Open Access Article
Open Access ArticleInterfacial energy as an approach to designing amphipathic surfaces during photopolymerization curing†
Sabrina J.
Curley
 and
Caroline R.
Szczepanski
and
Caroline R.
Szczepanski
 *
*
Department of Chemical Engineering & Materials Science, Michigan State University, East Lansing, MI 48824, USA. E-mail: szcz@msu.edu
First published on 18th April 2024
Abstract
Photopolymerization induced phase separation (PIPS) is a platform capable of creating heterogeneous materials from initially miscible resin solutions, where both the reaction's governing thermodynamics and kinetics significantly influence the resulting phase composition and morphology. Here, PIPS is used to develop materials in a single photopolymerization step that are hydrophobic on one face and hydrophilic on the other. These two faces possess a water contact angle difference of 50°, bridged by a bulk-scale chemical gradient. The impact of the PIPS-triggering inert additive is investigated by increasing the loading of poly(methyl methacrylate) (PMMA) in an acrylonitrile/1,6-hexanediol diacrylate comonomer resin. The extent of phase separation in the sample network depends on this loading, with increasing PMMA corresponding to macroscale domains that are more chemically and mechanically distinct. A significant period between the onsets of phase separation and reaction deceleration, determined using in situ FT-IR, facilitates this enhanced phase segregation in PMMA-modified samples. Spatially directed domain formation can be further promoted using multiple interface types in the sample mold, here, glass and stainless steel. With multiple interface types, interfacial rearrangements to minimize surface energy during resin photopolymerization result in a hydrophobic face that is nitrile-rich and a hydrophilic face that is nitrile-poor (e.g., acrylate-rich). Using this strategy, patterned wettability on a single face can also be engineered. This study illustrates the capabilities of PIPS for complex surface design and in applications requiring stark differences in surface character without sharp interfaces.
1 Introduction
Heterogeneity within the bulk volume of a polymer introduces a complexity not attainable through solely homogeneous materials, expanding the horizon of what is possible in soft matter research. Heterogeneous polymer systems are leveraged to increase toughness by modifying stress distributions throughout a bulk material1–3 and also to finely control gloss, antifouling, and extreme wettabilities4–6 not attainable using homogeneous counterparts. There exist a range of synthesis methods to produce and manipulate heterogeneity in polymeric materials, including but not limited to chemical reaction driven phase separation7–11 and the self assembly of block copolymers.12–15One type of chemical-reaction-driven phase separation is photoolymerization induced phase separation (PIPS), where the reaction of initially miscible components (monomers, inert additives, etc.) causes a decrease in Gibbs free energy during polymerization, promoting phase separation. While the reduction in Gibbs free energy during polymerization is a thermodynamic driving force promoting phase separation, with PIPS, the kinetics of polymerization also has a significant role, impacting the ease at which molecules diffuse to form distinct phase domains. As one example, if the reaction kinetics correspond with a rapid onset of gelation and/or vitrification of the network, the mobility of immiscible domains will be hindered and phase separation can be suppressed.16 The interplay between kinetics and thermodynamics is complex, with the relationship between the two discussed below.
The thermodynamic driving force for phase separation is ultimately dictated by the change in Gibbs free energy of mixing, ΔGmix, which is in turn determined by the system's enthalpy, ΔHmix, entropy, ΔSmix, and absolute temperature, T (eqn (1)). If the free energy (ΔGmix) of a system is negative, the mixture will remain in a well-mixed, single-phase state. However, a positive ΔGmix indicates that spontaneous de-mixing of the components is thermodynamically favorable. Enthalpic-driven phase separation occurs from a lack of compatibility between the mixture components, resulting in conditions where there is a higher preference for aggregation of similar species as opposed to equal mixing of different species. Alternatively, entropic-driven phase separation occurs from changes in the system's available degrees of freedom. The number of possible distinct configurations Ω available to a system influences ΔGmix in that more configurations enables a larger entropy, related through the Boltzmann constant kB (eqn (2)).17 In a polymerizing system, the growth of polymer chains from initial monomers gradually reduces the degrees of freedom, decreasing S.18 ΔGmix > 0 can be linked to the combination of decreased miscibility between developing species and a reduction of available macromolecular conformational states.19
| ΔGmix = ΔHmix − TΔSmix | (1) |
S = kB![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(Ω) ln(Ω) | (2) |
However, as mentioned, thermodynamic equilibrium as dictated by ΔGmix is not the only determining factor on the final morphology of phase separated polymer networks; reaction kinetics also play a significant role.20,21 The evolving physical state of a sample undergoing PIPS can constrain or limit phase separation by impacting the mobility of reactive species. Most significantly, cross-linking events and the introduction of inert additives will influence mobility within the system.10 Both a solution's initial viscosity as a function of its resin chemistry and its change in viscosity as monomer converts into polymer result in a wide range of time dependent viscosity environments that are possible in a polymerizing system.22 Since the viscosity environment impacts diffusion of immiscible species within a system, it has a direct influence on the final microstructure formed from PIPS. Transient changes to the local viscosity environment can be probed via reaction kinetics, typically via characterization of overall rate of polymerization (Rp). This type of kinetic analysis can identify the onset of diffusion–limitations during a polymerization, i.e. the moment where the viscosity environment is such that diffusion is limited for all reactive species. As highlighted in previous works, resin compositions and reaction conditions (e.g., rate of initiation) can be adjusted to tailor the final polymer morphology attained via phase separation.2,4,16 For instance, a system with a low initial resin viscosity will take longer to reach this upper limit viscosity threshold during polymerization, and thus can be employed to favor extensive phase diffusion.23 Understanding the impact of the available kinetic parameters can ultimately be a powerful tool, as manipulation of reaction kinetics is one strategy to tailor PIPS to yield diverse morphologies and phase structures, as phase separation can be halted or frozen in a non-equilibrium state.
This phase separation can come about by either spinodal decomposition or nucleation and growth, both of which are influenced by the thermodynamic and kinetic environment of the developing polymer. Both mechanisms can result in a multi-domain morphology but their associated thermodynamic stability and deviation from equilibrium differ from one another.24,25 On one hand, the nucleation and growth mechanism follows a meta-stable increase in domain size, while spinodal decomposition is inherently unstable and forms distinct domains from local fluctuations. When considering crosslinking polymerizations, domain development competes with the actively reacting network, as discussed in the kinetics section. Therefore, the phase separation mechanism can be manipulated via reaction kinetics, with slower rates allowing for a more equilibrium nucleation and growth approach while fast rates prevent thermodynamic equilibrium from being reached and instead favors spinodal decomposition.16 Manipulating the mechanism of phase separation presents an approach to tailor morphology, as nucleation and growth typically results in isolated domains within a matrix whereas spinodal decomposition is associated with more co-continuous morphologies.16,24
While controlling heterogeneity via phase separation involves complex interactions between thermodynamics and kinetics, the applications of these phenomena provide an avenue to create and tailor exemplary compositional gradients in materials. Such gradients can be leveraged for enhanced mechanical properties. A number of organisms in nature already make use of such gradients in their bodily systems across a range of length scales, including mussel filaments (centimeter), squid beaks (millimeter), and human teeth (micrometer).26–30 These gradients in a given mechanical property, such as modulus or toughness, can be facilitated by a gradient in chemical concentration.28,31 Having a chemical concentration change gradually over a distance as opposed to a sharp interface between the two endpoint concentrations has the benefits of reducing stresses that can lead to fracture as well as increasing overall toughness by preventing crack propagation.26,32 Despite the advantages associated with chemical gradients and heterogeneity in natural systems, there still exist challenges in creating similar gradients on the order of hundreds of microns in engineered materials. The majority of research in obtaining chemical gradients via phase separation focus largely on nanometer to micrometer thick samples.33,34 This limitation in length scale minimizes the benefit of such compositional gradients in terms of stress dissipation. Prior works have demonstrated that PIPS can be leveraged to engineer chemical gradients in phase separating resins by providing the relevant controls over the reaction, but such gradients have yet to be demonstrated on larger length scales.
This study demonstrates how PIPS can be employed to create macroscale chemical gradients in photopolymerized materials, ultimately creating surfaces with contrasting wettabilities. The rapidly formed acrylate networks with inert poly(methyl methacrylate) additions examined here develop chemical gradients without lengthy processing procedures. By varying the chemical component ratios and the UV curing conditions, the resins undergo macro-scale phase separation yielding chemical gradients spanning several hundred micrometers. Furthermore, spatially-directed bulk phase separation is achieved by tailoring the interfacial energies between the polymer resin and the sample mold configuration. This results in a final material where the location of phase domains is guided by the interfacial energies present between the developing polymer and the sample mold materials. Specifically, two different interfacial environments employed here (glass and stainless steel) impart hydrophobic character to one side of the formed polymer while the other possesses hydrophilic character. This work demonstrates the application of knowledge on thermodynamic compatibility between chemical species and the impact of reaction kinetics in developing phase separated polymer materials with macroscale gradients and dissimilar chemical domains. We develop spatially-resolved domains over large length-scales and generate stark differences in surface character from initially miscible resins in a single step procedure, opening avenues for streamlining complex surface manufacture.
2 Experimental
2.1 Materials
Co-monomer formulations consisted of acrylonitrile (AN, Sigma Aldrich) and 1,6-hexanediol diacrylate (HDDA, Sigma Aldrich), with HDDA acting as a cross-linker (Fig. 1). In certain resin formulations, an inert polymer additive, poly(methyl methacrylate) (PMMA; Mw = ∼120![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1, Sigma Aldrich) was included to promote phase separation during polymerization. 2,2-Dimethoxy-2-phenylacetophenone (DMPA, Sigma Aldrich) was employed in all formulations as photoinitiator (0.5 wt%). All chemicals were used as received and without further purification.
000 g mol−1, Sigma Aldrich) was included to promote phase separation during polymerization. 2,2-Dimethoxy-2-phenylacetophenone (DMPA, Sigma Aldrich) was employed in all formulations as photoinitiator (0.5 wt%). All chemicals were used as received and without further purification.
2.2 Resin preparation
Resin formulations in this study contained varying weight fractions (wt%) of components AN, HDDA, and PMMA, as described in Table 1. In all resins, DMPA loading was held at 0.5 wt% relative to the combined weight of the other species. To further increase the free energy in the system and promote phase separation, PMMA was incorporated in increasing amounts up to 20 wt%. For ease of reference throughout the following text, sample names are listed as AaXbPc, where A corresponds to acrylonitrile, X corresponds to the cross-linker 1,6-hexanediol diacrylate, and P corresponds to PMMA. The subscript values indicate the weight fractions present, where a and b are the wt% values of AN and HDDA relative to one another within the comonomer resin, respectively, and c is the wt% of PMMA relative to the whole resin sans DMPA.| AN (wt%) | HDDA (wt%) | PMMA (wt%) | DMPA (wt%) | |
|---|---|---|---|---|
| A70X30P0 | 69.65 | 29.85 | — | 0.50 |
| A70X30P5 | 66.17 | 28.86 | 4.98 | 0.50 |
| A70X30P10 | 62.69 | 26.86 | 9.95 | 0.50 |
| A70X30P20 | 55.72 | 23.88 | 19.90 | 0.50 |
To prepare resins, DMPA was first massed in a glass vial, and if necessary, PMMA was subsequently added to the same vial. These solids were then allowed to completely dissolve in the appropriate mass of AN and HDDA following the resin formulation. Haziness in the solution was not observed for any of the samples in the monomer state, indicating good mixing and macroscopic miscibility. After magnetic agitation for 30 minutes at ambient temperature, resins were degassed via N2 bubbling for 10 minutes prior to photopolymerization to eliminate any inhibition effects as a result of dissolved oxygen.
2.3 Viscosity measurement
The viscosity of the 4 A70X30Pc resins at ambient conditions was measured using a sine-wave vibration viscometer SV-10A (A&D Company Ltd, Tokyo, Japan). For each resin formulation, 15 mL was added to the well for measurement, with each formulation tested three times.2.4 Bulk photopolymerization
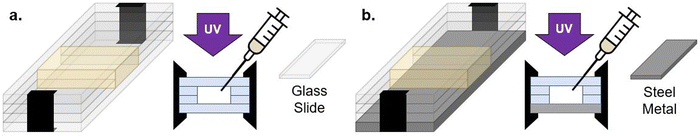
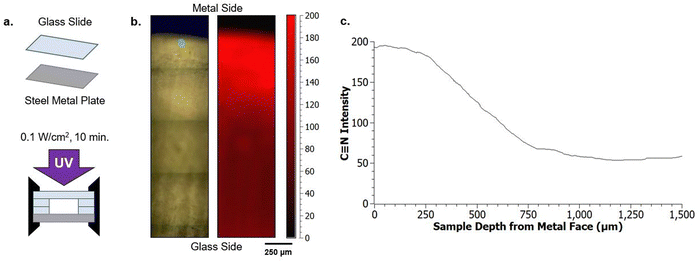
To investigate multiple length scales of phase separation behavior using mechanical testing and surface characterization techniques, rectangular samples were made. For these bulk sample photopolymerizations, 1 mL of the desired resin formulation was injected into a rectangular mold consisting of glass slides (two glass substrates with glass spacers), creating samples with approximate dimensions of 10 mm × 25 mm × 2 mm (length × width × thickness) (Fig. 2a). The glass mold was clamped together on both ends to prevent delamination. Samples were then immediately cured using a UV lamp (UVitron, Intelliry 600) with the 365 nm intensity (I0) value at the sample interface recorded using a handheld radiometer (Excelitas, OmiCure R2000 with Cure Site Detector attachment). Exposure time was 10 minutes with I0 = 0.1 W cm−2.To investigate directionally-guided macro phase separation, the molds used for bulk photopolymerization were modified to introduce a difference in interfacial energies at the mold interfaces. In these cases, bulk samples were made using a procedure similar to that described above, however one of the glass substrates was replaced with a stainless steel plate (Fig. 2b). Samples were oriented so that the metal substrate was on the bottom to allow UV light to pass through the upper glass substrate and interact with the resin to facilitate curing.
2.5 Dynamic mechanical analysis (DMA)
Thermo-mechanical properties of polymerized specimens without PMMA were assessed using a dynamic mechanical analyzer (DMA, TA Instruments, Discovery DMA850). Samples were measured in a three-point-bending configuration, with 15 mm between the outer points. An initial preload force of 0.01 N was selected, with a 20.0 μm amplitude and 1.0 Hz frequency selected for the duration of the run. A temperature ramp of 5.0 °C min−1 was employed to scan over a range of 20 °C to 200 °C. To ensure that residual, unreacted monomer in the photocured samples did not influence the collected data, all specimens were annealed for 1 hour at 150 °C prior to analysis. The annealing temperatures was chosen based on the reported Tg of poly(acrylonitrile) in the range of 75 °C to 105 °C.35,362.6 Raman spectroscopy
To better characterize chemical gradients, cross sections of polymerized samples were imaged using Raman spectroscopy (Horiba Jobin Yvon LabRAM HR). 2D chemical mapping of the cross sections allowed for confirmation of optically-observed phase separation. After an initial Si calibration, scans at 10× magnification were carried out over a 0.5 mm × 2.0 mm mapping area. A 785 nm laser was used with a 750 nm grating to excite the sample. Spectra were collected along a 25 × 100 point grid, collecting a total of 2500 data points. 2D maps were generated based off of the nitrile C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N functional group peak at 2250 cm−1, present only where polyacrylonitrile has formed in the sample. Raman mapping of the sample cross section was performed by stitching together multiple screening areas to compile the whole observed area.
N functional group peak at 2250 cm−1, present only where polyacrylonitrile has formed in the sample. Raman mapping of the sample cross section was performed by stitching together multiple screening areas to compile the whole observed area.
2.7 Small angle X-ray scattering (SAXS)
To check for nanoscale phase separation, 5 mm diameter, 0.5 mm thick sections were taken from bulk rectangle samples after curing to collect small angle scattering data (12-ID-B beamline, Advanced Photon Source, Argonne National Lab). The incident X-ray beam used was set at 13.3 keV (λ = 0.935 angstrom). The sample/detector distance was 2.1 m for all measurements, corresponding to a range of scattering wave vector (q) of 0.003–0.9 Å−1. A two-dimensional (2D) Pilatus 2 M detector was used. The scattering angles were calibrated with silver behenate and the absolute scattering intensity was determined from a glassy carbon standard. Polymer samples were secured to a solid sample plate using clear tape. SAXS measurements were taken of the clear tape by itself to provide a spectrum for background subtraction to yield the polymer material spectra. The corresponding d-spacing was calculated by taking the inverse of q and multiplying by a factor of 2π.2.8 Real time Fourier-transform infrared spectroscopy (FT-IR)
Real time Fourier-transform infrared spectroscopy (FTIR, ThermoNicolet, Nicolet iS50) was employed to monitor real-time photopolymerization kinetics of select formulations. For in situ photopolymerizations, the desired formulation was injected into a rectangular mold comprised of glass slides with a spacer of 0.1 mm thickness. The molds were designed to limit atmosphere exposure during testing; the glass slides were clamped together to prevent delamination. Samples were placed in the sample chamber with a UV light source (EFOS Ultracure 100ss Plus, λ ∼ 320–500 nm) equipped with a flexible light guide directed to irradiate the sample area (I0 = 0.01 W cm−2 measured at sample surface), to enable simultaneous UV exposure and collection of FTIR spectra. All samples were angled to ensure that the incoming UV light was perpendicular to the specimen while maintaining transmittance of the IR beam. This was done to mitigate the cosine error associated with light intensity experienced by non-perpendicular surfaces, where the intensity experienced at the surface decays as a Lambertian function with an increased change in the incident angle.37 The FTIR sample chamber was kept dark throughout the experiment by folding thick absorbent pads over the opening. Real-time conversion curves were generated by monitoring the integral area between 6100 cm−1 and 6240 cm−1, corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vinyl peak at 6165 cm−1.
C vinyl peak at 6165 cm−1.
2.9 Contact angle measurements
The wettability of sample surfaces was characterized via water contact angles (θ) measured using a goniometer (Kruss, Drop Shape Analyzer – DSA30). Prior to measurement, each side of the sample was washed with water for cleaning and gently wiped with a Kimwipe to dry. Contact angle values were calculated from a 2.0 μL sessile drop mechanically deposited by syringe. 5 images were collected per drop, and 5 drops were measured across the sample surface to obtain an average value. Contact angles were measured using an ellipse-tangent fit between the drop and surface. Surface energy values (γ) were calculated using the OWRK model pairing the water contact angle with a toluene contact angle to calculate polar and disperse contributions.38Surface free energy of initial comonomers were characterized using the pendant drop method, where 6.0 μL of monomer liquid was suspended from the deposition needle and calculated from the drop's shape factor.
3 Results and discussion
Phase separation in the 70 wt% acrylonitrile 30 wt% 1,6-hexanediol diacrylate system with increasing poly(methyl methacrylate) additions (denoted as A70X30Pc) was robustly investigated using optical (macro-imaging), thermomechanical (DMA), surface (contact angle), and spectroscopy (Raman, SAXS) characterization techniques. Combining the performed analyses enabled assembly of a full picture of PIPS. This determined how domain formation and its manipulation can be utilized to establish stark chemical gradients over large length scales using rapid photopolymerizations.3.1 PIPS system domain structures as a function of PMMA loading
To ensure that the PIPS observed in the platform studied here is due to the increase in entropy via addition of the inert prepolymer (PMMA), it was necessary to first ensure that the base copolymer matrix (AN:HDDA) formed a single-phase network. With this design, a homogeneous control (AN:HDDA network) is easily defined and the proclivity for phase separation is tailored via PMMA addition. It should be noted that crosslinked networks formed via free radical polymerization have an intrinsic degree of heterogeneity in their structure.39,40 The heterogeneity arises from the mechanism of nanosized gel formation, clusters of which form quickly after radical initiation. These local variations in network structure are different than discrete domains formed via phase separation observed in PIPS systems, and typically differ in length scales (nano vs. micro scale domains).Ultimately, the A70X30Pc comonomer system was chosen as the base formulation as it does not undergo phase separation during polymerization (e.g., A70X30P0 forms a random copolymer network). This selection of comonomer system was determined by investigating the photopolymerization behavior of a series of AN:HDDA copolymers (e.g., AxXyP0) consisting of 5 wt% to 100 wt% HDDA. The visual opacity of each copolymer network was used as an initial indicator for microscale phase separation2,41 (Fig. S1, ESI†). From this analysis, it was clear that an HDDA loading of 30 wt% (e.g., A70X30P0) presented the smallest fraction of crosslinker that did not have associated phase separation of the copolymer network.
Specifically, A70X30P0 samples were fully transparent after curing at I0 = 0.1 W cm−2, indicating a lack of phase separation at a domain size larger than the wavelength of the interacting visible light2,41 (Fig. 3ai). Furthermore, thermomechanical characterization via DMA testing revealed a single, symmetric peak in the tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ profile, indicative of a well-mixed homopolymer network with a single glass transition temperature (Tg = 110 °C, Fig. S2, ESI†). This Tg confirms that the base copolymer network is glassy at ambient conditions. Consistent with prior works highlighting nanoscale heterogeneities in networks formed via free-radical polymerization,39,40 some domains between 70 and 100 nm in size are observed in the A70X30Pc system via small-angle X-ray scattering (SAXS) Fig. S3 (ESI†). These local fluctuations in sample density are in line with previous descriptions of this behavior.42
δ profile, indicative of a well-mixed homopolymer network with a single glass transition temperature (Tg = 110 °C, Fig. S2, ESI†). This Tg confirms that the base copolymer network is glassy at ambient conditions. Consistent with prior works highlighting nanoscale heterogeneities in networks formed via free-radical polymerization,39,40 some domains between 70 and 100 nm in size are observed in the A70X30Pc system via small-angle X-ray scattering (SAXS) Fig. S3 (ESI†). These local fluctuations in sample density are in line with previous descriptions of this behavior.42
As demonstrated in prior works,22,43 the addition of an inert additive to a polymerizing resin can increase the viscosity environment, thus limiting reaction kinetics, while also modifying free energy conditions to promote phase separation. Implementing a similar design, in this study PMMA was employed as an inert additive based on its miscibility with the comonomers investigated (AN and HDDA), ensuring a stable and homogeneous precursor resin that does not phase separate prior to polymerization. PMMA loadings of 0 wt%, 5 wt%, 10 wt%, and 20 wt% were explored (i.e., A70X30P0, A70X30P5, A70X30P10, and A70X30P20). The influence of PMMA on the viscosity of the precursor resins was characterized to better understand the starting conditions for each formulation (Table 2). Unsurprisingly, viscosity increases with increased PMMA loading, resulting in a viscosity range from 0.66 mPa s for the comonomer control (A70X30P0) to 45.1 mPa s at maximum PMMA loading (A70X30P20).
| Viscosity (mPa s) | |
|---|---|
| A70X30P0 | 0.66 ± 0.03 |
| A70X30P5 | 1.61 ± 0.03 |
| A70X30P10 | 5.64 ± 0.06 |
| A70X30P20 | 45.1 ± 0.3 |
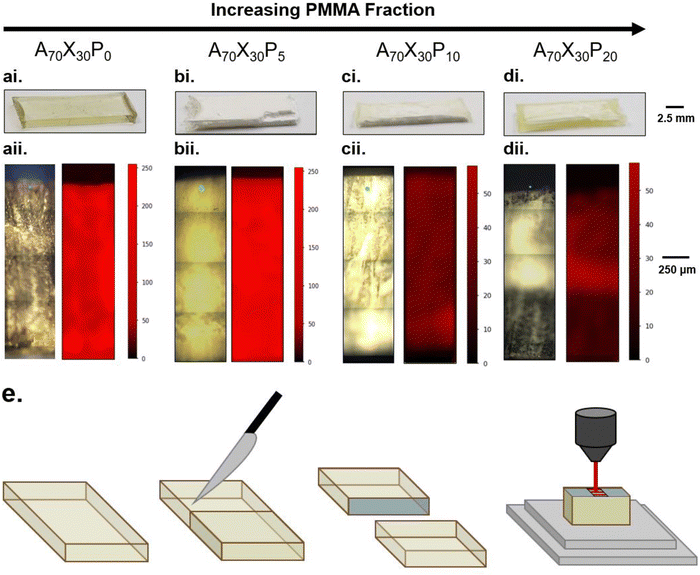
The integration of PMMA also impacts the compositional gradients that arise after photopolymerization, as depicted in Fig. 3. All resins were polymerized with standardized UV irradiation of 0.1 W cm−2 for 10 minutes. The compositional gradients that arise in PMMA-modified formulations, despite the initial precursor formulations being macroscopically homogeneous, confirms that PIPS occurs in this system. After photopolymerization, the control A70X30P0 sample is macroscopically homogeneous with yellow-tinted transparency (Fig. 3ai). Initial addition of PMMA (5 wt%, e.g. A70X30P5) changes the sample appearance dramatically. A70X30P5 is defined by a chalky, opaque white phase that is uniform throughout the macroscopic volume. Increasing the PMMA loading to 10 wt% (A70X30P10) results in a marbling appearance (Fig. 3cii). At this loading level, the sample is no longer uniform in appearance, indicating that distinct macroscale domains have formed, however, their spatial distribution is irregular. The transparent, yellow-tinted domains appear like those observed in the control comonomer sample and the opaque white domains appear like those observed in A70X30P5. The maximum PMMA loading studied here, (e.g., 20 wt%, A70X30P20) resulted in full phase separation, consisting of an exterior, yellow-tinted transparent region and an interior opaque white region (Fig. 3dii). The structure after polymerization of A70X30P20 is of particular interest, as stark phase domains on the macroscale have yet to be reported in rapidly-forming, glassy phase-separated materials.
Analyzing the cross-section of the samples in Fig. 3 revealed that the opaque domains (observed in A70X30P5, A70X30P10 and A70X30P20) had a rough, powdery texture similar to chalk. Meanwhile, the translucent domains (observed in A70X30P0, A70X30P10 and A70X30P20) were glassy, hard, and smooth with no discernible texture. This noted rigidity in the translucent domains and lack of such in the opaque domains indicate a difference in the relative degree of crosslinking present in those domains of the sample. Additionally, based on light scattering interactions, the opacity of the powdery region indicates a degree of microscale phase separation is likely occurring within these domains. These observations signify that two scales of phase separation occurring within a single sample: macro- and microscale.
Raman spectroscopy confirms the suspected compositional gradients made from optical observation of the A70X30Pc samples with varied PMMA loadings, particularly on the macroscale. 2D maps of the nitrile content were constructed from the exposed cross section of each sample (Fig. 3e), enabling classification of macroscopic domains as nitrile-rich or nitrile-poor. The nitrile functionality was chosen for mapping since it is a functional group unique to the acrylonitrile (AN) monomer within the resin system. In the Raman maps, regions with bright red indicate domains rich in poly(acrylonitrile) and thus dark regions correspond to domains with relatively lower local concentrations. A70X30P0 and A70X30P5 samples exhibit uniform concentrations of the nitrile group distributed across the entire sample volume (Fig. 3aii and bii). It is of note that though there are similar concentrations of C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N throughout these two samples, they have very different optical appearances, transitioning from completely clear to opaque with the initial addition of PMMA, which may indicate microscale variations within the sample volume. With higher PMMA loadings in A70X30P10 and A70X30P20, more distinct chemical segregation is apparent from the Raman maps. The dramatic interior/exterior bulk phase separation noted in A70X30P20 is verified to consist of two bulk domains with varying nitrile content (Fig. 3dii). The interior has a higher local concentration of nitrile groups and it is thus assumed that the acrylate species preferentially migrate to the glass mold interface. Additional experiments discussed in the next section leverage this observed chemical segregation to explicitly direct where domain formation occurs by changing the mold configuration used during curing.
N throughout these two samples, they have very different optical appearances, transitioning from completely clear to opaque with the initial addition of PMMA, which may indicate microscale variations within the sample volume. With higher PMMA loadings in A70X30P10 and A70X30P20, more distinct chemical segregation is apparent from the Raman maps. The dramatic interior/exterior bulk phase separation noted in A70X30P20 is verified to consist of two bulk domains with varying nitrile content (Fig. 3dii). The interior has a higher local concentration of nitrile groups and it is thus assumed that the acrylate species preferentially migrate to the glass mold interface. Additional experiments discussed in the next section leverage this observed chemical segregation to explicitly direct where domain formation occurs by changing the mold configuration used during curing.
Chemical segregation on a macroscopic length scale, as observed in Fig. 3d for the A70X30Pc system, is of particular note since prior studies have emphasized phase separation-driven polymer morphologies in submicron systems, primarily thin films with thicknesses less than 0.1 mm.16,33,34,44 Within these submicron examples, the difference in interfacial energies during fabrication, such as glass vs. exposed atmosphere, allows for a steep chemical gradient due to the extremely small distance between the two employed interfaces. For example, Vitale et al.33 demonstrated stark contrast in wettability on differing faces of photopolymerized films (10–200 μm) due to the segregation of siloxane oligomers throughout the film thickness. The results in Fig. 3 highlight how similar guiding principles, e.g. variations in surface energy, can be leveraged in larger samples, with thicknesses on the order of 102 μm. Specifically, the two prominent domains observed in A70X30P20, nitrile-rich interior and nitrile-poor exterior, are visible to the eye without the need of microscopy. Leveraging PIPS allows this patterning to span a sample volume of multi-millimeter thicknesses, which has yet to be highlighted.
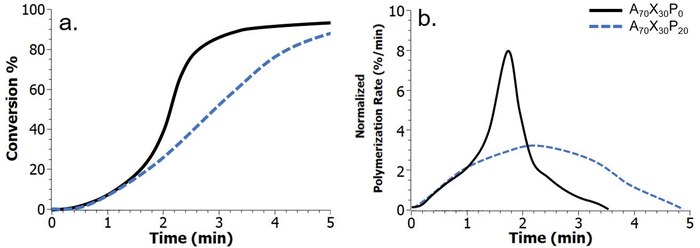
The observed macroscale phase segregation, particularly at higher PMMA loadings (e.g., A70X30P20) is significantly influenced by the kinetic environment. Specifically, macroscale phase separation is enabled by a decreased polymerization rate and delay in autodeceleration, predominantly due to the addition of the inert PMMA additive, which can be tracked via FTIR spectroscopy (Fig. 4). Real-time kinetic data including extent of double bond conversion and overall polymerization rate (Rp), which is taken as the first derivative of the conversion data, were characterized for both the control comonomer matrix – A70X30P0, and the A70X30P20 resin with macroscale phase separation. In this analysis, Rp was normalized by initial double bond concentration to account for the reduction in overall concentration of double bonds within the polymerizing system due to the introduction of inert PMMA. In the control comonomer resin A70X30P0, an initial sharp increase in double bond conversion, and thus an associated increase in Rp is observed throughout the first 1.5 minutes of polymerization. After this period, Rp decreases for the remainder of the reaction and the double-bond conversion eventually plateaus at approximately 90% (solid black series, Fig. 4b). This is consistent with autoacceleration behavior of free-radical photopolymerizations. Analysis of PMMA-modified resins (e.g., A70X30P20, dashed blue series Fig. 4) reveals a similar period of increasing Rp during the first minute of UV exposure. However, after 1 min the Rp behavior of A70X30P0 and A70X30P20 deviates. Ultimately, A70X30P20 has a longer period of autoacceleration (e.g., increasing Rp); an overall decrease in polymerization rate is not observed until much later (roughly 2.2 min) (Fig. 4b). This onset of autodeceleration, e.g. the period during the reaction where Rp steadily decreases, provides critical insight on diffusivity within the reactive medium. At the onset of autodeceleration, diffusion of all reactive species is significantly hindered, which manifests as a steady decline in observed reaction events.
Taking the maximum in Rp as the onset of deceleration,45,46 we estimate this transition occurs at 1.7 and 2.2 minutes after initial UV exposure for the A70X30P0 and A70X30P20 resins, respectively. This delay in onset of viscous and diffusive limitations within the reactive medium supports the stark phase segregation observed in Fig. 3d; there is ample time for phase separating domains to diffuse within the bulk system to minimize free energy prior to becoming kinetically trapped. It is important to note that the free-radical photopolymerizations employed here are exothermic in nature, impacting the morphology by both decreasing viscosity, which improves diffusion of components, and increasing mixing through the inverse relationship of temperature with the Flory–Huggins mixing parameter. The thermal evolution for select resins was characterized during curing and confirms the exothermic nature of the polymerizations (Fig. S4, ESI†). However, the results also highlight that a larger exotherm evolves in the control resin, A70X30P0, which experiences a larger maximum temperature (Tmax) during polymerization initiated by incident irradiation of 0.1 W cm−2. If phase domain formation was merely dictated by changes in the viscosity and the associated impact on diffusivity of immiscible domains in a polymerizing resin, one would expect a higher exotherm in samples with distinct, macroscale phase domains (e.g. PMMA-modified samples). However, since a larger exotherm arises in the non-phase separated control – A70X30P0, it is clear that other factors, including the evolution of Rp plays a significant role. It is difficult to fully resolve the two competing factors of viscosity and thermodynamic mixing (Flory–Huggins parameter) when considering the influence of temperature generated by the reaction, especially given that the Flory–Huggins parameter is also constantly changing due to propagation and termination events that impact molecular weight of resin constituents.
It is expected that other phase separating resins, i.e. A70X30P5 and A70X30P10, also have a delayed onset of autodeceleration. However, this was not probed directly as the phase separation in A70X30P5 and A70X30P10 resins resulted in samples that become IR opaque, thus giving only partial in situ FT-IR conversion curves (Fig. S5, ESI†). Cessation of IR transmission indicates the formation of domains capable of scattering IR irradiation. For both A70X30P5 and A70X30P10 samples, the IR signal from the measured 1620 nm wavelength (corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C vinyl peak at 6165 cm−1) stops approximately 1.4 minutes after initial UV light exposure, indicating that phase separation is ongoing at least by 15% conversion (Fig. S5a, ESI†) This is important, as it indicates that phase segregation on the microscale is ongoing prior to any observable onset of autodeceleration (e.g., decrease in Rp). An important distinction here is that phase domains of size below this threshold likely form at earlier timepoints in the reaction, but are not fully detected with this specific wavelength of irradiation. Even though complete, dynamic in situ FTIR measurements were not possible for A70X30P5 and A70X30P10, the extent of conversion for these two systems can still be estimated via gravimetric analysis. In brief, cured samples were placed in toluene for 2 days and dried, and the associated change in mass was recorded (Table S1, ESI†), yielding 75% and 71% conversion, respectively. This was also performed for the A70X30P0 and A70X30P20 resins, with final conversions matching those recorded from FT-IR (Fig. 4). The non-monotonic nature of these extents of conversion is related to the viscosities of the A70X30Pc resins. Formulations with higher initial viscosities enhance autoacceleration and thus enable higher extent of conversion, which is observed with A70X30P20 (Table 2).
C vinyl peak at 6165 cm−1) stops approximately 1.4 minutes after initial UV light exposure, indicating that phase separation is ongoing at least by 15% conversion (Fig. S5a, ESI†) This is important, as it indicates that phase segregation on the microscale is ongoing prior to any observable onset of autodeceleration (e.g., decrease in Rp). An important distinction here is that phase domains of size below this threshold likely form at earlier timepoints in the reaction, but are not fully detected with this specific wavelength of irradiation. Even though complete, dynamic in situ FTIR measurements were not possible for A70X30P5 and A70X30P10, the extent of conversion for these two systems can still be estimated via gravimetric analysis. In brief, cured samples were placed in toluene for 2 days and dried, and the associated change in mass was recorded (Table S1, ESI†), yielding 75% and 71% conversion, respectively. This was also performed for the A70X30P0 and A70X30P20 resins, with final conversions matching those recorded from FT-IR (Fig. 4). The non-monotonic nature of these extents of conversion is related to the viscosities of the A70X30Pc resins. Formulations with higher initial viscosities enhance autoacceleration and thus enable higher extent of conversion, which is observed with A70X30P20 (Table 2).
To address the impact of this developing opacity on the photopolymerization, and it's associated impact on the resulting phase domains, PIPS resins were optically monitored throughout curing with subsequent video analysis in ImageJ to relate opacity and polymerization rate (Fig. S6, ESI†). For samples A70X30P0 and A70X30P20, it has already been established that Rp is roughly the same at the beginning of the polymerization and these two curves do not deviate until after 1.1 minutes of UV exposure (Fig. 4b). However, characterization of the optical evolution during polymerization reveals that the onset of optical opacity for A70X30P20 occurs between 0.28 and 0.35 minutes (a similar onset in opacity is observed for A70X30P5 and A70X30P10, see Fig. S6, ESI†). Since Rp evolution does not deviate between the control comonomer system (A70X30P0) and samples undergoing PIPS (such as A70X30P20) during this initial period of increasing opacity (first 1.5 mins of polymerization), we conclude that the opacity is not a significant hindrance on the photopolymerization of these resins. Furthermore, given the rapid onset of opacity (e.g. slope of the absorbance data at the onset of opacity), we suspect that phase separation arises from an unstable state and follows the spinodal decomposition mechanism. This data does not point to a steady and consistent growth of scattering domains that would be expected from a nucleation and growth process.
To determine the influence of PMMA addition on the free energy state of each of the PIPS resins, theoretical estimates of each systems’ ΔGmix was performed (Table S2 and Fig. S7, ESI†). This was achieved by calculating the derivative of ΔGmix from (eqn (1)) at multiple points throughout the polymerization using estimations provided by the energy of cohesion for the developing molecules, with further details found in the Supplemental.47–50 The difference in 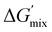 between the A70X30Pc resins is minimal when tracking the changing favorability for phase separation, particularly at high degrees of polymerization. Given that molecular weight builds rapidly in free-radical network polymerizations, the observed similarities in
between the A70X30Pc resins is minimal when tracking the changing favorability for phase separation, particularly at high degrees of polymerization. Given that molecular weight builds rapidly in free-radical network polymerizations, the observed similarities in 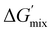 further emphasizes that the kinetic environment is a determining factor in the phase morphologies observed here.
further emphasizes that the kinetic environment is a determining factor in the phase morphologies observed here.
3.2 Guiding domain formation though use of glass and steel molds
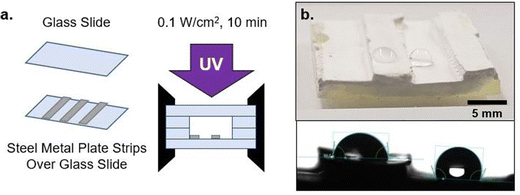
The phase separation behavior probed thus far uses glass as the surrounding mold, which results in nitrile-rich domains migrating towards the center of the sample. This migration, which reduces the contact between nitrile-domains and glass, becomes more significant with increasing PMMA loading (wt%) due to the reduced polymerization rate (Fig. 4b). To further explore the phase domain length scales possible from interface-driven phase segregation, curing molds were delibrately modified to have significant variations in surface energy within the mold geometry. Specifically, one substrate was modified to be a steel metal plate (Fig. 5a). This produced two types of interfaces: glass against resin and metal against resin.With this modification, directional segregation during the photopolymerization process driven by the surface energy imbalance from the two different mold materials was leveraged to design particular compositional gradients. With two different interfaces present, one resin/glass and the other resin/metal, the final morphology adopted by a resin undergoing PIPS minimizes the interfacial free energy at both faces (Fig. 5b and c). This is demonstrated by the polymerization of A70X30P5 in this configuration, where the separate mold materials further promote phase segregation and drive macroscale phase separation. This is in stark contrast to that observed during polymerization of A70X30P5 within a uniform curing mold (Fig. 3b). In the new configuration, the nitrile-rich domains are preferentially located near the metal interface (Fig. 5b), compared to being positioned in the center of the sample when only glass was used in the molds. This introduction of multiple interface types in the mold configuration serves as an additional driving force to promote phase separation.
This long-range phase segregation, which was not possible for the A70X30P5 sample when cured in a uniform glass mold, is achieved through deliberate selection of the mold materials. Specifically, incorporating a contacting mold surface with a characteristic surface energy can direct segregation of phase domains to minimize overall free energy.51,52 The surface energies (γ) of the two substrates (glass and steel) are sufficiently distinct, and they can be quantiatively determined using the OWRK model from water and toluene contact angles (θ) (eqn (3)).38 With this approach, a linear regression is used to calculate the polar (γp) and dispersive (γd) parts of the total substrate surface energy using two reference liquids (water and toluene) with known polar (γpL) and dispersive (γdL) components. Using this method, untreated glass had a γ of 72.4 mJ m−2, and the stainless steel a γ of 36.0 mJ m−2 (Table 3). These γ values are consistent with previous reports (Fig. S8, ESI†).53–58 The ratio between polar and dispersive components for the stainless steel and glass substrates are rather similar, with the greatest difference between these two substrates being the total net surface energy value. As such, the significant difference in total net surface energy suffices in driving phase segregation, not the specifics of polar vs dispersive character.
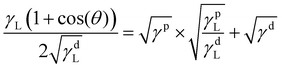 | (3) |
| Substrate | θ w (°) | θ t (°) | γ (mJ m−2) | γ p (mJ m−2) | γ d (mJ m−2) |
|---|---|---|---|---|---|
| Plain glass | 9.8 ± 0.5 | 9.8 ± 1.4 | 72.4 ± 0.6 | 44.3 ± 0.4 | 28.1 ± 0.2 |
| Stainless steel | 68.0 ± 5.9 | 9.8 ± 4.3 | 36.0 ± 6.7 | 17.8 ± 5.1 | 18.2 ± 1.6 |
Given the surface energy calculations (Table 3), the compositional gradients observed in the A70X30P5 sample (Fig. 5) are a result of minimizing system energy through selective migration of lower energy moieties to lower energy interfaces and likewise for higher energy moieties to higher energy interfaces. This phenomenon has previously been explored in both thin films from polymer melts and thin films from ongoing polymerization reactions.59–61 Directing phase separation in each of these scenarios is accomplished via deliberate selection of polymer components and substrates used for forming the thin film. By using different monolayer coatings of a substrate, researchers Genzer and Kramer could switch which of two polymer phases migrated to a substrate face depending on which polymer/substrate monolayer combination minimized interfacial free energy.59 These cited examples use the interplay between minimizing interfacial free energy and mixture phase separation to predict a polymer system's final morphology.
Here, nitrile groups migrating to the steel substrate while acrylate groups migrate to the glass substrate can be explained by the monomer surface energies relative to the two available mold interfaces. Pendant drop analysis of the individual monomers recorded γ of 9.5 mJ m−2 for AN and γ of 26.4 mJ m−2 for HDDA (Table 4). The lower energy AN monomer selectively diffuses to wet the lower energy metal substrate while the higher energy HDDA selectively diffuses to wet the higher energy glass substrate, forming a depthwise gradient in nitrile composition. The established chemical gradient also results in a mechanical gradient between the two sample faces. From characterization of the phases in Fig. 3, the nitrile-rich domains have a lower local modulus than the acrylate-rich domain. The acrylate crosslinker forms a more rigid network at the glass substrate interface compared to the monofunctional nitrile monomer. Compared to the results highlighted in Fig. 3, here a stark compositional gradient is achieved with a reduced inert additive loading (A70X30P5) due to the influence of the differing surface energy environments imparted by the mold materials (steel and glass). This is important, as it broadens the range of resin compositions over which macroscale, stark gradients can be achieved. Furthermore, the gradual chemical gradient accompanied with a gradual modulus change (e.g. variation in cross-linking density) has potential benefits in applications where biomimetic stress dissipation is desired.
| Comonomer | γ (mJ m−2) |
|---|---|
| AN | 9.5 ± 0.2 |
| HDDA | 26.4 ± 0.5 |
3.3 Tuning surface wettabilities as a function of PMMA loading
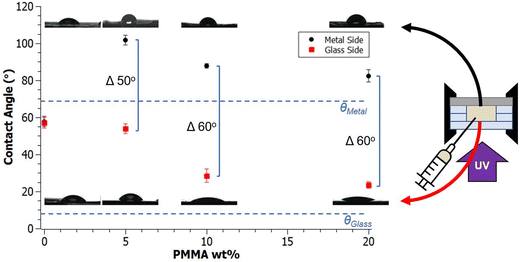
Introducing a mold interface with distinctly different surface energy from glass, such as metal explored here when curing A70X30Pc resins, drives the opaque nitrile-rich phase to the metal surface and the transparent nitrile-poor phase to the glass surface. In addition to the compositional gradients afforded by this design (Fig. 5b), this segregation also results in differing wettabilities on each face. As highlighted in Fig. 6, the measured contact angles of sample surfaces varied based on both the PMMA wt% loading and the surface energy of the mold interface (e.g., glass or metal).The control homopolymer network, A70X30P0 had a consistent water contact angle of 57° measured on both faces of the sample. Given that no macroscale phase separation occurs in this resin formulation, a uniform contact angle on all sample faces is not surprising. As PMMA is introduced to the resin system, a stark difference in wettability between the two sample faces emerges. The nitrile-rich phase of A70X30P5 had a water contact angle of 106° while the nitrile poor phase had a water contact angle of 53°. The two surfaces here straddle the transition from hydrophilic to hydrophobic behavior. A higher PMMA fraction in A70X30P10 drops the recorded contact angle values, reducing the metal side by ∼20° and the glass side ∼30° relative to A70X30P5. Increasing PMMA loading beyond 10 wt% in A70X30P20 does not appear to change the contact angle values significantly compared to the previous additions of PMMA. A70X30P5 possessed the most hydrophobic surface within the formulations explored.
Building upon this behavior, macroscale wettability design/patterning is possible via select application of steel plate sections on a glass substrate. This enables a single face of a sample to exhibit spatially-dependent wettability. Both hydrophilic and hydrophobic character can be imparted onto a given surface depending on the initial arrangement of the metal as depicted in Fig. 7. This mold arrangement illustrates that the phase separation in A70X30Pc is not limited to the z-direction depth, rather, discrete regions of a given face can have differing wettabilities.
The addition of PMMA produced a significant contact angle difference of ∼50°–60° between the two sample surfaces in the A70X30Pc PIPS systems. While changing the PMMA loading appears to shift the absolute value of the contact angles of the faces in contact with the glass and metal substrates, a similar difference between the two values is maintained. This demonstrates an opportunity to move the absolute values of the high and low contact angle values without sacrificing the distinct contrast in wettability between domains, maintaining a large window between the two faces. Increasing concentrations of PMMA lead to contact angles that trend towards the intrinsic contact angles of the glass and metal substrates, a phenomenon noted in literature focused on interfaces of polymer melts cast against different mold materials.44
From the contact angle behavior observed in Fig. 6, a balance is observed between introducing PMMA to induce PIPS and the associated decrease in the fraction of copolymer network in the overall formulation based on this modification. Specifically, higher PMMA loading increases the thermodynamic driving force for phase separation, however this comes at the cost of available nitrile content per volume. It is established that phase separation of the A70X30Pc resins yield chemically distinct domains. Furthermore, the specific compositions and concentrations of these domains depends on the PMMA loading fraction, as highlighted in Fig. 3e–h. Given that the nitrile-rich domains are responsible for the sample's hydrophobicity, the variable composition of the nitrile-rich phase as a function of PMMA loading conditions results in variable wettability. Increased PMMA loading leads to nitrile-poor domains having a greater composition of unbounded free polymer chains. The increase in free polymer chains facilitates an increased ability of the nitrile-poor domain to reorient chains and side groups to better match the surface of the mold material, resulting in differing wettability (Fig. 6). Polymer rearrangement at different interfaces has been documented in previous literature to have a significant impact on the final surface character for a given sample.44,62 A relevant example of this from Chihani et al. shows how the melt of a random copolymer containing both polar and non-polar functional groups had different ways of arranging itself at the interface depending on the polar character of said interface.
The ability of polymers present at the resin/mold interface to reorient in an attempt to increase compatibility between the sample and mold is supported by contact angle measurements taken 2 months after sample photopolymerization, removal from the curing mold, and storage under ambient conditions (Fig. S9, ESI†). After the elapsed time, samples with higher PMMA loading (e.g., A70X30P10, A70X30P20) have a larger increase between the initially measured contact angle associated with the glass substrate (e.g., hydrophilic side) and that measured 2 months later. For the A70X30P20 sample, this change was 24° to 62°, and for A70X30P10 a smaller change from 28° to 38° was observed. No significant change in the contact angle on the hydrophilic face was noted for A70X30P5. During curing in the mold, we suspect that HDDA domains initially migrate to the glass interface and reorient functional groups for interfacial energy reduction, yielding a relatively low contact angle. However, once this glass substrate is removed after curing and the sample is left untouched for several months, a gradual reorientation that minimizes the surface energy of the sample/air interface occurs. The new contact angle values measured on the hydrophilic face after 2 months can be rationalized when considering that acrylic polymers, such as PMMA are known to have water contact angles around 70° to 90°, a range supported by both previous literature and our own measurements of a pure PMMA film.63
At the same time, the contact angles for the hydrophobic faces (e.g. faces in contact with the steel substrate that are nitrile-rich) did not change for A70X30P10 and A70X30P20, but did for A70X30P5 where the contact angle dropped from 102° to roughly 85°. Still, the contact angles of the nitrile-rich domains remained hydrophobic and did not revert to the highly hydrophilic wettability as would be expected of generic poly(acrylonitrile). This is most likely due to the presence of the chains of PMMA present in the nitrile-rich domains, able to maintain hydrophobicity.
This concept of environmentally-influenced surface energy is demonstrated here by uniting the measured surface properties with the distribution of nitrile and acrylate groups within the A70X30Pc system sample volumes (Fig. 5c and 6). While pure poly(acrylonitrile) is commonly though of as a more hydrophilic polymer and pure PMMA is commonly thought of as a more hydrophobic polymer, the polymerization environment can modify how these polymer surfaces present themselves. It is possible for the intrinsic surface behavior of these polymers to be influenced by the substrate material as shown in Fig. 6. Referring to the kinetics studies of this system in Fig. 4, in phase-separated systems there is an enhanced period for domain formation allowing developing polymers to adopt an energy-minimizing arrangement prior to being kinetically trapped by the crosslinked network.
4 Conclusions
In this work, macroscale heterogeneity from homogeneous starting resins is demonstrated in nitrile-acrylate photopolymerizing systems, forming domains of differing wettability bridged by a bulk chemical gradient. The network morphology and resulting surface properties can be tailored by varying the polymerization mold materials and chemical ratios within a four component resin system. No macroscale phase separation occurred in the control sample A70X30P0, however, the addition of 5 wt%, 10 wt% and 20 wt% PMMA in the resins A70X30P5, A70X30P10, and A70X30P20 caused photoinitiated PIPS to occur. This separation is due to the increase in ΔGmix that occurs during the polymerization. The domains in A70X30Pc from PIPS were confirmed with optical microscopy and Raman spectroscopy, consisting of a transparent, glassy, nitrile-poor domain and an opaque, chalky, nitrile-rich phase. Under the highest PMMA loading condition, A70X30P20, macroscale phase separation was observed, with the chalky nitrile-rich phase preferentially migrating to the center of the sample surrounded by the glassy nitrile-poor phase on the exterior at the interface between the sample and the glass molds.Further experiments investigated the role of interfacial energies on resulting phase-separated morphology by modifying the mold materials used during curing. Differences in interfacial energies within the mold geometry provide an additional driving force to form distinct gradients via phase separation. Replacing one interface of the curing mold with a substrate of differing surface energy, such as stainless steel, spatially directed the resulting phase separation and created chemical gradients spanning 500 μm. In the case of A70X30P5, the guided phase separation resulted in a hydrophobic face with a contact angle of 101° from the nitrile-rich phase at the metal interface and a hydrophilic face with a contact angle of 53° from the nitrile-poor phase at the glass interface. The placement of these domains is driven by mitigation of the interfacial energy, with the low surface energy acrylonitrile (γ = 9.5 mJ m−2) migrating to the low energy metal surface (γ = 36.0 mJ m−2) and the high surface energy 1,6-hexanediol diacrylate (γ = 26.4 mJ m−2) migrating to the high energy glass surface (γ = 72.4 mJ m−2). The relationship between PMMA loading and wettability properties is explained by recognizing two aspects of this resin system: (1) there is a delay between the onset of phase separation and onset of deceleration, enabling domains to migrate within the sample volume, and (2) polymers at the mold interfaces are capable of orienting themselves to increase compatibility between the resin sample and the glass or metal surface, resulting in samples with a contact angle difference of ∼50°–60° between its two faces. These two findings allow for increased control over the polymerization design. Systems can be engineered to have a lengthened period prior to autodeceleration to allow for domain coalescence and significant phase separation by increasing the inert additive fraction, as demonstrated in A70X30P20 samples. Additionally, in systems where the delay is not as significant, changes in surface energy of the substrate can help further drive the phase separation as demonstrated in A70X30P5 using multiple interfaces, facilitating development of macroscale domains.
Future implementation of this work has potential for streamlining surface patterning while simultaneously reducing energy and waste associated with templating materials. Steel can be reclaimed and reformed into sheets much easier compared to frequently-used crosslinked silicone polymer photomasks.64–66 Using steel substrates as opposed to plastic photomasks can make the recycling and reuse of mold materials more efficient while still maintaining robust patterning abilities. The surface energies of the glass and metal used require no additional modification or functionalization to spatially direct bulk phase segregation. This approach serves as a facile method for developing macroscale chemical gradients across a range of wettability characteristics in a single polymerization procedure, without the need for separate steps of patterning and depositing.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by start-up funds from Michigan State University. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science user facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Furthermore, Prof. Shiwang Cheng is acknowledged for insightful feedback on SAXS results and analysis.References
- S. C. Ligon-Auer, M. Schwentenwein, C. Gorsche, J. Stampfl and R. Liska, Toughening of photo-curable polymer networks: a review, Polym. Chem., 2016, 7, 257–286 RSC.
- S. C. Leguizamon, J. Powers, J. Ahn, S. Dickens, S. Lee and B. H. Jones, Polymerization-Induced Phase Separation in Rubber-Toughened Amine-Cured Epoxy Resins: Tuning Morphology from the Nano-to Macro-scale, Macromolecules, 2021, 54(17), 7796–7807 CrossRef CAS.
- J. P. Gong, Why are double network hydrogels so tough?, Soft Matter, 2010, 6, 2583–2590 RSC.
- I. Calvez, C. R. Szczepanski and V. Landry, Hybrid Free-Radical/Cationic Phase-Separated UV-Curable System: Impact of Photoinitiator Content and Monomer Fraction on Surface Morphologies and Gloss Appearance, Macromolecules, 2022, 3129–3139 CrossRef CAS PubMed.
- Z. Li, G. Wang, Y. Wang and H. Li, Reversible Phase Transition of Robust Luminescent Hybrid Hydrogels, Angew. Chem., 2018, 2(130), 2216–2220 CrossRef.
- C. R. Szczepanski, T. Darmanin and F. Guittard, Spontaneous, Phase-Separation Induced Surface Roughness: A New Method to Design Parahydrophobic Polymer Coatings with Rose Petal-like Morphology, ACS Appl. Mater. Interfaces, 2016, 2(8), 3063–3071 CrossRef.
- F. Abedin, Q. Ye, H. J. Good, R. Parthasarathy and P. Spencer, Polymerization- and solvent-induced phase separation in hydrophilic-rich dentin adhesive mimic, Acta Biomater., 2014, 10, 3038–3047 CrossRef CAS PubMed.
- L. H. Sperling, Interpenetrating polymer networks and related materials, Springer Science & Business Media, 2012 Search PubMed.
- C. R. Szczepanski and J. W. Stansbury, Stress reduction in phase-separated, cross-linked networks: influence of phase structure and kinetics of reaction, J. Appl. Polym. Sci., 2014, 10, 131 Search PubMed.
- E. Hasa, J. P. Scholte, J. L. P. Jessop, J. W. Stansbury and C. A. Guymon, Kinetically Controlled Photoinduced Phase Separation for Hybrid Radical/Cationic Systems, Macromolecules, 2019, 4(52), 2975–2986, DOI:10.1021/acs.macromol.9b00177.
- H. Nakanishi, M. Satoh, T. Norisuye and Q. Tran-Cong-Miyata, Generation and Manipulation of Hierarchical Morphology in Interpenetrating Polymer Networks by Using Photochemical Reactions, Macromolecules, 2004, 11(37), 8495–8498, DOI:10.1021/ma048657i.
- F. S. Bates and G. H. Fredrickson, Block copolymers-designer soft materials, Phys. Today, 1999, 52, 32–38 CrossRef CAS.
- L. Cheng, J. W. Simonaitis, K. R. Gadelrab, M. Tahir, Y. Ding and A. Alexander-Katz, et al., Imparting Superhydrophobicity with a Hierarchical Block Copolymer Coating, Small., 2020, 16(1), 1905509, DOI:10.1002/smll.201905509.
- M. Li and C. K. Ober, Block copolymer patterns and templates, Mater. Today, 2006, 9(9), 30–39 CrossRef CAS.
- M. Byun, W. Han, B. Li, X. Xin and Z. Lin, An Unconventional Route to Hierarchically Ordered Block Copolymers on a Gradient Patterned Surface through Controlled Evaporative Self-Assembly, Angew. Chem., Int. Ed., 2013, 1(52), 1122–1127, DOI:10.1002/anie.201208421 Available from: .
- N. Kimura, K. Kawazoe, H. Nakanishi and T. Norisuye, Influences of wetting and shrinkage on the phase separation process of polymer mixtures induced by photopolymerization, pubsrscorg, 2013. Available from: https://pubs.rsc.org/en/content/articlehtml/2013/sm/c3sm51203g.
- E. T. Jaynes, Gibbs vs. Boltzmann entropies, Am. J. Phys., 1965, 33(5), 391–398 CrossRef CAS.
- C. R. Szczepanski and J. W. Stansbury, Accessing photo-based morphological control in phase-separated, cross-linked networks through delayed gelation, Eur. Polym. J., 2015, 67, 314–325 CrossRef CAS PubMed.
- G. E. Molau, Heterogeneous polymer systems. III. Phase separation in styrene-acrylonitrile copolymers, J. Polym. Sci., Part B: Polym. Lett., 1965, 3(12), 1007–1015 CrossRef CAS.
- L. Tian, B. Li, X. Li and Q. Zhang, Janus dimers from tunable phase separation and reactivity ratios, Polym. Chem., 2020, 11(28), 4639–4646 RSC.
- K. S. Anseth, C. M. Wang and C. N. Bowman, Reaction behaviour and kinetic constants for photopolymerizations of multi (meth) acrylate monomers, Polymer, 1994, 35(15), 3243–3250 CrossRef CAS.
- C. R. Szczepanski, C. S. Pfeifer and J. W. Stansbury, A new approach to network heterogeneity: polymerization induced phase separation in photo-initiated, free-radical methacrylic systems, Polymer, 2012, 53(21), 4694–4701 CrossRef CAS PubMed.
- C. R. Szczepanski and J. W. Stansbury, Modification of linear prepolymers to tailor heterogeneous network formation through photo-initiated polymerization-induced phase separation, Polymer, 2015, 6(70), 8–18 CrossRef PubMed.
- T. Inoue, Reaction-induced phase decomposition in polymer blends, Prog. Polym. Sci., 1995, 1(20), 119–153 CrossRef.
- A. S. Kulkarni and G. Beaucage, Reaction induced phase-separation controlled by molecular topology, Polymer, 2005, 46(12), 4454–4464 CrossRef CAS.
- A. Miserez, T. Schneberk, C. Sun, F. W. Zok and J. H. Waite, The transition from stiff to compliant materials in squid beaks, Science, 2008, 319(5871), 1816–1819 CrossRef CAS PubMed.
- J. D. Fox, J. R. Capadona, P. D. Marasco and S. J. Rowan, Bioinspired water-enhanced mechanical gradient nanocomposite films that mimic the architecture and properties of the squid beak, J. Am. Chem. Soc., 2013, 135(13), 5167–5174 CrossRef CAS PubMed.
- C. Sun and J. H. Waite, Mapping chemical gradients within and along a fibrous structural tissue, mussel byssal threads, J. Biol. Chem., 2005, 280(47), 39332–39336 CrossRef CAS PubMed.
- K. A. DeRocher, P. J. Smeets, B. H. Goodge, M. J. Zachman, P. V. Balachandran and L. Stegbauer, et al., Chemical gradients in human enamel crystallites, Nature, 2020, 583(7814), 66–71 CrossRef CAS PubMed.
- J. H. Waite, H. C. Lichtenegger, G. D. Stucky and P. Hansma, Exploring molecular and mechanical gradients in structural bioscaffolds, Biochemistry, 2004, 43(24), 7653–7662 CrossRef CAS PubMed.
- K. U. Claussen, T. Scheibel, H. W. Schmidt and R. Giesa, Polymer gradient materials: can nature teach us new tricks?, Macromol. Mater. Eng., 2012, 297(10), 938–957 CrossRef CAS.
- S. Suresh, Graded materials for resistance to contact deformation and damage, Science, 2001, 292(5526), 2447–2451 CrossRef CAS PubMed.
- A. Vitale, S. Touzeau, F. Sun and R. Bongiovanni, Compositional Gradients in Siloxane Copolymers by Photocontrolled Surface Segregation, Macromolecules, 2018, 51, 4023–4031 CrossRef CAS.
- Y. Gan, X. Jiang and J. Yin, Self-Wrinkling Patterned Surface of Photocuring Coating Induced by the Fluorinated POSS Containing Thiol Groups (F-POSS-SH) as the Reactive Nanoadditive, Macromolecules, 2012, 9(45), 7520–7526, DOI:10.1021/ma301439g.
- J. R. Rumble, CRC handbook of chemistry and physics, (Internet Version 2022), CRC Press, Taylor & Francis, Boca Raton, FL, 2022 Search PubMed.
- M. Minagawa, H. Kanoh, S. Tanno and M. Satoh, Glass-Transition Temperature (Tg) of Free-Radically Prepared Polyacrylonitrile by Inverse Gas Chromatography, 2. Molecular-Weight Dependence of Tg of Two Different Types of Aqueous Polymers, Macromol. Chem. Phys., 2002, 203(17), 2481–2487 CrossRef CAS.
- J. Michalsky, L. Harrison and W. Berkheiser III, Cosine response characteristics of radiometric and photometric sensors, State Univ. of New York, Albany, NY (United States). Atmospheric Sciences, 1992 Search PubMed.
- D. K. Owens and R. Wendt, Estimation of the surface free energy of polymers, J. Appl. Polym. Sci., 1969, 13(8), 1741–1747 CrossRef CAS.
- V. M. Litvinov and A. A. Dias, Real-Time NMR. How Fast Can We Do It?, Macromolecular Symposia, 2005, 230(1), 20–25, DOI:10.1002/masy.200551137.
- Z. Guo, H. Sautereau and D. Kranbuehl, Evidence for spatial heterogeneities observed by frequency dependent dielectric and mechanical measurements in vinyl/dimethacrylate systems, Polymer, 2005, 46(26), 12452–12459 CrossRef CAS.
- Y. Lin, E. Bilotti, C. W. Bastiaansen and T. Peijs, Transparent semi-crystalline polymeric materials and their nanocomposites: a review, Polym. Eng. Sci., 2020, 60(10), 2351–2376 CrossRef CAS.
- F. Di Lorenzo and S. Seiffert, Nanostructural heterogeneity in polymer networks and gels, Polym. Chem., 2015, 6(31), 5515–5528 RSC.
- G. L. Batch and C. W. Macosko, Kinetic model for crosslinking free radical polymerization including diffusion limitations, J. Appl. Polym. Sci., 1992, 44(10), 1711–1729 CrossRef CAS.
- R. Cuthrell, Environment-influenced surface layer in polymers, J. Appl. Polym. Sci., 1967, 11(8), 1495–1507 CrossRef CAS.
- C. S. Pfeifer, N. D. Wilson, Z. R. Shelton and J. W. Stansbury, Delayed gelation through chain-transfer reactions: mechanism for stress reduction in methacrylate networks, Polymer, 2011, 52(15), 3295–3303 CrossRef CAS PubMed.
- L. G. Lovell, J. W. Stansbury, D. C. Syrpes and C. N. Bowman, Effects of composition and reactivity on the reaction kinetics of dimethacrylate/dimethacrylate copolymerizations, Macromolecules, 1999, 32(12), 391321 CrossRef.
- Z. Wang, Q. Ran, R. Zhu and Y. Gu, Reaction-induced phase separation in a bisphenol A-aniline benzoxazine-N,N-(2,2,4-trimethylhexane-1,6-diyl)bis(maleimide)-imidazole blend: the effect of changing the concentration on morphology, Phys. Chem. Chem. Phys., 2014, 3(16), 5326–5331 RSC.
- D. W. V. Krevelen and K. T. Nijenhuis, Cohesive Properties and Solubility, Elsevier, 2009, pp. 189–227 Search PubMed.
- J. A. Lanasa, A. Neuman, R. A. Riggleman and R. J. Hickey, Investigating Nanoparticle Organization in Polymer Matrices during Reaction-Induced Phase Transitions and Material Processing, ACS Appl. Mater. Interfaces, 2021, 9(13), 42104–42113 CrossRef PubMed.
- J B, EH I, EA G, editors. v11 Exhibit 1011-Polymer Handbook. vol. 89. 4th edn. John Wiley Sons Inc, 2003.
- S. Ghaffari, P. K. Chan and M. Mehrvar, Long-range surface-directed polymerization-induced phase separation: a computational study, Polymers, 2021, 1(13), 1–19 Search PubMed.
- M. Tabatabaieyazdi, P. K. Chan and J. Wu, A computational study of long range surface-directed phase separation in polymer blends under a temperature gradient, Comput. Mater. Sci., 2016, 1(111), 387–394 CrossRef.
- D. Brian and M. Eslamian, Analysis of impact dynamics and deposition of single and multiple PEDOT:PSS solution droplets, Exp. Fluids, 2019, 60, 1–15 CrossRef CAS.
- K. Mader-Arndt, Z. Kutelova, R. Fuchs, J. Meyer, T. Staedler and W. Hintz, et al., Single particle contact versus particle packing behavior: model based analysis of chemically modified glass particles, Granular Matter, 2014, 16, 359–375 CrossRef CAS.
- M. Kruszelnicki, I. Polowczyk and P. B. Kowalczuk, Control of glass surface wettability via esterification with n-alkyl alcohols, Physicochem. Probl. Miner. Process., 2022, 58, 145147 CAS.
- S. Tang, O. J. Kwon, N. Lu and H. S. Choi, Surface free energy changes of stainless steel after one atmospheric pressure plasma treatment, Korean J. Chem. Eng., 2004, 21, 1218–1223 CrossRef CAS.
- J. Heil, B. Mohammadian, M. Sarayloo, K. Bruns and H. Sojoudi, Relationships between surface properties and snow adhesion and its shedding mechanisms, Appl. Sci., 2020, 10(16), 5407 CrossRef CAS.
- L. Boulange-Petermann, J. C. Joud and B. Baroux, Wettability parameters controlling the surface cleanability of stainless steel. Contact angle, wettability and adhesion Leiden, VSP, 2008, pp. 139–151 Search PubMed.
- J. Genzer and E. J. Kramer, Wetting of substrates with phase-separated binary polymer mixtures, Phys. Rev. Lett., 1997, 78(26), 4946 CrossRef CAS.
- T. Murashige, H. Fujikake, H. Sato, H. Kikuchi, T. Kurita and F. Sato, Polymer wall formation using liquidcrystal/polymer phase separation induced on patterned polyimide Films, Jpn. J. Appl. Phys., 2004, 43(12B), L1578 CrossRef CAS.
- N. Kimura, K. Kawazoe, H. Nakanishi, T. Norisuye and Q. Tran-Cong-Miyata, Influences of wetting and shrinkage on the phase separation process of polymer mixtures induced by photopolymerization, Soft Matter, 2013, 9(35), 8428–8437 RSC.
- T. Chihani, P. Bergmark and P. Flodin, Surface modification of ethylene copolymers moulded against different mould surfaces-1. Surface enrichment by functional groups, J. Adhesion Sci. Technol., 1993, 7(4), 327–341 CrossRef CAS.
- D. Briggs, H. Chan, M. Hearn, D. McBriar and H. Munro, The contact angle of poly(methyl methacrylate) cast against glass, Langmuir, 1990, 6(2), 420–424 CrossRef CAS.
- P. Zheng and T. J. McCarthy, Rediscovering silicones: molecularly smooth, low surface energy, unfilled, UV/vis-transparent, extremely cross-linked, thermally stable, hard, elastic pdms, Langmuir, 2010, 26(24), 18585–18590 CrossRef CAS PubMed.
- B. Rupasinghe and J. C. Furgal, Full circle recycling of polysiloxanes via room-temperature fluoride-catalyzed depolymerization to repolymerizable cyclics, ACS Appl. Polym. Mater., 2021, 3(4), 1828–1839 CrossRef CAS.
- G. Parker, Encyclopedia of materials: science and technology, Elsevier, 2001 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sm01528a |
| This journal is © The Royal Society of Chemistry 2024 |