An efficient Ni3S2–Ni electrode constructed by a one-step powder metallurgy approach for the hydrogen evolution reaction†
Yang
Zhao‡
a,
Xiaoqian
Shi‡
a,
Bin
Zhang
a,
Shizhong
Wei
 *a,
Jiping
Ma
a,
Jianbin
Lai
a,
Guangmin
Zhou
*a,
Jiping
Ma
a,
Jianbin
Lai
a,
Guangmin
Zhou
 b and
Huan
Pang
c
b and
Huan
Pang
c
aSchool of Materials Science and Engineering, Henan University of Science and Technology, Luoyang 471003, China. E-mail: wsz@haust.edu.cn
bShenzhen Geim Graphene Center, Tsinghua Shenzhen International Graduate School, Tsinghua University, Shenzhen, 518055, China
cSchool of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002, China. E-mail: panghuan@yzu.edu.cn
First published on 7th December 2023
Abstract
The production of green hydrogen has become one of the important parts of hydrogen energy in achieving zero carbon emission, and water electrolysis is an efficient and renewable strategy to produce green hydrogen. In recent years, many researchers have focused on the study of high-efficiency hydrogen evolution catalysts for water electrolysis. Apart from noble metal-based catalysts, non-noble Ni-based materials have been used to catalyze the hydrogen evolution reaction. However, the preparation of traditional Ni-based electrodes consists of multi-step synthesis processes. Here, a simple, time-saving and low-cost one-step powder metallurgy process using Ni and nano S powder as raw materials was innovatively proposed to prepare a mechanically stable and highly active Ni3S2–Ni electrode. Numerous Ni3S2 nano protuberances on the skeleton surface and strong metallurgical bonding between inter-connected Ni networks formed during the integrated in situ sintering process significantly improve the catalytic performance and durability. As a result, the Ni3S2–Ni-500-20 electrode exhibits high hydrogen evolution activity with an overpotential of only 157.8 mV at a current density of 100 mA cm−2 as well as superior durability with a constant current density of 260 mA cm−2 for 225 h. This work provides a feasible method for the one-step synthesis of transition metal compound-metal self-supporting water splitting electrodes with low-cost and high efficiency.
Introduction
With the continuous depletion of non-renewable fossil fuels and increasing environmental pollution, finding green and renewable energy sources to replace fossil energy is critical to future energy sustainability and the global environment.1–3 Hydrogen energy is considered to be an attractive sustainable energy with high mass energy density and zero-carbon nature.4–7 Hydrogen production is an essential part of hydrogen energy.8,9 Although water electrolysis is a simple method to produce green hydrogen,10–12 the sluggish reaction kinetics of the hydrogen evolution reaction (HER) limits its practical application. Therefore, exploring highly active catalysts to decrease the activation energy is highly imperative but challenging.Platinum (Pt) is one of the most important HER catalysts owing to its suitable free energy of hydrogen adsorption. However, as a noble metal catalyst, Pt is expensive and scarce, which limits its wide application, and thus it is urgent to find a cheap and renewable non-noble metal catalyst to replace Pt.13–15 In the last few decades, transition metals, alloys16,17 and transition metal compounds, such as transition metal borides,18 carbides,19,20 selenides,21,22 nitrides,23–25 oxides,26 phosphides,27,28 and sulfides,29,30 have been widely reported for the HER due to their unique electronic properties. For example, Cao et al.25 synthesized Mo(N)/Co nanosheets via hydrothermal synthesis, excessive corrosion, heat treatment and nitridation. Chen et al.20 prepared an IrNi@N,O–C catalytic electrode by combining hydrothermal synthesis with thermal annealing. The IrNi@N,O–C electrode effectively improves the hydrogen evolution reaction activity. Zhang and partners prepared NiS/Ni3S2 flakes on Ni foam through multi-step hydrothermal, thermal treatment and etching processes.31 In previous studies, the preparation of Ni foam and the decoration of Ni3S2 are separated, thus leading to a difficult control of interfacial structure between Ni3S2 and Ni. And the Ni3S2 catalyst was usually loaded on the surface of Ni foam by a multi-step and complex hydrothermal reaction, chemical vapor deposition or other processes.
To simplify the preparation process and reduce the preparation time and cost, a one-step powder metallurgy strategy was innovatively proposed to synthesize a Ni3S2–Ni self-supporting electrode. During the integrated sintering process, Ni could react with nano S and in situ transform into nano-Ni3S2. Meanwhile, Ni particles were connected to each other to form 3D porous Ni networks. As the size of nano S is much smaller than Ni, the formed nano-Ni3S2 is partially anchored on the Ni skeleton, ensuring a strong interface between Ni and Ni3S2. The one-step powder metallurgy strategy largely decreases the operation process and time. In addition, an electrode with a large size can be prepared, which is important for realizing the commercial application of the HER. Such a Ni3S2–Ni electrode with an intimate interface and nano Ni3S2 on the skeleton surface can effectively enhance the HER activity and exhibit long-term stability. As a consequence, the Ni3S2–Ni electrode shows excellent catalytic performance, achieving a current density of 100 mA cm−2 at an overpotential of 157.8 mV. More importantly, such an electrode can work stably at a current density of 260 mA cm−2 for 225 h.
Results and discussion
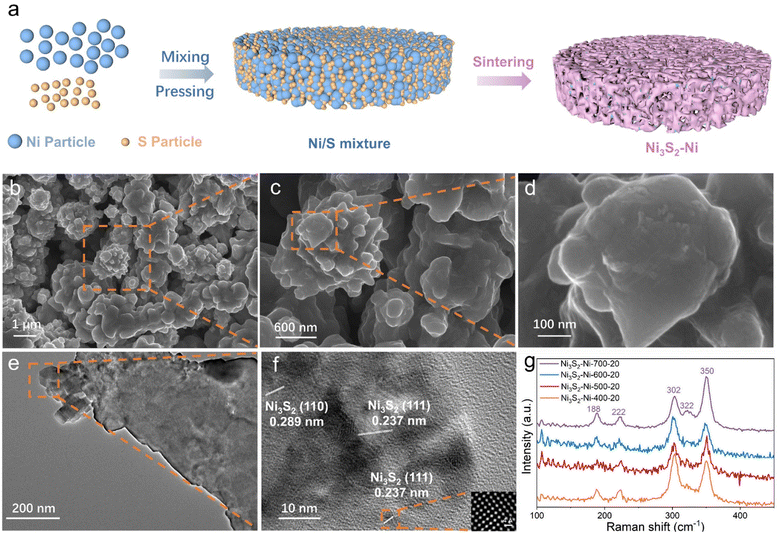
As illustrated in Fig. 1a, the Ni3S2–Ni-X-Y (X is the sintering temperature and Y is the S content) electrode was prepared by mixing, pressing and a one-step powder metallurgy method (Table S1†). In a typical preparation, Ni and nano S powder were first mixed uniformly, and then the Ni/S mixture was loaded into a stainless steel mold and pressed into a wafer using a hydraulic press (Fig. S1†). Finally, the wafer was sintered at different temperatures. As nano S shows a low melting point of 112.8 °C, during the sintering process, molten S could react with Ni to form Ni3S2 because of the high-temperature diffusion of Ni and S atoms. Meanwhile, Ni particles bond with each other to form an inter-connected Ni skeleton. As the size of nano S is smaller than that of Ni, a large number of Ni3S2 nano particles could be uniformly anchored on the Ni skeleton surface. The SEM image of the Ni3S2–Ni-500-20 electrode is shown in Fig. 1b, and the 3D inter-connected networks are formed with a pore size of ∼3 μm. Note that numerous irregular protuberances are distributed on the skeleton surface, which effectively increase the catalytically active sites (Fig. 1c and d). Moreover, such Ni3S2–Ni electrodes obtained by integrated sintering have superior electrical conductivity compared to Ni foam (Fig. S2†). The TEM image (Fig. 1e and f) confirms the existence of Ni3S2 protuberances. High-resolution TEM shows two different lattice spacings of 0.289 and 0.237 nm, which are assigned to the (110) and (111) planes of Ni3S2. Therefore, Ni3S2–Ni with highly active Ni3S2 protuberances on the skeleton surface can be successfully prepared by an integrated sintering strategy.When the S content was 20 mg, Ni3S2–Ni-X-20 electrodes with different sintering temperatures (400, 500, 600 and 700 °C) were synthesized to investigate their phase, surface chemical states and surface morphology. As shown in Fig. 1g. The Raman peaks at 188, 222, 302, 322, and 350 cm−1 correspond to the characteristic peaks of Ni3S2, proving that Ni3S2 exists on the surface of the Ni3S2–Ni-X-20 electrodes.14,32,33
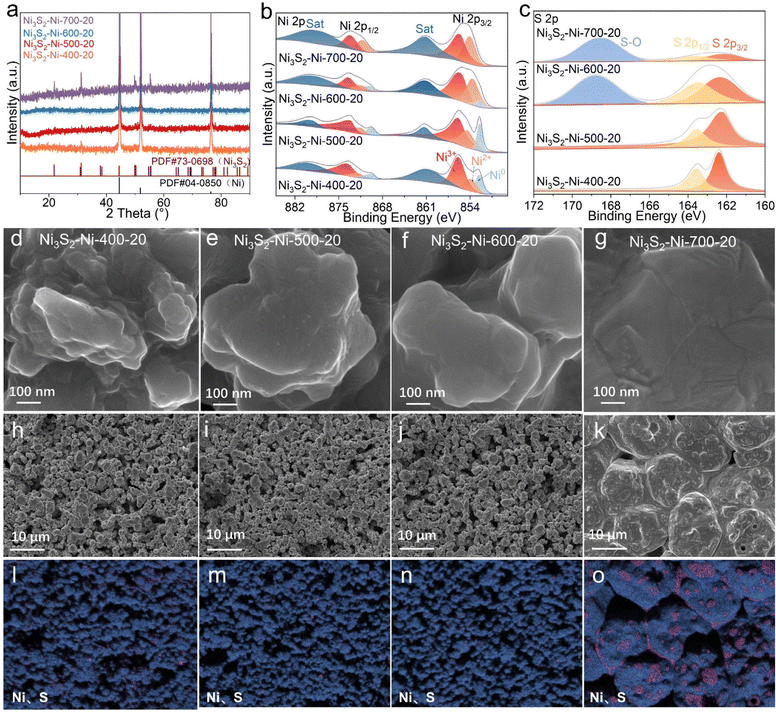
The XRD patterns of the Ni3S2–Ni-400-20, Ni3S2–Ni-500-20, Ni3S2–Ni-600-20, and Ni3S2–Ni-700-20 electrodes are displayed in Fig. 2a. The diffraction peaks of both Ni3S2 (PDF#73-0698) and Ni (PDF#04-0850) could be detected, confirming the formation of Ni3S2 on the Ni skeleton under different sintering temperatures. In addition, when the sintering temperature is 500 °C, the content of S also has a great influence on the diffraction peak intensity of Ni3S2. The diffraction peak intensities of Ni3S2 increase with increasing S content in Ni3S2–Ni-500-Y electrodes (Fig. S3†).
The surface chemical states of the electrode were analyzed by X-ray photoelectron spectroscopy (XPS). Fig. 2b shows the Ni 2p spectra of the Ni3S2–Ni-X-20 (X = 400, 500, 600, 700) electrodes, in which two peaks located at 855.9 and 873.9 eV are attributed to the Ni3+ 2p3/2 and Ni3+ 2p1/2 of Ni3S2.34 The binding energies of 854 and 872.3 eV correspond to Ni2+ of Ni 2p3/2 and Ni 2p1/2, respectively. The binding energies at 852.5 and 869.9 eV correspond to the Ni0 of Ni 2p3/2 and Ni 2p1/2, respectively.35–37 The Ni0 peak disappears at a high sintering temperature of 700 °C. Two peaks at 861 and 879.6 eV are satellite peaks. Two peaks with binding energies of 162.8 and 163.5 eV belong to the S 2p3/2 and S 2p1/2 of the Ni–S bond in the S 2p spectra, and the peak located at 168.9 eV is assigned to S–O (Fig. 2c).38,39 Notably, the Ni–S bond is detected in the Ni3S2–Ni-400-20, Ni3S2–Ni-500-20, Ni3S2–Ni-600-20 and Ni3S2–Ni-700-20 electrodes, confirming the existence of Ni3S2, while S–O can only be observed in Ni3S2–Ni-600-20 and Ni3S2–Ni-700-20, indicating their surface suffers from severe oxidation due to the high sintering temperature. In particular, the peak intensity of S–O in Ni3S2–Ni-700-20 is larger than that of Ni–S, leading to a poor catalytic performance.
The influence of sintering temperature on sample morphology was explored. Fig. 2d–g exhibit the SEM images of Ni3S2–Ni-400-20, Ni3S2–Ni-500-20, Ni3S2–Ni-600-20 and Ni3S2–Ni-700-20. When the sintering temperature is low (400 and 500 °C), numerous Ni3S2 irregular protuberances can be observed on the skeleton surface, which provide a large number of active sites. When the sintering temperature is 600 °C, the size of the protuberances increases, indicating the fusion of small protuberances, which decreases the active sites to some extent. When the sintering temperature is 700 °C, the protuberances completely disappear, leading to dramatic reduction in the number of active sites. Therefore, with increasing sintering temperature, the number of protuberances gradually decreases, and both Ni3S2 and Ni particles grow and aggregate together (Fig. 2h–k). Especially for Ni3S2–Ni-700-20, due to the high temperature, large particles are tightly connected and almost no pores exist. The corresponding elemental distribution demonstrates that Ni and S are evenly distributed at the low sintering temperature (400–600 °C), while the Ni and Ni3S2 particles tend to aggregate together at 700 °C (Fig. 2l–o). Above all, the morphology, phase, pore structure and surface chemical states of the Ni3S2–Ni-X-Y electrode can be effectively controlled by adjusting the sintering temperature and S content.
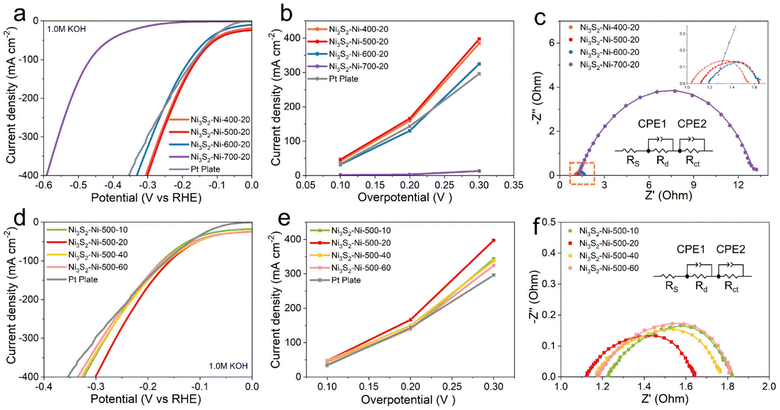
The catalytic HER performances of the different electrodes were tested in a 1.0 M KOH solution using a typical three-electrode system. As shown in Fig. 3a and b, when the current density is 100 mA cm−2, the overpotential of Ni3S2–Ni-400-20, Ni3S2–Ni-500-20, Ni3S2–Ni-600-20, Ni3S2–Ni-700-20 and Pt plate is 162.1, 157.8, 178.5, 465.1 and 165.1 mV, respectively. When the current density is 400 mA cm−2, the overpotential is 306.5, 301.3, 331.8, 1515.4 and 354 mV, respectively. Owing to the inter-connected porous skeleton with metallurgical bonding and the uniform distribution of nano Ni3S2 protuberances, the Ni3S2–Ni-500-20 exhibits superior catalytic performance to other electrodes. Ni3S2–Ni-700-20 displays the worst catalytic performance, which is ascribed to the absence of a porous structure and nano Ni3S2 protuberances. We compared the overpotential of the Ni3S2–Ni-500-20 electrode with those of other previously electrodes at a current density of 100 mA cm−2. The overpotential of the Ni3S2–Ni-500-20 electrode is 157.8 mV, while that of RCFP/NF,40 Fe-doped Ni3S2,34 Co–NiOOH/Ni3S2@NF,41 F, P–Fe3O4/IF,42 and Mo–NiS/Ni3S2-0.08S31 electrodes is 191, 254, 203, 179.5, and 230 mV respectively, confirming the excellent hydrogen evolution activity of the Ni3S2–Ni-500-20 electrode at high current density (Table S2†). To compare the charge transfer ability of different electrodes, the electrochemical impedance spectroscopy (EIS) results of the Ni3S2–Ni-400-20, Ni3S2–Ni-500-20, Ni3S2–Ni-600-20, and Ni3S2–Ni-700-20 electrodes are shown in Fig. 3c. The charge transfer resistances (Rct) of the corresponding equivalent circuits of the Ni3S2–Ni-400-20, Ni3S2–Ni-500-20, Ni3S2–Ni-600-20 and Ni3S2–Ni-700-20 electrodes is 0.1, 0.07, 0.39, and 1.93 Ω, respectively, which are consistent with the LSV results. The changes in Rct of different electrodes can be mainly ascribed to two factors (composition and structure of the electrodes). When the sintering temperature is 400 °C, the metallurgical bonding between the Ni network is insufficient, leading to weak charge transfer ability. When the sintering temperature increases to 600 or 700 °C, Ni particles agglomerate together and the electrode surface is oxidized (Fig. 2c). Especially for Ni3S2–Ni-700-20, nearly no pores exist and oxidation is serious, meanwhile, Ni and Ni3S2 aggregate together (Fig. 2k and o), which is harmful to the charge transfer, thus leading to inferior Rct of Ni3S2–Ni-700-20. When the sintering temperature is 500 °C, excellent charge transfer ability is obtained owing to the inter-connected porous structure and the uniform distribution of nano Ni3S2.
We also explored the effect of electrodes with different S contents (Ni3S2–Ni-500-10, 500–20, 500–40, 500–60) on electrochemical performance. As shown in Fig. 3d, only an appropriate S content (20 mg) is favorable for catalytic performance. Excessive or small S content could decrease the electrochemical performance of the electrodes. As the Ni3S2 plays a pivotal role in facilitating the hydrogen evolution reaction, inadequate S results in the scarcity of the Ni3S2 catalytic phase, thus leading to poor HER activity. However, excessive S content leads to the agglomeration and oversupply of Ni3S2 on the skeleton surface, which is harmful to the charge transfer due to the inherent non-metallic nature of Ni3S2, thus leading to inferior HER. Therefore, only optimized S content is beneficial for the HER. In addition, the influence of different sintering times on the electrochemical performance was also studied. The catalytic performance of the electrode held for 1 h is the best, and the catalytic performance decreases as the sintering time increases, which is due to the agglomeration of the particles (Fig. S4†).
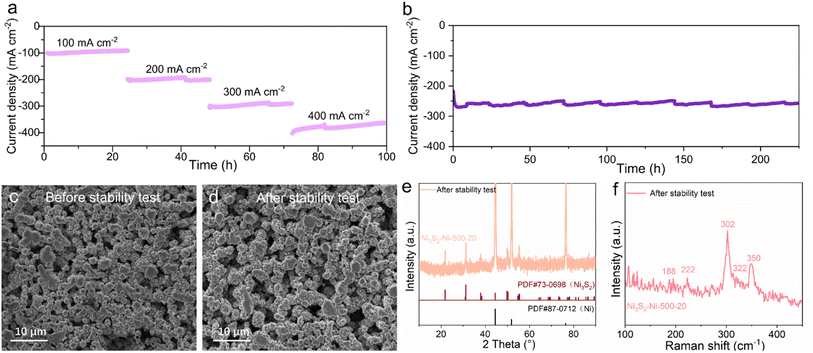
Stability is also an important standard for evaluating the electrochemical performance of electrodes. The stability of Ni3S2–Ni-500-20 at various current densities (100, 200, 300 and 400 mA cm−2) was tested for 24 h (Fig. 4a). Ni3S2–Ni-500-20 shows good stability from 100 to 400 mA cm−2. A long-time stability test was also conducted for Ni3S2–Ni-500-20 at 260 mA cm−2, as shown in Fig. 4b. Due to the consumption of electrolyte in the long-term test, some slight fluctuations were due to the addition of new electrolyte. The current density of the Ni3S2-500-20 electrode remains constant for 225 h, proving the good durability at large current density. SEM, XRD and Raman studies were conducted on the Ni3S2-500-20 electrode after the long-term stability test. The electrode after the stability test shows similar morphology to that of the pristine electrode (Fig. 4c and d). The diffraction peaks of XRD and Raman typical peaks of Ni3S2 exist clearly after continuous HER at large current density (Fig. 4e and f), demonstrating the superior mechanical stability of the electrode. This outstanding long-term stability at high current density makes the Ni3S2–Ni-500-20 electrode promising for successful application in the electrocatalytic hydrogen evolution industry.
Conclusions
In this paper, a porous Ni3S2–Ni self-supported electrode was prepared by a low-cost and high-efficiency one-step powder metallurgy method. Its phase, morphology, composition, and pore structure could be optimized by adjusting the sintering temperature and S content. A large number of nano Ni3S2 protuberances accompanying with the metallurgical bonding conductive network significantly increase the catalytic performance and long-term stability of the Ni3S2–Ni-500-20 electrode. As a consequence, such an electrode reveals high catalytic HER performance with a low overpotential of 157.8 mV at a current density of 100 mA cm−2. Moreover, the electrode can work stably for 225 h at a current density of 260 mA cm−2. This work provides a novel method to develop mechanically stable and highly active self-supporting electrodes for water electrolysis.Author contributions
Yang Zhao and Xiaoqian Shi conducted the electrode preparation and performed the physical characterization. Yang Zhao, Bin Zhang, Xiaoqian Shi, Jiping Ma and Jianbin Lai conceived the idea and carried out all the electrochemical characterization. All the authors discussed the final manuscript.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work is supported by the China Postdoctoral Science Foundation (2022M721029 and 2022M721030), the Key Scientific and Technological Project of Henan Province (No. 23A430019), and the Natural Science Foundation of Henan Province (no. 232300420308).Notes and references
- L. Chen and J. Shi, J. Mater. Chem. A, 2018, 6, 13538–13548 RSC.
- M. Liu, Z. Yao, J. Gu, C. Li, X. Huang, L. Zhang, Z. Huang and M. Fan, Chem. Eng. J., 2023, 461, 141918 CrossRef CAS.
- H. Zhang, T. Luo, Y. Chen, K. Liu, H. Li, E. Pensa, J. Fu, Z. Lin, L. Chai, E. Cortés and M. Liu, Angew. Chem., Int. Ed., 2023, 62, e202305651 CrossRef CAS PubMed.
- F. Dawood, M. Anda and G. M. Shafiullah, Int. J. Hydrogen Energy, 2020, 45, 3847–3869 CrossRef CAS.
- L. Kong, L. Li, G. Cai, C. Liu, P. Ma, Y. Bian and T. Ma, Int. J. Hydrogen Energy, 2021, 46, 2847–2861 CrossRef CAS.
- M. R. Maghami, R. Hassani, C. Gomes, H. Hizam, M. L. Othman and M. Behmanesh, Int. J. Hydrogen Energy, 2020, 45, 1499–1509 CrossRef CAS.
- S. Barua, A. Balciunaite, J. Vaiciunien, L. Tamasauskaite-Tamasiunaite and E. Norkus, Materials, 2022, 15, 215058 CrossRef PubMed.
- P. Nikolaidis and A. Poullikkas, Renewable Sustainable Energy Rev., 2017, 67, 597–611 CrossRef CAS.
- C. Cai, K. Liu, Y. Zhu, P. Li, Q. Wang, B. Liu, S. Chen, H. Li, L. Zhu, H. Li, J. Fu, Y. Chen, E. Pensa, J. Hu, Y. R. Lu, T. S. Chan, E. Cortés and M. Liu, Angew. Chem., Int. Ed., 2021, 61, e202113664 CrossRef PubMed.
- M. Asif, S. Sidra Bibi, S. Ahmed, M. Irshad, M. Shakir Hussain, H. Zeb, M. Kashif Khan and J. Kim, Chem. Eng. J., 2023, 473, 145381 CrossRef CAS.
- M. G. Rasul, M. A. Hazrat, M. A. Sattar, M. I. Jahirul and M. J. Shearer, Energy Convers. Manage., 2022, 272, 116326 CrossRef CAS.
- C. Li, Z. Wang, M. Liu, E. Wang, B. Wang, L. Xu, K. Jiang, S. Fan, Y. Sun, J. Li and K. Liu, Nat. Commun., 2022, 13, 3338 CrossRef PubMed.
- J. Leng, Z. Wang, J. Wang, H.-H. Wu, G. Yan, X. Li, H. Guo, Y. Liu, Q. Zhang and Z. Guo, Chem. Soc. Rev., 2019, 48, 3015–3072 RSC.
- Y. Zhao, S. Wei, L. Xia, K. Pan, B. Zhang, H. Huang, Z. Dong, H.-H. Wu, J. Lin and H. Pang, Chem. Eng. J., 2022, 430, 133040 CrossRef CAS.
- C. Cai, K. Liu, L. Zhang, F. Li, Y. Tan, P. Li, Y. Wang, M. Wang, Z. Feng, D. Motta Meira, W. Qu, A. Stefancu, W. Li, H. Li, J. Fu, H. Wang, D. Zhang, E. Cortés and M. Liu, Angew. Chem., Int. Ed., 2023, 62, e202300873 CrossRef CAS PubMed.
- H. Shi, X.-Y. Sun, S.-P. Zeng, Y. Liu, G.-F. Han, T.-H. Wang, Z. Wen, Q.-R. Fang, X.-Y. Lang and Q. Jiang, Small Struct., 2023, 4, 2300042 CrossRef CAS.
- K. Huang, J. Xia, Y. Lu, B. Zhang, W. Shi, X. Cao, X. Zhang, L. M. Woods, C. Han, C. Chen, T. Wang, J. Wu and Y. Huang, Advanced Science, 2023, 10, 2300094 CrossRef CAS PubMed.
- P. R. Jothi, Y. Zhang, J. P. Scheifers, H. Park and B. P. T. Fokwa, Sustainable Energy Fuels, 2017, 1, 1928–1934 RSC.
- J. Huang, W. Hong, J. Li, B. Wang and W. Liu, Sustainable Energy Fuels, 2020, 4, 1078–1083 RSC.
- S. Chen, S. Wang, P. Hao, M. Li, Y. Zhang, J. Guo, W. Ding, M. Liu, J. Wang and X. Guo, Appl. Catal., B, 2022, 304, 120996 CrossRef CAS.
- Z. Luo, L. Tong, Z. Lin, R. S. Amin, J. Ren, K. M. El-Khatib and C. Wang, Adv. Compos. Hybrid Mater., 2023, 6, 159 CrossRef CAS.
- J. Y. Kim, I. H. Kwak, I. S. Kwon, Q. A. Sial, J. Ihsan, G. M. Zewdie, J. Park and H. S. Kang, J. Mater. Chem. A, 2023, 11, 19619–19628 RSC.
- T. Xiong, J. Li, J. Chandra Roy, M. Koroma, Z. Zhu, H. Yang, L. Zhang, T. Ouyang, M. S. Balogun and M. Al-Mamun, J. Energy Chem., 2023, 81, 71–81 CrossRef CAS.
- T. Gao, K. S. Kumar, Z. Yan, M. Marinova, M. Trentesaux, M. A. Amin, S. Szunerits, Y. Zhou, V. Martin-Diaconescu, S. Paul, R. Boukherroub and V. Ordomsky, J. Mater. Chem. A, 2023, 11, 19338–19348 RSC.
- M. Cao, K. Liu, Y. Song, C. Ma, Y. Lin, H. Li, K. Chen, J. Fu, H. Li, J. Luo, Y. Zhang, X. Zheng, J. Hu and M. Liu, J. Energy Chem., 2022, 72, 125–132 CrossRef CAS.
- F. Hu, D. Yu, M. Ye, H. Wang, Y. Hao, L. Wang, L. Li, X. Han and S. Peng, Adv. Energy Mater., 2022, 12, 2200067 CrossRef CAS.
- J. Xia, K. Dhaka, M. Volokh, G. Peng, Z. Wu, Y. Fu, M. C. Toroker, X. Wang and M. Shalom, Sustainable Energy Fuels, 2019, 3, 2006–2014 RSC.
- M. Yu, X. Guo, X. Chang, X. Ma and M. Zhang, Sustainable Energy Fuels, 2022, 6, 5000–5007 RSC.
- W. He, F. Wang, Y. Gao, Q. Hao and C. Liu, Sustainable Energy Fuels, 2022, 6, 3852–3857 RSC.
- H.-J. Liu, W.-L. Yu, M.-X. Li, S.-Y. Dou, F.-L. Wang, R.-Y. Fan, Y. Ma, Y.-L. Zhou, Y.-M. Chai and B. Dong, Sustainable Energy Fuels, 2021, 5, 3428–3435 RSC.
- K. Zhang, Y. Duan, N. Graham and W. Yu, Appl. Catal., B, 2023, 323, 122144 CrossRef CAS.
- Z. Xu, Q. Chen, Q. Chen, P. Wang, J. Wang, C. Guo, X. Qiu, X. Han and J. Hao, J. Mater. Chem. A, 2022, 10, 24137–24146 RSC.
- L. Zeng, K. Sun, Z. Yang, S. Xie, Y. Chen, Z. Liu, Y. Liu, J. Zhao, Y. Liu and C. Liu, J. Mater. Chem. A, 2018, 6, 4485–4493 RSC.
- D. Li, W. Wan, Z. Wang, H. Wu, S. Wu, T. Jiang, G. Cai, C. Jiang and F. Ren, Adv. Energy Mater., 2022, 12, 2201913 CrossRef CAS.
- H. Zhang, C. Chen, X. Wu, C. Lv, Y. Lv, J. Guo and D. Jia, Small Methods, 2022, 6, 2200483 CrossRef CAS PubMed.
- M. Hu, Y. Qian, S. Yu, Q. Yang, Z. Wang, Y. Huang and L. Li, Small, 2023, 2305948 CrossRef PubMed.
- W. Y. An, H. Lee, S. R. Choi, S. Choi, H.-S. Cho, M. Choi and J.-Y. Park, J. Mater. Chem. A, 2023, 11, 5734–5745 RSC.
- Y. Li, Y. Duan, K. Zhang and W. Yu, Chem. Eng. J., 2022, 433, 134472 CrossRef CAS.
- P. Wang, T. Wang, R. Qin, Z. Pu, C. Zhang, J. Zhu, D. Chen, D. Feng, Z. Kou, S. Mu and J. Wang, Adv. Energy Mater., 2022, 12, 2103359 CrossRef CAS.
- K. Jang, H. Yoon, J. S. Hyoung, D. S. A. Pratama, C. W. Lee and D.-W. Kim, Appl. Catal., B, 2024, 341, 123327 CrossRef CAS.
- Z. Wu, Y. Feng, Z. Qin, X. Han, X. Zheng, Y. Deng and W. Hu, Small, 2022, 18, 2106904 CrossRef CAS PubMed.
- X.-Y. Zhang, F.-T. Li, R.-Y. Fan, J. Zhao, B. Dong, F.-L. Wang, H.-J. Liu, J.-F. Yu, C.-G. Liu and Y.-M. Chai, J. Mater. Chem. A, 2021, 9, 15836–15845 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3se01393f |
| ‡ Yang Zhao and Xiaoqian Shi contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |