 Open Access Article
Open Access ArticleDevelopment of a CO2-biomethanation reactor for producing methane from green H2†
Grégory
Cwicklinski
*,
Roger
Miras
,
Julien
Pérard
,
Clara
Rinaldi
,
Elisabeth
Darrouzet
and
Christine
Cavazza
 *
*
Univ. Grenoble Alpes, CEA, CNRS, IRIG, CBM, F-38000 Grenoble, France. E-mail: Gregory.cwicklinski@cea.fr; christine.cavazza@cea.fr
First published on 30th January 2024
Abstract
“Power-to-Methane” approaches allow the storage and transport of green methane, produced from renewable energy and any CO2 source. In nature, some microorganisms, namely methanogens, can grow on CO2 and H2 and produce pure methane via an ancestral process, the methanogenesis, under mild conditions (temperature, pressure, aqueous solvents…). These microorganisms are able to perform efficiently the Sabatier reaction (4H2 + CO2 → CH4 + 2H2O), using H2 and CO2 as sole energy and carbon sources. Here, we developed a biomethanation reactor to culitvate a pure culture of Methanococcus maripaludis, a mesophilic methanogen growing rapidly at ambient temperature. A modular scalable and frugal 2 L-bubble column bioreactor was constructed to operate efficiently and autonomously for several weeks under a wide range of conditions. High H2 conversion and methane yield higher than 90% could be reached. This high-performance, modular and robust bioreactor shows its potential for integration in outdoor systems coupling the conversion of alternative sources of green H2 to fossil-free methane.
Introduction
The use of renewable energies (EnR) for the production of electricity is a major challenge for the coming decades. Concomitantly the synthesis of green fuels and commodity products by reusing CO2 is also a major concern. In the latter case, syngas derived from biomass and wastes, a versatile substrate for the synthesis of a variety of products, would enable a gradual transition to more sustainable energy.1 Considering intermittent energies such as wind or solar energy, their utilization requires the development of new strategies for long-term storage. Among them, “Power-to-gas” processes allow the conversion of excess electricity produced from renewable sources into a gaseous energy carrier.2 Today, hydrogen is one of the leading options for storing EnR. However, the growing demand from industry and energy sectors requires the increase of green hydrogen production and the development of scale-up technologies. To this end, the cleanest way to produce hydrogen is by directly splitting water into hydrogen and oxygen, through “power-to-H2” processes. However, the use of hydrogen as an energy carrier presents a certain number of difficulties, such as its storage (gas under pressure at 700 bars, liquid at 20 K…). In addition, when mixed with natural gas, its extreme inflammability and corrosive properties impose, for safety reasons, an injection on the gas network limited to up to 20% in molar composition. Consequently, other ways of hydrogen storage are currently being studied, such as the use of “Liquid Organic Hydrogen Carrier” (LOHC) or its conversion into methane.3While not an ideal long-term solution, green methane is appealing to contribute to clean energy transition thanks to its easy storage and transportation in existing infrastructures. In “Power-to-Methane” strategies, methane is produced from H2 obtained from water electrolysis, combined to CO2, according to the Sabatier reaction: 4H2 + CO2 → CH4 + 2H2O. Currently, the production of green methane requires the development of CO2-methanation reactors, using either the so-called “catalytic methanation” or methanogenesis. On the one hand, the highly efficient catalytic methanation is based on the use of nickel catalysts, extremely sensitive to impurities (ex: H2S) and water, and occurring at high pressure (up to 40 bars) and temperature (200–600 °C).4 On the other hand, the production of biomethane by methane-producing microorganisms, namely methanogens, under mild conditions is an emerging field.5 Methanogens are ancestral obligate anaerobes representing a dominant group of Archaea. In these microorganisms, the only pathway for energy conservation is methanogenesis, generating methane as the final product.6 Among the three possible pathways for methanogenesis (depending on the substrate), the most energetically favorable one is the hydrogenotrophic one, present in all methanogens.7 In this case, methanogens can perform the Sabatier reaction to produce efficiently and quantitatively pure methane from hydrogen and carbon dioxide at room temperature and pressure.8 Recently, the development of an “ex situ” methanation process with a pure culture of a thermophilic evolved strain of Methanothermobacter thermautotrophicus, has been successfully commercialized by Electrochaea, for the conversion of CO2 from biosources or industrial exhaust.9 This has paved the way for the use of methanogens in “Power-to-methane” strategies.
However, the difficulty in the design of CO2-to-biomethane reactors lies in the control of redox potential (methanogenesis occurring at potentials below −300 mV), the absence of oxygen, the recovery of CH4 and the perfect distribution of gaseous substrates (H2 and CO2) in liquid growth media. To date, several types of bioreactors have been used for the cultures of methanogens. Continuous stirred-tank reactors (CSTR) are the most conventional ones, the mechanical stirring allowing an efficient gas-to-liquid mass transfer. However, the system is highly energy consuming.8,10 In the case of fixed-bed reactors (FBR), microorganisms are immobilized on a solid matrix, making it possible to easily renew the fresh growth medium in continuous cultures.11 However, few studies have been described with pure cultures of methanogens.12 Moreover, the imperfect distribution of the gaseous substrates creates pH gradients and pockets of gas, causing poor mass and heat transfer. On the other hand, membrane bioreactors (MR) allow the physical separation of immobilized microorganisms and nutrients. For example, hollow fiber membranes can supply methanogens by simple gas diffusion through the membrane, with instantaneous assimilation of the substrates. Pure methane is then released into the growth medium, separated from the H2/CO2 mixture by the biofilm. However, the affinities and physico-chemical interactions between the archaea and membranes, as well as biofilm formation are poorly characterized. Only one example is found in the literature with M. thermautotrophicus, with a methane evolution rate (MER) of 0.06 mol L−1 h−1 and a H2 conversion rate of about 90%.13 At last, bubble column reactors (BCR) have several interests for biological methanation. First, the design is relatively simple and easily scalable: the bioreactor is composed of a cylindrical tank containing the micro-organisms in which gas bubbles are diffused. Second, the absence of mechanical stirring decreases the energy consumption (PVR = 12.5–15.6 W h m−3vs. 50 W h m−3 in a CSTR). Third, the robustness of these bioreactors allows their use in diverse applications. However, the use of BCR with methanogens is poorly documented in the literature and limited to mixed microbial consortia in “in situ” biomethanation devices.14,15
Here, we have constructed a modular scalable 2L-bubble column bioreactor to grow a pure culture of Methanococcus maripaludis. Among methanogenic archaea, M. maripaludis is a well-described model organism that has the advantage of growing rapidly (with a growth rate of 0.346 h−1 under ambient conditions).16 To envisage the integration of a biomethane reactor into an outdoor device coupling the production of green H2 from intermittent energies to its conversion into methane, the bioreactor has been developed to operate efficiently under a wide range of conditions. Temperature variations, variable H2 flow rates and pressures or day/night intermittency (in the latter case mimicking solar H2) were then tested and their effect evaluated. We showed that this new bioreactor can work autonomously, and its robustness and modularity were proven by the production of high-quality methane in all tested conditions.
Results and discussion
Design of the bioreactor
To design an energy-efficient bioreactor, two important parameters need to be considered: heat transfer and mass transfer. In the specific case of anaerobic biological metabolism using H2 from renewable energy sources, the power consumption required for efficient mass transfer can be very high. The bioreactor must therefore minimize the required power density (power consumed by the process per unit volume – PVR in W h m−3). To do this, the system must achieve the lowest possible ratio between the power required to operate the bioreactor (PVR) and the system's ability to transfer a quantity of material from one phase to another (kLa).10 For the purposes of this work, the bubble column bioreactor (BCR) type was chosen. This choice was motivated by the fact that, unlike a continuously stirred bioreactor (CSTR), the bubble column has no mechanical agitator. This makes it less energy-intensive (PVRBCR ≈ 15 W h m−3vs. PVRCSTR ≈ 50 W h m−3).6 Moreover, the absence of an agitator makes its design simpler and more robust (no moving parts). Our BCR was also designed to be integrated into a dedicated experimental bench. The main objective of the bioreactor developed in this study is to enable efficient conversion of a hydrogen stream in a 0.5–2 L h−1 range. To enable the design of this device, two different approaches were implemented. The first one is an empirical approach, based on literature data. It enabled to determine the characteristic dimensions of the device, so that it could be rapidly manufactured. The second approach involved a numerical study to validate the design in terms of expected performance. The sizing of the bioreactor was first guided by the work of Goyal16 on M. maripaludis S2 strain. The authors report a MER of 9.24 mmol L−1 h−1. Considering an average growth of 0.9 OD units in the stationary phase,17 and assuming total conversion of hydrogen to methane, the minimum culture volume for a maximum hydrogen flow rate of QvH2,in = 2 L h−1 must therefore be Vmin = 2.7 L (eqn (1)). In eqn (1), the molar volume (Vm) of the gas used is 22.4 L mol−1, the stoichiometric ratio between H2 and CH4 in the Sabatier reaction equation is 4, and all gas volumes are considered under standard conditions of pressure (1 bar) and temperature (0 °C). | (1) |
From the volume obtained, it was therefore possible to determine the dimensions of the bubble column, aiming for a height/diameter ratio greater than 6 for optimum hydrodynamics.18 In our study, the bioreactor is 68.8 cm high and 7.2 cm in diameter, corresponding to an aspect ratio of 9.6 and a bubble column volume of 2.8 L. The bioreactor as built is shown in Fig. 1. It was designed to allow heat transfer via a jacketed heat exchanger (2) located around the bubble column (3) and mass transfer with sintered metal gas diffusers (5 and 7) located in the lower part of the bioreactor.
The reactor was constructed in stainless steel (304L) and has a borosilicate-viewing window (4) at the bottom of the column. The wall thickness was chosen for an operating pressure of up to 4 bar. The maximum permissible pressure is 11.5 bar. The seal is provided by an elastomeric hydraulic seal (Viton©, or FKM). The reaction chamber is equipped with 4 nozzles (6) allowing the insertion of removable probes (e.g. pH probe, redox…). The covers of the lower and upper parts of the reactor (1 and 8) are drilled with 5 holes for gas and liquid supply, pressure sensor and safety valve. Details of the bioreactor equipment are given in Table S1,† and its dimension is shown in Fig. S1.†
Validation of the reactor design by numerical simulation
The numerical model used for this study was designed to validate the bioreactor's sizing regarding expected performances. For this, an Eulerian approach was chosen, considerably less demanding in computing resources than commonly used methods such as direct numerical simulation (DNS) or Euler–Lagrange (E–L)-type approach.19 To carry out the sizing of the BCR, the choice of an axial dispersion model (ADM) was motivated by the introduction of a space dimension, unlike the more conventional model of perfectly agitated reaction (CSTR). This allows thus to evaluate, at any position of the BCR, the evolution of the methanation reaction.In the specific case of a BCR including a mass transfer term and a reaction term, the ADM 1D model can be written as follows for each “i” constituent:
 | (2) |
Note that the continuity equation proposed here, in addition to the advection and dispersion terms commonly encountered in ADM models, includes two source terms. The first term corresponds to mass transfer between the gas and liquid phases where the methanation reaction takes place. The second is a reaction term corresponding to the conversion of gaseous substrates (H2 and CO2) into products (H2O and CH4) by the methanation reaction. However, determining the reaction rate of methanogens is difficult to determine experimentally, and no values are available in the literature. This is why, in line with Jud's work on the role of hydrogen mass transfer in methanogen growth kinetics,20 we proposed to replace this term by an apparent reaction rate, assimilated to the diffusion rate of the limiting gas. Thus, considering the mass transfer of hydrogen in the liquid phase as limiting and the soluble hydrogen instantly consumed by the microorganisms (CL,H2 = 0), it is possible to express the apparent reaction rate, with respect to each constituent (eqn (3)).
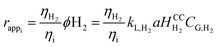 | (3) |
The generalized expression (eqn (2)) can be simplified for each of the two phases by considering the liquid phase as immobile and the only one undergoing a reaction.21 The generalized expression can thus be written for the liquid and gas phases, respectively:
 | (4) |
 | (5) |
All the correlations, expressions and experimentally determined values used in the model are given in Table S2.† The model proposed here was tested to evaluate the achievable performances of the bioreactor under standard conditions. The results obtained after a transient period of about 10 minutes suggest that the composition of the gas leaving the bioreactor reaches a value of around YCH4,out = 85% methane per volume. Considering the methanation reaction as total (χCH4:H2 = 1), and the expression of the material balance at the reactor terminals, it is possible to determine the total gas flow rate at the bioreactor outlet (eqn (6)): Qv,tot,out = 0.23 L h−1. This, under the conditions defined for this simulation, gives a productivity value: MERl = 4.5 mmolCH4 L−1 h−1.
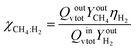 | (6) |
Experimental setup
The bioreactor was then integrated into a finely instrumented experimental bench to study the biological methanation. The experimental set-up (Fig. S1, Tables S3–S4†) is made up of three sub-assemblies: a reagent feed section (red), a reaction section (green) and a production section for analyzing the gases produced from the culture (blue). This bench, which can be used to study the methanation reaction over variable times, has been fully automated and allows acquiring the measurements for all the studies carried out (Fig. 2). | ||
| Fig. 2 Experimental setup of the bioreactor: the reagent feed section is framed in red, the reaction section in green and the production section in blue. | ||
The system is fed with a commercial mixture of hydrogen and carbon dioxide in a stoichiometric ratio (80% H2/20% CO2). This mixture, expanded (A) to operating pressure (approximately 2 bar abs), is injected into the column at a flow rate regulated by a thermal mass flow meter (B). In this part of the equipment, the flow rates, pressures, and feed temperatures of the mixture are measured and recorded. The gas mixture is injected into the bioreactor through a porous metal vessel (C), allowing the generation of gas bubbles with a diameter of approximately 0.17 mm. The desired working pressure is maintained in the bioreactor with an adjustable valve (N) located on the exhaust line. The reaction part of the bioreactor (J) is filled with growth medium. The temperature of this culture medium is maintained by a double envelope and a Peltier/Seebeck effect thermal control module (I and G), developed in the laboratory (Fig. S2, Table S5†), with a power of 120 W. In order to study the impact of the gas residence time in the bioreactor, the reaction section is equipped with a recirculation loop for the gases present in the headspace. This loop is equipped with a liquid gas separator (K), a condenser (L) and a microvolumetric pump (E) to enable moisture-free gas to be reinjected at the bottom of the column. The recirculated gas is re-injected into the column through a metal porous tube (D) to obtain gas bubbles with a diameter of around 0.5 mm. The instrumentation in this part of the system is essentially intended for biological monitoring of the process. The bioreactor is equipped with a probe for measuring pH, oxido-reduction potential (ORP) and temperature. The bioreactor is also equipped with a device comprising a turbidity sensor for measuring the microorganism concentration. This low-cost device was developed in the laboratory and the turbidity measured correlated with the measurement of absorbance at 600 nm in order to follow precisely methanogen growth the inside the bioreactor (Fig. S2, Table S5†).
The production part of the experimental bench is entirely dedicated to analyzing the gases coming from the bioreactor. Gas flow and gas composition are measured using a thermal mass flowmeter (8) and infrared spectrometry (Non Dispersive Infra-Red) (11 and 12) respectively. As these two technologies are sensitive to the presence of water, a drying cartridge (O) is positioned upstream. As well as the feed gas, the pressure and temperature of the outlet gases are also measured and recorded. Finally, to avoid any risk of contamination of the device by oxygen, a hydraulic valve (F) is positioned at the gas outlet.
Start-up of the bioreactor under standard growth conditions
Standard conditions for starting a culture in the bioreactor are defined as a temperature of 37 °C, a pressure of 2 bar, a flow of 1 L h−1 of a 80% H2/20% CO2 gas mixture and a culture volume of 2 liters. To ensure proper mixing and maximize conversion of the gases fed, the top gases in the bioreactor are partially re-injected at the bottom of the column using the recirculation loop. The recirculation flow applied is approximately 10 L h−1.The nature of the growth medium directly affects the growth of M. maripaludis. Two different growth media were compared: rich medium (RM) and minimal medium (MM). The growth rate of M. maripaludis is higher in the RM with a maximum OD600 of 1.6 compared to 1.1 in MM (Fig. S4(a)†). On the other hand, the results show that the production of CH4 is comparable for the two media (Fig. S4(b) and (c)†). The MM medium was selected for several reasons: first, the absence of other carbon sources than CO2 significantly reduces the risk of contaminations in the bioreactor. Second, minimizing the number and concentration of the nutrients simplifies the experimental procedures. Third, the supply of carbon and energy sources solely by gas injection allows the growth of M. maripaludis in an autonomous mode. The addition of CO2 in the growth medium results in a decrease in pH that prevent the growth of M. maripaludis for which the maximum growth is between pH 7.0 and 8.5. To avoid the use of a pH control system, HEPES buffer was added in MM medium to maintain a pH value above 7.0. The MM + HEPES medium was therefore retained for the rest of the study. The bioreactor was filled with 2 L of MM + HEPES and subsequently saturated with the gas mix. NaOH and Na2S were then added to stabilize the pH and the ORP, respectively.22 After this equilibration phase (about 5 hours), a pH around 7.0 and a redox potential at −380 mV were reached (Fig. 3(a) and (b)). The bioreactor was then inoculated with a 20 mL stationary phase culture of M. maripaludis. After 12 h of culture, the ORP continually decreased to reach a value of −513 mV at t23h (Fig. 3(b)). At the same time, the OD600nm slightly increased (Fig. 3(c)), these two parameters attesting thus the good start of the growth of the microorganisms. It is worth mentioning that at t24h, a decrease in gas flow at the bioreactor outlet was observed, indicating the conversion by M. maripaludis of H2/CO2 into CH4 (detected by NDIR) and H2O. In Fig. 3(d) and (e), an inflexion point is observed at t = 38 h. This is due to the variation in the number of moles produced along the methanation reaction. Actually, the functioning pressure is not regulated and depends on the inlet and outlet gas flow. For example, in the exponential growth phase, the gaseous substrates (H2 and CO2) were rapidly consumed leading to a drastic diminution of the number of moles of gas present in the bioreactor, which has an impact on the bioreactor pressure and the outlet flow. This observation indicates that conversion kinetics are at their maximum, and provides important information for the development of continuous cultures where DO would be regulated at the maximum production threshold. At t60h, the bioreactor reached a steady state, with a stable OD600nm at 0.7. The composition of the gas produced is around 90% methane by volume (Fig. 3(f)), with a Yrel conversion efficiency of 0.8. At the early stationary phase, the methane evolution rate (MER) was 3.5 mmol L−1 h−1.
 | ||
| Fig. 3 Monitoring of operating conditions: pH (a); ORP (b); OD600 (c); pressure inside the bioreactor (d); gas outlet flow (e); composition of the offgas (f). | ||
The results of this study show that the bioreactor can be used to grow M. maripaludis. Culture monitoring can be carried out simply using basic instruments commonly used in bioprocessing (pH and ORP probe, flowmeter), without the need for more complex measurements (OD probe, gas analyzer). In this study, the time required to reach nominal operation is estimated at around 60 hours. From this point onwards, studies of resilience to operating variations can be carried out.
Evaluation of operating variables on methane production
With the aim of integrating this bioreactor in demonstrators in autonomous mode, variations are likely to occur during its use. In this study, several operating parameters were selected (listed in Table 1) and their effect on the bioreactor performance evaluated. Some parameters were deliberately defined for all the tests. These included the strain of methanogen, the culture medium, the volume of culture (Vclt = 2.02 L), the inoculum injected (1% of the culture volume) and the recirculation rate (Qv recirculation about 10 L h−1). The bioreactor was first placed in the steady state described above. For each trial, the measurements were recorded continuously for short trials (less than 100 hours) and punctually for long trials (Fig. S7–S11†). The performance indicator values given in Table 2 are expressed as the average of the values measured after 50 hours of experiment.| Operating variables | Inlet gas flow (H2/CO2) (L h−1) | Working pressure (bar abs) | Working temperature (°C) | |
|---|---|---|---|---|
| #1 | Reference | 1.0 | 1.9 | 34.5 |
| #2 | Room temperature | 1.0 | 2.4 | 23.1* |
| #3 | Gas supply flow rate | 2.0* | 2.4 | 36.7 |
| #4 | High pressure | 1.0 | 4.3* | 33.9 |
| #5 | Low pressure | 1.0 | 1.2* | 36.6 |
| #6 | Day cycle | 1.0* | 2.0 | 36.6 |
| Night cycle | 0.0* | |||
| #7 | Long-term culture | 0.5* | 1.5 | 35 |
| Experiment | Value after 50 hours of experiment | Total time (h) | |||||
|---|---|---|---|---|---|---|---|
| Y CH4 (%) | Y CH4,rel | MER (mmol L−1 h−1) | MP° (mmol L−1 Lgas inlet−1) | pH | OD600 | ||
| #1 | 91 | 0.90 | 4.04 | 4.04 | 6.83 | 0.91 | 50:00 |
| #2 | 93 | 0.92 | 4.08 | 4.08 | 7.02 | 0.94 | 89:00 |
| #3 | 87 | 0.93 | 8.22 | 4.11 | 7.20 | 0.90 | 62:00 |
| #4 | 90 | 0.93 | 4.05 | 4.05 | 7.09 | 1.02 | 86:58 |
| #5 | 78 | 0.94 | 4.15 | 4.15 | 7.23 | 0.68 | 87:00 |
| #6 | 88 | 0.88 | 3.91 | 3.91 | 6.83 | 0.76 | 100:56 |
| #7 | 91 | 0.87 | 2.14 | 4.28 | 6.72 | — | 848:42 |
The results of experiment #2 show that after the start-up phase at 37 °C, the bioreactor was able to operate at room temperature without thermal regulation (Fig. S5†), while continuing to produce methane (MERlt=50h = 4.9 mmol L−1 h−1; Fig. 4). It can also be seen that during this period, the concentration of microorganism remains relatively stable (Fig. S6†). This observation is in agreement with the capacity of M. maripaludis of growing between 20 and 40 °C.16 This experiment also highlights the fact that in the tested conditions, the methane production is not affected by a decrease in temperature over 50 h, which would allow an outdoor utilisation with a minimal temperature regulation. The experiment carried out with a gas feed rate of 2 L h−1 (experiment #3) indicates that in addition to achieving a good conversion efficiency the methane production in the bioreactor is double. Furthermore, observation of methane production normalised by the gas flow rate fed into the bioreactor (MP°) shows that, whatever the feed rate, production is approximately 4 × 10−3 moles of methane per litre of culture medium and per litre of gas supplied. It is also possible to compare the MER, expressed in cell dry weight (MER), obtained for this test with that reported by Goyal.16 Thus, for this experiment, the MER is 26.57 mmol gDCW−1 h−1, compared to that of the work carried out by Goyal which is 27.19 mmol gDCW−1 h−1. This observation suggests that, under these conditions, the bioreactor operates at its maximum performance. Nevertheless, further experiments with higher gas supply flow rates need to be conducted to explore the possibility of further increasing this MER value. In comparison, the most widely used methanogen in biological methanation, namely the optimized thermophile Methanothermobacter marburgensis can reach an outstanding MER of 476.50 mmol L−1 h−1 with OD600 reaching around 7 and a high gassing rate (vvm) of 2 L gas L medium min−1.23 However, in these conditions, the conversion rate into CH4 reached only 13.5%. Conversely, yields of up to around 96% can be reached with the same strain but at low vvm (0.01) in a 25 L-reactor with a MER of 7.96,24 comparable to the values obtained in experiment 2. As expected, the MER and the conversion efficiency are highly dependent on the H2/CO2 inflow rate. It suggests that there is still room of improvement with the mesophile M. maripaludis in further studies to reach the optimized conditions. Experiments #4 and #5, which evaluated the impact of pressure variations on bioreactor performance, show that pressure has little influence. However, the methane composition of the gas produced at low pressure is less than 80%vol, which can be explained by the lower concentration of dissolved gas at low pressure. These tests show that the bioreactor is able to accept variations in the hydrogen feed stream, with negligible changes in methane production and quality. Experiment #6 was carried out to simulate day–night cycle operation (12 h/12 h) without hydrogen supply during the night phases. During the night phases, the bioreactor operates in “batch” mode, without power or production. Performance measurements are therefore obtained during the day phases. The results of the experiment show that, despite this alternation, the performance measurements are very comparable to those obtained during the reference test (Table 2) and that the MER achieved is almost 4 mmol h−1 L−1. Finally, observation of all the performance criteria monitored (Fig. 4) shows, for experiment #7, that the bioreactor is capable of efficiently converting hydrogen into methane over a long period. At the end of operation (t = 848 h), the bioreactor showed a methane concentration in the gas produced of 96%, a conversion efficiency of 0.93 and a methane evolution rate of 2.04 mmol h−1 L−1. This observation shows that, under these operating conditions and in this culture medium, the biological methanation process can be carried out efficiently, without any external intervention (no addition of pH or ORP corrector, no addition of nutrient other than continuously fed H2/CO2, no renewal of culture medium).
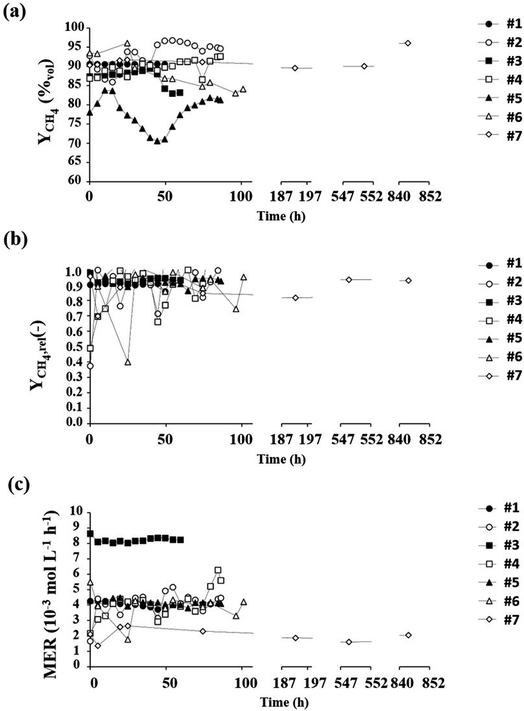 | ||
| Fig. 4 Evolution of methane composition in outlet gases (a). Evolution of the conversion of hydrogen into methane (b). Evolution of methane rate production (MER) (c). | ||
The results, demonstrate that the designed bioreactor allows for a good and relatively stable conversion of hydrogen to methane compared to the reference test (experiment #1). As expected, fluctuations are observed in experiment 6 with intermittent H2 feeding during day/night cycles. Over the same period, in all the experiments, whatever the operating variable studied, conversion efficiencies between 0.88 and 0.97 were achieved for outlet gas compositions between 78% and 93% methane.
Experimental
Strain and growth media
Pure culture of M. maripaludis JJ (DSM2067) was obtained from the DSMZ microbial open collections. The rich medium (RM) containing peptone and yeast extract, is the reference DSMZ growth medium 141 for the selected strain. The compositions of the Wolin's vitamin solution, Modified Wolin's mineral and “trace elements” solution are available on the DSMZ website https://www.dsmz.de/. The minimum medium (MM) is a modified 141 DSMZ medium (adapted from Goyal et al.14). The composition is the following: KCl (0.34 g L−1), MgCl2 hexahydrate (4.00 g L−1), MgSO4 heptahydrate (3.45 g L−1), NH4Cl (0.25 g L−1), CaCl2 (0.14 g L−1), NaCl (18.00 g L−1), K2HPO4 (0.14 g L−1), trace elements (10 mL L−1), resazurin (stock: 1 g l−1, 0.5 mL L−1), ammonium iron(II) sulfate (stock: 1 g L−1, 1 mL L−1), Na2S (0.5 g L−1). The only energy and carbon sources came from the H2/CO2 gas mix injected into the cultures. The MM + HEPES medium was supplemented with 50 mM HEPES, pH 8.5.Bioreactor inoculation
Pre-cultures were obtained in 3 steps, giving a final pre-culture volume of 500 mL. 2 mL of a glycerol stock stored at −80 °C were used to inoculate 5 mL of rich medium in Hungate tubes. 5 mL of this culture was then supplemented with 10 mL of MM + HEPES. Finally, 10 mL of cultures were used to inoculate 490 mL of MM + HEPES in a 500 mL DURAN® GL45 Pressure Plus flask. All cultures were incubated at 37 °C at 150 rpm and saturated every 24 h with a gas mixture of 80% H2/20% CO2 at a pressure of 2 bar. For each step, a culture with an OD600 between 0.6 and 1 was used. Bioreactor cultures were carried out in 2 liters of MM + HEPES inoculated at 1:100. The standard conditions to start a culture in the bioreactor are defined as a temperature of 37 °C, a 2 bar pressure and an 80% H2/20% CO2 flow of 1 L h−1.Performance evaluation
To assess the reactor performances, several criteria were observed: the methane composition of the gas collected at the bioreactor outlet (YCH4), the relative methane yield (eqn (7)), the methane evolution rate (eqn (8)) and the methane production normalised by the quantity of gas fed (eqn (9)). Methane composition was measured directly at the bioreactor outlet using infrared spectroscopy (NDIR). This measurements were obtained using an NDIR sensor, smartGAZ® FlowEVO. The relative methane yield YCH4,rel is defined as the yield of mole of methane produced per mole of hydrogen introduced in a stoichiometric ratio, the methane evolution rate (MER) describes the total amount of methane produced by the system and the normalised methane production (MP°) is none other than the quantity of methane produced by the system in relation to the quantity of gas supplied.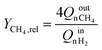 | (7) |
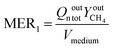 | (8) |
 | (9) |
Conclusions
Today, biomethane is mainly generated from anaerobic digestion of organic wastes by consortia of microorganisms, producing biogas as the final product. This biogas, rich in CO2 requires an energy-intensive upgrading step to enable its use. On the other hand, highly pure biomethane can be produced by cultures of methanogens growing on a mixture of H2 and CO2. Still in its infancy, this biological methanation process is a promising technology for both energy storage and CO2 conversion. In addition, it can be used to upgrade biogas, utilizing CO2 in excess combined to an external source of H2 in ex situ technologies.25Among biological methanation systems described in the literature,26,27 CSTR are the most commonly found. In most of the cases, H2 diffusion in liquid media remains the major drawback and little information is available on the system efficiency and optimization. Moreover, shear forces in CSTR possibly inhibit the growth of microorganisms.28 Here, we have designed an optimized bubble column reactor (BCR) for the pure culture of M. maripaludis taking into account two important parameters: heat transfer and mass transfer, while minimizing the required power density. Compared to commercially developed bioreactors, here, the BCR was equipped with a recirculation loop, enhancing the gas residence time inside the culture. Moreover, with a power consumption of around 120 W, this bioreactor is remarkably energy-efficient. The absence of mobile parts, as in CSTR, makes also the bioreactor mechanically more robust. With the aim of coupling this bioreactor with green H2 production in outdoor demonstrators, day/night intermittency, temperature and gas flow variations, and resistance and stability over time of the cultures were considered.
While pure cultures are often less robust and resilient than mixed consortia routinely used in biogas plants, several methanogens, mainly thermophiles, renown for their high performances28 have been used and optimized in CO2-based biological methane production (namely CO2-BMP).29 Conversely, few examples are described in the literature with pure cultures of mesophilic methanogens. We showed in this study that a culture of M. maripaludis is able to produced highly pure methane with conversion rates reaching more than 90% whatever the operating conditions. Our results also show that in conditions that favour high yields of CH4 (low vvm) for Methanothermobacter marburgensis, M. maripaludis can be quite competitive In further studies, other mesophilic methanogenic strains will be tested and their performance compared to well-described thermophilic ones. The conditions will also be optimized (pressure, gassing rate,…) to determine the trade-off between the quality and quantity of methane produced under autonomous conditions. Importantly, the bioreactor used in this study will allow to assay the effect of “contaminants” in the input gases (for e.g. O2 in the hydrogen, CO in the CO2 or even H2S) both in term of methanogen growth and CH4 production in the perspective of using this setup to upgrade biogases and produce non-fossil grid-quality gases.
In the present study, we have constructed a modular, robust, frugal and high-performance BCR, optimized for the production of green methane from alternative green H2 sources. Interestingly, the bioreactor was shown to work autonomously over 35 days without loss of performances. The culture also tolerated H2 starvation and the absence of thermal regulation over 50 h. Recently, this new bioreactor was coupled to an integrated photo-electrochemical cell for H2 production (manuscript under revision). This outdoor device was selected as one of the three finalists in the EIC Horizon Prize “fuel from the sun” international competition (https://sunergy-initiative.eu/eic-horizon-prize-on-artificial-photosynthesis-2022), demonstrating its potential in green methane production from solar H2.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors wish to acknowledge the DRF-Impulsion call 2019, funded by the CEA and the circular carbon economy program launched by the CEA in 2019. This work was supported by the Agence Nationale de la Recherche through the LabEx ARCANE program (ANR-11-LABX-0003-01) and the Graduate School on Chemistry, Biology and Health of Univ. Grenoble Alpes CBH-EUR-GS (ANR-17-EURE-0003).Notes and references
- A. Giuliano, E. Catizzone and C. Freda, Int. J. Environ. Res. Public Health, 2021, 18(2), 8072 CrossRef PubMed.
- M. Gotz, J. Lefebvre, F. Mors, A. McDaniel Koch, F. Graf, S. Bajohr, R. Reimert and T. Kolb, Renewable Energy, 2016, 85, 1371–1390 CrossRef.
- K. Ghaib and F. Z. Ben-Fares, Renewable Sustainable Energy Rev., 2018, 81, 433–446 CrossRef CAS.
- D. Katla, D. Węcel, M. Jurczyk and A. Skorek-Osikowska, Energy, 2023, 263, 125881 CrossRef CAS.
- J. Zabranska and D. Pokorna, Biotechnol. Adv., 2018, 36, 707–720 CrossRef CAS PubMed.
- N. R. Buan, Emerging Top. Life Sci., 2018, 2, 629–646 CrossRef CAS PubMed.
- Z. Lyu, N. Shao, T. Akinyemi and W. B. Whitman, Curr. Biol., 2018, 28, R727–R732 CrossRef CAS PubMed.
- M. Thema, T. Weidlich, M. Hörl, A. Bellack, F. Mörs, F. Hackl, M. Kohlmayer, J. Gleich, C. Stabenau, T. Trabold, M. Neubert, F. Ortlo, R. Brotsack, D. Schmack, H. Huber, D. Hafenbradl, J. Karl and M. Sterner, Energies, 2019, 12, 1–32 Search PubMed.
- L. T. Angenent, J. G. Usack, T. Sun, C. Fink and B. Molitor, Resour. Recover. from Water, 2022, pp. 141–158 Search PubMed.
- S. Rittmann, A. Seifert and C. Herwig, Crit. Rev. Biotechnol., 2015, 35, 141–151 CrossRef CAS PubMed.
- J. B. Restrepo, J. A. Bustillo, A. J. Bula and C. D. Paternina, Processes, 2021, 9, 1–15 CrossRef.
- R. B. Carneiro, G. M. Gomes, M. Zaiat and Á. J. Santos-Neto, J. Environ. Manage., 2022, 317, 115388 CrossRef CAS PubMed.
- H. S Jee, N. Nishio and S. Nagai, Biotechnol. Lett., 1988, 10, 243–248 CrossRef.
- I. Bassani, P. G. K ougias and I. Angelidaki, Bioresour. Technol., 2016, 221, 485–491 CrossRef CAS PubMed.
- M. A. Voelklein, D. Rusmanis and J. D. Murphy, Appl. Energy, 2019, 235, 1061–1071 CrossRef CAS.
- N. Goyal, M. Padhiary, I. A. Karimi and Z. Zhou, Microb. Cell Fact., 2015, 14, 1–9 CrossRef PubMed.
- C. H. Vo, N. Goyal, M. Kraft and I. A. Karimi, Chem. Eng. Sci., 2023, 278, 118910 CrossRef CAS.
- M. B. Moo-Young, in Encyclopedia of Physical Science and Technology, 3rd edn, 2020, pp. 1–23 Search PubMed.
- M. Bothe, Experimental Analysis and Modeling of Industrial Two-Phase Flows in Bubble Column Reactors, 2016 Search PubMed.
- G. Jud, K. Schneider and R. Bachofen, J. Ind. Microbiol. Biotechnol., 1997, 19, 246–251 CrossRef CAS.
- L. Han and M. H. Al-Dahhan, Chem. Eng. Sci., 2007, 62, 131–139 CrossRef CAS.
- S. Fetzer and R. Conrad, Arch. Microbiol., 1993, 160, 108–113 CrossRef CAS.
- A. Abdel Azim, C. Pruckner, P. Kolar, R.-S. Taubner, D. Fino, G. Saracco, F. L. Sousa and S. K.-M. R. Rittmann, Bioresour. Technol., 2017, 241, 775–786 CrossRef CAS PubMed.
- M. P. Hoffarth, T. Broeker and J. Schneider, Fermentation, 2019, 5, 56 CrossRef CAS.
- A. Thapa, H. Jo, U. Han and S. K. Cho, Biotechnol. Adv., 2023, 68, 108218 CrossRef CAS PubMed.
- D. Rusmanis, R. O'Shea, D. M. Wall and J. D. Murphy, Bioengineered, 2019, 10, 604–634 CrossRef CAS PubMed.
- H. Jiang, W. Hao, Y. Li and H. Zhou, J. Cleaner Prod., 2022, 370, 133518 CrossRef CAS.
- A. H. Seifert, S. Rittmann and C. Herwig, Appl. Energy, 2014, 132, 155–162 CrossRef CAS.
- S. K.-M. R. Rittmann, A. H. Seifert and S. Bernacchi, Appl. Energy, 2018, 216, 751–760 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se01550e |
| This journal is © The Royal Society of Chemistry 2024 |

