Biocrude production via hydrothermal liquefaction of Canadian lignocellulosic residues for sustainable transportation: screening, catalytic effect, and modelling†
J. G. B.
Churchill
 a,
V. B.
Borugadda
a,
V. B.
Borugadda
 ab and
A. K.
Dalai
ab and
A. K.
Dalai
 *a
*a
aCatalysis and Chemical Reaction Engineering Laboratories, Department of Chemical and Biological Engineering, University of Saskatchewan, S7N 5A9, Canada. E-mail: akd983@mail.usask.ca; Tel: +1 (306) 966-4771
bTidewater Renewables Ltd, Calgary, T2P 0B4, Canada
First published on 22nd October 2024
Abstract
Ten Canadian-grown lignocellulosic agro-forestry residues were screened for non-catalytic, catalytic, and composition effects in biocrude production via hydrothermal liquefaction. Evaluation of Canadian agricultural residue availability indicated a significant variation, with wheat straw determined to be the most abundant at 38.3 million metric tonnes annually, while flax straw and dried distillers' grains had limited availability for a hypothetical biorefinery. Comparing K2CO3 + Fe catalyst and non-catalytic screening revealed a pronounced catalytic effect for softwoods over straws and hardwood due to higher lignin content. Trends included increase in biocrude and oxygen content with holocellulose, while higher lignin tended to decrease oxygen content of the biocrude. Catalytically, pig manure performed poorly with the lowest biocrude yield (9.3 wt%) while dried distillers' grains was desired due to high biocrude yield (25.3 wt%) with the lowest oxygen content (10.2 wt%). Barley among straws and aspen among woods were promising based on high catalytic biocrude yields (23.3 & 26.5 wt%) and moderate oxygen content (20.8 & 21.4 wt%). Catalytic effects for both straw and wood included changes to product yields, increase in degree of degradation, energy recovery, and biocrude volatility, as well as a decrease in biocrude acidity, density, and heteroatoms. A fibre-based multiple linear regression model had a strong fit (R2adjusted = 0.87) for catalytic biocrude yield, with positive contribution in the order of extractives > cellulose > hemicellulose > lignin, while volatile matter had the strongest individual correlation to catalytic biocrude yield (R2 = 0.94). Next steps in HTL optimization and biocrude upgrading were identified to advance the feasibility of lignocellulosic biocrude production for sustainable transportation fuel production through integration with existing crude oil refineries.
1. Introduction
Transportation fuels make up nearly 30% of all global energy consumption,1 while accounting for 37% of CO2 emissions in 2021, maintaining the highest reliance on fossil fuels of any energy sector, as seen in Fig. 1.2 As the transportation industry is falling behind on sustainability, renewable energy solutions in the form of biofuels are seen as more important than ever before. The dire environmental impacts, increased prices, and future decline in fossil fuel supplies have pushed researchers to investigate sustainable sources of energy. Countries like Canada are striving for carbon neutrality within the next 30 years and have passed initial legislation such as the Renewable Fuels Regulations, Pan-Canadian Approach to Pricing Carbon Pollution, Clean Fuels Regulations, and Clean Fuels Fund to increase biofuel demand and reduce fossil fuels.3 Sustainable transportation fuel production is one of the most needed solutions to meet worldwide sustainability goals in the transport sector. | ||
| Fig. 1 Reliance of global energy sectors on non-renewable energy – 2009–2019.4 | ||
Biomass-derived biofuels are a particularly promising solution for resource-rich countries like Canada, utilizing already well-established fossil fuel infrastructure and engines.5,6 Biomass sources for fuels have gained interest as they sequester carbon during growth to create a renewable carbon cycle when combusted.6 First-generation biomass has shown early promise in sustainable biofuel production, however, the demand for these edible biomasses competes directly with vital food and feed industries that are predicted to only become more strained with future societal growth.7,8 Cost and sustainability advantages have led researchers to shift focus towards second-generation feedstock, often a waste product of first-generation, for advanced biomass-to-bioenergy technology. Second-generation biomass includes non-edibles such as agricultural residues (straws, husks, stalks, and hulls) that are widely available even after fulfilling the demand for livestock and tillage purposes.9 Despite the potential of second-generation renewable fuel, the development of production technology is in its beginnings and faces major technological and economical challenges in commercialization.7
Hydrothermal liquefaction (HTL) is an emerging technology that is suitable for second-generation biomass with any water content.6 The HTL process uses moderate to high temperature and pressure in the range of 240–550 °C and 5–25 MPa, while exploiting water's unique properties when approaching and at supercritical conditions (lower viscosity, surface tension, polarity, and dielectric constant) to decompose, depolymerize, and recombine biomass into organics such as desired hydrocarbons in the form of a biocrude.10,11 Additionally, this use of water in HTL allows for the energy-efficient decomposition of biomass at lower temperatures compared to the alternative higher intensity for effective pyrolysis (370–700 °C).12 With more advantages through wide feedstock acceptance, less intensive pretreatment, and higher quality fuel-precursor products over other biofuel technologies like pyrolysis or fermentation, several drawbacks exist for HTL. HTL biocrude still differs greatly from desired fossil fuel characteristics by primarily oxygen content, associated with higher acidity, higher viscosity, lower heating value, and a need for individual upgradation to meet firm transportation regulations.5,13 As an emerging technology with low technological readiness, the lack of direct feedstock comparisons for HTL processing is apparent and needed before effective optimization, industrial implementation, and economic considerations.5,14
To address the gap in feedstock comparisons for HTL studies using abundant yet under-utilized Canadian bio-residues, this study aimed to screen agricultural and forestry residues for suitability in biocrude production with potential for upgradation to transportation fuels. Lignocellulosic agricultural residues were assessed in terms of availability in Canada, biocrude yield, and biocrude oxygen content through catalytic/non-catalytic HTL reactions. The effect of homogeneous potassium carbonate in combination with heterogeneous iron as a catalyst to improve the HTL of lignocellulosic residues was also investigated. In addition, linear regression models were explored to relate feedstock composition to biocrude production to better understand the complex HTL process.
2. Availability of Canadian agricultural residues
Exporting millions of tonnes of grain annually, resource-abundant western Canada accounts for 83% of crop production primarily in the Canadian prairie provinces of Alberta, Saskatchewan, and Manitoba.9 This agricultural-rich region of Canada has the potential to be a key supplier of biofuels. Agricultural biomass has the benefit of being more accessible than forestry residues, as widespread farming equipment like combines, trucks, and bailers exist to bundle and transport the residues from less remote locations. Historically, excess agriculture residues are worked into the field as a soil-enricher, economic livestock feed, and insulator for harsh Canadian winters, however appropriate residue management is needed as sometimes straw is even illegally burned in the case of fibrous flax straw that has damaged equipment.15,16 The main use of second-generation agricultural residues is agriculture-related (roughly 95%), while there is a small construction market for biomass-based building materials (roughly 5%).16 Despite applications, annually available residues were estimated to be 6.6 million tonnes from barley, 37.1 million tonnes from spring wheat, and most abundantly 43.4 million tonnes from canola within the prairies (Alberta, Saskatchewan, and Manitoba) in 2020.9 Despite promising availability, sustainability, and cost over first-generation biomass, second-generation biomass still faces challenges in logistics, being a new area of fuel sourcing with little industrial implementation.5 There may be unrealized impacts on future soil conditions and crop yields if all available straws are utilized in prairies, an area that requires further large-scale study. The agricultural residue regions of the prairies are vast as depicted in Fig. 2. | ||
| Fig. 2 Significant agricultural residue production of the prairie provinces.9 | ||
Focusing on the production of biocrude from straw, availability is an important factor considered when screening feedstocks for commercialization. Previous studies have estimated the availability of agricultural residues but are now outdated with the continual development and changing trends of the Canadian agriculture industry. There are various methodologies for estimating agricultural residue availability, with no direct reporting by Statistics Canada. The availability of the 4 straws screened in this study were estimated based on methodology used by Li et al.,17 presented in Table 1. This estimation is deemed particularly accurate over other methods by incorporating known straw-to-grain ratios and 2022 statistics for seed production, straw requirements via tillage, and regional livestock feeding/bedding needs. Agreeing with previous studies, wheat straw is overwhelmingly the most available straw at nearly 40 million tonnes per year. The availability of canola and barley straw is notably close despite much larger annual seeding area of canola straw (8.6 million ha) compared to barley straw (2.6 million ha), attributed to the average barley straw yield per area (3.8 tonne per ha) being nearly double that of canola straw (2.1 tonne per ha).18 Unlike other straws, flax straw has notably too limited of availability for supplying a hypothetical biorefinery, with a study by Zheng and Qiu,9 hypothesizing an annual minimum biorefinery feed of 2.38 million tonnes for economic feasibility.
| Type of straw | Annual availability (metric tonnes) |
|---|---|
| Wheat | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 353 353![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Canola | 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 654 654![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Barley | 6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 893 893![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Flax | 455![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
The availability of agricultural by-products like dried distillers' grains (spent grain) and pig manure are not as well-known, with less accurate methods to estimate compared to straw. It was estimated in 2020 by Renewable Industries Canada that 810 million litres of bioethanol are produced from spent grain annually in western Canada alone.19 This translates to an annual estimate of 960![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 metric tonnes of spent grain on the reported basis of 434 kg of spent grain producing 366 L of ethanol.20 However, given the high nutrient content of spent grain there is a large animal feed market, with some regions of Canada even importing spent grain from the states due to lack of availability.21 This demand for spent grain raises the feedstock's price and significantly reduces its availability, making it unsuitable for large-scale use as a sole HTL biorefinery feed. As for pig manure, it is estimated that there are nearly 14.17 million hogs in Canada that each produce approximately 1 metric tonne of manure annually for a yearly total of 14.17 million tonnes.22,23 Despite manure being generally regarded as a waste-product, there is a market for the product as a low-cost alternative fertilizer that can be stored and placed in fields.23 Given the large production and low-cost of manure from the large livestock industry of Canada, exact manure availability is unclear but likely suitable to feed a hypothetical HTL biorefinery.
500 metric tonnes of spent grain on the reported basis of 434 kg of spent grain producing 366 L of ethanol.20 However, given the high nutrient content of spent grain there is a large animal feed market, with some regions of Canada even importing spent grain from the states due to lack of availability.21 This demand for spent grain raises the feedstock's price and significantly reduces its availability, making it unsuitable for large-scale use as a sole HTL biorefinery feed. As for pig manure, it is estimated that there are nearly 14.17 million hogs in Canada that each produce approximately 1 metric tonne of manure annually for a yearly total of 14.17 million tonnes.22,23 Despite manure being generally regarded as a waste-product, there is a market for the product as a low-cost alternative fertilizer that can be stored and placed in fields.23 Given the large production and low-cost of manure from the large livestock industry of Canada, exact manure availability is unclear but likely suitable to feed a hypothetical HTL biorefinery.
3. Experimental
3.1. Materials
Canadian agricultural wheat, barley, canola, and flaxseed straws are collected from local farmers in rural areas near Saskatoon, Saskatchewan, Canada. Pig manure and dried distillers' grains were obtained from producers near Calgary, Alberta. Canadian wood chip samples were sourced from Alberta forests and supplied by industrial partners (Tidewater Renewables Ltd). The residues were ground and sieved into <1.7 mm particle size using lab-scale knife mill (Retsch GmbH, 5657 HAAN, western Germany). ACS reagent-grade acetone was purchased from Caledon Laboratory Chemicals (ON, Canada) and used as the biocrude-extraction and washing solvent. The catalysts in this experiment were used as received with K2CO3 and Fe (powder) both obtained from Fischer Scientific (Toronto, Canada). Water used in this experiment was purified deionized water produced and provided by the University of Saskatchewan Engineering Building.3.2. Hydrothermal liquefaction and product separation
A 0.97 liter high-pressure Parr batch reactor (Parr series no. 4520 HP) was used to conduct all the HTL experiments, capable of withstanding up to 350 °C and a pressure of 2900 psi. The reactor is managed by a standard 4848 Parr reactor controller. The select feedstock powder is made to a slurry within the reactor vessel by addition of a predetermined amount of water. Initially, the reactor is pressurized to 150 psi (∼1 MPa) with nitrogen before being vented to atmospheric pressure and then purged to 250 psi (1.4 MPa) (corresponding to a reaction pressure of ∼10 MPa), while also performing a leak test of the system. A typical HTL screening run includes 40 g of biomass, 400 mL of water (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 biomass
10 biomass![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) water (B/W) ratio), and 2 g of each catalyst (5 wt% catalyst loading in terms of biomass). The inert and pressurized reactor begins heating at approximately 5 °C min−1 to the specified temperature via an electrical heating jacket. When heating begins the agitator is turned on and set to ∼1000 rpm to ensure uniform mixing throughout the reaction. Once the set temperature is reached the reaction temperature is maintained for the 30 minutes reaction time. At the end of the reaction time cooling begins by removing the heating jacket and quenching the reactor with buckets of cold tap water as well as a fan.
water (B/W) ratio), and 2 g of each catalyst (5 wt% catalyst loading in terms of biomass). The inert and pressurized reactor begins heating at approximately 5 °C min−1 to the specified temperature via an electrical heating jacket. When heating begins the agitator is turned on and set to ∼1000 rpm to ensure uniform mixing throughout the reaction. Once the set temperature is reached the reaction temperature is maintained for the 30 minutes reaction time. At the end of the reaction time cooling begins by removing the heating jacket and quenching the reactor with buckets of cold tap water as well as a fan.
After cooling to room temperature, HTL product separation is outlined in the flow diagram of Fig. 3. The gas phase is first vented and collected via Tedlar gas sampling bags. Before further separation, the reactor is removed and weighed with its liquid and solid content for comparison to the empty reactor weight for weight lost to gas formation and biocrude residuals on the agitator. Due to the inherent immiscibility of the polar aqueous phase with biocrude, the liquid and solid reactor contents are vacuum filtered with Whatman® Grade 202 (pore size 15–19 μm) filter paper, collecting the aqueous phase as a filtrate and bio crude-containing hydrochar as a retentate. To ensure minimal loss of biocrude, residual biocrude is washed from the reactor vessel walls and agitator via the acetone solvent and extracted separately for mass balance calculations. The retentate hydrochar-biocrude slurry is mixed with acetone (relative polarity 0.355) to achieve a hydrochar/biocrude mass-to-solvent volume ratio of at least 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10. The biocrude–hydrochar-solvent mixture is heated to 50 °C, maintaining constant agitation to ensure effective extraction of biocrude from the hydrochar. After 1 hour, the solvent-extracted biocrude is separated from the solid hydrochar via vacuum filtration with Whatman® Grade 202 filter paper. The hydrochar is then dried in an oven for a minimum of 6 hours at 105 °C before weighing. The much more volatile solvent is removed from the biocrude using a rotary evaporator and water bath at 50 °C, reducing pressure to 58 millibars (atmospheric equivalent of 130 °C), removing the solvent/moisture as recommended by ASTM D2892. The remaining dark sticky liquid (semi-solid at room temperature) is weighed for biocrude yield. For reproducibility, triplicates of select catalytic and noncatalytic runs were conducted to determine reproducibility based on standard statistical error.
10. The biocrude–hydrochar-solvent mixture is heated to 50 °C, maintaining constant agitation to ensure effective extraction of biocrude from the hydrochar. After 1 hour, the solvent-extracted biocrude is separated from the solid hydrochar via vacuum filtration with Whatman® Grade 202 filter paper. The hydrochar is then dried in an oven for a minimum of 6 hours at 105 °C before weighing. The much more volatile solvent is removed from the biocrude using a rotary evaporator and water bath at 50 °C, reducing pressure to 58 millibars (atmospheric equivalent of 130 °C), removing the solvent/moisture as recommended by ASTM D2892. The remaining dark sticky liquid (semi-solid at room temperature) is weighed for biocrude yield. For reproducibility, triplicates of select catalytic and noncatalytic runs were conducted to determine reproducibility based on standard statistical error.
The product yields, conversion, and energy recovery of the HTL process are expressed on a dry basis of biomass and calculated as the following:
 | (1) |
 | (2) |
| Aqueous yield (wt%) = 100 − biocrude (wt%) − hydrochar (wt%) − gas (wt%) | (3) |
 | (4) |
| Degree of degradation (wt%) = 100 − hydrochar (wt%) | (5) |
 | (6) |
3.3. Characterization techniques
Ultimate analysis (elemental C, H, N, S, and O) of the feedstocks, biocrudes, and hydrochars was performed by a PerkinElmer Elementar CHNSO analyzer (Vario EL III, Elementar Americas Inc., NJ). Carbon, hydrogen, nitrogen, and sulphur were directly measured through the instrument while oxygen was calculated by difference (eqn (7)).| O (wt%) = 100 − [C (wt%) + H (wt%) + N (wt%) + S (wt%) + ash (wt%)] | (7) |
Instead of using one of the several empirical formulas based on elemental analysis for reporting HHV of feedstocks, biocrude, and hydrochar,24 a more accurate method via oxygen bomb calorimeter (Parr 6400 Calorimeter, IL, USA) was used based on ASTM D5865.25 The proximate analysis of biomass feedstocks is determined according to ASTM D3173 (moisture), ASTM D3174 (ash), and ASTM D3175 (volatile matter), while fixed carbon is assumed by difference (eqn (8)).26–28
| Fixed carbon (wt%) = 100 − [moisture (wt%) + ash (wt%) + volatile matter (wt%)] | (8) |
Fibre content of lignocellulosic feeds was analyzed by the ANKOM method using an ANKOM 200 Fiber Analyzer (ANKOM Technology, Macedon, NY). Triplicates of the Ankom 200 5, 6, and 8 methods based on the Van Soest method were performed and averaged to ensure repeatability.29,30 Determining neutral detergent fibre (NDF), acid detergent fibre (ADF), and acid detergent lignin (ADL), the extractives, cellulose, hemicellulose, and lignin were determined for the feeds viaeqn (9)–(12).
| Extractives (wt%) = 100 − NDF (wt%) | (9) |
| Cellulose (wt%) = ADF (wt%) − ADL (wt%) | (10) |
| Hemicellulose (wt%) = NDF (wt%) − ADF (wt%) | (11) |
| Lignin (wt%) = ADL (wt%) − ash (wt%) | (12) |
Density of the biocrude and aqueous phase was measured via mass and volume measurements, determined by filling and weighing 0.6 mL centrifuge tubes with biocrude in the controlled temperature environment of a lab. Moisture content of the biocrude was determined by Karl-Fischer (KF) Coulometric titration using a Mettler-Toledo DL32 Karl-Fischer Coulometer (Mettler-Toledo, LLC, Columbus, OH, USA). Biocrude was dissolved in HPLC grade methanol (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, wt. to vol. ratio; Fisher Scientific, Canada) to prepare the solutions for moisture analysis titration with Hydranal™-Coulomat AG (Honeywell Fluka TM, Canada) reagent (anolyte solution). Measuring biocrude acidity was conducted by volumetric KOH base titration, following ASTM D664 but with ethanol instead of propan-2-ol for better solubility with biocrude.31 This standard uses a potentiometric approach through measuring pH with an electrode, alternative to a pH indicator that can be challenging to see with dark shaded biocrude solutions.
10, wt. to vol. ratio; Fisher Scientific, Canada) to prepare the solutions for moisture analysis titration with Hydranal™-Coulomat AG (Honeywell Fluka TM, Canada) reagent (anolyte solution). Measuring biocrude acidity was conducted by volumetric KOH base titration, following ASTM D664 but with ethanol instead of propan-2-ol for better solubility with biocrude.31 This standard uses a potentiometric approach through measuring pH with an electrode, alternative to a pH indicator that can be challenging to see with dark shaded biocrude solutions.
The compositional analysis of the biocrude is analyzed by gas chromatography mass spectrometry (GC-MS) using a Trace 1310 Gas Chromatograph and a TSQ Duo Mass 19 Spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Biocrude GC-MS samples were dissolved in HPLC-grade acetone extraction solvent. With a helium flowrate of 1.2 mL min−1, the samples are injected under a split ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with a split flow of 60 mL min−1 at 250 °C. Oven temperature was initially kept at 40 °C for 1 minute before being increased to 150 °C at a rate of 5 °C min−1 then further increased to 320 °C at a rate of 10 °C min−1, and finally holding at the final temperature for 5 minutes. The standard NIST (National Institute of Standards and Technology) library from the ChromeleonTM 7.2 Chromatography Data System (CDS) software was used to analyze the resulting mass spectral data between 50 and 650 m/z. Inert and oxidative thermal stability profiles of the biocrude were assessed by thermogravimetric analysis (TGA 8000; PerkinElmer, Llantrisant, USA), where the biocrude underwent heating from room temperature to 1000 °C (10 °C min−1) in an inert nitrogen atmosphere (30 mL min−1) as well as room temperature to 600 °C (10 °C min−1) in an oxidative air atmosphere (30 mL min−1). 600 °C was selected for the oxidative TGA analysis as this is the maximum capability of the equipment to safely run under air atmosphere.
1 with a split flow of 60 mL min−1 at 250 °C. Oven temperature was initially kept at 40 °C for 1 minute before being increased to 150 °C at a rate of 5 °C min−1 then further increased to 320 °C at a rate of 10 °C min−1, and finally holding at the final temperature for 5 minutes. The standard NIST (National Institute of Standards and Technology) library from the ChromeleonTM 7.2 Chromatography Data System (CDS) software was used to analyze the resulting mass spectral data between 50 and 650 m/z. Inert and oxidative thermal stability profiles of the biocrude were assessed by thermogravimetric analysis (TGA 8000; PerkinElmer, Llantrisant, USA), where the biocrude underwent heating from room temperature to 1000 °C (10 °C min−1) in an inert nitrogen atmosphere (30 mL min−1) as well as room temperature to 600 °C (10 °C min−1) in an oxidative air atmosphere (30 mL min−1). 600 °C was selected for the oxidative TGA analysis as this is the maximum capability of the equipment to safely run under air atmosphere.
The surface characteristics of the hydrochar were analyzed by Brauner–Emmett–Teller (BET) and Barrett–Joyner–Halenda (BJH) methods, specifically analyzing the desorption isotherm for surface area, pore size, and pore volume. To ensure complete removal of residual oil and other volatile contaminants, the hydrochar was pyrolyzed under a nitrogen atmosphere for 3 hours at 400 °C. A Micromeritics ASAP 2020 surface area and porosity analyzer (Micromeritics Instrument Corporation, Norcross, GA, USA) was used to conduct BET analysis via the adsorption and desorption of N2 at 77 K. Components of the HTL gas phase were identified and quantified via gas chromatograph (Agilent 7890A, DE, USA). The gas was fed through a desiccant tube to ensure the samples were moisture-free.
4. Research and discussion
4.1. Feedstock characterizations
Several characterizations were completed of the 6 agricultural residues, including 4 straws and 2 commercial agriculture by-products, as well as 4 forestry wood feeds. Ultimate analysis revealed that forestry residues have minimal amounts of nitrogen (<0.2 wt%) and sulphur content (0.0 wt%) compared to that of agricultural residues (0.1–4.0 & 0.1–0.6 wt%). Ash content of the agricultural residues (3.9–17.4 wt%) was much higher than forestry residues (0.5–1.1 wt%), potentially having catalytic benefit or detriment for HTL biocrude formation.32 The high ash of canola straw and pig manure is particularly concerning for contaminating biocrude. Fibre analysis revealed that spent grain had the highest extractives content (39.5 wt%), ideal for conventional biofuel applications and expected due to high amounts of lipids, protein, and simple sugars. Softwood residues were particularly higher in lignin with lower extractives than agricultural residues, reflected by woody biomass' rigid nature. The lone hardwood aspen was comparable in lignin content to straws while maintaining low extractives, reporting the highest cellulose content (56.0 wt%). More energy is potentially recoverable from the woods compared to most agricultural feedstocks, established by heating values of the woody biomass (18.1–18.8 MJ kg−1) typically higher than straws (16.3–18.2 MJ kg−1), assumed to be due to lower heteroatom and ash content. The aforementioned trends are confirmed by findings from similar biomass utilization studies summarized in Table 2. Differences from literature can be attributed to geographical variances in procured feedstocks and growing conditions/seasons, validated by observed difference among the reported studies,47,52 even with biomass procured from the same Saskatoon region.36,37,39 Further differences in fibre characterizations could stem from different methods, such as some studies using NREL instead of the ANKOM method.36,37| Biomass | HHV (MJ kg−1) | C (wt%) | H (wt%) | N (wt%) | S (wt%) | O (wt%) | Moisture (wt%) | Ash (wt%) | Volatile matter (wt%) | Fixed carbon (wt%) | Cellulose (wt%) | Hemi-cellulose (wt%) | Lignin (wt%) | Extractives (wt%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| a Friedl's formula used for calculating HHV based on CHNSO data.50 | |||||||||||||||
| Pig manure | 14.2 | 44.8 | 5.8 | 2.5 | 0.6 | 28.9 | 12.2 | 17.4 | 46.1 | 24.3 | 16.2 | 5.7 | 26.3 | 22.3 | — |
| — | — | — | 4.3 | 0.6 | — | — | — | — | — | 13.9 | 20.5 | 6.4 | 59.2 | 32 | |
| 7.2 | 33.5 | 6.2 | 2.8 | — | 57.5 | 20.0 | 22.3 | 77.7 | — | 15.1 | 19.9 | 0.9 | 41.8 | 33 | |
| Spent grain | 19.1 | 48.4 | 7.2 | 4.0 | 0.2 | 35.7 | 5.2 | 4.5 | 74.7 | 15.6 | 17.0 | 25.8 | 13.2 | 34.4 | — |
| 21.0 | 50.3 | 6.4 | 3.9 | 0.0 | 39.2 | 1.4 | 7.6 | 63.0 | 24.4 | 23.6 | 15.5 | 25.2 | 28.1 | 34 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||||||
| Straws | |||||||||||||||
| Wheat | 17.6 | 45.7 | 6.5 | 1.6 | 0.2 | 40.9 | 8.6 | 5.1 | 66.7 | 19.6 | 31.4 | 22.2 | 15.1 | 17.6 | — |
| 20.3 | 41.6 | 6.1 | 0.1 | 0.1 | 52.1 | 6.0 | 1.3 | 78.3 | 14.4 | 34.6 | 29.3 | 21.3 | 7.5 | 35 | |
| 19.8 | 45.4 | 6.2 | 0.1 | 0.0 | 48.3 | 6.3 | 1.3 | 78.4 | 14.0 | 35.4 | 29.7 | 20.8 | 6.5 | 36 | |
| 17.4 | 42.8 | 5.5 | 0.5 | 0.0 | 51.2 | 5.9 | 5.8 | 71.4 | 17.0 | — | — | — | — | 37 | |
| Barley | 17.4 | 50.1 | 6.1 | 0.2 | 0.1 | 39.1 | 7.7 | 4.4 | 68.8 | 19.2 | 43.6 | 28.1 | 8.0 | 8.2 | — |
| — | — | — | — | — | — | 6.7 | 2.2 | — | — | 33.3 | 20.4 | 17.1 | 20.3 | 38 | |
| 15.7 | 41.4 | 6.2 | 0.6 | 0.0 | 51.7 | 6.9 | 9.8 | 78.5 | 4.8 | 32.5 | 25.7 | 23.0 | 2.1 | 35 | |
| 17.4 | 44.7 | 6.3 | 0.5 | 0.6 | 48.0 | 6.2 | 4.3 | 78.0 | 11.5 | 46.0 | 23.0 | 15.0 | 11.7 | 39 | |
| Canola | 18.2 | 44.6 | 6.5 | 2.5 | 0.6 | 37.0 | 8.9 | 8.8 | 64.6 | 17.7 | 32.9 | 12.0 | 13.0 | 24.4 | — |
| — | — | — | — | — | — | 6.7 | 2.1 | — | — | 42.4 | 16.4 | 14.2 | 18.2 | 38 | |
| Rapeseed | 12.0 | 45.5 | 3.6 | 2.6 | 0.6 | 47.6 | 0.0 | 5.7 | 75.8 | 18.5 | 7.1 | 31.3 | 30.3 | 25.6 | 40 |
| Canola | 18.9 | 46.3 | 6.8 | 0.9 | 0.4 | 45.6 | — | — | — | — | 46.2 | 29.2 | 14.2 | — | 41 |
| Flax | 16.3 | 47.0 | 6.1 | 0.1 | 0.1 | 42.8 | 8.1 | 3.9 | 72.7 | 15.4 | 39.9 | 26.3 | 7.5 | 14.3 | — |
| 17.0 | 43.1 | 6.2 | 0.7 | 0.1 | 49.9 | 7.9 | 3.0 | 80.3 | 8.8 | 28.7 | 26.8 | 22.5 | 11.1 | 35 | |
| 17.2 | 47.8 | 6.4 | 0.1 | 0.0 | 45.7 | 8.1 | 3.1 | 79.9 | 8.9 | 32.3 | 27.5 | 20.1 | 8.9 | 36 | |
| 17.3 | 47.8 | 5.4 | 0.8 | 0.2 | 43.2 | 9.3 | 2.6 | 83.3 | 4.8 | 53.8 | 17.1 | 23.3 | 5.8 | 42 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||||||||||||
| Woods | |||||||||||||||
| Aspen | 18.1 | 47.0 | 6.5 | 0.1 | 0.0 | 45.3 | 8.4 | 1.1 | 76.8 | 13.7 | 56.0 | 16.3 | 13.0 | 5.3 | — |
| 20.1a | 50.4 | 6.2 | 0.2 | 0.0 | 43.2 | — | 0.5 | — | — | 47.1 | 19.6 | 22.1 | 6.6 | 43 | |
| 18.6a | 47.0 | 6.0 | 0.1 | 0.0 | 43.9 | — | 0.7 | 83.0 | 16.0 | — | — | — | — | 44 | |
| Pine | 18.6 | 48.6 | 6.6 | 0.0 | 0.0 | 44.2 | 6.8 | 0.6 | 77.6 | 15.0 | 43.9 | 14.9 | 27.1 | 6.7 | — |
| 19.6 | 49.0 | 6.4 | 0.1 | 0.0 | 44.4 | 5.8 | 1.5 | 82.4 | 10.3 | 39.0 | 34.0 | 12.0 | 7.7 | 35 | |
| 19.8 | 46.8 | 6.1 | 0.0 | 0.0 | 47.0 | 9.3 | <0.1 | 70.0 | 20.7 | — | — | — | — | 45 | |
| 17.6 | 51.3 | 5.5 | 0.1 | 0.0 | 42.6 | — | 0.6 | 78.5 | 21.0 | 65.1 | 30.3 | 4.1 | 46 | ||
| Tamarack | 18.0 | 48.8 | 6.5 | 0.1 | 0.0 | 44.1 | 9.6 | 0.5 | 74.6 | 15.4 | 43.5 | 12.5 | 23.5 | 10.5 | — |
| 24.6a | 55.2 | 9.9 | 0.7 | 0.0 | 31.0 | — | 4.2 | 69.5 | 26.3 | — | — | — | — | 47 | |
| 19.6a | 49.1 | 6.3 | 0.5 | 0.0 | 41.1 | 15.0 | 1.4 | 66.5 | 17.1 | 40.6 | 26.6 | 24.2 | 7.3 | 48 | |
| Spruce | 18.8 | 48.2 | 6.5 | 0.0 | 0.0 | 44.6 | 9.1 | 0.7 | 75.5 | 14.7 | 47.3 | 13.8 | 25.0 | 4.1 | — |
| 19.2 | 47.1 | 6.1 | 0.0 | 0.0 | 46.8 | 8.0 | <0.1 | 70.0 | 21.3 | — | — | — | — | 45 | |
| 19.6 | 51.7 | 6.3 | 0.3 | 0.0 | 41.7 | — | — | 71.1 | 28.9 | 51.2 | 21.0 | 27.8 | — | 49 | |
4.2. Hydrothermal liquefaction products
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 B/W ratio, 5 wt% K2CO3 + 5 wt% Fe, 10 MPa reaction pressure, <1.7 mm biomass particle size, and biocrude extraction via acetone. These conditions were selected based on the review of common optimal HTL conditions for agro-forestry residues, with the moderate values of optimal parameters selected, like residence time and temperature, when a range of values was reported in literature.12,53–55 In particular, the combination of acidic, heterogeneous, and zerovalent Fe catalyst with homogeneous and alkaline K2CO3 was selected for screening due to the favourable effect of each catalyst individually and synergistically on biocrude yield and quality.47,56,57 Acetone is very common among biocrude extraction solvents for its high volatility, moderate polarity, and low cost, however the ideal extraction solvent for biocrude yield can depend on the feedstock and HTL conditions, with future solvent optimization planned.12,54 Due to biocrude's inherent difference in quality from crude oil streams, there is a preference to favour biocrude yield over its oxygen content in this screening as the deoxygenation will be explored by upgradation regardless. The lowest-lignin aspen hardwood had the highest biocrude yield (26.5 wt%), while maintaining a comparable oxygen content (21.4 wt%) to other wood biocrudes from catalytic HTL (ranging 20.4–22.4 wt%). With unoptimized conditions for screening, these biocrude yield and oxygen results are both in the similar 10 s to 20 s of other screening work.58,59 Among straws, the lowest-lignin flax had the highest catalytic biocrude yield (25.3 wt%) but with the downside of unfavourably higher oxygen content (24.4 wt%). Among catalytic wood and straw, a positive trend between biocrude yield and oxygen content was observed, with an increase in biocrude yield tending to produce biocrude with higher oxygen content. Pig manure had the lowest yield, as expected with its higher unconvertable ash content, while spent grain had the highest yield and lowest oxygen content due to its high oil-containing extractives content. Overall, the high reported oxygen content of the biocrude (10.2–28.4 wt%) would have major challenges in integration with crude oil refinery streams (oxygen content <2 wt%) due to immiscibility, higher viscosity, and oxidative instability, requiring further upgradation techniques.55,60
10 B/W ratio, 5 wt% K2CO3 + 5 wt% Fe, 10 MPa reaction pressure, <1.7 mm biomass particle size, and biocrude extraction via acetone. These conditions were selected based on the review of common optimal HTL conditions for agro-forestry residues, with the moderate values of optimal parameters selected, like residence time and temperature, when a range of values was reported in literature.12,53–55 In particular, the combination of acidic, heterogeneous, and zerovalent Fe catalyst with homogeneous and alkaline K2CO3 was selected for screening due to the favourable effect of each catalyst individually and synergistically on biocrude yield and quality.47,56,57 Acetone is very common among biocrude extraction solvents for its high volatility, moderate polarity, and low cost, however the ideal extraction solvent for biocrude yield can depend on the feedstock and HTL conditions, with future solvent optimization planned.12,54 Due to biocrude's inherent difference in quality from crude oil streams, there is a preference to favour biocrude yield over its oxygen content in this screening as the deoxygenation will be explored by upgradation regardless. The lowest-lignin aspen hardwood had the highest biocrude yield (26.5 wt%), while maintaining a comparable oxygen content (21.4 wt%) to other wood biocrudes from catalytic HTL (ranging 20.4–22.4 wt%). With unoptimized conditions for screening, these biocrude yield and oxygen results are both in the similar 10 s to 20 s of other screening work.58,59 Among straws, the lowest-lignin flax had the highest catalytic biocrude yield (25.3 wt%) but with the downside of unfavourably higher oxygen content (24.4 wt%). Among catalytic wood and straw, a positive trend between biocrude yield and oxygen content was observed, with an increase in biocrude yield tending to produce biocrude with higher oxygen content. Pig manure had the lowest yield, as expected with its higher unconvertable ash content, while spent grain had the highest yield and lowest oxygen content due to its high oil-containing extractives content. Overall, the high reported oxygen content of the biocrude (10.2–28.4 wt%) would have major challenges in integration with crude oil refinery streams (oxygen content <2 wt%) due to immiscibility, higher viscosity, and oxidative instability, requiring further upgradation techniques.55,60
Pig manure had a contrary catalytic effect than typically observed among feeds in Fig. 5, decreasing biocrude yield and increasing oxygen content (11.0 to 9.3 wt%; 14.0 to 16.9 wt%). Having a considerably higher ash content (17.4 wt%) and moisture content (12.2 wt%) while holding the lowest volatile matter (46.1 wt%) compared to other feedstocks screened, pig manure's biocrude forming components are dilute and create a low potential for biocrude-forming capabilities.61,65 With the addition of catalysts in this study, the biocrude-forming components are further diluted and isolated, averting from the threshold volatile solids concentration needed for biocrude formation. Given the high concentration of alkaline metals (Na, K, Ca, etc.) and carbonates already present in pig manure ash, it is likely that the catalytic effect by addition of these similar compounds causes saturation during HTL that nullifies the effects observed by other feedstocks.65 Similarly, Canola straw by far had the highest ash content (8.8 wt%) among feeds other than pig manure and likely had only a small increase in biocrude yield (1.5 wt%) and decrease in oxygen content (1.3 wt%) because of a similar saturation of the catalyst with inherent ash components.
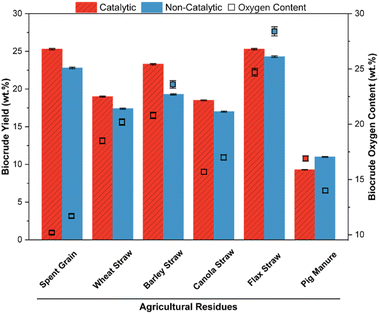 | ||
| Fig. 5 Biocrude yield and oxygen content of agricultural residues screened with and without catalyst. | ||
The combined effect of the K2CO3 and Fe catalysts had a larger impact on forestry feedstocks' biocrude yield and oxygen content. As opposed to straws, the higher lignin-content softwoods (23.5–27.1 wt%) with lower proportions of cellulose and hemicellulose to lignin, observed the greatest reduction in biocrude oxygen from catalyst addition, ranging in reduction of 4.1–7.0 wt% in oxygen content difference. Fig. 6 outlines the effect of catalyst on the wood feeds, also increasing biocrude yield for each feedstock by significant amounts (2.7–10.2 wt%). There are more studies utilizing woods for HTL than agricultural straws with a reported wide range of biocrude yield (3.7–53.3 wt%) and biocrude quality when comparing wood studies for both catalytic and non-catalytic HTL.66–68 This further emphasizes the impact of the several key operating conditions during HTL that need to be optimized and similar for HTL studies to even begin to be comparable. With the use of moderate conditions in this study, there is no surprise that the catalytic (24.5–26.5 wt%) and non-catalytic (14.8–23.8 wt%) wood biocrude yields ranged near the middle of reported HTL studies.66 Notably, the biocrude derived from higher-lignin (23.5–27.1 wt%) softwoods was more impacted between catalytic and non-catalytic runs than the lower-lignin (13.0 wt%) hardwood aspen as well as lower-lignin agricultural straws (7.5–15.1 wt%) with biocrude increases of 9.0–10.2 wt% indicating the reaction pathway for degradation of softwood lignin into biocrude is significantly targeted by the catalyst. The catalytic HTL pathway opens the otherwise untapped biocrude potential of inherently lower-oxygen and higher-carbon lignin.69 Simultaneously the catalyst still increases deoxygenation reactions (decarboxylation, dehydration, decarbonylation, etc.) of all fibre fragments, explaining the observation of a significant 1.8–6.1 wt% decrease in biocrude oxygen content for the high-lignin (>23.5 wt%) softwoods.70 Given the complexity and harsh conditions of HTL with current capabilities, the exact non-catalytic and catalytic mechanisms and degradation pathways occurring during the reaction are limited in understanding with these generalized trends serving as a potential starting point for further agro-forestry catalytic HTL compound studies.
Among specific compounds identified, guaiacol (phenol, 2-methoxy-) was identified as the most abundant for catalytic barley straw biocrude (15.0% total area), while prevalent as the second most for non-catalytic barley straw and catalytic aspen (9.2% & 8.9%), as well as third for non-catalytic aspen (6.1%). Syringol (phenol, 2,6-dimethoxy-) was also very prevalent as the most abundant biocrude compound for non-catalytic barley straw (10.6%) as well as both catalytic and non-catalytic aspen wood (16.6% & 17.2%). Both guaiacol and syringol formation pathways from lignin are well known through thermochemical and/or alkaline-induced aryl ether cleavages of the appropriately-named guaiacyl and syringyl subunits, consistent with production during HTL conditions.72,73 It was noted that guaiacol content was increased likely at the expense of syringol during catalytic HTL as syringol can further degrade to guaiacol through hydrodeoxygenation of a methoxy group that is induced through the reductive hydrogen production of the Fe catalyst used.72 Other phenols commonly identified across the four biocrudes included 4-ethyl-2-methoxy-phenol, 4-ethyl-phenol, nortrachelogenin, and phenol, most of which are identified in similar lignocellulosic HTL studies.57,74,75
Both catalytic barley straw and aspen wood biocrudes contained higher amounts of hydrocarbons and lower amounts of carboxylic acids, likely indicative of decarboxylation reaction pathways promoted during catalytic HTL and further improved biocrude quality. Large portions of each biocrude included carboxylic acids: primarily myristic, palmitic, and oleic long-chain fatty acids (C14–C18), derived from degradation reactions such as hydrolysis, retro-aldol, and dehydration of hemicellulose, cellulose, as well as select extractives (sugars and lipids).76 The derivation of carboxylic acids from cellulose has shown to be promoted during alkaline-aqueous conditions, prevalent initially in catalytic HTL with K2CO3 and potentially explaining the only small decrease in carboxylic acids between catalytic and non-catalytic runs despite decarboxylation promoted further along catalytic HTL reactions.55 Under neutral and acidic conditions in part created by carboxylic acid formation, hemicellulose and cellulose tend to form more ketones, aldehydes, and alcohols through several hydrolysis, dehydration, transformation, recombination, and cyclization reactions, as reflected by these significant compounds in GC-MS results.76–78 As the second largest compositional group, significant ketone components among the biocrude samples included 2,2-methyl-cyclopenten-1-one, 2,3-dimethyl-2-cyclopenten-1-one, 1-(4-hydroxy-3,5-dimethoxyphenyl)-ethanone, 1-(2,6-dihydroxy-4-methoxyphenyl)-ethanone, 1-(4-hydroxy-3-methoxyphenyl)-2-propanone, most of which are prevalent in similar lignocellulosic HTL studies.57,79,80
Detection of significant ethers was specific to aspen wood biocrudes, likely since these ether compounds are derived from the more prevalent aryl–ether bonds (β-O-4 & α-O-4) unique to the lignin of lignocellulosic biomass that is 62.5% higher in aspen wood than barley straw.69 Aspen's higher lignin content may also contribute to the lack of significant non-phenolic alcohols (relative peak area >1%) present in the aspen wood biocrudes despite 6–8% of the barley straw biocrude containing these compounds. With more biocrude-forming reaction pathways from lignin that primarily form phenolics, ketones, and ethers during aspen wood HTL, it is likely that the alcohol compounds derived from cellulose and hemicellulose become diluted and joined the portion of unidentified compounds.77 As for barley straw, like most agricultural residues, it contains small but significant protein and ash content, leading to nitrogen compounds specific to the biocrude (mainly fatty amines) that are nonexistent in forestry biocrude.78,81 The Maillard reaction between proteins and sugars is one prevalent mechanism to form the amides detected in barley straw biocrude. Moreover, lipids prevalent in the straw extractives and differing from wood feedstocks likely led to a small number of esters prevalent only in the catalytic barley straw biocrude.81 Overall, the number of compounds identified during GC-MS was a good representation of each sample's biocrude, containing primarily oxygenated compounds and accounting for the majority of the peak areas for each (>73%).
 | ||
| Fig. 8 TGA of barley straw and aspen wood biocrude under (a) nitrogen and (c) air atmosphere with respective absolute DTGA curves (b and d). | ||
The approximate fuel distributions of the biocrudes show a distinct trend between catalytic and non-catalytic HTL, with some key differences between barley straw and aspen wood biocrude. Considering common fuel fractions based on volatility, the gasoline range (100–190 °C) was relatively small compared to other fractions only making up between 6.2 and 9.6 wt% of samples in Fig. 8(a), suggesting the majority of the biocrude lacked volatility and low molecular weight compounds as confirmed by the high onset temperatures as well as GC-MS results of Section 4.2.3. From non-catalytic to catalytic runs, an increase in the gasoline phase was observed with barley straw biocrude improving from 7.4 to 9.6 wt% and aspen wood from 6.2 to 7.0 wt%. This fraction was likely composed of the light-ends of ketones, alcohols, and hydrocarbons, with potentially small portions of aldehydes, carboxylic acids, and phenols. Significant compounds include 2-methyl-2-cyclopenten-1-one, 2,3-dimethyl-2-cyclopenten-1-one, 3-methyl-1-pentyne, and phenol identified by GC-MS of Section 4.2.3 as well as reported by Pedersen et al.13 Between barley straw and aspen wood, it is also noticeable that the gasoline phase is larger for barley straw and increases more with catalyst, likely due to the higher portion of alcohols and carboxylic acids present in non-catalytic barley straw, shown to catalytically convert to more volatile compounds like hydrocarbons. The diesel phase (190–340 °C) of Fig. 8(a) was more than double the gasoline phase for all biocrudes, making up a relatively consistent portion between 18.3 and 21.7 wt%. It was observed that the aspen wood diesel fraction increased with catalytic HTL from 18.3 to 20.3 wt%, following the trend of increased volatility due to further decomposition and deoxygenation during catalytic HTL. However, the barley straw had a slight decrease from 21.7 to 20.1 wt% in diesel fraction, possibly due to the loss of the diesel phase for the increase to the gasoline phase, while the vacuum gas oil range (340–538 °C) remained unaffected in contributing to the diesel phase. This diesel phase likely contained a combination of lighter phenols like guaiacol and syringol previously identified in Section 4.2.3 as well as heavier long-chain hydrocarbons (such as undecane and 2,9-dimethyl-decane), carboxylic acids (tetradecanoic acid and n-hexadecanoic acid), alcohols (2-hexyl-1-octanol and 2-hexyl-1-decanol), ketones (2-dodecanone and 2-tetradecanone), and aldehydes (3-hydroxy-4-methoxy-benzaldehyde).
The phase affected the most by the catalyst was overwhelmingly vacuum residue (>538 °C) of Fig. 8(a), decreasing from 37.3 to 11.0 wt% for barley straw and 39.3 to 16.8 wt% for aspen wood biocrude. Just as the metallic and alkaline catalysts are known to enhance decomposition pathways to reduce solid products, heavy non-volatile residues made up of largely unidentified multi-ring aromatics as well as long-chain oxygenated compounds from biocrude phase repolymerization are significantly reduced and instead contribute to the vacuum gas oil phase.56,62 The decomposition and partial deoxygenation of heavy biocrude residues that make up the majority of non-catalytic biocrude significantly increase vacuum gas oil to become the majority composition for catalytic biocrude. Barley straw vacuum gas oil increased from 33.2 to 58.9 wt% while aspen wood increased from 35.4 to 55.3 wt%, primarily made up of relatively stable and non-volatile phenolics with multiple functional groups, as well as heavier aldehydes and ketones. Biocrude GC-MS (Section 4.2.3) identified significant compounds in this phase such as syringylacetone, homosyringaldehyde, 1-(2,6-dihydroxy-4-methoxyphenyl)-ethanone, 1-(4-hydroxy-3,5-dimethoxyphenyl)-ethanone, 1-(4-hydroxy-3-methoxyphenyl)-2-propanone, 1,1′-tetradecylidenebis-benzene, palmitic, and oleic acid. Overall the volatility of the selected biocrude was lower compared to similar studies involving lignocellulosic biomass with and without alkaline catalyst. Seehar et al.83 & Hoffmann et al.84 have reported 50–60 wt% of the biocrude aligning with desired gasoline to diesel fractions (<340 °C), indicating the need for further optimization of the screened HTL conditions, as there is likely potential to improve biocrude quality.
Observation of the DTGA curves of Fig. 8(b) and (d) aligns with the TGA findings, showing the majority non-volatility and high molecular weight components of the biocrude have similar volatility, leading to large mass loss rates at vacuum gas oil temperatures. The largest rates of biocrude mass loss ramp up in groupings representing similar volatility compounds in each distinct fuel distribution, as mentioned. The largest consistent rates of mass loss were in the range of vacuum gas oils for catalytic biocrude and vacuum residue for non-catalytic biocrude, coinciding with each biocrude's largest equivalent fuel fraction. It can be observed that some large but sudden spikes are observed near the start of the inert vacuum gas oil region (∼350–375 °C) for only the non-catalytic biocrudes, likely coinciding with palmitic and oleic acid that is particularly large in the non-catalytic biocrude fractions and is the only major compound identified in this region of volatility (boiling points: 351 & 360 °C, respectively). The oxidative DTGA observed three main stages of mass loss among all biocrudes, partly aligning with the conventional fuel fractions mentioned and giving an idea of the combustion characteristics.54 The gasoline and diesel fractions are over a smaller range and compose the first range of elevated oxidative degradation rate starting at 125–135 °C before dropping off around 315–330 °C and then increasing again over 350–450 °C in the vacuum gas oil range. Meanwhile the largest overall rates were observed towards the end of the vacuum gas oil range for catalytic biocrudes (475–550 °C) or further into the vacuum residue range for non-catalytic biocrude, confirming the large non-volatile components of the biocrude due to high molecular weight components. These high temperatures needed for the largest biocrude fuel portions to combust are particularly undesirable as the heating conditions needed are inefficient and particularly inconvenient for transportation systems, suggesting further biocrude upgrading through chemical methods like hydrotreatment is needed to improve combustion characteristics.82
| Characterization | Barley straw | Aspen wood | ||
|---|---|---|---|---|
| Catalytic | Non-catalytic | Catalytic | Non-catalytic | |
| Degree of degradation (wt%) | 83.4 ± 0.3 | 77.3 ± 0.4 | 88 ± 0.8 | 79 ± 0.3 |
| Energy recovery (%) | 41.4 ± 0.4 | 32.4 ± 0.7 | 47.3 ± 0.1 | 39.9 ± 0.4 |
| HHV (MJ kg−1) | 30.8 ± 0.2 | 29.2 ± 0.6 | 30.9 ± 0.1 | 29.2 ± 0.3 |
| C (wt%) | 71.4 ± 0.1 | 68.9 ± 0.7 | 71.5 ± 0.4 | 69 ± 0.8 |
| H (wt%) | 7.3 ± 0.0 | 7.0 ± 0.0 | 7.1 ± 0.0 | 6.8 ± 0.3 |
| N (wt%) | 0.4 ± 0.0 | 0.4 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.1 |
| S (wt%) | 0.1 ± 0.0 | 0.1 ± 0.1 | 0.0 ± 0.0 | 0.1 ± 0.0 |
| O (wt%) | 20.8 ± 0.1 | 23.6 ± 0.5 | 21.4 ± 0.4 | 24.1 ± 0.5 |
| Moisture (wt%) | 0.1 ± 0.0 | 0.2 ± 0.0 | 0.5 ± 0.0 | 0.6 ± 0.0 |
| Density (kg m−3@ 22 °C) | 1035 ± 15 | 1100 ± 10 | 1045 ± 15 | 1095 ± 15 |
| TAN (mg KOH g−1) | 25.3 ± 0.9 | 70.6 ± 2.6 | 28.7 ± 1 | 57.1 ± 2.1 |
Catalytic and non-catalytic biocrude quality displayed advantageous properties compared to alternative pyrolysis studies of similar lignocellulosic feeds, verifying the potential and advantages of HTL.12 More energy-intensive pyrolysis bio-oil (550 °C) deviates from this study's biocrude with higher yield (60–68 wt%) but undesirable higher oxygen (31.9–35.3 wt%), lower heating value (23.7–24.0 MJ kg−1), higher density (1120–1150 kg m−3), and much higher water content (25.7–27.3 wt%).86 Yet, the quality of the catalytic biocrude still differs from conventional crude oil properties in terms of oxygen (<2.0 wt%), heating value (40.7–42.0 MJ kg−1), density (803.8–1007.7 kg m−3), and TAN (<2.27 mg KOH g−1).87,88 The main cause is high oxygen content, verified by a trend of oxygenated compounds via GC-MS (Section 4.2.3). These oxygenated compounds possess high acidity, viscosity, density, and lower heating value, making the reduction of biocrude oxygen content key to improving its quality and removing barriers for transport, storage, and integration with conventional crude oil.12,87 Given this overarching effect of oxygen on biocrude quality, it is logical that the catalyzed decarboxylation pathways lowering oxygen content in the biocrude possess consistently improved properties. Zhu et al.57 with similar barley straw feed and HTL conditions have reported biocrude with oxygen content of 29.75 wt% for non-catalytic & 23.18 wt% for catalytic, suggesting improved quality in this work's biocrude potentially due to the use of not only an alkaline but additionally a metallic catalyst that the compared study did not consider. Yu et al.75 reported aspen wood biocrude as low as 10.7 wt% in terms of oxygen however it should be noted that the HTL was performed at a much greater temperature (∼400 °C), shown to favour deoxygenation at the expense of biocrude yield.12 Despite the option to improve biocrude quality through optimization, there is still further processing needed in the form of upgrading biocrude properties before co-processing with conventional crude oil is feasible. All of the biocrude's moisture content was relatively low (<0.6 wt%), desirable for avoiding microbial growth that can reduce quality and cause problems for handling, transport, and catalytic upgrading.89
| Characterization | Barley straw | Aspen chips | |||
|---|---|---|---|---|---|
| Catalytic | Non-catalytic | Catalytic | Non-catalytic | ||
| a CHNSO data is reported on an ash-free basis. | |||||
| Hydrochar | |||||
| HHV (MJ kg−1) | 18.1 ± 0.6 | 26.6 ± 0.6 | 21.3 ± 0.1 | 28.5 ± 0.6 | |
| Ca (wt%) | 78.7 ± 1.5 | 79.3 ± 1.2 | 89.6 ± 0 | 82.6 ± 0.3 | |
| Ha (wt%) | 6.2 ± 0.1 | 5.6 ± 0.1 | 6.8 ± 0 | 5.6 ± 0 | |
| Na (wt%) | 1.1 ± 0.1 | 1.3 ± 0.0 | 0.2 ± 0.0 | 0.3 ± 0.2 | |
| Sa (wt%) | 0.1 ± 0.1 | 0.2 ± 0.0 | 0.1 ± 0.0 | 0.2 ± 0.1 | |
| Oa (wt%) | 13.9 ± 1.7 | 13.6 ± 1.3 | 3.4 ± 0.1 | 11.3 ± 0.5 | |
| Ash (wt%) | 58.7 ± 0.7 | 13.4 ± 0.4 | 46.9 ± 0.8 | 10.6 ± 0.2 | |
| BET surface area (m2 g−1) | 17.3 ± 0.6 | 73.7 ± 2.5 | 2.5 ± 0.1 | 280.8 ± 9.7 | |
| Average pore size (nm) | 241 ± 8.7 | 29.2 ± 1.1 | 212.3 ± 7.7 | 52.8 ± 1.9 | |
| Average pore volume (cm3 g−1) | 0.07 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.02 ± 0.00 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Aqueous phase | |||||
| Density (kg m−3@22 °C) | 1073 ± 8 | 1083 ± 8 | 1085 ± 10 | 1080 ± 5 | |
It was observed that the non-catalytic hydrochar for both barley straw and aspen wood had different surface properties than catalytic counterparts, with much higher BET surface area and lower average pore sizes. The catalytic hydrochar structures likely underwent collapsing leading to their lower porosity as more decomposition as well as less repolymerization reactions occur during catalytic HTL, creating a weaker carbon structure with more volatile biocrude components present.96 Average pore volume was relatively constant among the aspen wood hydrochar, however catalytic barley straw had an average pore volume size 7 times larger than that of non-catalytic barley straw. A possible explanation is that the pore volume is a cumulation for pores between 1.7 nm and 300 nm, where non-catalytic barley straw hydrochar with the lowest average pore size of 29.2 nm may have significant pores below the range measured. Lignocellulosic hydrochar studies have reported non-activated hydrochars to be typically low-porosity with BET surface areas under 4 m2 g−1, while this study produced notably porous hydrochars well above this range as high as 280.8 m2 g−1.91,96,97 However, it is important to note that the hydrochars were pyrolyzed at 400 °C to remove residual oils, known to physically improve the surface area of the char to as high as 438 m2 g−1.97
For applications in adsorption pollutant removal, like water treatment or CO2 capture, mesoporous (2–50 nm) average pore sizes like the non-catalytic barley straw (and nearly non-catalytic aspen wood) are ideal for high recovery and capacity.96,98 The particularly low heteroatom content of the catalytic aspen hydrochar (<3.7 wt%) indicates low polarity and hydrophobicity, relating to lower degradability that is lucrative for catalyst supports, electrochemicals, soil enrichment, and solid fuel applications.97 Studies have utilized chemical and physical activation techniques to further improve hydrochar's surface properties with surface areas even beyond 1000 m2 g−1, higher than some commercial adsorbents/catalyst supports and having the potential to improve these industrial processes.92,97 The mesoporous (2–50 nm) network of hydrochar pores combined with moderate volume (>0.01 cm3 g−1) and surface area (2–300 m2 g−1) is also being used in innovative electrochemical devices such as bio-supercapacitors and bio-battery production.97 The larger gigaporous (>200 nm) materials, such as the hydrochars derived from both catalytic HTL, lack adsorption qualities and are more suited for applications not reliant on surface properties, such as a soil enhancement (erosion prevention and fertilizer) or solid energy source (bio-coal).97 Valuing sustainability considerations, future work will investigate the suitability of integrating activated HTL hydrochar as a by-product catalyst support in upgrading.
Other than surface properties, the non-catalytic hydrochar also exhibited particularly high HHV (26.6–28.5 MJ kg−1), 52.9% and 57.5% higher than their respective biomass, having potential as a solid energy source.97 Hydrothermal carbonization coal with an HHV of 22.3 MJ kg−1 was considered a candidate for energy production in paper mills, making the non-catalytic hydrochars of this study (>26.6 MJ kg−1) lucrative for energy applications.97 The catalytic hydrochars observed lower HHV likely due to high-ash deposition and higher conversion of energy-dense fibres like lignin while reducing the presence of high viscosity, density, and HHV vacuum residue biocrude that partly linger within hydrochar, even after solvent extraction.97 This is confirmed by TGA results of Section 4.2.4 reporting significantly less vacuum residue range in catalytic biocrudes, as well as the significant decrease in carbon and hydrogen content of the catalytic hydrochar while increasing heteroatom content that align with less biocrude presence.
The density of the aqueous phase (1073–1085 kg m−3) was consistently higher than water, as suspected due to the presence of dissolved organics like organic acids or phenols that have higher density than water. The HTL aqueous phase typically contains a high amount of organics (sugars, alcohols, ketones, etc.) as well as nutrients (phosphate, nitrate, ammonia), characterized by a high chemical oxygen demand and toxicity unsuitable for the environment, but potentially valorizable for integrated thermochemical processing, such as hydrogen gas production from supercritical water gasification.99 Further investigation of the aqueous phase is recommended for potential applications in biochemicals as well as a recyclable solvent for improving HTL biocrude production.100
4.3. Effects of biomass fibre composition on biocrude production
Given the diverse composition of lignocellulosic residues available in Canada, possible relations of fibre content to HTL biocrude were examined. As discussed in Section 4.1, the straw feedstocks generally have higher ash and extractives content with lower lignin, observed to cause higher non-catalytic biocrude yields than the softwoods (i.e. other than aspen wood) but less yield increase from the catalyst, leading to generally lower catalytic yields. Among the barley and flax straw with higher holocellulose (cellulose + hemicellulose) and subsequently lower lignin and ash content, higher biocrude yields but also higher biocrude oxygen contents are observed. The particularly high ash and lignin canola and wheat straw may have lower biocrude yield and oxygen content due to less content of the more-degradable and higher-oxygen content cellulose/hemicellulose.105 The more than three times higher ash content of agricultural residues (>3.9 wt%) compared to forestry residues (<1.1 wt%) can also contribute to generally lower catalytic biocrude yields of agricultural HTL when compared, likely forming a diluting effect as described in Section 4.2.2, or even inducing physical swamping of the heterogeneous Fe catalyst to significantly reduce its activity.106 Canola notably had the highest ash, sulphur, and nitrogen content of straws, leading to the lowest biocrude yield with the highest sulphur (0.4 wt% versus <0.1 wt%) and nitrogen (2.6 wt% versus <1.0 wt%) contents, particularly undesirable for further upgradations where nitrogen compounds have shown to be least reactive and rate-limiting for removal among heteroatoms.107 The ratio of cellulose (C) to hemicellulose (H) content in straws was also noted as a potentially positive indicator of biocrude yield by the work of Tian et al.,58 however this study shows no relation between this ratio and biocrude yield (catalytic nor non-catalytic) as biocrude yields follow the order of canola (C/H: 2.74) < wheat (1.41) < barley (1.55) < flax (1.52).Although trends are observed between the straw fibre composition and the biocrude yield as well as oxygen content, there are differences not fully explained by the fibre content summarized in Fig. 11. For example, the fibre content of the barley and flax straw is closely comparable in terms of cellulose (47.2 to 43.4 wt%), hemicellulose (30.4 to 28.6 wt%), and lignin (8.7 to 8.2 wt%), however the non-catalytic (19.3 versus 23.3 wt%) and catalytic (24.3 versus 25.3 wt%) biocrude yields differ as if these feeds have greater compositional differences. Additionally, the oxygen content of flax straw biocrude was notably the highest for catalytic and non-catalytic straws (24.4 and 28.4 wt%) while barley remained significantly lower (20.8 and 23.6 wt%). As barley and flax straw had the highest ratios of higher oxygen content sugar-polymers (hemicellulose and cellulose) to lignin, these components are proportionately targeted more during HTL for degradation and hence higher-oxygen biocrude production. Although the higher and different extractives as well as marginally lower ash content of the flax straw contributes to the differences among biocrude produced between barley and flax, these significant variations are also likely due to key differences in the straw fibre complex of the oilseed flax crop and cereal barley crop. Specifically, cellulose, the largest fibre component of both flax and barley straw, is approximately 66.2 wt% crystalline in whole flax straw while barley straw is reportedly only 42 wt%.108,109 Flax is known for its long uninterrupted inner crystalline-cellulose chains (74.4 wt% crystalline) and rigid outer high-lignin structure (shive), lending to a physically tougher fibrous straw that is less amorphous and may uniformly hydrolyze for decomposition into biocrude components at harsher points of the reaction, leaving less time for key deoxygenation reactions to occur on the hydrolyzed cellulose fragments.109 Similar to the amorphous nature of hemicellulose's branch structure causing it to have a lower thermal degradation temperature than cellulose, amorphous cellulose has shown to degrade at 313 °C while crystalline is higher at 333 °C, causing less oxygenated biocrude formation and higher hydrochar remains.105,109 Key differences like cellulose crystallinity, extractives content, and inter-fibre interactions may have an impact on HTL biocrude making a fibre-based prediction of the yield and oxygen content less precise.
 | ||
| Fig. 11 Summarized fibre composition of agro-forestry feedstocks with their respective biocrude yield and oxygen content. | ||
Differing in composition trends from straws, the wood feedstocks had particularly low ash and extractives with higher lignin content, as discussed in Section 4.1. Under non-catalytic conditions the softwood feedstocks had lower biocrude yield than the straws with high oxygen content, likely due to the lack of extractives and undegraded lignin, causing the biocrude to be primarily derived from cellulose and hemicellulose. The wood feeds had similar high-holocellulose content (>60 wt%) to barley and flax straw that also had particularly high oxygen content biocrudes, summarized in Fig. 11. This trend is further seen with the lower holocellulose wheat and canola straw having lower oxygen content and the lowest holocellulose spent grain and pig manure having the lowest oxygen content. As discussed in Section 4.2.2, a trend between higher lignin and biocrude yield increase from the catalyst was observed, supported by the high-lignin softwood feedstocks having the largest increase in biocrude yield while the hardwood aspen (13.0 wt%) observed a smaller increase similar to straws. The lignin-derived compounds simply contain less oxygen and likely form lower-oxygen biocrude compounds compared to some hemicellulose and cellulose fragments. With similar comparisons of flax and barley straw to other straw biomass, aspen hardwood had the lowest lignin among woods while maintaining the highest holocellulose content out of all biomass, observed as lucrative among lignocellulosic feeds and agreeing with previous work.110–112 Hence, aspen wood had the highest catalytic biocrude yield (26.5 wt%) with moderate oxygen content (21.4 wt%) compared to the softwoods and straws. The wood-derived biocrude had low nitrogen (<0.3 wt%) and sulphur (∼0 wt%) content, consistent with the low heteroatom content of the wood feeds and desirable for further biocrude upgrading.
The degradation temperature range of hemicellulose and cellulose are reported at 220–315 °C and 315–400 °C, respectively, while lignin has a much wider range of 160–900 °C with the components typically being more thermodynamically stable.105 Considering the lignocellulosic fibre degradation temperatures, it is likely that most if not all the hemicellulose and cellulose are degraded through at least initial hydrolysis into aqueous, biocrude, or gas products during the 300 °C and 10 MPa HTL conditions, while a significant portion of lignin remains as a solid hydrochar product in non-catalyzed environments. Compared to the glycosidic bonds of hemicellulose and cellulose that are susceptible to both acid and alkaline degradation, the more chemically inert aryl–ether bonds that link lignin are mainly susceptible to degrade in strong alkaline conditions created by K2CO3.57 The key to increasing biocrude yield is through unlocking lignin's potential by cleaving its aryl–ether bonds to produce significant phenolic and ketone groups, as seen with higher lignin components generally observing greater biocrude yield increase with alkaline catalyst similarly reported by Bhaskar et al.113 Given that the degradation of many extractives, cellulose, and hemicellulose generates acids,90 alkaline catalysts are key like the K2CO3 used in this work. Otherwise, acidic conditions can be induced early into HTL without an alkaline catalyst, preventing the degradation of lignin, and also catalyzing condensation and repolymerization reactions for lower overall biocrude formation.70
4.4. Fibre-based model for estimating catalytic biocrude production
Among the 10 lignocellulosic feedstocks there are a broad range of fibre contents including cellulose (17.9–61.1 wt%), hemicellulose (6.5–30.4 wt%), lignin (8.2–29.1 wt%), and extractives (4.1–36.2 wt%) that have impact on the HTL process. Although the trends and literature discussed are useful, the creation of a fibre-based model to predict biocrude yield would be even more beneficial for future HTL lignocellulosic feedstock considerations. A multiple linear regression model was developed with Origin data analysis software to predict catalytic biocrude yield based on cellulose (C), hemicellulose (H), lignin (L), and extractives (E) of a lignocellulosic feed.114 To avoid the potentially unnecessary complexity of the model, only individual linear effects were used with the assumption that there were no significant interaction terms among the variables representing fibre content. The catalytic biocrude yield model represented the data with a strong fit, indicated by an adjusted coefficient of determination (R2adjusted) equivalent to 0.87. The model had small and unpredictable residuals in predicting biocrude yield, with each of the 10 feedstocks being predicted with residuals of 1.4 wt% or less except for wheat straw, which had a 3.3 wt% difference. The model accounted for 87.4% of the biocrude yield variance among the differing fibre-composition feeds of this study, a strong predictor for lignocellulosic HTL at these set conditions. Given this model is only applicable at these conditions, and can only be verified at these conditions, a more flexible and robust model that includes HTL conditions is recommended, such as the one developed by Yang et al.115 for model compounds. The model represented by eqn (13) indicates that all the lignocellulosic components positively contributed to biocrude yield with varying effects in the order of extractives > cellulose > hemicellulose > lignin based on the individual coefficients. Single linear regression was performed on each fibre in relation to catalytic biocrude yield summarized in Table 5, determining that no individual fibre had a particularly strong relation compared to the overall model as cellulose had the highest coefficient of determination (R2) at 0.64. No sole fibre component is a strong indicator for catalytic biocrude yield as all components have contrasting influences, only captured accurately by the overall model that includes all fibres and hence has the highest adjusted coefficient of fit.| Catalytic biocrude yield (wt%) = 0.932 (E) + 0.889 (C) + 0.865 (H) + 0.766 (L) − 59.978 | (13) |
| R2adjusted = 0.87 |
| Residue | Pig manure | Spent grain | Canola straw | Wheat straw | Barley straw | Flax straw | Pine wood | Tamarack wood | Spruce wood | Aspen wood |
|---|---|---|---|---|---|---|---|---|---|---|
| Experimental biocrude yield (wt%) | 9.3 | 25.3 | 18.5 | 19.0 | 23.3 | 25.3 | 24.0 | 25.3 | 25.5 | 26.5 |
| Predicted biocrude yield (wt%) | 8.6 | 23.9 | 19.5 | 22.3 | 23.2 | 24.2 | 24.7 | 25.4 | 24.3 | 25.9 |
| Individual models | Cellulose | Hemicellulose | Lignin | Extractives | ||||||
| R 2 | 0.64 | 0.52 | 0.32 | 0.46 | ||||||
Despite catalytic biocrude being modelled reasonably well, a model for the non-catalytic biocrude based on fibre content was found to be an ineffective fit with an R2adjusted of 0.68. The unpredictability of the non-catalytic biocrude could be due to the complex inter-fibre bonding and interactions during thermochemical degradation that may need non-linear or interactive effects to be better modelled. These interactions are overcome and have less influence during the more effective decomposition that occurs in catalytic HTL of lignocellulosic residues.116 Multiple linear regression of the fibre content concerning biocrude oxygen content was also investigated with neither catalytic and non-catalytic biocrude oxygen content forecast well at R2adjusted of 0.66 and 0.61, respectively. Similar to the non-catalytic biocrude, oxygen content may not be predictable by multiple linear regression without interaction terms due to the intricacy of deoxygenation pathways that occur through HTL including cleavages, decarboxylation, decarbonylation, dehydration, and hydrodeoxygenation among others. Much more complex compositional-based models for predicting biocrude yield and other HTL products have been utilized by Wang90 and Subramanya et al.,117 reporting reasonable prediction and understandable error due to HTL complexity. HTL complexities include competing simultaneous reactions, forward/reverse kinetics, interaction effects between components, strong influence of other process conditions, etc. The use of assistive-AI like machine learning and other innovative techniques is recommended to further investigate the challenge of HTL product prediction.
Although no sole fibre content overwhelmingly predicted biocrude yield across the 10 different lignocellulosic feedstocks, volatile matter was a predictable indicator of catalytic biocrude yield when considering proximate analysis. Similar to the fibre model, volatile matter was found to have strong R2 correlation of 0.94 for catalytic biocrude, while non-catalytic biocrude lacked a linear trend with a mere R2 of 0.31. Visual representation of the volatile matter's relation to catalytic biocrude yield as well as the lack of relation for non-catalytic biocrude is highlighted in Fig. 12, including the model equations developed by linear regression. Volatile matter had a strong positive relation with catalytic biocrude yield, more desirable than the multi-variable fibre model due to fit and simplicity, suggesting that the more volatile matter a lignocellulosic residue contains the higher catalytic biocrude yield it will produce during HTL. This correlation aligns with the trend observed by Mishra & Mohanty,118 as volatile components more readily decompose and deoxygenate to biocrude, however the creation of a representative equation to predict biocrude yield from lignocellulosic volatile matter could not be found among literature. Visual representation of the volatile matter's relation to catalytic biocrude yield as well as the lack of relation for non-catalytic biocrude is highlighted in Fig. 12, including the model equations developed by linear regression.
 | ||
| Fig. 12 Linear regression models of catalytic (a) and non-catalytic (b) biocrude yield as a function of volatile matter of lignocellulosic residues. | ||
5. Conclusions
Among the 10 Canadian lignocellulosic residues screened, significant differences in biocrude yield and quality were identified due to compositional differences. Investigating Canadian availability of the agriculture residues through statistics and demand for other applications, the availability of flax straw and spent grain was far too low for a hypothetical biorefinery, while wheat straw was identified as the overwhelmingly most available with approximately 38.3 million metric tonnes available in 2022. Comparison of catalytic and non-catalytic HTL screening showed varying desirable effects of the catalyst to increase biocrude yield and reduce oxygen for the residues, except for pig manure due to high ash and low volatiles. The catalyst had a particularly larger impact on biocrude yield for high-lignin softwood feedstocks compared to straws and hardwood, likely due to increase in decomposition of chemically-resistant lignin. Observational trends of higher holocellulose content contributing to higher oxygen content in the biocrude was identified, while higher lignin had an opposite effect. Based on biocrude yield and oxygen considerations, barley among straws and aspen among woods had the most desirable biocrudes screened and were selected for further analysis.Barley straw had a catalytic yield of 23.3 wt% with 20.8 wt% oxygen, while aspen wood had a catalytic yield of 26.5 wt% with 21.4 wt% oxygen, both largely made up of phenols. Major catalytic effects for both the straw and wood feed were desirable including increased biocrude and gas yields with lower hydrochar yield, increased hydrocarbon content by reducing oxygenated compounds, increased biocrude volatility by a decrease in low-volatility vacuum residues, increased HHV, higher degree of degradation and energy recovery, lower acidity, and lower density. A fibre-based linear regression model of catalytic biocrude yield was created with a strong fit of R2adjusted = 0.87, indicating a positive contribution of each fibre to biocrude yield in the order of extractives > cellulose > hemicellulose > lignin. Both biocrude oxygen contents, as well as non-catalytic biocrude yield, did not show significant fits by fibre-based multiple linear regression, however volatile matter had the strongest correlation to catalytic biocrude yield out of any single characteristic, with R2 = 0.94.
As oxygen content of the biocrude decreases the quality of the biocrude while increasing polarity for reduced miscibility and integration with conventional crude oil, the reduction of high oxygen content reported for all biocrudes in this work (as well as increasing yield) is a priority of HTL. Given screening at these identical conditions, further optimization of the key HTL parameters and their effect on HTL biocrude is considered for future study. Additional considerations for biomass pretreatment and biocrude-extracting solvent, as well as biocrude upgrading will be investigated due to commercial significance to produce sustainable transportation fuels.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
John Churchill: conceptualization, data curation, formal analysis, investigation, methodology, validation, visualization, writing – original draft, writing – review & editing. Venu Borugadda: conceptualization, writing – review & editing, supervision. Ajay Dalai: conceptualization, funding acquisition, project administration, writing – review & editing, supervision.Conflicts of interest
The authors declare that no conflicts, personal or financial, appear to influence this work.Acknowledgements
This work was financially supported by Tidewater Renewables Ltd. Additionally, this work was supported financially by the Natural Sciences and Engineering Research Council of Canada (NSERC). The workspace and facilities provided by the University of Saskatchewan is greatly acknowledged. GC-MS conducted by the Saskatchewan Structural Sciences Centre (SSSC) is acknowledged and appreciated. The ability to copyright and use Fig. 1 and 2 from the work of REN21 as well as Yanan Zheng & Professor Feng Qiu, respectively, is acknowledged and greatly appreciated.References
- Energy Institute, Exploring Energy Topics – Transport, https://www.energyinst.org/exploring-energy/topic/transport#, accessed 22 April 2023.
- International Energy Agency, Transport – Improving the sustainability of passenger and freight transport, https://www.iea.org/topics/transport, accessed 22 April 2023.
- Government of Canada, Net-Zero Emissions by 2050, https://www.canada.ca/en/services/environment/weather/climatechange/climate-plan/net-zero-emissions-2050.html, accessed 22 April 2023.
- REN21, Global Overview, Renewables 2022 Global Status Report, REN21, Paris, 2022, ch. 1, pp. 43–43 Search PubMed.
- H. Kargbo, J. S. Harris and A. N. Phan, Renewable Sustainable Energy Rev., 2021, 135, 110168 CrossRef.
- K. Alper, K. Tekin, S. Karagöz and A. J. Ragauskas, Sustainable Energy Fuels, 2020, 4, 4390–4414 RSC.
- A. Muscat, E. M. de Olde, I. J. M. de Boer and R. Ripoll-Bosch, Glob. Food Secur., 2020, 25, 100330 CrossRef.
- L. Vevere, A. Fridrihsone, M. Kirpluks and U. Cabulis, Polymers, 2020, 12, 2706 CrossRef PubMed.
- Y. Zheng and F. Qiu, Biomass Bioenergy, 2020, 140, 105669 CrossRef.
- S. N. Sahu, N. K. Sahoo, S. N. Naik and D. M. Mahapatra, Bioreactors: Sustainable Design and Industrial Applications in Mitigation of GHG Emissions, 2020, pp. 195–213 Search PubMed.
- S. S. Toor, L. Rosendahl and A. Rudolf, Energy, 2011, 36, 2328–2342 CrossRef.
- R. K. Mishra, V. Kumar, P. Kumar and K. Mohanty, Fuel, 2022, 316, 123377 CrossRef.
- T. H. Pedersen, C. U. Jensen, L. Sandström and L. A. Rosendahl, Appl. Energy, 2017, 202, 408–419 CrossRef.
- W. U. Rahman, M. Patel, V. Kurian and A. Kumar, Energy Convers. Manage., 2022, 267, 115877 CrossRef.
- S. Sokhansanj, S. Mani, M. Stumborg, R. Samson and J. Fenton, Can. Biosyst. Eng., 2006, 48, 39–44 Search PubMed.
- S. González-García, L. Luo, M. T. Moreira, G. Feijoo and G. Huppes, Biomass Bioenergy, 2012, 36, 268–279 CrossRef.
- X. Li, E. Mupondwa, S. Panigrahi, L. Tabil, S. Sokhansanj and M. Stumborg, Renewable Sustainable Energy Rev., 2012, 16, 2954–2965 CrossRef.
- Statistics Canada, Crop production: Visualization tool, https://www150.statcan.gc.ca/n1/pub/71-607-x/71-607-x2020025-eng.htm, accessed 30 September 2023.
- Renewable Industries Canada, Map – Renewable Industries Canada, https://ricanada.org/industry-map/, accessed 31 December 2023.
- M. Navaratnasamy, L. Papworth and J. Jones, Integrating Biogas, Confined Feedlot Operations and Ethanol Production, 2014 Search PubMed.
- The Western Producer, Canadian feeders imports more dried distillers grain from U.S. | The Western Producer, https://www.producer.com/news/canadian-feeders-imports-more-dried-distillers-grain-from-u-s/, accessed 31 December 2023.
- Statistics Canada, Hogs/Pork – agriculture.canada.ca, https://agriculture.canada.ca/en/sector/animal-industry/red-meat-and-livestock-market-information/hogs-pork, accessed 31 December 2023.
- Agriculture and Agri-Food Canada, Research Strategy for Hog Manure Management in Canada, 1998 Search PubMed.
- S. Acar, A. Ayanoglu and A. Demirbas, Energy Educ. Sci. Technol., Part A, 2012, 28, 749–758 Search PubMed.
- ASTM, Standard Test Method for Gross Calorific Value of Coal and Coke, 2019 Search PubMed.
- ASTM, Standard Test Method for Moisture in the Analysis Sample of Coal and Coke, 2017 Search PubMed.
- ASTM, Standard Test Method for Ash in the Analysis Sample of Coal and Coke from Coal, 2020 Search PubMed.
- ASTM, Standard Test Method for Volatile Matter in the Analysis Sample of Coal and Coke, 2020 Search PubMed.
- O. Fadele, I. N. A. Oguocha, A. G. Odeshi, M. Soleimani and L. G. Tabil, Cellulose, 2019, 26, 9463–9482 CrossRef.
- F. Pattnaik, S. Nanda, V. Kumar, S. Naik and A. K. Dalai, Fuel, 2022, 311, 122618 CrossRef.
- ASTM, Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration, 2019 Search PubMed.
- D. Xu, Y. Wang, G. Lin, S. Guo, S. Wang and Z. Wu, Renew. Energy, 2019, 138, 1143–1151 CrossRef.
- S. Chen, W. Liao, C. Liu, Z. Wen, R. Kincaid and J. Harrison, Value-Added Chemicals from Animal Manure, U.S. Department of Energy, 2003 Search PubMed.
- S. Xiu, A. Shahbazi, V. Shirley and D. Cheng, J. Anal. Appl. Pyrolysis, 2010, 88, 73–79 CrossRef.
- A. Lorente, J. Remón, V. L. Budarin, P. Sánchez-Verdú, A. Moreno and J. H. Clark, Energy Convers. Manage., 2019, 185, 410–430 CrossRef.
- S. Naik, V. V. Goud, P. K. Rout, K. Jacobson and A. K. Dalai, Renew. Energy, 2010, 35, 1624–1631 CrossRef.
- R. Azargohar, K. L. Jacobson, E. E. Powell and A. K. Dalai, J. Anal. Appl. Pyrolysis, 2013, 104, 330–340 CrossRef.
- S. Das, A. Mathanker, D. Pudasainee, M. Khan, A. Kumar and R. Gupta, Int. J. Energy a Clean Environ., 2022, 23, 31–45 CrossRef.
- P. Adapa, L. Tabil and G. Schoenau, Biosyst. Eng., 2009, 104, 335–344 CrossRef.
- Z. Zhu, L. Rosendahl, S. S. Toor and G. Chen, Sci. Total Environ., 2018, 630, 560–569 CrossRef PubMed.
- Z. Xiao, Q. Wu, X. Zheng, L. Zhang, D. Zou, B. Chen, B. Wang and F. Liu, Ind. Crops Prod., 2022, 188, 115564 CrossRef.
- K. Khandelwal, S. Nanda, P. Boahene and A. K. Dalai, Int. J. Hydrogen Energy, 2024, 49, 1518–1527 CrossRef.
- J. A. Okolie, S. Nanda, A. K. Dalai and J. A. Kozinski, Energy Convers. Manage., 2020, 208, 112545 CrossRef.
- T. H. Pedersen, I. F. Grigoras, J. Hoffmann, S. S. Toor, I. M. Daraban, C. U. Jensen, S. B. Iversen, R. B. Madsen, M. Glasius, K. R. Arturi, R. P. Nielsen, E. G. Søgaard and L. A. Rosendahl, Appl. Energy, 2016, 162, 1034–1041 CrossRef.
- M. Patel, A. O. Oyedun, A. Kumar and R. Gupta, Waste Biomass Valorization, 2019, 10, 2745–2760 CrossRef CAS.
- N. Harun and M. Afzal, Adv. Environ. Biol., 2015, 9, 34–41 Search PubMed.
- B. Zhao, H. Li, H. Wang, Y. Hu, J. Gao, G. Zhao, M. B. Ray and C. C. Xu, Renew. Energy, 2021, 176, 543–554 CrossRef CAS.
- R. W. Bryers, Prog. Energy Combust. Sci., 1996, 22, 29–120 CrossRef CAS.
- H. S. Du, X. Y. Li, X. Y. Ren and Y. X. Han, Adv. Mater. Res., 2013, 774–776, 503–507 CAS.
- A. Demirbas, Energy Sources, Part A, 2017, 39, 592–598 CrossRef CAS.
- A. Friedl, E. Padouvas, H. Rotter and K. Varmuza, Anal. Chim. Acta, 2005, 544, 191–198 CrossRef CAS.
- F. Hardi, M. Mäkelä and K. Yoshikawa, Energy Procedia, 2017, 105, 75–81 CrossRef.
- R. Kaur, B. Biswas, J. Kumar, M. K. Jha and T. Bhaskar, Ind. Crops Prod., 2020, 149, 112359 CrossRef CAS.
- R. Chand, V. Babu Borugadda, M. Qiu and A. K. Dalai, Appl. Energy, 2019, 254, 113679 CrossRef CAS.
- I. A. Basar, H. Liu, H. Carrere, E. Trably and C. Eskicioglu, Green Chem., 2021, 23, 1404–1446 RSC.
- B. de Caprariis, I. Bavasso, M. P. Bracciale, M. Damizia, P. De Filippis and M. Scarsella, J. Anal. Appl. Pyrolysis, 2019, 139, 123–130 CrossRef CAS.
- Z. Zhu, S. S. Toor, L. Rosendahl, D. Yu and G. Chen, Energy, 2015, 80, 284–292 CrossRef CAS.
- Y. Tian, F. Wang, J. O. Djandja, S. L. Zhang, Y. P. Xu and P. G. Duan, Fuel, 2020, 265, 116946 CrossRef CAS.
- H. Wang, M. Zhang, X. Han, Y. Zeng and C. C. Xu, Biomass Bioenergy, 2023, 173, 106810 CrossRef CAS.
- V. B. Borugadda, R. Chand and A. K. Dalai, Energy Convers. Manage., 2020, 222, 113186 CrossRef CAS.
- W. T. Chen, W. Qian, Y. Zhang, Z. Mazur, C. T. Kuo, K. Scheppe, L. C. Schideman and B. K. Sharma, Algal Res., 2017, 25, 297–306 CrossRef.
- Y. Chen, X. Cao, S. Zhu, F. Tian, Y. Xu, C. Zhu and L. Dong, Bioresour. Technol., 2019, 278, 92–98 CrossRef CAS.
- A. A. Shah, K. Sharma, M. S. Haider, S. S. Toor, L. A. Rosendahl, T. H. Pedersen and D. Castello, Processes, 2022, 10, 207 CrossRef CAS.
- Y. Wu, H. Wang, H. Li, X. Han, M. Zhang, Y. Sun, X. Fan, R. Tu, Y. Zeng, C. C. Xu and X. Xu, Renew. Energy, 2022, 196, 462–481 CrossRef CAS.
- B. He, Y. Zhang, Y. Yin, T. L. Funk and G. L. Riskowski, Journal of the ASABE, 2001, 697, 697–701 Search PubMed.
- M. K. Jindal and M. K. Jha, Rev. Chem. Eng., 2016, 32, 459–488 CAS.
- Y. Qian, C. Zuo, J. Tan and J. He, Energy, 2007, 32, 196–202 CrossRef CAS.
- S. Karagöz, T. Bhaskar, A. Muto, Y. Sakata and M. A. Uddin, Energy Fuels, 2004, 18, 234–241 CrossRef.
- M. E. Jazi, G. Narayanan, F. Aghabozorgi, B. Farajidizaji, A. Aghaei, M. A. Kamyabi, C. M. Navarathna and T. E. Mlsna, SN Appl. Sci., 2019, 1, 1–19 Search PubMed.
- B. Hao, D. Xu, G. Jiang, T. A. Sabri, Z. Jing and Y. Guo, Green Chem., 2021, 23, 1562–1583 RSC.
- D. A. Nelson, S. D. Landsman and P. M. Molton, Carbohydr. Res., 1984, 128, 356–360 CrossRef CAS.
- K. Venkatesan, J. V. J. Krishna, S. Anjana, P. Selvam and R. Vinu, Catal. Commun., 2021, 148, 106164 CrossRef CAS.
- U. Tyagi, V. Singh, S. P. Singh and N. Anand, BioEnergy Res., 2023, 17, 403–418 CrossRef.
- K. Tekin, S. Karagöz and S. Bektaş, Fuel Process. Technol., 2013, 110, 17–23 CrossRef CAS.
- J. Yu, P. Biller, A. Mamahkel, M. Klemmer, J. Becker, M. Glasius and B. B. Iversen, Sustainable Energy Fuels, 2017, 1, 832–841 RSC.
- S. Yin and Z. Tan, Appl. Energy, 2012, 92, 234–239 CrossRef CAS.
- J. Yang, Q. (Sophia) He, H. Niu, K. Corscadden and T. Astatkie, Appl. Energy, 2018, 228, 1618–1628 CrossRef CAS.
- Z. Wang, Reaction mechanisms of hydrothermal liquefaction of model compounds and biowaste feedstocks, University of Illinois, 2012 Search PubMed.
- G. Haarlemmer, C. Guizani, S. Anouti, M. Déniel, A. Roubaud and S. Valin, Fuel, 2016, 174, 180–188 CrossRef CAS.
- Y. Chen, L. Dong, J. Miao, J. Wang, C. Zhu, Y. Xu, G. Y. Chen and J. Liu, Bioresour. Technol., 2019, 294, 122148 CrossRef CAS.
- X. Wang, Z. Yang, X. Liu, G. Huang, W. Xiao and L. Han, Waste Manage., 2020, 110, 87–97 CrossRef CAS.
- X. Ren, J. Meng, A. M. Moore, J. Chang, J. Gou and S. Park, Bioresour. Technol., 2014, 152, 267–274 CrossRef CAS PubMed.
- T. H. Seehar, S. S. Toor, A. A. Shah, T. H. Pedersen and L. A. Rosendahl, Energies, 2020, 13, 3114 CrossRef CAS.
- J. Hoffmann, C. U. Jensen and L. A. Rosendahl, Fuel, 2016, 165, 526–535 CrossRef CAS.
- F. Marrakchi, S. Sohail Toor, A. Haaning Nielsen, T. Helmer Pedersen and L. Aistrup Rosendahl, Chem. Eng. J., 2023, 452, 139293 CrossRef CAS.
- T. N. Trinh, P. A. Jensen, D. J. Kim, N. O. Knudsen, H. R. Sørensen and S. Hvilsted, Energy Fuels, 2013, 27, 1399–1409 CrossRef CAS.
- M. Fahim, T. Al-Sahhaf and A. Elkilani, Fundamentals of Petroleum Refining, Elsevier, Oxford, 1st edn, 2010 Search PubMed.
- B. A. Akash and J. O. Jaber, Energy Sources, 2003, 25, 1171–1182 CrossRef CAS.
- S. R. Westbrook, in Significance of Tests for Petroleum Products, ed. S. J. Rand, West Conshohocken, 7th edn, 2003, pp. 63–81 Search PubMed.
- H. Wang, Hydrothermal liquefaction (HTL) of lignocellulosic biomass for biocrude production: Reaction kinetics and corrosion-resistance performance of candidate alloys for reactors, Western University, 2023 Search PubMed.
- M. Jayathilake, S. Rudra, N. Akhtar and A. A. Christy, Materials, 2021, 14, 3024 CrossRef CAS PubMed.
- X. Zhu, Y. Liu, F. Qian, C. Zhou, S. Zhang and J. Chen, ACS Sustain. Chem. Eng., 2015, 3, 833–840 CrossRef CAS.
- K. Gallucci, L. Taglieri, A. A. Papa, F. Di Lauro, Z. Ahmad and A. Gallifuoco, Appl. Sci., 2020, 10, 1879 CrossRef CAS.
- S. Masoumi, V. B. Borugadda and A. K. Dalai, Biorefinery of Alternative Resources: Targeting Green Fuels and Platform Chemicals, 2020, pp. 249–270 Search PubMed.
- P. Roy, H. Jahromi, S. Adhikari, Y. Zou Finfrock, T. Rahman, Z. Ahmadi, M. Mahjouri-Samani, F. Feyzbar-Khalkhali-Nejad and T. S. Oh, Energy Convers. Manage., 2022, 252, 115131 CrossRef CAS.
- D. Congsomjit and C. Areeprasert, Biomass Convers. Biorefin., 2021, 11, 2569–2584 CrossRef CAS.
- S. Masoumi, V. B. Borugadda, S. Nanda and A. K. Dalai, Catalysts, 2021, 11, 939 CrossRef CAS.
- H. Bayat, M. Dehghanizadeh, F. Omar Holguin, U. Jena and C. E. Brewer, ASABE 2020 Annual International Meeting, 2020, p. 1 Search PubMed.
- K. Khandelwal and A. K. Dalai, Int. J. Hydrogen Energy, 2024, 49, 577–592 CrossRef CAS.
- P. Biller, R. B. Madsen, M. Klemmer, J. Becker, B. B. Iversen and M. Glasius, Bioresour. Technol., 2016, 220, 190–199 CrossRef CAS PubMed.
- A. J. Hunt, E. H. K. Sin, R. Marriott and J. H. Clark, ChemSusChem, 2010, 3, 306–322 CrossRef CAS PubMed.
- Q. Liu, R. Xu, C. Yan, L. Han, H. Lei, R. Ruan and X. Zhang, Bioresour. Technol., 2021, 340, 125630 CrossRef CAS.
- W. Yang, X. Li, Z. Li, C. Tong and L. Feng, Bioresour. Technol., 2015, 196, 99–108 CrossRef CAS.
- A. M. Scheer, C. Mukarakate, D. J. Robichaud, M. R. Nimlos and G. B. Ellison, J. Phys. Chem. A, 2011, 115, 13381–13389 CrossRef CAS PubMed.
- H. Yang, R. Yan, H. Chen, D. H. Lee and C. Zheng, Fuel, 2007, 86, 1781–1788 CrossRef CAS.
- G. Yildiz and W. Prins, Energy Fuels, 2023, 37, 805–832 CrossRef CAS.
- C. Zhu, O. Y. Gutiérrez, D. M. Santosa, M. Flake, R. Weindl, I. Kutnyakov, H. Shi and H. Wang, Appl. Catal., B, 2022, 307, 121197 CrossRef CAS.
- M. A. M. Rodrigues, J. W. Cone, A. H. Van Gelder, J. C. Sequeira, A. M. Fonseca, L. M. M. Ferreira and C. A. Sequeira, J. Sci. Food Agric., 2003, 83, 652–657 CrossRef CAS.
- A. G. Barneto, C. Vila, J. Ariza and T. Vidal, Cellulose, 2011, 18, 17–31 CrossRef CAS.
- S. Karagöz, T. Bhaskar, A. Muto and Y. Sakata, Fuel, 2005, 84, 875–884 CrossRef.
- S. J. Kim and B. H. Um, Renew. Energy, 2020, 160, 612–622 CrossRef CAS.
- A. Demirbaş, Energy Sources, 2005, 27, 1235–1243 CrossRef.
- T. Bhaskar, A. Sera, A. Muto and Y. Sakata, Fuel, 2008, 87, 2236–2242 CrossRef CAS.
- OriginLab Corporation, Origin(Pro), Northampton, MA, USA, 2023 Search PubMed.
- J. Yang, Q. (Sophia) He, K. Corscadden, H. Niu, J. Lin and T. Astatkie, Appl. Energy, 2019, 233–234, 906–915 CrossRef CAS.
- J. Yu, N. Paterson, J. Blamey and M. Millan, Fuel, 2017, 191, 140–149 CrossRef CAS.
- S. M. Subramanya, N. Rios, A. Kollar, R. Stofanak, K. Maloney, K. Waltz, L. Powers, C. Rane and P. E. Savage, Energy Fuels, 2023, 37, 6619–6628 CrossRef CAS.
- S. Mishra and K. Mohanty, Energy Convers. Manage., 2020, 204, 112312 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4se00878b |
| This journal is © The Royal Society of Chemistry 2024 |






