Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: The thermodynamics and kinetics of depolymerization: what makes vinyl monomer regeneration feasible?
Victoria
Lohmann
a,
Glen R.
Jones
a,
Nghia P.
Truong
ab and
Athina
Anastasaki
*a
aLaboratory of Polymeric Materials, Department of Materials, ETH Zürich, Vladimir-Prelog-Weg 5, 8093 Zürich, Switzerland. E-mail: athina.anastasaki@mat.ethz.ch
bMonash Institute of Pharmaceutical Sciences, Monash University, 399 Royal Parade, Parkville, VIC 3152, Australia
First published on 30th January 2024
Abstract
Correction for ‘The thermodynamics and kinetics of depolymerization: what makes vinyl monomer regeneration feasible?’ by Victoria Lohmann et al., Chem. Sci., 2024, 15, 832–853, https://doi.org/10.1039/D3SC05143A.
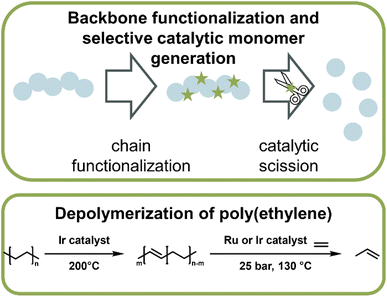
The authors regret the mislabelling of Fig. 4 as Fig. 5 (and vice versa) and Fig. 15 as Fig. 16 (and vice versa), resulting in discrepancies between text references and the figures. The correctly labelled figures are shown below:
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © The Royal Society of Chemistry 2024 |