Open Access Article
Open Access ArticleSimultaneous coupling of metal removal and visual detection by nature-inspired polyphenol-amine surface chemistry†
Helen H.
Ju
 ,
Hong K.
Park
,
Hong K.
Park
 ,
Jingxian
Wu
,
Yu Ri
Nam
,
Eunu
Kim
,
Jeongin
Seo
,
Jingxian
Wu
,
Yu Ri
Nam
,
Eunu
Kim
,
Jeongin
Seo
 and
Haeshin
Lee
and
Haeshin
Lee
 *
*
Department of Chemistry, KAIST (Korea Advanced Institute of Science and Technology), Daehak-ro, Yuseong-gu, Daejeon 34141, Republic of Korea. E-mail: haeshin@kaist.ac.kr
First published on 10th February 2024
Abstract
The interplay between polyphenols, amines, and metals has broad implications for surface chemistry, biomaterials, energy storage, and environmental science. Traditionally, polyphenol–amine combinations have been recognized for their ability to form adhesive, material-independent thin layers that offer a diverse range of surface functionalities. Herein, we demonstrate that a coating of tannic acid (TA) and polyethyleneimine (PEI) provides an efficient platform for capturing and monitoring metal ions in water. A unique feature of our PEI/TA-coated microbeads is the ‘Detection-Capture’ (Detec-Ture) mechanism. The galloyl groups in TA coordinate with Fe(III) ions (capture), initiating their oxidation to gallol–quinone. These oxidized groups subsequently react with PEI amines, leading to the formation of an Fe(II/III)–gallol–PEI network that produces a vivid purple color, thereby enabling visual detection. This mechanism couples metal capture directly with detection, distinguishing our approach from existing studies, which have either solely focused on metal removal or metal detection. The metal capturing capacity of our materials stands at 0.55 mg g−1, comparable to that of established materials like alginate and wollastonite. The detection sensitivity reaches down to 0.5 ppm. Our findings introduce a novel approach to the utility of metal–polyphenol–amine networks, presenting a new class of materials suited for simultaneous metal ion detection and capture in environmental applications.
Introduction
The development of polyphenolic chemistry has significantly advanced the field of surface science. Initiated by the pioneering study on substrate-independent surface chemistry facilitated by polydopamine coatings,1 a plethora of subsequent studies have emerged, exploring diverse polyphenolic moieties to broaden the scope of surface functionalities. Among these, Sileika et al. have developed multifunctional surface chemistry, utilizing trihydroxybenzene (i.e., galloyl) functional groups, such as pyrogallol (PG) and tannic acid (TA).2 This material-independent coating has been applied to a wide array of organic and inorganic substrates, including poly(carbonate), poly(tetrafluoroethylene), stainless steel, metal nanoparticles, and others, demonstrating antibacterial and antioxidant properties.The interplay between amines and polyphenols plays a pivotal role in insect biochemistry.3 For instance, the initial sclerotization of a fragile cuticle layer leads to a mechanically robust structure via oxidative catecholamine cross-linking as it ages.4 Studies have elucidated how amine-rich histidyl residues in cuticular proteins form cross-links with catechol-derived phenolic quinones, serving as the backbone of insect exoskeletal cuticles.5,6 Inspired by this natural phenomenon, scientists have engineered biomimetic polyphenol–amine chemistry at interfaces. Hong et al. demonstrated the chemical synergy between amine and galloyl groups using a model small molecule, 5-pyrogallol 2-aminoethane (PAE).7 The incorporation of primary amine substantially enhanced the coating stability across various materials compared to coatings with amine-absent galloyl molecules, like tannic acid. Moreover, Hong et al. extended their work beyond examining the inter-molecular attributes of amine-containing polymers and phenolic molecules. By using catechol/catecholamine small molecules and poly(ethyleneimine) (PEI), they illustrated an intermolecular, bio-mimetic oxidative interfacial cross-linking reaction that mimics an insect's protective exoskeleton.8 Their findings suggest that catechol/catecholamine cross-linking is particularly effective at air–water interfaces where oxygen is rich, explaining the increased mechanical rigidity of external cuticle layers compared to inner layers. Sequentially, Park et al. developed a protective coating using gallolamine chemistry at the air–water interface.9 This synthetic, exoskeleton-like coating exhibited resilient properties under harsh external conditions such as dehydration, shrinkage, and temperature variations.
Although the synergy between polyphenols and amines offers remarkable stability at the interface, the interaction of polyphenols with metal ions offers an additional avenue of exploration. A notable example of this area was introduced by Ejima et al. through the development of metal–phenolic networks (MPNs).10 They reported the chelation chemistry between phenolic galloyl ligands, such as tannic acid, and inorganic cross-linkers like ferric ions, leading to the formation of various structured metal–galloyl frameworks. However, a limitation of MPNs is their reliance on the metal cross-linker for structure formation. Nature offers examples of similar phenol–metal interactions, evident in siderophore-mediated iron chelation systems. For instance, enterobactin is secreted by E. coli to secure iron,11 while another siderophore, cyclic trichrysobactin, is produced by the plant Dickeya chrysanthemi to protect itself against pathogens.12 Both siderophores employ multiple phenolic (catechol) moieties for robust interaction with metal ions.
While the fields of polyphenol–amine and polyphenol–metal surface chemistries have evolved largely in parallel, integrated studies embracing all three components–polyphenols, amines, and metals–are scarce. This gap in the literature presents an opportunity for potential studies to harness the synergistic potentials of these interactions. Building on this insight, we propose that a robust, conformal coating derived from polyphenol–amine (specifically, TA–PEI) interactions could serve as an effective platform for sequestration of iron ions in water with real-time monitoring capabilities. We introduce a unique surface chemistry, termed ‘Detec-Ture’, for simultaneous detection and capture. This dual-function mechanism sets our approach apart from existing studies, which traditionally emphasize either metal removal or detection. Upon iron–ion binding, a redox coupling facilitates iron reduction (III to II) and galloyl's oxidation to galloyl–quinone, further prompting amine–galloyl covalent reactions. The resulting metal–galloyl–amine networks manifest distinctive coating, enabling concurrent metal removal and visual detection. Iron, although non-toxic, presents its unique set of challenges due to its abundance and difficulty in removal. Its widespread presence in drinking water systems necessitates effective methods to control its concentration in water. Our gallolamine-functionalized surface introduces a groundbreaking technique to regulate and monitor waterborne iron concentration, suggesting potential applications in addressing diseases stemming from iron overload/accumulation,13 such as secondary hemochromatosis. Notably, our methodology addresses iron concentration without relying on energy-intensive reverse osmosis or intricate pH control methods.14,15 In a testament to its efficacy, PEI/TA-coated beads were able to visually detect iron from a 10 ppm Fe(III) solution in just 20 seconds, boasting a sensitivity threshold down to 1 ppm. Furthermore, one gram of PEI/TA-coated beads adsorbed 0.55 mg of iron ions, showcasing the rapid and robust adsorption capabilities of our formulation.
Experimental
Materials
Poly(ethyleneimine) (branched, M.W. = 750![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), tannic acid (M.W. = 1701.20), and iron(III) chloride (M.W. = 162.20) were ordered from Sigma Aldrich, South Korea. Sephadex G-75 (particle size = 90–280 μm (wet)) was ordered from GE Healthcare, Sweden. An Econo-Pac® chromatography column (pore size = 30 μm) was ordered from Bio-Rad, South Korea. Teabag was purchased from a dollar store, South Korea.
000), tannic acid (M.W. = 1701.20), and iron(III) chloride (M.W. = 162.20) were ordered from Sigma Aldrich, South Korea. Sephadex G-75 (particle size = 90–280 μm (wet)) was ordered from GE Healthcare, Sweden. An Econo-Pac® chromatography column (pore size = 30 μm) was ordered from Bio-Rad, South Korea. Teabag was purchased from a dollar store, South Korea.
PEI/TA coating procedures
PEI and TA were separately dissolved in double-distilled water (DDW). The final concentration of both PEI and TA was 0.01 wt%. Dextran bead powder (10 mg) was swollen and washed with 500 mL of DDW ahead of coating. Excessive DDW was drained. Subsequently, PEI solution (500 mL) was poured to the swollen bead, and the bead/PEI solution was gently shaken for 5 minutes. The excessive PEI solution was drained, and DDW (500 mL) was poured for washing. After 30 seconds of gentle shaking, DDW was drained. Subsequently, a TA solution (500 mL) was poured on the PEI-coated bead, and the PEI-coated bead/TA solution was gently shaken for 5 minutes. The excessive TA solution was drained, and DDW (500 mL) was poured for washing. After 30 seconds of gentle shaking, DDW was drained. The PEI/TA-coated beads were used in a variety of subsequent experiments.Characterization of PEI/TA coatings
Fe–ion adsorption experiment
Fe–ion adsorption test
PEI/TA-coated beads (wet = 15.618 g; dry = 1.937 g) were immersed in a 10 ppm Fe(III) solution (300 mL) and stirred at 100 rpm. The Fe(III) concentration was measured using ICP-OES (5110 ICP-OES, Agilent) at 10-second intervals for the first 2 minutes, then every minute from 2–10 minutes, and finally every 10 minutes from 10–30 minutes.Application of PEI/TA-coated beads
Results and discussion
The PEI/TA-coated beads were prepared by the following method. Cross-linked dextran beads, specifically Sephadex G-75, were added to a PEI solution (0.01 wt%). The volume ratio of the coating solution to the swollen bead was 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. Coatings were carried out for 5 minutes, and excess PEI was washed with distilled water. Then, identical coating and washing procedures were applied to the TA coating step (0.01 wt%).
3. Coatings were carried out for 5 minutes, and excess PEI was washed with distilled water. Then, identical coating and washing procedures were applied to the TA coating step (0.01 wt%).
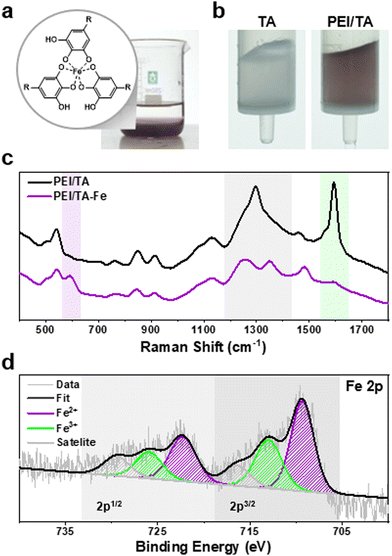
To confirm the PEI/TA coating as depicted in Fig. 1a, we analyzed the unmodified, PEI-coated, and PEI/TA-coated beads using X-ray photoelectron spectroscopy (XPS). The presence of nitrogen-1s (N1s) photoelectrons indicated successful PEI and PEI/TA-functionalization. Given the surface sensitivity of XPS, where the probes can detect roughly up to 20 nm from the atop surface, the N1s peak from PEI might be slightly decreased due to the presence of TA that was coated over the PEI layer. As anticipated, the bare bead displayed no N1s peak (Fig. 1b, left). In contrast, the bead coated solely with PEI exhibited the N1s peaks. The N1s peak deconvolution reveals that the peak at 399.06 eV corresponds to primary amine (C–NH2, yellow), 400.37 eV corresponds to secondary amine (C–NH–C, purple), and the one at 401.52 eV corresponds to tertiary amine (C–N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C, green).16,17 The N1s spectrum of the PEI/TA-coated bead displayed peaks mirroring those from PEI. Moreover, the intensity of the N1s peak observed in the PEI/TA bead was slightly decreased compared to the one observed in the PEI-coated bead. This decrease was attributed to the surface sensitivity of the XPS technique (<20 nm), probing the ad-layer formation of tannic acid. The chemical reactivity of the galloyl group to the amine moiety results in the generation of secondary amine peaks at 400.3 eV (by Michael addition) and/or imine peaks at 401.5 eV (Schiff base formation).16 In this case, the peak intensities corresponding to secondary amine and imine peaks should be increased relative to the peak ratio observed from PEI only (primary
C, green).16,17 The N1s spectrum of the PEI/TA-coated bead displayed peaks mirroring those from PEI. Moreover, the intensity of the N1s peak observed in the PEI/TA bead was slightly decreased compared to the one observed in the PEI-coated bead. This decrease was attributed to the surface sensitivity of the XPS technique (<20 nm), probing the ad-layer formation of tannic acid. The chemical reactivity of the galloyl group to the amine moiety results in the generation of secondary amine peaks at 400.3 eV (by Michael addition) and/or imine peaks at 401.5 eV (Schiff base formation).16 In this case, the peak intensities corresponding to secondary amine and imine peaks should be increased relative to the peak ratio observed from PEI only (primary![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) secondary
secondary![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) tertiary = 1
tertiary = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.32
0.32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.71, Fig. 1b, middle). However, the galloyl–amine reaction occurring between TA and PEI is inconclusive, considering the slight increase in the primary: secondary: tertiary ratio (1
0.71, Fig. 1b, middle). However, the galloyl–amine reaction occurring between TA and PEI is inconclusive, considering the slight increase in the primary: secondary: tertiary ratio (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.44
0.44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.85, Fig. 1b, right) during the coating layer formation.
0.85, Fig. 1b, right) during the coating layer formation.
To validate the successful coating of PEI/TA on the beads, confocal fluorescence microscopy was employed. A previous study has elucidated that the oxidation of TA emits fluorescence signals.18 Similar to this study, the PEI/TA-coated beads exhibit a fluorescence signal (Fig. 1c, right) due to the unavoidable oxidation of the galloyl group in the presence of dissolved oxygen as well as chemical reactions with amine groups present in PEIs. The uncoated beads showed no fluorescence signal (Fig. 1c, left).
The gallol moieties are known for their metal chelating abilities with various toxic heavy metal ions, including Pb(II),19–21 Mn(II),22 and Cu(II).23 However, their potential in addressing the removal or control of the predominant metal ion, Fe(II/III), remains relatively unexplored.24 Instead, iron ions act either as oxidants for polyphenolic molecules, or vice versa, with polyphenols reducing Fe(III) to Fe(II),25 or as coordinating metals to establish a thin metal–organic layer,26 as illustrated in Fig. 2a. As highlighted earlier, the interplay between polyphenols and amines, specifically the tannic acid–PEI combination, might provide a practical scaffold for sequestering metal ions in aqueous systems. In fact, PEI effectively stabilizes the TA–Fe ion chelated complex.27 To substantiate this hypothesis, a column packed with PEI/TA-coated beads (3 mg, wet weight) was prepared to elute 5 mL of a 10 ppm Fe(III) ion solution down the column. The post-treatment concentration of Fe(III) was measured to be 3.65 × 10−7 moles. The noticeable shift to a reddish-purple color in the beads after iron-binding has visually confirmed the effective removal of iron from water.
TA is known to establish an octahedral coordination around iron ions.26 To validate whether TA coordinates with iron ions via the chelation mechanism, Raman spectroscopy was performed on PEI/TA-coated beads before and after treatment with an Fe(III) solution. These beads were freeze-dried for two days prior to analysis. A Raman shift indicating TA's phenyl ring vibrations typically appear from ∼1250 to ∼1500 cm−1. Concurrently, shifts associated with TA–Fe coordination generally appear between ∼550 and ∼650 cm−1.28,29 As demonstrated in Fig. 2c, the spectrum of PEI/TA (the black line) exhibited a single, broad peak at 1295.92 cm−1, attributed to phenyl vibrations. This peak bifurcated into two narrower peaks at 1257.35 and 1348.66 cm−1 upon Fe(III) binding (the purple line). Also, the characteristic TA–Fe coordination peak emerged distinctly at 591.29 cm−1 exclusively for PEI/TA-Fe beads, highlighting the chelation of Fe ions by TA. The results showed that the Fe(III) removal mechanism is based on galloyl–Fe metal–phenolic coordination. Also, the Raman spectrum of the PEI/TA bead exhibited a sharp peak at 1593.2 cm−1. This peak, when juxtaposed with the amine peak from pure PEI (∼1689 cm−1),30 was shifted to slightly lower wave numbers, indicating the interactions with the phenolic hydroxyl groups of TA. Notably, the intensity of this peak was significantly decreased upon introducing iron ions to the PEI/TA beads, demonstrating the close interactions among the amine–iron ion–galloyl groups. This finding further provides an explanation (that will be explained later in Fig. 3d and 4, top and bottom photos) for the more pronounced color change observed in PEI/TA-coated beads compared to solely TA-coated beads when iron ions are present.
 | ||
| Fig. 4 The ‘Detec-Ture’ mechanism explaining how to couple metal removal and visual detection through the interplay of polyphenol–metal–amine. | ||
The role of TA as a reducing agent has been well-documented.31 Given the antioxidant properties of TA, we hypothesized that a considerable amount of the Fe ions would be reduced from Fe(III) to Fe(II) during the elution process. To verify this, we analyzed the iron (Fe2p) scan of the identical XPS samples. If our hypothesis is correct, peaks corresponding to Fe(II) and Fe(III) would be observed with a binding energy shift of about 3–4 eV for Fe2p photoelectrons, where Fe(II) 2p3/2: ∼709–711 eV and Fe(III) 2p3/2: ∼711–714 eV.32,33 As shown in Fig. 2d (right), the Fe(II) 2p3/2 peak was detected at 709.3 eV (purple), and the Fe(III) 2p3/2 peak was detected 713.0 eV (green). Similarly, Fe(II) and Fe(III) photoelectron peaks for Fe2p1/2 co-existed at 722.3 eV and 725.9 eV with a binding energy difference of 3.6 eV. These results confirm that the bound Fe(III) ions were reduced by redox coupling of TA upon coordination.
With the confirmation of successful iron adsorption capabilities, the sensitivity of removal–detection coupling was evaluated in real-time by the development of a deep-blue color upon Fe(III) coordination to TA/PEI. The precipitation model eliminates spatial constraints, facilitating more extensive contact between the beads and metal ions. First, 25 g of wet PEI/TA beads was dispersed in double-distilled water (DDW), stirring the bead solution at 300 rpm. As shown in Fig. 3a and b, binding of iron ions (equivalent to metal removal from water) simultaneously developed a deep purple color within a minute (5 ppm). Rapid color development was observed in just 20 seconds for a 5 ppm solution (Fig. 3a). The removal–detection coupling was able to monitor the capturing reaction for a sub-ppm concentration, 0.5 ppm. Because of the low concentration, the color development took a longer time of 40 seconds for visual detection (Fig. 3b). Compared to existing ppm-level colorimetric detection methods,34,35 the PEI/TA beads have achieved superior detection sensitivity down to the ppb-level. When operated under the ‘tea-bag’ configuration, there was a marginal reduction in the metal removal capacity to approximately ∼0.38 mg g−1. This decrease might be attributed to spatial limitations experienced by the beads, in contrast to the unconstrained environment of the precipitation method.
While numerous existing chemical adsorption methodologies predominantly focus on the mere removal of metal ions, our polyphenol-amine chemistry offers a compelling difference. Specifically, it allows real-time visualization of metal removal as illustrated in Fig. 3c. The system's efficacy and sensitivity notably exceeded our initial expectations.
During the exposure of the tea-bag to a 10 ppm of Fe(III) solution, the beads underwent a discernible shift to a light pink hue in only 20 seconds. This hue intensified to deeper pink within 4 minutes, as evidenced by the last photograph in Fig. 3c. The inherent sensitivity of our polyphenol–gallol chemistry is underscored by its capacity to monitor metal–ion removal even from water with mere 0.5 ppm Fe(III) (Fig. 3b). In a comparative assessment, TA-only-coated beads (Fig. 4, top) displayed nearly no color change. In contrast, the PEI/TA-coated beads manifested a distinct purple color (Fig. 4, bottom), enabling the visual detection of iron ions.
Based on the results presented so far, the intricate chemical interplay among polyphenol, metal, and PEI can be elucidated as outlined in Fig. 4. Initially, the galloyl group's reductive capability results in the coexistence of both oxidation states of iron ions (II/III) (i). The electronic state is corroborated by XPS results (Fig. 2d). Subsequently, the oxidized galloyl–quinone (iia) undergoes a chemical reaction with the amines in PEI (iib). This leads to the formation of dual-ligand-associated coordination (–N–Fe–galloyl–) bonds (iii), forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (PEI
1 (PEI![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Fe(II/III)
Fe(II/III)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TA) ternary complex. Additionally, there exists coordination solely mediated by TA's galloyl ligands (iv), forming a binary complex with a maximum ratio of 1
TA) ternary complex. Additionally, there exists coordination solely mediated by TA's galloyl ligands (iv), forming a binary complex with a maximum ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 (Fe(II/III): TA). The interplay between amine and polyphenol intensifies the resulting coloration. As depicted in the provided photographs, a vivid deep purple color emerges upon the reaction involving PEI–gallol (lower). In contrast, a lighter purple shade was observed when only TA coated the bead surfaces (upper). This distinction clarifies why the PEI/TA-coated beads exhibited enhanced detection sensitivity down to a 1 ppm Fe solution. The feature was not observed in TA-coated beads (Fig. 3d).
3 (Fe(II/III): TA). The interplay between amine and polyphenol intensifies the resulting coloration. As depicted in the provided photographs, a vivid deep purple color emerges upon the reaction involving PEI–gallol (lower). In contrast, a lighter purple shade was observed when only TA coated the bead surfaces (upper). This distinction clarifies why the PEI/TA-coated beads exhibited enhanced detection sensitivity down to a 1 ppm Fe solution. The feature was not observed in TA-coated beads (Fig. 3d).
Determining the iron removal capacity of PEI/TA-coated beads is crucial. For this purpose, an approach involving an excessive exposure of metal ions to the beads was considered appropriate. Thus, we adopted a precipitation method, wherein an excess of iron (10 ppm, 300 mL) was introduced to the beads. The iron solution was collected at 10-second intervals for the first 2 minutes, then every minute from 2–10 minutes, and finally every 10 minutes from 10–30 minutes. Subsequent ICP analysis of these solutions revealed that the iron binding capacity of the PEI/TA-beads was approximately 0.55 mg g−1. When juxtaposed with prior research Fe adsorbents – specifically, alginate (∼0.38 mg g−1, pH = 4)36 and wollastonite (∼0.05 mg g−1, pH = 4),37 − the PEI/TA beads demonstrated commendable iron removal. Also, the binding of Fe(III) onto the PEI/TA beads follows a pseudo-second order kinetic model rather than a pseudo-first order model. The equation for pseudo-second order kinetics38 is:
 | (1) |
Utilizing the experimental outcomes shown in Table S1 (ESI†), a dot plot (time vs. t/qt) can be drawn (Fig. 4a, red). When performing linear regression, a nearly perfect fit with an R2 value of 0.999 was observed. In contrast, the pseudo-first order kinetics model did not provide a well-fitting result. The corresponding model equation is:
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t | (2) |
To further understand the adsorption mechanism of PEI/TA-coated beads toward Fe ions, two representative adsorption isotherm models were considered. The Langmuir isotherm model depicts Fe ion adsorption in a monolayer with uniform binding energy on a homogeneous surface, while the Freundlich isotherm suggests multi-layer adsorption on a heterogeneous surface.37,39 The linear equations for Langmuir and Freundlich isotherm models are presented in eqn (3) and (4), respectively.
 | (3) |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kf − n Kf − n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce | (4) |
Conclusions
In the evolving landscape of polyphenol–amine and polyphenol–metal surface chemistries, our work stands out by integrating polyphenols, amines, and metals for concurrent detection and capture of iron ions. The ‘Detec-Ture’ mechanism we introduce exhibits both real-time monitoring and sequestration, filling a noted gap in existing research. This approach efficiently addresses the challenges posed by abundant iron in water without relying on energy-intensive or complex methods. Impressively, PEI/TA-coated beads rapidly detected iron ions, with a sensitivity reaching 0.5 ppm, and displayed robust adsorption capabilities. Our findings not only showcase the potential of polyphenol–amine–metal networks but also illuminate a promising path for future endeavors in water purification and iron overload disease mitigation.Author contributions
H. J. investigation, methodology, visualization, writing – original draft, and writing – review and editing; H. K. P. investigation, methodology, and visualization; J. W. methodology, visualization, and software; Y. R. N. software; E. K. methodology and visualization; J. S. methodology and visualization; H. L. conceptualization, funding acquisition, methodology, project administration, supervision, writing – original draft, and writing – review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Research Foundation of Korea (NRF) grants funded by the Government of Korea (MSIT): Center for Multiscale Chiral Architectures, Science Research Center (SRC) Program (2018R1A5A1025208) and the Food and Drug Administration of South Korea (21173MFDS562).References
- H. Lee, S. M. Dellatore, W. M. Miller and P. B. Messersmith, Science, 2007, 318, 426–430 CrossRef CAS PubMed.
- T. S. Sileika, D. G. Barrett, R. Zhang, K. H. Lau and P. B. Messersmith, Angew. Chem., Int. Ed., 2013, 52, 10766–10770 CrossRef CAS PubMed.
- H. Lee, J. Rho and P. B. Messersmith, Adv. Mater., 2009, 21, 431–434 CrossRef CAS PubMed.
- K. Lee, E. Prajatelistia, D. S. Hwang and H. Lee, Chem. Mater., 2015, 27, 6478–6481 CrossRef CAS.
- R. Xu, X. Huang, T. L. Hopkins and K. J. Kramer, Insect Biochem. Mol. Biol., 1997, 27, 101–108 CrossRef CAS.
- K. J. Kramer, M. R. Kanost, T. L. Hopkins, H. Jiang, Y. C. Zhu, R. Xu, J. L. Kerwind and F. Turecek, Tetrahedron, 2001, 57, 385–392 CrossRef CAS.
- S. Hong, J. Yeom, I. T. Song, S. M. Kang, H. Lee and H. Lee, Adv. Mater. Interfaces, 2014, 1, 1400113 CrossRef.
- S. Hong, C. F. Schaber, K. Dening, E. Appel, S. N. Gorb and H. Lee, Adv. Mater., 2014, 26, 7581–7587 CrossRef CAS PubMed.
- H. K. Park, D. Lee, H. Lee and S. Hong, Mater. Horiz., 2020, 7, 1387–1396 RSC.
- H. Ejima, J. J. Richardson, K. Liang, J. P. Best, M. P. V. Koeverden, G. K. Such, J. Cui and F. Caruso, Science, 2013, 341, 154–157 CrossRef CAS PubMed.
- K. N. Raymond, E. A. Dertz and S. S. Kim, Proc. Natl. Acad. Sci. U. S. A., 2003, 100, 3584–3588 CrossRef CAS PubMed.
- G. P. Maier, M. V. Rapp, J. H. Waite, J. N. Israelachvili and A. Butler, Science, 2015, 349, 628–632 CrossRef CAS PubMed.
- C. C. Hsu, N. H. Senussi, K. Y. Fertrin and K. V. Kowdley, Hepatol. Commun., 2022, 6, 1842–1854 CrossRef CAS PubMed.
- I. Radovenchyk, O. Ivanenko, M. K. Victorivna and V. Radovenchyk, Ecol. Eng. Environ. Technol., 2023, 24, 163–172 CrossRef PubMed.
- M. D. Víctor-Ortega, J. M. Ochando-Pulido and A. Martínez-Ferez, J. Ind. Eng. Chem., 2016, 36, 298–305 CrossRef.
- M. Shin, J. H. Choi, K. Kim, S. Kim and H. Lee, ACS Appl. Mater. Interfaces, 2021, 13, 10741–10747 CrossRef CAS PubMed.
- J. Lee, E. Park, A. Fujisawa and H. Lee, ACS Appl. Mater. Interfaces, 2021, 13, 21703–21713 CrossRef CAS PubMed.
- Y. Zhao, L. Xu, F. Kong and L. Yu, Chem. Eng. J., 2021, 416, 129090 CrossRef CAS.
- S. Y. Lee, Y. J. Jeong and W. H. Park, Chemosphere, 2022, 307, 135719 CrossRef CAS PubMed.
- S. Jiang, S. Li, P. Zhang, H. Miao, P. Jiang and Y. Leng, J. Environ. Chem. Eng., 2022, 10, 108670 CrossRef CAS.
- K. W. Huang, C. J. Yu and W. L. Tseng, Biosens. Bioelectron., 2010, 25, 984–989 CrossRef CAS PubMed.
- A. Üçer, A. Uyanik and Ş. F. Aygün, Sep. Purif. Technol., 2006, 47, 113–118 CrossRef.
- W. Wei, J. Li, X. Han, Y. Yao, W. Zhao, R. Han, S. Li, Y. Zhang and C. Zheng, Sci. Total Environ., 2021, 778, 146189 CrossRef CAS PubMed.
- A. E. Fazary, M. Taha and Y. H. Ju, J. Chem. Eng. Data, 2009, 54, 35–42 CrossRef CAS.
- S. Berto and E. Alladio, Front. Chem., 2020, 8, 614171 CrossRef CAS PubMed.
- T. K. Ross and R. A. Francis, Corros. Sci., 1978, 18, 351–361 CrossRef CAS.
- Y. Wang, Y. Zhang, C. Hou, F. He and M. Liu, New J. Chem., 2016, 40, 781–788 RSC.
- Q. Li, D. G. Barrett, P. B. Messersmith and N. Holten-Andersen, ACS Nano, 2016, 10, 1317–1324 CrossRef CAS PubMed.
- A. Espina, M. V. Canamares, Z. Jurasekova and S. Sanchez-Cortes, ACS Omega, 2022, 7, 27937–27949 CrossRef CAS PubMed.
- C. Chen, F. Chen, L. Liu, J. Zhao and F. Wang, Electrochim. Acta, 2019, 326, 134964 CrossRef CAS.
- S. M. Burkinshaw and N. Kumar, Dyes Pigm., 2009, 80, 53–60 CrossRef CAS.
- M. A. Rahim, H. Ejima, K. L. Cho, K. Kempe, M. Müllner, J. P. Best and F. Caruso, Chem. Mater., 2014, 26, 1645–1653 CrossRef CAS.
- C. M. Maerten, L. Lopez, P. Lupattelli, G. Rydzek, S. Pronkin, P. Schaaf, L. Jierry and F. Boulmedais, Chem. Mater., 2017, 29, 9668–9679 CrossRef CAS.
- A. Choodum, W. Sriprom and W. Wongniramaikul, Spectrochim. Acta, Part A, 2019, 208, 40–47 CrossRef CAS PubMed.
- M. F. Filgueiras and E. M. Borges, J. Chem. Educ., 2022, 99, 2067–2078 CrossRef CAS.
- A. Asthana, R. Verma, A. K. Singh, M. A. B. H. Susan and R. Adhikari, Macromol. Symp., 2016, 366, 42–51 CrossRef CAS.
- A. K. Singh, D. P. Singh, K. K. Pandayb and V. N. Singh, J. Chem. Tech. Biotechnol., 1988, 42, 39–49 CrossRef CAS.
- Y. S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- O. P. Murphy, M. Vashishtha, P. Palanisamy and K. V. Kumar, ACS Omega, 2023, 8, 17407–17430 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3sm01363d |
| This journal is © The Royal Society of Chemistry 2024 |