Mayer, A. Asteriadi, C. N. Likos, F. Sciortino, J. Roovers, H. Iatrou, N. Hadjichristidis, P. Tartaglia, H. Lowen and D. Vlassopoulos, Phys. Rev. Lett., 2005, 95, 268301 CrossRef CAS PubMed.
- C. Mayer, E. Zaccarelli, E. Stiakakis, C. N. Likos, F. Sciortino, A. Munam, M. Gauthier, N. Hadjichristidis, H. Iatrou, P. Tartaglia, H. Lowen and D. Vlassopoulos, Nat. Mater., 2008, 7, 780 CrossRef CAS PubMed.
- C. M. Likos, Phys. Rep., 2001, 348, 267 CrossRef CAS.
- L. Berthier, A. J. Moreno and G. Szamel, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2010, 82, 060501(R) CrossRef PubMed.
- L. Wang, Y. Duan and N. Xu, Soft Matter, 2012, 8, 11831 RSC.
- M. Schmiedeberg, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2013, 87, 052310 CrossRef PubMed.
- R. Miyazaki, T. Kawasaki and K. Miyazaki, Phys. Rev. Lett., 2016, 117, 165701 CrossRef PubMed.
- X. Sun, H. Zhang, L. Wang, Z. Zhang and Y. Ma, Chin. Phys. B, 2020, 129, 126201 CrossRef.
- R. Miyazaki, T. Kawasaki and K. Miyazaki, J. Chem. Phys., 2019, 150, 074503 CrossRef PubMed.
- Y. Nie, J. Liu, J. Guo and N. Xu, Nat. Commun., 2020, 11, 3198 CrossRef CAS PubMed.
- J. Guo, Y. Nie and N. Xu, Soft Matter, 2021, 17, 3397 RSC.
- M. P. Allen and D. J. Tildesley, Computer Simulation of Liquids, Oxford University Press, Oxford, 1987 Search PubMed.
- W. Kob and H. C. Andersen, Phys. Rev. Lett., 1994, 73, 1376 CrossRef CAS PubMed.
- N. Lačević, F. W. Starr, T. B. Schrøder and S. C. Glotzer, J. Chem. Phys., 2003, 119, 7372 CrossRef.
- D. Coslovich and C. M. Roland, J. Chem. Phys., 2009, 131, 151103 CrossRef CAS PubMed.
- C. Zhao, K. Tian and N. Xu, Phys. Rev. Lett., 2011, 106, 125503 CrossRef PubMed.
- N. Xu, T. K. Haxton, A. J. Liu and S. R. Nagel, Phys. Rev. Lett., 2009, 103, 245701 CrossRef PubMed.
- L. Wang, P. Guan and W. H. Wang, J. Chem. Phys., 2016, 145, 034505 CrossRef PubMed.
- L. Wang, N. Xu and P. Guan, Phys. Rev. Lett., 2018, 120, 125502 CrossRef CAS PubMed.
- J. Zhang, W. Zheng, S. Zhang, D. Xu, Z. Jiang and N. Xu, Sci. Adv., 2021, 7, eabg6766 CrossRef PubMed.
- M. Zu, J. Liu, H. Tong and N. Xu, Phys. Rev. Lett., 2016, 117, 085702 CrossRef PubMed.
- S. Prestipino, F. Saija and P. V. Giaquinta, Phys. Rev. Lett., 2011, 106, 235701 CrossRef PubMed.
- Y. D. Fomin, N. V. Gribova, V. N. Ryzhov, S. M. Stishov and D. Frenkel, J. Chem. Phys., 2008, 129, 064512 CrossRef PubMed.
- J. C. Pamies, A. Cacciuto and D. Frenkel, J. Chem. Phys., 2009, 131, 044514 CrossRef PubMed.
- D. E. Dudalov, Y. D. Fomin, E. N. Tsiok and V. N. Ryzhov, Soft Matter, 2014, 10, 4966 RSC.
- N. Xu, Chin. J. Polym. Sci., 2019, 37, 1065 CrossRef CAS.
- M. Zu, T. Peng and N. Xu, Nat. Commun., 2017, 8, 2089 CrossRef PubMed.
- W. L. Miller and A. Cacciuto, Soft Matter, 2011, 7, 7552 RSC.
- J. Russo, F. Romano and H. Tanaka, Phys. Rev. X, 2018, 8, 021040 CAS.
| This journal is © The Royal Society of Chemistry 2024 |