 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Simple and complex coacervation in systems involving plant proteins
Nirzar
Doshi
ab,
Wei
Guo
cd,
Feipeng
Chen
d,
Paul
Venema
b,
Ho Cheung
Shum
 cd,
Renko
de Vries
cd,
Renko
de Vries
 *a and
Xiufeng
Li
*a and
Xiufeng
Li
 *ce
*ce
aPhysical Chemistry and Soft Matter, Wageningen University and Research, Wageningen 6708 WE, The Netherlands. E-mail: renko.devries@wur.nl
bLaboratory of Physics and Physical Chemistry of Foods, Wageningen University, Bornse Weilanden 9, 6708, WG, Wageningen, The Netherlands
cAdvanced Biomedical Instrumentation Centre, Hong Kong Science Park, New Territories, Shatin, Hong Kong, China
dDepartment of Mechanical Engineering, The University of Hong Kong, Pokfulam Road, Hong Kong, China
eCollege of Food Science and Technology, Nanjing Agricultural University, Nanjing, 210095, Jiangsu, China. E-mail: xiufeng.li@njau.edu.cn
First published on 1st February 2024
Abstract
Plant-based foods are gaining popularity as alternatives to meat and dairy products due to sustainability and health concerns. As a consequence, there is a renewed interest in the phase behaviour of plant proteins and of mixtures of plant proteins and polysaccharides, in particular in the cases where coacervation is found to occur, i.e., liquid–liquid phase separation (LLPS) into two phases, one of which is rich in biopolymers and one of which is poor in biopolymer. Here we review recent research into both simple and complex coacervation in systems involving plant proteins, and their applications in food- as well as other technologies, such as microencapsulation, microgel production, adhesives, biopolymer films, and more.
Introduction
Driven by, among others, concerns about sustainability and health there is increased interest of consumers in plant-based foods as replacements for meat- and dairy products. As a consequence, there is great interest in current food technology research, both commercial and academic, in the extraction and processing of plant proteins, and in using them to formulate new and attractive plant-based food products.1–3Understanding the physical characteristics of plant proteins, such as their solubility, phase behaviour and interactions can be extremely helpful for improving their extraction and processing, and for formulating new products incorporating plant proteins.
With this in mind, we here review recent literature on the phase behaviour of plant proteins, both the fundamentals and applications in food technology. The focus is on Liquid-Liquid Phase Separation (LLPS) into biopolymer-rich and biopolymer-poor phases, sometimes also called coacervation. The term coacervation, coined by H. G. Bungenberg de Jong and H. R. Kruyt, delineates the separation process into two liquid layers in colloidal systems.4 As usual, we distinguish between simple coacervation, where LLPS is driven by attractive interactions of a single macromolecular species (Fig. 1A), and complex coacervation, where LLPS is driven by attractive interactions between two or more different macromolecular species (Fig. 1B), typically electrostatic interactions caused by opposite net charges on the biopolymers. LLPS and coacervation are closely related phenomena that many studies treat as synonyms. However, in this review, we only focus on simple and complex coacervation, which result in the formation of a polymer-rich phase and a polymer-dilute phase. We exclude another type of LLPS, segregative phase separation, such as that of polyethylene glycol (PEG) and dextran,5 from the scope of this review.
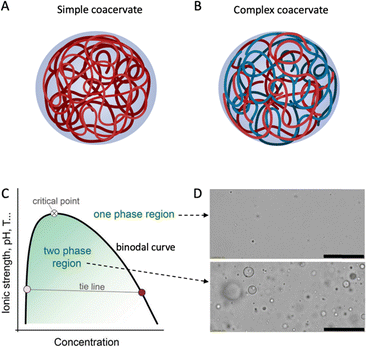 | ||
| Fig. 1 Schematic drawing of (A) simple and (B) complex coacervate droplets consisting of one or more entities. Created with BioRender.com. (C) Schematic phase diagram of simple and complex coacervates. Reproduced from6 with permission from the Royal Society of Chemistry, copyright 2021. (D) Optical microscopy images of a coacervate system in its one-phase region (top) or two-phase region (bottom). Scale bars are 50 μm. Reprinted from7 with permission from the American Chemical Society, copyright 2020. | ||
Whereas both simple and complex coacervation is a topic with a long history in the physical chemistry of biopolymers,8,9 including food biopolymers,10–12 the topic has recently also come to the fore in molecular biology with the discovery that LLPS underpins the formation of the many types of intracellular membrane-less organelles.6,13
Historically, most applications of LLPS in food technology have made use of complex coacervation, typically (but not exclusively) positively charged proteins with negatively charged polysaccharides, under acidic conditions.12 Not surprisingly therefore, with the strong recent growth of interest in plant proteins, many researchers have tried to identify and study systems of plant proteins and polysaccharides that exhibit complex coacervation and have considered food technology applications of these novel complex coacervates. This is the first topic that we will review.
Next, it is important to recognize that many of the plant proteins currently being used in food technology belong to the class of storage proteins of grains and legumes, and as such, have some common characteristics. In particular, many of the storage proteins of legumes are poorly soluble around their isoelectric points and exhibit pH-, salt-, and temperature-dependent LLPS. For example, the major storage proteins of grains, the prolamins, are generally insoluble in aqueous solvents and show LLPS in mixed solvents.7 While this behaviour has been well known from a fundamental point of view, applications in food technology of the simple coacervation of the storage proteins of grains and legumes are only now starting to be explored. This is the second topic that we will review.
Since most literature we consider is on the storage proteins of grains and legumes that are so crucial in food technology, in this review, we restrict ourselves to this class of proteins and refer to them simply as “plant proteins”. The outline of the review is as follows. First, we introduce biopolymer LLPS phenomena and terminology. Next, we review the recent literature on LLPS of plant proteins that focuses mostly on the phase separation per se, rather than on applications. In a further section, the content encompasses industries beyond food applications, emphasizing the broader need for other sectors to adopt sustainable methods. We close off with some observations about major challenges and opportunities in this field.
Liquid–liquid phase separation (LLPS)
Liquid–liquid phase separation of a homogeneous biopolymer solution results in two immiscible liquid phases, in which a dense biopolymer phase coexists with a dilute biopolymer phase. As shown by the generic phase diagram in Fig. 1C, the one-phase and two-phase regions are separated by a coexistence curve, which is known as the binodal in physical chemistry. The (overall) biopolymer concentration is on the x-axis, whereas parameters that control the interaction strength (ionic strength, pH, T) are on the y-axis. Below the binodal curve, the dense polymer phase exists as a dispersion of spherical liquid droplets (Fig. 1D, bottom) that can coalesce to eventually lead to macroscopic phase separation. Above the binodal curve, biopolymers are homogeneously dispersed in the solution (Fig. 1D, top). The nature of the weak attractions between the dissolved biopolymers that drive phase separation vary but certainly include electrostatic- and hydrophobic interactions and these, in turn, depend on the physical–chemical parameters such as the ionic strength, pH and temperature that are used as interaction strength parameters in the generic phase diagram (Fig. 1C).As Fig. 1C shows, the interaction strength determines the width of the two-phase region. Polymer concentration may also affect the interaction strength; however, it is not simple or linear, depending on various factors such as the type of polymers and interactions. For example, for a polymer solution with oppositely charged polyelectrolytes, the interaction strength usually increases with concentration at low polymer levels, as more polymer chains can bind.14 Coacervation occurs when the system reaches a minimum saturation concentration. Increasing polymer concentration enhances the interaction strength, resulting in a larger coexistence region and a higher critical point. However, after reaching a maximum, the interaction strength may decline with concentration, due to the repulsion between the polymers (e.g., same-charged polymers) and the entropy of mixing. The polymers tend to mix randomly to increase the entropy. Frequently, experimental coacervate studies focus on the low concentration regime, due to the limited solubility of food proteins in water.
For food applications, complex coacervates formed by proteins (mostly highly soluble proteins of animal origin) and anionic polysaccharides have received most of the attention. The earlier work on this topic has been summarized in several reviews. Turgeon et al.10 and Schmitt et al.11 thoroughly discussed fundamentals and applications of complex coacervates of proteins and polysaccharides. The review of de Vries et al.12 emphasizes that a liquid coacervate phase usually consists of weak polyelectrolytes, whereas strong polyelectrolytes often lead to the formation of solid precipitates. Pathak et al. summarized the roles of various interactions, such as electrostatic, surface patch, hydrogen bonding, and hydrophobic interactions, in coacervation, as well as the effects of ionic strength and heat on these interactions.15 More recently, Nickerson et al.16 and Li et al.17 also reviewed emerging work on plant protein–polysaccharide combinations that lead to complex coacervation. Zheng et al. examined the relaxation dynamics and molecular architectures of biopolymer complexes to clarify the distinction between liquid coacervates and solid precipitates, and to correct the misuse of the term coacervation.18
As opposed to complex coacervation, simple coacervation, also known as self-coacervation, has not been studied extensively with respect to its food applications, but there are several interesting recent developments that we will review here. It is well known that many plant storage proteins exhibit simple coacervation. Numerous studies have investigated LLPS, or coacervation, for the storage proteins from the seeds of soy, pea, mung bean, fava, wheat, and corn.7,19–24
Major classes of plant proteins known to exhibit simple coacervation are especially the globulins from legumes and the prolamins from grains. For globulins that exhibit coacervation, it is typically found to be sensitive to salt, suggesting that electrostatic interactions play a key role.24,25 For prolamins in mixed solvents the main parameter is the solvent composition.7,26–29 Since these are typically quite hydrophobic and insoluble in most aqueous solvents, in this case, it appears that solvation effects, such as hydrophobic interactions, are more important. While these general points appear to be clear, there is no detailed understanding of the molecular interactions that drive LLPS in plant storage proteins, yet obtaining such understanding could open doors for developing more innovative applications of plant protein simple coacervation.19,24,30 Next, we first provide overviews of the main systems for which LLPS involving plant storage proteins (either complex- or simple coacervation) has been studied. After that, we turn to applications.
Complex coacervation
Liquid–liquid phase separation of plant storage proteins (both complex coacervation and simple coacervation) is strongly affected by not only the species of plant from which the protein is extracted but also by how it is extracted. This latter dependency is not always clear from the literature, hence we briefly discuss this point first. After that we discuss the plant storage proteins for which complex coacervation has been studied, organized by protein source and nature of extraction.Impact of extraction procedures on state and phase behaviour of plant storage proteins
Many proteins from animal sources have been successfully used to formulate complex coacervates, and have been widely applied in the food industry, such as core–shell microcapsules made of gelatin-Arabic gum complex coacervates.31–33 In trying to develop applications of coacervation involving plant proteins, it is often suggested that protein solubility is the biggest challenge. Numerous studies have emphasized the crucial role of forming intermolecular soluble complexes, a step that precedes coacervation and is essential for the upcoming LLPS.34,35However, the nature of animal proteins, like whey protein and collagen (gelatin), is similar to that of plant storage proteins, like albumins and globulins, in terms of their hydrophobic amino acid content percentage. For example, Fig. 2 shows the fraction of hydrophobic residues (alanine, valine, leucine, and glycine) for a range of proteins. Except for the corn prolamin zein, which is quite hydrophobic and insoluble in most aqueous solvents, the fraction of non-polar amino acids in most plant proteins is not necessarily higher than for gelatin and whey, two animal proteins that are widely used for formulating complex coacervates.
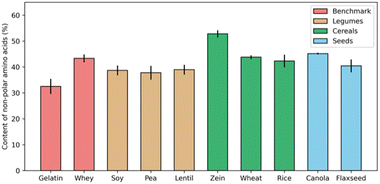 | ||
| Fig. 2 Percentage of non-polar amino acids in total protein contents from different sources. For each protein source, data was collected from three independent studies, and error bars indicate the standard deviation.28,36–51 | ||
Clearly, of course, the fraction of hydrophobic amino acids is not a very precise predictor of solubility. In particular, the extent to which the proteins self-assemble into higher-order structures may also play a role. This is known to be important, especially for plant globulins, which exhibit a range of association states depending on solution conditions.52
Traditional categories for storage plant proteins derive from the different solvents that have been used to extract and fractionate them: the so-called Osborne classification method distinguishes water-soluble albumins from salt-soluble globulins, ethanol-soluble gliadins and prolamins, and acid- or alkali-soluble glutelins. The extraction processes themselves, however, may have a strong effect on the structures of the plant storage proteins as extracted, and this, in turn, may be expected to have a strong impact on their phase behaviour including LLPS. Processing steps that have been implicated in plant protein denaturation are for example various precipitation- and drying steps.53
Therefore in this review, we try to distinguish between (1) plant storage proteins isolated at the lab scale, which can often be of high purity and have a low degree of denaturation, and (2) concentrates of plant storage proteins that have often been obtained with mild processes such as dry fractionation that lead to a low degree of denaturation but also a rather low purity, and (3) isolates of plant storage proteins which have high purity but which often also have a high degree of denaturation due to harsh processing steps. With this in mind, we next discuss complex coacervation for plant storage proteins isolated from legumes, oil seeds, and cereals, respectively.
Legume proteins
The seeds of leguminous plants such as soy, peas, and lentils are an important source of plant proteins. Despite variations across different species of crops, salt-soluble globulins (60–80%) and water-soluble albumins (10–25% of total storage protein) are the most abundant protein fractions in legumes.54–56 As mentioned, isolates of these proteins are often poorly soluble. These powders form dispersions with a broad range of particle sizes and typically do not form liquid complex coacervates when complexed with oppositely charged polyelectrolytes.Instead, to form complex coacervates many groups start from either flours or concentrates and perform lab-scale extractions. Typically, alkali extraction is used, often an appropriate amount of salt is also added to enhance the solubility of the globulins.57,58 After removing insoluble components through centrifugation, and dialysis to remove the salts, the proteins are acid precipitated and freeze-dried.
The salt removal is crucial since complex coacervation is opposed by the addition of salt which screens the electrostatic interactions driving the phase separation. Indeed, as reported by Li et al.,59 the critical salt (NaCl) concentrations, above which no complexes are formed, for soy protein extracts and pea protein extracts with Arabic gum were found at 151 mM and 160 mM, respectively, when the total polymer concentrations were kept at 1.2 mg mL−1.
Lab scale isolates of legume seed proteins prepared as explained above often have low degrees of denaturation. Such protein isolates obtained from soybean, pea, and lentils have been shown to form complex coacervates with various polysaccharides, including sugar beet pectin,60–62 κ-carrageenan and gellan gum,63 Arabic gum,64–70 and alginate71 at pH 3 to 5, or with chitosan72–75 at around pH 6.
The lab scale isolates are however not very representative of the protein ingredients used currently in the food industry. With this in mind, Li et al.59 explored the idea of directly complexing polysaccharides to legume proteins in impure protein concentrates. Since most natural polysaccharides are anionic, and hence allow only for complex coacervation at low pH, acid extraction was used, and Arabic gum was added directly. The impure nature of the extract did not prevent complex coacervation from occurring. As shown in Fig. 3, the acid-extracted soy proteins formed liquid coacervates with Arabic gum at pH 3, and this coacervate tended to wet the surface of oil droplets. In contrast, alkali-extracted pea proteins formed solid precipitates with chitosan at pH 5.8. Other authors72–75 report that complex coacervates were formed from chitosan and acid-extracted soy proteins, rather than solid precipitate particles, and this illustrates the tricky point that coacervation is a sensitive function of the state of the proteins and also for example of the precise degree of deacetylation of the chitosan.
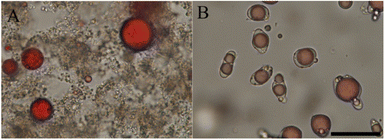 | ||
| Fig. 3 Bright-field microscopic graphs of complex formation and their interfacial affinity on oil droplets. (A) Chitosan with alkali-extracted pea protein at pH 5.8. (B) Arabic gum with acid-extracted soy protein at pH 3. Oil droplets were dyed in red, and the scale bar is 50 μm. Reprinted from59 with permission from the American Chemical Society, copyright 2021. | ||
An interesting counterexample to the general case that denaturation of plant proteins (such as in industrial plant protein isolates) prevents complex coacervates from forming is provided by Chourpa et al. They report that pea globulins partially lost their beta-sheets at pH 2.75, yet this was the pH where the strongest interaction with Arabic gum was found and liquid coacervates still occurred.76
Heat treatments are key steps in food processing, hence it is interesting to study the interplay between heat-induced protein aggregation and complex coacervation. We have already discussed the general observation that liquid coacervates typically do not form from industrial plant protein isolates. In the study of Dong et al.,70 the authors compared the rheological properties of complex coacervates composed of Arabic gum and soy protein isolates that were either preheated or not. Fig. 4 shows that following our general expectation that liquid coacervates only form for proteins that are mostly native and non-aggregated, samples with preheated protein complexed with Arabic gum are distinctly gel-like, which was also borne out by more detailed rheological characterization.
 | ||
| Fig. 4 Photographs showing the state of complexes formed by Arabic gum with soy protein (left), soy protein heated at 85 °C (middle) and 95 °C (right). Reprinted from70 with permission from Wiley, copyright 2019. | ||
Oil seed proteins
Industries typically produce commercial seed proteins from oil crops as byproducts, but these seed proteins have undergone harsh processing and hence are not suited for studying complex coacervation, in view of its sensitivity to the structure of the proteins. In studies on complex coacervation involving oil seed proteins, most research groups isolate the proteins at a lab scale. Similar to storage proteins from legume proteins, the major storage protein fractions in oilseeds are salt-soluble globulins and water-soluble albumins, and they can be extracted in similar ways from (defatted) seed flour. Indeed, seed proteins from canola, rapeseed, flaxseed, and chia have been frequently reported to form complex coacervates with anionic polysaccharides, such as alginate,77 (κ-, ι- and λ-type) carrageenan,78 chia seed gum,79 flaxseed gum,80 Arabic gum,81,82 and pectin.83 For this set of reports, however, it is less clear whether the complexes that were formed were truly complex coacervates: mostly only turbidity measurements were reported, possibly along with optical microscopy. High turbidity and spherical particle morphologies do not necessarily exclude the possibility that the complexes that are formed are not liquid, but rather solid-like. For example, Nickerson et al.82 reported that canola protein isolate forms complexes with Arabic gum at acidic pH, forming a material that has storage moduli higher than loss moduli over a broad frequency range, contrary to bulk coacervate phases which typically are only weakly viscoelastic.84It is not always easy to demonstrate the liquid-like nature of complexes, especially if bulk phase separation is not attained. Possible ways are for example demonstrating droplet coalescence or spreading of droplets on surfaces.85
Cereal proteins
Corn and wheat are major sources of cereal proteins. Compared with proteins obtained from legumes, corn and wheat usually contain fewer albumins and globulins, but more prolamins, which are more hydrophobic. Indeed, prolamins (gliadin and glutenin) represent around 80% of the total protein content in wheat flour, and more than 50% of corn proteins are prolamins (corn prolamins are known as zein).86 These proteins are largely insoluble in aqueous solvents and hence there are very few convincing reports of complex coacervation of prolamins in purely aqueous solvents.An exception worth mentioning is the report of Boury et al. The key point is that while at moderate pH values solubility of prolamins in aqueous solvents is very low, at extreme pH, the solubility of prolamins in aqueous solvents can be appreciable. Boury et al. reported that alpha-gliadin (30 KDa) obtained from wheat proteins by chromatography can form liquid coacervate droplets with Arabic gum around pH 3, and these liquid droplets spread at an oil–water interface. Hence it seems like at least under some conditions, prolamins have the potential to be used in formulating complex coacervates in aqueous solutions.65 This finding certainly encourages the community to also further explore the potential of prolamins for complex coacervation.
Furthermore, the phase behaviors of zein with various polyanions, such as pectin,87 dsDNA,88 agar,89 and LAPONITE®,34 were investigated in ethanol-containing solutions. It was found that ethanol enhances the solubility of zein, allowing the complexes to retain more solvent molecules and remain fluidity. As observed in most complex coacervate systems in pure solvents,12 the optimal coacervation condition is usually achieved at charge balance. These studies offer a method to obtain complex coacervates of hydrophobic proteins like zein by adding a miscible organic solvent.
Some other reports are available on complex particles composed of polysaccharides and zein or gliadin,90–92 but as for the case of the oil seed proteins, in these cases it was unclear whether the particles were liquid coacervate droplets or solid complex particles. Next, we give an overview of the systems involving plant storage proteins for which simple coacervation has been studied.
Simple coacervation
For simple coacervation of plant seed storage proteins, the best-studied cases are the purified soy proteins glycinin and β-conglycinin, hence these are discussed first. Next, we consider simple coacervation for less purified soy protein and other legume proteins, such as peas, mung beans, and fava beans. Finally, we review simple coacervation of cereal proteins, such as prolamins from wheat, corn, and rice, in mixed solvents.Purified soy proteins
Soy protein primarily comprises the globular storage proteins glycinin and β-conglycinin. The phase behaviour of purified glycinin and β-conglycinin has been studied quite extensively in recent years.In the Osborne classification, the plant seed globulins are classified as “salt soluble”. While a minimum amount of salt appears to be necessary to obtain LLPS, further increase of salt indeed opposes phase separation and promotes solubility. Coming from the alkaline side, a decrease in the pH promotes LLPS. At pH values close to the iso-electric point, globulins may also enter solid–liquid phase equilibria (precipitate), a phenomenon used in their large-scale isolation in industry. Finally, higher temperatures oppose LLPS. These tendencies are nicely illustrated, for example, by the recent work of Chen et al. for purified soy glycinin.19
Early work by Tolstoguzov and coworkers explained the salt dependence of the critical temperature for LLPS of plant seed globulins in terms of molecular theories of electrostatic dipole–dipole interactions.93,94 Globulins typically exist as oligomers of disulphide bridged acidic- and basic chains, hence strong dipolar interactions may indeed be expected, and these are screened at high salt. More straightforward is the pH-dependence, which can be understood as being due to a balance between the attractive interactions that drive coacervation (whether of dipolar or other origins) and the electrostatic monopole-monopole repulsions, which oppose coacervation, and which will be stronger away from the isoelectric point.
In their recent work on soy glycinin, Chen et al. emphasize several additional molecular features of soy glycinin that may play a role.19 In particular, they emphasize that the acidic polypeptides have disordered hypervariable regions of significant hydrophobicity19 which may contribute to glycinin self-coacervation.95,96 Finally, Chen et al. also emphasize the work of Lakemond et al.25 who find whereas at neutral pH, soy glycinin mostly exists as hexamers, the equilibrium shifts towards trimers when decreasing the pH. Additionally, at very low ionic strengths, the acidic chains appear to be increasingly hidden and the basic sidechain is increasingly exposed. Since the hypervariable regions of the acidic chains are implicated in self-coacervation, this suggests a mechanism for why very low ionic strength opposes LLPS.19
Some of the temperature dependence of the LLPS may be due to the temperature dependence of the dipolar interactions as originally suggested by Popello.93,94 If hydrophobic interactions, for example of the hypervariable regions of the acidic chains, are also important, as suggested by Chen et al.,19 this may provide another explanation of the temperature dependence.
Mixtures of pure soy proteins, crude soy protein extracts
Industrially produced protein powders, including those from legumes, are complex mixtures containing different protein fractions. For example, soy protein powder contains a combination of globulins, glycinin and β-conglycinin, while pea and mung protein powders especially contain legumin and vicilin. To understand the behaviour of these protein mixtures, it is essential to also investigate the phase behaviour of mixed systems.LLPS in soy protein mixtures was first reported for crude soy protein mixtures extracted directedly from defatted soy flour at alkaline pH.97 This study investigated the effect of pH on the precipitation of soy protein on acidification after alkaline extraction. The authors report the occurrence of micrometre-sized droplets of concentrated soy protein when acidifying down to pH values in the range pH 6.8–5.7. Acidification to lower pH values, closer to the protein isoelectric points, led to solid–liquid phase separation (aggregation/precipitation) rather than to LLPS.
Later studies considered more purified mixtures of glycinin and β-conglycinin to study the role of each of these proteins in the LLPS of the crude flours.97–99 The main conclusion appears to be that glycinin more readily exhibits LLPS than β-conglycinin, consistent with the earlier observation that the protein droplets in acidified soy flour protein extracts mainly seemed to consist of glycinin.97
It is crucial to note that phase behaviour is sensitive to the details of the secondary, tertiary, and quaternary structure of the proteins, hence it may be expected that LLPS is a sensitive function of the processing history of the proteins or protein mixtures. Indeed, this is now clear from several studies that focus on soy glycinin.100,101 These show that prolonged incubation of alkaline protein extracts at low salt (several days) can lead to the dissociation of hexamers into trimers, which in turn leads to aggregation rather than LLPS after pH-adjustment. A similar effect was demonstrated when reducing agents were used to cleave the disulfide linkages that maintain the quaternary structure of the glycinin hexamers.
These observations demonstrate the importance of protein structure (at all levels) in determining plant storage protein LLPS. It appears that when starting from commercially available protein ingredients, LLPS is best obtained by acidifying alkaline extracts obtained from crude flours of mildly processed concentrates, rather than from the more extensively processed isolates.
Other legume proteins
Next to the best-studied cases of soy glycinin and β-conglycinin, liquid–liquid phase separation has been observed for a wide range of plant globulins, from a wide range of legume sources. Here we provide a short list without going into much detail.Very thorough initial work on purified globulins from broad beans was performed by Tolstoguzov et al.93,94 this work includes a detailed theoretical interpretation in terms of statistical-thermodynamical theories of phase separation.
Another source for which extensive studies have been performed is yellow pea.24,102 Observations for crude mixtures of mildly extracted proteins24,102 are very similar to those for soy. In a study where a range of extraction and purification methods were compared, Kornet et al. clearly showed that only mild extractions preserved the LLPS, whereas no LLPS was observed for yellow pea globulins extracted using methods known to lead to structural changes, denaturation, and aggregation, such as isoelectric precipitation at low pH.24
Yet another crop of increasing importance for producing industrial plant proteins is fava beans. To ultimately develop protein microcapsules, Zhao demonstrated LLPS for purified fava legumins.103
Finally, crude mixtures of alkaline-extracted mung bean proteins have also been shown to be able to lead to LLPS when acidified.20 Proteins were extracted from mung bean flour enriched in protein by dry fractionation. Whereas LLPS in crude mixtures of alkaline extracted soy- and yellow pea proteins leads to the formation of protein droplets of many micrometres in size, for the case of mung bean instead it was found that there were only a few droplets that reached micrometre size. Instead, most droplets were much smaller and had sizes significantly below a micron.
In summary, globulins from many legumes (including soy, pea, mung, and fava bean) have been shown to exhibit LLPS, strongly suggesting that this behaviour should be more or less general across an even wider array of plant seed globulins than discussed here, and should be connected to shared biochemical and structural features of these plant seed globulin proteins.
Cereal proteins
Prolamins from the seeds of cereals such as corn, wheat, rice etc. are also known to exhibit LLPS. These proteins are generally insoluble in aqueous solvents (except sometimes at extreme pH values) but do dissolve in mixed solvents such as water/ethanol, or water/propylene glycol. LLPS typically occurs for a narrow range of solvent compositions, that are in between the regions of protein solubility and insolubility.Prolamin LLPS and its applications have been studied especially for zein from corn, for a range of solvent systems, including water/propylene glycol,7 as well as alcohols such as methanol, ethanol, and isopropyl alcohol.21 A unique feature of the self-coacervation of zein is that, presumably due to the strong hydrophobic driving force for coacervation, it is much less pH sensitive than self-coacervation for globulins, where changing the electrostatic interactions has a drastic impact on the phase separation. Consequently, in phase phase-separated system, prolamin droplets can have a wide range of surface charges, from positive to negative, which has important consequences for properties such as wetting of these droplets on surfaces.7,21
Wheat is another cereal with seeds of high prolamin content. Gliadins from wheat have also been investigated for their capacities to form simple coacervates.22,23 These studies indicate that for gliadin extracted from commercial gluten, as compared to zein, a relatively high protein concentration and relatively low salt concentration are required to form coacervates.22 Coacervation was also found to depend more strongly on pH than for zein23 suggesting that the balance of hydrophobic and electrostatic forces driving and/or opposing phase separation is different for these two prolamins.
Applications
Applications of (associative) polymer LLPS in nature and technology are extremely varied but all derive from a few of their basic physical properties. For example, strong partitioning effects due to the very different compositions of concentrated and excess phases are exploited in purification processes or membrane-less organelles, low interfacial tension against the excess phase is a key driver for their excellent wetting properties, and for the high stability of dispersions of small droplets of concentrated phase in the excess phase. Additionally, the combination of good wetting and high viscosity makes them useful as adhesives and glues. Note that in many applications, such as microencapsulation or glues, the concentrated phase is an intermediate stage that is useful for processing, but which is later cured into a solid phase.As mentioned at the beginning of the review, there is a clear need for more sustainable production of foods, as well as for more sustainable non-food materials. Therefore, it is only logical that there is much activity on exploring whether plant biopolymers, including plant proteins, could substitute for less sustainable polymers in technological applications of coacervation. Here we review recent work on a number of these applications, viz., microencapsulation, the production of microgels, adhesive glues and thin films.
Microencapsulation
A classic application of coacervation is microencapsulation, for example for flavours and fragrances.31,32 Preparing core–shell microcapsules typically involves four steps: formation of an oil-in-water emulsion, LLPS of the continuous phase, shell formation, and shell hardening. The key point is that when inducing LLPS in the continuous phase, the (very small) droplets of the condensed phase will adhere to and spread on the surface of the (somewhat larger) oil droplets.The microencapsulation process is a dynamic process in which the solvent quality of the polymers in the continuous phase is gradually reduced to induce the LLPS. It involves interactions and transport phenomena among three phases: oil, water, and coacervate. Erni and Dardelle discuss how wetting phenomena and coacervate viscoelasticity affect this process.33 They show that core–shell structures do not form by spontaneous spreading of coacervate droplets at the oil/water interface, but rather by sequential deposition and coalescence of individual droplets. Hence, not only surface tensions play a role but also coacervate viscoelasticity and interactions between coacervate droplets that may either favour or oppose their coalescence.
Li et al. reported a simple method to extract soluble proteins from legume flours at acidic pH.59,104 The extracted proteins are not further purified but instead directly used to form coacervates via the addition of oppositely charged polysaccharides such as Arabic gum. The coacervates can be used to create core–shell microcapsules in the presence of oil droplets (Fig. 3B). The authors demonstrated that the coacervate shell can be cured by simply heating above the denaturation temperature of the legume proteins.
Simple coacervation of plant storage proteins has also been exploited for microencapsulation. Another study by Li et al. showed that zein, the major prolamin from corn, can form a simple coacervate in a mixture of water and propylene glycol and that the resulting coacervate droplets wet oil/water interfaces (Fig. 5A).7 Unlike complex coacervates, which are nearly neutral in charge, simple coacervate droplets of zein can have a significant net charge. In the study, it was found that while wetting of the oil/water interface by the coacervate was favoured thermodynamically, kinetic limitations prevented capsule formation, except in a narrow range of pH values around pH 8. The explanation suggested by the authors was that capsule formation cannot occur at high droplet charges since then coacervate droplet coalescence is too slow, while on the other hand, at a coacervate droplet charge that was too low, extensive coacervate droplet coalescence was observed to occur in the bulk phase rather than at the oil/water interface.
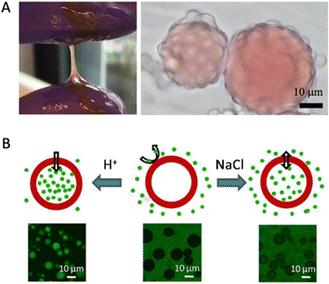 | ||
| Fig. 5 (A) Left: Photograph of zein simple coacervate phase in bulk; Right: Micrograph of zein simple coacervate droplets sitting on oil droplets. Oil droplets are dyed with Oil Red O. Reproduced from7 with permission from the American Chemical Society, copyright 2020. (B) Illustration of ionic strength and pH-responsive permeability of soy glycinin microcapsules. The green colour represents FITC-dextran (2000 kDa). Reproduced from105 with permission from the American Chemical Society, copyright 2018. | ||
By exploiting the subtle temperature dependence of the LLPS of plant seed globulins, one can also create core–shell microcapsules in the absence of oil droplets as a template. A precondition is that one should work in conditions where phase separation occurs at room temperature, but not at a higher temperature (that is still below the temperature for protein denaturation and aggregation). Then, by tuning the rate of increase of temperature, one can induce the partial dissolution of coacervate droplets, leading to the formation of (solvent-filled) vacuoles inside the coacervate droplets. Further temperature increases lead to protein denaturation and aggregation and cure these hollow structures.
Chen and Nicolai et al. studied how to form core–shell microcapsules from soy glycinin via simple coacervation.19,25,102,105 When heated above 60 °C, they observed that coacervate droplets turned into hollow core–shell microcapsules. The temperature history (heating rate, temperatures) strongly affected whether hollow structures were formed or not, as reported by Cochereau et al.102 Chen et al. tested how ionic strength and pH changed the permeability of the core–shell microcapsules after formation.105 They reported that the capsules remained intact between pH 1 and 11.5 and that their permeability was a function of pH and salt, such that changes in these parameters could be used for loading and unloading cargo (Fig. 5B).
Microgels
As reviewed elsewhere,106 protein microgels, which have also been called protein particles or protein colloids, have a range of suggested applications, such as fat replacers or as ingredients in plant-based beverages. Except under the special conditions reviewed above, heating protein coacervate droplets will normally lead to the formation of protein microgels. Indeed, in the studies on microcapsule formation from soy glycin reviewed above, in many cases, rather than microcapsules, microgel formation was also observed to occur.With the specific aim of creating a low-viscosity mung bean protein ingredient, Yang et al. studied microgel formation by heating coacervate droplets formed after alkaline protein extraction of mung bean flour, followed by slow acidification.20 In this case it was found that the coacervate droplets and hence also the resulting microgels, had sizes in the submicron range, which is very favorable for beverage applications. Also, it was found that it was possible to spray dry the microgels, and that spray drying did not affect the size of the microgels (after resuspension).
Adhesives and films
There has been much activity in the area of coacervate-based (underwater) glues, inspired by biological coacervate glues of for example mussels and sandcastle worms.107,108 Several investigations have appeared in which plant proteins feature as components in glue systems.109–111 Very closely related are polymer films obtained by spreading and drying coacervates. Also, here it appears that processing from the coacervate state may offer functional benefits. These two applications are discussed next.One unique feature of coacervate-based adhesives is that they can be applied in humid and underwater environments, such as the human body. In biomedical applications, various types of coacervates have been explored as biomimetic adhesives for the treatment of bone fractures. Quek and Wattanutchariya investigated the potential of using zein coacervates as a bone haemostatic agent and bone filler in surgery.109 As we previously mentioned, zein has low solubility in water and can form coacervates by simply mixing with binary solvents containing ethanol and water. Therefore, zein coacervates could be easily prepared during surgery and used to adhere to bone surfaces and stop bleeding. The authors demonstrated that zein coacervates degrade in solutions containing lysozyme, a common enzyme in biological fluids, indicating that the material is bioresorbable and can be removed by cell-mediated degradation. For non-biomedical applications, zein has also been explored as a bio-based alternative to petroleum-derived adhesives. Schmidt et al. showed that high-strength adhesives can be obtained from zein protein cross-linked with plant phenolics.110,111 Phenolics are a group of compounds found in some plants and animals that can enhance the adhesion of proteins to various surfaces. The glues exhibited strong adhesion strength on different surfaces (wood, steel, and plastic). Notably, although the authors did not use the term coacervation in their studies, they dissolved zein protein in water/ethanol binary solvents and then dried the samples. The samples likely went through the coacervation stage before they completely dried, since ethanol evaporates faster than water.
Edible polymer films or coatings made of plant-based ingredients have also received increasing attention in the food industry. These films could play a pivotal role in long-term food preservation. Coacervation appears to be a convenient strategy to tune the appearance and performance of the film, such as mechanical strength and flexibility, opacity, and water and oxygen permeability. Maria et al. investigated edible films obtained from complex coacervates of soy protein and carboxymethyl cellulose.112 This study showed that increasing the polysaccharide concentration resulted in an increased tensile strength of all composite films. Furthermore, coacervation conditions resulted in more brittle and heterogeneous films. Film opacity was also found to be variable depending on the pH conditions, showing higher opacity under coacervation conditions compared to films from alternative processing routes. Furthermore, higher carboxymethyl cellulose concentration resulted in a reduced oxygen-permeable film. In another study focusing on complex coacervates of soy protein isolate and high-methylated pectin, the authors113 found that when the pH was reduced to acidic pH, the transparency of soy protein isolate films was compromised. The addition of pectin to soy protein isolate films did not notably affect their elasticity, solubility, or permeability to water vapour. However, it did contribute to an enhancement in tensile strength.
Conclusions and perspectives
A crucial point when trying to explore the coacervate properties of plant seed storage proteins is that this is probably best done for sources in which the protein secondary, tertiary, and quaternary structures have not (yet) been compromised by extensive processing. While many papers are using lab-extracted plant proteins, these are not very representative of commercially available plant protein sources. This does not mean that commercial applications of plant storage protein coacervates cannot be developed, since coacervation can also be achieved in impure mixtures with moderate contents of native proteins, such as concentrates.There is a very broad range of technological applications that have been explored for polymer coacervates and many of these, plant storage proteins could be sustainable replacements for example for synthetic polymers. Complex coacervates of plant biopolymers are an especially popular topic, but research on the fundamentals and applications of simple coacervation of plant storage protein is rapidly gaining traction.
Many opportunities remain in this area and have hardly been explored yet, for example, spinning114,115 and bioprinting,116,117 both of which may benefit from the unique physical properties of coacervates and the sustainability of plant biopolymers.
Author contributions
Conceptualization: N. D., R. d. V., X. L.; funding acquisition: R. d. V., H. C. S.; supervision: R. d. V.; visualization: N. D., X. L.; writing – original draft: N. D., W. G., F. C., R. d. V., X. L.; writing – review & editing: All authors reviewed and edited the manuscript.Conflicts of interest
H. C. S. is a scientific advisor of EN Technology Limited, Microdiagnostics Limited, PharmaEase Tech Limited, and Upgrade Biopolymers Limited, and also a managing director of the research center, namely Advanced Biomedical Instrumentation Center Limited. X. L. is a cofounder and director of Upgrade Biopolymers Limited. The works in the paper are however not directly related to the core businesses of these companies. The remaining authors declare no competing interests.Acknowledgements
X. L., W. G., and H. C. S. were funded by Health@InnoHK program of the Innovation and Technology Commission of the Hong Kong SAR Government. This work is part of the project ‘Clean label solutions for structuring plant based foods’ co-financed by the Top Consortium for Knowledge and Innovation Agri & Food by the Dutch Ministry of Economic Affairs under contract number LWV20.68. We thank Qin Li for useful discussions on the use of coacervates in producing biopolymer-based films.References
- A. Tamayo Tenorio, J. Gieteling, G. A. H. de Jong, R. M. Boom and A. J. van der Goot, Food Chem., 2016, 203, 402–408 CrossRef CAS PubMed.
- A. J. van der Goot, P. J. M. Pelgrom, J. A. M. Berghout, M. E. J. Geerts, L. Jankowiak, N. A. Hardt, J. Keijer, M. A. I. Schutyser, C. V. Nikiforidis and R. M. Boom, J. Food Eng., 2016, 168, 42–51 CrossRef.
- B. L. Dekkers, R. M. Boom and A. J. van der Goot, Trends Food Sci. Technol., 2018, 81, 25–36 CrossRef CAS.
- H. G. Bungenberg de Jong and H. R. Kruyt, Proc. K. Ned. Akad. Wet, 1929, 32, 849–856 Search PubMed.
- F. Chen, X. Li, Y. Yu, Q. Li, H. Lin, L. Xu and H. C. Shum, Nat. Commun., 2023, 14(1), 2793 CrossRef CAS PubMed.
- M. Abbas, W. P. Lipiński, J. Wang and E. Spruijt, Chem. Soc. Rev., 2021, 50, 3690–3705 RSC.
- X. Li, P. Erni, J. Van Der Gucht and R. De Vries, ACS Appl. Mater. Interfaces, 2020, 12, 57 Search PubMed.
- I. K. Voets, A. de Keizer and M. A. Cohen Stuart, Adv. Colloid Interface Sci., 2009, 147–148, 300–318 CrossRef CAS PubMed.
- A. H. Hofman, I. A. van Hees, J. Yang and M. Kamperman, Adv. Mater., 2018, 30, 1704640 CrossRef PubMed.
- S. L. Turgeon, C. Schmitt and C. Sanchez, Curr. Opin. Colloid Interface Sci., 2007, 12, 166–178 CrossRef CAS.
- C. Schmitt and S. L. Turgeon, Adv. Colloid Interface Sci., 2011, 167, 63–70 CrossRef CAS PubMed.
- C. G. de Kruif, F. Weinbreck and R. de Vries, Curr. Opin. Colloid Interface Sci., 2004, 9, 340–349 CrossRef CAS.
- D. Bracha, M. T. Walls and C. P. Brangwynne, Nat. Biotechnol., 2019, 37, 1435–1445 CrossRef CAS PubMed.
- E. Spruijt, A. H. Westphal, J. W. Borst, M. A. Cohen Stuart and J. van der Gucht, Macromolecules, 2010, 43, 6476–6484 CrossRef CAS.
- J. Pathak, E. Priyadarshini, K. Rawat and H. B. Bohidar, Adv. Colloid Interface Sci., 2017, 250, 40–53 CrossRef CAS PubMed.
- S. N. Warnakulasuriya and M. T. Nickerson, J. Sci. Food Agric., 2018, 98, 5559–5571 CrossRef CAS PubMed.
- B. Muhoza, B. Qi, J. D. Harindintwali, M. Y. Farag Koko, S. Zhang and Y. Li, Food Hydrocolloids, 2022, 124, 107239 CrossRef CAS.
- J. Zheng, P. Van der Meeren and W. Sun, Aggregate, 2023, e449 CrossRef.
- N. Chen, Z. Zhao, Y. Wang and R. Dimova, ACS Macro Lett., 2020, 9, 1844–1852 CrossRef CAS PubMed.
- Q. Yang, P. Venema, E. van der Linden and R. de Vries, Food Hydrocolloids, 2023, 139, 108541 CrossRef CAS.
- L. Muthuselvi and A. Dhathathreyan, Colloids Surf., B, 2006, 51, 39–43 CrossRef CAS PubMed.
- M. C. Mauguet, J. Legrand, L. Brujes, G. Carnelle, C. Larre and Y. Popineau, J. Microencapsulation, 2002, 19, 377–384 CrossRef CAS PubMed.
- M. G. Herrera and V. I. Dodero, Self-assembly of Gliadin protein modulated by pH, 2013.
- R. Kornet, S. L. Roozalipour, P. Venema, A. J. van der Goot, M. B. Meinders and E. van der Linden, Food Hydrocolloids, 2022, 125, 107379 CrossRef CAS.
- N. Chen, M. Zhao, T. Nicolai and C. Chassenieux, Biomacromolecules, 2017, 18, 2064–2072 CrossRef CAS PubMed.
- C. D. Evans and R. H. Manley, Ind. Eng. Chem., 1944, 36, 408–410 CrossRef CAS.
- C. D. Evans and R. H. Manley, Ind. Eng. Chem., 1941, 33, 1416–1417 CrossRef CAS.
- R. Shukla and M. Cheryan, Ind. Crops Prod., 2001, 13, 171–192 CrossRef CAS.
- R. H. Manley and C. D. Evans, Ind. Eng. Chem., 1943, 35, 661–665 CrossRef CAS.
- H. B. Bohidar and B. Mohanty, Phys. Rev. E: Stat., Nonlinear, Soft Matter Phys., 2004, 69, 21902 CrossRef CAS PubMed.
- G. Dardelle, M. Jacquemond and P. Erni, Adv. Mater., 2017, 29, 1606099 CrossRef PubMed.
- P. Erni, G. Dardelle, M. Sillick, K. Wong, P. Beaussoubre and W. Fieber, Angew. Chem., Int. Ed., 2013, 52, 10334–10338 CrossRef CAS PubMed.
- G. Dardelle and P. Erni, Adv. Colloid Interface Sci., 2014, 206, 79–91 CrossRef CAS PubMed.
- P. Tiwari, I. Bharti, H. B. Bohidar, S. Quadir, M. C. Joshi and N. Arfin, ACS Omega, 2020, 5, 33064–33074 CrossRef CAS PubMed.
- K. Kaibara, T. Okazaki, H. B. Bohidar and P. L. Dubin, Biomacromolecules, 2000, 1, 100–107 CrossRef CAS PubMed.
- D. S. Kalman, Foods, 2014, 3, 394–402 CrossRef PubMed.
- S. Wu, X. Wang, W. Qi and Q. Guo, Trends Food Sci. Technol., 2019, 92, 184–193 CrossRef CAS.
- P. Kaushik, K. Dowling, S. McKnight, C. J. Barrow, B. Wang and B. Adhikari, Food Chem., 2016, 197, 212–220 CrossRef CAS PubMed.
- S. H. Tan, R. J. Mailer, C. L. Blanchard and S. O. Agboola, J. Food Sci., 2011, 76, R16–R28 CrossRef CAS PubMed.
- E. Kuznetsova, L. Shayapova, E. Klimova, G. Nasrullaeva, J. Brindza, M. Stolyarov, G. Zomiteva, T. Bychkova and V. Gavrilina, Potravinarstvo, 2019, 13, 1 CrossRef PubMed.
- R. S. Bhatty, Can. Inst. Food Sci. Technol. J., 1988, 21, 144–160 CrossRef CAS.
- T. Kumagai, H. Kawamura, T. Fuse, T. Watanabe, Y. Saito, T. Masumura, R. Watanabe and M. Kadowaki, J. Nutr. Sci. Vitaminol., 2006, 52, 467–472 CrossRef CAS PubMed.
- F. F. Shih and K. W. Daigle, J. Am. Oil Chem. Soc., 2000, 77, 885–889 CrossRef CAS.
- T. L. Pownall, C. C. Udenigwe and R. E. Aluko, J. Agric. Food Chem., 2010, 58, 4712–4718 CrossRef CAS PubMed.
- P. Leterme, T. Monmart and E. Baudart, J. Sci. Food Agric., 1990, 53, 107–110 CrossRef CAS.
- L. B. Matthews, M. E. Kunkel, J. C. Acton, A. A. Ogale and P. L. Dawson, Food Nutr Sci Search PubMed.
- Y. Pranoto, M. Istigani, U. Santoso, L. A. Lestari, Y. Erwanto and A. Rohman, KnE Life Sci., 2016, 92–97 CrossRef.
- H. Yahdiana, J. Irwandi and A. Effionora, Int. Food Res. J., 2018, 25, 275–281 Search PubMed.
- R. Hafidz, C. M. Yaakob, I. Amin and A. Noorfaizan, Int. Food Res. J., 2011, 18, 787–791 Search PubMed.
- U. J. Gunnerud, C. Heinzle, J. J. Holst, E. M. Östman and I. M. E. Björck, PLoS ONE, 2012, 7(9), e44731 CrossRef CAS PubMed.
- M. Claessens, Dietary proteins: their effect on insulin and glucagons in relation to body weight management, Universitaire Pers Maastricht, 2008 Search PubMed.
- C. Schmitt, L. Bovetto, J. Buczkowski, G. De Oliveira Reis, P. Pibarot, L. Amagliani and J. Dombrowski, Curr. Opin. Colloid Interface Sci., 2021, 56, 101510 CrossRef CAS.
- W. H. van Megen, J. Agric. Food Chem., 1974, 22, 126–129 CrossRef CAS PubMed.
- Z. Yi-Shen, S. Shuai and R. Fitzgerald, Food Nutr. Res., 2018, 62 Search PubMed.
- W. Kim, Y. Wang and C. Selomulya, Trends Food Sci. Technol., 2020, 105, 261–272 CrossRef CAS.
- D. Chéreau, P. Videcoq, C. Ruffieux, L. Pichon, J. C. Motte, S. Belaid, J. Ventureira and M. Lopez, OCL: Oilseeds Fats, Crops Lipids, 2016, 23(4), D406 CrossRef.
- X. D. Sun and S. D. Arntfield, Food Res. Int., 2010, 43, 509–515 CrossRef CAS.
- H. Hu, T. Fan, X. Zhao, X. Zhang, Y. Sun and H. Liu, J. Food Sci. Technol., 2017, 54, 2833–2841 CrossRef CAS PubMed.
- X. Li, J. van der Gucht, P. Erni and R. de Vries, ACS Appl. Mater. Interfaces, 2021, 13, 37598–37608 CrossRef CAS PubMed.
- Y. Lan, J.-B. Ohm, B. Chen and J. Rao, Food Hydrocolloids, 2020, 101, 105556 CrossRef CAS.
- Y. Lan, B. Chen and J. Rao, Food Hydrocolloids, 2018, 80, 245–253 CrossRef CAS.
- X. Qi, Y. Lan, J.-B. Ohm, B. Chen and J. Rao, Food Funct., 2021, 12, 8907–8919 RSC.
- F. N. A. Aryee and M. T. Nickerson, Int. J. Food Sci. Technol., 2014, 49, 65–71 CrossRef CAS.
- S. Liu, C. Elmer, N. H. Low and M. T. Nickerson, Food Res. Int., 2010, 43, 489–495 CrossRef CAS.
- V. Ducel, J. Richard, P. Saulnier, Y. Popineau and F. Boury, Colloids Surf., A, 2004, 232, 239–247 CrossRef CAS.
- D. R. Klassen and M. T. Nickerson, Food Res. Int., 2012, 46, 167–176 CrossRef CAS.
- S. Liu, Y.-L. Cao, S. Ghosh, D. Rousseau, N. H. Low and M. T. Nickerson, J. Agric. Food Chem., 2010, 58, 552–556 CrossRef CAS PubMed.
- S. Liu, N. H. Low and M. T. Nickerson, J. Agric. Food Chem., 2009, 57, 1521–1526 CrossRef CAS PubMed.
- D. Dong, Y. Hua, Y. Chen, X. Kong, C. Zhang and Q. Wang, J. Agric. Food Chem., 2013, 61, 3934–3940 CrossRef CAS.
- D. Dong and B. Cui, J. Food Process Eng., 2019, 42, e13196 CrossRef.
- K. J. Klemmer, L. Waldner, A. Stone, N. H. Low and M. T. Nickerson, Food Chem., 2012, 130, 710–715 CrossRef CAS.
- C. Elmer, A. C. Karaca, N. H. Low and M. T. Nickerson, Food Res. Int., 2011, 44, 1441–1446 CrossRef CAS.
- Y. Yuan, Z.-L. Wan, S.-W. Yin, Z. Teng, X.-Q. Yang, J.-R. Qi and X.-Y. Wang, Food Hydrocolloids, 2013, 31, 85–93 CrossRef CAS.
- Q. Zhang, H. Dong, J. Gao, L. Chen and T. Vasanthan, Carbohydr. Polym., 2020, 250, 116925 CrossRef CAS PubMed.
- Q. Zhang, B. Jeganathan, H. Dong, L. Chen and T. Vasanthan, Food Chem., 2021, 344, 128569 CrossRef CAS PubMed.
- I. Chourpa, V. Ducel, J. Richard, P. Dubois and F. Boury, Biomacromolecules, 2006, 7, 2616–2623 CrossRef CAS PubMed.
- D. R. Klassen, C. M. Elmer and M. T. Nickerson, Food Chem., 2011, 126, 1094–1101 CrossRef CAS.
- A. K. Stone, L. Cheung, C. Chang and M. T. Nickerson, Food Res. Int., 2013, 54, 195–202 CrossRef CAS.
- Y. P. Timilsena, B. Wang, R. Adhikari and B. Adhikari, Food Hydrocolloids, 2016, 52, 554–563 CrossRef CAS.
- P. Kaushik, K. Dowling, C. J. Barrow and B. Adhikari, Food Res. Int., 2015, 72, 91–97 CrossRef CAS.
- F. Plati, C. Ritzoulis, E. Pavlidou and A. Paraskevopoulou, Int. J. Biol. Macromol., 2021, 182, 144–153 CrossRef CAS PubMed.
- A. K. Stone, A. Teymurova and M. T. Nickerson, Food Biophys., 2014, 9, 203–212 CrossRef.
- A. K. Stone, A. Teymurova, C. Chang, L. Cheung and M. T. Nickerson, Food Sci. Biotechnol., 2015, 24, 1209–1218 CrossRef CAS.
- E. Spruijt, M. A. Cohen Stuart and J. Van Der Gucht, Macromolecules, 2013, 46, 1633–1641 CrossRef CAS.
- W. Guo, D. Ji, A. B. Kinghorn, F. Chen, Y. Pan, X. Li, Q. Li, W. T. S. Huck, C. K. Kwok and H. C. Shum, J. Am. Chem. Soc., 2023, 145, 2375–2385 CrossRef CAS PubMed.
- S. Uthayakumaran and C. Wrigley, in Cereal Grains, ed. C. Wrigley, I. Batey and D. Miskelly, Woodhead Publishing, 2nd edn, 2017, pp. 91–134 Search PubMed.
- P. Kaushik, K. Rawat, V. K. Aswal, J. Kohlbrecher and H. B. Bohidar, Soft Matter, 2018, 14, 6463–6475 RSC.
- P. K. Pandey, P. Kaushik, K. Rawat, V. K. Aswal and H. B. Bohidar, Soft Matter, 2017, 13, 6784–6791 RSC.
- P. Kaushik, K. Rawat, V. K. Aswal, J. Kohlbrecher and H. B. Bohidar, Carbohydr. Polym., 2019, 224, 115150 CrossRef PubMed.
- C.-R. Su, Y.-Y. Huang, Q.-H. Chen, M.-F. Li, H. Wang, G.-Y. Li and Y. Yuan, LWT, 2021, 139, 110591 CrossRef CAS.
- X. Ren, T. Hou, Q. Liang, X. Zhang, D. Hu, B. Xu, X. Chen, M. Chalamaiah and H. Ma, Food Chem., 2019, 279, 223–230 CrossRef CAS PubMed.
- G. Chen, S. Dong, Y. Chen, Y. Gao, Z. Zhang, S. Li and Y. Chen, Food Hydrocolloids, 2020, 107, 105943 CrossRef CAS.
- I. A. Popello, V. V. Suchkov, V. Y. Grinberg and V. B. Tolstoguzov, J. Sci. Food Agric., 1991, 54, 239–244 CrossRef CAS.
- I. A. Popello, V. V. Suchkov, V. Y. Grinberg and V. B. Tolstoguzov, J. Sci. Food Agric., 1990, 51, 345–353 CrossRef CAS.
- B. S. Schuster, G. L. Dignon, W. S. Tang, F. M. Kelley, A. K. Ranganath, C. N. Jahnke, A. G. Simpkins, R. M. Regy, D. A. Hammer, M. C. Good and J. Mittal, Proc. Natl. Acad. Sci. U. S. A., 2020, 117, 11421–11431 CrossRef CAS PubMed.
- A. Majumdar, P. Dogra, S. Maity and S. Mukhopadhyay, J. Phys. Chem. Lett., 2019, 10, 39 Search PubMed.
- D. Y. M. Lui, J. D. Litster and E. T. White, Am. Inst. Chem. Eng. J., 2007, 53, 514–522 CrossRef CAS.
- N. Chen, M. Zhao, C. Chassenieux and T. Nicolai, Food Hydrocolloids, 2016, 56, 417–424 CrossRef CAS.
- N. Chen, T. Nicolai, C. Chassenieux and Y. Wang, Food Hydrocolloids, 2020, 105, 105853 CrossRef.
- W. J. Wolf, J. J. Rackis, A. K. Smith, H. A. Sasame, G. E. Babcock Yol, B. W. J. Wolf and G. E. Babcock, Arch. Biochem. Biophys., 1958, 18, 1179 Search PubMed.
- W. J. Wolf3 and D. R. Briggs, Arch. Biochem. Biophys., 1958, 76, 377–393 CrossRef PubMed.
- R. Cochereau, T. Nicolai, C. Chassenieux and J. V. C. Silva, Colloids Surf., A, 2019, 562, 213–219 CrossRef CAS.
- H. Zhao, X. Zhou, J. Wang, X. Ma, M. Guo and D. Liu, Food Hydrocolloids, 2021, 112, 106207 CrossRef CAS.
- J. van der Gucht, R. de Vries, X. Li, G. Dardelle, P. Erni, L. Ouali and N. Thiebaut, 2023, US Pat., Appl. No. 17/995,566.
- N. Chen, J. Zhang, L. Mei and Q. Wang, Langmuir, 2018, 34, 9711–9718 CrossRef CAS PubMed.
- D. Sağlam, P. Venema, E. van der Linden and R. de Vries, Curr. Opin. Colloid Interface Sci., 2014, 19, 428–437 CrossRef.
- H. J. Kim, B. Yang, T. Y. Park, S. Lim and H. J. Cha, Soft Matter, 2017, 13, 7704–7716 RSC.
- R. J. Stewart, Appl. Microbiol. Biotechnol., 2011, 89, 27–33 CrossRef CAS PubMed.
- T. Quek and W. Wattanutchariya, Mater. Today: Proc., 2019, 16, 1929–1934 Search PubMed.
- G. Schmidt, B. R. Hamaker and J. J. Wilker, Adv. Sustainable Syst., 2018, 2, 1700159 CrossRef.
- G. Schmidt, K. H. Smith, L. J. Miles, C. K. Gettelfinger, J. A. Hawthorne, E. C. Fruzyna and J. J. Wilker, Adv. Sustainable Syst., 2022, 6, 2100392 CrossRef CAS.
- M. M. G. de Oliveira, K. de Souza Silva and M. A. Mauro, Food Biophys., 2021, 16, 214–228 CrossRef.
- L. R. Amado, K. de S. Silva and M. A. Mauro, J. Appl. Polym. Sci., 2020, 137, 48732 CrossRef CAS.
- X. Meng, Y. Du, Y. Liu, E. B. Coughlin, S. L. Perry and J. D. Schiffman, Macromolecules, 2021, 54, 5033–5042 CrossRef CAS.
- J. Sun, G. Monreal Santiago, W. Zhou, G. Portale and M. Kamperman, ACS Sustainable Chem. Eng., 2022, 10, 15968–15977 CrossRef CAS PubMed.
- M. Khoonkari, J. Es Sayed, M. Oggioni, A. Amirsadeghi, P. Dijkstra, D. Parisi, F. Kruyt, P. van Rijn, M. K. Włodarczyk-Biegun and M. Kamperman, Adv. Mater., 2023, 35, 2210769 CrossRef CAS PubMed.
- S. Zhang, C. Qi, W. Zhang, H. Zhou, N. Wu, M. Yang, S. Meng, Z. Liu and T. Kong, Adv. Mater., 2023, 35, 2209263 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |





